Abstract
Basophilia is an established prognostic variable in Ph-chromosome+ chronic myeloid leukemia (CML). However, in CML, basophils are often immature and thus escape microscopic quantification. We have previously shown that tryptase is produced and secreted by immature CML basophils. In the current study, serum samples of 79 CML patients (chronic phase=CP, n=69; accelerated/blast phase=AP/BP, n=10) treated with BCR/ABL inhibitors, were analyzed for their tryptase content. Serum-tryptase levels at diagnosis were found to correlate with basophil counts and were higher in AP/BP patients (median tryptase: 29.9 ng/mL) compared to patients with CP (11.7 ng/mL; p<0.05). In 20/69 patients with CP, progression occurred. The progression-rate was higher in patients with tryptase >15 ng/mL (31%) compared to those with normal tryptase levels (9%, p<0.05). To validate tryptase as new prognostic variable, we replaced basophils by tryptase levels in the EUTOS score. This modified EUTOS-T score was found to predict progression-free and event-free survival significantly better, with p values of 0.000064 and 0.00369, respectively, compared to the original EUTOS score (progression-free survival: p=0.019; event-free survival: p=0.156). In conclusion, our data show that the serum-tryptase level at diagnosis is a powerful prognostic biomarker in CML. Inclusion of tryptase in prognostic CML scores may improve their predictive value.
Keywords: CML, tryptase, survival, scoring system, prognostication
Introduction
The BCR/ABL tyrosine kinase inhibitors (TKI) have substantially improved the prognosis of patients with chronic myeloid leukemia (CML). These drugs produce major cytogenetic (MaCyR) and molecular (MMR) responses in a majority of patients with chronic phase (CP) CML [1-9]. Imatinib remains the golden standard of first-line treatment in CML. However, a number of patients fail to achieve a durable MaCyR during imatinib [10-12]. In these patients, second- or third generation TKI can be administered [12,13]. Moreover, for young and fit patients stem cell transplantation (SCT) has to be considered. However, SCT is associated with considerable morbidity and mortality and several of the new TKI have major side effects. Therefore, it is of importance to identify ‘high risk patients’ who may benefit from upfront-use of second-line TKI, SCT or from an early switch from first- to second-line drugs.
Several scoring systems have been developed in order to predict the response to therapy and survival in CML, including the Sokal and Hasford score [14,15]. Although both scores were established based on data from patients treated with hydroxyurea, busulfan or interferon-alpha, recent studies have shown that these scores are also predictive in patients treated with imatinib [16,17]. Likewise, the Sokal score is of prognostic value in patients who receive imatinib after interferon-alpha or a 2nd-line TKI after imatinib [18,19]. The newly developed EUTOS score is the first scoring system that has been established in patients receiving TKI [20]. Basophilia is one of the most significant and well established risk factors in CML and was therefore included in both, the Hasford score and in the EUTOS score [14,20]. However, the total basophil-compartment of the CML clone often exceeds the morphologically identifiable fraction of basophils which is a critical point because basophilia is employed as key prognostic parameter in the above mentioned scores.
In order to overcome this problem, we screened for potential markers of immature basophils. Tryptase is a serine protease primarily expressed by mast cells and immature basophils. We have previously shown that immature CML basophils express and release tryptase and that elevated serum-tryptase levels are found in a subset of patients with CML at diagnosis [21-23]. However, so far, only little is known about the prognostic value of tryptase in patients with CML. In the present study, we provide evidence that the serum tryptase level is a highly prognostic biomarker in freshly diagnosed patients with CML.
Patients and methods
Patients
Seventy-nine patients with Ph+ CML seen at our institution who received first line treatment with TKI were examined retrospectively. Sixty-nine had CML-CP, 9 accelerated phase (AP) CML and one blast phase (BP) CML. Seventy-six patients received imatinib (400 mg/day, n=74 or 600 mg/day, n=2), two CP-patients received dasatinib (100 mg/day) and one nilotinib (600 mg/day). Parameters recorded at diagnosis included complete blood counts (CBC) and differential counts, bone marrow (bm) morphology and histology, karyotyping according to standard techniques [24], BCR-ABL transcript levels, and serum-tryptase levels. The patients’ characteristics are shown in Supplemental Table S1. In all patients, the Hasford score, Sokal score, and EUTOS score, were calculated. Follow-up investigations were based on the recommendations of the European-Leukemia-Net (ELN) [25,26]. If necessary, FISH and/or BCR/ABL mutation analyses were performed. All patients gave written informed consent before blood donation and bm puncture. The study was approved by the Local Ethics Committee of the Medical University of Vienna.
Tryptase measurements
Serum-tryptase levels were measured by a commercial fluoroenzyme-immunoassay (FIA, Thermo Fisher Scientific, Uppsala, Sweden) as described [21-23]. In all CML patients, serum tryptase levels were measured prior to therapy.
qPCR of BCR/ABL and tryptase mRNA levels in peripheral blood (pb) cells
BCR-ABL transcripts were quantified by real-time qPCR according to standard methods using the Ipsogen BCR-ABL Mbcr Kit (Quiagen, Hilden, Germany) and the LightCycler 2.0-System (Roche, Mannheim, Germany) [27]. BCR/ABL levels were expressed as percent of ABL after adjusting PCR data according to the international scale (IS) [28]. In a subgroup of our CML patients (CP, n=36; AP, n=7), tryptase mRNA levels were determined. Tryptase mRNA expression was normalized to ABL copy numbers. Detailed information is provided in the Supplemental materials.
Statistical analyses
Differences between patients’ groups according to various risk-factors and scores were calculated by Kruskal-Wallis or Mann-U-test. To weigh different overlapping prognostic variables, a canonical analysis of the relationship of these parameters was applied. Progression was defined as hematologic, cytogenetic (reappearance of BCR/ABL) or molecular (>1 log increase after major molecular response) relapse, or appearance of additional chromosomal abnormalities in BCR/ABL-positive or BCR/ABL-negative cells according to ELN guidelines [26]. Detailed information is provided in the Supplemental materials.
Establisment of the EUTOS-T score
Based on the correlation between basophils and tryptase and the superior prognostic value of tryptase levels, we established a new EUTOS score system in which basophil counts were replaced by tryptase levels. Like in the EUTOS score, spleen size was measured in centimeters below the costal margin. Spleen size and tryptase were weighed. According to this calculation the formula of the EUTOS Score was adapted as follows: serum-tryptase (ng/ml) + 5* spleen size. The cutoff for this EUTOS-T score was determined by the relationship between the score and the zero residuals [29]. A score of 80 points or less indicated low risk and more than 80 points high risk disease.
Results
Survival in TKI-treated patients with CML
The median follow up period was 3.5 years (range 0.04 to 12.76 years). OS in all patients was 67% at 5 years, the PFS reached a plateau at 75% after 3.6 years. In CML-CP patients, the OS was 88% after 5 years and the PFS reached a plateau at 75% after 3.6 years. When applying the EUTOS score in our CML-CP patients, significant differences among the two groups were found for PFS (p=0.019; Figure 1), but not for OS (p>0.05). We also confirmed the prognostic value of the Sokal score (PFS p=0.042; OS p=0.012) and the Hasford score (PFS, p=0.042; OS, p=0.050).
Figure 1.
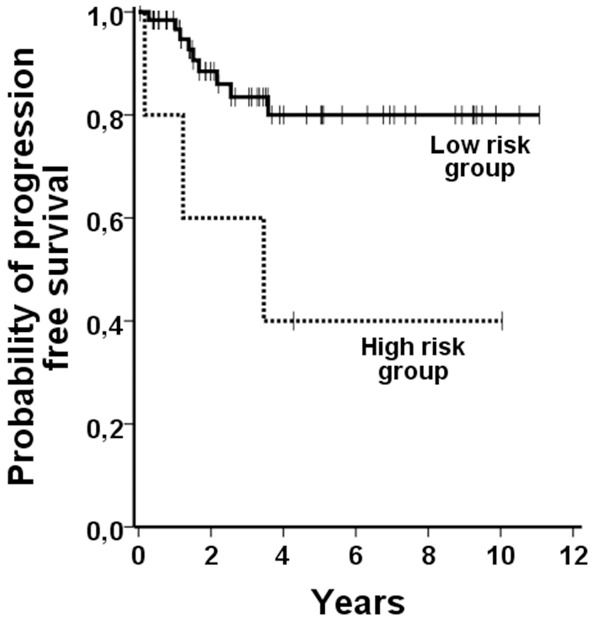
Survival according the EUTOS sore. When EUTOS low and high risk patients were compared with regard to PFS, the differences observed were found to be statistically significant.
Serum-tryptase levels in various phases of CML
The median serum-tryptase level in all patients was 12.5 ng/mL (range: 1.4-67.7 ng/mL). In 33 patients (41.8%), elevated serum-tryptase levels (i.e. >15 ng/mL) were detected, and 46 (58.2%) patients presented with normal enzyme levels. Significantly higher median tryptase levels were recorded in patients with advanced CML (CML-AP/CML-BP: 29.9 ng/mL; range 8.1-67.7) compared to patients with CML-CP (11.7 ng/mL, range 1.4-65.5; p<0.05; Supplemental Figure 1A). Serum-tryptase levels >15 ng/mL were found in 38% of CP-patients and in 70% of advanced CML patients. Significant differences in tryptase levels were also found when comparing prognostic risk groups of the Sokal (low, 6.3 ng/mL; intermediate, 10.7 ng/mL; high, 24.7 ng/mL), Hasford (low, 8.5 ng/mL; intermediate, 14.7 ng/mL; high 20.2 ng/mL), and EUTOS Score (low, 10.3 ng/mL; high, 33.2 ng/mL; p<0.05; Supplemental Figure 1B-D).
Identification of serum-tryptase as independent prognostic variable in CML
Twenty-six of our CML-CP patients (38%) had elevated serum-typtase levels, and 43 (62%) normal enzyme levels. In the cohort with high-tryptase, 30.8% (8/26) of the patients had a disease progression compared to 9.3% (4/43) in the normal serum-tryptase group (p<0.05). The 5-year PFS was 86% and 61% in the low and high tryptase group, respectively (Figure 2A). Marked differences were also seen when calculating EFS. Here, an event occurred in 42.3% (11/26) of patients with elevated serum-tryptase and 18.6% (8/43) of patients with normal tryptase. The 5-year EFS rates were 75% and 54% in the low and high tryptase group, respectively (Figure 2B; p=0.052). No significant differences in OS were found (p>0.05). Of the 11 patients (median age 75 years at diagnosis) who died during the observational period, six patients were in the high-tryptase group and five in the low-tryptase group. The 5-year OS rates were 87% for both the tryptase ≤15 ng/mL and the tryptase >15 ng/mL group (Figure 2C).
Figure 2.
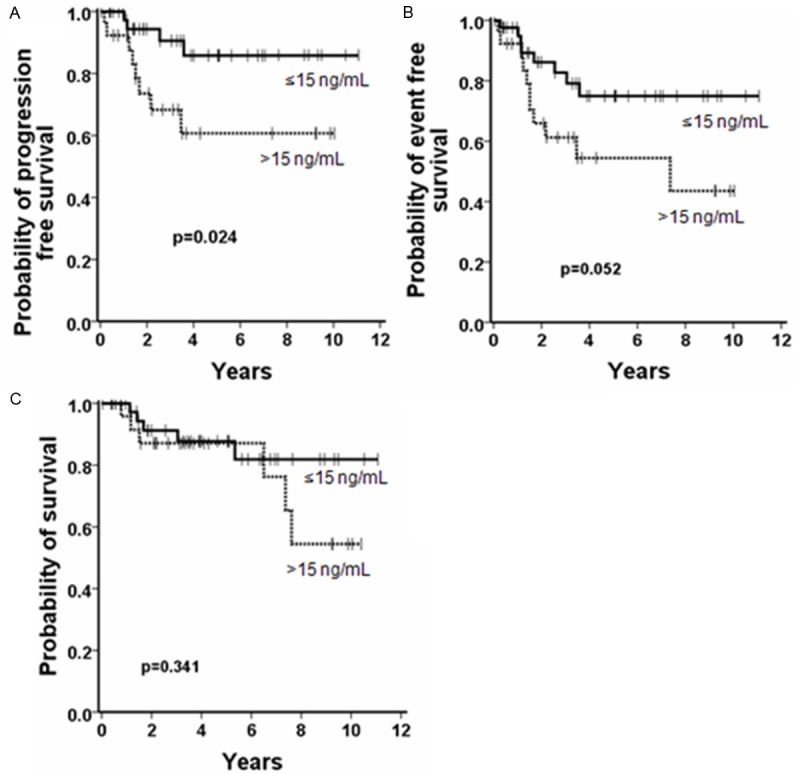
Influence of serum-tryptase levels on the survival in patients with CP-CML. Patients with normal serum-tryptase levels (≤15 ng/mL; solid line) and patients with elevated serum-tryptase levels (>15 ng/mL, dashed line) were compared. Significant differences in PFS were found (A; p<0.05). With regard to the EFS a markedly difference was found but the p value was 0.052 (B). Differences in OS rates were not statistically significant (C).
Comparison of tryptase with other prognostic variables
We found a positive correlation between serum-tryptase levels and other prognostic variables including basophils, peripheral blast cell counts, and bm blasts in CML-CP. Canonical analysis of the relationship of these parameters and PFS showed that tryptase had the highest weight. Multivariate analysis, including other pb-parameters i.e. white blood cell counts, platelet counts, percentage of eosinophils, and hemoglobin together with tryptase showed, that tryptase was an independent prognostic variable with regard to PFS.
We also measured tryptase mRNA levels in circulating leukocytes of 43 patients (CP, n=36; AP, n=7). In all CML patients tested pb-cells expressed tryptase mRNA. As expected, tryptase mRNA levels varied from patient to patient. As expected, tryptase mRNA levels correlated with serum-tryptase levels as assessed by Pearson correlation (r=0.56; p<0.001) (Supplemental Figure 2).
Transcript levels of BCR/ABL were also measured in the follow up and compared to tryptase levels. In both groups of patients (those with elevated and those with normal tryptase), a marked decrease in BCR/ABL was observed over time. However, in the group of patients with tryptase levels ≤15 ng/ml, the decrease of BCR/ABL was faster during the first year when compared to patients with serum-tryptase levels >15 ng/ml. These differences in the BCR/ABL transcript levels in the two groups of patients were found to be statistically significant at 6, 9 and 12 months (Figure 3).
Figure 3.
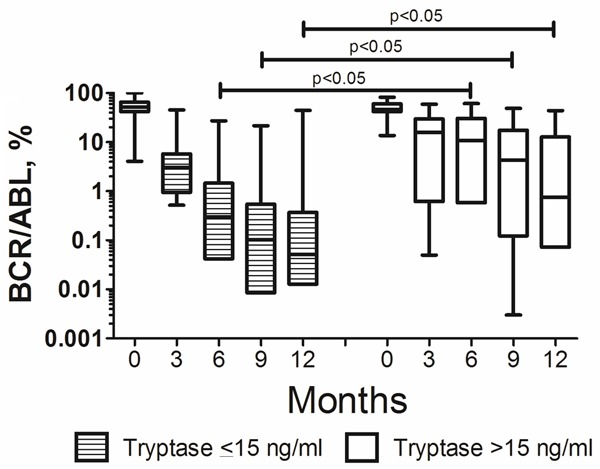
Follow up of bcr/abl levels in patients with normal and elevated serum tryptase levels. The bcr/abl levels decresed markedly in both, patients with normal (filled boxes) and elevated serum tryptase levels (empty boxes). However, The differences between the two groups with regard to transcript levels of bcr/abl observed at 6, 9 and 12 months were found to be statistically significant (p<0.05).
A proposed score including tryptase as prognostic variable: EUTOS-T
To evaluate whether the prognostic value of the EUTOS score in CML-CP would increase by including tryptase, we replaced the percentage of basophils by serum-tryptase levels. Using this modified EUTOS-T score, 61 patients with CML in CP (88.4%) were classified as low risk and 8 patients (11.6%) were considered high risk. Significant differences were found between the two risk groups in both, the PFS and EFS (p<0.0001; Figure 4). The 5-year PFS rates were 84% and 0% for the low and high risk group, respectively. Of all patients, 11.5% (8/61) of the low risk group and 62.5% (5/8) of the high risk group progressed. EFS rates at 5 years were 75% and 0% in low and high risk patients, respectively. Of all patients with 21.3% (13/61) in the low risk group developed an event and 75% (6/8) in the high risk group. No significant difference was found between these two groups with regard to OS.
Figure 4.
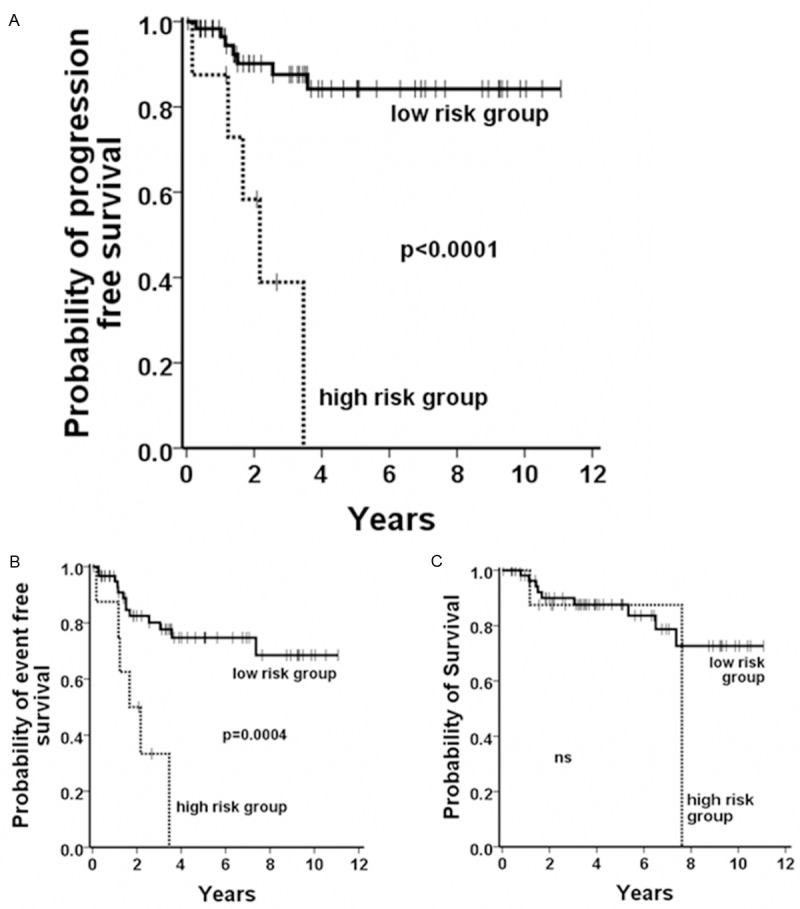
Survival according the EUTOS-T sore. When EUTOS-T low and high risk patients were compared with regard to PFS (A), and EFS (B) the differences observed were found to be statistically significant. No differences could be observed calculating the probability of OS (C).
Discussion
The BCR/ABL TKI have significantly improved the survival in patients with CML. The rate of complete cytogenetic response increased from 30% (with interferon-alpha) to >70% with imatinib [1-8,30,31]. However, still several patients are unresponsive to imatinib or relapse. Recently, second- and third-generation TKI have been developed and have shown beneficial results in untreated and imatinib-resistant CML [6,7]. However, due to their side effects, these novel TKI cannot be offered to all patients. Currently available guidelines suggest that these drugs should be reserved for those who are at high risk for disease-progression [10-12]. For patients who are resistant to novel TKI or present with BP, hematopoietic SCT remains a treatment option [32,33]. Whereas in advanced CML it is straightforward to offer SCT, the treatment algorithm in CP is more complex. Although several scores have been established in the past, it is difficult to estimate the exact risk of progression during TKI therapy.
Basophilia is one of the most powerful predictive markers in CML [14,20]. However, the identification and quantification of immature basophils may be difficult, since the cells often exhibit little or no granulation. Tryptase, a serine protease expressed by mast cells and immature basophils has been suggested as potential marker of the total basophil burden in CML. In contrast to mature basophils, immature (CML) basophils are able to synthesize and to release substantial amounts of tryptase [21]. Indeed, elevated tryptase levels were found in CML [22] which was confirmed in the current study. Moreover, pb leukocytes in CML expressed substantial amounts of tryptase mRNA. Other blood leukocytes do not express tryptase under physiologic conditions [34-35]. However, immature myeloid cells can produce substantial amounts of tryptase and the enzyme is elevated in patients with acute myeloid leukemia, myelodysplastic syndromes and chronic eosinophilic leukemia [22,35-38].
Elevated serum-tryptase levels were detected more frequently in patients with advanced phase CML compared to patients with CP CML. The fact, that an elevated tryptase level is of prognostic significance was confirmed by the observation that enzyme levels were significantly higher in high risk patients than in low risk patients in prognostic scoring systems, including the Sokal, Hasford, and EUTOS score. Moreover, CP patients with elevated tryptase levels had a significantly higher progression- and event rate compared to patients with normal tryptase levels. Finally, the decrease in the BCR/ABL, one of key markers indicating responses in CML patients in the follow up, was faster and deeper in the group of patients with normal serum tryptase levels compared to those with elevated enzyme levels. An interesting finding was that tryptase was not of predictive value for OS. Nevertheless, a `late drop` in the survival curve in the high tryptase group and a higher mortality-rate in this group was observed. In this regard, it is noteworthy that several high risk patients switched from one TKI to another and that this may explain the discrepancy in the prognostic value of tryptase regarding PFS and OS. In line with this hypothesis, the majority of our CML patients who progressed and then switched the TKI is still alive. In order to detect an influence of an elevated tryptase level on survival, a longer observation period may be necessary. Overall, tryptase at diagnosis seems to be a strong prognostic parameter predicting disease progression in CML and could thus be of importance in the decision to treat patients with CP CML.
Although we found a correlation between the percentage of pb basophil counts and serum tryptase levels, there are patients with high tryptase levels and a rather low basophil count and vice versa. This difference may be explained by the fact that tryptase is primarily expressed in immature basophils, which are often difficult to identify due to their hypo-granulated (or even non-granulated) cytoplasm. Therefore, the total basophil burden can be underestimated in such patients. On the other hand, the percentage of basophils in the pb may not always reflect the total basophil body burden. Tryptase measurement may thus be a preferable marker in CML.
We also analyzed the prognostic value of tryptase concerning PFS and EFS. So far, the only established scoring system developed for patients receiving TKI is the EUTOS score. The PFS of our patients differed significantly between the high and low risk group according to the EUTOS score which is in line with other publications [19,39]. In an attempt to improve the prognostication by EUTOS, we replaced the percentage of basophils with serum tryptase level. Indeed, with a cut off of 80 ng/ml, this modified EUTOS-T score was able to distinguish significantly between low and high risk patients regarding EFS and PFS.
In conclusion, tryptase is a new robust prognostic marker in CML. Elevated serum-tryptase levels are of prognostic significance for PFS and EFS. Moreover, when used to replace the percentage of basophils in the EUTOS score, tryptase appears to improve this scoring system, most probably by better reflecting the total basophil burden (mature+immature basophils) in these patients.
Acknowledgements
This work was supported by the Austrian Science Funds, SFB grant F4704-B20.
Disclosure of conflict of interest
WRS received honoraria from Novartis, TP, GH, SH, CS, CM, and MK have nothing to disclose. PV is a consultant of Novartis, received research support from Novartis and received honoraria from Novartis, BMS, Pfizer and Ariad.
Supporting Information
References
- 1.Druker BJ, Talpaz M, Resta DJ, Peng B, Buchdunger E, Ford JM, Lydon NB, Kantarjian H, Capdeville R, Ohno-Jones S, Sawyers CL. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med. 2001;344:1031–1037. doi: 10.1056/NEJM200104053441401. [DOI] [PubMed] [Google Scholar]
- 2.O’Brien SG, Guilhot F, Larson RA, Gathmann I, Baccarani M, Cervantes F, Cornelissen JJ, Fischer T, Hochhaus A, Hughes T, Lechner K, Nielsen JL, Rousselot P, Reiffers J, Saglio G, Shepherd J, Simonsson B, Gratwohl A, Goldman JM, Kantarjian H, Taylor K, Verhoef G, Bolton AE, Capdeville R, Druker BJ. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2003;348:994–1004. doi: 10.1056/NEJMoa022457. [DOI] [PubMed] [Google Scholar]
- 3.Hughes TP, Kaeda J, Branford S, Rudzki Z, Hochhaus A, Hensley ML, Gathmann I, Bolton AE, van Hoomissen IC, Goldman JM, Radich JP. Frequency of major molecular responses to imatinib or interferon alfa plus cytarabine in newly diagnosed chronic myeloid leukemia. N Engl J Med. 2003;349:1423–1432. doi: 10.1056/NEJMoa030513. [DOI] [PubMed] [Google Scholar]
- 4.Talpaz M, Silver RT, Druker BJ, Goldman JM, Gambacorti-Passerini C, Guilhot F, Schiffer CA, Fischer T, Deininger MW, Lennard AL, Hochhaus A, Ottmann OG, Gratwohl A, Baccarani M, Stone R, Tura S, Mahon FX, Fernandes-Reese S, Gathmann I, Capdeville R, Kantarjian HM, Sawyers CL. Imatinib induces durable hematologic and cytogenetic responses in patients with accelerated phase chronic myeloid leukemia: results of a phase 2 study. Blood. 2002;99:1928–1937. doi: 10.1182/blood.v99.6.1928. [DOI] [PubMed] [Google Scholar]
- 5.Druker BJ, Sawyers CL, Kantarjian H, Resta DJ, Reese SF, Ford JM, Capdeville R, Talpaz M. Activity of specific inhibitor of the BCR-ABL tyrosine kinase in the blast crisis of chronic myeloid leukemia and acute lymphoblastic leukemia with the Philadelphia chromosome. N Engl J Med. 2001;344:1038–1042. doi: 10.1056/NEJM200104053441402. [DOI] [PubMed] [Google Scholar]
- 6.Kantarjian H, Shah NP, Hochhaus A, Cortes J, Shah S, Ayala M, Moiraghi B, Shen Z, Mayer J, Pasquini R, Nakamae H, Huguet F, Boqué C, Chuah C, Bleickardt E, Bradley-Garelik MB, Zhu C, Szatrowski T, Shapiro D, Baccarani M. Dasatinib versus Imatinib in Newly Diagnosed Chronic-Phase Chronic Myeloid Leukemia. N Engl J Med. 2010;362:2260–2270. doi: 10.1056/NEJMoa1002315. [DOI] [PubMed] [Google Scholar]
- 7.le Coutre P, Ottmann OG, Giles F, Kim DW, Cortes J, Gattermann N, Apperley JF, Larson RA, Abruzzese E, O’Brien SG, Kuliczkowski K, Hochhaus A, Mahon FX, Saglio G, Gobbi M, Kwong YL, Baccarani M, Hughes T, Martinelli G, Radich JP, Zheng M, Shou Y, Kantarjian H. Nilotinib (formerly AMN107), a highly selective BCR-ABL tyrosine kinase inhibitor, is active in patients with imatinib-resistant or -intolerant accelerated-phase chronic myelogenous leukemia. Blood. 2008;111:1834–1839. doi: 10.1182/blood-2007-04-083196. [DOI] [PubMed] [Google Scholar]
- 8.Giles FJ, le Coutre PD, Pinilla-Ibarz J, Larson RA, Gattermann N, Ottmann OG, Hochhaus A, Radich JP, Saglio G, Hughes TP, Martinelli G, Kim DW, Novick S, Gillis K, Fan X, Cortes J, Baccarani M, Kantarjian HM. Nilotinib in imatinib-resistant or imatinib-intolerant patients with chronic myeloid leukemia in chronic phase: 48-month follow-up results of a phase II study. Leukemia. 2013;27:107–112. doi: 10.1038/leu.2012.181. [DOI] [PubMed] [Google Scholar]
- 9.Quintás-Cardama A, Kantarjian H, O’brien S, Borthakur G, Bruzzi J, Munden R, Cortes J. Pleural effusion in patients with chronic myelogenous leukemia treated with dasatinib after imatinib failure. J. Clin. Oncol. 2007;25:3908–3914. doi: 10.1200/JCO.2007.12.0329. [DOI] [PubMed] [Google Scholar]
- 10.Aichberger KJ, Herndlhofer S, Schernthaner GH, Schillinger M, Mitterbauer-Hohendanner G, Sillaber C, Valent P. Progressive peripheral arterial occlusive disease and other vascular events during nilotinib therapy in CML. Am J Hematol. 2011;86:533–539. doi: 10.1002/ajh.22037. [DOI] [PubMed] [Google Scholar]
- 11.Valent P. Severe adverse events associated with the use of second-line BCR/ABL tyrosine kinase inhibitors: preferential occurrence in patients with comorbidities. Haematologica. 2011;96:1395–1397. doi: 10.3324/haematol.2011.052076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Breccia M, Latagliata R, Stagno F, Luciano L, Gozzini A, Castagnetti F, Fava C, Cavazzini F, Annunziata M, Russo Rossi A, Pregno P, Abruzzese E, Vigneri P, Rege-Cambrin G, Sica S, Pane F, Santini V, Specchia G, Rosti G, Alimena G. Charlson comorbidity index and adult comorbidity evaluation-27 scores might predict treatment compliance and development of pleural effusions in elderly patients with chronic myeloid leukemia treated with second-line dasatinib. Haematologica. 2011;96:1457–1461. doi: 10.3324/haematol.2011.041251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cortes J, Kantarjian H. Advanced-phase chronic myeloid leukemia. Semin Hematol. 2003;40:79–86. doi: 10.1053/shem.2003.50005. [DOI] [PubMed] [Google Scholar]
- 14.Hasford J, Pfirrmann M, Hehlmann R, Allan NC, Baccarani M, Kluin-Nelemans JC, Alimena G, Steegmann JL, Ansari H. A New Prognostic Score for Survival of Patients With Chronic Myeloid Leukemia Treated With Interferon Alfa. Writing Committee for the Collaborative CML Prognostic Factors Project Group. J Natl Cancer Inst. 1998;90:850–858. doi: 10.1093/jnci/90.11.850. [DOI] [PubMed] [Google Scholar]
- 15.Sokal JE, Cox EB, Baccarani M, Tura S, Gomez GA, Robertson JE, Tso CY, Braun TJ, Clarkson BD, Cervantes F. Prognostic discrimination in “good-risk” chronic granulocytic leukemia. Blood. 1984;63:789–799. [PubMed] [Google Scholar]
- 16.Druker BJ, Guilhot F, O’Brien SG, Gathmann I, Kantarjian H, Gattermann N, Deininger MW, Silver RT, Goldman JM, Stone RM, Cervantes F, Hochhaus A, Powell BL, Gabrilove JL, Rousselot P, Reiffers J, Cornelissen JJ, Hughes T, Agis H, Fischer T, Verhoef G, Shepherd J, Saglio G, Gratwohl A, Nielsen JL, Radich JP, Simonsson B, Taylor K, Baccarani M, So C, Letvak L, Larson RA. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355:2408–2417. doi: 10.1056/NEJMoa062867. [DOI] [PubMed] [Google Scholar]
- 17.Hochhaus A, O’Brien SG, Guilhot F, Druker BJ, Branford S, Foroni L, Goldman JM, Müller MC, Radich JP, Rudoltz M, Mone M, Gathmann I, Hughes TP, Larson RA. Six-year follow-up of patients receiving imatinib for the first-line treatment of chronic myeloid leukemia. Leukemia. 2009;23:1054–1061. doi: 10.1038/leu.2009.38. [DOI] [PubMed] [Google Scholar]
- 18.Marin D, Marktel S, Bua M, Szydlo RM, Franceschino A, Nathan I, Foot N, Crawley C, Na Nakorn T, Olavarria E, Lennard A, Neylon A, O’Brien SG, Goldman JM, Apperley JF. Prognostic factors for patients with chronic myeloid leukaemia in chronic phase treated with imatinib mesylate after failure of interferon alfa. Leukemia. 2003;17:1448–1453. doi: 10.1038/sj.leu.2402996. [DOI] [PubMed] [Google Scholar]
- 19.Milojkovic D, Nicholson E, Apperley JF, Holyoake TL, Shepherd P, Drummond MW, Szydlo R, Bua M, Foroni L, Reid A, Khorashad JS, de Lavallade H, Rezvani K, Paliompeis C, Goldman JM, Marin D. Early prediction of success or failure of treatment with second-generation tyrosine kinase inhibitors in patients with chronic myeloid leukemia. Haematologica. 2010;95:224–231. doi: 10.3324/haematol.2009.012781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hasford J, Baccarani M, Hoffmann V, Guilhot J, Saussele S, Rosti G, Guilhot F, Porkka K, Ossenkoppele G, Lindoerfer D, Simonsson B, Pfirrmann M, Hehlmann R. Predicting complete cytogenetic response and subsequent progression-free survival in 2060 patients with CML on imatinib treatment: the EUTOS score. Blood. 2011;118:686–692. doi: 10.1182/blood-2010-12-319038. [DOI] [PubMed] [Google Scholar]
- 21.Samorapoompichit P, Kiener HP, Schernthaner GH, Jordan JH, Agis H, Wimazal F, Baghestanian M, Rezaie-Majd A, Sperr WR, Lechner K, Valent P. Detection of tryptase in cytoplasmic granules of basophils in patients with chronic myeloid leukemia and other myeloid neoplasms. Blood. 2001;98:2580–2583. doi: 10.1182/blood.v98.8.2580. [DOI] [PubMed] [Google Scholar]
- 22.Sperr WR, El-Samahi A, Kundi M, Girschikofsky M, Winkler S, Lutz D, Endler G, Rumpold H, Agis H, Sillaber C, Jäger U, Valent P. Elevated tryptase levels selectively cluster in myeloid neoplasms: a novel diagnostic approach and screen marker in clinical haematology. Eur J Clin Invest. 2009;39:914–923. doi: 10.1111/j.1365-2362.2009.02184.x. [DOI] [PubMed] [Google Scholar]
- 23.Wimazal F, Walchshofer S, Baghestanian M, Chott A, Sperr WR, Kopp C, Sillaber C, Semper H, Horny HP, Tröndle U, Födinger M, Schwarzinger I, Lechner K, Valent P. Detection of mi transcription factor (MITF) mRNA in a case of myelodysplastic syndrome and bone marrow mastocytosis. Wien Klin Wochenschr. 1998;110:79–88. [PubMed] [Google Scholar]
- 24.ISCN: An international system for human cytogenetic nomenclature. Cytogenet Cell Genet. 1994;31:5–23. doi: 10.1159/000131621. [DOI] [PubMed] [Google Scholar]
- 25.Baccarani M, Saglio G, Goldman J, Hochhaus A, Simonsson B, Appelbaum F, Apperley J, Cervantes F, Cortes J, Deininger M, Gratwohl A, Guilhot F, Horowitz M, Hughes T, Kantarjian H, Larson R, Niederwieser D, Silver R, Hehlmann R. Evolving concepts in the management of chronic myeloid leukemia: recommendations from an expert panel on behalf of the European LeukemiaNet. Blood. 2006;108:1809–1820. doi: 10.1182/blood-2006-02-005686. [DOI] [PubMed] [Google Scholar]
- 26.Baccarani M, Cortes J, Pane F, Niederwieser D, Saglio G, Apperley J, Cervantes F, Deininger M, Gratwohl A, Guilhot F, Hochhaus A, Horowitz M, Hughes T, Kantarjian H, Larson R, Radich J, Simonsson B, Silver RT, Goldman J, Hehlmann R. Chronic myeloid leukemia: an update of concepts and management recommendations of European LeukemiaNet. J. Clin. Oncol. 2009;27:6041–6051. doi: 10.1200/JCO.2009.25.0779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bolufer P, Sanz GF, Barragán E, Sanz MA, Cervera J, Lerma E, Senent L, Moreno I, Planelles MD. Rapid quantitative detection of BCR-ABL transcripts in chronic myeloid leukemia patients by real-time reverse transcriptase polymerase-chain reaction using fluorescently labeled probes. Haematologica. 2000;85:1248–1254. [PubMed] [Google Scholar]
- 28.Branford S, Fletcher L, Cross NC, Müller MC, Hochhaus A, Kim DW, Radich JP, Saglio G, Pane F, Kamel-Reid S, Wang YL, Press RD, Lynch K, Rudzki Z, Goldman JM, Hughes T. Desirable performance characteristics for BCR-ABL measurement on an international reporting scale to allow consistent interpretation of individual patient response and comparison of response rates between clinical trials. Blood. 2008;112:3330–3338. doi: 10.1182/blood-2008-04-150680. [DOI] [PubMed] [Google Scholar]
- 29.Therneau TM. Extending the Cox model. Technical Report Series, Section of Biostatistics, Mayo Clinic, Rochester, Minnesota, Technical Report number. 1996;58:1–63. [Google Scholar]
- 30.Baccarani M, Rosti G, de Vivo A, Bonifazi F, Russo D, Martinelli G, Testoni N, Amabile M, Fiacchini M, Montefusco E, Saglio G, Tura S. A randomized study of interferon-alpha versus interferon-alpha and low-dose arabinosyl cytosine in chronic myeloid leukemia. Blood. 2002;99:1527–1535. doi: 10.1182/blood.v99.5.1527. [DOI] [PubMed] [Google Scholar]
- 31.Hehlmann R, Saussele S. Treatment of Chronic Myeloid Leukemia in Blast Crisis. Haematologica. 2008;93:1765–1769. doi: 10.3324/haematol.2008.001214. [DOI] [PubMed] [Google Scholar]
- 32.Guilhot F, Apperley J, Kim DW, Bullorsky EO, Baccarani M, Roboz GJ, Amadori S, de Souza CA, Lipton JH, Hochhaus A, Heim D, Larson RA, Branford S, Muller MC, Agarwal P, Gollerkeri A, Talpaz M. Dasatinib induces significant hematologic and cytogenetic responses in patients with imatinib-resistant or -intolerant chronic myeloid leukemia in accelerated phase. Blood. 2007;109:4143–4150. doi: 10.1182/blood-2006-09-046839. [DOI] [PubMed] [Google Scholar]
- 33.le Coutre P, Ottmann OG, Giles F, Kim DW, Cortes J, Gattermann N, Apperley JF, Larson RA, Abruzzese E, O’Brien SG, Kuliczkowski K, Hochhaus A, Mahon FX, Saglio G, Gobbi M, Kwong YL, Baccarani M, Hughes T, Martinelli G, Radich JP, Zheng M, Shou Y, Kantarjian H. Nilotinib (formerly AMN107), a highly selective BCR-ABL1 tyrosine kinase inhibitor, is active in patients with imatinib-resistant or-intolerant accelerated-phase chronic myelogenous leukemia. Blood. 2008;111:1834–1839. doi: 10.1182/blood-2007-04-083196. [DOI] [PubMed] [Google Scholar]
- 34.Schwartz LB. Tryptase, a mediator of human mast cells. J Allergy Clin Immunol. 1990;864:594–598. doi: 10.1016/s0091-6749(05)80222-2. [DOI] [PubMed] [Google Scholar]
- 35.Sperr WR, Jordan JH, Fiegl M, Escribano L, Bellas C, Dirnhofer S, Semper H, Simonitsch-Klupp I, Horny HP, Valent P. Serum tryptase levels in patients with mastocytosis: correlation with mast cell burden and implication for defining the category of disease. Int Arch Allergy Immunol. 2002;128:136–141. doi: 10.1159/000059404. [DOI] [PubMed] [Google Scholar]
- 36.Schwartz LB. Clinical utility of tryptase levels in systemic mastocytosis and associated hematologic disorders. Leuk Res. 2001;25:553–562. doi: 10.1016/s0145-2126(01)00020-0. [DOI] [PubMed] [Google Scholar]
- 37.Sperr WR, Jordan JH, Baghestanian M, Kiener HP, Samorapoompichit P, Semper H, Hauswirth A, Schernthaner GH, Chott A, Natter S, Kraft D, Valenta R, Schwartz LB, Geissler K, Lechner K, Valent P. Expression of mast cell tryptase by myeloblasts in a group of patients with acute myeloid leukemia. Blood. 2001;98:2200–2209. doi: 10.1182/blood.v98.7.2200. [DOI] [PubMed] [Google Scholar]
- 38.Klion AD, Noel P, Akin C, Law MA, Gilliland DG, Cools J, Metcalfe DD, Nutman TB. Elevated serum tryptase levels identify a subset of patients with a myeloproliferative variant of idiopathic hypereosinophilic syndrome associated with tissue fibrosis, poor prognosis, and imatinib responsiveness. Blood. 2003;101:4660–4466. doi: 10.1182/blood-2003-01-0006. [DOI] [PubMed] [Google Scholar]
- 39.Jabbour E, Cortes J, Nazha A, O’Brien S, Quintas-Cardama A, Pierce S, Garcia-Manero G, Kantarjian H. EUTOS score is not predictive for survival and outcome in patients with early chronic phase chronic myeloid leukemia treated with tyrosine kinase inhibitors: a single institution experience. Blood. 2012;119:4524–4526. doi: 10.1182/blood-2011-10-388967. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


