Abstract
Recent studies have suggested that elevated gonadotropins contribute to ovarian epithelial tumor (OET) cell proliferation. However, the cellular effects of luteinizing hormone, a member of gonadotropins, on OET proliferation are controversial. Our previous work showed that luteinizing hormone has no effect on cell proliferation, but the molecular mechanism of such finding remains to be clarified. Considering that the cell growth in various types of tumors has been associated with regulations of prohibitin and matrix metalloproteinases, we aim to investigate a possible regulatory role of luteinizing hormone on prohibitin and matrix metalloproteinases to determine the roles of these molecules in OET proliferation. We found that LH stimulation resulted in a dose-dependent expression of prohibitin and MMPs and time-dependent phosphorylations of ERK and AKT. Blocking MAPK or PI3K/AKT signaling could attenuate LH-induced prohibitin and MMPs expression. Additionally, the depletion of prohibitin reduced the level of MMPs expression, and increased prohibitin expression abolished the positive effect of LH-induced MMP-9 on cellular growth. Therefore, we conclude that LH is able to up-regulate both prohibitin and MMP-9 in OET cells without the cellular growth effect due to opposing biologic functions for cell proliferation between these two molecules. The opposing cellular growth function between prohibitin and MMP-9 is a novel finding. Regulation of either molecule may be useful for future targeted therapy for ovarian epithelial cancers.
Keywords: Prohibitin, MMP-2, MMP-9, LH, proliferation
Introduction
Ovarian epithelial cancer (OEC) is one of the leading causes of cancer-related deaths among all gynecological malignancies. However, the precise biologic mechanisms of ovarian epithelial carcinogenesis are poorly understood. The gonadotropins, follicle-stimulating hormone (FSH) and luteinizing hormone (LH), are crucial regulators in steroidogenesis and gametogenesis. In the last few decades, there has been increasing evidence to suggest that the hormonal environment of the normal ovarian surface epithelium (OSE) and ovarian epithelial tumor (OET) cells is associated with the development and progression of ovarian cancer. From an epidemiologic point of view, exposure to excess gonadotropins has been proposed as a risk factor for ovarian cancer [1], especially in postmenopausal women or in women who have received treatment for induction of ovulation [2,3]. Alternatively, multiple pregnancies, breast-feeding, oral contraceptives, and estrogen replacement therapy, have shown to reduce the risk for ovarian cancer due to reduced exposure to gonadotropins [4,5]. However, reports on the association of gonadotropins with the proliferation of OSE and OEC cells are controversial. Increased cellular proliferation was observed after FSH and/or LH stimulation in some studies [6,7]. In other studies, however, there was no report of such an association [8,9] or even reduced cellular proliferation [6,9]. Such a controversial finding indicates that the role of gonadotropins in the etiology of OEC development and progression is far more complicated than what has been previously proposed. Therefore, there is a critical need to better understand the exact roles of gonadotropins and related molecular mechanisms in OEC development.
In our previous work, the proliferative cellular effects were not observed when the OET cells were treated with various doses of LH. However, LH, as a member of the glycoprotein hormones family, could induce prohibitin expression, a potential tumor suppressor gene [10]. The prohibitin gene encodes a protein of 275 amino acids that is highly conserved. In some cancers, prohibitin was found to have anti-proliferative activity by causing cell cycle arrest at G1/S [11,12]. Besides prohibitin, MMP-2 and MMP-9 were also up-regulated by LH/hCG, which contributed to increased invasion in the trophoblast and endometrial cancer cells [13,14]. Both proteins belong to matrix metalloproteinases (MMPs) that are involved in tissue remodeling and morphogenesis by degrading components of the extracellular matrix. Increased activity of these enzymes could accelerate tumor growth, migration, and the spread of cancer cell by damaging the extracellular matrix barriers [15]. Moreover, MMPs are also associated with tumor proliferation [16,17]. Previous work showed MMP-2 siRNA treatment to significantly decrease cellular growth in keratinocytes [18] and overexpression of MMP-9 resulted in rapid proliferation of cervical cancer cells [17]. These roles were reported to rely on the activation of signal pathways such as protein kinase A [14] and AKT [13]. However, it is not clear if there is a similar regulatory pattern by LH in ovarian cancer. To further explore the cellular and molecular mechanisms of LH on OET cell proliferation, the current study is designed from the following aspects: 1) the effect of LH on prohibitin and MMPs expression and signaling; 2) whether the opposite roles of prohibitin and MMPs on cellular growth has any affect on the proliferative activity of LH.
Materials and methods
Ovarian epithelial tumor cell culture and treatment
The MCV152 cell line was derived from SV40-transformed serous papillary cystadenoma and transfected with telomerase hTERT [6]. The cells were known to contain gonadotropin receptors that respond to gonadotropin stimulation. Cells were maintained in Eagle’s minimal essential medium (MEM). The SKOV-3 cells from an ovarian serous carcinoma cell line were purchased from the American Type Tissue Collection (Manassas, VA) and cultured in 1:1 DMEM/F12. Both cell lines were cultured in medium supplemented with 100 U/ml penicillin, 100 ug/ml streptomycin, and 10% fetal bovine serum. Cultures were maintained at 37°C in a humidified incubator containing 95% room air and 5% CO2.
To investigate the effects of LH on the expression of prohibitin, MMP-2, and MMP-9, both MCV152 and SKOV3 cells were seeded in 100 mm dishes. When it reached 60% confluence, the mediums were replaced with serum-free opti-MEM and cultured for an additional 24 hours. The mediums were followed by treatment with LH at the concentrations of 5, 25, 50, 100, 200, 400 mIU/ml for 48 hours, respectively. PBS instead of LH was used as a negative control. In order to determine the special signal pathways related to LH, the cells were changed with serum-free opti-MEM and incubated for 1 hour prior to 50 mIU/ml LH for 15 minutes, 30 minutes, and 1 hour. Cells were incubated by inhibitors of PI3K/AKT and ERK1/2 (LY294002, U0126) for 1 hour before the addition of LH or PBS for another 48 hours. In the end, cells were collected for Western blot analysis.
RNA extraction and real time PCR
To determine the suitability of cell systems for analyses of LH-dependent effects, the real time PCR was performed to investigate the LH receptor in MCV152 and SKOV3 cells. Total RNA was isolated according to the protocol of the RNeasy micro Kit (Qiagen Company, Frankfurt, Federal Republic of Germany). 2.5 μg RNA was reverse transcribed into cDNA in a 20 μl reaction as described previously [6]. Briefly, samples were incubated at 37°C for 1 hour in 20 μl of 10 mM TRIS (pH 8.4), 50 mM KCl, 2 mM MgCl2, 1 mM DTT, 5 units of ribonuclease inhibitor, 0.5 mM dNTP, 100 pmol of oligo-d(T) 18, and 200 units of M-MLV reverse transcriptase (Promega Company, USA). The reaction was terminated by heating for 5 minutes at 95°C. Reverse transcribed products were used for real time PCR amplification. The primers, designed to specifically amplify the LHR, are as follows: sense, 5’-TCA ATG TGG TGG CCT TCT TCA TA-3’; antisense, 5’-TTG GCA CAA GAA TTG ATG GGA TA-3’. The length of amplification was 233bp. The PCR conditions were as follows: 95℃ for 45 seconds followed by 55°C for 45 seconds, 72°C for 45 seconds for 30 cycles, and then 95°C for 10 minutes. GAPDH served as a loading control.
Western blot analyses
All of the cells collected were lysed in lysis buffer (0.5% Nonidet P(NP)-40, 10 mM Tris, pH 7.4, 150 mM NaCl, 1 mM ethylene diamine tetra-acetic acid [EDTA], 1 mM Na3VO4) containing protease inhibitors (1 mM phenylmethylsulfonyl fluoride [PMSF]). Proteins were loaded on a 10% or 12% SDS-PAGE and followed by transfer to polyvinylidene fluoride (PVDF) membranes before being blocked in 5% non-fat milk in 10 mM Tris, pH 7.5, 100 mM NaCl, 0.1% (w/v) Tween-20 for 2 hours. The anti-human MMP-2 and MMP-9 rabbit polyclonal antibody was diluted at 1:1000 while the anti-human prohibitin mouse monoclonal antibody was diluted at 1:400 (Labvision Corporation–NeoMarkers, Fremont, CA), p-AKT, AKT, ERK1/2 rabbit polyclonal antibody 1:1000, p-ERK1/2 1:10000, and incubated at 4°C overnight. This was followed by a 1 hour incubation period with the appropriate secondary antibody. The anti-β-actin mouse monoclonal antibody was diluted at 1:10000 for sample loading control. The blots were further developed using the chemiluminescence reagent (SuperSignal West Pico, Rockford, IL). Software Image Quant was used for densitometry analysis of the scanned images (Molecular Dynamics, Inc).
RNA interference and MTT assay
The siRNA against prohibitin used the short RNA duplexes with 21 oligos designed to target sequence (5’-CCC AGA AAT CAC TGT GAA ATT-3’), as r(CAG AAA UCA CUG UGA AAU U)dTdT and r(AAU UUC ACA GUG AUU UCU G)dGdG (Qiagen Company, Frankfurt, Federal Republic of Germany). As a control (siCon), it has no homology to known mammalian genes (Qiagen Company, Frankfurt, Federal Republic of Germany). Cellular proliferation after prohibitin silencing was evaluated by 3-(4, 5-dimethylthiazol-2-yl)-2, 5-Diphenyltetrazolium (MTT) assay. Briefly, MCV152 cells were seeded into 96-well plates at 1 × 104/well in a final volume of 200 μl and grew to a confluence of 30-50% after 24 hours of antibiotics-free incubation. Then transfection was carried out using HiperFect Transfection Reagent (Qiagen company, Frankfurt, Federal Republic of Germany) according to the manufacturer’s recommendation. The cells were then incubated in MEM with 10% FBS for 48 hours followed by LH stimulation at 50 mIU/ml for 24 hours. After, 20 μl of MTT solution (5 mg/ml in PBS) was added to each well. After 4 hours of incubation at 37°C, the culture medium was removed and 200 μl dimethyl sulfoxide (DMSO) was added to dissolve the formazan. Finally, its density was read at 570 nm using a microplate reader each in six wells. Prohibitin knockdown was again verified by Western blot. The investigations of MMP-2 and MMP-9 on proliferation were carried out according to above description. Non-modified and high performance purified siRNA duplexes were obtained from QIAGEN. The constructs were directed against the following sequences: 5’-CAGGCTCTTCTCCTTTCACAA-3’ for knocking down MMP-2 and 5’-ACGGCTTGCCCTGGTGCAGTA-3’ for knocking down MMP-9 in MCV152 cells.
To examine the relationship between PHB and MMPs, their expressions were evaluated after the prohibitin knockdown was performed. The MCV152 and SKOV3 cells were seeded in 100 mm dishes and cultured for 24 hours. siPHB short sequence was then transfected into the cells with HiperFect Transfection Reagent (Qiagen company, Frankfurt, Federal Republic of Germany) when these cells reached 50% confluence. After culturing for an additional 48 hours, the cells were collected. The knockdown rate of prohibitin and the expression of MMP-2 and MMP-9 were evaluated by Western blot.
Statistical analyses
Cell proliferation and the band density of Western blot were analyzed with the Student’s t-test. The statistical significance of the results was assessed using SPSS 11.5 software with P < 0.05 considered significant
Results
Expression of LHR in ovarian epithelial tumor cells
Real time PCR was performed to measure the expression of LHR in MCV152 and SKOV3 cells. As shown in Figure 1, both cell lines expressed LHR. Despite slightly elevated LHR expression in MCV152 cells, there is no significant difference between the both cell lines. The presence of LHR indicates that both cell lines respond to LH stimulation.
Figure 1.
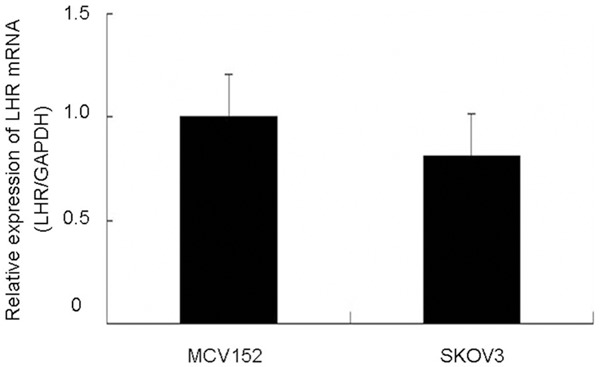
Real time PCR determine the expression of LH receptor in MCV152 and SKOV3 cells.
LH induces PHB and MMPs expressions
As seen in Figure 2, an increased dose of LH resulted in significant up-regulations of prohibitin, MMP-2, and MMP-9 proteins, in a dose-dependent manner. In MCV152 cells, the expressions of prohibitin and MMP-9 reached peak levels with the addition of LH at 50 mIU/ml for 48 hours, while peak levels of MMP-2 appeared with LH at 100 mIU/ml (Figure 2A). Similar expression profiles appeared in SKOV3 cells (Figure 2B).
Figure 2.
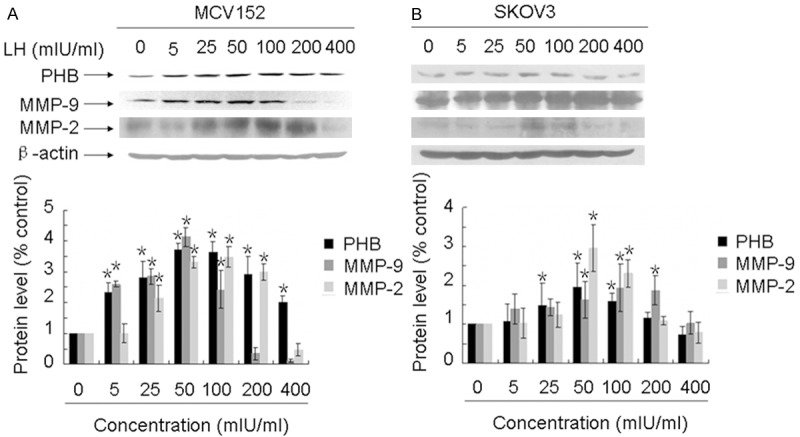
The expression of prohibitin, MMP-2 and MMP-9 was LH-dependent. Serum-free cultures were treated with different doses of LH for 48 h (from 5 to 400 mIU/ml), preparation of protein lysates and Western blot analyses were performed as described in Materials and Methods. A. Shows the LH dose-response of prohibitin, MMP-2 and MMP-9 expression in MCV152 cells. β-actin was used as a loading control. B. Shows the LH dose-response of prohibitin, MMP-2 and MMP-9 expression in SKOV3 cells. β-actin was also used as a loading control. *represents the results of comparison with control group, which is statistically significant (P< 0.05).
LH activates MAPK and PI3K/AKT signal pathways and mediates PHB and MMPs expressions via these signal pathways
Critical signal transduction pathways related to LH in OEC development were further investigated. As seen in Figure 3A, 50 mIU/ml LH induced phosphorylation of ERK and AKT with peak levels at 60 minutes of treatment in MCV152 cells. Activated AKT (p-AKT/AKT) and ERK (p-ERK/ERK) with a clear increase of more than three-fold were observed, whereas in SKOV3 cells, the maximum increment of phosphorylated ERK and AKT was observed with LH for 30 minutes, 12-fold for AKT (p-AKT/AKT) and 3-fold for ERK (p-ERK/ERK) when compared with controls. The data is summarized in Figure 3B. Subsequently, the relationship between these effectors, including PHB and MMPs, and the involved signal pathways was further examined. As seen in Figure 4, the expressions of PHB, MMP-2, and MMP-9 were significantly reduced when cells were pretreated alone with U0126 or LY294002. Moreover, in both MCV152 and SKOV3 cells, LH-induced the up-regulation of MMPs and PHB was down-regulated by both inhibitors.
Figure 3.
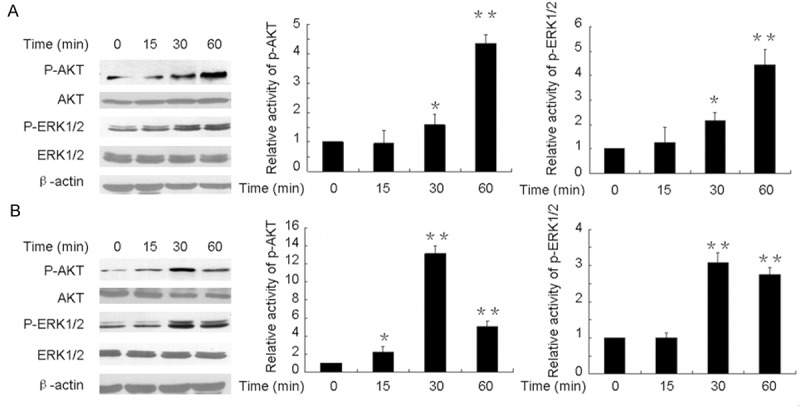
Western blot analyses showing LH dependent phosphorylation of AKT and ERK in MCV152 and SKOV3 cells. Both of the cells were treated with 50 mIU/ml LH for indicated time periods in serum-free cultures. The harvested cells were lysed and Western blot analyses were performed as Materials and Methods described. β-actin was used as a loading control. PBS treatment served as a negative control. Each experiment repeated three times. A. LH-dependent activation of ERK and AKT in MCV152 cells. B. LH-dependent activation of ERK and AKT in SKOV3 cells. *represents the results of comparison between different time point of LH treatment with control, which is statistically significant (P < 0.05). **P < 0.01.
Figure 4.
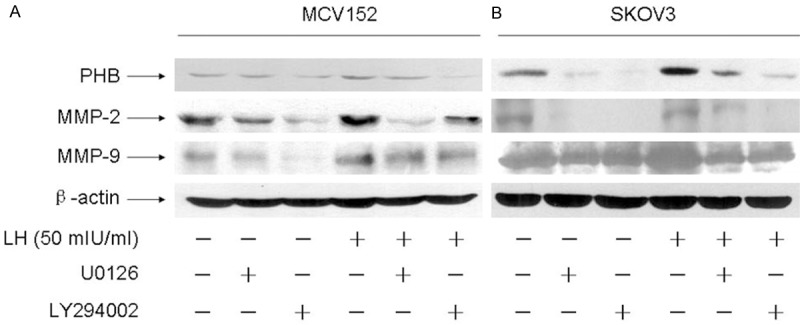
LH regulated PHB, MMP-2 and MMP-9 protein expression through AKT and ERK1/2 signal pathway. The cells were treated with or without 50 mIU/ml LH for 48 h in serum-free cultures after pre-treatment with 10 μmol/L U0126 and LY294002 for 1 h, the protein expressions were investigated by western blot analysis. PBS treatment was used as a negative control. A. Shows ERK1/2 signaling inhibitor U0126 and AKT signaling inhibitor LY294002 block the up-regulation of prohibitin, MMP-2 and MMP-9 induced by LH in MCV152 cells. B. Shows ERK1/2 signaling inhibitor U0126 and AKT signaling inhibitor LY294002 block the up-regulation of prohibitin, MMP-2 and MMP-9 induced by LH in SKOV3 cells.
Knocking down PHB attenuates MMP2 and MMP9 expressions
Although MMP-2, MMP-9, and prohibitin are target molecules of LH, the relationship between the effectors has not been revealed. An RNAi approach was used to explore this problem. As described in Figure 5, the inhibition rate of prohibitin was 90% in MCV152 cells and 73% in SKOV3 cells. Knockdown of prohibitin resulted in a sharp decrease of MMP-2 and MMP-9 expression levels. MMP-2 was decreased by 88% in MCV152 cells and 31% in SKOV3 cells. Similar inhibition patterns of MMP-9 were observed; MMP-9 level was reduced by 87% in MCV152 and 29% in SKOV3 cells accompanying prohibitin deficiency.
Figure 5.
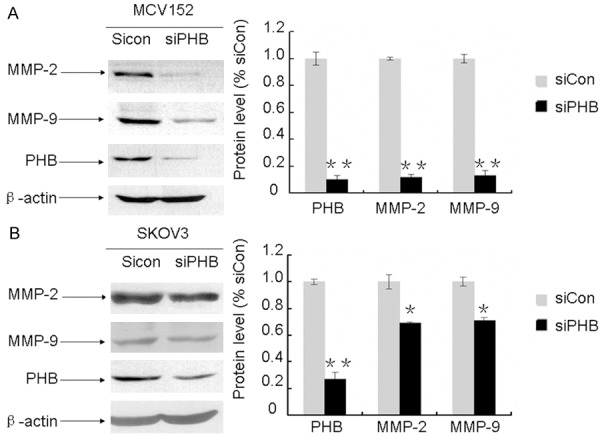
Silence of prohibitin reduced the expression of MMP-2 and MMP-9 in MCV152 and SKOV3 cells. MCV152 and SKOV3 cells were plated in 60mm dishes and cultured for 24 h, the siCon and siPHB RNA sequence were transfected into these cells when they reached 50% confluence and cultured for another 48 h, the changes of these proteins were examined with Western blot analysis. β-actin was used to evaluate protein loading. Representative examples (n = 3) are shown. A. Effect of knockdown of prohibitin on the expression of MMP-2 and MMP-9 in MCV152 cells. B. Effect of knockdown of prohibitin on the expression of MMP-2 and MMP-9 in SKOV3 cells. * or **represents the results of comparison with control, which is statistically significant (*P < 0.05; **P< 0.01).
Effect of PHB on ovarian epithelial cell growth
Given that LH regulates prohibitin, we investigated the effect of prohibitin silencing on cell growth in MCV152 cells. Compared with the control group (siCon), cells transfected with siPHB resulted in a clear reduction of prohibitin expression. After cells were transfected by siPHB, they were stimulated by LH to examine for any changes in proliferative effect . As expected, prohibitin expression was higher in the siPHB- and siCon-transfected cells when compared to the corresponding groups without LH stimulation (Figure 6A). The MTT assay was subsequently used to examine the cellular growth effect in prohibitin-depleted cells. The data showed an increase in MCV152 cells after prohibitin ablation. However, regardless of siPHB- or siCon-transfected cells, there was no significant difference in total cell numbers between the groups with or without LH treatment. This was consistent with our previous finding that LH itself does not have an inhibitory role in OET cell proliferation. The data is summarized in Figure 6B.
Figure 6.
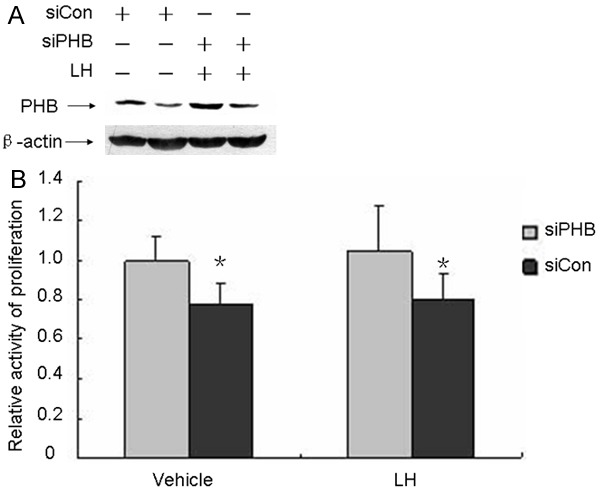
Effects of RNA interference on prohibitin expression and cell proliferation.MCV152 cells were seeded and cultured in 60 mm dishes and 96-well plate, respectively, the siPHB and siCon sequence were transfected into MCV152 cells through HiperFect transfection reagents when these cells reach 50% confluence and cultured for additional 24 h, then they were expose to 50 mIU/ml LH or absence LH for additional 48 h, the proliferation was determined by MTT assay and the knockdown rate of prohibitin was evaluated through western blot assay. A. Effects of RNA interference on prohibitin expression. B. Effects of RNA interference on cell proliferation.
The opposite roles of PHB and MMP-9 on proliferation contribute to LH’s failure to enhance OEC proliferation
To explore the molecular mechanism that LH has no effect on cell proliferation, the effects of MMP-9 and MMP-2 on cellular growth were investigated with MTT assay. It was observed that cellular proliferation activity significantly decreased after knocking down MMP-9. The MMP-9 knock-down cell population, even with additional LH treatment, showed lower cellular growth than that of the control. Cellular growth was slightly increased with the LH treatment compared to the MMP-9 knock-down cell population alone (Figure 7). As seen in Figure 8, compared with the control group, the loss of prohibitin resulted in approximately 20% cell population growth. However, silencing both prohibitin and MMP-9 decreased cellular growth more than prohibitin deletion alone. This indicates that the down-regulation of MMP-9 enhances the effects of prohibitin deletion and both contribute decreased cellular proliferation. The results are summarized in Figure 8.
Figure 7.
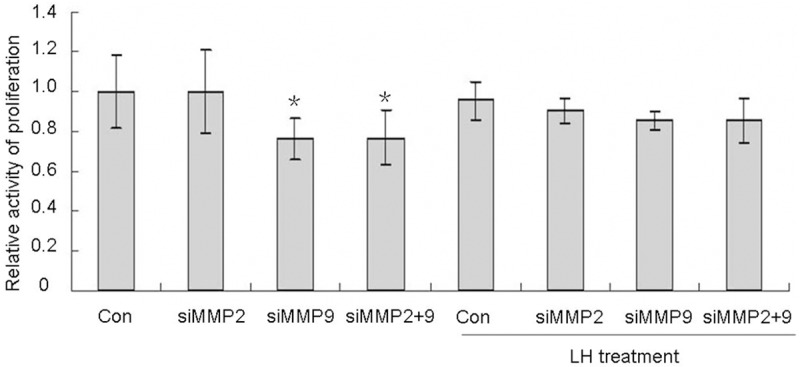
Effects of knocking down MMP-2 and MMP-9 on cell proliferation. MCV152 cells were seeded in 96-well plate with a concentration of 1000/well, RNA interference and MTT assay were done as described in Material and Methods. *represents the results of comparison between different treatments and control group, which is statistically significant (P < 0.05).
Figure 8.
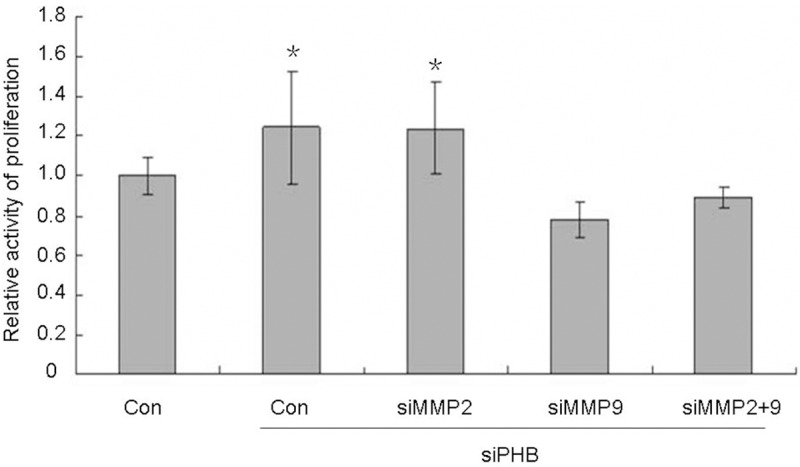
MMP-9 attenuate the inhibition of prohibitin on cell proliferation. MCV152 cells were seeded in 96-well plate with a concentration of 1000/well, RNA interference and MTT assay were done as described in Material and Methods. *p < 0.05, when the group of RNA interference compared with control group.
Discussion
Despite tremendous progress in the study of ovarian epithelial cancer etiology, the detailed mechanisms remain unclear. The gonadotropin theory has been proposed to explain the reason of OEC occurrence, which suggests that that elevated serum gonadotropins contributes significantly to OEC development [7]. Despite the suggested role of gonadotropins in the pathogenesis of ovarian cancer, different research groups have shown dramatically different effects of LH on OEC proliferation. Our previous work suggested that LH has no effect and even may have an opposing effect of FSH on OEC cell proliferation [6]. However, it remains unclear what are the molecular mechanisms for the role of LH in this particular setting.
In this study, we found that treatment with LH resulted in a significant change of prohibitin, MMP-2, and MMP-9 protein levels (Figure 2). This shows that prohibitin is one of the target genes of LH. As other studies have reported, prohibitin plays an important role in OET cell proliferation. Additionally, the silence of PHB resulted in enhanced cell proliferative activity (Figure 6). In our current work, dose-dependent expression profiles of MMP-2 and MMP-9 induced by LH were also observed. Previous studies have focused on exploring the roles of MMPs in tumor invasion and migration. Furthermore, much research only emphasizes the enhanced proteolytic activities of MMPs induced by LH rather than addressing the cellular proliferative activity. In our current study, knocking down MMP-9 resulted in a significantly reduced cell population. However, a similar effect on cell proliferation was not observed when MMP-2 was deleted (Figure 8).
Though both prohibitin and MMP-9 are related to cell proliferation, the relationship between these two molecules is still not clear. Johanna Prast et al. reported that LH/hCG up-regulated MMP-2 and MMP-9 in SGHPL-5 cells and different EVT pools, which resulted in an enhanced invasion [13]. Though this effect was observed, it is unclear if LH directly interacts with MMP-2/MMP-9 or is medicated by other molecules. In our current work, we found that the knockdown of prohibitin could down-regulate both MMP-2 and MMP-9 (Figure 5). Prohibitin protein level was paralleled with the expression of MMP-2 and MMP-9 when an increased dose of LH was given (Figure 2). These results indicate that prohibitin is an important mediator for LH regulating MMP-2 and MMP-9 expressions. Therefore, these observations may imply that prohibitin promotes the progression of ovarian cancer. Krishnaraj previously observed that prohibitin is required for Ras-induced Raf-MEK-ERK activation and epithelial cell migration. They reported that prohibitin is indispensable for the activation of the Raf-MEK-ERK pathway by Ras, whereby direct interaction with prohibitin is required for C-Raf activation. Moreover, in prohibitin-deficient cells, the adhesion complex proteins cadherin and catenin relocated to the plasma membrane and thereby stabilized adherens junctions [19]. They also reported that short interfering RNA (siRNA) mediated the silencing of prohibitin expression, resulting in an increased intercellular adhesion and formation of cell clusters in various tumor cell lines. Prohibitin-silenced cells failed to migrate in response to epidermal growth factor on collagen suggesting that prohibitin is obligatory for cell migration [20]. The above work confirmed that prohibitin is involved in ovarian cancer development and progress, which is different from previous reports. Commonly, prohibitin is regarded as a candidate of tumor suppressor gene by arresting the cell cycle and blocking cellular growth. However, recent work also addressed the contribution of prohibitin to the development of drug resistance and promoted survival of ovarian cancer cells [21]. Based on this data, although it was suspected that prohibitin played a dual role in OEC development, the detailed mechanism requires further study. Prohibitin is shown to be involved in invasion and provides an interesting connection between prohibitin and MMP-2/MMP-9. This is perhaps the difference versus the effect of FSH on invasion in ovarian cancer development because FSH does not induce prohibitin expression [10].
Although less than 0.1% of the total cellular protein, kinase and phosphatase enzymes play a pivotal role in conducting signals to control cell growth or death. Previous, researchers have tried to investigate the signaling involved in the regulation of LH on the expressions of MMP-2 and MMP-9. It was reported that inhibition of ERK or AKT could diminish hCG-induced MMP-2 expression, which demonstrated that hCG promoted trophoblast invasion and migration depending on activation of ERK and AKT signaling [13]. In this work, both signal pathways were investigated. Western blot analyses revealed the LH-dependent activation of phosphorylation of ERK and AKT in a time-dependent manner, suggesting that LH, when bound to its receptor, activated both ERK and AKT signal pathways. Treatment of cultures with the specific MEK inhibitor U0126 sharply reduced the prohibitin, MMP-2 and MMP-9 proteins in the presence or absence of LH. Similarly, LH-induced prohibitin, MMP-2, and MMP-9 were attenuated in the presence of PI3K/AKT inhibitor LY294002. These results further confirmed that LH regulated prohibitin, MMP-2, and MMP-9 through ERK and PI3K/AKT signal pathways.
Previous studies have also reported the cellular growth effect of LH [22-31]. The increased proliferation of OSE and cancer cells was observed and the inhibitory effect was also noticed, though the reason for the discrepancy in results is not clear. In this study, we found that LH failed to promote proliferation in MCV152 cells. This was consistent with our previous finding that LH itself does not have a promotion role in OET cell proliferation. However, knocking down prohibitin resulted in significant enhanced proliferation with or without LH treatment compared with the cells transfected with siCon. Prohibitin was reported to regulate the cell cycle and cell growth, thus increased expression of prohibitin could inhibit cell proliferation. In this project, the loss of prohibitin significantly increased the cell number due to its inability to arrest the cell cycle. Many more cells went through cell cycle checkpoints and into S and G2/M phases, resulting in an increased cell population. In addition to the role prohibitin plays in regulating the expression of MMPs and invasion, it also displays an anti-cancer effect. We believe that prohibitin plays different roles during different stages of OEC development. A positive promotion effect perhaps is mainly present in the stages of invasion and metabasis. We also noticed treatment with LH resulted in a significant increase of prohibitin in the control group. However, an obvious reduction of cellular proliferation wasn’t observed with LH treatment. We believe that perhaps the balance between prohibitin and MMPs is responsible for the absent effects of LH on cell growth. On one hand, we observed that LH up-regulated prohibitin and overexpression of prohibitin blocking cell growth. In fact, LH has no effect on cellular proliferation. On the other hand, LH enhanced the MMPs levels and these activated enzymes could accelerate tumor growth by damaging components of the extracellular matrix [15]. Moreover, it was demonstrated that MMPs increased vascular smooth muscle cell proliferation via elevation of beta-catenin signaling and cyclin D1 expression [32]. Previous work also stated overexpression of MMP-9 enhanced proliferation of cervical cancer cells [17]. Perhaps the later results partly dismiss the inhibitory effect of cell proliferation induced by overexpression of prohibitin with LH treatment. In our study, this kind of hypothesis was confirmed, as shown in Figure 7. The loss of MMP-9 resulted in significantly decreased MCV152 proliferation, whereas the silence of MMP-2 failed to affect cellular growth. The down regulation of both MMP-2 and MMP-9 reduced proliferation, which showed little different from the effects of down regulation MMP-9 alone. This indicates that MMP-9 perhaps plays an important role in proliferation whereas perhaps MMP-2 mainly contributes to invasion. As described in Figure 8, the increased cell population induced by knocking down prohibitin was significantly attenuated by MMP-9-depletion, which implies MMP-9 and prohibitin have an opposite role in cellular growth. Perhaps the neutralizing effect of MMP-9 and prohibitin was responsible lack of effect of LH on cell growth.
Based on these findings, we propose a model of OEC development as follows: The binding of LH to LHR activates signal pathways of ERK1/2 and PI3K/AKT, which in turn results in increased expressions of prohibitin and MMPs. LH fails to enhance the proliferation in MCV152 cells, which is consistent with the previous report that LH alone has no effect on cell growth. This can be interpreted by the presence of prohibitin and MMP-9 induced by LH. Their neutralization leads to comparative cell numbers’ variation with LH treatment. In cells with loss of prohibitin, the addition of LH caused increased cell growth as a result of MMP-9.
Taken together, prohibitin and MMPs are regulators associated with LH in OEC development, which depend on signal pathways of ERK1/2 and PI3K/AKT. Moreover, prohibitin plays an inhibitory role to balance the effect of MMP-9 on cellular growth despite its promotion of MMP-2 and MMP-9. Accordingly, LH alone has few effects in OEC development. More detailed studies are necessary to define the mechanism involved by prohibitin on MMPs.
Acknowledgements
HCZ is a student of Basis Tucson North High School. She was doing her summer internship in 2014 when she involved the research project. The work was supported by grants from the National Natural Science Foundation of China (NSFC No. 81202044 and No. 81370074), the Science and Technology Commission of Shanghai Municipality (STCSM No. 12ZR1447600), the Shanghai Municipal Public Health Bureau (XYQ2013119), the grant of “Chenxin Plan (Young Star Project)” from Shanghai Jiao Tong University to ZZ, and the grant (200902) from Shanghai First Maternity and the Infant hospital to HL. This work was also partially supported by Better Than Ever grant, Arizona Cancer Center, University of Arizona to WZ. We thank Ms. Lucy Han for her thorough review and the critics of the manuscript.
Disclosure of conflict of interest
None.
References
- 1.Choi JH, Choi KC, Auersperg N, Leung PC. Gonadotropins activate proteolysis and increase invasion through protein kinase A and phosphatidylinositol 3-kinase pathways in human epithelial ovarian cancer cells. Cancer Res. 2006;66:3912–3920. doi: 10.1158/0008-5472.CAN-05-1785. [DOI] [PubMed] [Google Scholar]
- 2.Venn A, Watson L, Bruinsma F, Giles G, Healy D. Risk of cancer after use of fertility drugs with in-vitro fertilisation. Lancet. 1999;354:1586–1590. doi: 10.1016/S0140-6736(99)05203-4. [DOI] [PubMed] [Google Scholar]
- 3.Brekelmans CT. Risk factors and risk reduction of breast and ovarian cancer. Curr Opin Obstet Gynecol. 2003;15:63–68. doi: 10.1097/00001703-200302000-00010. [DOI] [PubMed] [Google Scholar]
- 4.Daly M, Obrams GI. Epidemiology and risk assessment for ovarian cancer. Semin Oncol. 1998;25:255–264. [PubMed] [Google Scholar]
- 5.Gnagy S, Ming EE, Devesa SS, Hartge P, Whittemore AS. Declining ovarian cancer rates in U. S. women in relation to parity and oral contraceptive use. Epidemiology. 2000;11:102–105. doi: 10.1097/00001648-200003000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Zheng W, Lu JJ, Luo F, Zheng Y, Feng Y, Felix JC, Lauchlan SC, Pike MC. Ovarian epithelial tumor growth promotion by follicle-stimulating hormone and inhibition of the effect by luteinizing hormone. Gynecol Oncol. 2000;76:80–88. doi: 10.1006/gyno.1999.5628. [DOI] [PubMed] [Google Scholar]
- 7.Ohtani K, Sakamoto H, Kikuchi A, Nakayama Y, Idei T, Igarashi N, Matukawa T, Satoh K. Follicle-stimulating hormone promotes the growth of human epithelial ovarian cancer cells through the protein kinase C-mediated system. Cancer Lett. 2001;166:207–213. doi: 10.1016/s0304-3835(00)00713-8. [DOI] [PubMed] [Google Scholar]
- 8.Wright JW, Toth-Fejel S, Stouffer RL, Rodland KD. Proliferation of rhesus ovarian surface epithelial cells in culture: lack of mitogenic response to steroid or gonadotropic hormones. Endocrinology. 2002;143:2198–2207. doi: 10.1210/endo.143.6.8848. [DOI] [PubMed] [Google Scholar]
- 9.Ivarsson K, Sundfeldt K, Brannstrom M, Hellberg P, Janson PO. Diverse effects of FSH and LH on proliferation of human ovarian surface epithelial cells. Hum Reprod. 2001;16:18–23. doi: 10.1093/humrep/16.1.18. [DOI] [PubMed] [Google Scholar]
- 10.Jia L, Yi XF, Zhang ZB, Zhuang ZP, Li J, Chambers SK, Kong BH, Zheng W. Prohibitin as a novel target protein of luteinizing hormone in ovarian epithelial carcinogenesis. Neoplasma. 2010;58:104–109. doi: 10.4149/neo_2011_02_104. [DOI] [PubMed] [Google Scholar]
- 11.Nuell MJ, Stewart DA, Walker L, Friedman V, Wood CM, Owens GA, Smith JR, Schneider EL, Dellorco R, Lumpkin CK, Danner DB, Mcclung JK. Prohibitin, an Evolutionarily Conserved Intracellular Protein That Blocks DNA-Synthesis in Normal Fibroblasts and Hela-Cells. Mol Cell Biol. 1991;11:1372–1381. doi: 10.1128/mcb.11.3.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dell’Orco RT, McClung JK, Jupe ER, Liu XT. Prohibitin and the senescent phenotype. Exp Gerontol. 1996;31:245–252. doi: 10.1016/0531-5565(95)02009-8. [DOI] [PubMed] [Google Scholar]
- 13.Prast J, Saleh L, Husslein H, Sonderegger S, Helmer H, Knofler M. Human chorionic gonadotropin stimulates trophoblast invasion through extracellularly regulated kinase and AKT signaling. Endocrinology. 2008;149:979–987. doi: 10.1210/en.2007-1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dabizzi S, Noci I, Borri P, Borrani E, Giachi M, Balzi M, Taddei GL, Marchionni M, Scarselli GF, Arcangeli A. Luteinizing hormone increases human endometrial cancer cells invasiveness through activation of protein kinase A. Cancer Res. 2003;63:4281–4286. [PubMed] [Google Scholar]
- 15.Rasmussen HS, McCann PP. Matrix metalloproteinase inhibition as a novel anticancer strategy: a review with special focus on batimastat and marimastat. Pharmacol Ther. 1997;75:69–75. doi: 10.1016/s0163-7258(97)00023-5. [DOI] [PubMed] [Google Scholar]
- 16.Wu TF, Wu H, Wang YW, Chang TY, Chan SH, Lin YP, Liu HS, Chow NH. Prohibitin in the pathogenesis of transitional cell bladder cancer. Anticancer Res. 2007;27:895–900. [PubMed] [Google Scholar]
- 17.Sairam MR, Jiang LG, Yarney TA, Khan H. Follitropin signal transduction: Alternative splicing of the FSH receptor gene produces a dominant negative form of receptor which inhibits hormone action. Biochem Biophys Res Commun. 1996;226:717–722. doi: 10.1006/bbrc.1996.1419. [DOI] [PubMed] [Google Scholar]
- 18.Xue M, Jackson CJ. Autocrine actions of matrix metalloproteinase (MMP)-2 counter the effects of MMP-9 to promote survival and prevent terminal differentiation of cultured human keratinocytes. J Invest Dermatol. 2008;128:2676–2685. doi: 10.1038/jid.2008.136. [DOI] [PubMed] [Google Scholar]
- 19.Rajalingam K, Wunder C, Brinkmann V, Churin Y, Hekman M, Sievers C, Rapp UR, Rudel T. Prohibitin is required for Ras-induced Raf-MEK-ERK activation and epithelial cell migration. Nat Cell Biol. 2005;7:837–843. doi: 10.1038/ncb1283. [DOI] [PubMed] [Google Scholar]
- 20.Rajalingam K, Rudel T. Ras-Raf signaling needs prohibitin. Cell Cycle. 2005;4:1503–1505. doi: 10.4161/cc.4.11.2142. [DOI] [PubMed] [Google Scholar]
- 21.Gregory-Bass RC, Olatinwo M, Xu W, Matthews R, Stiles JK, Thomas K, Liu D, Tsang B, Thompson WE. Prohibitin silencing reverses stabilization of mitochondrial integrity and chemoresistance in ovarian cancer cells by increasing their sensitivity to apoptosis. Int J Cancer. 2008;122:1923–1930. doi: 10.1002/ijc.23351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parrott JA, Doraiswamy V, Kim G, Mosher R, Skinner MK. Expression and actions of both the follicle stimulating hormone receptor and the luteinizing hormone receptor in normal ovarian surface epithelium and ovarian cancer. Mol Cell Endocrinol. 2001;172:213–222. doi: 10.1016/s0303-7207(00)00340-3. [DOI] [PubMed] [Google Scholar]
- 23.Weiss G, Skurnick JH, Goldsmith LT, Santoro NF, Park SJ. Menopause and hypothalamic-pituitary sensitivity to estrogen. JAMA. 2004;292:2991–2996. doi: 10.1001/jama.292.24.2991. [DOI] [PubMed] [Google Scholar]
- 24.Wimalasena J, Dostal R, Meehan D. Gonadotropins, estradiol, and growth factors regulate epithelial ovarian cancer cell growth. Gynecol Oncol. 1992;46:345–350. doi: 10.1016/0090-8258(92)90230-g. [DOI] [PubMed] [Google Scholar]
- 25.Gubbay O, Guo W, Rae MT, Niven D, Howie AF, McNeilly AS, Xu L, Hillier SG. Anti-inflammatory and proliferative responses in human and ovine ovarian surface epithelial cells. Reproduction. 2004;128:607–614. doi: 10.1530/rep.1.00272. [DOI] [PubMed] [Google Scholar]
- 26.Edmondson RJ, Monaghan JM, Davies BR. Gonadotropins mediate DNA synthesis and protection from spontaneous cell death in human ovarian surface epithelium. Int J Gynecol Cancer. 2006;16:171–177. doi: 10.1111/j.1525-1438.2006.00274.x. [DOI] [PubMed] [Google Scholar]
- 27.Kurbacher CM, Jager W, Kurbacher JA, Bittl A, Wildt L, Lang N. Influence of human luteinizing hormone on cell growth and CA 125 secretion of primary epithelial ovarian carcinomas in vitro. Tumour Biol. 1995;16:374–384. doi: 10.1159/000217954. [DOI] [PubMed] [Google Scholar]
- 28.Tashiro H, Miyazaki K, Okamura H, Iwai A, Fukumoto M. c-myc over-expression in human primary ovarian tumours: its relevance to tumour progression. Int J Cancer. 1992;50:828–833. doi: 10.1002/ijc.2910500528. [DOI] [PubMed] [Google Scholar]
- 29.Kuroda H, Mandai M, Konishi I, Yura Y, Tsuruta Y, Hamid AA, Nanbu K, Matsushita K, Mori T. Human chorionic gonadotropin (hCG) inhibits cisplatin-induced apoptosis in ovarian cancer cells: possible role of up-regulation of insulin-like growth factor-1 by hCG. Int J Cancer. 1998;76:571–578. doi: 10.1002/(sici)1097-0215(19980518)76:4<571::aid-ijc21>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 30.Kuroda H, Mandai M, Konishi I, Tsuruta Y, Kusakari T, Kariya M, Fujii S. Human ovarian surface epithelial (OSE) cells express LH/hCG receptors, and hCG inhibits apoptosis of OSE cells via up-regulation of insulin-like growth factor-1. Int J Cancer. 2001;91:309–315. doi: 10.1002/1097-0215(200002)9999:9999<::aid-ijc1060>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 31.Wimalasena J, Meehan D, Dostal R, Foster JS, Cameron M, Smith M. Growth factors interact with estradiol and gonadotropins in the regulation of ovarian cancer cell growth and growth factor receptors. Oncol Res. 1993;5:325–337. [PubMed] [Google Scholar]
- 32.Dwivedi A, Slater SC, George SJ. MMP-9 and -12 cause N-cadherin shedding and thereby {beta}-catenin signalling and vascular smooth muscle cell proliferation. Cardiovasc Res. 2008;81:178–86. doi: 10.1093/cvr/cvn278. [DOI] [PubMed] [Google Scholar]


