Abstract
Rotaviruses are the leading etiological agents of acute gastroenteritis in infants and young children worldwide. These nonenveloped viruses enter cells using different types of endocytosis and, depending on the virus strain, travel to different endosomal compartments before exiting to the cytosolic space. In this Gem, we review the viral and cellular factors involved in the different stages of a productive virus cell entry and share with the readers the journey that we have taken into the cell to learn about virus entry.
INTRODUCTION
Rotaviruses (RVs) are nonenveloped viruses, members of the family Reoviridae, and the leading etiological agents of viral gastroenteritis. In vivo, these viruses infect primarily mature enterocytes in the intestinal epithelium. They are composed of a triple-layered protein capsid that surrounds the viral genome. The outermost layer is formed by VP7, which makes the smooth surface of the virus, and by the spike protein VP4, which functions as the virus attachment protein. VP4 is cleaved by trypsin into two subunits, VP8 and VP5, and this cleavage is required for the virus to enter the cell (1).
The initial step in a viral infection is the attachment of the virus to specific receptors on the cell surface, an interaction that frequently triggers cellular signaling cascades that facilitate either virus entry or replication. In most instances, regardless of whether the virus is enveloped or not, virus internalization proceeds through an endocytic pathway that delivers the viral particle to early endosomes (EEs), characterized by the presence of the GTPase Rab5 and early endosomal antigen 1 (EEA1) (2). Some viruses then traffic from these EE compartments to late endosomes (LEs), which are enriched in the GTPase Rab7 (2). The switch from Rab5 to Rab7 occurs via formation of hybrid endosomes that carry both Rab GTPases in separate domains (2) and are known as maturing endosomes (MEs) (3). Some MEs contain intraluminal vesicles (ILVs) that are formed by the endosomal sorting complex required for transport (ESCRT) machinery (4).
The study of RV entry and vesicular traffic has been challenging and its advancement slow because a robust reverse genetic system to manipulate the genome of the virus is lacking. This limitation has been partially overcome, however, by use of RNA interference (RNAi) technology to explore the function of individual viral and cellular genes involved in this process, as well as by determination of the three-dimensional structures of the RV surface proteins.
ATTACHMENT AND POSTATTACHMENT INTERACTIONS
RV cell entry is a multistep process involving cellular glycans for cell binding and several coreceptors during postattachment steps (5) (Fig. 1, step a). The VP8 domain of VP4 mediates the initial interaction of the virus with the cell surface, whereas VP5 and likely the surface glycoprotein VP7 interact with downstream coreceptors (5). Several glycans have been identified as receptors for rotavirus; RV strains were classified initially as neuraminidase (NA) sensitive or NA resistant, depending on their susceptibility to the treatment of cells with NA. Some animal RV strains require sialic acid (SA) for attachment, whereas other animal RV and most human RV strains are NA resistant (5). More recently, RV strains whose infectivity was initially thought to be independent of SA were shown to bind to internal SAs, which are not trimmed by the activity of NAs. In addition, it was recently found that many human RV strains bind human histo-blood group antigen. Some of them have been shown to have an H-type binding specificity, while others bind A-type glycans (6).
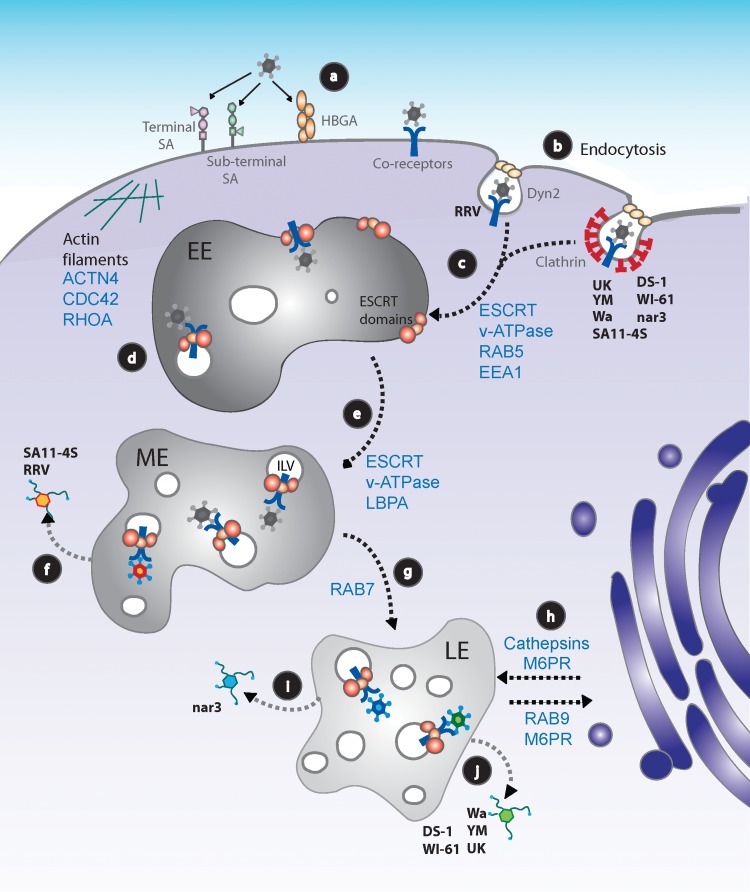
FIG 1.
Working model for rotavirus cell entry into MA104 cells. (a) RVs attach to the cell surface through different glycans, depending on the virus strain. After initial binding, the virus interacts with several coreceptors concentrated at lipid rafts. All known coreceptors are represented as a single blue Y symbol. (b) RVs are internalized into cells by clathrin-dependent or -independent endocytic pathways, depending on the virus strain. (c) Regardless of the endocytic pathway used, all RV strains reach EEs in a process that depends on RAB5, EEA1, and probably on HRS and the vacuolar ATPase (v-ATPase) (11). (d) At the EE, the virus probably begins to be internalized into the endosomal lumen through the action of VPS4A. (e) EEs progress to MEs, with a progressive decrease in pH and intraendosomal calcium concentration through the function of the v-ATPase; during this process the formation of ILVs increases. (f) E-P rotaviruses RRV and SA11-4S reach the cytoplasm from MEs. (g) GTPase Rab7 participates in the formation of LE compartments; ILVs increase in number. (h) The stability and function of LEs depend on the arrival of cellular factors (e.g., cathepsins) from the trans-Golgi network, traffic that is mediated by M6PRs, and the GTPase Rab9, among other factors. (i) L-P RV strains reach late endosomes. RV nar3's exit from LEs requires the function of Rab9. (j) RV strains UK, Wa, WI61, DS-1, and YM require, in addition to Rab9, the function of the CD-M6PR and the activity of cathepsins to productively infect cells. (f, i, j) The cytosolic double-layered particles begin transcribing the RV genome to continue the replication cycle of the virus. The different colors of the viruses represent those strains that exit from MEs (orange) or from LEs that do not require cathepsins (blue) or form LEs, but do require the activity of CD-M6PR and cathepsins (green). HBGA, human histo-blood group antigen; LBPA, lysobisphosphatidic acid.
After initial binding to the cells, RVs have been proposed to interact with integrins α2β1, αVβ3, and αXβ2 and with the heat shock cognate protein hsc70, although the order of interaction and whether these interactions are sequential or alternative are not known. In the particular case of the simian RV strain RRV, it was shown that some of these interactions occur sequentially (5). Not all RV strains require integrins, while all the strains tested require hsc70 for efficient cell infection (7). Ganglioside GM1, integrin subunits α2 and β3, and hsc70 are localized in detergent-resistant membrane domains in MA104 (epithelial monkey kidney) cells, and the integrity of these domains is fundamental for RRV cell entry (5). In addition, it has recently been found that the tight-junction proteins JAM-A, occludin, and ZO-1 are important for cell entry of some RV strains (21). Furthermore, gangliosides were also reported to play a role during virus entry at a postattachment step (8). Whether all the described molecules work in concert or represent alternative routes of entry remains to be determined. It is noteworthy that blocking the interaction of RV with each of the proposed receptors and coreceptors, through the use of either proteases, antibodies, peptides, sugar analogues, competing proteins, or small interfering RNAs (siRNAs), decreases less than 1 log the infectivity of the virus. These findings are puzzling and suggest that either a more relevant entry factor has not been found for RV, the virus has the plasticity to use more than one route of entry, or the entry factors for RV are redundant.
ENDOCYTOSIS
RVs were originally proposed to enter cells via direct penetration at the plasma membrane; however, recent findings have shown that the virus enters cells by endocytosis, and different RV strains use different endocytic pathways (7) (Fig. 1, step b). Most RVs tested, including human (Wa, DS-1, WI69) and animal (UK, YM, SA11-4S, nar3) strains, enter cells by clathrin-mediated endocytosis (7, 9). In contrast, the simian RRV strain follows an endocytic pathway that is independent of clathrin and caveolin but depends on the presence of dynamin 2, the small GTPases RhoA and Cdc42, actinin-4, and cholesterol on the cell surface (10, 11). The requirement for cholesterol and dynamin is also shared by those RVs that are internalized into MA104 cells by clathrin-dependent endocytosis (7), although contradictory results were recently reported in Madin-Darby canine kidney cells (12). Interestingly, the glycan used by RVs to attach to the cell surface does not determine the endocytic pathway employed, since both NA-resistant and -sensitive strains, as well as RVs that interact with blood group antigens, can enter through clathrin-mediated endocytosis (9). Furthermore, recent findings showed that the spike protein VP4 defines the endocytic pathway used (13). In this regard, it is remarkable that a single amino acid substitution (K187R) in VP8 changes the route of entry of RRV from one that is clathrin independent to one that is clathrin dependent (13). The RRV variant bearing this mutation, named nar3, also changes from an NA-sensitive phenotype to an NA-resistant one, and it attaches to the cell surface through an interaction between VP5 and integrin α2β1 (14).
INTRACELLULAR VESICULAR TRAFFIC
Regardless of the nature of the cell surface receptor and the endocytic pathway used for cell internalization (7, 9, 11, 13, 15), all RV strains seem to converge in EEs during cell entry (Fig. 1, step c), since their infectivity depends on the activities of Rab5 and EEA1 (11, 13, 15). Also, all RVs tested seem to require a functional ESCRT system in MA104 as well as in colon carcinoma human Caco-2 cells, since silencing the expression of components of each of the four ESCRT complexes reduces virus infectivity (11) (Fig. 1, step d). ILVs play an important role during virus entry, since viral infectivity is inhibited by siRNAs against VPS4A, the ESCRT-associated ATPase involved in fission of ILVs, as well as by incubation of cells with an antibody that blocks the phospholipid lysobisphosphatidic acid (LBPA), a specific cellular component found in the membranes of ILVs and crucial for ILV formation. An important question that remains to be answered is how the ESCRT machinery is involved in RV cell entry and why the formation of ILVs is required (11). We suggest two possible scenarios. In the first, ILVs may be the site where an RV coreceptor becomes enriched and clustered, and this clustering may be required to prime a conformational change of VP4 to induce disruption of the EE membrane. In the second scenario, the requirement for the ESCRT machinery and the formation of ILVs may result from the need to either attenuate or activate a signaling cascade. Many cellular signaling events are governed by internalization of ligand-activated receptors by endocytosis. However, receptors can still signal in early signaling endosomes. In this case, signal transduction ends when the receptor complex is sequestered by ILVs into endosomes. In contrast, in the case of canonical WNT signal transduction, it has been reported that sequestration of an enzyme from the cytosol inside ILVs activates the signaling pathway (see references in reference 11). Thus, an exciting possibility is that ILV formation is needed for RV entry to regulate a signaling event required for efficient virus replication. In contrast with these observations, a recent report suggested that in BSC-1 cells, RRV enters the cytoplasmic space from endocytic vesicles without reaching EEs (16).
After reaching MEs, RV strains follow different routes to enter the cytoplasm. RV strains RRV and SA11-4S presumably exit from MEs (Fig. 1f), as judged by the fact that their infectivity does not depend on Rab7 (11, 13). On the other hand, the infectivity of all other RV strains tested, including nar3, depends on the expression of this GTPase, suggesting that these viruses continue a deeper journey into the cell to reach LEs (13). This endosomal compartment likely provides the optimal environment for Rab7-dependent strains to enter the cytosol. In this regard, Rab7-dependent RVs behave as late-penetration (L-P) viruses, while simian RV strains RRV and SA11-4S can be considered early-penetration (E-P) viruses (17). Remarkably, as noted above for the role of VP4 in defining the endocytic route, the differential trafficking of the RVs RRV (E-P), UK, and nar3 (L-P) is also determined by the spike protein VP4, and the single amino acid difference in the VP4 protein of nar3 from that of RRV dictates not only the pathway of endocytosis used but also its behavior as an L-P virus (13).
REQUIREMENT FOR CATHEPSINS
Newly synthesized lysosomal acid hydrolases are delivered from the trans-Golgi network to endosomes by mannose-6-phosphate receptors (M6PRs), and recycling of M6PRs back to the Golgi network depends on the small GTPase Rab9 (18) (Fig. 1, step h). Among the hydrolases transported to endosomes/lysosomes from the trans-Golgi network are cysteine cathepsins. RV strains that reach LEs depend on a functional Rab9a GTPase to infect the cell, and all of them, with the exception of nar3, also require the activity of the cation-dependent M6PR (CD-M6PR). In turn, these viruses also require the activity of cysteine cathepsins B, L, and S (13). Endolysosomal cysteine cathepsins are also required for processing the capsid or the surface proteins of other viruses, such as reovirus, Ebola virus, severe acute respiratory syndrome (SARS) coronavirus, and Nipah virus, and these cleavages are necessary for these viruses to be infectious (see references in reference 13). Based on the observation of the role of cathepsin proteases in other virus systems, it is tempting to speculate that these endosomal proteases process one or both RV surface proteins to facilitate the exit of transcriptionally active double-layered particles into the cytoplasm.
EXIT FROM THE ENDOSOMAL COMPARTMENT
During their vesicular trafficking, viruses are exposed to modifications in the endosomal environment, such as a drop in luminal pH, a decrease in calcium concentration, the exchange of membrane components, the formation of additional ILVs, and the acquisition of lysosomal components, among other factors that might induce conformational changes in the viral particle to promote the delivery of the viral genome or nucleocapsid into the cytoplasm (19). Little is known about the mechanism through which RVs exit the endosomal compartments, but several of the mentioned factors might be involved in this process.
It has been reported that preventing endosomal acidification with NH4Cl and other weak bases does not block the infection of RRV, but it reduces the infectivity of RVs UK, Wa, and TFR-41 (7). These findings support the idea that, in contrast to RRV, L-P RVs may require the low pH of LEs to enter the cell. Based on structural cryo-electron microscopy and crystallography data of the VP5 domain of VP4, it has been proposed that to exit the endosomal compartment, the spike protein VP4 undergoes an initial conformational change, triggered by an unknown factor. Calcium decrease in turn promotes the release of the smooth VP7 surface layer in the endosome, which is believed to cause a more drastic rearrangement of VP5 to a fold-back conformation that leads to the interaction of a hydrophobic domain of this VP4 subunit with the endosomal membrane to disrupt it, a process that is followed by escape of the double-layered virus particles into the cytosol to begin transcribing the virus genome (20) (Fig. 1, step f and steps i to j). Thus, it is likely that the vesicular compartments from which different RV strains escape to the cytoplasm provide the specific luminal conditions required for the conformational changes of VP4 to occur. All together, these observations suggest that RV exit from the endosomal compartment depends most likely on a combination of factors, such as the interplay between pH and calcium concentration, as well as on cysteine proteases and possibly other uncharacterized cellular factors.
Despite the recent advances in the knowledge of the entry process of RVs during cell infection, many questions still remain to be answered. Importantly, most studies on RV biology have been carried out in nonpolarized MA104 cells, since these are the most permissive cells for RV replication, but it remains to be determined if all the events observed in cultured cells occur in intestinal enterocytes during a natural infection. Further structural studies of the surface proteins of the virus and their interaction with cellular receptors/coreceptors, virus tracking by live-cell imaging systems coupled with novel superresolution microscopy techniques at a nanoscale dimension, and single-cell studies will be important tools to advance this field. The continuous use of RNAi, the recently described clustered regularly interspaced short palindromic repeat (CRISPR)/CAS9 technology to knock out cellular genes, and eventually the development of a robust reverse genetic system will also be important. Whatever the answers might be, the available data show that RVs are versatile, highly evolved viruses that take advantage of different cellular processes to their benefit.
ACKNOWLEDGMENTS
We apologize to all colleagues whose work could not be referenced because of length restrictions.
Work in our laboratory relevant to this article was supported by grants 153639 and 221019 from the National Council for Science and Technology (Conacyt), Mexico, and grant IG-200114 from DGAPA-UNAM.
We dedicate this article to Pedro Romero, whose technical skills supported many of the findings referenced here and who left us prematurely early this year.
REFERENCES
- 1.Estes MK, Greenberg HB. 2013. Rotaviruses, p 1347–1401 InKnipe DM, Howley PM (ed), Fields virology, vol 2 Wolters Kluwer, Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 2.Stenmark H. 2009. Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol 10:513–525. doi: 10.1038/nrm2728. [DOI] [PubMed] [Google Scholar]
- 3.Mercer J, Schelhaas M, Helenius A. 2010. Virus entry by endocytosis. Annu Rev Biochem 79:803–833. doi: 10.1146/annurev-biochem-060208-104626. [DOI] [PubMed] [Google Scholar]
- 4.Henne WM, Buchkovich NJ, Emr SD. 2011. The ESCRT pathway. Dev Cell 21:77–91. doi: 10.1016/j.devcel.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 5.Lopez S, Arias CF. 2006. Early steps in rotavirus cell entry. Curr Top Microbiol Immunol 309:39–66. [DOI] [PubMed] [Google Scholar]
- 6.Venkataram Prasad BV, Shanker S, Hu L, Choi J M, Crawford SE, Ramani S, Czako R, Atmar RL, Estes MK. 2014. Structural basis of glycan interaction in gastroenteric viral pathogens. Curr Opin Virol 7:119–127. doi: 10.1016/j.coviro.2014.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gutierrez M, Isa P, Sanchez-San Martin C, Perez-Vargas J, Espinosa R, Arias CF, Lopez S. 2010. Different rotavirus strains enter MA104 cells through different endocytic pathways: the role of clathrin-mediated endocytosis. J Virol 84:9161–9169. doi: 10.1128/JVI.00731-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martinez MA, Lopez S, Arias CF, Isa P. 2013. Gangliosides have a functional role during rotavirus cell entry. J Virol 87:1115–1122. doi: 10.1128/JVI.01964-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diaz-Salinas MA, Romero P, Espinosa R, Hoshino Y, Lopez S, Arias CF. 2013. The spike protein VP4 defines the endocytic pathway used by rotavirus to enter MA104 cells. J Virol 87:1658–1663. doi: 10.1128/JVI.02086-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sanchez-San Martin C, Lopez T, Arias CF, Lopez S. 2004. Characterization of rotavirus cell entry. J Virol 78:2310–2318. doi: 10.1128/JVI.78.5.2310-2318.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Silva-Ayala D, Lopez T, Gutierrez M, Perrimon N, Lopez S, Arias CF. 2013. Genome-wide RNAi screen reveals a role for the ESCRT complex in rotavirus cell entry. Proc Natl Acad Sci U S A 110:10270–10275. doi: 10.1073/pnas.1304932110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wolf M, Vo PT, Greenberg HB. 2011. Rhesus rotavirus entry into a polarized epithelium is endocytosis dependent and involves sequential VP4 conformational changes. J Virol 85:2492–2503. doi: 10.1128/JVI.02082-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diaz-Salinas MA, Silva-Ayala D, Lopez S, Arias CF. 2014. Rotaviruses reach late endosomes and require the cation-dependent mannose-6-phosphate receptor and the activity of cathepsin proteases to enter the cell. J Virol 88:4389–4402. doi: 10.1128/JVI.03457-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lopez S, Arias CF. 2004. Multistep entry of rotavirus into cells: a Versaillesque dance. Trends Microbiol 12:271–278. doi: 10.1016/j.tim.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 15.Wolf M, Deal EM, Greenberg HB. 2012. Rhesus rotavirus trafficking during entry into MA104 cells is restricted to the early endosome compartment. J Virol 86:4009–4013. doi: 10.1128/JVI.06667-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abdelhakim AH, Salgado EN, Fu X, Pasham M, Nicastro D, Kirchhausen T, Harrison SC. 2014. Structural correlates of rotavirus cell entry. PLoS Pathog 10:e1004355. doi: 10.1371/journal.ppat.1004355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lozach PY, Huotari J, Helenius A. 2011. Late-penetrating viruses. Curr Opin Virol 1:35–43. doi: 10.1016/j.coviro.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 18.Braulke T, Bonifacino JS. 2009. Sorting of lysosomal proteins. Biochim Biophys Acta 1793:605–614. doi: 10.1016/j.bbamcr.2008.10.016. [DOI] [PubMed] [Google Scholar]
- 19.Tsai B. 2007. Penetration of nonenveloped viruses into the cytoplasm. Annu Rev Cell Dev Biol 23:23–43. doi: 10.1146/annurev.cellbio.23.090506.123454. [DOI] [PubMed] [Google Scholar]
- 20.Settembre EC, Chen JZ, Dormitzer PR, Grigorieff N, Harrison SC. 2011. Atomic model of an infectious rotavirus particle. EMBO J 30:408–416. doi: 10.1038/emboj.2010.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Torres-Flores JM, Silva-Ayala D, Espinoza MA, López S, Arias CF. The tight junction protein JAM-A functions as coreceptor for rotavirus entry into MA104 cells. Virology, in press. [DOI] [PubMed] [Google Scholar]