Abstract
Toll-like receptor 7 and Myd88 are required for antiretroviral antibody and germinal center responses, but whether somatic hypermutation and class-switch recombination are required for antiretroviral immunity has not been examined. Mice deficient in activation-induced cytidine deaminase (AID) resisted Friend virus infection, produced virus-neutralizing antibodies, and controlled viremia. Passive transfer demonstrated that immune IgM from AID-deficient mice contributes to Friend virus control in the presence of virus-specific CD4+ T cells.
TEXT
Susceptibilities to retroviral infections differ within a single host species as exemplified by the presence of slow or rapid progressors and elite controllers among individuals infected with human immunodeficiency virus (1). Friend virus (FV) infection of adult immunocompetent mice is useful to study the genetic, molecular, and cellular bases of resistance against retroviruses (2, 3). FV consists of replication-competent Friend murine leukemia virus (F-MuLV) and defective spleen focus-forming virus (SFFV). In C57BL/6 (B6) mice homozygously possessing the resistant Fv2 allele (4), not only is SFFV-induced proliferation of infected erythroid progenitor cells limited, but they are also actively eliminated by cellular immune responses (5). CD4+ T cells are required for SFFV elimination, while B-lymphocytes are required for F-MuLV elimination (5, 6). FV-susceptible (BALB/c × B6)F1 (CB6F1) mice rapidly develop splenomegaly and succumb to leukemia within 2 months postinfection (pi), but they can be protected against FV by a single immunization with an F-MuLV-encoded CD4+ T cell epitope, Env462–479 (7). This vaccine-induced protection also requires B-lymphocytes, while FV-infected cells are eliminated in the absence of CD8+ T cells (8).
For the production of FV-neutralizing antibodies (Ab), CD11c+ dendritic cells and Myd88 that transduces signals from Toll-like receptors (TLR) are crucial (9). TLR7 senses retroviral entry (10) and inhibits virus replication over the first 5 days of infection through rapid production of nonneutralizing IgM (11). TLR7-deficient mice failed to generate germinal centers and IgM-negative (IgM−) B cells upon FV infection (12), suggesting that immunoglobulin somatic hypermutation (SHM) and class switch recombination (CSR) are associated with neutralizing Ab production and FV control. To examine directly if SHM and CSR are required for FV neutralization, we utilized mice deficient in activation-induced cytidine deaminase (AID), a B cell-specific enzyme required for SHM and CSR (13).
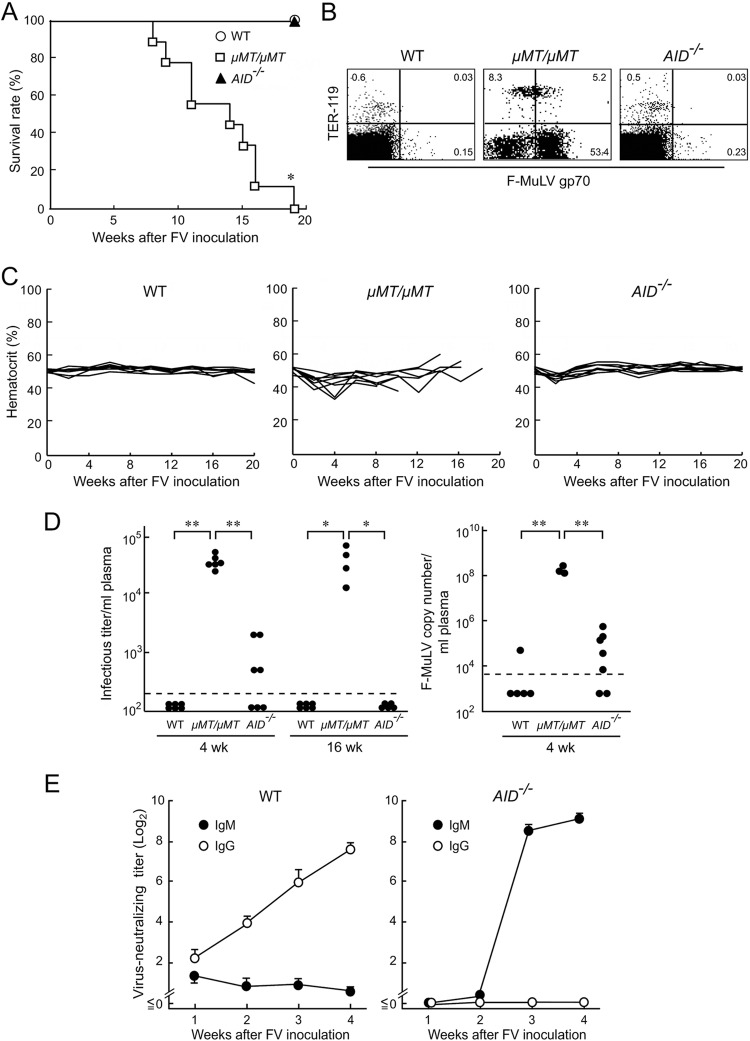
AID-deficient (AID−/−) B6 and BALB/c mice were purchased from Riken BioResource Center, Tsukuba, Japan, and bred and maintained along with B cell-deficient μMT/μMT mice (5). Animal experiments were carried out in accordance with governmental and institutional regulations. To examine if SHM and CSR are required for Fv2-associated resistance, we infected wild-type (WT), μMT/μMT, and AID−/− B6 mice with 5,000 spleen focus-forming units (SFFU) of FV that was free of lactate dehydrogenase-elevating virus (5). All μMT/μMT mice died within 20 weeks pi (Fig. 1A) without developing polycythemia (Fig. 1C), while AID−/− mice remained resistant. F-MuLV gp70 expression on expanding TER-119− cells consistent with the development of F-MuLV-induced myeloid leukemia (5) was evident in μMT/μMT but not in AID−/− mice (Fig. 1B). Thus, AID-mediated SHM and CSR are not required for F-MuLV elimination. Plasma viremia and virus-neutralizing Ab were detected as described previously (14). While μMT/μMT mice remained viremic even at 16 weeks pi, AID−/− mice cleared viremia, albeit less quickly than the WT mice did (Fig. 1D). As virus particles in the plasma might not be detected by the above-described focus-formation assays in the presence of antiviral Ab, viral RNA was extracted from 20 μl of each plasma sample using an RNeasy Plus kit (Qiagen, Valencia, CA) and eluted into 40 μl of RNase-free water, and 11 μl of each eluate was used for cDNA synthesis using the oligo(dT)20 primer and a SuperScript III first-strand synthesis system (Life Technologies, Carlsbad, CA). Real-time PCR assays for the quantification of the F-MuLV genome were performed as described previously (5). At 4 weeks pi, the level of the F-MuLV genome was below the limit of detection in 4 of the 5 infected WT mice, while low levels of the F-MuLV genome were detectable in the plasma samples from infected AID−/− mice (Fig. 1D, right panel). However, the average plasma number of F-MuLV genome was significantly lower in infected AID−/− than in the μMT/μMT mice and was not different from the number seen in the WT mice. Thus, AID−/− B6 mice can control viremia. AID−/− mice possessed high titers of virus-neutralizing Ab at 3 weeks pi (Fig. 1E), clearly indicating that IgM produced in the absence of AID can neutralize F-MuLV.
FIG 1.
AID−/− B6 mice resist FV infection. (A and C) AID−/− (n = 9), μMT/μMT (n = 8), and wild-type (WT, n = 8) B6 mice were inoculated with 5,000 spleen focus-forming units (SFFU) of FV. Their survival was monitored for 20 weeks, and hematocrit values were measured every 2 weeks after FV inoculation as described previously (5). Each line in panel C shows time-dependent changes in hematocrit values in an individual mouse. *, significantly different from the survival curves of two other groups (P < 0.0001) by Mantel-Cox test. (B) Spleen cells were prepared from AID−/−, μMT/μMT, and WT B6 mice at 12 weeks after FV inoculation, stained with F-MuLV gp70-specific monoclonal antibody (MAb) 720 (18) along with anti-TER-119 MAb (BD Biosciences, San Jose, CA), and analyzed by flow cytometry as described previously (5). Representative dot plots are shown. Percentages of cells that were positive for the indicated antigens are shown in each corresponding quadrant. (D) (Left panel) Plasma viral loads detectable as infectious foci at 4 and 16 weeks after FV inoculation were determined as described previously (14). Each closed circle represents the actual value obtained from an individual mouse. The dashed line indicates the limit of detection at 200 focus-forming units (FFU)/ml. One-way analysis of variance (ANOVA) and Tukey's post hoc tests were performed for multiple comparisons (*, P < 0.01; **, P < 0.001). (Right panel) DNA-free viral RNA was extracted from plasma samples and cDNA synthesized. The cDNAs (5 μl each) generated as described above were mixed with 20 μl of a master mixture containing 2× Platinum Quantitative PCR SuperMix-UDG with ROX (Life Technologies), 5 pmol of F-MuLV-specific primers (5), and 2.5 pmol of the TaqMan probe. Standard curves obtained by using a plasmid containing the F-MuLV env gene as a template were linear over the range of 10 to 106 copies. With an equivalent of 1.375 μl plasma per reaction mixture, the limit of detection in this assay was 7,272 copies per ml plasma (dashed line). Each closed circle represents the actual value obtained from an individual mouse. **, P < 0.0001 < α3(0.05) = 0.017 (by Student's t test performed with Bonferroni's correction for multiple comparisons). (E) Changes in serum F-MuLV-neutralizing titers of either the IgM class or IgG class in AID−/− (n = 6) or WT (n = 5) mice after FV inoculation. Mice were bled and neutralizing titers were determined as described previously (8, 14). Each data point shows the mean ± the standard error of the mean (SEM) calculated with values obtained from the five or six mice described above. For panels A, B, D, and E, similar results were obtained in two separate experiments.
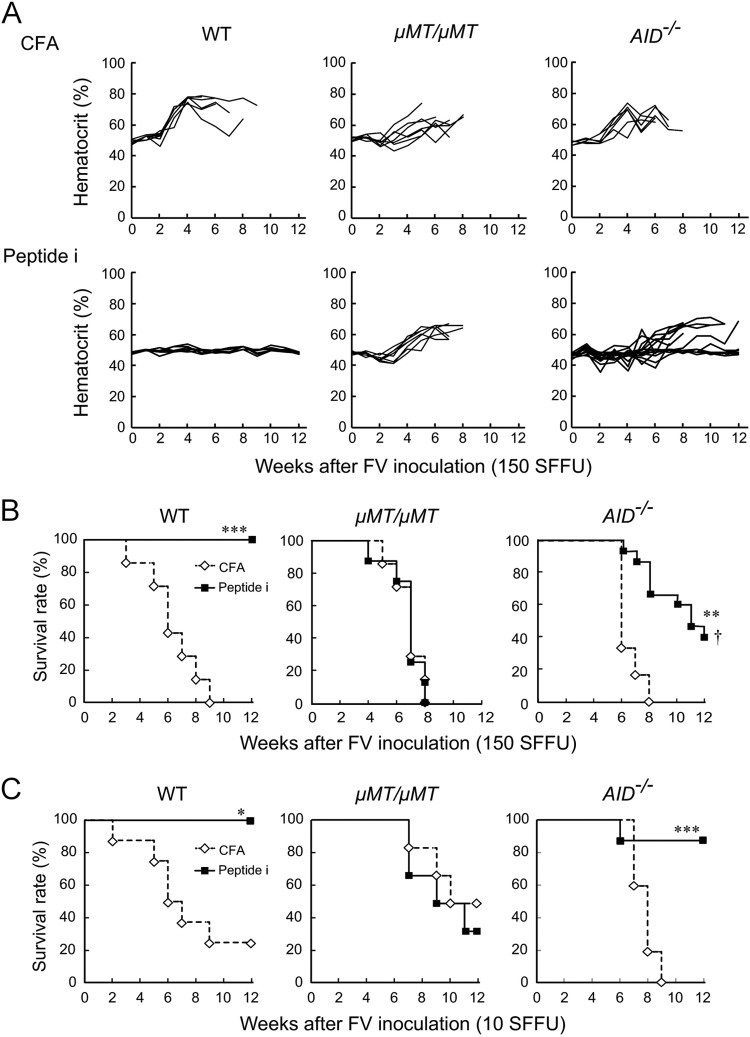
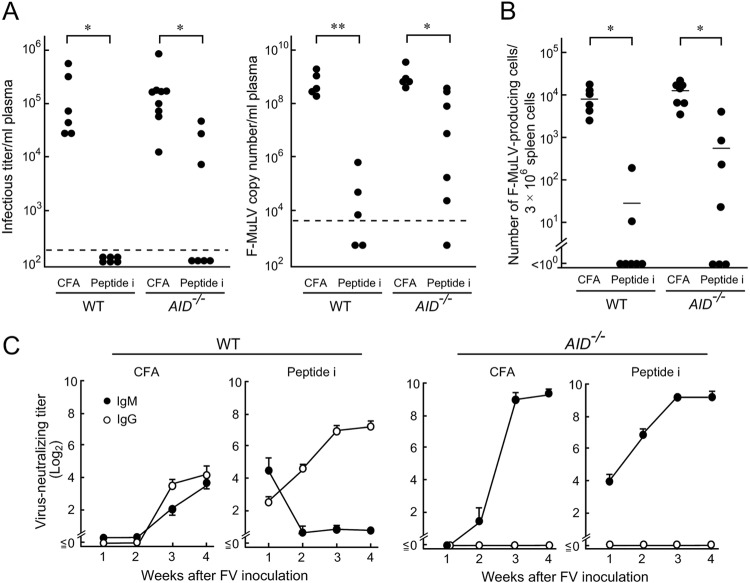
We next examined if SHM and CSR are required for vaccine-induced protection of FV-susceptible mice. As previously described (7, 8), WT CB6F1 mice were 100% protected by a single peptide immunization, while μMT/μMT mice died regardless of the presence or absence of vaccination. About half of the vaccinated AID−/− mice were free from polycythemia and survived past 12 weeks pi (Fig. 2A and B), indicating that SHM and CSR may contribute to but are not absolutely required for peptide-induced protection of CB6F1 mice against FV. To confirm that AID−/− mice can control FV after peptide immunization, we reduced the challenge dose to 10 SFFU, which still killed most of the unimmunized CB6F1 mice by 12 weeks pi (Fig. 2C). While no effect of peptide immunization was observed in μMT/μMT mice, 90% of AID−/− mice were protected against the low-dose challenge. Levels of viremia determined by both fluorescence focus assays and the enumeration of F-MuLV genomic RNAs and numbers of FV infectious centers were all significantly lower in peptide-immunized than in unimmunized animals even in the absence of AID (Fig. 3A and B), and virus-neutralizing IgM became detectable in immunized AID−/− mice as quickly as in the WT mice (Fig. 3C).
FIG 2.
Effects of peptide immunization on FV-induced disease development in CB6F1 mice lacking AID. AID−/−, μMT/μMT, and WT CB6F1 mice were either immunized with 10 μg per mouse of the oligopeptide that harbors the F-MuLV env gene-encoded helper T cell epitope (HPPSYVYSQFEKSYRHKR [Peptide i]) emulsified in complete Freund's adjuvant (CFA) or given CFA alone as described previously (8). Three weeks later, they were challenged with 150 (A and B) or 10 (C) SFFU of FV, and their survival and hematocrit values were monitored for 12 weeks. Each line in panel A shows time-dependent changes in hematocrit values in an individual mouse. Similar results were obtained in two separate experiments. (A and B) The numbers of animals in each group were as follows: for the WT mice, 7 given CFA and 6 given peptide i; for the μMT/μMT mice, 7 CFA and 6 peptide i, and for the AID−/− mice, 6 CFA and 15 peptide i. (C) The numbers of animals in each group were as follows: for the WT mice, 6 CFA and 6 peptide i; for the μMT/μMT mice, 8 CFA and 9 peptide i; and for the AID−/− mice, 5 CFA and 9 peptide i. Statistically significant differences between the peptide-immunized and unimmunized groups were detected by the Mantel-Cox test (*, P < 0.005; **, P < 0.0005; ***, P < 0.0001). †, significant differences in survival between the immunized AID−/− and immunized WT mice by the Mantel-Cox test (P < 0.05).
FIG 3.
Plasma viral loads and FV-neutralizing Ab titers in AID-deficient CB6F1 mice immunized with peptide i. AID−/− and WT CB6F1 mice were either immunized with 10 μg per mouse of peptide i plus CFA or given CFA alone and were challenged with 150 SFFU of FV 3 weeks later. (A and B) Plasma viral loads detectable as infectious foci and as F-MuLV RNA copy numbers and the numbers of F-MuLV infectious centers in the spleen were determined at 2 weeks after FV inoculation as described for Fig. 1 and elsewhere (5, 8). Each closed circle represents the actual value obtained from an individual mouse, and the horizontal bars in panel B indicate the mean values for each group. The dashed lines in panel A indicate the limit of detection at 200 FFU/ml or 7,272 copies per ml plasma. Significant differences were detected by Mann-Whitney or Wilcoxon signed-rank tests for non-Gaussian distributions or by Welch's t test for Gaussian distributions (*, P < 0.05; **, P < 10−5). (C) Serum titers of virus-neutralizing IgM or IgG were measured at the indicated time points for a period of 4 weeks after FV inoculation as described previously (8, 14). Each data point shows the mean ± SEM calculated with data from five to six mice per group.
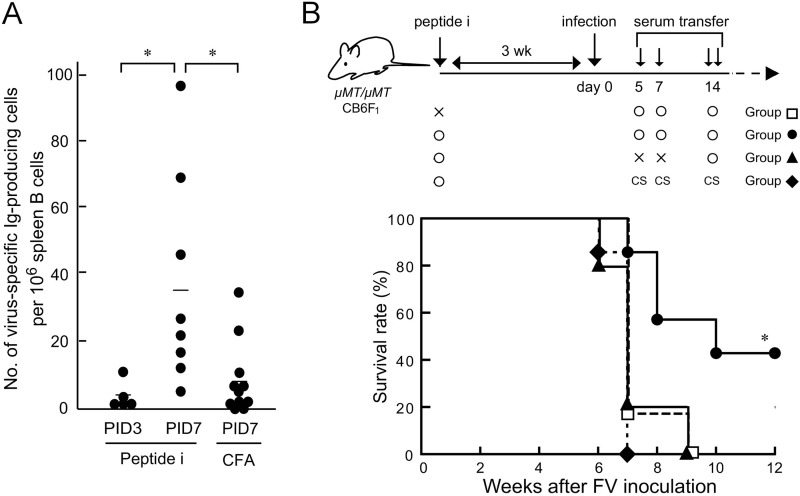
To examine directly if IgM produced in the absence of AID can contribute to FV control, passive transfer was attempted. AID−/− CB6F1 mice were subjected to peptide immunization and challenged with FV, and sera were collected at 3 weeks pi and subjected to heat inactivation (14). To reproduce Ab production in infected mice, we examined the kinetics of anti-FV Ab production. F-MuLV particles were purified (15) and adsorbed onto a nitrocellulose membrane (Multiscreen-HA; Millipore, Bedford, MA). A serially diluted spleen cell suspension was added to the membrane-bottomed wells and incubated for 18 h, followed by Ab detection with horseradish peroxidase-conjugated anti-mouse IgM (SouthernBiotech, Birmingham, AL) and 3-amino-9-ethylcarbazole. In peptide-immunized AID−/− mice, cells producing anti-F-MuLV IgM were detected at 3 days pi, and the cell numbers became significantly higher than those in unimmunized mice at 7 days pi (Fig. 4A). F-MuLV-neutralizing serum Ab started to be detected in peptide-immunized AID−/− mice at 6 days pi with an average titer of 22.5 (n = 5). Therefore, we started injecting B cell-deficient CB6F1 mice with the pooled sera on day 5 pi (Fig. 4B). Prior to serum transfer, recipients were either immunized with the peptide or given adjuvant alone and challenged with 150 SFFU FV 3 weeks later. Peptide-immunized μMT/μMT recipient mice given immune sera from the WT mice possessed undetectable or very low levels of FV infectious centers and normal spleen sizes at 4 weeks pi, indicating the efficacy of the adoptive immunization described above (data not shown). Immunized mice given naive sera all died by 7 weeks pi, while a significantly higher proportion of those given the immune sera from AID−/− mice survived past 12 weeks pi (Fig. 4B), reproducing the partial protection of immunized AID−/− mice (Fig. 2B). A previous report demonstrated the requirement of host T cells for passive immunotherapy with FV-neutralizing monoclonal IgG (16). Similarly, no protection was observed when the recipients were not previously immunized. Serum transfer on day 14 pi alone was ineffective.
FIG 4.
Passive transfer of IgM produced in the absence of AID into B cell-deficient mice. (A) AID−/− CB6F1 mice were either immunized with peptide i or given CFA alone and were infected with FV as described for Fig. 2. The numbers of cells producing FV-specific IgM in spleens at 3 days after FV infection (PID3) and 7 days after FV infection (PID7) were determined by enzyme-linked immunospot assays. Each closed circle represents the actual value obtained from an individual mouse, and horizontal bars indicate the mean values for each group. *, significantly different (P < 0.05) by Welch's t test. (B) Sera were collected at 3 weeks after infection from AID−/− CB6F1 mice that had been immunized with peptide i and challenged with 150 SFFU of FV 3 weeks later. The pooled immune sera showed F-MuLV-neutralizing titers of 28 to 29. B cell-deficient μMT/μMT mice were either immunized with peptide i or given CFA alone and inoculated with 150 SFFU of FV 3 weeks later (day 0). Infected μMT/μMT mice were given 125 μl of the pooled immune sera described above once intravenously on day 5, 500 μl once intraperitoneally (i.p.) on day 7, and 500 μl twice i.p. in the morning and evening of day 14 after FV infection. Control mice were given sera from unimmunized and uninfected AID−/− CB6F1 mice (control sera [CS]) on the same schedule. A separate group of mice received only the immune sera on day 14. Survival was monitored for 12 weeks after FV infection. Similar data were obtained in two separate experiments. *, significantly different from the survival curves of three other groups (P < 0.01) by the Mantel-Cox test.
As a recent report (17) has indicated that an apolipoprotein B mRNA editing enzyme, catalytic polypeptide 3 ([APOBEC3]), expressed in B6 mice can complement AID in generating SHM and as we performed the present study in the presence of B6-derived APOBEC3, the IgM Ab we detected in AID−/− mice might not necessarily have been unmutated, although whether APOBEC3 alone induces significant SHM in the absence of AID has not been examined. Nevertheless, it is now clear that, although TLR7-Myd88 signaling is required for anti-FV Ab production and germinal center responses (9–12), AID-mediated SHM and CSR are not absolutely required, and IgM produced in the absence of AID can still contribute to spontaneous and vaccine-induced resistance to FV infection.
ACKNOWLEDGMENTS
Animal experiments and flow cytometry were supported by members of the Central Research Facilities, Kinki University Faculty of Medicine. AID-deficient B6 (BRC 00897) and BALB/c (BRC 00901) mice were kindly provided by Tasuku Honjo through the Riken BioResource Center with the support of the National BioResource Project of the Ministry of Education, Culture, Sports, Science and Technology of Japan (MEXT). We thank J. B. Dowell for critical reading and correction of the manuscript.
This work was supported in part by Grants-in-Aid for Scientific Research (KAKENHI and the Anti-aging Center Project) from MEXT and those from the Ministry of Health, Labor and Welfare of Japan for research on HIV/AIDS.
REFERENCES
- 1.Miyazawa M, Lopalco L, Mazzotta F, Lo Caputo S, Veas F, Clerici M. 2009. The “immunologic advantage” of HIV-exposed seronegative individuals. AIDS 23:161–175. doi: 10.1097/QAD.0b013e3283196a80. [DOI] [PubMed] [Google Scholar]
- 2.Chesebro B, Miyazawa M, Britt WJ. 1990. Host genetic control of spontaneous and induced immunity to Friend murine retrovirus infection. Annu Rev Immunol 8:477–499. doi: 10.1146/annurev.iy.08.040190.002401. [DOI] [PubMed] [Google Scholar]
- 3.Miyazawa M, Tsuji-Kawahara S, Kanari Y. 2008. Host genetic factors that control immune responses to retrovirus infections. Vaccine 26:2981–2996. doi: 10.1016/j.vaccine.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 4.Persons DA, Paulson RF, Loyd MR, Herley MT, Bodner SM, Bernstein A, Correll PH, Ney PA. 1999. Fv2 encodes a truncated form of the Stk receptor tyrosine kinase. Nat Genet 23:159–165. doi: 10.1038/13787. [DOI] [PubMed] [Google Scholar]
- 5.Tsuji-Kawahara S, Kawabata H, Matsukuma H, Kinoshita S, Chikaishi T, Sakamoto M, Kawasaki Y, Miyazawa M. 2013. Differential requirements of cellular and humoral immune responses for Fv2-associated resistance to erythroleukemia and for the regulation of retrovirus-induced myeloid leukemia development. J Virol 87:13760–13774. doi: 10.1128/JVI.02506-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsuji-Kawahara S, Miyazawa M. 2014. Elimination of Friend retrovirus in the absence of CD8+ T cells. J Virol 88:1854–1855. doi: 10.1128/JVI.03271-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miyazawa M, Fujisawa R, Ishihara C, Takei YA, Shimizu T, Uenishi H, Yamagishi H, Kuribayashi K. 1995. Immunization with a single T helper cell epitope abrogates Friend virus-induced early erythroid proliferation and prevents late leukemia development. J Immunol 155:748–758. [PubMed] [Google Scholar]
- 8.Kawabata H, Niwa A, Tsuji-Kawahara S, Uenishi H, Iwanami N, Matsukuma H, Abe H, Tabata N, Matsumura H, Miyazawa M. 2006. Peptide-induced immune protection of CD8+ T cell-deficient mice against Friend retrovirus-induced disease. Int Immunol 18:183–198. doi: 10.1093/intimm/dxh361. [DOI] [PubMed] [Google Scholar]
- 9.Browne EP, Littman DR. 2009. Myd88 is required for an antibody response to retroviral infection. PLoS Pathog 5:e1000298. doi: 10.1371/journal.ppat.1000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kane M, Case LK, Wang C, Yurkovetskiy L, Dikiy S, Golovkina TV. 2011. Innate immune sensing of retroviral infection via Toll-like receptor 7 occurs upon viral entry. Immunity 35:135–145. doi: 10.1016/j.immuni.2011.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Browne EP. 2013. Toll-like receptor 7 inhibits early acute retroviral infection through rapid lymphocyte responses. J Virol 87:7357–7366. doi: 10.1128/JVI.00788-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Browne EP. 2011. Toll-like receptor 7 controls the anti-retroviral germinal center response. PLoS Pathog 7:e1002293. doi: 10.1371/journal.ppat.1002293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Muramatsu M, Kinoshita K, Fagarasan S, Yamada S, Shinkai Y, Honjo T. 2000. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell 102:553–563. doi: 10.1016/S0092-8674(00)00078-7. [DOI] [PubMed] [Google Scholar]
- 14.Tsuji-Kawahara S, Chikaishi T, Takeda E, Kato M, Kinoshita S, Kajiwara E, Takamura S, Miyazawa M. 2010. Persistence of viremia and production of neutralizing antibodies differentially regulated by polymorphic APOBEC3 and BAFF-R loci in Friend virus-infected mice. J Virol 84:6082–6095. doi: 10.1128/JVI.02516-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ishihara C, Miyazawa Nishio MJ, Chesebro B. 1991. Induction of protective immunity to Friend murine leukemia virus in genetic nonresponders to virus envelope protein. J Immunol 146:3958–3963. [PubMed] [Google Scholar]
- 16.Hasenkrug KJ, Brooks DM, Chesebro B. 1995. Passive immunotherapy for retroviral disease: influence of major histocompatibility complex type and T-cell responsiveness. Proc Natl Acad Sci U S A 92:10492–10495. doi: 10.1073/pnas.92.23.10492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Halemano K, Guo K, Heilman KJ, Barrett BS, Smith DS, Hasenkrug KJ, Santiago ML. 2014. Immunoglobulin somatic hypermutation by APOBEC3/Rfv3 during retroviral infection. Proc Natl Acad Sci U S A 111:7759–7764. doi: 10.1073/pnas.1403361111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robertson MN, Miyazawa M, Mori S, Caughey B, Evans LH, Hayes SF, Chesebro B. 1991. Production of monoclonal antibodies reactive with a denatured form of the Friend murine leukemia virus gp70 envelope protein: use in a focal infectivity assay, immunohistochemical studies, electron microscopy and Western blotting. J Virol Methods 34:255–271. doi: 10.1016/0166-0934(91)90105-9. [DOI] [PubMed] [Google Scholar]