Abstract
Objective: To clarify the value of postoperative adjuvant transcatheter arterial chemoembolization (TACE) for resectable multiple hepatocellular carcinoma beyond the Milan criteria. Background: Patients with multiple HCC have been shown to have a worse survival after a partial hepatectomy (PH) because of the high incidence of intrahepatic tumor recurrence. Postoperative adjuvant TACE is an optional strategy for HCC patients with a high recurrence risk. Its effects and range of applications are debatable. Methods: This retrospective study enrolled 135 HCC patients with resectable multiple hepatocellular carcinoma beyond the Milan criteria, and those patients underwent a hepatectomy with/without postoperative adjuvant TACE from Jan. 2004 to Dec. 2008. The patients were divided to the PH cohort or the PH+TACE cohort. The prognosis measures were the disease-free survival (DFS) and overall survival (OS) from the date of treatment. Univariate and multivariate analyses were used to assess the prognostic factors associated with DFS and OS, using the Cox proportional hazards model. Results: The 1-, 2-, and 5-year DFS and OS for the PH+TACE group differed significantly from the PH group (p = 0.004, p = 0.002, respectively). Multivariate analysis revealed that the significant independent risk factors associated with the DFS and OS were postoperative TACE treatment (p = 0.002, p = 0.001, respectively) and the number of tumors (p = 0.006, p = 0.037, respectively). Conclusions: Our results show that postoperative adjuvant treatment resulted in delayed intrahepatic recurrence and better survival for patients with resectable multiple hepatocellular carcinoma beyond the Milan criteria. Postoperative adjuvant TACE should be regarded as a common strategy for patients with resectable multiple HCC beyond the Milan criteria.
Keywords: TACE, multiple hepatocellular carcinoma, Milan criteria, recurrence, prognosis
Introduction
Hepatocellular carcinoma (HCC) is one of the most common malignancies globally and a common cause of cancer-related mortality, particularly in East Asian countries [1,2]. Surgical resection, transplantation, and ablation are treatments that offer a high complete response rate and potential for cure [3]. The most commonly used curative therapy for HCC is a partial hepatectomy (PH), which has good results for small HCC [4]. However, the treatment for multiple HCC remains controversial.
The Barcelona Clinic Liver Cancer (BCLC) staging system is thought to offer the best stage classification and guidance for HCC treatment allocation [5,6]. The Milan Criteria is used to determine patients for liver transplantation and yields positive results for a solitary HCC up to 5 cm in size or for multiple HCC, up to 3 tumors of up to 3 cm in size for each tumor [7]. The BCLC staging system recommends transcatheter arterial chemoembolization (TACE) for intermediate-stage multiple HCC (BCLC stage B), which is beyond the Milan Criteria [6]. Most groups always restrict the indication for PH to patients with one tumor because multifocality is associated with high recurrence and impaired survival, particularly for multiple HCC. Recently, a randomized comparative trial [8] has demonstrated that PH resulted in better prognosis than conventional TACE for patients with resectable multiple HCC beyond the Milan Criteria, while similar result has also been reported by previous retrospective studies [9,10]. The high incidence of intrahepatic tumor recurrence is showed to be the major challenge in improving the OS of multiple HCC patients [8,11]. PH could be followed by other rational adjuvant therapies to address the micro-metastases left in the liver remnant, to prevent intrahepatic tumor recurrence and to improve the results of liver resection [8].
Transarterial chemoembolization (TACE) is a non-curative treatment that improves the survival of HCC patients [3]. Randomized controlled trials demonstrates that the survival of patients with unresectable tumors is better after the administration of TACE than with conservative treatment [12]. However, the efficacy of TACE as adjuvant therapy following hepatectomy remains controversial. Because of the problem of multiple HCC treatments and the debate regarding the role of postoperative adjuvant TACE, we conducted this retrospective study to compare the prognosis of patients treated by PH+TACE or PH and to determine the role of postoperative adjuvant TACE for multiple HCC beyond the Milan criteria.
Patients and methods
Patients
This retrospective study examined the data collected on patients diagnosed with HCC and treated by PH at Zhongshan Hospital, Fudan University between Jan. 2004 and Dec. 2008. The entry criteria included the following: 1) primary multiple HCC beyond the Milan criteria; 2) curative resection of tumor lesions; 3) no lymph node involvement; 4) no vascular invasion; and 5) no distant metastasis. The exclusion criteria included the following: 1) accompaniment by other types of malignancies; 2) intrahepatic recurrence within two months after surgery, which ensured that postoperative adjuvant TACE was impossible; and 3) loss to follow-up. All the pathologic specimens were reviewed by at least 2 pathologists to confirm the diagnosis of HCC. The histologic grade of tumor cells differentiation was assessed according to the Edmondson grading system, and the liver function stage was assigned using the Child-Pugh scoring system.
Postoperative TACE was performed approximately 1-2 months after the hepatic resections.
Clinicopathological factors potentially related to recurrence and survival were selected in this study on the basis of previous study [13], including age (≤ 53 or > 53, 53 years was the median age of HCC patients this study), gender (male or female), Child score (A or B), cirrhosis (yes or no), the total tumor diameter, the largest tumor diameter, the number of tumor nodules (2 or > 2), the presence of a tumor capsule (yes or no), the differentiation of tumor cells (Edmondson classification I/II or III/IV), and the preoperative laboratory values including the serum alanine aminotransferase concentration (ALT ≤ 75 U/L or > 75 U/L), serum a-fetoprotein concentration (AFP ≤ 400 ng/mL or > 400 ng/mL), and serum γ-glutamyl transpeptidase concentration (GGT ≤ 50 U/L or > 50 U/L), using the upper limit of the normal values in our hospital as the cutoff values for the laboratory parameters.
Follow-up
The patients were followed regularly in the outpatient clinic and were monitored prospectively for recurrence according to a standard protocol that included serum AFP quantification and ultrasound or contrast CT images. The follow up occurred every 2 months during the first postoperative year and at least every 3 to 6 months thereafter. The serum AFP, abdominal ultrasonograph and chest radiograph were monitored at each follow-up visit. A CT scan of the abdomen was performed every 6 months [13]. The survival time was calculated from the date of surgery to the deadline for follow-up or date of death.
The diagnosis and treatment of recurrent tumors
In cases of suspected recurrent or metastatic lesions, further investigations consisting of computed tomography and/or hepatic angiography were conducted. Necessary biopsies were performed, and the diagnosis of tumor recurrence was based on cytological/histological evidence or on the non-invasive diagnostic criteria for HCC used by the EASL [6]. The number and the location of recurrent tumors were recorded at the time of the first diagnosis of recurrence. The patients with recurrence were treated with curative and/or non-curative treatments to improve survival. The curative therapies considered included surgical resection, liver transplantation and percutaneous radiofrequency ablation. The non-curative treatments included TACE, percutaneous ethanol injection (PEI), radiotherapy or systemic therapy.
Statistical methods
The continuous variables were expressed as the mean (± sd) or median (range), as appropriate. The categorical variables were compared by the χ2 test or Fisher’s exact test, and the continuous variables by the student’s t-test. The p values of < 0.05 were considered significant. Univariate survival analysis was performed using the Kaplan-Meier method, and the significance of the difference between the groups was analyzed with the log-rank test. The relative prognostic significance of the variables in predicting OS and DFS was assessed by Cox proportional hazards regression models. The statistical analyses of the data were performed using SPSS 21 for Windows (SPSS, Chicago, IL).
Results
Patients
Among the HCC patients who underwent hepatectomy at our department from Jan. 2004 to Dec. 2008, 135 patients were included our study based on the inclusion and exclusion criteria and those patients were divided into the PH cohort and the PH+TACE cohort. Our study included 74 patients in the PH+TACE cohort and 61 patients in the PH cohort.
The baseline characteristics were well matched between our two groups. Among them, 126 (93.33%) patients were positive for the hepatitis B surface antigen, and all the patients were negative for the hepatitis C antibody. 110 patients (81.48%) were diagnosed to be accompanied with cirrhosis (Table 1).
Table 1.
Clinical features
| Characteristics | PH (n = 61) | PH+TACE (n = 74) | p value |
|---|---|---|---|
| Age (years) (≤ 53/> 53) | 26/35 | 41/33 | 0.461 |
| Sex (male/female) | 57/4 | 67/7 | 0.540 |
| HBsAg (positive/negative) | 58/3 | 68/6 | 0.460 |
| Cirrhosis (positive/negative) | 49/12 | 61/13 | 0.754 |
| Child-Pugh classification (A/B) | 56/5 | 70/4 | 0.518 |
| AFP (ng/ml) (≤ 400/> 400) | 37/24 | 47/27 | 0.733 |
| GGT (≤ 50 U/L vs > 50 U/L) | 10/51 | 19/55 | 0.191 |
| ALT (U/L) (≤ 75 vs >75) | 47/14 | 62/12 | 0.323 |
| Tumor capsule (positive vs negative) | 35/26 | 38/36 | 0.484 |
| Tumor differentiation (I/II vs III/IV) | 42/19 | 53/21 | 0.726 |
| Total tumor diameter (cm) (mean ± sd) | 5.54 ± 3.13 | 5.68 ± 2.83 | 0.127 |
| tumor biggest diameter (cm) (mean ± sd) | 8.26 ± 3.45 | 8.43 ± 3.46 | 0.773 |
| Number of tumor (2/≥ 3) | 38/23 | 45/29 | 0.860 |
In the PH cohort, the number of tumours per patient was as follows: 2 lesions (n = 38), 3 lesions (n = 14), 4 lesions (n = 4), 5 lesions (n = 3), and 6 lesions (n = 2). The primary surgeries included left hepatectomies (n = 8), right hepatectomies (n = 30), and 2-segment resections (n = 23). Of the 43 (70.49%) patients having recurrent HCC in the PH group, 5 patients underwent additional surgery, and 25 patients received TACE or/and PEI.
Specifically, in the PH+TACE group, the distribution of the number of lesions was as follows: 2 lesions (n = 45), 3 lesions (n = 19), 4 lesions (n = 8), and 6 lesions (n = 2). The primary surgeries included left hepatectomies (n = 5), right hepatectomies (n = 40), and 2-segment resections (n = 29). Of the 47 (63.51%) patients with recurrent HCC in this group, 6 patients underwent additional surgery, which included live hepatectomy (n = 4), live transplantation (n = 1), live hepatectomy + radiofrequency ablation (n = 1), 27 patients received TACE or/and PEI, and 1 patient received IFN-α treatment.
Follow-up
The deadline for follow-up was June 2013, at which time, 107 patients (79.26 %) had died. In the PH cohort, 48 patients succumbed to tumor related causes, and 7 patients succumbed to non tumor-related causes. In the PH+TACE cohort, 40 patients succumbed to tumor-related causes, and 12 patients succumbed to non tumor-related causes.
Survival
In the PH+TACE cohort, the 1-, 2-, and 5-year cumulative incidence of intrahepatic recurrence was 24.32%, 38.11%, and 63.61%, respectively. The corresponding figures for the PH cohort were 45.56%, 64.16%, and 76.18%, respectively. The PH+TACE cohort had significantly different cumulative incidences of intrahepatic recurrence than did the PH cohort (log-rank test, χ2 = 8.406, p = 0.004) (Figure 1A). Additionally, the cumulative incidence of intrahepatic recurrence was significantly different between the PH+TACE cohort and the PH cohort with 2 tumors (χ2 = 4.468, p = 0.035) (Figure 1B) and for patients with more than 2 tumors (χ2 = 5.002, p = 0.025) (Figure 1C), respectively.
Figure 1.
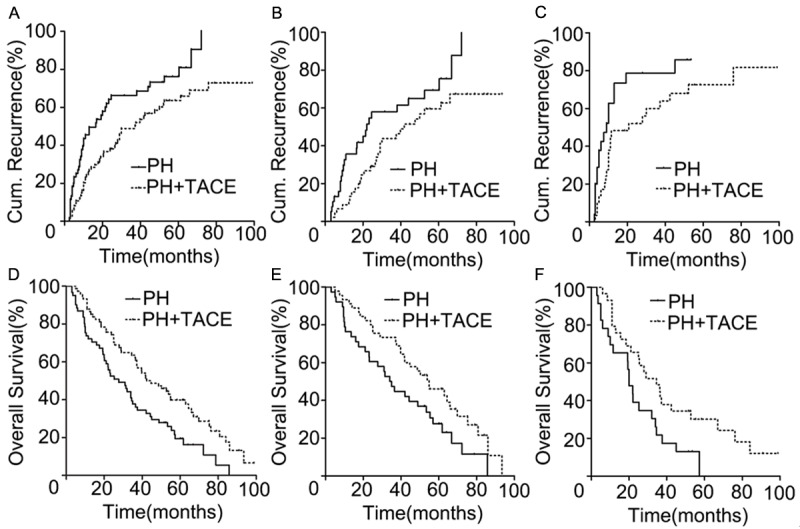
Disease-Free Survival and Overall survival curves for PH+TACE and PH group. A. The PH+TACE group showed significantly better cumulative incidence of intrahepatic recurrence than the PH group (p = 0.004). B, C. cumulative incidence of intrahepatic recurrence curves for patients with 2 tumors (p = 0.035) and > 2 tumors (p = 0.025) after PH with/without TACE. D. The PH+TACE group showed significantly better overall survival than the PH group (p = 0.002,). E, F. Overall survival curves for patients with 2 tumors (p = 0.026) and > 2 tumors (p = 0.027) after PH with/without TACE.
The 1-, 2-, and 5-year overall survival rates and median survival were, 72.13%, 52.46%, and 19.41%, respectively, and 27.67 months (range 3-85.80 months) in the PH cohort, which was significantly different from the OS and median survival rates in the PH+TACE cohort (87.83%, 74.32%, and 39.86%), respectively, and 44.67 months (range 5.03-98.89 months; χ2 = 9.435, p = 0.002, Figure 1D). The OS was significantly different between the PH+TACE group and the PH group with 2 tumors (χ2 = 4.950, p = 0.026) (Figure 1E) and for patients with more than 2 tumors (χ2 = 4.904, p = 0.027) (Figure 1F).
Univariate analysis revealed the factors that significantly influenced the DFS and OS were the postoperative TACE treatment and the number of tumors. With multivariate analysis, the postoperative TACE treatment and the number of tumors were shown to be the significant DFS and OS factors (Tables 2 and 3).
Table 2.
Univariate and multivariate analysis of prognostic factors of DFS
| Variable | Univariate | Multivariate | ||
|---|---|---|---|---|
|
| ||||
| χ2 value (Log-rank) | p value | HR (95% Cl) | p value | |
| Treatment (PH+TACE vs PH) | 8.406 | 0.004 | 0.333-0.777 | 0.002 |
| Number of tumor (> 2 vs 2) | 6.237 | 0.013 | 1.185-2.788 | 0.006 |
| The biggest tumor diameter (cm) (≤ 5 vs > 5) | 0.357 | 0.550 | - | n.a. |
| Total tumor diameter (cm) (≤ 8 vs > 8) | 0.001 | 0.981 | - | n.a. |
| Cirrhosis (positive vs negative) | 0.487 | 0.485 | - | n.a. |
| Age (years) (≤ 53 vs > 53) | 1.840 | 0.175 | 0.506-1.164 | 0.213 |
| ALT (U/L) (≤ 75 vs > 75) | 0.015 | 0.903 | - | n.a. |
| GGT (≤ 50 U/L vs > 50 U/L) | 0.786 | 0.375 | - | n.a. |
| AFP (ng/ml) (≤ 400 vs > 400) | 0.023 | 0.879 | - | n.a. |
| Tumor capsule (positive vs negative) | 0.225 | 0.635 | - | n.a. |
| Tumor differentiation (III/IV vs I/II) | 1.599 | 0.206 | - | n.a. |
| Tumor location (same vs different) | 0.177 | 0.674 | - | n.a. |
n.a. not applicable.
Table 3.
Univariate and multivariate analysis of prognostic factors of OS
| Variable | Univariate | Multivariate | ||
|---|---|---|---|---|
|
| ||||
| χ2 value (Log-rank) | p value | HR (95% Cl) | p value | |
| Treatment (PH+TACE vs PH) | 9.435 | 0.002 | 0.344-0.760 | 0.001 |
| Number of tumor (> 2 vs 2) | 4.997 | 0.025 | 1.027-2.335 | 0.037 |
| GGT (≤ 50 U/L vs > 50 U/L) | 1.308 | 0.253 | - | n.a. |
| The biggest tumor diameter (cm) (≤ 5 vs >5) | 0.204 | 0.652 | - | n.a. |
| Tumor capsule (positive vs negative) | 0.464 | 0.496 | - | n.a. |
| Total tumor diameter (cm) (≤ 8 vs > 8) | 1.708 | 0.182 | 0.820-1.860 | 0.313 |
| Cirrhosis (positive vs negative) | 1.305 | 0.253 | - | n.a. |
| Age (years) (≤ 53 vs > 53) | 0.850 | 0.356 | - | n.a. |
| ALT (U/L) (≤ 75 vs > 75) | 0.346 | 0.557 | - | n.a. |
| AFP (ng/ml) (≤ 400 vs > 400) | 1.124 | 0.289 | - | n.a. |
| Tumor differentiation (III/IV vs I/II) | 0.287 | 0.592 | - | n.a. |
| Tumor location (same vs different) | 0.173 | 0.678 | - | n.a. |
n.a. not applicable.
Discussion
Despite great treatment advances, the prognosis of HCC is poor, particularly for multiple-tumor patients beyond the Milan Criteria. Retrospective and randomized studies have demonstrated that PH resulted in better survival than TACE for patients with resectable multiple HCC beyond the Milan Criteria [8-10]. However, patients with multiple tumors have a worse prognosis than those with a solitary tumor after surgery [3,14]. The high recurrence rate in PH patients is the major impediment to improving survival.
Image-guided transcatheter treatments are based on selective intravascular delivery of drugs into the arterial vessels that nourish the tumor and are considered in patients with large cancers or multifocal disease that is not amenable to curative treatments. The only option that has shown survival benefit is TACE, which combines an injection of chemotherapeutic agents with obstruction of the arterial blood supply [3,15,16]. Besides, researchers have also investigated whether the combination of TACE with molecular-targeted agents might delay tumor progression and improve survival [17,18].
Although several studies have been conducted to clarify the role of postoperative TACE for HCC, its survival benefit remains controversial. Indeed, our previous study suggested that postoperative TACE could only provide survival benefit in HCC patients with risk factors for residual tumor [19]. Unfortunately, the role of postoperative TACE for resectable multiple HCC patients is still unconvincing. Besides, a randomized controlled trial showed that hepatectomy with adjuvant TACE, compared with hepatectomy alone, efficaciously and safely improved the survival outcomes of Stage IIIA HCC patients. But the proportion of HCC cases with portal vein or hepatic vein invasion was 41.74% in that study [20], whereas our study focused on resectable multiple HCC without vein invasion. Thus, the clinicopathological factors of HCC in the two studies were significantly different.
Our study retrospectively analyzed the clinical data of 135 cases of multiple HCC; one patient group underwent PH plus postoperative adjuvant TACE, whereas one group underwent PH without postoperative adjuvant TACE. This retrospective study showed PH plus postoperative TACE to be superior to PH as a routine procedure for resectable multiple HCC beyond the Milan Criteria. PH surgery could be sequentially treated with postoperative TACE, which was attempted to eradicate the residual tumors. Postoperative adjuvant TACE delayed 1-, 2- and 5-year recurrence and improved the overall survival rates of resectable multiple HCC beyond the Milan Criteria. Consistent with previous reports [21,22] most of patients with recurrence were treated with subsequent multidisciplinary aggressive treatments which are demonstrated to play a significant role in obtaining good OS.
The number of tumors, which predicts the degree of intrahepatic spread, is an independent risk factor of HCC [10,23-25]. Although more than one-half of our patients had two tumors, the recurrence and OS rates were significantly different between the PH cohort and the PH+TACE cohort, no matter what the number of tumors. Consistent with some previous reports [10,25], in the multivariate analysis, we identified the number of tumors as an independent risk factor associated with the DFS and OS of these HCC patients.
However, our study did not show that the Child-Pugh liver functional status was a significant prognostic factor of DFS and OS for the multiple HCC patients beyond the Milan criteria. The small sample size might explain this result. There are only 9 (6.67%) patients having Child-Pugh B. In addition, most patients (124/135, 91.85%) in our series were male, resulting in the analysis of the gender influence on the recurrence and OS rates being unauthentic, which is a limitation of this study. Additionally, our results should be further confirmed by multicenter-randomized comparative trials.
In conclusion, postoperative adjuvant TACE could delay tumor recurrence and improve the survival of patients with resectable multiple HCC beyond the Milan Criteria. Postoperative adjuvant TACE and the number of tumors were showed to be independent risk factors of the DFS and OS for this cohort. Postoperative adjuvant TACE should be regarded as a common strategy to achieve good outcomes in patients with resectable multiple HCC beyond the Milan criteria.
Acknowledgements
This study was supported by the National Key Sci-Tech Project (2012ZX10002011-002), the National Natural Science Foundation of China (81472840, 81172023 and 81071741) and the Shanghai Municipal Natural Science Foundation (14ZR1405800, 11ZR1428300, 114119a5000).
Disclosure of conflict of interest
There is no conflicted interesting.
References
- 1.Zheng F, Liao YJ, Cai MY, Liu YH, Liu TH, Chen SP, Bian XW, Guan XY, Lin MC, Zeng YX, Kung HF, Xie D. The putative tumour suppressor microRNA-124 modulates hepatocellular carcinoma cell aggressiveness by repressing ROCK2 and EZH2. Gut. 2012;61:278–289. doi: 10.1136/gut.2011.239145. [DOI] [PubMed] [Google Scholar]
- 2.Li T, Dong ZR, Guo ZY, Wang CH, Tang ZY, Qu SF, Chen ZT, Li XW, Zhi XT. Aspirin enhances IFN-α-induced growth inhibition and apoptosis of hepatocellular carcinoma via JAK1/STAT1 pathway. Cancer Gene Ther. 2013;20:366–374. doi: 10.1038/cgt.2013.29. [DOI] [PubMed] [Google Scholar]
- 3.Alejandro Forner, Josep M Llovet, Jordi Bruix. Hepatocellular carcinoma. Lancet. 2012;379:1245–1255. [Google Scholar]
- 4.Bruix J, Llovet JM. Prognostic prediction and treatment strategy in hepatocellular carcinoma. Hepatology. 2002;35:519–524. doi: 10.1053/jhep.2002.32089. [DOI] [PubMed] [Google Scholar]
- 5.Bruix J, Sherman M. Practice Guidelines Committee, American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, Christensen E, Pagliaro L, Colombo M, Rodés J EASL Panel of Experts on HCC. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421–430. doi: 10.1016/s0168-8278(01)00130-1. [DOI] [PubMed] [Google Scholar]
- 7.Lau WY, Lai EC. Salvage surgery following downstaging of unresectable hepatocellular carcinoma-a strategy to increase resectability. Ann Surg Oncol. 2007;14:3301–3309. doi: 10.1245/s10434-007-9549-7. [DOI] [PubMed] [Google Scholar]
- 8.Yin L, Li H, Li AJ, Lau WY, Pan ZY, Lai EC, Wu MC, Zhou WP. Partial hepatectomy vs. transcatheter arterial chemoembolization for resectable multiple hepatocellular carcinoma beyond Milan criteria: A RCT. J Hepatol. 2014;61:82–88. doi: 10.1016/j.jhep.2014.03.012. [DOI] [PubMed] [Google Scholar]
- 9.Inoue K, Nakamura T, Kinoshita T, Konishi M, Nakagohri T, Oda T, Takahashi S, Gotohda N, Hayashi T, Nawano S. Volume reduction surgery for advanced hepatocellular carcinoma. J Cancer Res Clin Oncol. 2004;130:362–366. doi: 10.1007/s00432-004-0566-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nagashima J, Okuda K, Tanaka M, Sata M, Aoyagi S. Prognostic benefit in cytoreductive surgery for curatively unresectable hepatocellular carcinoma-comparison to transcatheter arterial chemoembolization. Int J Oncol. 1999;15:1117–1123. doi: 10.3892/ijo.15.6.1117. [DOI] [PubMed] [Google Scholar]
- 11.Chen MF, Tsai HP, Jeng LB, Lee WC, Yeh CN, Yu MC, Hung CM. Prognostic factors after resection for hepatocellular carcinoma in noncirrhotic livers: univariate and multivariate analysis. World J Surg. 2003;27:443–447. doi: 10.1007/s00268-002-6708-7. [DOI] [PubMed] [Google Scholar]
- 12.Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolization improves survival. Hepatology. 2003;37:429–442. doi: 10.1053/jhep.2003.50047. [DOI] [PubMed] [Google Scholar]
- 13.Li T, Qin LX, Gong X, Zhou J, Sun HC, Qiu SJ, Ye QH, Wang L, Fan J. Hepatitis B virus surface antigen-negative and hepatitis C virus antibody-negative hepatocellular carcinoma: clinical characteristics, outcome, and risk factors for early and late intrahepatic recurrence after resection. Cancer. 2013;119:126–135. doi: 10.1002/cncr.27697. [DOI] [PubMed] [Google Scholar]
- 14.Colombo M, Raoul JL, Lencioni R, Galle PR, Zucman-Rossi J, Bañares R, Seehofer D, Neuhaus P, Johnson P. Multidisciplinary strategies to improve treatment outcomes in hepatocellular carcinoma: a European perspective. Eur J Gastroenterol Hepatol. 2013;25:639–651. doi: 10.1097/MEG.0b013e32835e33bb. [DOI] [PubMed] [Google Scholar]
- 15.Llovet JM, Real MI, Montaña X, Planas R, Coll S, Aponte J, Ayuso C, Sala M, Muchart J, Solà R, Rodés J, Bruix J Barcelona Liver Cancer Group. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734–1739. doi: 10.1016/S0140-6736(02)08649-X. [DOI] [PubMed] [Google Scholar]
- 16.Lo CM, Ngan H, Tso WK, Liu CL, Lam CM, Poon RT, Fan ST, Wong J. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35:1164–1171. doi: 10.1053/jhep.2002.33156. [DOI] [PubMed] [Google Scholar]
- 17.Bargellini I, Sacco R, Bozzi E, Bertini M, Ginanni B, Romano A, Cicorelli A, Tumino E, Federici G, Cioni R, Metrangolo S, Bertoni M, Bresci G, Parisi G, Altomare E, Capria A, Bartolozzi C. Transarterial chemoembolization in very early and early-stage hepatocellular carcinoma patients excluded from curative treatment: a prospective cohort study. Eur J Radiol. 2012;81:1173–1178. doi: 10.1016/j.ejrad.2011.03.046. [DOI] [PubMed] [Google Scholar]
- 18.Song MJ, Chun HJ, Song do S, Kim HY, Yoo SH, Park CH, Bae SH, Choi JY, Chang UI, Yang JM, Lee HG, Yoon SK. Comparative study between doxorubicin-eluting beads and conventional transarterial chemoembolization for treatment of hepatocellular carcinoma. J Hepatol. 2012;57:1244–1250. doi: 10.1016/j.jhep.2012.07.017. [DOI] [PubMed] [Google Scholar]
- 19.Ren ZG, Lin ZY, Xia JL, Ye SL, Ma ZC, Ye QH, Qin LX, Wu ZQ, Fan J, Tang ZY. Postoperative adjuvant arterial chemoembolization improves survival of hepatocellular carcinoma patients with risk factors for residual tumor: a retrospective control study. World J Gastroenterol. 2004;10:2791–4. doi: 10.3748/wjg.v10.i19.2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhong C, Guo RP, Li JQ, Shi M, Wei W, Chen MS, Zhang YQ. A randomized controlled trial of hepatectomy with adjuvant transcatheter arterial chemoembolization versus hepatectomy alone for Stage III A hepatocellular carcinoma. J Cancer Res Clin Oncol. 2009;135:1437–1445. doi: 10.1007/s00432-009-0588-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheng YC, Chen TW, Fan HL, Yu CY, Chang HC, Hsieh CB. Transarterial chemoembolization for intrahepatic multiple recurrent HCC after liver resection or transplantation. Ann Transplant. 2014;19:309–316. doi: 10.12659/AOT.890505. [DOI] [PubMed] [Google Scholar]
- 22.Yamamoto N, Okano K, Kushida Y, Deguchi A, Yachida S, Suzuki Y. Clinicopathology of recurrent hepatocellular carcinomas after radiofrequency ablation treated with salvage surgery. Hepatol Res. 2014;44:1062–1071. doi: 10.1111/hepr.12223. [DOI] [PubMed] [Google Scholar]
- 23.Zhao WC, Fan LF, Yang N, Zhang HB, Chen BD, Yang GS. Preoperative predictors of microvascular invasion in multinodular hepatocellular carcinoma. Eur J Surg Oncol. 2013;39:858–864. doi: 10.1016/j.ejso.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 24.Kim BK, Han KH, Park YN, Park MS, Kim KS, Choi JS, Moon BS, Chon CY, Moon YM, Ahn SH. Prediction of microvascular invasion before curative resection of hepatocellular carcinoma. J Surg Oncol. 2008;97:246–252. doi: 10.1002/jso.20953. [DOI] [PubMed] [Google Scholar]
- 25.Hao K, Luk JM, Lee NP, Mao M, Zhang C, Ferguson MD, Lamb J, Dai H, Ng IO, Sham PC, Poon RT. Predicting prognosis in hepatocellular carcinoma after curative surgery with common clinicopathologic parameters. BMC Cancer. 2009;9:389. doi: 10.1186/1471-2407-9-389. [DOI] [PMC free article] [PubMed] [Google Scholar]


