Abstract
Background: Dysregulation of BCL6 plays critical oncogenic roles and facilitates tumorigenesis in various malignancies. However, whether the aberrant expression of BCL6 in ovarian carcinoma is associated with malignancy, metastasis or prognosis remains unknown. Our study aimed to investigate the expression of BCL6 in ovarian carcinoma and its possible correlation with clinicopathological features as well as patient survival to reveal its biological effects in ovarian tumor progression. Methods: Immunochemistry analysis was performed in 105 cases of ovarian carcinoma covering the histological types of serous, endometrioid and clear cell. Spearman analysis was used to calculate the correlation between pathological parameters and the expression of BCL6. Kaplan–Meier method and Cox proportional hazards analysis were used to analyze the disease-specific survival (DSS) and disease-free survival (DFS). We also assessed whether overexpression and knockdown of BCL6 influence in vitro cell proliferation, cell cycle progression, as well as tumor cell invasion and migration. Results: The expression of BCL6 was higher in all three major kinds of ovarian cancer in comparison with paratumorous epithelium. BCL6 expression was tightly correlated with FIGO staging, lymph node metastasis and recurrence. Higher expression of BCL6 led to a significantly poorer DSS and DFS and multivariate analysis revealed that BCL6 was an independent risk factor of DSS and DFS. Enforced overexpression of BCL6 in ovarian tumor cells stimulated proliferation by inducing G1–S transition, and promoted tumor cell invasion and migration. Conversely, RNA interference–mediated silencing BCL6 expression inhibited proliferation by altered cell cycle progression and reduced the ability of the cells to migrate, and invade the extracellular matrix in culture. Conclusions: Our study suggests that the inappropriate activation of BCL6 predicts poor prognosis and promotes tumor progression in ovarian carcinoma. Targeting BCL6 could be a novel therapeutic choice for treating ovarian carcinoma patients.
Keywords: BCL6, ovarian carcinoma, prognosis, proliferation, invasion
Introduction
Ovarian carcinoma, as the leading malignancy of female congenital system with a high mortality worldwide [1], its high invasiveness and recurrence cause a total crude 5-year survival of only 35% [2]. Searching the prognostic predictor of ovarian carcinoma could be not only helpful with the clinical prevention but also suggestive of oncological pharmacology.
B-cell CLL/lymphoma 6 (BCL6), a highly conserved zinc finger transcriptional factor identified from lymphoma [3,4], has earned a reputation as an oncogene in human cancers by negative regulating p53 [5-8]. In 2009 Hirata for the first time reported that BCL6 was overexpressed in gastric cancers [9], bringing a speculation that BCL6 exerted its oncogenic functions in somatic malignancies other than lymphomas, which were subsequently confirmed by series of researches in breast cancer [10,11]. However, whether the aberrant expression of BCL6 in ovarian carcinoma is associated with malignancy, metastasis or prognosis remains unknown.
The primary aim of this present study was to investigate whether BCL6 is detectable and altered in ovarian cancer tissues compared with adjacent normal tissues. Then, we investigated the potential relationship between BCL6 levels and existing clinicopathological features of ovarian cancer, such as tumor size, histologic types, FIGO stage, the status of lymphatic metastasis, remote metastasis, recurrence and prognosis. We also assessed whether BCL6 influences in vitro cell proliferation, migration or invasion.
Materials and methods
Patients collections and immunochemistry
A total of 105 female patients (aged 24~59 years; mean, 37.3 years) with ovarian carcinoma were selected from Gynecology and Obstetrics Hospital of Fudan University, Shanghai, China from 2009-2013. None of patients had received preoperative chemotherapy. The collected clinicopathological features included tumor size, histological types, FIGO stage, lymph node metastasis, remote metastasis, and recurrence. All the follow-up information was got from the Follow-up Center of Gynecology and Obstetrics Hospital of Fudan University, Shanghai, China. All patients were staged based on the International Federation of Gynecology and Obstetrics (FIGO) staging system [12]. The follow-up interval was from the date of surgery to the date of death or the last clinical investigation. This study was approved by The Clinical Research Ethics Committee of Gynecology and Obstetrics Hospital of Fudan University. Written informed consent was obtained from all participants.
Consecutive paraffin sections of these cases were prepared and incubated overnight at 37°C with primary antibodies against BCL6 at a 1:400 dilution. Standard avidin-biotin immunohistochemical analysis of the sections was done. The staining results were evaluated by at least three certificated pathologists and the staining in the tumor cells was scored as 0 (no staining for nucleus despite of the staining result of cytoplasm or membrane), 1 (weak staining for nucleus (and cytoplasm) and area of positive staining < 10%), 2 (moderate staining for nucleus (and cytoplasm) and area of positive staining among 10%-50%), and 3 (strong staining for nucleus (and cytoplasm) and area of positive staining > 50%) [10,13]. The cases with score 0-1 were considered as low and those with score 2-3 were considered as high [10,13].
Cell lines and culture conditions
Human ovarian carcinoma cell lines NIH: OVCAR3, 3AO, A2780, CAOV3, ES-2 and SKOV3 were purchased from the Fudan University IBS cell bank, Shanghai, China. All cells were grown and maintained in RPMI-1640 medium (Gibco, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS) (Gibco, Carlsbad, CA, USA) and 1% penicillin and streptomycin (Sigma, St. Louis, MO, USA), maintain at 37°C in a humidified atmosphere with 5% CO2.
Antibodies and reagents
For antibodies used: BCL6 (#5650), HA-tag (6E2, #2367), CyclinB1 (V152, #9869), Cdc25B (#9525), N-Cadherin (D4R1H, #13116), MMP2 (D8N9Y, #13132), MMP9 (D6O3H, #13667) and GAPDH (D16H11, #5174) was from Cell Signaling, Boston, Massachusetts, USA; For reagents used: Lipofectamine 3000 transfection reagent (Lot. 11668-027 Invitrogen, Carlsbad, CA, USA). RIPA Lysis buffer (Lot. 89901, Thermo Scientific, USA). The pcDNA3.1-HA-BCL6 was a gift from Dr. Q.W. of Nanjing agriculture University, Nanjing, China. The siRNAs of BCL6 (5’-CGGCUCAAUAACAUCGUUATT-3’, 5’-UAACGAUGUUAUUGAGCCGTT-3’) and scramble siRNA were purchase from Ribobio, Guangzhou, China.
Proliferation and cell cycle assays
Cells were seeded in 96- or 6-well plates 24 h before the experiment. ES-2 and SKOV3 cells were transfected with pcDNA3.1-HA-BCL6 or negative control; CAOV3 cells were transfected with siBCL6 or scramble siRNA).
Proliferation was measured using the CCK-8 kit (Dojindo, Japan) and EdU DNA imaging kit (Invitrogen, Life technology, USA). The CCK8 assay counts the living cells in different time points and the EdU imaging system helps to visualize the difference of cell growth. Approximately transfected 3.5 × 103 cells in 100 μl were incubated in triplicate in 96-well plates. At 0, 24, 48, 72 and 96 h, the CCK-8 reagent (10 μl) was added to each well and incubated at 37°C for 3 h. The optical density at 450 nm was measured using an automatic microplate reader (Synergy4; BioTek, Winooski, VT, USA). For EdU DNA assay, 72 h after transfection, cells were incubated with 10 μM EdU solution for 2 h and fixed with 3.7% Formaldehyde (Sigma) and penetrated with 0.5% triton X-100 (Sigma) for 20 minutes. Then the cells were stained with EdU/Alexia Fluor Azide 594 for 30 minutes followed by Hoechst 33342 (1:2000) for another 30 minutes and then imaged at 100× and counted 200× under the immunofluorescence microscopy (IX51, Olympus, Japan).
For cell cycle analysis, cells were collected 48 hours post-transfection and fixed with ethanol for 24 h. The cells were washed by PBS twice and then stained with propidium iodide (PI, Calbiochem) for 20 minutes and subjected to flow cytometry analysis. Representative data from one of three independent experiments are shown.
Cell invasion detection
The Transwell assay was used to assess cell invasion with the transwell system of Corning co. Ltd., USA. The lower chambers were pre-coated with 100 μl Matrix gel (#354234, BD Bioscience, USA) for 30min. 24h after transfection, cells were seeded on the upper chamber at a density of 3.0 × 104 cells/well in serum-free medium. Medium containing 20% fetal bovine serum medium was applied to the lower chamber as chemo-attractant. After 24 h incubation at 37°C, non-invasive cells remaining on the upper surface of the membrane were removed by wiping with cotton-tipped swabs. Cells which invaded through the matrix gel and adhered to the lower surface of the filter were fixed with Ethanol, stained with 0.5% crystal violet, photographed at 200×, and counted at 400× under microscope (BX51, Olympus, Japan).
Cell migration detection
The in vitro wound-healing assay was used to assess cell motility. Transfected cells were plated at equal density in 6-well plates and grown to confluence. Wounds were then generated with a sterile pipette tip, cells were rinsed two times with PBS and serum-free culture medium was added. Photos were taken at 24 h at 100× under microscope (BX51, Olympus, Japan).
Western blotting
The cell lysates were added with sample buffer and boiled at 95°C for 5 min. The samples were transferred to SDS-PAGE at 80V for 3 h and then transferred to PVDF membranes for another 2 h. The membranes then were blocked by 5% BSA for 30 min and incubated with primary antibodies at 4°C overnight and washed by 1% TBST for three times followed by incubated with secondary antibodies for 1 h. The final detection of the substrates was performed with the ECL system (#32209, Thermo Fisher, USA).
Total RNA isolation and RT-qPCR
Total RNA was isolated from cells by Trizol (Invitrogen, Life technology, USA). The RT and qPCR reactions were performed as previously described [14], and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an endogenous control to normalize the data. Primer sequences used in this study were the following: 5’-TACAGGATCATTGGCTACACACC-3’ (forward) and 5’-GGTCACATCGCTCCAGACT-3’ (reverse) for MMP2, 5’-TGTACCGCTATGGTTACACTCG-3’ (forward) and 5’-GGCAGGGACAGTTGCTTCT-3’ (reverse) for MMP9, 5’-GGAGTCGAGACATCTTGACTGA-3’ (forward) and 5’-ATGAGGACCGTTTTATGGGCT-3’ (reverse) for BCL6, 5’-GCACCAGGTTTGGAATGGG-3’ (forward) and 5’-CATGTTGGGAGAAGGGGTG-3’ (reverse) for N-Cadherin.
Statistical analysis
Each experiment was repeated 3 times, and the data are presented as the mean with error bars indicating the standard deviation. All statistical analyses were performed using SPSS 20.0 (IBM, SPSS, Chicago, IL, USA). Student’s t-test and one-way ANOVA were used in either 2 or multiple groups for statistical significance. Spearman rank order was used to analyze the correlations between clinical parameters and expressions of BCL6 of immunochemistry; DFS and DSS curves were calculated with the Kaplan-Meier method and were analyzed with the log-rank test. The DFS rate was calculated from the date of surgery to the date of progression (local and/or distal tumor recurrence) or to the date of death. The DSS rate was defined as the length of time between the diagnosis and death or last follow-up. Univariate analysis and multivariate models were fit using a Cox proportional hazards regression model. A P value under 0.05 was considered significant.
Results
BCL6 was upregulated in malignant ovarian epithelial cancer
First we conducted immunochemistry in 105 patients with malignant ovarian epithelial cancer to investigate whether BCL6 could be detected and whether its expression was altered in cancer tissue. Specific BCL6 staining was observed scattered in the nucleus and cytoplasm of cancer cells in 96 of 105 cases of all three major histological types (serous, endometrioid and clear cell types), whereas no staining was observed in paratumorous epithelium (Figure 1A). In the cases with carcinoma of clear cell types the staining of BCL6 was positive in membrane and nucleus due to the occupation of cytoplasm by glycogen (Figure 1A). Furthermore, BCL6 expression levels were compared in cancer tissue samples according to FIGO stages. As shown in Figure 1B, the expression of BCL6 was doubtfully positive in Stage I cancer but got intense along with the advance of FIGO stage.
Figure 1.
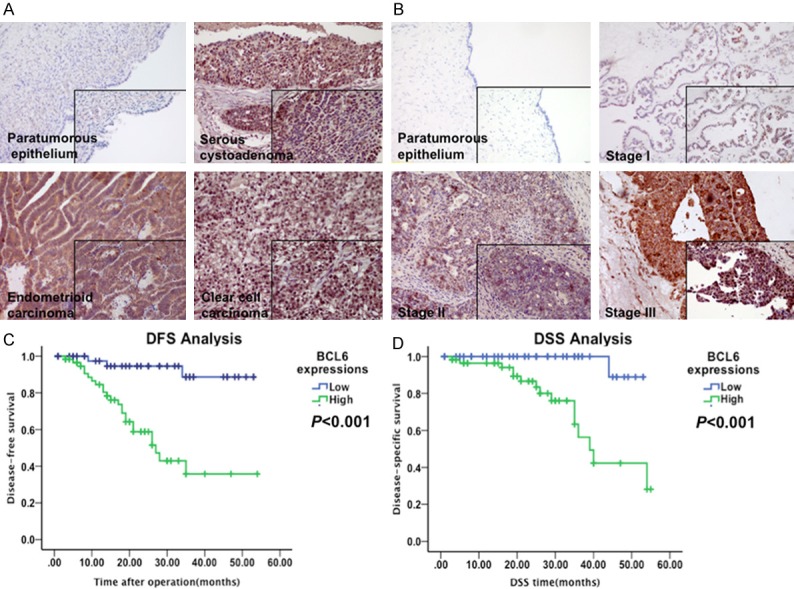
BCL6 was highly expressed and predicted poor prognosis in ovarian carcinoma. A: Representative immunostaining results of BCL6 in paratumorous epithelium, serous, endometrioid and clear cell carcinoma. B: Representative immunostaining results of BCL6 in paratumorous epithelium, malignant carcinoma of Stage I, II and III. C, D: Kaplan-Meier disease-free survival (DFS) (C) and disease-specific survival (DSS) (D) curves of patients with different expressions of BCL6 in ovarian carcinoma (Low vs. High).
BCL6 upregulation correlated with clinicopathologic characteristics and the survival of patients with ovarian carcinoma
Next we correlated BCL6 expression levels with the clinicopathological status of patients with ovarian carcinoma (Table 1). The expression levels of BCL6 were upregulated in tumors with a higher tumor burden, as defined by a larger tumor size (P = 0.05), more advanced FIGO stage (P < 0.001), presence of lymph node metastasis (P = 0.21), as well as the existence of recurrence (P = 0.001) (Table 1). However, there was no significant correlation between the expression of BCL6 and age, histological classification or remote metastasis.
Table 1.
The relationship between clinicoathological parameters and expression of BCL6
| Clinicopathological Features | N | % | BCL6 Expression | Rho value | P value | ||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| 0 | 1 | 2 | 3 | ||||||
| All cases | 105 | 100 | 19 | 24 | 22 | 40 | |||
| Age (years) | < 40 | 59 | 32.17 | 14 | 16 | 6 | 23 | 0.125 | 0.203 |
| (Average 57.6) | ≥ 40 | 46 | 57.14 | 5 | 8 | 16 | 17 | ||
| Tumor size | < 5 cm | 57 | 54.29 | 9 | 20 | 13 | 15 | 0.191 | 0.05* |
| ≥ 5 cm | 48 | 45.71 | 7 | 10 | 7 | 24 | |||
| Histological types | Serous | 43 | 40.92 | 10 | 8 | 3 | 22 | -0.166 | 0.091 |
| Endometrioid | 31 | 29.52 | 2 | 9 | 6 | 14 | |||
| Clear Cell | 31 | 19.52 | 4 | 14 | 11 | 3 | |||
| FIGO Stage | I | 47 | 42.86 | 13 | 21 | 3 | 10 | 0.43 | < 0.001* |
| II | 32 | 30.48 | 3 | 4 | 8 | 17 | |||
| III, IV | 26 | 24.76 | 0 | 5 | 9 | 12 | |||
| Lymph node metastasis | - | 89 | 84.76 | 16 | 27 | 16 | 30 | 0.225 | 0.021* |
| + | 16 | 15.24 | 0 | 3 | 4 | 9 | |||
| Remote metastasis | - | 103 | 98.1 | 16 | 30 | 20 | 37 | 0.159 | 0.106 |
| + | 2 | 1.9 | 0 | 0 | 0 | 2 | |||
| Recurrence | - | 78 | 74.3 | 18 | 28 | 10 | 25 | 0.308 | 0.001* |
| + | 27 | 25.7 | 1 | 2 | 10 | 14 | |||
N, number of patients;
p < 0.05.
When linked to the prognosis, the Kaplan-Meier analysis using the log-rank test showed that patients with high levels of BCL6 expression (n = 62) had significantly shorter DFS (P < 0.001; Figure 1C) and DSS (P < 0.001; Figure 1D) than those with low levels of BCL6 expression (n = 43). A univariate Cox analysis showed that FIGO stage, lymph node metastasis, and BCL6 expression were correlated with the survival (Tables 2 and 3). Multivariate analysis using the Cox proportional hazard model demonstrated that BCL6 expression were independent risk factors for DFS (P = 0.009, Table 2) and DSS (P = 0.011, Table 3) in addition to lymph node metastasis. Notably, FIGO staging was also an independent risk factor for DFS but not for DSS (Tables 2 and 3). These results identified the overexpression of BCL6 in ovarian carcinoma seemed to be a risk factor predicting poor survival, suggesting that overexpression of BCL6 likely contributes to ovarian carcinoma pathogenesis and might represent a prognostic biomarker for the disease.
Table 2.
The Univariate and multivariate Cox proportional analysis of disease-free survival of cases with ovarian carcinoma
| Variables | Categories | Univariate analysis | P value | Multivariate analysis | P value | ||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| HR | 95% CI | HR | 95% CI | ||||
| Age | < 40 | 0.975 | 0.934-1.018 | 0.25 | |||
| ≥ 40 | |||||||
| FIGO stage | I | 3.806 | 2.227-6.504 | < 0.001* | 2.162 | 1.047-4.465 | 0.037* |
| II | |||||||
| III-IV | |||||||
| Tumor size | < 5 cm | 0.624 | 0.292-1.336 | 0.223 | |||
| ≥ 5 cm | |||||||
| Lymph node metastasis | - | 7.902 | 3.650-17.107 | < 0.001* | 2.754 | 1.005-7.548 | 0.049* |
| + | |||||||
| BCL6 expression | Low | 8.992 | 2.680-30.162 | < 0.001* | 5.407 | 1.528-19.130 | 0.009* |
| High | |||||||
HR, Hazard ratio, CI, confidence interval,
P < 0.05.
Table 3.
The Univariate and multivariate Cox proportional analysis of disease-specific survival of cases with ovarian carcinoma
| Variables | Categories | Univariate analysis | P value | Multivariate analysis | P value | ||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| HR | 95% CI | HR | 95% CI | ||||
| Age | < 40 | 0.976 | 0.920-1.035 | 0.416 | |||
| ≥ 40 | |||||||
| FIGO stage | I | 3.425 | 1.730-6.778 | < 0.001* | |||
| II | |||||||
| III-IV | |||||||
| Tumor size | < 5 cm | 1.493 | 0.529-4.209 | 0.449 | |||
| ≥ 5 cm | |||||||
| Lymphnode metastasis | - | 6.808 | 2.480-18.692 | 0.001* | 8.752 | 2.976-25.737 | 0.001* |
| + | |||||||
| BCL6 expression | Low | 2.908 | 1.498-5.648 | 0.002* | 15.011 | 1.869-120.587 | 0.011* |
| High | |||||||
HR, Hazard ratio, CI, confidence interval,
P < 0.05.
Identification of the efficiencies of BCL6 overexpression and knockdown
The baseline BCL6 expressions was examined in six ovarian cancer cell lines including ES-2, CAOV3, NIH:OVCAR3, 3AO, SKOV3 and A2780 by both Western blotting and RT-qPCR (Figure 2A, 2B). It turned out that BCL6 were expressed in all six ovarian tumor cell line and its expression was lower in both ES-2 and SKOV3 while higher in CAOV3 (Figure 2A, 2B). Then both ES-2 and SKOV3 cells were transfected with pcDNA3.1-HA-BCL6 for 48 h and CAOV3 cells with siBCL6 for 24 h followed by immunoblotting and RT-qPCR. The mRNA levels of BCL6 were significantly increased in pcDNA3.1-HA-BCL6 transfecting SKOV3 and ES-2 cells compared with the mock or the vector-transfecting counterparts (Figure 2C, 2D). While in CAOV3 cells the BCL6 was decreased in the cells transfected with siRNA both on the protein and the mRNA levels (Figure 2C, 2D).
Figure 2.
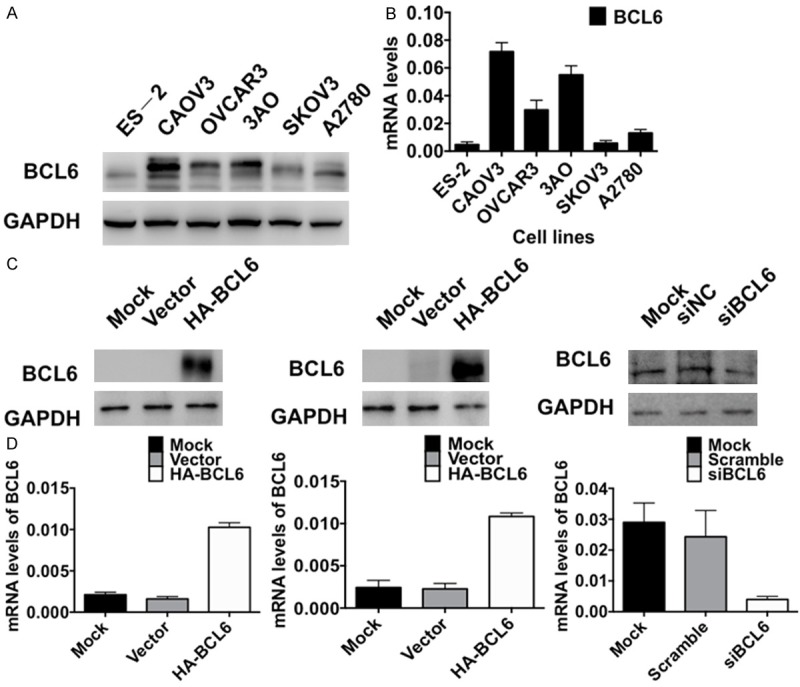
Efficiencies of overexpression and knockdown of BCL6 in ovarian carcinoma. A: The baseline expressions of BCL6 protein in six ovarian tumor cell lines detected by Western blotting. B: The baseline mRNA levels of BCL6 in six ovarian tumor cell lines detected by RT-qPCR. C: The expressions of BCL6 protein in SKOV3 and ES-2 cells transfected with pcDNA3.1-HA-BCL6 and in CAOV3 cells transfected with siBCL6 detected by Western blotting. D: The mRNA levels of BCL6 in SKOV3 and ES-2 cells transfected with pcDNA3.1-HA-BCL6 and in CAOV3 cells transfected with siBCL6 detected by RT-qPCR.
BCL6 promotes ovarian tumor cell proliferation
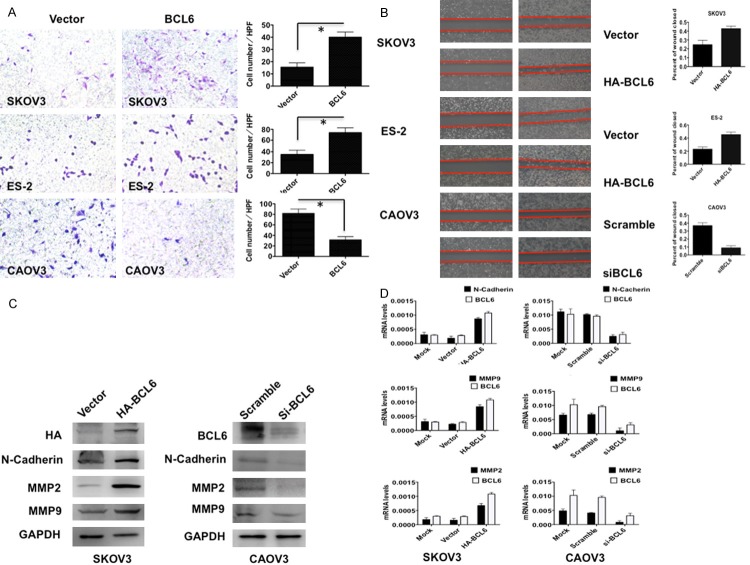
To observe the influence of BCL6 on tumor cell proliferation, we conducted the CCK8 cell counting and EdU imaging assays. The CCK8 assay showed that the tumor cell growth of the BCL6-overexpressing SKOV3 and ES-2 cells was significantly accelerated compared with that of the controls (Figure 3A). While in CAOV3 cells, knockdown of BCL6 by siRNA effectively inhibited the cell proliferation (Figure 3A). The EdU imaging assay visualized the cell growth especially the cell population of S phase at 72 h after transfection. Again in BCL6-overexpressing SKOV3 and ES-2 cells, more living cells and more cells of S phase were observed than in controls, while knockdown of BCL6 by siRNA caused a sharp reduction of living cells in CAOV3 (Figure 3B, p < 0.01). These results suggested that BCL6 promoted cell proliferation in ovarian carcinoma in vitro.
Figure 3.
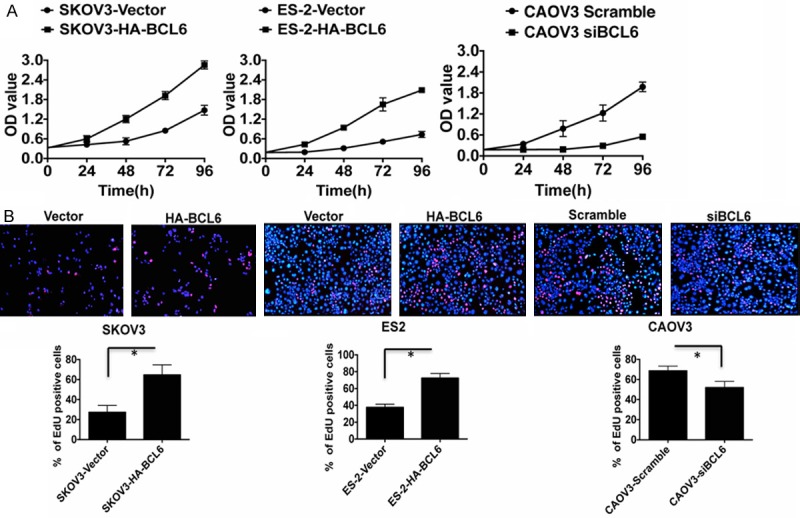
BCL6 stimulated tumor cell proliferation in ovarian carcinoma. A: The CCK8 cell counting assays revealed cell growth curves of indicated cells. B: The EdU imaging results revealed cell growth curves of indicated cells (The scale bars = 100 μm). The EdU positive cells were counted under 200× by immunofluorescence microscope. *p < 0.05.
BCL6 facilitated cell cycle progression in ovarian carcinoma cells
To dissect the biologic events accompanying the alterations of cell proliferation caused by BCL6, fluorescence-activated cell sorting (FACS) was applied to analyze changes of DNA content throughout various phases of the cell cycle. The FACS found a significantly decreased proportion of cells in the G1 phase but elevated in the S phases in BCL6-overexpressing SKOV3 and ES-2 cells compared with controls (Figure 4A, p < 0.01). In contrast, knockdown of BCL6 by siRNA in CAOV3 led to an increase of cells in the G1 phase but reduction in S phases (Figure 4A, p < 0.01). The Western blotting results also showed that overexpression of BCL6 in SKOV3 cells induced the expression of CyclinB1 and Cdc25B (Figure 4B top). On the contrary, knockdown of BCL6 by siRNA in CAOV3 caused a reduction of CyclinB1 and Cdc25B (Figure 4B bottom). Taken together, these results suggested that BCL6 promoted cell cycle progression by inducing G1-S transition in ovarian carcinoma.
Figure 4.
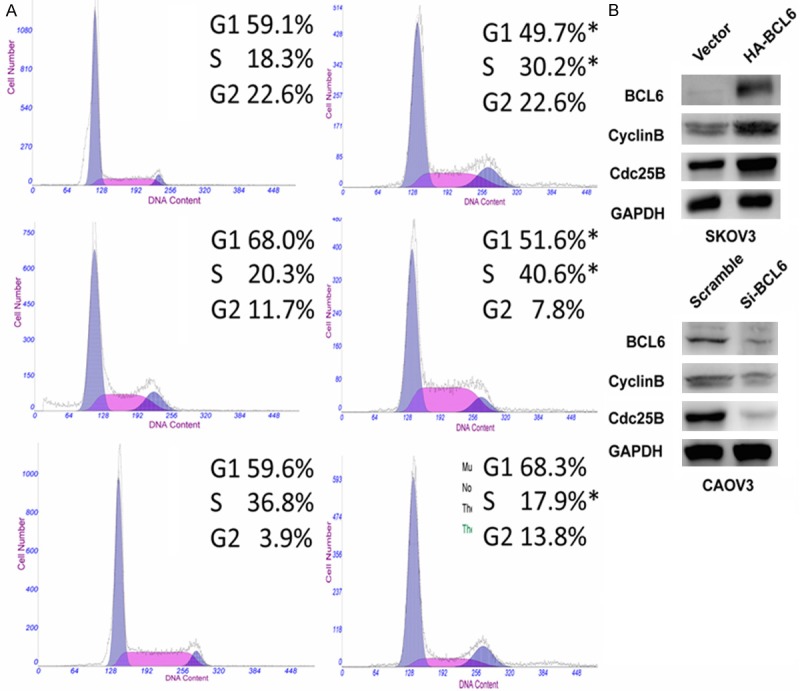
BCL6 promoted cell cycle progression in ovarian carcinoma. A: Representative Flow-cytometric images with determination of proportion of indicated cells in distinct cell-cycle phases. *p < 0.05. B: The Western blotting results of CyclinB1 and Cdc25B in indicated cells. GAPDH was used as reference.
BCL6 enhanced tumor cell migration and invasion in vitro
To determine whether BCL6 promoted tumor cell invasion and migration in ovarian carcinoma, we performed Transwell and wound-healing assays. Overexpression of BCL6 in both SKOV3 and ES-2 cells resulted in a significant increase in the ability of the cells to migrate (Figure 5A, p < 0.01) and invade through an extracellular matrix (Figure 5B, p < 0.01). while knockdown of BCL6 effectively abolished the mobility (Figure 5A, p < 0.01) and invasiveness (Figure 5B, p < 0.01) of CAOV3 cells. Accordingly, the Western blotting and RT-qPCR results supported that overexpression of BCL6 in SKOV3 cells induced the expression invasion-promoting genes such as N-Cadherin, MMP2 and MMP9, while knockdown of BCL6 in CAOV3 cells suppressed the expressions of these genes both on the mRNA and protein levels (Figure 5C, 5D). Collectively, these data suggested that BCL6 induced tumor cell migration and invasion in ovarian carcinoma.
Figure 5.
BCL6 induced tumor cell invasion and migration in ovarian carcinoma. A: Representative images (left) and quantification (right) of transwell invasion assays for indicated cells. (Scale bars = 50 μm). *p < 0.01. B: Representative images (left) and quantification (right) of wound-healing assays for indicated cells. (Scale bars = 400 μm). *p < 0.01. C: The immunoblotting results of N-Cadherin, MMP2 and MMP9 in indicated cells. GAPDH was used as reference. D: The mRNA levels of N-Cadherin, MMP2 and MMP9 in indicated cells. GAPDH was used as reference.
Discussion
As far as it is concerned, our study was the first one to explore the expression of BCL6 and its biological functions in ovarian carcinoma. We confirmed that BCL6 was overexpressed in three major subtypes of ovarian epithelial cancers, clinically, high expression levels of BCL6 correlate with ovarian carcinoma DFS and DSS, might represent a novel prognostic indicator for patients with ovarian carcinoma. In addition, BCL6 contributes to the malignant characters of ovarian cancer cells through involvement in diverse cellular processes, including proliferation, migration and invasion.
Just as its name suggested, BCL6 was firstly found in malignant lymphomas especially DLBCL [15-17]. In the past five years researchers gradually found that BCL6 could also be in detected in somatic malignancies, which is found to be highly expressed in cancer tissues than the corresponding noncancerous tissues in gastric cancers [9] and primary breast tumors [11,18,19]. Similarly, the present study showed that higher levels of BCL6 staining were observed in all three major histological types of ovarian epithelial cancer tissue than in para-tumorous epithelium, suggesting that the elevation of BCL6 may also be an important molecular event in somatic malignancies other than lymphomas.
The correlation of BCL6 upregulation with a higher tumor burden including larger tumor size and presence of lymph node metastasis in our study suggested that BCL6 may facilitate tumor progression in ovarian carcinoma mainly via stimulating tumor growth and tumor invasion, which was supported by the results of in vitro experiments. Considering that the FIGO staging was majorly concerned about tumor invasiveness [12], it was not surprising that the expression of BCL6 got intense along with the advance of stages and was tightly correlated with FIGO staging. Indeed, BCL6 might push the advance of FIGO staging by inducing tumor migration and invasion in ovarian carcinoma. Therefore, it was reasonable to find that overexpression of BCL6 suggested a poorer DFS and DSS in patients with ovarian carcinoma. Taken together, monitoring the levels of BCL6 might be an effective method in the follow-up of ovarian carcinoma.
Recently BCL6 was reported to exert a “hit-and-run” mechanism in DLBCL, which mean that even transient overexpression of BCL6 can obviously trigger the oncogenicity of DLBCL [16]. Our study identified that transient overexpression of BCL6 could significantly upregulating CyclinB1 and Cdc25B to accelerate the cell cycle progression, thus promoting the tumor cell proliferation. Moreover, N-Cadherin, MMP2 and MMP9 were crucial in tumor invasion as well as epithelial-mesenchymal transition (EMT) [20-22]. Thus the elevation of these three invasion-related genes both on the mRNA and protein levels might explain why overexpression of BCL6 facilitated tumor migration and invasion. Future studies might focus on the specific mechanisms of BCL6 in the development of ovarian carcinoma, and its potential interactions with other EMT-related genes such as E-Cadherin [20,23,24], Snail [24,25] and FOXM1 [13,26].
Finally, knocking down of BCL6 potently suppressed the tumor-promoting effects of BCL6 in CAOV3 cells. These results on one hand inversely supported that overexpression of BCL6 should be responsible for the tumor progression in ovarian carcinoma, and on the other hand suggested that suppression of BCL6 could be an effective therapeutic choice in clinical practice. Further studies might consider BCL6 as a major molecular target in future pharmacological designing. Indeed, Cerchietti et al. [27] found that a small-molecular peptide inhibitor that bond to the BTB negative groove of BCL6 could inhibited the tumorigenicity of DLBCL in xenograft models, which shed a light on developing effective BCL6-inhibitors in ovarian cancer.
Conclusions
Our study suggests that BCL6 predicts poor prognosis and promotes tumor progression in ovarian carcinoma. BCL6 might be applied in clinical practice as a biomarker to monitor the invasiveness of tumor and to predict the prognostic risk of patients. Targeting BCL6 could be a therapeutic choice in ovarian carcinoma and the BCL6 inhibitors might be considered in future pharmacological designing.
Acknowledgements
This study was supported by National Clinical Key Discipline (2013-2015), Priority of Shanghai key discipline of medicine (2013-2015), Shanghai R&D public service platform construction projects (12DZ2295100), National Natural Science Foundation of China (81071791), Clinical Key Discipline Fund by Ministry of Health (2010-2012), and Shanghai Science and Technology Development Fund (Basic Research Major Project, No. 10DJ1400500). We thank the faculty of department of pathology, Gynecology & Obstetrics Hospital of Fudan University to prepare the paraffin sections for immunochemistry.
Disclosure of conflict of interest
The authors declare no conflict of interest.
References
- 1.Lazarov N, Lazarov L, Lazarov S. [Tumor size and prognosis for patients with epithelial ovarian carcinoma at early stage] . Akush Ginekol (Sofiia) 2013;52:10–12. [PubMed] [Google Scholar]
- 2.Anuradha S, Webb PM, Blomfield P, Brand AH, Friedlander M, Leung Y, Obermair A, Oehler MK, Quinn M, Steer C, Jordan SJ. Survival of Australian women with invasive epithelial ovarian cancer: a population-based study. Med J Aust. 2014;201:283–288. doi: 10.5694/mja14.00132. [DOI] [PubMed] [Google Scholar]
- 3.Lo Coco F, Ye BH, Lista F, Corradini P, Offit K, Knowles DM, Chaganti RS, Dalla-Favera R. Rearrangements of the BCL6 gene in diffuse large cell non-Hodgkin’s lymphoma. Blood. 1994;83:1757–1759. [PubMed] [Google Scholar]
- 4.Ye BH, Chaganti S, Chang CC, Niu H, Corradini P, Chaganti RS, Dalla-Favera R. Chromosomal translocations cause deregulated BCL6 expression by promoter substitution in B cell lymphoma. EMBO J. 1995;14:6209–6217. doi: 10.1002/j.1460-2075.1995.tb00311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ritz O, Rommel K, Dorsch K, Kelsch E, Melzner J, Buck M, Leroy K, Papadopoulou V, Wagner S, Marienfeld R, Bruderlein S, Lennerz JK, Moller P. STAT6-mediated BCL6 repression in primary mediastinal B-cell lymphoma (PMBL) Oncotarget. 2013;4:1093–1102. doi: 10.18632/oncotarget.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kusam S, Munugalavadla V, Sawant D, Dent A. BCL6 cooperates with CD40 stimulation and loss of p53 function to rapidly transform primary B cells. Int J Cancer. 2009;125:977–981. doi: 10.1002/ijc.24450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Basso K, Dalla-Favera R. BCL6: master regulator of the germinal center reaction and key oncogene in B cell lymphomagenesis. Adv Immunol. 2010;105:193–210. doi: 10.1016/S0065-2776(10)05007-8. [DOI] [PubMed] [Google Scholar]
- 8.Phan RT, Dalla-Favera R. The BCL6 proto-oncogene suppresses p53 expression in germinal-centre B cells. Nature. 2004;432:635–639. doi: 10.1038/nature03147. [DOI] [PubMed] [Google Scholar]
- 9.Hirata Y, Ogasawara N, Sasaki M, Mizushima T, Shimura T, Mizoshita T, Mori Y, Kubota E, Wada T, Tanida S, Kataoka H, Kamiya T, Higashiyama S, Joh T. BCL6 degradation caused by the interaction with the C-terminus of pro-HB-EGF induces cyclin D2 expression in gastric cancers. Br J Cancer. 2009;100:1320–1329. doi: 10.1038/sj.bjc.6605010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu Q, Liu X, Yan H, He YH, Ye S, Cheng XW, Zhu GL, Wu WY, Wang XN, Kong XJ, Xu XC, Lobie PE, Zhu T, Wu ZS. B-cell lymphoma 6 protein stimulates oncogenicity of human breast cancer cells. BMC Cancer. 2014;14:418. doi: 10.1186/1471-2407-14-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walker SR, Liu S, Xiang M, Nicolais M, Hatzi K, Giannopoulou E, Elemento O, Cerchietti L, Melnick A, Frank DA. The transcriptional modulator BCL6 as a molecular target for breast cancer therapy. Oncogene. 2014 doi: 10.1038/onc.2014.61. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zeppernick F, Meinhold-Heerlein I. The new FIGO staging system for ovarian, fallopian tube, and primary peritoneal cancer. Arch Gynecol Obstet. 2014;290:839–42. doi: 10.1007/s00404-014-3364-8. [DOI] [PubMed] [Google Scholar]
- 13.Kong FF, Qu ZQ, Yuan HH, Wang JY, Zhao M, Guo YH, Shi J, Gong XD, Zhu YL, Liu F, Zhang WY, Jiang B. Overexpression of FOXM1 is associated with EMT and is a predictor of poor prognosis in non-small cell lung cancer. Oncol Rep. 2014;31:2660–2668. doi: 10.3892/or.2014.3129. [DOI] [PubMed] [Google Scholar]
- 14.Xiao X, Wang L, Wei P, Chi Y, Li D, Wang Q, Ni S, Tan C, Sheng W, Sun M, Zhou X, Du X. Role of MUC20 overexpression as a predictor of recurrence and poor outcome in colorectal cancer. J Transl Med. 2013;11:151. doi: 10.1186/1479-5876-11-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ci W, Polo JM, Cerchietti L, Shaknovich R, Wang L, Yang SN, Ye K, Farinha P, Horsman DE, Gascoyne RD, Elemento O, Melnick A. The BCL6 transcriptional program features repression of multiple oncogenes in primary B cells and is deregulated in DLBCL. Blood. 2009;113:5536–5548. doi: 10.1182/blood-2008-12-193037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Green MR, Vicente-Duenas C, Romero-Camarero I, Long Liu C, Dai B, Gonzalez-Herrero I, Garcia-Ramirez I, Alonso-Escudero E, Iqbal J, Chan WC, Campos-Sanchez E, Orfao A, Pintado B, Flores T, Blanco O, Jimenez R, Martinez-Climent JA, Criado FJ, Cenador MB, Zhao S, Natkunam Y, Lossos IS, Majeti R, Melnick A, Cobaleda C, Alizadeh AA, Sanchez-Garcia I. Transient expression of Bcl6 is sufficient for oncogenic function and induction of mature B-cell lymphoma. Nat Commun. 2014;5:3904. doi: 10.1038/ncomms4904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thieblemont C, Briere J. MYC, BCL2, BCL6 in DLBCL: impact for clinics in the future? Blood. 2013;121:2165–2166. doi: 10.1182/blood-2013-01-480392. [DOI] [PubMed] [Google Scholar]
- 18.Tran TH, Utama FE, Lin J, Yang N, Sjolund AB, Ryder A, Johnson KJ, Neilson LM, Liu C, Brill KL, Rosenberg AL, Witkiewicz AK, Rui H. Prolactin inhibits BCL6 expression in breast cancer through a Stat5a-dependent mechanism. Cancer Res. 2010;70:1711–1721. doi: 10.1158/0008-5472.CAN-09-2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sato T, Tran TH, Peck AR, Girondo MA, Liu C, Goodman CR, Neilson LM, Freydin B, Chervoneva I, Hyslop T, Kovatich AJ, Hooke JA, Shriver CD, Fuchs SY, Rui H. Prolactin suppresses a progestin-induced CK5-positive cell population in luminal breast cancer through inhibition of progestin-driven BCL6 expression. Oncogene. 2014;33:2215–2224. doi: 10.1038/onc.2013.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zuo JH, Zhu W, Li MY, Li XH, Yi H, Zeng GQ, Wan XX, He QY, Li JH, Qu JQ, Chen Y, Xiao ZQ. Activation of EGFR promotes squamous carcinoma SCC10A cell migration and invasion via inducing EMT-like phenotype change and MMP-9-mediated degradation of E-cadherin. J Cell Biochem. 2011;112:2508–2517. doi: 10.1002/jcb.23175. [DOI] [PubMed] [Google Scholar]
- 21.Zhang X, Liu G, Kang Y, Dong Z, Qian Q, Ma X. N-cadherin expression is associated with acquisition of EMT phenotype and with enhanced invasion in erlotinib-resistant lung cancer cell lines. PLoS One. 2013;8:e57692. doi: 10.1371/journal.pone.0057692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tao T, Shi Y, Han D, Luan W, Qian J, Zhang J, Wang Y, You Y Chinese Glioma Cooperative Group (CGCG) TPM3, a strong prognosis predictor, is involved in malignant progression through MMP family members and EMT-like activators in gliomas. Tumour Biol. 2014;35:9053–9. doi: 10.1007/s13277-014-1974-1. [DOI] [PubMed] [Google Scholar]
- 23.Lee YH, Albig AR, Regner M, Schiemann BJ, Schiemann WP. Fibulin-5 initiates epithelial-mesenchymal transition (EMT) and enhances EMT induced by TGF-beta in mammary epithelial cells via a MMP-dependent mechanism. Carcinogenesis. 2008;29:2243–2251. doi: 10.1093/carcin/bgn199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Y, Shi J, Chai K, Ying X, Zhou BP. The Role of Snail in EMT and Tumorigenesis. Curr Cancer Drug Targets. 2013;13:963–972. doi: 10.2174/15680096113136660102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gras B, Jacqueroud L, Wierinckx A, Lamblot C, Fauvet F, Lachuer J, Puisieux A, Ansieau S. Snail family members unequally trigger EMT and thereby differ in their ability to promote the neoplastic transformation of mammary epithelial cells. PLoS One. 2014;9:e92254. doi: 10.1371/journal.pone.0092254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Y, Yao B, Wang Y, Zhang M, Fu S, Gao H, Peng R, Zhang L, Tang J. Increased FoxM1 expression is a target for metformin in the suppression of EMT in prostate cancer. Int J Mol Med. 2014;33:1514–1522. doi: 10.3892/ijmm.2014.1707. [DOI] [PubMed] [Google Scholar]
- 27.Cerchietti LC, Ghetu AF, Zhu X, Da Silva GF, Zhong S, Matthews M, Bunting KL, Polo JM, Fares C, Arrowsmith CH, Yang SN, Garcia M, Coop A, Mackerell AD Jr, Prive GG, Melnick A. A small-molecule inhibitor of BCL6 kills DLBCL cells in vitro and in vivo. Cancer Cell. 2010;17:400–411. doi: 10.1016/j.ccr.2009.12.050. [DOI] [PMC free article] [PubMed] [Google Scholar]