Abstract
2-amino-1-methyl-6-phenylimidazo[4,5b]pyridine (PhIP) is a dietary mutagenic carcinogen that has been shown not only to induce the formation of DNA adducts, but is capable of inducing tumors in the colon, mammary, and prostate glands. The normal development and maturation of the prostate gland, as well as early progression of prostate cancer, is dependent on androgens acting on the androgen receptor (AR). The actual mechanism by which PhIP interacts with our biological system and its potential interaction at the AR has yet to be fully defined. Here, we describe our work in evaluating the molecular events associated with PhIP-mediated disruption of AR function in LNCaP human prostate cancer cells. We demonstrate, by molecular docking simulation, that PhIP and its metabolite can bind to the ligand-binding domain (LBD). The binding competes with dihydrotestosterone (DHT) in the native AR binding cavity of the receptor. In vitro assays show that PhIP increase AR protein expression in LNCaP cells and alters its responsiveness through PSA protein and mRNA expression. We propose that the mechanism for the tissue-specific carcinogenicity seen in the rat prostate tumors and the presumptive human prostate cancer associated with the consumption of well-done meat may be mediated by this receptor activation. Our results indicate that PhIP may play an important role in modifications of AR function.
Keywords: 2-amino-1-methyl-6-phenylimidazo[4, 5b]pyridine (PhIP), dietary carcinogenesis, molecular modeling, prostate cancer
Introduction
Prostate cancer is the second most commonly diagnosed cancer among men globally. Epidemiological studies have shown changes in prostate cancer incidence among immigrant populations, which suggest that lifestyle factors including diet may be a cause of disease onset [1-3]. It is well documented that diet plays a major role in the etiology and prevention of cancer. One dietary modification that is frequently found among immigrants from eastern to western countries is an increase in the consumption of cooked muscle meats [1,4]. The well-done cooked meats are known to contain potent mutagens and carcinogens [5]. The correlation between increase cancer risk and meat preparation has been linked to the production of heterocyclic amines (HCAs), a group of compounds formed during high temperature cooking of meat [6].
Through diet humans are habitually exposed to varying amounts of food-derived compounds. The major groups of heterocyclic amines found in cooked meats have been identified as compounds with a quinolone, quinoxaline, or pyridine moiety [7]. The most abundant HCA found in the human diet is 2-amino-1-methyl-6-phenylimidazo[4,5b] pyridine (PhIP), it has been shown to produce tissue specific cancer in colon, mammary, and prostate glands. The highest levels of PhIP can be found in grilled or fried meats. PhIP is naturally formed during the cooking process through heat-dependent condensation of creatinine and phenylalanine, two natural components of muscle meats [8]. Human intake varies with food type and cooking conditions and is estimated to range from the nano to tens of micrograms per day [9].
It is known that PhIP itself is not genotoxic, but requires metabolic activation to a direct acting mutagenic species like most environmental carcinogens [10]. The major pathway for bioactivation has been said to involve cytochrome P450 (CYP) and other liver enzymes. The metabolic activation of PhIP occurs in two phases. First, during Phase I metabolism PhIP is oxidized via CYP1A2 to a hydroxylated intermediate, 2-hydroxyamino-1-methyl-6-phenylimidazo[4,5b] pyridine (N2-hydroxy-PhIP). N2-hydroxy-PhIP is then converted via Phase II to a more biologically reactive form. Through esterification it generates O-sulfonyl and O-acetyl esters, which have the capacity to bind DNA and cellular proteins. The end result of PhIP has been shown to present DNA strand breaks, sister chromatid exchanges and form DNA adducts [11].
In addition to many carcinogenic factors, hormones play a role in the turmorgenesis of the prostate gland. Androgens serve as the most important ligands for prostate cancer, its growth and development is dependent on the presence of androgens and the androgen receptor (AR). The AR is a nuclear receptor, which is activated by the binding of androgenic hormones in the cytoplasm and then translocating into the nucleus. This study seeks to evaluate whether PhIP and N2-hydroxy-PhIP can interfere with androgenic functions and regulate gene expression through binding to the androgen receptor (structures shown in Table 1). We analyzed the binding profiles of PhIP and N2-hydroxy-PhIP at the AR using the molecular modeling programs.
Table 1.
Theoretical affinity of PhIP and N-OH-PhIP with respect to the androgen receptor (3L3X)
| No. | Compound | AScore (kcal/mol) |
|---|---|---|
| 1 | Dihydrotestosterone | -13.531 |
| 2 | PhIP | -11.4022 |
| 3 | N-OH-PhIP | -11.093 |
| 4 | 4-OH-PhIP | -9.0353 |
Materials and methods
Chemicals and reagents
RPMI-1640 medium was purchased from ATCC (Manassas, VA). Phenol red-free RPMI-1640 medium, fetal bovine serum, penicillin-streptomycin, 0.25% trypsin-EDTA, dimethylsulfoxide (DMSO), phosphate buffed saline, and Hanks’ Balanced Salt Solution (HBSS) were purchased from Sigma-Aldrich Inc. (St. Louis, MO). PhIP (2-amino-1-methyl-6-phenylimidazo[4,5b]pyridine) was purchased from Toronto Research Chemicals (Toronto, Ontario, Canada). N2-hydroxy-PhIP (2-hydroxyamino-1-methyl-6-phenylimidazo[4,5b] pyridine) was purchased from MRIGlobal Chemical Carcinogen Repository (Kansas City, MO).
Cell culture
LNCaP human prostate cancer cells were purchased from ATCC (Manassas, VA), and were maintained in 75 cm2 flasks with RPMI-1640 growth medium supplemented with 10% fetal bovine serum and penicillin-streptomycin in a 37°C humidified, 95% air to 5% CO2 atmosphere. Growth medium was replaced every three days and subculture was performed biweekly between a 1:3-1:6 ratio upon 60-80% confluency with 0.25% trypsin-EDTA.
Cell treatment
Once the cells had attached, and reached 70-80% confluency, the cells were then supplemented with experimental RPMI-1640 medium containing reduced-serum (2.5%).
Stock solutions for PhIP and N2-hydroxy-PhIP were prepared and constituted in 10% DMSO and stored at 4 and -80°C, respectively, until use. A stock solution of PhIP was prepared at 60 mM; N2-hydroxy-PhIP was prepared at 10 mM. On the day of treatment, working stock solutions were prepared at concentrations of 1-10 µM of N2-hydroxy-PhIP in intervals of 1 µM, and 1-300 µM of PhIP in intervals of 10 µM. DMSO was added as a positive control for all concentrations, with no treatment having over 0.1% DMSO.
Docking
The compounds were docked into the androgen receptor-binding site using ArgusLab 4.0.1 and the Molegro Virtual Docker (MVD), programs used for predicting the most likely conformation of how a ligand will bind to a macromolecule. MVD is a precise semi-flexible molecular docking program that is based on a differential evolution algorithm; the solution of the algorithm takes into account the sum of the intermolecular interaction energy between the ligand and the protein, and the intramolecular interaction energy of the ligand. The accuracy of MVD is higher compared with other dock software. Three-dimensional structures of compounds PhIP, N2-hydroxy-PhIP and 4-hydroxy-PhIP were built using the Chem Draw 7.0 software, and overall geometry optimizations were performed. Each compound was first saved as a MDL Molfile and opened in ArgusLab. Procedures for the docking of substrates were essentially as previously described [12]. Crystal coordinates of the androgen receptor were taken from the Protein Data Bank (code: 3L3X). Each compound was made into a ligand using the “Make a Ligand Group from this Residue” option of the ArgusLab software. The docking between the binding site and each ligand was performed using the “Dock a ligand” option. A maximum number of 500 poses was set in order to increase the binding precision.
Western blot
To determine if PhIP affects the cellular AR and PSA protein levels, Western blotting of whole cell extracts from PhIP or N2-hydroxy-PhIP-treated LNCaP cells was performed. LNCaP cells were propagated in serum-reduced media for 24-48 hr before treatment with PhIP or N2-hydroxy-PhIP for 24, 48 and 72 hr. Cells were then pelleted and lysed in a RIPA buffer solution containing 1 mM of protease inhibitor cocktail (Sigma) for one hour. Lysates were subjected to brief sonication and clarified by centrifugation. For immunoblots, 50-70 μg of whole-cell lysate were loaded and subjected to SDS-PAGE. After electrophoresis, proteins were transferred to nitrocellulose membranes for blotting. The membrane then incubated with 5% milk (a form of blocking buffer) for 2 hr at room temperature and then incubated with 1:1000 dilution of primary antibodies to AR, PSA or GAPDH. The blot was washed three times with 1x PBS + 0.1% Tween 20, pH 7.5, incubated with a 1:2,000 dilution of secondary antibody for 1 hr, and developed with a Superscript Chemiluminescense detection system. Quantitation of the Western blot was performed using a VersaDoc scanner and Quantity One (Bio Rad) software. Results for quantitative comparisons of AR and PSA protein levels are expressed as means ± S.E.M for three separate experiments, and levels were compared to the control group.
RNA extraction and RT-PCR for PSA mRNA
LNCaP cells were treated with PhIP and/or N2-hydroxy-PhIP at the respective concentrations and time-points. Total RNA was extracted from the LNCaP cells via Trizol reagent. Genomic DNA was later removed from the RNA product utilizing a DNase treatment/removal reagent. cDNA synthesis was carried out using iScript cDNA synthesis kit and total RNA (1 µg) measured by the NanoDrop system, and used for the reverse transcription reaction. Isolated RNA with a A260/A280 ratio of 1.8 or greater was then utilized for cDNA synthesis. The RNA (10 µg) was measured by cDNA synthesis and carried out using iScript cDNA synthesis kit for the reverse transcription reaction.
Real-time PCR was performed in an iCycler thermal cycler with MyiQ detection system utilizing the iQ SYBR Green supermix. The GAPDH product was used as a control to assure that an equal amount of RNA was used. PCR reactions contained a cDNA template corresponding to 0.125 µg RNA for PSA and 25 µl of 2× SYBR Green PCR Master Mix (Applied Biosystems), forward and reverse primers in a total volume of 50 µl. PCR samples were incubated at 95°C for 3 min, followed by 40 cycles at 95°C for 10 secs and 55°C for 45 secs. Following data acquisition, PCR cycle threshold (Ct) values determined for each PCR reaction. The quantities of PSA and GAPDH mRNA in cultured LNCaP cells were calculated based on standard curves that were generated using known quantities of PSA and GAPDH RNA standards. Primers for mRNA amplification GAPDH, forward 5’-AAGGTGAAGGTCGGAGTCAAC-3’, reverse 5’-GGGGTCATTGATGGCAACAATA-3’; PSA forward 5’-ACAAAAGCGTGATCTTGCTGG-3’, reverse 5’-CTGACCTGAAATACCTGGCCT-3’.
Statistical analysis
The data are presented as mean ± S.E.M. (n = 3 independent experiments) Statistical comparisons between two groups were performed using a one-way analysis of variance (ANOVA) followed by Bonferroni’s Multiple Comparison Test; the significance levels were defined as *P < 0.05, **P < O.O1, ***P < 0.001 vs. Control. Statistical analysis was conducted with GraphPad Prism 5 software.
Data analysis
The crystal structure of the androgen receptor was used as the basis of the docking experiments. PhIP and N2-hydroxy-PhIP were imported into the docking program, assigned bonds, hybridization and explicit hydrogens if missing, and charges and flexible torsions with MVD. Water molecules in the protein structure were excluded from the docking experiments. The interaction modes of PhIP and N2-hydroxy-PhIP with the androgen receptor active sites were determined as the protein-ligand complex. Docking calculations were carried out using ArgusLab default settings and procedures. For the Molegro program the potential binding sites of the androgen receptor were calculated by using the built-in cavity detection algorithm. The active sites used in the docking studies were defined in a region of a 10.0 Å radius from the centroid of the DHT ligand. Between 10-150 poses were manipulated and analyzed with the MolDock (MD) scores and AScore of ArgusLab.
All other docking analysis parameters were set as previously described [12]. Spacing between the grid points was set at 0.4 Å. “ArgusDock” and “Dock” were chosen as the docking engine for the simulations and calculation type, respectively. “Flexible” was chosen for the ligand and “AScore” for the scoring function. The binding energies for the ligands were calculated with parameters from the AScore.prm file using the AScore function. Ligands that were previously defined, from ligand setup, were then docked and the AScore energies recorded. Poses were rank-ordered by docking energy and the pose with the lowest energy was chosen as the predicted receptor-bound conformation of the ligand. Molecular viewing was conducted with Visual Molecular Dynamics (VMD) software [13].
Results
Androgens have been shown to contribute and be a significant factor in prostate cancer and its aggressiveness. Previous studies have shown that PhIP is capable of causing tissue specific tumors in animal models. The mechanism for this selectivity has not yet fully been elucidated and is usually attributed to differences in organ metabolism. For those heterocyclic amines that are formed in well-done meat, the ability to either bind to the androgen receptor and activate or inhibit an androgenic response will have a major impact on its carcinogenicity. Hence, we investigated the interactions of PhIP and N2-hydroxy-PhIP with the androgen receptor using molecular modeling programs.
Binding simulation with argusLab 4.0.1
The in silico analysis of the HCAs revealed compounds with AScore docking energies ranging from -13.53 – -9.035 kcal/mole based on 1000 posed (Table 1). The entire AR-LDB was used in the docking calculations by including it in all the atom interaction grids. Using molecular docking simulations, we have revealed a possible binding mode of PhIP to the AR based on the predicted binding free energy when compared to the endogenous ligand dihydrotestosterone (DHT). As shown in Figures 1 and 3, both PhIP and N2-hydroxy-PhIP showed similar binding modes to that of DHT being that these two molecules docked into the same cavity of the androgen receptor with high affinity. 4-OH-PhIP was also docked to the AR LBD but showed lower binding affinity when compared to DHT (Figure 2). Models of each exogenous ligand (PhIP, N2-hydroxy-PhIP, and 4-hydroxy-PhIP) were prepared for the docking studies. Docking to the active site of the androgen receptor was conducted as described in the methods section. The visualizations were created using Visual Molecular Dynamics (VMD). The active site and ligands are displayed in the Licorice visualization. The active site amino acids are shown with the coloring method: Name (carbon atoms in blue, oxygen in red, sulfur in yellow, nitrogen in dark blue, fluoride in green, hydrogen in white) and material: Transparent. PhIP is shown with the coloring method: Name and material: Opaque.
Figure 1.
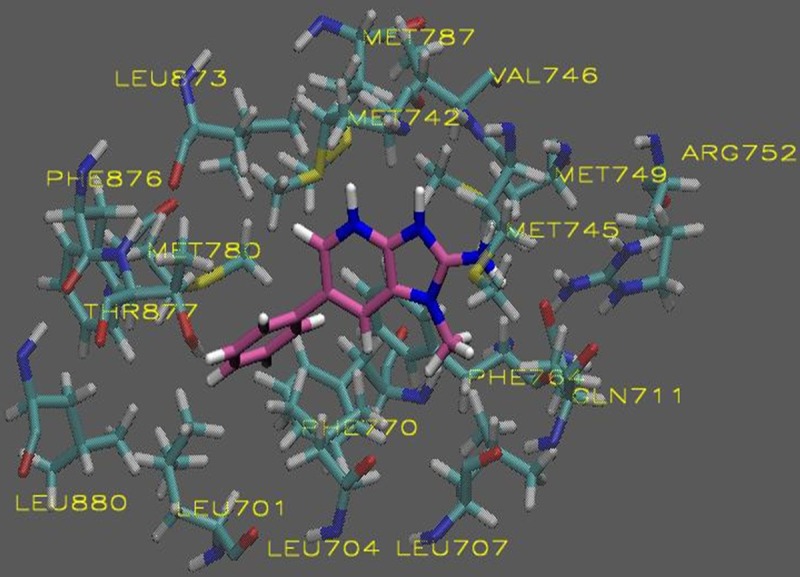
Molecular visualization of PhIP in the active site of the androgen receptor.
Figure 3.
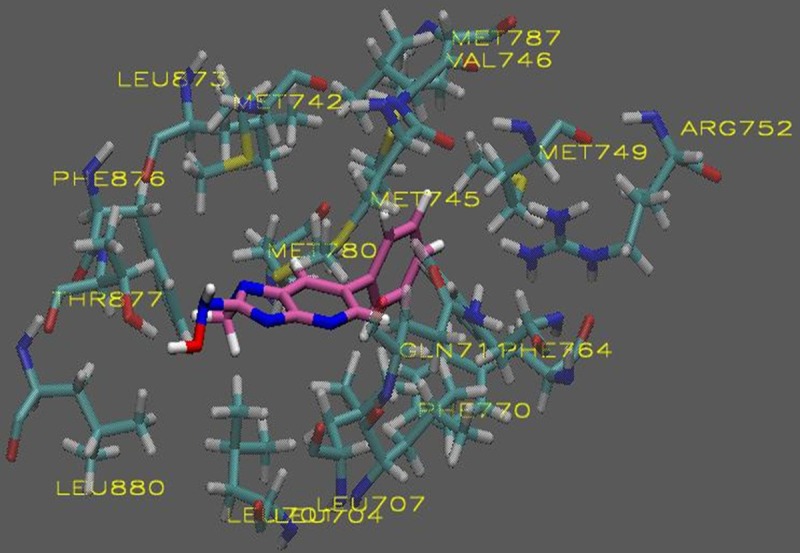
Molecular visualization of N-OH-PhIP in the active site of the androgen receptor.
Figure 2.
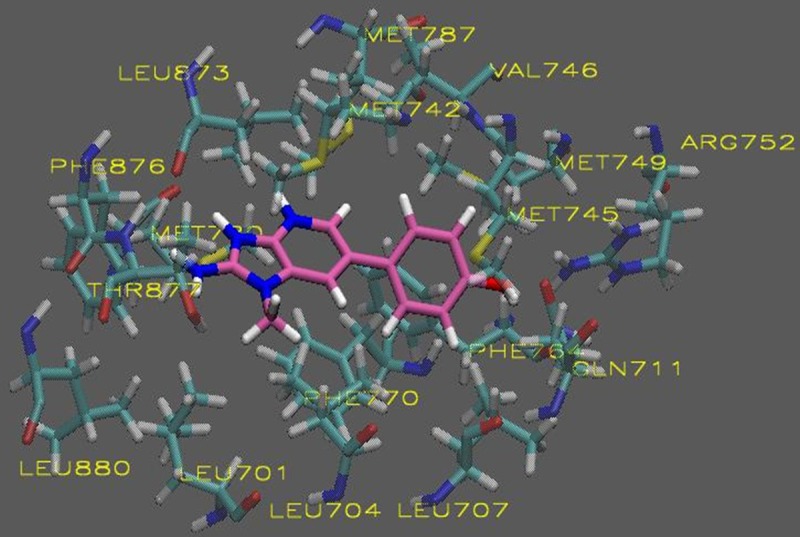
Molecular visualization of 4-OH-PhIP in the active site of the androgen receptor.
Western
To determine if PhIP affects the cellular AR and PSA protein levels, Western blotting of whole cell extracts from PhIP or N2-hydroxy-PhIP-treated LNCaP cells was performed. Levels of AR protein expression may influence androgen-responsiveness and the results in Figures 4 and 5 demonstrate levels of AR and PSA protein in LNCaP cells after various treatments.
Figure 4.
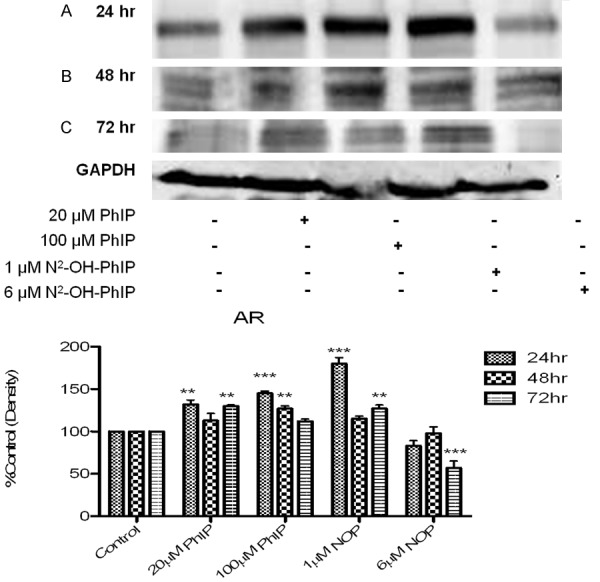
Effect of PhIP and N2-OH-PhIP on protein levels of AR. LNCaP cells were treated with 20 or 100 μM PhIP and 1 or 6 μM N2-OH-PhIP for (A) 24, (B) 48 or (C) 72 hr. Whole cell lysates were analyzed by Western blot analysis as described in section 4.2.5. Antibodies were used to detect the AR proteins. GAPDH served as a loading control. Significant effect compared to control is indicated with an asterisk (** indicates P < 0.01; ***P < 0.001).
Figure 5.
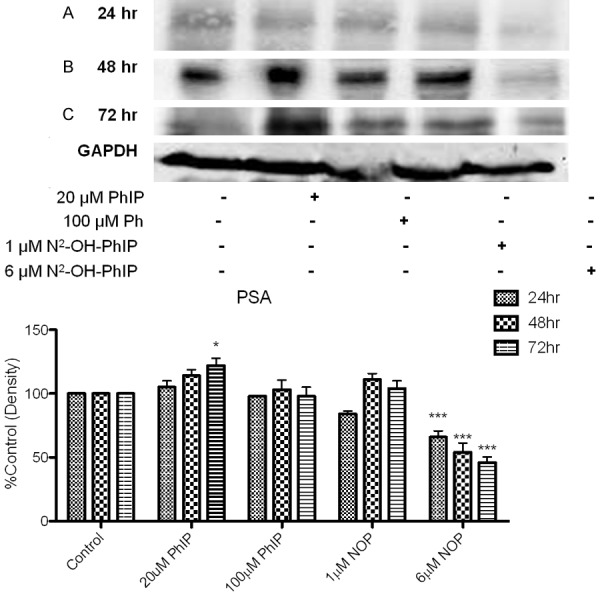
Effect of PhIP and N2-OH-PhIP on protein levels of PSA. LNCaP cells were treated with 20 or 100 μM PhIP and 1 or 6 μM N2-OH-PhIP for (A) 24, (B) 48 or (C) 72 hr. Whole cell lysates were analyzed by Western blot analysis as described in section 4.2.5. Antibodies were used to detect the PSA proteins. GAPDH served as a loading control. Significant effect compared to control is indicated with an asterisk (* indicates P < 0.05; ***P < 0.001).
The data revealed that treatments with PhIP or N2-hydroxy-PhIP differentially modulated AR protein levels in LNCaP cells. These studies illustrated the influence of ligand-induced changes in AR protein expression and the magnitude of hormone-induced transactivation. The expression of AR was significantly increased by 20 μM PhIP (125 ± 1.25%), 100 μM PhIP (133 ± 0.987%), 1 μM N2-hydroxy-PhIP (167 ± 4.32%) and decreased with 6 μM N2-hydroxy-PhIP (78 ± 3.938%) after 24 hr treatments over control levels (Figure 4), significance is noted by the p < 0.05 or greater. After 48 hr, the PhIP and N2-hydroxy-PhIP’s effects on the expression of AR were slightly lowered compared to 24 hr treatment and continued to decrease at 72 hr following 20 μM and 100 μM PhIP; however, these levels remained significantly higher than the control. N2-hydroxy-PhIP exhibited a significant decrease in AR expression with 6 μM N2-hydroxy-PhIP (74.53 ± 4.32%) treatments as shown in Figure 4. The ability of PhIP and N2-hydroxy-PhIP to regulate transcription and PSA protein levels were investigated and revealed that 20 μM of PhIP was able to significantly increase PSA protein expression; 6 μM N2-hyrdoxy-PhIP presented a significant decrease in PSA across all time points (Figure 5). Taken together, these results suggest that PhIP directly regulates the stability of AR and PSA proteins, moderating their expressions.
RT-PCR
Binding of ligand to the AR may not by itself be sufficient to elicit androgenic responses, there has to be transformation and translocation of the AR, followed by binding of coactivators and subsequent transcription. Receptor ligand binding assays cannot account for such steps and, for this reason, RT-PCR was chosen to assess androgenic activity. Previous studies reported that AR-responsiveness was confirmed by induction of PSA mRNA. The effects of PhIP or N2-hydroxy-PhIP on the induction of PSA mRNA activity were compared in LNCaP cells after 24, 48 and 72 hr. Results of Figure 6 show that 20 and 100 μM PhIP and 1 and 6 μM N2-hydroxy-PhIP produce PSA mRNA alterations in LNCaP cells. PSA levels across all time points were found to be lower when compared to control; however, as the time increased there was a returned increase in mRNA expression.
Figure 6.
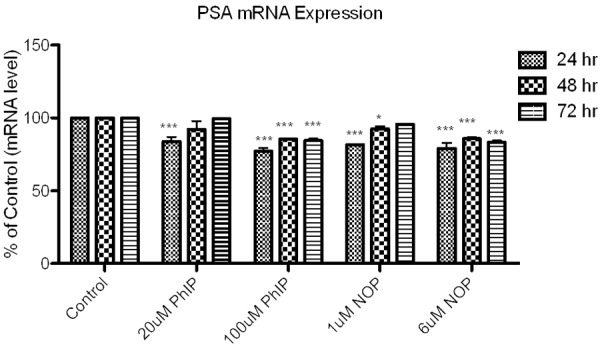
Compilation of all time-points and mRNA expression mRNA expression of PSA in LNCaP cells following treatment of PhIP and N2-OH-PhIP following 24, 48 and 72 hr. Significant effect compared to control are indicated with an asterisk (* indicates P < 0.05; ***P < 0.001).
PSA mRNA expression was significantly decreased by 20 μM PhIP (83 ± 3.075%), 100 μM PhIP (77 ± 2.457%), 1 μM N2-hydroxy-PhIP (81.6 ± 0.490%) and 6 μM N2-hydroxy-PhIP (78 ± 3.938%) after 24 hr treatments when compared to control levels (Figure 4). After 48 hr the sensitivity of PhIP and N2-hydroxy-PhIP to the expression of PSA were slightly lowered compared to control following 20 μM PhIP (92.01 ± 5.725%) and showed significant reduction with 100 μM PhIP (85.38 ± 0.33), 1 μM N2-hydroxy-PhIP (92.38 ± 1.744%) and 6 μM N2-hydroxy-PhIP (85.65 ± 1.055%) treatments as shown in Figure 4. To further assess the effect of prolonged PhIP exposure, cells treated for 72 hr were examined (Figure 4) and there was a significant decrease in mRNA expression following 100 μM PhIP (84.42 ± 1.52) and 6 μM N2-hydroxy-PhIP (83.4 ± 1.087%) treatment compared to control.
Comparing all time-points of PhIP and N2-hydroxy-PhIP exposure, there were considerable reductions in the expression of PSA (Figure 6 and Table 2). As the time of exposure increased there were marginal to modest changes in PSA expression with 48 and 72 hr exposure of LNCaP cells to PhIP and N2-hydroxy-PhIP. Statistically significant decreases, p < 0.05 and 0.001, were detected following one-way ANOVA analysis in the magnitude of PhIP or N2-hydroxy-PhIP down-regulation of PSA expression compared to controls.
Table 2.
Relative expression of PSA mRNA
| 24 hr | 48 hr | 72 hr | |
|---|---|---|---|
| 20 μM PhIP | 83.7 ± 3.08 | 92.09 ± 5.72 | 99.39 ± 0.77 |
| 100 μM PhIP | 77.04 ± 2.46 | 85.38 ± 0.33 | 84.42 ± 1.52 |
| 1 μM NOHPhIP | 81.6 ± 0.49 | 92.38 ± 1.74 | 95.63 ± 0.36 |
| 6 μM NOHPhIP | 78.92 ± 3.93 | 85.65 ± 1.06 | 83.39 ± 1.09 |
Discussion
It is well recognized that diet plays an important role in cancer development and that Westernized diet may be a reason for the geographical bias development of hormone responsive tumors [14,15]. Previous studies have shown that PhIP is capable of causing tissue specific tumors in animal models [16]. The mechanism for this selectivity has not yet fully been elucidated and is usually attributed to differences in organ metabolism. After many studies have shown positive correlations regarding the potential estrogenicity of PhIP and its relevance to tumor progression [17], and others confirming that PhIP activates ER and stimulates cell growth [7], we are stating that PhIP is capable of having potential anti-androgenicity. The goal of the present study was to obtain information on the molecular mechanisms of PhIP-mediated effects in prostate cancer.
The androgen receptor (AR) is a member of the nuclear receptor superfamily and like the other nuclear receptors, it is made up of three main functional domains: a mutable N-terminal domain (NTD), a well-maintained DNA-binding domain (DBD), and a ligand-binding domain (LBD) [18,19]. After binding of an androgen to its LBD, AR translocates to the nucleus, where it directly interacts with DNA as a homodimer, at the androgen response elements (ARE) found in the regulatory regions of target genes. Findings from this study add to the growing body of knowledge about food choice and diet-related health crises. The idea of PhIP having androgenic effects through binding to the androgen receptor is quite feasible, this being that PhIP and androgens are structurally quite similar. This is the receptor through which androgens mainly elicit its androgenic activity and responses such as increase in cell proliferation and viability [20].
To elucidate the molecular mechanism of action by which PhIP and N2-hydroxy-PhIP affect tumor growth, we investigated the interactions of these compounds with the androgen receptor using MVD that provides a novel optimization technique shown to yield higher docking accuracy than other docking products. We have shown through computational docking that, with the human androgen receptor (AR), PhIP and the hydroxylated metabolite of PhIP N2-hydroxy-PhIP binds favorably into the AR ligand-binding domain binding site. The AScore binding energies for the compounds suggested that it is likely, based on high-throughput site localization that the relative interactions with the receptor active site are of high affinity. Although the calculated probability of PhIP is lowered compared to DHT, this binding competes with DHT in the native binding cavity of the androgen receptor. Compared to PhIP, both the N2-hydroxy- and 4-hydroxy PhIP metabolites rotated by 180° with the phenyl ring pointing toward residues THR877 and MET780. As shown in Figures 1 and 3, PhIP and N2-hydroxy-PhIP have similar binding modes with the androgen receptor. They were recognized and bound to the same site of the androgen receptor, which may explain how these compounds are target-specific in its tumorigenesis. PhIP and/or N2-hydroxy-PhIP compete for the same sites to which DHT and other androgens would normally bind; therefore, the natural actions of these hormones would be inhibited.
We, therefore, examined the ability of PhIP to induce biochemical changes that may be associated with androgen-like mediated proliferation or cytotoxic effects. This data demonstrate that varying treatments of PhIP, and constituently N2-hydryoxy-PhIP, differentially modulate AR protein levels in LNCaP cells. This also affects the ligand-induced changes in the degree of hormone-induced transactivation, via PSA expression. Consistent with its activation of the AR, PhIP up-regulated PSA expression. The up-regulation of AR in prostate cancer cells is considered to be mainly due to transcriptional events and is regarded as a marker of androgen responsiveness.
Further evidence that PhIP and N2-hydroxy-PhIP acts as a component in regulating AR responsiveness is provided by its effect on PSA mRNA expression. We have shown that the inhibitory effect of the parent compound and metabolite on PSA mRNA activity. Given our results that PhIP regulates PSA mRNA as well as protein levels, and AR protein levels, it remains to be investigated whether the changes observed at the protein level are a consequence of variations of transcriptional modulators that cooperate with PSA. Alternatively, we may consider that AR regulation occurs also at a translational or post-translational level.
In conclusion, PhIP remains an area of concern because of human exposure and its mutagenic potential. Based on the present studies, PhIP and N2-hydroxy-PhIP are capable of disrupting endogenous androgen function, and therefore prostate cell growth, through its conceivable manipulation of the androgen receptor. PhIP and its metabolite possess androgenic activity at low concentrations and amplifies the AR, supporting the idea that exposure to PhIP, even at low doses could result in androgenic effects. At high concentrations N2-hydroxy-PhIP significantly decreased AR expression. Together, these results imply that this dietary constituent may play an active role in both activating and inhibiting hormone sensitive cancer, such as prostate cancer.
Acknowledgements
Research reported in this publication was supported by the National Institute on Minority Health and Health Disparities of the National Institutes of Health under award numbers P20MD006738 (P20 Center of Excellence) and G12MD007582 (RCMI). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosure of conflict of interest
None.
References
- 1.Sugimura T. Food and cancer. Toxicology. 2002;181-182:17–21. doi: 10.1016/s0300-483x(02)00250-0. [DOI] [PubMed] [Google Scholar]
- 2.Felton JS, Knize MG. New mutagens from cooked food. Prog Clin Biol Res. 1990;347:19–38. [PubMed] [Google Scholar]
- 3.Kolonel LN, Nomura AM, Cooney RV. Dietary fat and prostate cancer: current status. J Natl Cancer Inst. 1999;91:414–428. doi: 10.1093/jnci/91.5.414. [DOI] [PubMed] [Google Scholar]
- 4.Lauber SN, Gooderham NJ. The cooked meat-derived mammary carcinogen 2-amino-1-methyl-6-phenylimidazo[4,5-b] pyridine promotes invasive behaviour of breast cancer cells. Toxicology. 2011;279:139–145. doi: 10.1016/j.tox.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 5.Bennion BJ, Cosman M, Lightstone FC, Knize MG, Montgomery JL, Bennett LM, Felton JS, Kulp KS. PhIP carcinogenicity in breast cancer: computational and experimental evidence for competitive interactions with human estrogen receptor. Chem Res Toxicol. 2005;18:1528–1536. doi: 10.1021/tx0501031. [DOI] [PubMed] [Google Scholar]
- 6.Sugimura T, Wakabayashi K, Nakagama H, Nagao M. Heterocyclic amines: Mutagens/carcinogens produced during cooking of meat and fish. Cancer Sci. 2004;95:290–299. doi: 10.1111/j.1349-7006.2004.tb03205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lauber SN, Ali S, Gooderham NJ. The cooked food derived carcinogen 2-amino-1-methyl-6-phenylimidazo[4,5-b] pyridine is a potent oestrogen: a mechanistic basis for its tissue-specific carcinogenicity. Carcinogenesis. 2004;25:2509–2517. doi: 10.1093/carcin/bgh268. [DOI] [PubMed] [Google Scholar]
- 8.Kulp KS, Knize MG, Fowler ND, Salmon CP, Felton JS. PhIP metabolites in human urine after consumption of well-cooked chicken. J Chromatogr B Analyt Technol Biomed Life Sci. 2004;802:143–153. doi: 10.1016/j.jchromb.2003.09.032. [DOI] [PubMed] [Google Scholar]
- 9.Knize MG, Kulp KS, Salmon CP, Keating GA, Felton JS. Factors affecting human heterocyclic amine intake and the metabolism of PhIP. Mutat Res. 2002;506-507:153–162. doi: 10.1016/s0027-5107(02)00162-8. [DOI] [PubMed] [Google Scholar]
- 10.Han JF, He XY, Herrington JS, White LA, Zhang JF, Hong JY. Metabolism of 2-amino-1-methyl-6-phenylimidazo[4,5-b] pyridine (PhIP) by human CYP1B1 genetic variants. Drug Metab Dispos. 2008;36:745–752. doi: 10.1124/dmd.107.016824. [DOI] [PubMed] [Google Scholar]
- 11.Aboyade-Cole A, Darling-Reed S, Oriaku E, McCaskill M, Thomas R. Diallyl sulfide inhibits PhIP-induced cell death via the inhibition of DNA strand breaks in normal human breast epithelial cells. Oncol Rep. 2008;20:319–323. [PMC free article] [PubMed] [Google Scholar]
- 12.Yanamala N, Tirupula KC, Klein-Seetharaman J. Preferential binding of allosteric modulators to active and inactive conformational states of metabotropic glutamate receptors. BMC Bioinformatics. 2008;9(Suppl 1):S16. doi: 10.1186/1471-2105-9-S1-S16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Humphrey W, Dalke A, Schulten K. VMD: visual molecular dynamics. J Mol Graph. 1996;14:33–38. 27–38. doi: 10.1016/0263-7855(96)00018-5. [DOI] [PubMed] [Google Scholar]
- 14.Hsing AW, Tsao L, Devesa SS. International trends and patterns of prostate cancer incidence and mortality. Int J Cancer. 2000;85:60–67. doi: 10.1002/(sici)1097-0215(20000101)85:1<60::aid-ijc11>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 15.Shirai T, Asamoto M, Takahashi S, Imaida K. Diet and prostate cancer. Toxicology. 2002;181-182:89–94. doi: 10.1016/s0300-483x(02)00260-3. [DOI] [PubMed] [Google Scholar]
- 16.Shirai T, Sano M, Tamano S, Takahashi S, Hirose M, Futakuchi M, Hasegawa R, Imaida K, Matsumoto K, Wakabayashi K, Sugimura T, Ito N. The prostate: a target for carcinogenicity of 2-amino-1-methyl-6-phenylimidazo[4, 5-b] pyridine (PhIP) derived from cooked foods. Cancer Res. 1997;57:195–198. [PubMed] [Google Scholar]
- 17.Gooderham NJ, Zhu H, Lauber S, Boyce A, Creton S. Molecular and genetic toxicology of 2-amino-1-methyl-6-phenylimidazo[4,5-b] pyridine (PhIP) Mutat Res. 2002;506-507:91–99. doi: 10.1016/s0027-5107(02)00155-0. [DOI] [PubMed] [Google Scholar]
- 18.Jenster G, van der Korput HA, van Vroonhoven C, van der Kwast TH, Trapman J, Brinkmann AO. Domains of the human androgen receptor involved in steroid binding, transcriptional activation, and subcellular localization. Mol Endocrinol. 1991;5:1396–1404. doi: 10.1210/mend-5-10-1396. [DOI] [PubMed] [Google Scholar]
- 19.Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schutz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, Evans RM. The nuclear receptor superfamily: the second decade. Cell. 1995;83:835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakai Y, Nelson WG, De Marzo AM. The dietary charred meat carcinogen 2-amino-1-methyl-6-phenylimidazo[4,5-b] pyridine acts as both a tumor initiator and promoter in the rat ventral prostate. Cancer Res. 2007;67:1378–1384. doi: 10.1158/0008-5472.CAN-06-1336. [DOI] [PubMed] [Google Scholar]


