Abstract
Osteolytic bone disease in multiple myeloma (MM) is associated with upregulated osteoclast activity. Macrophage inflammatory protein-1α (MIP-1α) is crucially involved in the development of osteolytic bone lesions in MM. We previously reported that minodronate inhibited lipopolysaccharide-induced MIP-1α secretion in mouse myeloma cells. However, it remains unknown whether bisphosphonates and statins inhibit MIP-1α secretion by human MM cells. In present study, we investigated whether bisphosphonates and statins had any inhibitory effect on MIP-1α secretion by human myeloma cells and the mechanism underlying this effect. In this study, we found that bisphosphonates and statins inhibited MIP-1α mRNA and MIP-1α secretion and suppressed extracellular signal-regulated kinase 1/2 (ERK1/2) and Akt phosphorylation by inhibiting Ras prenylation. Moreover, bisphosphonates and statins suppressed the expression of acute myeloid leukemia-1A (AML-1A) mRNA, a MIP-1α transcription factor. These results indicate that bisphosphonates and statins suppress the Ras/mitogen-activated protein kinase kinase/ERK/AML-1A and Ras/phosphatidylinositol-3 kinase/Akt/AML-1A pathways, thereby inhibiting MIP-1α secretion by MM cells. Therefore, use of MIP-1α expression inhibitors such as bisphosphonates and statins may provide a new therapeutic approach to inhibiting tumour progression and bone destruction in MM patients.
Keywords: Bisphosphonate, statin, MIP-1α, AML-1A, myeloma, Ras, ERK, Akt
Introduction
Multiple myeloma (MM) is an incurable plasma cell neoplasm that accounts for 13% of all the haematological malignancies [1,2]. MM patients are at an increased risk of infection, anaemia, thrombocytopenia, renal failure, and bone disease. Osteolytic bone disease occurs in 70-80% of MM patients and results in severe bone pain, pathological fractures, paralysis through nerve compression, hypercalcemia, and death [2]. Bone destruction results from highly localised osteoclastic bone resorption induced by the osteoclastic stimulatory factors secreted by MM cells and bone marrow stromal cells that are adjacent to the MM cells [3]. Studies have implicated several cytokines as potential factors responsible for MM-related bone destruction, including interleukin-1β (IL-1β), IL-6, and tumour necrosis factor-α. However, none of these cytokines has been found to be consistently elevated in the peripheral blood or bone marrow plasma of MM patients [4,5].
Macrophage inflammatory protein-1α (MIP-1α) is expressed and secreted by the myeloma cells freshly isolated from patients and by human myeloma cell lines [6-9]. MIP-1α is elevated in the bone marrow plasma of patients with MM than in the bone marrow plasma of patients with other lymphoid hematologic malignancies and healthy donors [6,8]. It has been indicated that MIP-1α stimulates chemotaxis of human osteoclast precursors, promotes the expression of the receptor activator of NF-κB ligand by osteoblastic and interstitial cells, and acts directly on the osteoclasts to promote osteoclastogenesis and osteoclastic bone resorption [10-14]. Moreover, MIP-1α accelerates cell growth in MM cell lines [15,16]. It is further possible that osteoclastic activity will be inhibited by the use of a neutralising antibody to control MIP-1α. It has been confirmed that bone resorption is inhibited in severe combined immunodeficient mice through the inhibition of MIP-1α by using an antisense strand [17]. Accordingly, it is possible that MIP-1α inhibition has the potential to serve as a therapeutic method for inhibiting tumour cell growth or preventing bone destruction.
Bisphosphonates are structural analogues of pyrophosphoric acid that are used as therapeutic agents for osteoporosis and hypercalcemia through the apoptotic induction of osteoclasts [18]. Nitrogen-containing bisphosphonates have an inhibitory effect on farnesyl pyrophosphate (FPP) synthase and geranylgeranyl pyrophosphate (GGPP) synthase in the mevalonate pathway; such inhibition of the intermediates in this pathway results in disruption of protein prenylation that is vital to the function of essential signaling proteins such as small GTPases (Ras and Rho) [19-21]. Statins, which are inhibitors of 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase (the rate-limiting enzyme in cholesterol synthesis), suppress cell proliferation, and induce apoptosis in various cell types such as osteoclasts and various tumour cells, by inhibiting GGPP biosynthesis and Ras prenylation [21-24].
If the MIP-1α released from the myeloma cells can be inhibited by bisphosphonates and statins that act not only on osteoclasts but also on tumour cells, it would be possible to inhibit the growth of myeloma cells or prevent osteolysis. We previously reported that minodronate inhibited lipopolysaccharide-induced MIP-1α secretion in mouse myeloma [16]. However, it remains unknown whether bisphosphonates and statins can inhibit MIP-1α secretion by human MM cells. Moreover, the mechanism underlying this suppression is yet to be elucidated. Therefore, the use of bisphosphonates and statins may possibly prolong lives, alleviate osteolytic pain, or contribute to improved quality of life for patients. Thus, in this study, we investigated whether bisphosphonates and statins can inhibit MIP-1α secretion by the human myeloma cells and the mechanism possibly underlying this inhibition.
Materials and methods
Materials
Minodronate was supplied from Astellas Pharmaceutical Co., Ltd. (Tokyo, Japan). Alendronate was purchased from Sigma (St. Louis, MO, USA). Fluvastatin and simvastatin were purchased form Calbiochem (San Diego, CA, USA), and dimethyl sulfoxide (DMSO). This reagent was dissolved in phosphate buffer saline (PBS; 0.05 M, pH 7.4, Wako, Osaka, Japan), filtrated through syringe filters (0.45 μm, IWAKI GLASS, Tokyo, Japan) and used for various assays described below.
Mevalonic acid lactone (MVA), FPP, GGPP, squalene, isopentenyladenine, ubiquinone, and dolichol were purchased from Sigma. MVA, FPP and GGPP were dissolved in dry ethanol. U0126 and LY294002 were purchased from Promega (Madison, WI, USA) and dissolved in DMSO. Rapamycin and Y27632 were purchased form Wako (Tokyo, Japan) and dissolved in DMSO. And then, these dissolved reagents were resuspended in PBS (0.05 M, pH 7.4), filtrated through syringe filters (0.45 μm, IWAKI GLASS) before use.
Cell culture
IM9 cells, RPMI8226 cells, and HS-sultan cells, were obtained from Health Science Research Resources Bank (Osaka, Japan). ARH77 cells were obtained from DS Pharma biomedical (Osaka, Japan). These human myeloma cell lines were cultured in RPMI1640 medium (Sigma) supplemented with 10% fetal calf serum (Gibco, Carlsbad, CA, USA), 100 μg/mL penicillin (Gibco), 100 U/mL streptomycin (Gibco), and 25 mM HEPES (pH 7.4; Wako) in an atmosphere containing 5% CO2.
Enzyme-linked immunosorbent assay (ELISA)
Cells were cultured at 1 × 104 cells/mL and conditioned media were harvested. The MIP-1α levels were measured using Quantikine MIP-1α enzyme immunoassay kits (R & D Systems, Minneapolis, MN, USA), according to the manufacturer’s instructions.
Quantitative real-time polymerase chain reaction (PCR)
Total RNA was isolated using TRIZOL (Invitrogen; Carlsbad, CA). One microgram of purified total RNA was used for the real-time PCR analysis with the SuperScript First-Strand Synthesis System (Invitrogen). cDNA was subjected to quantitative real-time PCR by using SYBR Premix Ex Taq (Takara Biomedical; Siga, Japan) and the Thermal Cycler Dice Real Time system (Takara Biomedical) in a 96-well plate according to the manufacturer’s instructions. The PCR conditions for glyceraldehyde-3-phosphate dehydrogenase (GAPDH), MIP-1α, AML-1A, CREB, NF-AT, and C/ERBβ were 94°C for 2 min; followed by 40 cycles of 94°C for 0.5 min, 50°C for 0.5 min, and 72°C for 0.5 min. The following primers were used: for MIP-1α, 5’-CAG CGA GTA CCA GTC CCT TTT-3’ (5’-primer); 5’-CCT CGC TGC CTC CAA GA-3’ (3’-primer), for AML-1A, 5’-CTG GTC ACT GTG ATG GCT GG-3’ (5’-primer); 5’-CTG CCT TAA CAT CTC CAG GG-3’ (3’-primer), for CREB, 5’-GGC CTG CAA ACA TTA ACC AT-3’ (5’-primer); 5’-ACG ACA CTC TCG AGC TGC TT-3’ (3’-primer), for NF-AT, 5’-TCC TCG CAT CGA GAT AAC CT-3’ (5’-primer); 5’-ACG ACA CTC TCG AGC TGC TT-3’ (3’-primer), for C/ERBβ, 5’-ACA GCG ACG AGT ACA AGA TCC-3’ (5’-primer); 5’-GCA GCT GCT TGA ACA AGT TCC-3’ (3’-primer), and for GAPDH, 5’-ACT TTG TCA AGC TCA TTT-3’ (5’-primer), 5’-TGC AGC GAA CTT TAT TG-3’ (3’-primer). As an internal control for each sample, the GAPDH gene was used for standardization. Cycle threshold (Ct) values were established, and the relative difference in expression from GAPDH expression was determined according to the 2-∆∆Ct method of analysis and compared to the expression in control cells.
Cell viability
Cells (2 × 103 cells/well) were plated in 96-well plates and incubated with various concentration of minodronate, alendronate, fluvastatin, and simvastatin. After incubation for 5 days, cells were stained with trypan blue dye, and the number of stained cells was counted.
Western blotting
Cells treated under various conditions were lysed with a lysis buffer (20 mM Tris-HCl (pH 8.0), 150 mM NaCl, 2 mM EDTA, 100 mM NaF, 1% NP40, 1 μg/mL leupeptin, 1 μg/mL antipain, and 1 mM phenylmethylsulfonyl fluoride), and the protein concentrations of the resulting cell lysates were determined using a BCA protein assay kit (Pierce, Rockford, IL, USA). The membrane fraction of cells was extracted with the ProteoExtract Native Membrane Protein Extraction Kit (Calbiochem, San Diego, CA, USA). An aliquot of each extract (containing 40 μg protein) was fractionated by electrophoresis in sodium dodecyl sulfate -polyacrylamide gel and transferred to a polyvinyl difluoride membrane (Amer-sham, Arlington Heights, IL, USA). Membranes were blo-cked with a solution containing 3% skim milk and then incubated overnight at 4°C with the following antibodies: anti-phospho-p44/42 mitogen-activated protein kinase (MAPK; ERK1/2) (Thr202/Tyr204) antibody, anti-phospho-Akt (Ser473) antibody, anti-phospho-p38MAPK (Thr180/Thr182) antibody, anti-phospho-mTOR (Ser2448) antibody, anti-p44/42 MAPK (ERK1/2) antibody, anti-Akt antibody, anti-p38MAPK antibody, anti-mTOR antibody, I-κB antibody (Cell Signaling Technology, Beverly, MA, USA), anti-Ras antibody (clone RAS-10), anti-Rho antibody (clone 55) (Upstate Biology, Charlottesville, VA, USA), Na/K-ATPase (Santa Cruz Biotechnologies, CA, USA), and anti-β-actin antibody (Sigma). Subsequently, the membranes were incubated for 1 h at room temperature with anti-rabbit IgG sheep antibody or anti-mouse IgG sheep antibody coupled to horseradish peroxidase (Amer-sham). Reactive proteins were visualized using a chemiluminescence (ECL-plus) kit (Amersham) according to the manufacturer’s instructions.
Statistical analysis
All results are expressed as means and S.D. of several independent experiments. Multiple comparisons of the data were done by ANOVA with Dunnet’s test. P values less than 5% were regarded as significant.
Results
Expression and secretion of MIP-1α in MM cell lines
To assess whether different MM cell lines express MIP-1α mRNA, we analysed MIP-1α mRNA expression by real time PCR. MIP-1α mRNA expression was detected on IM9 cells and ARH77 cells (Figure 1A). To confirm that IM9 cells, ARH77cells, RPMI8226 cells, and HS-Sultan cells secrete MIP-1α, cells were seeded at a concentration of 1 × 104 cells/mL in conditioned media. MIP-1α secretion was measured in 48-h culture supernatants by ELISA. MIP-1α secretion was detectable in IM9 cells and ARH77 cells (Figure 1B).
Figure 1.
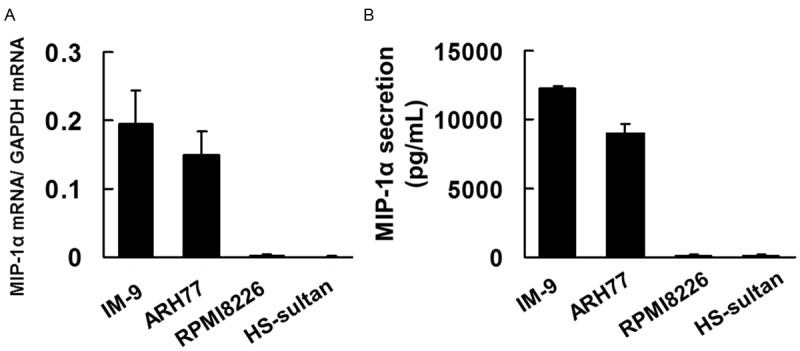
Multiple cell lines myeloma express MIP-1α. A. A total RNA was extracted, and MIP-1α mRNA levels were determined by real-time PCR. The results are expressed as treated over control ratio after correction to GAPDH mRNA levels. B. Cells (1 × 104 cells per well) were incubated for 48 h. Culture supernatant was collected and analyzed for MIP-1α by enzyme-linked immunosorbent assay (ELISA). The results are representative of five independent experiments.
Cytotoxicity toward IM9 cells and ARH77 cells
The cytotoxic effects of minodronate, alendronate, fluvastatin, and simvastatin on IM9 cells were measured by Trypan blue dye exclusion assay. The results showed that minodronate and simvastatin did not affect cell viability at a concentration of 0.5 μM to 1 μM for 5 days (Figure 2A, 2D). We also found that alendronate did not affected cell viability at a concentration of 1 μM to 5 μM for 5 days (Figure 2B). Fluvastatin did not affect cell viability at a concentration of 0.1 μM to 0.5 μM for 5 days (Figure 2C). On the basis of these results, we selected 1 μM as the effective concentration of minodronate; 5 μM, of alendronate; 0.5 μM, of fluvastatin; and 1 μM, of simvastatin that did not show cytotoxicity toward IM9 cells.
Figure 2.
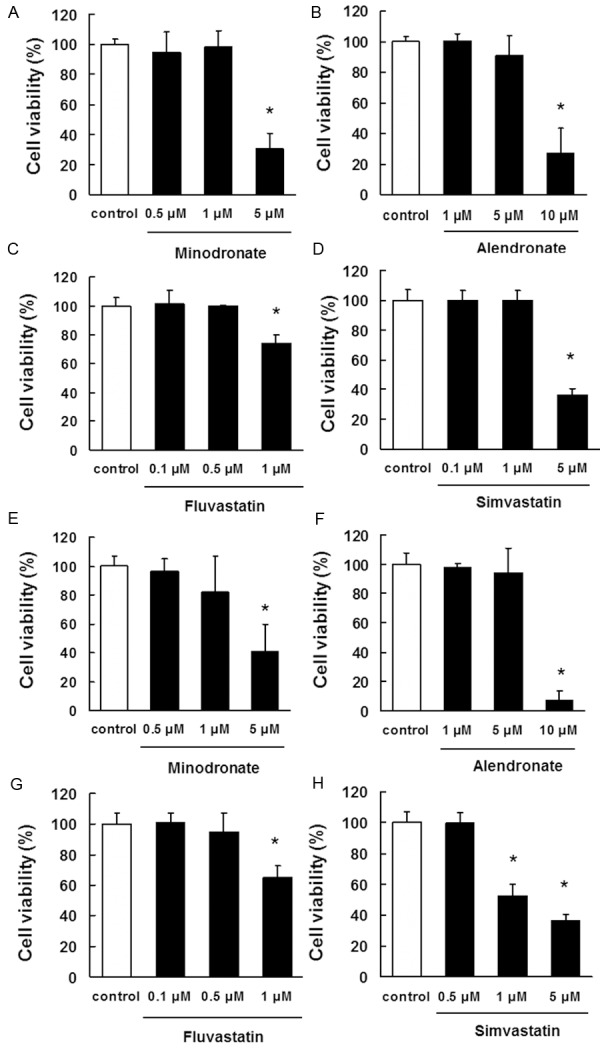
Determination of the appropriate concentrations of bisphosphonates and statins that are not cytotoxic to IM9 cells and ARH77 cells. IM9 cells (A-D) and ARH77 cells (E-H) were incubated in 96-well plates for 24 h and then treated with various concentrations of (A, E) minodronate, (B, F) alendronate, (C, G) fluvastatin, or (D, H) simvastatin. After 5 days, cell viability was quantified by trypan blue dye assays. The results are representative of 5 independent experiments. *p < 0.01 vs. the controls (ANOVA with Dunnet’s test).
Next, we examine the cytotoxic effects of minodronate, alendronate, fluvastatin, and simvastatin on ARH77 cells. The results showed that minodronate did not affect cell viability at a concentration of 0.5 μM to 1 μM for 5 days (Figure 2E). We also found that alendronate did not affected cell viability at a concentration of 1 μM to 5 μM for 5 days (Figure 2F). Fluvastatin and simvastatin did not affect cell viability at a concentration of 0.5 μM for 5 days (Figure 2G, 2H). On the basis of these results, we selected 1 μM as the effective concentration of minodronate; 5 μM, of alendronate; 0.5 μM, of fluvastatin; and 0.5 μM, of simvastatin that did not show cytotoxicity toward ARH77 cells.
Inhibitory effects of bisphosphonates and statins on MIP-1α mRNA expression and secretion by IM9 cells and ARH77 cells
To determine whether bisphosphonates and statins inhibited MIP-1α mRNA expression by IM9 cells and ARH77 cells, we treated IM9 cells or ARH77 cells with bisphosphonates and statins. As shown in Figure 3A and 3C, bisphosphonates and statin downregulated MIP-1α mRNA expression. Bisphosphonates and sta-tins also suppressed the secretion of MIP-1α in IM9 cells and ARH77 cells (Figure 3B and 3D).
Figure 3.
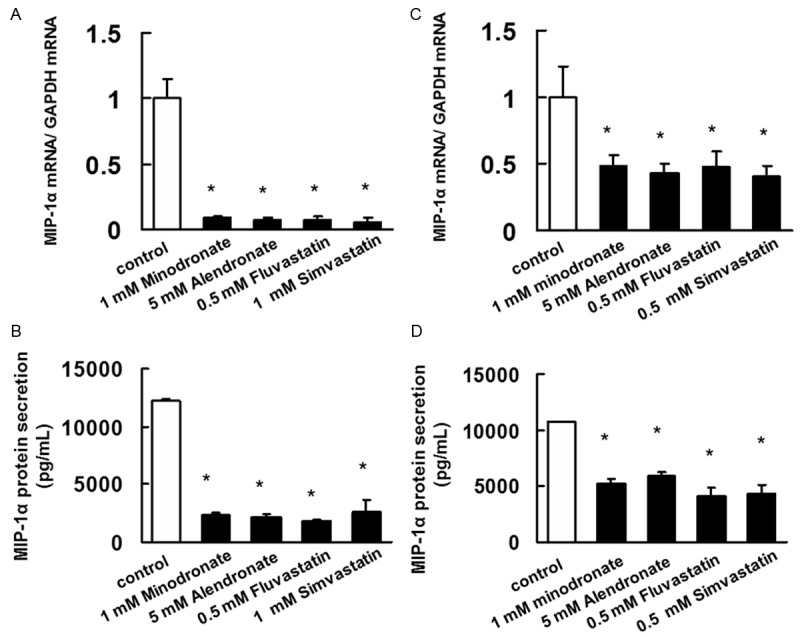
Inhibitory effect of bisphosphonates and statins on the mRNA expression and protein secretion of MIP-1α. (A, C) A total RNA was extracted, and MIP-1α mRNA levels were determined by real-time PCR. (A) IM-9 cells and (C) ARH77 cells treated with minodronate, alendronate, fluvastatin, or simvastatin for 3 days. The results are representative of 5 independent experiments. *P < 0.01, as compared to controls (ANOVA with Dunnett’s test). (B) IM-9 cells and (D) ARH77 cells were treated with minodronate, alendronate, fluvastatin, or simvastatin for 3 days. MIP-1α concentration of the cell culture supernatants was assayed using ELISA. minodronate, alendronate, fluvastatin, or simvastatin suppressed MIP-1α secretion in IM-9 cells and ARH77 cells. The results are representative of five independent experiments. *p < 0.01 vs. the controls (ANOVA with Dunnet’s test).
Confirmation of the inhibitory effect of bisphosphonates and statins plus mevalonate pathway intermediates on MIP-1α secretion by IM9 cells
We administered bisphosphonates and statins in combination with mevalonate pathway intermediates to investigate whether the reduced MIP-1α secretion observed in IM9 cells was due to bisphosphonate- or statin-induced inhibition of the intermediates of the mevalonate biosynthesis pathway.
Bisphosphonates inhibited MIP-1α secretion, but when used in combination with FPP or GGPP, MIP-1α secretion was restored to the degree observed in control (0.1% DMSO-treated) cells (Figure 4A and 4B). This finding suggests that the inhibition of MIP-1α secretion by IM9 cells treated with bisphosphonates was due to the inhibition of FPP or GGPP biosynthesis. Statins inhibited MIP-1α secretion, whereas statins in combination with MVA, FPP, or GGPP led to the restoration of MIP-1α secretion to the degree observed in control cells (Figure 4C and 4D). This finding suggests that the inhibition of MIP-1α secretion in IM9 cells treated with statins was due to the inhibition of FPP or GGPP biosynthesis.
Figure 4.
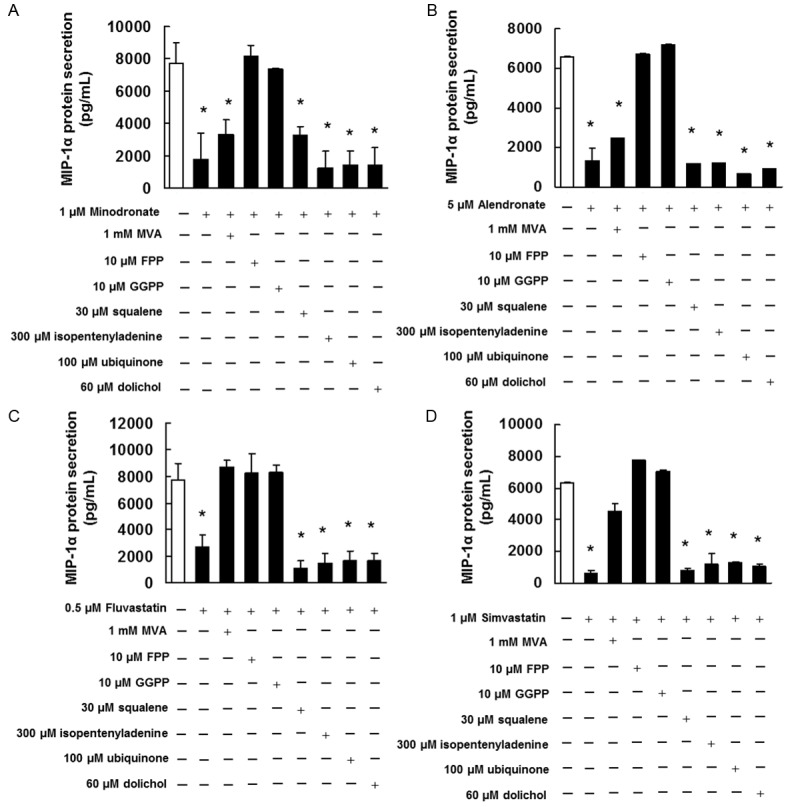
Effect of MIP-1α secretion in IM-9 cells by intermediate of the mevalonate pathway. IM9 cells were pretreated with 1 mM MVA, 10 μM FPP, 10 μM GGPP, 30 μM squalene, 300 μM isopentenyladenine, 100 μM ubiquinone, or 60 μM dolichol for 4 h and then treated with (A) 1 μM minodronate, (B) 5 μM alendronate, (C) 0.5 μM fluvastatin, or (D) 1 μM simvastatin for 72 h. Culture supernatant was collected and analyzed for MIP-1α by ELISA. These results are representative of 5 independent experiments. *p < 0.01 vs. the controls (ANOVA with Dunnet’s test).
Bisphosphonates and statins suppress Ras and Rho prenylation in IM9 cells
To demonstrate whether bisphosphonates and statins inhibit the functions of Ras and Rho by suppressing their prenylation, the protein samples were subjected to a standard western blot assay to detect the presence of small GTPases in both the membrane and the cytoplasm lysates of IM9 cells incubated with or without bisphosphonates and statins. Membrane localization of Ras or Rho proteins significantly decreased in the bisphosphonate- and statin-treated cells compared to the control cells. In contrast, the cytoplasmic localization of Ras or Rho proteins increased in the reagent-treated cells compared to the control cells (Figure 5A and 5C).
Figure 5.
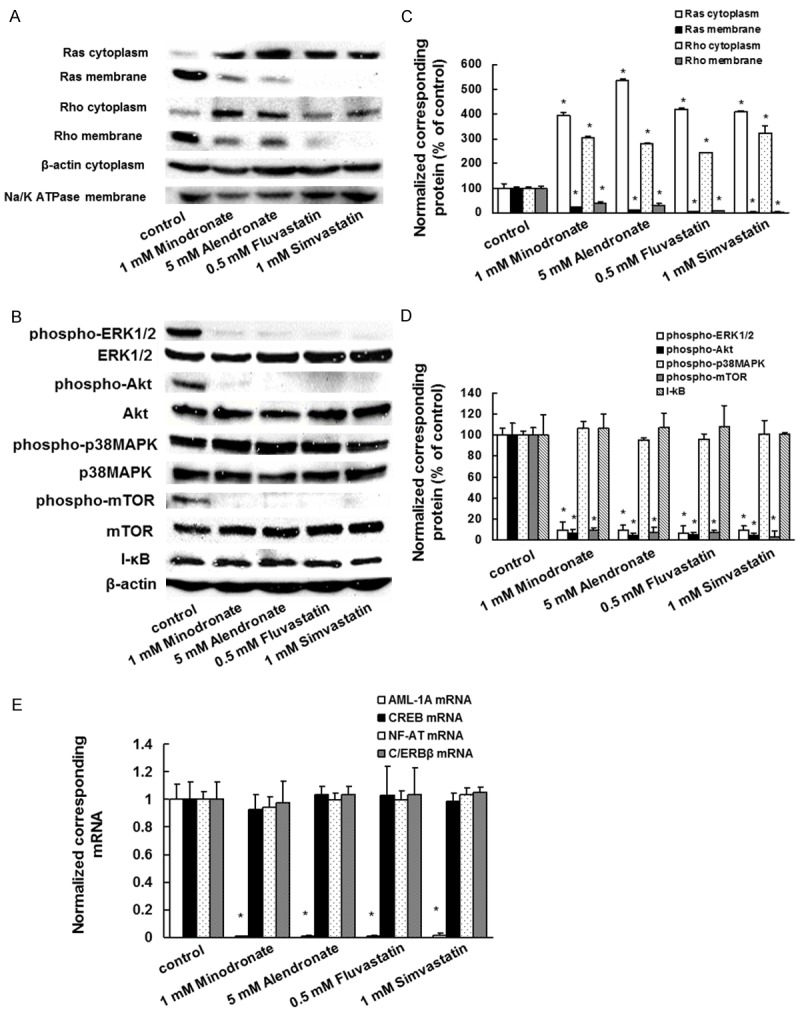
Effect of bisphosphonates and statins on Ras/Rho membrane localization, activation of signaling molecules, and expression of AML-1A mRNA in IM9 cells. The cells were incubated with 1 μM minodronate, 5 μM alendronate, 0.5 μM fluvastatin, or 1 μM simvastatin for 3 days. A. The cytoplasmic fractions and membrane fractions were extracted and then subjected to SDS-PAGE/immunoblotting with anti-Ras and anti-Rho antibodies. Anti-Na/K-ATPase antibody or Anti-β-actin antibody was used as the membrane or cytoplasm internal standard, as the primary antibody to detect Na/K-ATPase or β-actin protein. B. The cytoplasmic fractions were extracted and then subjected to SDS-PAGE/immunoblotting with antibodies against phosphorylated ERK1/2 (phospho-ERK1/2), phosphorylated Akt (phospho-Akt), phosphorylated p38MAPK (phospho-p38MAPK), I-κB, phosphorylated mTOR (phospho-mTOR), ERK, Akt, p38MAPK, mTOR, and β-actin. C. Quantification of the amount of Ras or Rho, normalized to the amounts of the corresponding proteins, respectively. The results are representative of 5 independent experiments. *P < 0.01, as compared to controls (ANOVA with Dunnett’s test). D. Quantification of the amount of phospho-ERK1/2, phospho-Akt, phospho-p38MAPK, phospho-mTOR, or I-κB, normalized to the amounts of the corresponding proteins, respectively. The results are representative of 5 independent experiments. *P < 0.01, as compared to controls (ANOVA with Dunnett’s test). E. A total RNA was extracted, and AML-1A, CREB, NF-AT, and C/ERBβ mRNA levels were determined by real-time PCR. IM-9 cells treated with minodronate, alendronate, fluvastatin, or simvastatin for 3 days. The results are representative of 5 independent experiments. *P < 0.01, as compared to controls (ANOVA with Dunnett’s test).
Bisphosphonates and statins suppress ERK1/2, Akt, and mTOR activation in IM9 cells
We further investigated whether bisphosphonates and statins inhibit signal transduction factors that are downstream of Ras and found that they suppressed ERK1/2, Akt, and mTOR activation. There was no substantial change in the level of phosphorylated p38MAPK or expression of I-κB in the bisphosphonate- and statin-treated cells compared to that in the control cells (Figure 5B and 5D).
Bisphosphonates and statins inhibit AML-1A mRNA expression
Next, we investigated whether bisphosphonates and statins suppress the transcription factor for MIP-1α expression. Real time PCR analyses revealed that bisphosphonates and statins induced markedly decreased expression of AML-1A mRNA. There were no substantial changes in the cyclic adenosine monophosphate response element-binding (CREB), NF-AT, or C/ERBβ mRNA expression levels in the bisphosphonate- and statin-treated cells compared to those in the control cells (Figure 5E).
Inhibitory effect of U0126 and LY294002 on MIP-1α and AML-1A mRNA expression and MIP-1α secretion
Our results indicated that the inhibitory effects of bisphosphonates and statins on MIP-1α expression are exerted via the inhibition of Ras/MEK/ERK, Ras/PI3K/Akt, Ras/mTOR, and Rho/ROCK signalling pathways. Therefore, we treated IM9 cells with U0126 (MEK inhibitor), LY294002 (PI3K inhibitor), Y27632 (ROCK inhibitor), and rapamycin (mTOR inhibitor) to determine whether the suppression of ERK, Akt, ROCK, or mTOR would result in MIP-1α expression inhibition. Administration of U0126 or LY294002 inhibited MIP-1α and AML-1A mRNA expression and MIP-1α secretion even at concentrations of these reagents that did not show anti-proliferative effects on IM9 cells (Figure 6). However, neither Y27632 nor rapamycin affected MIP-1α and AML-1A mRNA expression or MIP-1α secretion (Figure 6).
Figure 6.
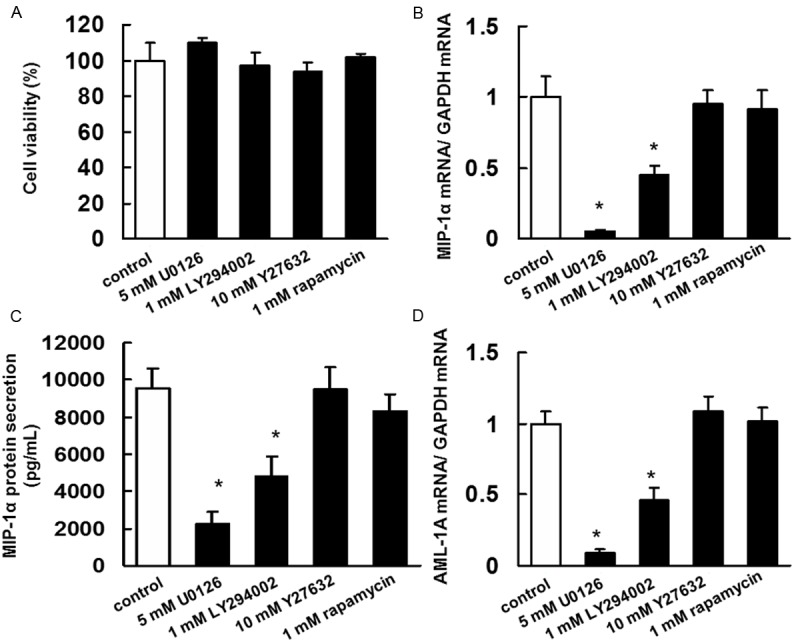
Inhibitory effect of U0126, LY294002, Y27632, or rapamycin on the secretion of MIP-1α and AML-1A mRNA expression in IM9 cells. A. Determination of the appropriate concentrations of U0126, LY294002, Y27632, or rapamycin that are not cytotoxic to IM9 cells. IM9 cells were incubated in 96-well plates for 24 h and then treated with 5 μM U0126, 1 μM LY294002, 10 μM Y27632, or 1 μM rapamycin. After 5 days, cell viability was quantified by trypan blue dye assays. The results are representative of 5 independent experiments. B. A total RNA was extracted, and MIP-1α mRNA levels were determined by real-time PCR. IM-9 cells treated with U0126, LY294002, Y27632, or rapamycin for 3 days. The results are representative of 5 independent experiments. *P < 0.01, as compared to controls (ANOVA with Dunnett’s test). C. IM-9 cells were treated with U0126, LY294002, Y27632, or rapamycin for 3 days. MIP-1α concentration of the cell culture supernatants was assayed using ELISA. The results are representative of five independent experiments. *p < 0.01 vs. the controls (ANOVA with Dunnet’s test). D. A total RNA was extracted, and AML-1A mRNA levels were determined by real-time PCR. IM-9 cells treated with U0126, LY294002, Y27632, or rapamycin for 3 days. The results are representative of 5 independent experiments. *P < 0.01, as compared to controls (ANOVA with Dunnett’s test).
Discussion
In the present study, we demonstrated that bisphosphonates and statins inhibit MIP-1α expression and secretion by suppressing FPP and GGPP biosynthesis in human MM cells. We also found that bisphosphonates and statins suppressed Ras and Rho prenylation in our experimental model. It has been reported that statins decrease Ras and Rho prenylation by suppressing FPP and GGPP biosynthesis in myeloma cells [25]. We also previously indicated that minodronate and mevastatin induced apoptosis by inhibiting GGPP biosynthesis and Ras prenylation [20,21,24,26]. Collectively, these findings suggest that bisphosphonate- and statin-induced inhibition of small GTPase prenylation through FPP and GGPP biosynthesis plays an important role in the suppression of MIP-1α secretion by MM cells.
Activation of Ras can lead to the activation of MAPKs, the PI3K/Akt pathway, and the PI3K/mTOR pathway [27]. MAPKs are serine/threonine kinases that are present in most cell types and can activate MIP-1α gene expression [28]. The PI3K/Akt pathway has also been reported to regulate MIP-1α expression [28]. Our results clearly demonstrate that bisphosphonates and statins induce decreases in ERK1/2, Akt, and mTOR phosphorylation. Moreover, we observed that U0126 (MEK1/2 inhibitor) and LY294002 (PI3K inhibitor) inhibited MIP-1α mRNA expression in IM9 cells. However, rapamycin (mTOR inhibitor) and Y27632 (ROCK inhibitor) had no affect on MIP-1α and AML-1A mRNA expression or MIP-1α secretion. MIP-1α expression can be induced by various growth factors and cytokines, including acidic fibroblast growth factor and IL-6 [29,30]. These inductions require activation of MAPKs or the PI3K/Akt pathway [31]. Therefore, our present findings indicate that bisphosphonates and statins inhibit MIP-1α expression by suppressing Ras/MEK/ERK and Ras/PI3K/Akt pathways.
MIP-1α expression is related to AML-1A, a transcription factor located downstream of ERK1/2 [32,33]. It has been reported that AML-1A regulates the upregulation of MIP-1α gene promoter activity. We found that bisphosphonates and statins inhibited AML-1A mRNA expression. Moreover, U0126 and LY294002 also inhibited the expression of AML-1A mRNA. These findings suggest that bisphosphonates and statin suppressed MIP-1α expression by inhibiting AML-1A mRNA expression.
In the previous study, we have shown that minodronate inhibited LPS-induced MIP-1α secretion by inhibiting Ras/ERK and Ras/Akt pathways. However, the detailed mechanism by which bisphosphonates and statins suppress MIP-1α secretion by myeloma cells, though, has not been clearly elucidated. The findings of this study suggest that bisphosphonates and statin suppress MIP-1α expression by inhibiting Ras/MEK/ERK/AML-1A and Ras/PI3K/Akt/AML-1A pathways. Our results also indicate that AML-1A expression possibly is regulated by ERK1/2 and Akt activation.
In conclusion, our data showed that bisphosphonates and statins inhibited MIP-1α expression by suppressing Ras/MEK/ERK/AML-1A and Ras/PI3K/Akt/AML-1A pathways in human MM cells. Therefore, the use of MIP-1α expression inhibitors such as bisphosphonates and statins may provide a new therapeutic approach for inhibiting tumour progression and bone destruction in MM patients.
Acknowledgements
This work was supported in part by a Grant-in-Aid for Scientific Research (C) from the Japan Society for the Promotion of Science (JSPS) and by Ministry of Education, Culture, Sports, Science, and Technology (MEXT)-Supported Program for the Strategic Research Foundation at Private Universities, 2014-2018.
Disclosure of conflict of interest
The authors have no competing interest.
References
- 1.Bergsagel D. The incidence and epidemiology of plasma cell neoplasms. Stem Cells. 1995;13:1–9. [PubMed] [Google Scholar]
- 2.Callander NS, Roodman GD. Myeloma bone disease. Semin Hematol. 2001;38:276–285. doi: 10.1016/s0037-1963(01)90020-4. [DOI] [PubMed] [Google Scholar]
- 3.Roodman GD. Mechanisms of bone lesions in multiple myeloma and lymphoma. Cancer. 1997;15:1557–1563. doi: 10.1002/(sici)1097-0142(19971015)80:8+<1557::aid-cncr5>3.3.co;2-k. [DOI] [PubMed] [Google Scholar]
- 4.Anderson K. Advances in the biology of multiple myeloma: therapeutic applications. Semin Oncol. 1999;26:10–22. [PubMed] [Google Scholar]
- 5.Kiss TL, Lipton JH, Bergsagel DE, Meharchand JM, Jamal N, Minden MD, Messner HA. Determination of IL6, IL1, and IL4 in the plasma of patients with multiple myeloma. Leuk Lymphoma. 1994;14:335–340. doi: 10.3109/10428199409049687. [DOI] [PubMed] [Google Scholar]
- 6.Abe M, Hiura K, Wilde J, Moriyama K, Hashimoto T, Ozaki S, Wakatsuki S, Kosaka M, Kido S, Inoue D, Matsumoto T. Role for macrophage inflammatory protein (MIP)-1alpha and MIP-1beta in the development of osteolytic lesions in multiple myeloma. Blood. 2002;100:2195–2202. [PubMed] [Google Scholar]
- 7.Chauhan D, Auclair D, Robinson EK, Hideshima T, Li G, Podar K, Gupta D, Richardson P, Schlossman RL, Krett N, Chen LB, Munshi NC, Anderson KC. Identification of genes regulated by dexamethasone in multiple myeloma cells using oligonucleotide arrays. Oncogene. 2002;21:1346–1358. doi: 10.1038/sj.onc.1205205. [DOI] [PubMed] [Google Scholar]
- 8.Choi SJ, Cruz JC, Craig F, Chung H, Devlin RD, Roodman GD, Alsina M. Macrophage inflammatory protein 1-alpha is a potential osteoclast stimulatory factor in multiple myeloma. Blood. 2000;96:671–675. [PubMed] [Google Scholar]
- 9.Uneda S, Hata H, Matsuno F, Harada N, Mitsuya Y, Kawano F, Mitsuya H. Macrophage inflammatory protein-1 alpha is produced by human multiple myeloma (MM) cells and its expression correlates with bone lesions in patients with MM. Br J Haematol. 2003;120:53–55. doi: 10.1046/j.1365-2141.2003.04040.x. [DOI] [PubMed] [Google Scholar]
- 10.Han JH, Choi SJ, Kurihara N, Koide M, Oba Y, Roodman GD. Macrophage inflammatory protein-1alpha is an osteoclastogenic factor in myeloma that is independent of receptor activator of nuclear factor kappaB ligand. Blood. 2001;97:3349–3353. doi: 10.1182/blood.v97.11.3349. [DOI] [PubMed] [Google Scholar]
- 11.Oba Y, Lee JW, Ehrlich LA, Chung HY, Jelinek DF, Callander NS, Horuk R, Choi SJ, Roodman GD. MIP-1alpha utilizes both CCR1 and CCR5 to induce osteoclast formation and increase adhesion of myeloma cells to marrow stromal cells. Exp Hematol. 2005;33:272–278. doi: 10.1016/j.exphem.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 12.Tsubaki M, Kato C, Manno M, Ogaki M, Satou T, Itoh T, Kusunoki T, Tanimori Y, Fujiwara K, Matsuoka H, Nishida S. Macrophage inflammatory protein-1alpha (MIP-1alpha) enhances a receptor activator of nuclear factor kappaB ligand (RANKL) expression in mouse bone marrow stromal cells and osteoblasts through MAPK and PI3K/Akt pathways. Mol Cell Biochem. 2007;304:53–60. doi: 10.1007/s11010-007-9485-7. [DOI] [PubMed] [Google Scholar]
- 13.Tsubaki M, Kato C, Isono A, Kaneko J, Isozaki M, Satou T, Itoh T, Kidera Y, Tanimori Y, Yanae M, Nishida S. Macrophage inflammatory protein-1α induces osteoclast formation by activation of the MEK/ERK/c-Fos pathway and inhibition of p38MAPK/IRF-3/IFN-β pathway. J Cell Biochem. 2010;111:1661–1672. doi: 10.1002/jcb.22907. [DOI] [PubMed] [Google Scholar]
- 14.Votta BJ, White JR, Dodds RA, James IE, Connor JR, Lee-Rykaczewski E, Eichman CF, Kumar S, Lark MW, Gowen M. CKbeta-8 [CCL23] , a novel CC chemokine, is chemotactic for human osteoclast precursors and is expressed in bone tissues. J Cell Physiol. 2000;183:196–207. doi: 10.1002/(SICI)1097-4652(200005)183:2<196::AID-JCP6>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 15.Lentzsch S, Gries M, Janz M, Bargou R, Dörken B, Mapara MY. Macrophage inflammatory protein 1-alpha (MIP-1 alpha) triggers migration and signaling cascades mediating survival and proliferation in multiple myeloma (MM) cells. Blood. 2003;101:3568–3573. doi: 10.1182/blood-2002-08-2383. [DOI] [PubMed] [Google Scholar]
- 16.Tsubaki M, Kato C, Nishinobo M, Ogaki M, Satou T, Ito T, Kusunoki T, Fujiwara K, Yamazoe Y, Nishida S. Nitrogen-containing bisphosphonate, YM529/ONO-5920, inhibits macrophage inflammatory protein 1 alpha expression and secretion in mouse myeloma cells. Cancer Sci. 2008;99:152–158. doi: 10.1111/j.1349-7006.2007.00651.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choi SJ, Oba Y, Gazitt Y, Alsina M, Cruz J, Anderson J, Roodman GD. Antisense inhibition of macrophage inflammatory protein 1-alpha blocks bone destruction in a model of myeloma bone disease. J Clin Invest. 2001;108:1833–1841. doi: 10.1172/JCI13116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fleisch H. Bisphosphonates: mechanisms of action. Endocr Rev. 1998;19:80–100. doi: 10.1210/edrv.19.1.0325. [DOI] [PubMed] [Google Scholar]
- 19.Caraglia M, Santini D, Marra M, Vincenzi B, Tonini G, Budillon A. Emerging anti-cancer molecular mechanisms of aminobisphosphonates. Endocr Relat Cancer. 2006;13:7–26. doi: 10.1677/erc.1.01094. [DOI] [PubMed] [Google Scholar]
- 20.Tsubaki M, Satou T, Itoh T, Imano M, Yanae M, Kato C, Takagoshi R, Komai M, Nishida S. Bisphosphonate- and statin-induced enhancement of OPG expression and inhibition of CD9, M-CSF, and RANKL expressions via inhibition of the Ras/MEK/ERK pathway and activation of p38MAPK in mouse bone marrow stromal cell line ST2. Mol Cell Endocrinol. 2012;361:219–231. doi: 10.1016/j.mce.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 21.Nishida S, Matsuoka H, Tsubaki M, Tanimori Y, Yanae M, Fujii Y, Iwaki M. Mevastatin induces apoptosis in HL60 cells dependently on decrease in phosphorylated ERK. Mol Cell Biochem. 2005;269:109–114. doi: 10.1007/s11010-005-3086-0. [DOI] [PubMed] [Google Scholar]
- 22.Woo JT, Kasai S, Stern PH, Nagai K. Compactin suppresses bone resorption by inhibiting the fusion of prefusion osteoclasts and disrupting the actin ring in osteoclasts. J Bone Miner Res. 2000;15:650–662. doi: 10.1359/jbmr.2000.15.4.650. [DOI] [PubMed] [Google Scholar]
- 23.Yanae M, Tsubaki M, Satou T, Itoh T, Imano M, Yamazoe Y, Nishida S. Statin-induced apoptosis via the suppression of ERK1/2 and Akt activation by inhibition of the geranylgeranyl-pyrophosphate biosynthesis in glioblastoma. J Exp Clin Cancer Res. 2011;30:74. doi: 10.1186/1756-9966-30-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsubaki M, Itoh T, Satou T, Imano M, Komai M, Ogawa N, Mukai J, Nishida S. Nitrogen-containing bisphosphonates induce apoptosis of hematopoietic tumor cells via inhibition of Ras signaling pathways and Bim-mediated activation of the intrinsic apoptotic pathway. Biochem Pharmacol. 2013;85:163–172. doi: 10.1016/j.bcp.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 25.Cafforio P, Dammacco F, Gernone A, Silvestris F. Statins activate the mitochondrial pathway of apoptosis in human lymphoblasts and myeloma cells. Carcinogenesis. 2005;26:883–891. doi: 10.1093/carcin/bgi036. [DOI] [PubMed] [Google Scholar]
- 26.Nishida S, Fujii Y, Yoshioka S, Kikuichi S, Tsubaki M, Irimajiri K. A new bisphosphonate, YM529 induces apoptosis in HL60 cells by decreasing phosphorylation of single survival signal ERK. Life Sci. 2003;73:2655–2664. doi: 10.1016/s0024-3205(03)00664-7. [DOI] [PubMed] [Google Scholar]
- 27.Jiang BH. Liu LZ. Role of mTOR in anticancer drug resistance: perspectives for improved drug treatment. Drug Resist Updat. 2008;11:63–76. doi: 10.1016/j.drup.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Song X, Tanaka S, Cox D, Lee SC. Fcgamma receptor signaling in primary human microglia: differential roles of PI-3K and Ras/ERK MAPK pathways in phagocytosis and chemokine induction. J Leukoc Biol. 2004;75:1147–1155. doi: 10.1189/jlb.0403128. [DOI] [PubMed] [Google Scholar]
- 29.Masih-Khan E, Trudel S, Heise C, Li Z, Paterson J, Nadeem V, Wei E, Roodman D, Claudio JO, Bergsagel PL, Stewart AK. MIP-1alpha (CCL3) is a downstream target of FGFR3 and RAS-MAPK signaling in multiple myeloma. Blood. 2006;108:3465–3471. doi: 10.1182/blood-2006-04-017087. [DOI] [PubMed] [Google Scholar]
- 30.Smith RE, Strieter RM, Phan SH, Lukacs N, Kunkel SL. TNF and IL-6 mediate MIP-1alpha expression in bleomycin-induced lung injury. J Leukoc Biol. 1998;64:528–536. [PubMed] [Google Scholar]
- 31.Liu S, Ma Z, Cai H, Li Q, Rong W, Kawano M. Inhibitory effect of baicalein on IL-6-mediated signaling cascades in human myeloma cells. Eur J Haematol. 2010;84:137–144. doi: 10.1111/j.1600-0609.2009.01365.x. [DOI] [PubMed] [Google Scholar]
- 32.Choi SJ, Oba T, Callander NS, Jelinek DF, Roodman GD. AML-1A and AML-1B regulation of MIP-1alpha expression in multiple myeloma. Blood. 2003;101:3778–3783. doi: 10.1182/blood-2002-08-2641. [DOI] [PubMed] [Google Scholar]
- 33.Lee JW, Chung HY, Ehrlich LA, Jelinek DF, Callander NS, Roodman GD, Choi SJ. IL-3 expression by myeloma cells increases both osteoclast formation and growth of myeloma cells. Blood. 2004;103:2308–2315. doi: 10.1182/blood-2003-06-1992. [DOI] [PubMed] [Google Scholar]


