Abstract
Background and Objectives: To elucidate diagnostic criteria, clinicopathological features and clinical outcome in patients with esophageal gastrointestinal stromal tumors (GIST), representing an extremely rare subform of GIST with an estimated incidence of about 0.1 to 0.3 per million people. Patients and methods: Esophageal GIST cases from the Ulmer GIST registry consisting of 1077 cases were pooled with case reports and case series of esophageal GIST extracted from MEDLINE. Data were compared with those from 683 cases with gastric GIST from the Ulmer GIST registry. Results: In comparison to gastric GIST, esophageal GIST (n = 55) occurred significantly more frequent in men (p = 0.035) as well as in patients younger than 60 at diagnosis (p < 0.001). Primary tumor sizes were significantly larger (p < 0.001), thereby resulting more frequently in a high-risk classification (OR = 4.53, CI 95% 2.41-8.52, p < 0.001). The 5-year rates of disease-specific survival (DSS), disease-free survival (DFS), and overall survival (OS) were 50.9%, 65.3% and 48.3%, respectively. The prognosis of esophageal GIST was less favorable compared with gastric GIST (DSS: p < 0.001, HR = 0.158, 95% CI: 0.087-0.288; DFS: p = 0.023, HR 0.466, 95% CI: 0.241-0.901; OS p = 0.003, HR = 0.481, 95% CI: 0.294-0.785; univariate Cox model) after a median follow-up time of 28 months (range 1.9 to 202). Mutational analysis for KIT showed more frequently wild-type status in esophageal GIST (OR = 10.13, CI 95% 3.02-33.96, p < 0.001). Conclusions: Esophageal GIST differ significantly from gastric GIST in respect to clinicopathological features and clinical outcome. To optimize treatment options further prospective data on patients with esophageal GIST are urgently warranted.
Keywords: GIST, gastrointestinal stromal tumor, esophagus, prognosis, outcome, mutation analysis
Introduction
Gastrointestinal stromal tumors (GIST) are the most common mesenchymal neoplasms of the gastrointestinal tract with an annual incidence of 7 to 20 per million [1-6]. There is substantial evidence that GISTs differentiate parallel to the gut pacemaker cells, the interstitial cells of Cajal suggesting an origin from the Cajal cells or their progenitor cells [7-9]. Despite prognostic relevance of metastases at primary stage and tumor rupture, risk stratification in GIST is related to tumor size, mitotic rate and as recently recognized also to tumor location. The majority of GISTs are located in the stomach (60-70%) and the small intestine (25-30%), whereas GISTs of the colo-rectum (up to 5%) and extra-gastrointestinal manifestations (< 5%) are less common [10-12]. Esophageal GIST is a very rare entity of GIST and represents < 1% of all cases. Therefore data on clinicopathological characteristics and clinical outcome are limited. The aim of the present study was to elucidate comprehensively demographic and clinicopathological features, diagnostic procedures and data on treatment and outcome in patients with GIST and esophageal manifestation.
Material and methods
GISTs cases of the esophagus were extracted from the Ulmer GIST registry and in addition from the literature. The multicenter Ulmer GIST registry comprises 1077 patients retrospectively collected from 18 collaborative oncological centres in Southern-Germany between 2004 and 2012. As previously outlined [13], data registration of the Ulmer GIST registry is strictly based on clearly defined methodological criteria, such as Strengthening of the Reporting of Observational Studies in Epidemiology (STROBE) Statement and the User’s Guide to Registries Evaluating Patient Outcomes [14-17]. Literature search of MEDLINE was performed for all articles published from 1993 through 2013, using the following MeSH (Medical Subject Heading) terms: esophagus, gastrointestinal stromal tumor, esophageal GIST, outcome, clinicopathological features, clinical manifestation and related articles respectively.
To this end a total of 55 GIST patients with esophageal localization were identified (Figure 1). From the Ulmer registry seven patients with GIST of the esophagus were extracted (0.65%). Clinical manifestations, diagnostic, localization, pathological findings including mutational analysis and treatments were evaluated retrospectively, the outcome was recorded prospectively. Clinical data were collected from medical history given by the patients, from hospital records and pathology reports or as outlined in published reports. Due to data acquisition, completeness of data is limited (see Table 1). The diagnosis of GIST was based on well-established international criteria [18,19] using histo-morphological findings (i.e. cellular spindle/epithelioid/mixed cell tumors), immunohistochemical staining (expression of KIT/CD117, or PDGFRA) and facultatively mutational analysis. MEDLINE search resulted in 19 case reports [20-36] and 3 case series including 29 patients [37-39]. All selected esophageal GIST cases were compared with 683 GIST patients of the stomach, extracted from the multi-centric Ulmer GIST registry. Regarding clinical outcome, analyses were performed for disease-specific-survival (DSS), disease-free-survival (DFS) and overall-survival (OS). Immunohistomical features if available comprised CD117/KIT, CD34, actin, desmin, vimentin, and S100.
Figure 1.
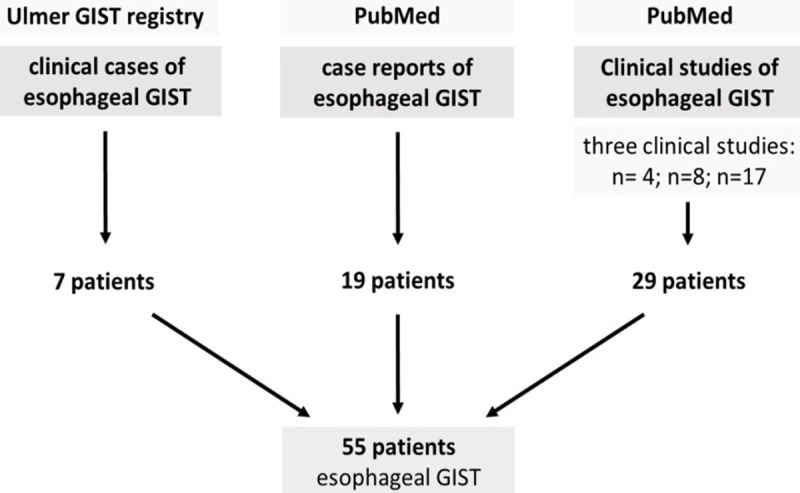
Schematic diagram regarding selection of esophageal GIST patients.
Table 1.
Demographic and clinicopathological data of 55 patients with esophageal GIST
| Parameter | Study cohort of esophageal GIST | ||
|---|---|---|---|
| Age (∑ = 55) | |||
| Mean (yr, ± SD) | 60.3 (11.9) | ||
| Median (yr, range) | 61.0 (21.0; 87.9) | ||
| Sex (∑ = 55) | n | % | |
| Female | 19 | 34.5 | |
| Male | 36 | 65.5 | |
| Localization (∑ = 55) | |||
| Middle 1/3 | 2 | 4.0 | |
| Middle/lower 1/3 | 2 | 4.0 | |
| Lower 1/3 | 46 | 92.0 | |
| Not definable | 5 | - | |
| Tumor size (∑ = 52) | |||
| Mean (cm, ± SD) | 8.0 (4.8) | ||
| Median (cm, range) | 7.35 (0.2; 25.0) | ||
| Mitotic rate (∑ = 41) | |||
| Mean (per 50HPF, ± SD) | 13.4 (18.2) | ||
| Median (per 50HPF, range) | 5.5 (0; 79) | ||
| Risk after Fletcher et al. (∑ = 46) | n | % | |
| High | 25 | 56.8 | |
| Intermediate | 10 | 22.7 | |
| Low | 6 | 13.6 | |
| Very Low | 3 | 6.8 | |
| Operative therapy (∑ = 33) | |||
| Enucleation | 14 | 42.4 | |
| Esophagectomy | 19 | 57.6 | |
| TKI/Imatinib (∑ = 55) | |||
| Yes/No | 6/49 | 10.9/89.1 | |
| Histological subtype (∑ = 43) | |||
| Spindle cell/Epithelioid or mixed | 35/8 | 81.4/18.6 | |
| Immunohistochemistry | pos | neg | |
| (∑ = 53) | c-kit | 53 | 0 |
| (∑ = 46) | CD34 | 45 | 1 |
| (∑ = 22) | Aktin | 7 | 15 |
| (∑ = 10) | Desmin | 4 | 6 |
| (∑ = 4) | Vimentin | 4 | 0 |
| (∑ = 34) | S100 | 0 | 34 |
| Mutational status (∑ = 14) | n | % | |
| c-kit | 8 | 57.1 | |
| PDGFRα | 0 | 0.0 | |
| wild type | 6 | 42.9 | |
| Symptoms | |||
| (∑ = 50) | Symptoms at diagnosis | 38 | 76.0 |
| Incidental | 12 | 24.0 | |
| (∑ = 49) | Dysphagia | 26 | 53 |
| Weight loss | 10 | 20 | |
| Bleeding | 6 | 12 | |
| Abdominal pain | 4 | 8 | |
| Nausea | 3 | 6 | |
| Cough | 3 | 6 | |
| Vomiting/Reflux/Night sweat | 1/1/1 | 2/2/2 | |
| Follow up time | |||
| Mean (m, ± SD)/median (m, range) | 48.2 (46.6)/28.0 (1.9; 202.0) | ||
| Survival rates | % | ||
| 1-/3-/5-year DSS | 97.5/76.3/50.9 | ||
| 1-/3-/5-year DFS | 86.6/65.3/65.3 | ||
| 1-/3-/5-year OS | 95.2/72.4/48.3 | ||
| Survival data | n | % | |
| (∑ = 55) | Recurrence or metastases | 14 | 25.5 |
| (∑ = 55) | Exitus letalis overall | 18 | 32.7 |
| (∑ = 54) | Exitus letalis GIST depend. | 15 | 27.3 |
HPF, high power field; m, month; SD, standard deviation; yr, year; DSS, disease specific survival; DSF, disease free survival; OS, overall survival.
Two-sided χ²-test or Fisher’s exact test were applied, as appropriate, to check for differences of qualitative demographic, clinical and clinicopathological parameters between the independent study-cohorts. Alternatively, two-sided t-test or Wilcoxon test were applied in case of quantitative parameters. Estimates for disease-free-survival (DFS), disease-specific-survival (DSS) and overall-survival (OS) were obtained by the Kaplan-Meier method and differences between Kaplan-Meier curves were investigated by the log-rank test. For analysis of DSS non GIST-related deaths were censored. If applicable, the Hazard Ratio (HR) and 95% confidence interval (95% CI) were calculated regarding tumor-related death and tumor recurrence and/or metastasis by applying univariate Cox proportional hazards regression models. To prove the most relevant findings of those univariate and Kaplan-Meier analyses, an additional multivariate Cox proportional hazards regression model has been established considering the variables age, gender, size of primary tumor and mitotic rate. Statistical analysis was performed using SPSS V19.0 (SPSS Inc., USA). A p-value ≤ 0.05 was considered as significant. The study was approved by the independent institutional ethics committee of the university of Ulm (Study-No: 90 & 91/2006). All patients gave written formed consent.
Results
Descriptive and clinical data of extracted esophageal GIST cases are given in Table 1 and Supplemental Table 1. Mean follow-up-time for all 55 patients was 48.2 months (SD ± 46.6). The male to female ratio was 1.9:1 (male n = 36, female n = 19). The median age was 61.0 years (range 21.0 to 87.9 years). Nearly half of the patients (49.1%) were younger than 60 years, 16.4% were younger than 50 years. Regarding risk classification for GIST 57% (CI 95% 0.44, 0.70; n = 25) of the patients were classified as high risk according to Fletcher et al. [40] whereas similar figures were found for intermediate (23%, n = 10) and low/very low risk (21%, n = 9) patients. Immunohistochemical analyses were performed and available most frequently for CD117/KIT (n = 53) and CD34 (n = 46) while staining for actin (n = 22), desmin (n = 10) and S100 (n = 34) was done only for selected cases. CD117/Kit and CD34 staining was positive for all cases in 100% and 98%, respectively. In contrast immunohistochemistry for actin and desmin was positive in 32% and 40%, respectively and negative for S100 in all cases investigated. Data on mutational analysis for gain of function variants were available only in 14 GIST cases resulting in seven patients carrying a mutation in exon 11 of KIT, and one patient in exon 13. The remaining six patients were KIT wild type. PDGFRα variants were not detected in these 14 cases.
With regard to clinical features, 46 of 50 esophageal GIST were localized in the distal esophagus, 38 of 50 patients (76%, CI 95% 0.64; 0.88) presented with clinical symptoms. Interestingly, 12 of 50 patients (24%, CI 95% 0.1; 0.36) were detected incidentally. The most common symptom was dysphagia (26/49 = 53%, CI 95% 0.39; 0.67) whereas 10/49 patients reported weight loss. Other symptoms occurred in descending order: 6/49 gastrointestinal bleeding, hemoptysis or melena, 4/49 abdominal pain, 3/49 nausea, 3/49 cough, 1/49 vomiting, 1/49 gastro-esophageal reflux and 1/49 night sweat. Data on operative procedures were limited and only available for n = 33/55 patients (66%). 14 patients of them (42%, CI 95% 0.28-0.56) received local excision/enucleation of the tumor (range of tumor size: 1.8 cm; 12.5 cm), whereas partial esophagectomy/oesophagogastrostomy was performed in 19 patients (58%, CI 95% 0.41; 0.75). Patients with local excisions showed a significantly better outcome (DSS: p = 0.035, log-rank-test; OS: p = 0.035, log-rank-test; DFS: p = 0.049, log-rank-test; p = 0.07, Cox model; HR = 0.232, 95% CI: 0.05-1.13) in comparison to patients with more radical surgery. Regarding OS and DSS, calculation of the corresponding OR or HR failed since only censored events were observed in the enucleation group. Imatinib application was reported in 6/55 patients. The median follow-up time was 28.0 months (range 1.9 to 202). Of the 55 GIST patients 14 (25%, CI 95% 0.14; 0.36) and 18 (33%, CI 95% 0.21; 45) cases showed disease progression (recurrence or metastasis) or died, respectively. GIST-related deaths occurred in 15 cases (27%, CI 95% 0.15; 0.39).
For comparative evaluation, registry data of 683 patients with GIST of the stomach were used. Main results are given in Tables 1 and 2 and Supplemental Table 1. The male to female ratio was 1:1 (male n = 346, female n = 337). The median age was 68.7 years (range 12.8 to 94.8). 25% (n=165) of patients were younger than 60 years, 10% (n = 64) younger than 50 years. Immunohistochemical staining was positive in 97% (n = 597/613) and 96% (n = 471/496) of cases for CD117/KIT and CD34, respectively. Actin staining was positive in 32% (n = 112/347), desmin staining in 16% (n = 53/325) and S100 immunohistochemistry in 13% (n = 48/363). The median follow-up time was 49.2 months (range 0.0 to 271.4). Disease progression (recurrence or metastasis) and GIST-related deaths were found in 13% (n = 91/683) and 6% (41/683) of patients, respectively.
Table 2.
Comparison of selected clinicopathological parameters between esophageal and gastric GIST
| Basic parameters | Esophagus n = 55 | Stomach n = 683 | p-value |
|---|---|---|---|
| Age at diagnosis | |||
| Mean (yr, ± SD) | 60.3 (11.9) | 66.8 (12.6) | < 0.001 (t-test) |
| Median (yr, range) | 61.0 [21.0; 87.9] | 68.7 [12.8; 94.8] | |
| Size of primarius | |||
| Mean (cm, ± SD) | 8.0 (4.8) | 5.1 (4.6) | < 0.001 (t-test) |
| Median (cm, range) | 7.35 [0.2;25.0] | 4.0 [0.1; 32.0] | |
| Mitotic rate per 50 HPF | |||
| Mean (per 50HPF, ± SD) | 13.4 (18.2) | 8.0 (28.1) | 0.005 (Wilcoxon test) |
| Median (per 50HPF, range) | 5.5 [0.0; 79.0] | 2.0 [0.0; 500.0] | |
| Follow-up-time (months) | |||
| Mean (m, ± SD) | 48.2 (46.6) | 59.3 (46.4) | - |
| Median (m, range) | 28.0 [1.9; 202.0] | 49.2 [0.0; 271.4] |
When we compared data regarding GIST of the stomach vs. esophageal GIST, some significant differences were found. GIST of the esophagus was significantly associated with male gender (OR = 1.8, CI 95% 1.03; 3.28, p = 0.034, χ²-test) and overall the mean age at diagnosis was significantly lower (p < 0.001, t-test). In more detail patients were more often younger than 60 years at date of diagnosis (OR = 2.85, CI 95% 1.62; 5.03, p < 0.001, χ²-test). Generally, the primary tumor size was significantly larger (p < 0.001, t-test). Tumor sizes in esophageal GIST were increased (> 5 cm, OR = 4.03, CI 95% 2.14; 7.58, p < 0.001, χ²-test; > 10 cm, OR = 3.51, CI 95% 1.88; 6.56, p < 0.001, χ²-test), thereby resulting more frequently in a high-risk classification according to Fletcher et al. (OR = 4.53, CI 95% 2.41; 8.52, p < 0.001, χ²-test). Mutational analysis for KIT showed more frequently wild-type status for esophageal vs. gastric GIST (OR = 10.13, CI 95% 3.02; 33.96, p < 0.001, χ²-test). No significant differences were found regarding histological subtypes, and immunohistochemical staining for CD117/KIT, CD34, aktin, and desmin.
Regarding clinical outcome, we performed Kaplan-Meier survival analyses (Figures 2, 3 and 4) to elucidate differences between esophageal and gastric GIST. Consistently data for disease-specific- (DSS), disease-free- (DFS) as well as overall-survival (OS) were significantly less favor in patients with esophageal GIST in comparison to gastric GIST. 1-, 3-, and 5-years DSS rates were 97.5% (95% CI: 92.6; 100), 76.3% (95% CI: 60.6; 92.0) and 50.9% (95% CI: 31.1; 71.0) in patients with esophageal GIST compared to 97.7% (95% CI: 96.3; 99.1), 94.9% (95% CI: 92.9; 96.9) and 92.6% (95% CI: 90.1; 95.1) in patients with gastric GIST (p < 0.001, log-rank-test; p < 0.001, cox model; HR = 0.158 95% CI: 0.087; 0.288), respectively. 1-, 3-, and 5-years DFS rates were 86.6% (95% CI: 75.6; 97.6), 65.3% (95% CI: 45.1; 85.5) and 65.3% (95% CI: 45.1; 85.5) in patients with esophageal GIST compared to 91.4% (95% CI: 89.0; 93.8), 88.0% (95% CI: 85.3; 90.7) and 86.1% (95% CI: 83.0; 89.2) in patients with gastric GIST (p = 0.018, log-rank-test; p = 0.023, cox model; HR = 0.466, 95% CI: 0.241; 0.901). 1-, 3-, and 5-years OS rates were 95.2% (95% CI: 88.7; 100), 72.4% (95% CI: 56.7; 88.1) and 48.3% (95% CI: 29.3; 67.3) in patients with esophageal GIST compared to 93.1% (95% CI: 90.9; 95.3), 84.6% (95% CI: 81.5; 87.7) and 77.1% (95% CI: 73.2; 81.0) in patients with gastric GIST (p = 0.003, log-rank-test; p = 0.003, cox model; HR 0.481, 95% CI: 0.294; 0.785). Results of the multivariate Cox models are given in Table 3.
Figure 2.
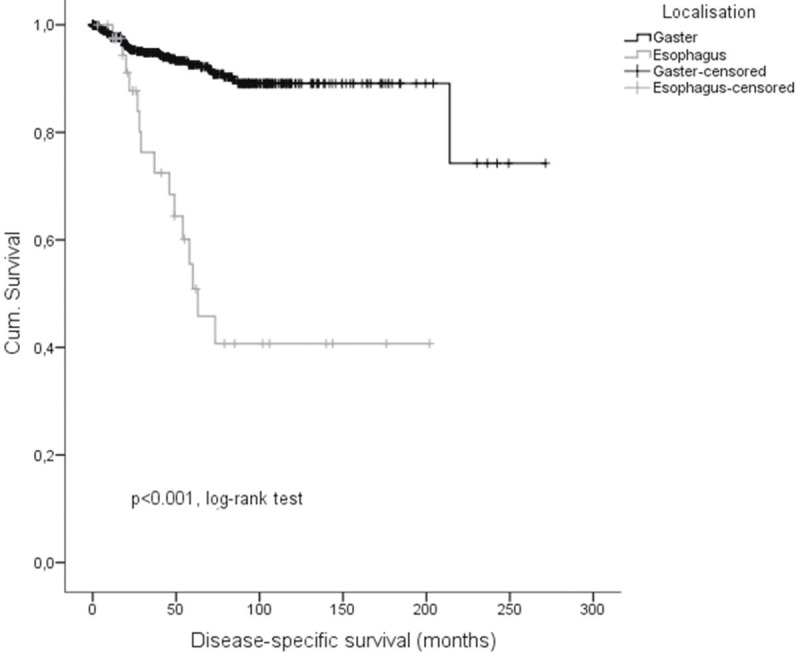
Disease-specific-survival of esophageal and gastric GIST.
Figure 3.
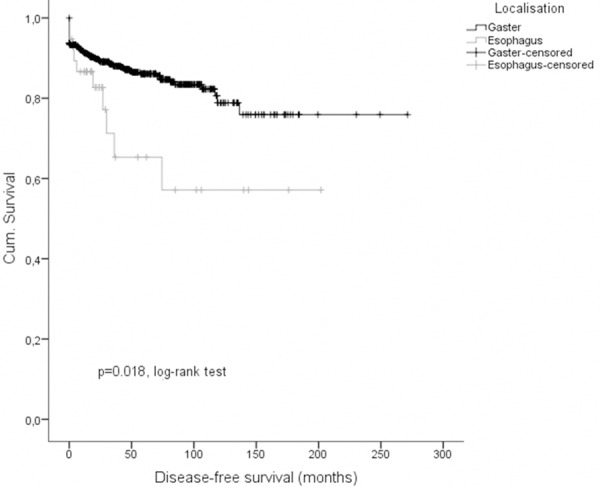
Disease-free-survival of esophageal and gastric GIST.
Figure 4.
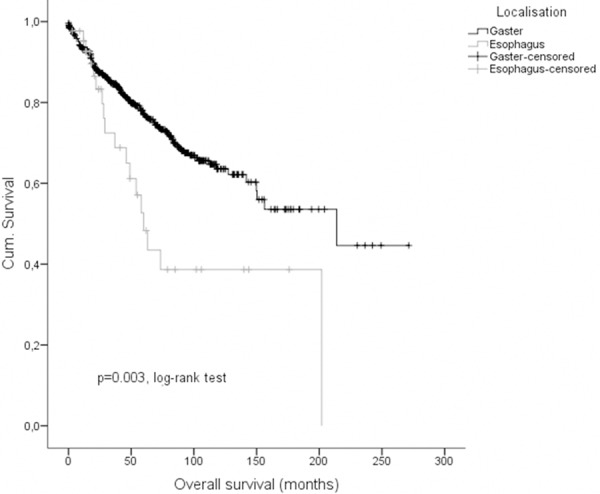
Overall-survival of esophageal and gastric GIST.
Table 3.
Comparative survival analyses of esophageal and gastric GIST using univariate & multivariate cox regression models
| (ntotal = 55/683) | Esophagus | Stomach | Univariate Cox model | Multivariate Cox model |
|---|---|---|---|---|
|
|
|
|
|
|
| % (n) | % (n) | p-, HR (95% CI) | p-, HR (95% CI) | |
| Disease-specific-survival | ||||
| 1-yr-DSS | 97.5 (40) | 97.7 (490) | p < 0.001, HR 0.158 (0.087; 0.288) | p < 0.001, HR 0.132 (0.062; 0.282) |
| 3-yr-DSS | 76.3 (20) | 94.9 (355) | ||
| 5-yr-DSS | 50.9 (12) | 92.6 (236) | ||
| 10-yr-DSS | 40.7 (4) | 89.1 (49) | ||
| Disease-free-survival | ||||
| 1-yr-DFS | 86.6 (31) | 91.4 (466) | p = 0.023, HR 0.466 (0.241; 0.901) | p = 0.140, HR 0.566 (0.266; 1.204) |
| 3-yr-DFS | 65.3 (12) | 88.0 (328) | ||
| 5-yr-DFS | 65.3 (9) | 86.1 (214) | ||
| 10-yr-DFS | 57.1 (4) | 78.8 (42) | ||
| Overall-survival | ||||
| 1-yr-OS | 95.2 (40) | 93.1 (513) | p = 0.003, HR 0.481 (0.294; 0.785) | p < 0.001, HR 0.284 (0.158; 0.510) |
| 3-yr-OS | 72.4 (20) | 84.6 (372) | ||
| 5-yr-OS | 48.3 (12) | 77.1 (246) | ||
| 10-yr-OS | 38.6 (4) | 63.6 (52) |
yr, year; DSS, disease specific survival; DSF, disease free survival; OS, overall survival; p, p-value; HR, hazard ratio. Multivariate Cox proportional hazards regression model has been adjusted considering the variables age, gender, size of primary tumor & mitotic rate.
Discussion
Gastrointestinal stromal tumors located in the esophagus constitute a very rare subform of GISTs with limited data on their demographic and clinic-pathological features. Therefore, we evaluated data of 55 pooled esophageal GISTs (Figure 1) from our Ulmer GIST registry and the literature with regard to clinical symptoms, diagnostic features, risk factors, treatment and outcome. The current study represents the largest analysis of esophageal GIST estimating an annual incidence of about 0.1 to 0.3 per million (approximately 8-12 per year in Germany). The present study indicates some characteristics significantly associated with esophageal GIST.
In comparison to the most common GIST of the stomach, esophageal GIST occurred significantly more frequent in men (p = 0.035) as well as in patients younger than 60 at diagnosis (p < 0.001). The significant predominance of men within the 5th decade in esophageal GIST is in accordance with previous published data [38,39,41]. However, despite 25% of incidental, asymptomatic tumors at diagnosis, 75% of patients with esophageal GIST present most commonly with dysphagia (51%), weight loss (20%) and bleeding (10%) [20-39,42]. With regard to cell morphology the majority (81%, CI 95% 0.71; 0.91) of esophageal GISTs show spindle cell morphology which is comparable to data from the literature [42]. With a 100% positivity of KIT expression and 98% of CD34 expression, GIST of the esophagus seem to have an immune-profile similar to their gastric counterparts.
With a mean tumor size of 8.0 cm and a mean mitotic rate of 13/50 HPF, esophageal GISTs are significantly larger and show a higher mitotic rate than GIST of the stomach [20-39]. Hence, esophageal GIST are generally classified more frequently as high risk GIST according to Fletcher et al. (56.8% versus 22.5%, p < 0.001, χ²-test). Regarding mutational status, data are limited and therefore conclusion should be drawn with caution. Only in 14/55 GIST patients the mutation status of KIT was available. Eight had KIT gain of function variants and none PDGFRα mutations. A wild type frequency of 42.9% is remarkably higher compared to GISTs from other sites.
With a 5 year DSS, DFS, and OS of 50.9%, 65.3% and 48.3% esophageal GIST present a significantly worse prognosis in comparison to GIST of the stomach (HR = 0.158, 95% CI: 0.087; 0.288, p < 0.001; HR = 0.466, 95% CI: 0.241; 0.901, p = 0.023; HR = 0.481, 95% CI: 0.294; 0.785, p = 0.003; - univariate Cox models). In contrast, Tran et. al. report a 5 year survival rate of 14%, but they do not differentiate DSS, DFS and OS and the acquisition of patients was performed in the pre imatinib-era [4]. Nevertheless, the majority reports from the literature support a higher malignant potential of esophageal GIST with a high risk for metastases and/or tumor recurrence and unfavorable outcome with a high mortality rate [38]. Most likely poor outcome in esophageal GIST is related to the above described significant higher rate of large tumor size and higher mitotic rate. Definite cellular mechanisms need to be addressed in future work.
Regarding the management of esophageal GIST three pillars need to be considered and linked together: i) appropriate pre-therapeutic histological diagnostics including biopsies, ii) alternative surgical procedures (i.e. radical resection vs. local tumor excision/enucleation), and iii) administration of tyrosine kinase inhibitors (e.g. imatinib) in different settings (i.e. neo-adjuvant, adjuvant, additive). Since controlled trials for esophageal GIST are missing due to the low incidence, neither the best surgical procedure, nor the impact of adjuvant or neo-adjuvant tyrosine kinase inhibitor therapy is well established. Currently, complete surgical elimination of the tumor appears to be the only curative therapeutic option in the management of non-metastatic, resectable esophageal GIST [43]. The outcome after local tumor enucleation compared to post-esophagectomy seems to be more favorable as mentioned above, however the mean tumor size in the enucleation group 5.8 cm [1.8-12.5] was significantly smaller with subsequent lower risk classification. Nevertheless, the decision which surgical procedure should be performed in esophageal GIST is still discussed controversially [41,44-47]. Driven by the goal to achieve R0 resection by highest radicality, local tumor enucleation might be limited and primary radical surgical resection may be the treatment of choice, as appropriate, combined with tryosine kinase inhibitor therapy [46,47]. With regard to post-surgical morbidity and mortality, the local tumor enucleation seems a probate and less traumatic option, particularly in patients with significant comorbidities [37,38,41,44]. As long as the tumor is entirely eliminated with intact pseudo tumor capsule and without tumor spread (R0), tumor up to a size of 12.5 cm are reported to be safely enucleated [37]. Generally, enucleation of esophageal GIST are recommended for smaller tumors (2 to 5 cm) [37,41], whereas esophagectomy should be performed for GIST above 9 cm in size [38]. In all cases between, the surgical procedure should be chosen based on the patient’s individual surgical risk under consideration of underlying comorbidities [37,38,41,44].
About 25% of mesenchymal esophageal tumors are GIST [39]. The role of pre-therapeutic histological and genetic diagnosis is judged individually, as it is essential for neo-adjuvant or dose adjusted TKI treatment. Ultrasound guided fine needle aspiration or core biopsy is reported to be a secure procedure and enables differentiation of mesenchymal tumors including GIST [37,48,49]. Whether biopsy induced scars may complicate subsequent tumor enucleation is under debate [41]. Indicators for preoperative biopsies are tumors above 2 cm in size with observed enlargement and/or intended neo-adjuvant TKI treatment [37,41,42,48,49]. In the presented study only in six of 55 patients, the application of imatinib was reported. Currently the ESMO-guidelines recommend adjuvant imatinib treatment at least for high-risk GIST based on the mutational status [43]. However, it has been reported that in large tumors neo-adjuvant application of imatinib may be beneficial too with regard to surgical and oncological outcome of these patients [37,41].
The presented study lacks systematic prospective data acquisition and therefore in part completeness of data is limited. The heterogeneity of data selection based on registry data, case reports and small case series does not exclude some selection bias. Nevertheless, our work provides important information of the largest cohort of esophageal GIST so far. Esophageal GIST differ significantly from gastric GIST in respect to clinicopathological features and clinical outcome. To optimize treatment options further prospective data on patients with esophageal GIST are urgently warranted.
Acknowledgements
We do thank Annette Blatz (University of Ulm, Germany) for editorial assistance and Karl and Annemarie Schmieder (Schwäbisch Gmünd, Germany) for data management. MS was in part supported by the Robert Bosch Stiftung, Stuttgart, Germany and the IZEPHA grant Tübingen-Stuttgart #8-0-0. KK and MiSc had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Disclosure of conflict of interest
The authors have no conflict of interest or financial disclosure to declare.
Abbreviations
- yr
years
- mo
month
- DFS
disease-free-survival
- DSS
disease-specific-survival
- HPF
high power field
- SD
standard deviation
- GIST
gastrointestinal stromal tumor
- TKI
tyrosine kinase inhibitor
Supporting Information
References
- 1.Monges G, Bisot-Locard S, Blay JY, Bouvier AM, Urbieta M, Coindre JM, Scoazec JY. The estimated incidence of gastrointestinal stromal tumors in France. Results of PROGIST study conducted among pathologists. Bull Cancer. 2010;97:E16–22. doi: 10.1684/bdc.2010.1041. [DOI] [PubMed] [Google Scholar]
- 2.Nilsson B, Bumming P, Meis-Kindblom JM, Oden A, Dortok A, Gustavsson B, Sablinska K, Kindblom LG. Gastrointestinal stromal tumors: the incidence, prevalence, clinical course, and prognostication in the preimatinib mesylate era--a population-based study in western Sweden. Cancer. 2005;103:821–829. doi: 10.1002/cncr.20862. [DOI] [PubMed] [Google Scholar]
- 3.Steigen SE, Bjerkehagen B, Haugland HK, Nordrum IS, Loberg EM, Isaksen V, Eide TJ, Nielsen TO. Diagnostic and prognostic markers for gastrointestinal stromal tumors in Norway. Mod Pathol. 2008;21:46–53. doi: 10.1038/modpathol.3800976. [DOI] [PubMed] [Google Scholar]
- 4.Tran T, Davila JA, El-Serag HB. The epidemiology of malignant gastrointestinal stromal tumors: an analysis of 1,458 cases from 1992 to 2000. Am J Gastroenterol. 2005;100:162–168. doi: 10.1111/j.1572-0241.2005.40709.x. [DOI] [PubMed] [Google Scholar]
- 5.Tryggvason G, Kristmundsson T, Orvar K, Jonasson JG, Magnusson MK, Gislason HG. Clinical study on gastrointestinal stromal tumors (GIST) in Iceland, 1990-2003. Dig Dis Sci. 2007;52:2249–2253. doi: 10.1007/s10620-006-9248-4. [DOI] [PubMed] [Google Scholar]
- 6.Tzen CY, Wang MN, Mau BL. Spectrum and prognostication of KIT and PDGFRA mutation in gastrointestinal stromal tumors. Eur J Surg Oncol. 2008;34:563–568. doi: 10.1016/j.ejso.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 7.Hirota S, Isozaki K, Moriyama Y, Hashimoto K, Nishida T, Ishiguro S, Kawano K, Hanada M, Kurata A, Takeda M, Muhammad Tunio G, Matsuzawa Y, Kanakura Y, Shinomura Y, Kitamura Y. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279:577–580. doi: 10.1126/science.279.5350.577. [DOI] [PubMed] [Google Scholar]
- 8.Kindblom LG, Remotti HE, Aldenborg F, Meis-Kindblom JM. Gastrointestinal pacemaker cell tumor (GIPACT): gastrointestinal stromal tumors show phenotypic characteristics of the interstitial cells of Cajal. Am J Pathol. 1998;152:1259–1269. [PMC free article] [PubMed] [Google Scholar]
- 9.Miettinen M, Sarlomo-Rikala M, Lasota J. Gastrointestinal stromal tumors: recent advances in understanding of their biology. Hum Pathol. 1999;30:1213–1220. doi: 10.1016/s0046-8177(99)90040-0. [DOI] [PubMed] [Google Scholar]
- 10.Miettinen M, Lasota J, Sobin LH. Gastrointestinal stromal tumors of the stomach in children and young adults: a clinicopathologic, immunohistochemical, and molecular genetic study of 44 cases with long-term follow-up and review of the literature. Am J Surg Pathol. 2005;29:1373–1381. doi: 10.1097/01.pas.0000172190.79552.8b. [DOI] [PubMed] [Google Scholar]
- 11.Miettinen M, Makhlouf H, Sobin LH, Lasota J. Gastrointestinal stromal tumors of the jejunum and ileum: a clinicopathologic, immunohistochemical, and molecular genetic study of 906 cases before imatinib with long-term follow-up. Am J Surg Pathol. 2006;30:477–489. doi: 10.1097/00000478-200604000-00008. [DOI] [PubMed] [Google Scholar]
- 12.Woodall CE 3rd, Brock GN, Fan J, Byam JA, Scoggins CR, McMasters KM, Martin RC 2nd. An evaluation of 2537 gastrointestinal stromal tumors for a proposed clinical staging system. Arch Surg. 2009;144:670–678. doi: 10.1001/archsurg.2009.108. [DOI] [PubMed] [Google Scholar]
- 13.Kramer K. Establishment of a multi-centric GIST registry and oncologic network. 2012 [Google Scholar]
- 14.Glicklich RE, Dreyer NA, editors. Registries for Evaluating Patient Outcomes: A User’s Guide. Agency for Healthcare Research and Quality (US); 2010. [PubMed] [Google Scholar]
- 15.Vandenbroucke JP, von Elm E, Altman DG, Gotzsche PC, Mulrow CD, Pocock SJ, Poole C, Schlesselman JJ, Egger M. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. Epidemiology. 2007;18:805–835. doi: 10.1097/EDE.0b013e3181577511. [DOI] [PubMed] [Google Scholar]
- 16.Vandenbroucke JP, von Elm E, Altman DG, Gotzsche PC, Mulrow CD, Pocock SJ, Poole C, Schlesselman JJ, Egger M. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. PLoS Med. 2007;4:e297. doi: 10.1371/journal.pmed.0040297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vandenbroucke JP, von Elm E, Altman DG, Gotzsche PC, Mulrow CD, Pocock SJ, Poole C, Schlesselman JJ, Egger M. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. Ann Intern Med. 2007;147:W163–194. doi: 10.7326/0003-4819-147-8-200710160-00010-w1. [DOI] [PubMed] [Google Scholar]
- 18.Blay JY, Wardelmann E. Gastrointestinal stromal tumors: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23(Suppl 7):vii49–55. doi: 10.1093/annonc/mds252. [DOI] [PubMed] [Google Scholar]
- 19.Miettinen M, Sobin LH, Lasota J. Gastrointestinal stromal tumors of the stomach: a clinicopathologic, immunohistochemical, and molecular genetic study of 1765 cases with long-term follow-up. Am J Surg Pathol. 2005;29:52–68. doi: 10.1097/01.pas.0000146010.92933.de. [DOI] [PubMed] [Google Scholar]
- 20.Axel J, Weickert U, Dancygier H. [Gastrointestinal tumor (GIST) of the esophagus in a 34-year-old man: clubbed fingers and alopecia arealis as an early paraneoplastic phenomenon] . Dtsch Med Wochenschr. 2005;130:2380–2383. doi: 10.1055/s-2005-918579. [DOI] [PubMed] [Google Scholar]
- 21.Ertem M, Baca B, Dogusoy G, Erguney S, Yavuz N. Thoracoscopic enucleation of a giant submucosal tumor of the esophagus. Surg Laparosc Endosc Percutan Tech. 2004;14:87–90. doi: 10.1097/00129689-200404000-00008. [DOI] [PubMed] [Google Scholar]
- 22.Fang FC, Tzao C, Cheng YL, Chan DC, Nieh S, Lee SC. Surgical treatment of gastrointestinal stromal tumor in the esophagus: report of three cases. Z Gastroenterol. 2007;45:1252–1256. doi: 10.1055/s-2007-963428. [DOI] [PubMed] [Google Scholar]
- 23.Gouveia AM, Pimenta AP, Lopes JM, Capelinha AF, Ferreira SS, Valbuena C, Oliveira MC. Esophageal GIST: therapeutic implications of an uncommon presentation of a rare tumor. Dis Esophagus. 2005;18:70–73. doi: 10.1111/j.1442-2050.2005.00446.x. [DOI] [PubMed] [Google Scholar]
- 24.Graham J, Debiec-Rychter M, Corless CL, Reid R, Davidson R, White JD. Imatinib in the management of multiple gastrointestinal stromal tumors associated with a germline KIT K642E mutation. Arch Pathol Lab Med. 2007;131:1393–1396. doi: 10.5858/2007-131-1393-IITMOM. [DOI] [PubMed] [Google Scholar]
- 25.Hamada S, Itami A, Watanabe G, Nakayama S, Tanaka E, Hojo M, Yoshizawa A, Hirota S, Sakai Y. Intracranial metastasis from an esophageal gastrointestinal stromal tumor. Intern Med. 2010;49:781–785. doi: 10.2169/internalmedicine.49.3124. [DOI] [PubMed] [Google Scholar]
- 26.Iijima S, Maesawa C, Sato N, Ikeda K, Inaba T, Akiyama Y, Ishida K, Saito K, Masuda T. Gastrointestinal stromal tumour of the oesophagus: significance of immunohistochemical and genetic analyses of the c-kit gene. Eur J Gastroenterol Hepatol. 2002;14:445–448. doi: 10.1097/00042737-200204000-00020. [DOI] [PubMed] [Google Scholar]
- 27.Kaida H, Ishibashi M, Yuzuriha M, Kurata S, Arikawa S, Kawahara A, Uozumi J, Uchida M, Kobayashi M, Hirose Y, Fujita H, Kage M, Hayabuchi N. Glucose transporter expression of an esophageal gastrointestinal tumor detected by F-18 FDG PET/CT. Clin Nucl Med. 2010;35:505–509. doi: 10.1097/RLU.0b013e3181e05d79. [DOI] [PubMed] [Google Scholar]
- 28.Manu N, Richard P, Howard S. Bleeding esophageal GIST. Dis Esophagus. 2005;18:281–282. doi: 10.1111/j.1442-2050.2005.00480.x. [DOI] [PubMed] [Google Scholar]
- 29.Ozan E, Oztekin O, Alacacioglu A, Aykas A, Postaci H, Adibelli Z. Esophageal gastrointestinal stromal tumor with pulmonary and bone metastases. Diagn Interv Radiol. 2010;16:217–220. doi: 10.4261/1305-3825.DIR.1861-08.2. [DOI] [PubMed] [Google Scholar]
- 30.Padula A, Chin NW, Azeez S, Resetkova E, Andriko JA, Miettinen M. Primary gastrointestinal stromal tumor of the esophagus in an HIV-positive patient. Ann Diagn Pathol. 2005;9:49–53. doi: 10.1053/j.anndiagpath.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 31.Papaspyros S, Papagiannopoulos K. Gastrointestinal stromal tumor masquerading as a lung neoplasm. A case presentation and literature review. J Cardiothorac Surg. 2008;3:31. doi: 10.1186/1749-8090-3-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Portale G, Zaninotto G, Costantini M, Rugge M, Pennelli GM, Rampado S, Bocus P, Ancona E. Esophageal GIST: case report of surgical enucleation and update on current diagnostic and therapeutic options. Int J Surg Pathol. 2007;15:393–396. doi: 10.1177/1066896907302366. [DOI] [PubMed] [Google Scholar]
- 33.Sakai M, Kato H, Saito K, Tanaka N, Inose T, Kimura H, Miyazaki T, Kuwano H. Clinical applications of 18F-fluorodeoxyglucose positron emission tomography in gastrointestinal stromal tumor of the esophagus. Int Surg. 2008;93:209–213. [PubMed] [Google Scholar]
- 34.Spinelli GP, Miele E, Tomao F, Rossi L, Pasciuti G, Zullo A, Zoratto F, Nunnari J, Pisanelli GC, Tomao S. The synchronous occurrence of squamous cell carcinoma and gastrointestinal stromal tumor (GIST) at esophageal site. World J Surg Oncol. 2008;6:116. doi: 10.1186/1477-7819-6-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamada H, Shinohara T, Yokoyama K, Takasu K, Fujimori Y, Yamagishi K. Thoracoscopic enucleation of esophageal gastrointestinal stromal tumor using prone positioning in a patient with severe chronic obstructive lung disease. J Laparoendosc Adv Surg Tech A. 2011;21:635–639. doi: 10.1089/lap.2011.0264. [DOI] [PubMed] [Google Scholar]
- 36.Al-Salam S, El-Teraifi HA, Taha MS. Could imatinib replace surgery in esophageal gastrointestinal stromal tumor. Saudi Med J. 2006;27:1236–1239. [PubMed] [Google Scholar]
- 37.Blum MG, Bilimoria KY, Wayne JD, de Hoyos AL, Talamonti MS, Adley B. Surgical considerations for the management and resection of esophageal gastrointestinal stromal tumors. Ann Thorac Surg. 2007;84:1717–1723. doi: 10.1016/j.athoracsur.2007.05.071. [DOI] [PubMed] [Google Scholar]
- 38.Jiang P, Jiao Z, Han B, Zhang X, Sun X, Su J, Wang C, Gao B. Clinical characteristics and surgical treatment of oesophageal gastrointestinal stromal tumours. Eur J Cardiothorac Surg. 2010;38:223–227. doi: 10.1016/j.ejcts.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 39.Miettinen M, Sarlomo-Rikala M, Sobin LH, Lasota J. Esophageal stromal tumors: a clinicopathologic, immunohistochemical, and molecular genetic study of 17 cases and comparison with esophageal leiomyomas and leiomyosarcomas. Am J Surg Pathol. 2000;24:211–222. doi: 10.1097/00000478-200002000-00007. [DOI] [PubMed] [Google Scholar]
- 40.Fletcher CD, Berman JJ, Corless C, Gorstein F, Lasota J, Longley BJ, Miettinen M, O’Leary TJ, Remotti H, Rubin BP, Shmookler B, Sobin LH, Weiss SW. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum Pathol. 2002;33:459–465. doi: 10.1053/hupa.2002.123545. [DOI] [PubMed] [Google Scholar]
- 41.Lee HJ, Park SI, Kim DK, Kim YH. Surgical resection of esophageal gastrointestinal stromal tumors. Ann Thorac Surg. 2009;87:1569–1571. doi: 10.1016/j.athoracsur.2009.01.051. [DOI] [PubMed] [Google Scholar]
- 42.Demetri GD, von Mehren M, Antonescu CR, DeMatteo RP, Ganjoo KN, Maki RG, Pisters PW, Raut CP, Riedel RF, Schuetze S, Sundar HM, Trent JC, Wayne JD. NCCN Task Force report: update on the management of patients with gastrointestinal stromal tumors. J Natl Compr Canc Netw. 2010;8(Suppl 2):S1–41. doi: 10.6004/jnccn.2010.0116. quiz S42-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gastrointestinal stromal tumors: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23(Suppl 7):vii49–55. doi: 10.1093/annonc/mds252. [DOI] [PubMed] [Google Scholar]
- 44.Coccolini F, Catena F, Ansaloni L, Lazzareschi D, Pinna AD. Esophagogastric junction gastrointestinal stromal tumor: resection vs enucleation. World J Gastroenterol. 2010;16:4374–4376. doi: 10.3748/wjg.v16.i35.4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dumont AG, Rink L, Godwin AK, Miettinen M, Joensuu H, Strosberg JR, Gronchi A, Corless CL, Goldstein D, Rubin BP, Maki RG, Lazar AJ, Lev D, Trent JC, von Mehren M. A nonrandom association of gastrointestinal stromal tumor (GIST) and desmoid tumor (deep fibromatosis): case series of 28 patients. Ann Oncol. 2012;23:1335–1340. doi: 10.1093/annonc/mdr442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peparini N, Carbotta G, Chirletti P. Enucleation for gastrointestinal stromal tumors at the esophagogastric junction: is this an adequate solution? World J Gastroenterol. 2011;17:2159–2160. doi: 10.3748/wjg.v17.i16.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peparini N, Chirletti P. Tumor rupture during surgery for gastrointestinal stromal tumors: pay attention! World J Gastroenterol. 2013;19:2009–2010. doi: 10.3748/wjg.v19.i12.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stelow EB, Jones DR, Shami VM. Esophageal leiomyosarcoma diagnosed by endoscopic ultrasound-guided fine-needle aspiration. Diagn Cytopathol. 2007;35:167–170. doi: 10.1002/dc.20606. [DOI] [PubMed] [Google Scholar]
- 49.Stelow EB, Stanley MW, Mallery S, Lai R, Linzie BM, Bardales RH. Endoscopic ultrasound-guided fine-needle aspiration findings of gastrointestinal leiomyomas and gastrointestinal stromal tumors. Am J Clin Pathol. 2003;119:703–708. doi: 10.1309/UWUV-Q001-0D9W-0HPN. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


