Abstract
Lung cancer (LCa) is one of the most common and deadly malignancies in elderly patients. During the course of the disease, these patients frequently present with lower respiratory tract infection. Therefore, this study aims to investigate the clinical features of lower respiratory tract infection in elderly LCa patients and evaluate the impact on overall survival rate. Clinical and laboratory data were analyzed retrospectively for a total of 1936 patients that were over 60-years-old. Patients were classified into three groups based on pulmonary diseases: Group 1, lung cancer (LCa); Group 2, chronic obstructive pulmonary disease (COPD); and Group 3, other medical diseases without pulmonary problems (OMD). Univariate and multivariate analysis were used to evaluate related risk factors of infections and prognostic factors. The infection rate of the LCa group (46.25%) was significantly higher than the COPD (31.40%) and OMD (23.33%) groups. Polymicrobial infections were most prevalent in the LCa group (28.75%), which far exceeded the prevalence in COPD (11.05%) and OMD (4.44%) groups. In LCa patients, the most frequent pathogens were Gram-negative bacteria (44.87%), followed by fungi (34.62%) and Gram-positive bacteria (20.51%), the major pattern of polymicrobial infections was mixed Gram-negative bacteria and fungi (43.48%). Multivariate analysis revealed that COPD, pleural effusion, anatomical type, low cellular immune function, and length of hospital stay were related risk factors of lower respiratory tract infection in elderly LCa patients. A multivariate Cox proportional hazards regression model revealed that age, stage of TNM, surgical resection, antitumor therapy, lower respiratory tract infection, COPD, and pleural effusion were independent prognostic factors for cancer-related death. Patients who received effective antimicrobial treatment had a better outcome than those who did not respond to antimicrobial drugs (HR = 0.458, P < 0.05). Understanding lower respiratory tract infection in elderly LCa patients is vital if we are to set up corresponding measures and to target effective antimicrobial treatment.
Keywords: Lung cancer, infection, risk factors, prognosis
Introduction
Lung cancer (LCa) is the leading cause of cancer-related mortality worldwide [1]. The incidence in the elderly has been increasing rapidly in the last few years [2], with over half of 500,000 patients diagnosed annually with LCa worldwide being over the age of 70 [3,4]. Despite the rising incidence of LCa with age, a substantial number of elderly LCa patients have distant tumor spread at diagnosis because of atypical presentation, which results in lower histological confirmation rates [5,6], less accurate staging [7], and fewer opportunities of surgical resection. Therefore, chemotherapy and radiation are the anticancer treatments of choice. Individuals suffering from LCa can expect a high symptom burden, particularly from fatigue and breathlessness [8,9], together with the highest rates of co-morbidities found among all tumors [10,11], including cardiovascular disease (23%), chronic obstructive airways disease (COPD) (22%), and other malignancies (15%) [11]. Indeed, with advancing age the likelihood that at least one significant medical illness will co-exist increases substantially; the risk of developing respiratory infections and pneumonia further rises when various conditions are present such as malnutrition, obstructive lung disease, congestive heart failure, diabetes mellitus, renal disease, or immunosuppressive therapy [12-14]. Numerous factors can predispose elderly LCa patients to develop lower respiratory tract infection, and damage to anatomical barriers that offer the opportunity for pathogen is common after chemotherapy, radiation therapy, inflammation, or invasive procedures (e.g., bronchoscopy and tracheal intubation).
LCa patients suffer frequent infections that not only thwart the effect of oncological treatment but also affect overall survival [15-18]. Fever, as the most constant and often the only indicator of infection, is hard to distinguish from other causes, including malignant disease, drugs, allergic reactions, or thromboembolic events [19]. With subtle or absent respiratory symptoms, diagnosis of infection can often be delayed, which can readily lead to increased morbidity and mortality for elderly individuals. Specific microbiological diagnosis is necessarily established for respiratory tract infection. However, few studies have specifically documented microbiological infections in elderly LCa patients. Therefore, the aim of this study is to more precisely delineate the microbiological characteristics of lower respiratory tract infection and associated risk factors in elderly LCa patients and to evaluate the infection and antimicrobial treatment contributing to survival rate.
Materials and methods
Study participants
The medical records of a total of 1936 patients over 60-years-old who hospitalized in the Shanghai Sixth People’s Hospital between January 2011 and January 2014 were retrospectively reviewed. All the patients were classified into the following three groups: Group 1, patients with LCa confirmed by clinical symptoms and radiological or cytological laboratory findings (few had pathological evidence taken from bronchoscopy or by CT-guided fine needle biopsy); Group 2, patients with COPD as identified by physical examination, chest radiograph, and the demonstration of airflow obstruction by spirometry (the spirometric finding of a post-bronchodilator forced expiratory volume in 1 second (FEV1) to forced vital capacity (FVC) ratio of less than 0.70 is accepted as being diagnostic of significant airflow obstruction) [20,21]; Group 3, patients with other medical diseases (OMD), including diabetes mellitus, cardiovascular disease, cerebrovascular disease, digestive disease, or other medical diseases (no incidence of hematologic malignancy, solid tumor malignancy, history of pulmonary disease including bronchitis, COPD and tuberculosis).
Infection diagnosis standard
A case definition for typical infection includes the following findings: (i) the clinical manifestations of respiratory infection, and/or (ii) a positive test result for the presence of bacteria isolated from sputum samples obtained from lower respiratory tract. Infections occurring in other locations, such as generalized septicemia or urinary tract infection, were not included in the present study.
The success of antimicrobial therapy was defined according to Kern’s criteria: no fever for three successive days, the absence of clinical signs, or the eradication of an identified pathogen. The data collected by the physician in charge of all the patients was based on results provided by the microbiology laboratory and the infectious disease consultant [22].
Samples collection
Samples were obtained from patients suspected to have infection. Sputum sample collection was performed according to standard procedures. Sputum was sampled early in the morning, before the patient had anything to eat or drink. First, patient’s mouth was rinsed with water to decrease mouth bacteria and dilute saliva. Then, through a deep cough, the patient coughed up sputum from within the chest. Taking deep breaths and lowering the head was observed to help bring up the sputum. The sputum sample must not be held in the mouth but immediately spat into a sterile container.
Covariates used in the analyses
Demographic information (age, gender), anatomical type, stage of TNM, surgical resection, antitumor therapy including chemotherapy and radiation, cellular immune function measured by T lymphocyte subpopulation (normal CD4: 25.8~41.6% and normal CD8: 18.1~29.6%) and natural killer (NK) cell (normal: 8.1~25.6%), invasive procedures, co-morbidities, length of hospital stay and duration of illness (time from diagnosis to death) were used as covariates in analyzing related risk factors of infections and prognostic factors.
Statistical methods
Statistical calculations were performed using the Statistical Package for the Social Sciences (SPSS) software, version 16 (SPSS Inc., Chicago, IL, USA) and GraphPad Prism 5.1 (GraphPad, San Diego, CA, USA). Binary variables were compared by the Pearson’s chi-squared test. Multivariate analyses using logistic regression were performed to evaluate the risk factors for lower respiratory tract infection. The prognostic influence of variables on survival was analyzed using the log-rank test for univariate analysis and the Cox proportional hazards model for multivariate analysis. An alpha level of P < 0.05 was considered as statistically significant.
Results
Patient characteristics
Over a period of three years, 160 LCa patients over 60-years-old were hospitalized in the Department of Oncology at the Shanghai No. 6 People’s Hospital (8.26% of the total of 1,936 elderly patients during that period). Patient characteristics are reported in Table 1. Of 160 LCa patients, 76 presented with central type pulmonary carcinoma, while 84 patients had peripheral carcinoma. The patients tended to present with more advanced stages of cancer (stage I, n = 16; stage II, n = 23; stage III, n = 55; and stage IV, n = 66). The following comorbidities and/or potentially predisposing conditions were found when diagnosing the infectious episode [22]: surgical resection (n = 39), antitumor therapy (n = 65), low cellular immune function (n = 104), invasive procedures (n = 58), length of hospital stay of > 14 days (n = 65).
Table 1.
Characteristics of the study group
| Item | LCa | COPD | OMD |
|---|---|---|---|
| Number of patients | 160 | 516 | 1260 |
| Gender (Male/Female) | 108/52 | 291/225 | 658/602 |
| Median age (y) | 75.0 | 70.2 | 73.6 |
| Comorbidity | |||
| COPD* | 75 | 516 | 0 |
| Cardiovascular disease | 49 | 117 | 329 |
| Diabetes mellitus | 41 | 81 | 486 |
| Pleural effusion | 58 | 79 | 43 |
| Low cellular immune function (CD4 < 25.8%, CD8 < 18.1%, NK < 8.1%) | 104 | 156 | 252 |
| Invasive procedures | 58 | 77 | 168 |
| Length of hospital stay of > 14 days | 65 | 102 | 241 |
COPD: chronic obstructive pulmonary disease.
Infection pattern
We found lower respiratory tract infection presented more frequently in the LCa group than the COPD group (46.25% vs 31.40%; x2 = 7.721; P < 0.05) and the OMD group (46.25% vs 23.33%; x2 = 7.721; p < 0.05). The data showed that the LCa group (46/160, 28.75%) was associated with higher rates of polymicrobial infections than the COPD group (57/516, 11.05%) and the OMD group (56/1260, 4.44%). There were 159 documented polymicrobial infections; the distribution of all groups is shown in Figure 1. The patterns of polymicrobial infections in the LCa group were as follows: mixed Gram-negative bacteria and fungi (20/46, 43.48%), mixed bacteria and fungi (10/46, 21.74%), mixed Gram-negative and Gram-positive species (8/46, 17.39%), mixed Gram-positive bacteria and fungi (5/46, 10.87%), and mixed different Gram-negative species (3/46, 6.52%). In the COPD group, mixed different Gram-negative bacteria (21/57, 36.84%) and mixed Gram-negative bacteria and fungi (17/57, 29.82%) occurred more often. The majority pattern of polymicrobial infections in OMD group was mixed Gram-negative and Gram-positive species (21/56, 37.50%).
Figure 1.
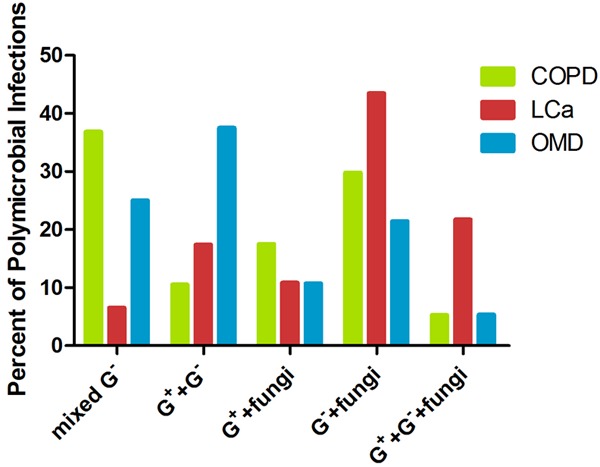
The principal patterns of polymicrobial infections among 3 groups: LCa, COPD and OMD. *G+, Gram-positive bacteria; G-, Gram-negative bacteria.
Pathogens
Bacterial infection accounted for 74.77% of infectious episodes with a total of 765 microorganisms. The most frequent pathogens were Gram-negative bacteria (402/765, 52.55%) followed by fungi (193/765, 25.23%) and Gram-positive bacteria (170/765, 22.22%). The documented pathogens based on the lower respiratory tract infection are reported in Table 2.
Table 2.
Documented pathogens based on the site of lower respiratory tract infection
| LCa | COPD | OMD | ||||
|---|---|---|---|---|---|---|
|
|
||||||
| Pathogens | No. | % | No. | % | No. | % |
| Staphylococcus aureus | 17 | 10.90 | 16 | 6.35 | 46 | 12.89 |
| Acinetobacter baumannii | 10 | 6.41 | 9 | 3.57 | 28 | 7.84 |
| Streptococcus pneumoniae | 3 | 1.92 | 5 | 1.98 | 14 | 3.92 |
| Other Gram-positive bacilli | 2 | 1.28 | 3 | 1.19 | 17 | 4.76 |
| Klebsiella pneumoniae | 20 | 12.82 | 30 | 11.90 | 39 | 10.92 |
| Pseudomonas aeruginosa | 18 | 11.54 | 33 | 13.10 | 60 | 16.81 |
| Acinetobacter baumannii | 12 | 7.69 | 24 | 9.52 | 41 | 11.48 |
| Escherichia coli | 6 | 3.85 | 17 | 6.75 | 22 | 6.16 |
| Enterobacter cloacae | 3 | 1.92 | 10 | 3.97 | 9 | 2.52 |
| Stenotrophomonas maltophilia | 4 | 2.56 | 9 | 3.57 | 7 | 1.96 |
| Other Gram-negative bacilli | 7 | 4.49 | 6 | 2.38 | 25 | 7.00 |
| Candida albicans | 31 | 19.87 | 42 | 16.67 | 33 | 9.24 |
| Other Fungi | 23 | 14.74 | 48 | 19.04 | 16 | 4.48 |
| Total | 156 | 100 | 252 | 100 | 357 | 100 |
In the LCa group, the most frequent pathogens were Candida albicans, Klebsiella pneumoniae, Pseudomonas aeruginosa and Staphylococcus aureus. Major isolated pathogens in the COPD group were similar to the LCa group and included Candida albicans, Pseudomonas aeruginosa, and Klebsiella pneumoniae. However, there were more Staphylococcus aureus observed in the OMD group, and the percentage of Candida albicans (9.24%) was significantly less than in the LCa group (19.87%) and the COPD group (16.67%).
Risk factors of infection
We compared clinical data between LCa patients with infection (Infection (+)) and those without infection (Infection (-)). In univariate analysis, the baseline characteristics were similar between the two groups. Patients with central type pulmonary carcinoma were more likely to catch infections than those with peripheral carcinoma (P = 0.002). LCa patients with COPD had a significantly higher infection rate than those patients without a co-morbidity (P < 0.001). In contrast, those associated with cardiovascular disease and/or diabetes mellitus did not differ significantly between Infection (+) and Infection (-). Pleural effusion and lower cellular immune function were statistically different between the two groups (P = 0.001 and P = 0.022). Invasive procedures and length of hospital stay > 14 days had a significant impact on the Infection (+) group (both P < 0.001). Age, stage of TNM, surgical resection, and antitumor therapy including chemotherapy and radiation did not differ significantly between the two groups (Table 3).
Table 3.
Risk factors of LCa patients with lower respiratory tract infection
| Variables | Infection (+) | Infection (-) | P value |
|---|---|---|---|
| Age | |||
| 60-69 | 26 | 27 | 0.843 |
| 70-79 | 25 | 29 | |
| ≥ 80 | 23 | 30 | |
| COPD | 47 | 28 | < 0.001 |
| Cardiovascular disease | 22 | 27 | 0.820 |
| Diabetes mellitus | 21 | 20 | 0.459 |
| Tumor stage | |||
| I, II | 18 | 21 | 0.989 |
| III, IV | 56 | 65 | |
| Anatomical type | |||
| Peripheral carcinoma | 29 | 55 | 0.002 |
| Central type pulmonary carcinoma | 45 | 31 | |
| Treatment | |||
| Surgical resection | 21 | 18 | 0.274 |
| Antitumor therapy | 36 | 29 | 0.055 |
| Invasive procedures | 39 | 19 | < 0.001 |
| Pleural effusion | 37 | 21 | 0.001 |
| Low cellular immune function | 55 | 49 | 0.022 |
| Length of hospital stay of > 14 days | 43 | 22 | <0.001 |
Multivariate analysis showed that COPD, pleural effusion, anatomical type, cellular immune function, and length of hospital stay other than invasive procedures were independent risk factors of infection in elderly LCa patients (Table 4).
Table 4.
Logistic model of risk factors of LCa patients with lower respiratory tract infection
| Variables | OR | 95% CI | P value |
|---|---|---|---|
| COPD | 5.807 | 2.458-13.719 | < 0.001 |
| Pleural effusion | 2.401 | 1.053-5.476 | 0.037 |
| Central type pulmonary carcinoma | 0.439 | 0.198-0.973 | 0.043 |
| Invasive procedures | 1.034 | 0.438-2.442 | 0.939 |
| Low cellular immune function | 4.676 | 1.974-11.079 | < 0.001 |
| Length of hospital stay of > 14 days | 4.844 | 2.089-11.231 | < 0.001 |
Survival analysis
The median follow-up time of elderly LCa patients was 19.0 months (range, 2-71 months). A total of 105 of 160 elderly LCa patients (65.63%) died during the follow-up period; the median survival time (MST) of the group was 13.0 months (range, 3-52 months). The 1-, 2- and 3-year overall survival (OS) rates for all the LCa patients were 57.0%, 24.6%, and 17.0%, respectively. However, for Infection (+) patients, the 1-, 2- and 3-year OS rates were 40.1%, 11.6%, and 7.7%, respectively. The MST was in excess of 9.0 months. It is noteworthy that the MST in patients with polymicrobial infections was significantly shorter than patients with simple infection (8.0 vs 15.0 months, P = 0.003) and patients without infection (8.0 vs 16.0 months, P < 0.001) (Figure 2A).
Figure 2.
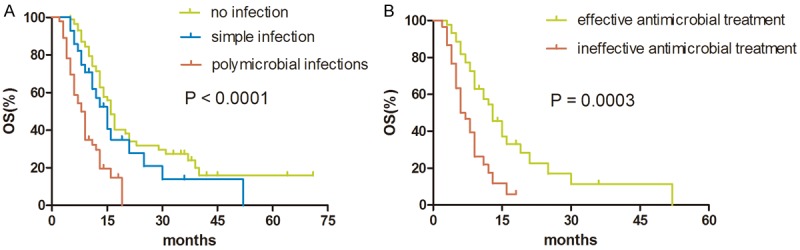
A. Survival difference among 3 groups: patients without infection, patients with simple infection and polymicrobial infections; B. Survival difference between infection (+) patients with effective and ineffective antimicrobial treatment.
To better understand the risk factors associated with improved outcomes, univariate and multivariate analyses were performed using relevant clinical parameters. Univariate analyses revealed that age, COPD, pleural effusion, stage of TNM, anatomical type, surgical resection, antitumor therapy, cellular immune function, and infection were prognostic factors (Table 5). In the Cox regression analysis, infection was the most important prognostic factor with an HR of 3.284 (P < 0.001). From multivariate analysis, age (HR = 1.789; P < 0.001), COPD (HR = 1.576; P = 0.049), pleural effusion (HR = 1.998; P = 0.002), stage (HR = 2.566; P = 0.001), surgical resection (HR = 0.468; P = 0.006), and antitumor therapy (HR = 0.375; P < 0.001) were found to be independent predictors of overall survival (Table 6).
Table 5.
Univariate analyses of factors in relation to LCa survival
| Variables | No. of patients | MST | P value |
|---|---|---|---|
| Age | |||
| 60-69 | 53 | 17.0 | < 0.001 |
| 70-79 | 54 | 14.0 | |
| ≥ 80 | 53 | 9.0 | |
| Gender | |||
| Male | 108 | 13.0 | 0.868 |
| Female | 52 | 13.0 | |
| COPD | |||
| Yes | 75 | 11.0 | 0.001 |
| No | 85 | 16.0 | |
| Cardiovascular disease | |||
| Yes | 49 | 13.0 | 0.436 |
| No | 111 | 14.0 | |
| Diabetes mellitus | |||
| Yes | 41 | 13.0 | 0.448 |
| No | 118 | 13.0 | |
| Pleural effusion | |||
| Yes | 58 | 9.0 | < 0.001 |
| No | 102 | 15.0 | |
| Tumor stage | |||
| I, II | 39 | 30.0 | < 0.001 |
| III, IV | 121 | 11.0 | |
| Anatomical type | |||
| Peripheral carcinoma | 84 | 15.0 | 0.206 |
| Central type pulmonary carcinoma | 76 | 12.0 | |
| Surgical resection | |||
| Yes | 39 | 21.0 | 0.001 |
| No | 121 | 13.0 | |
| Antitumor therapy | |||
| Yes | 65 | 16.0 | < 0.001 |
| No | 95 | 11.0 | |
| Low cellular immune function | |||
| Yes | 104 | 13.0 | 0.006 |
| No | 56 | 16.0 | |
| Infection | |||
| Yes | 74 | 9.0 | < 0.001 |
| No | 86 | 16.0 |
Table 6.
Multivariate analyses of factors in relation to LCa survival
| Variables | HR | 95% CI | P value |
|---|---|---|---|
| Infection | 3.284 | 2.002-5.387 | < 0.001 |
| Age | 1.789 | 1.359-2.354 | <0.001 |
| COPD | 1.576 | 1.002-2.477 | 0.049 |
| Pleural effusion | 1.998 | 1.285-3.106 | 0.002 |
| Stage | 2.566 | 1.440-4.570 | 0.001 |
| Surgical resection | 0.468 | 0.271-0.807 | 0.006 |
| Antitumor therapy | 0.375 | 0.232-0.606 | < 0.001 |
In Infection (+) patients, antimicrobial treatment was a significant predictor of improved outcomes. Patients who received effective antimicrobial treatment (44/74) presented MST 13.0 months, which was statistically significantly longer than the MST of 6.0 months in patients who did not respond to antimicrobial drugs (30/74) (P < 0.001). The survival difference at two years between the two groups was 28.3% (54.6% vs 26.3%) (Figure 2B).
Discussion
In our cohort of 1936 hospitalized patients over 60-years-old, 530 episodes of lower respiratory tract infection were documented. The most prominent group of infection was LCa group. In our study, 46.25% of LCa patients experienced infections, and 28.75% of these patients were associated with polymicrobial infections. Meanwhile, we found that Gram-negative bacteria accounted for the majority of documented pathogens in LCa patients, and the major pattern of polymicrobial infections was mixed Gram-negative bacteria and fungi. This pattern was very different as compared to the COPD and OMD groups. The most predominant pathogen in our LCa patients were Candida albicans, followed by Klebsiella pneumoniae, Pseudomonas aeruginosa, and Staphylococcus aureus, which each accounted for approximately 10% of the documented microorganisms. However, in the literature, Haemophilus influenzae, Kleb-siella pneumoniae, Pseudomonas aeruginosa, and Enterobacter cloacae have been documented in lung infections in up to 68% of cases, and Staphylococcus aureus has also been frequently found [15,23,24].
The present study revealed that COPD, pleural effusion, central type pulmonary carcinoma, length of hospital stay > 14 days, and low cellular immune function were independent risk factors of lower respiratory tract infection in elderly LCa patients. These patients often represent an invariable population with COPD, and in these individuals, mucociliary clearance is significantly impaired, while bacterial colonization with Streptococcus pneumoniae or Hemophilus influenzae is common [19]. As a result, acute tracheobronchitis preceding acute exacerbations of COPD is more common and serious in LCa patients than in patients without pulmonary diseases. Malignant pleural effusion is a common concomitant phenomenon of LCa; invasive procedures include thoracentesis, tunneled indwelling pleural catheters, and tube thoracostomy are considered appropriate options for pleural effusion, which are also considered a risk factor of infection. Central type pulmonary carcinoma is more easily complicated with obstructive pneumonia and bronchial obstruction, which can further result in atelectasis and lung collapse. Subsequently, microorganism colonization can lead to infection.
Lower respiratory tract defenses against infection include mechanical defenses such as cough, the barrier function of mucus and epithelium, and mucociliary clearance which, in concert with innate immune responses, help clear aspirated or inhaled substances including infectious agents [25]. However, both non-immune and immune defenses tend to decline with advancing age; naive T lymphocyte subpopulation and NK cell gradually decline. The underlying malignancy itself or antitumor therapy, including chemotherapy, radiation therapy and corticosteroids, can lead to immune defects. It was worth noting that in our results lower cellular immune function was a significant predictor in univariate analysis but was rejected by multivariate analysis. This inconsistent finding may be the result of selection bias because most LCa patients have suppressed cellular immunity but few take immunotherapy. In addition, elderly residents of long-term care facilities have a high incidence of infection with case fatality rates that range up to 40% [26,27]. Regular lengthy in-hospital stays lead to changes and colonization of endogenous microflora, which, in combination with obstruction of natural passages by neoplasm, facilitate microorganism proliferation and result in increased morbidity and mortality [28,29].
In our analysis of 160 elderly LCa patients, multiple factors were associated with overall survival rate. Older age, COPD, pleural effusion, and advanced stage (stage III, IV) indicated very poor prognosis, while early stage (stage I, II), surgical resection and antitumor therapy had favorable impact on survival. Most of these variables are consistent with prior studies and provided additional evidence to support the benefit of current therapy. However, lower respiratory tract infection and antimicrobial treatment were found to be independent predictors of survival.
Increased patient age and advanced stage are frequently associated with significantly worse outcomes in our study; this is consistent with previous reports [30-33]. Although earlier studies have identified gender as an important prognostic factor [30,32,33], being male was not associated significantly with a better outcome than being female. The reasons for discrepancies between our findings and those of other studies may reflect different sample sizes and study designs. In one study of 100 patients of who died of LCa in America [31], the most common immediate cause of death was attributed to respiratory failure in 38% of cases, probably because most patients had lung diseases besides cancer. Therefore, the impairment of lung function caused by the malignancy was aggravated by other lung diseases. In our study, 82.5% of patients had comorbidities, including COPD and pleural effusion, which placed the patients at a high risk of respiratory infection, dyspnea, and reduced physical capability. Respiratory failure is a function of not only a single lung disease, but rather a set of them. The frequency and contribution of surgery is evident in the outcomes and survival of elderly LCa patients. A retrospective review of elderly patients showed a non-significant difference in operative mortality for patients aged < 69 years, 70-79 years, and > 80 years of 1.6%, 4.2% and 2.8%, respectively. However, pneumonectomy was significantly associated with mortality in the elderly [34]. In Osaki et al. Study [35] of 851 LCa patients (mean age = 65.4 years) in Japan, there has been an increase in the mean age of patients undergoing surgery over the last two decades in combination with an increase in 5 year survival and lower operative mortality. A subset of patients in this study received radiotherapy to the chest, bone, or brain in addition to chemotherapy. We also found that active antitumor therapy (chemotherapy and radiotherapy) was associated with improved survival, which is consistent with previous studies [36-38].
Among the long-term survivors, many elderly LCa patients experienced lower respiratory tract infection. The clinical manifestations of most patients with polymicrobial infections will eventually progress (after an initial response to antimicrobial treatment), and the patients will succumb to severe pneumonia accompanied by respiratory failure. In our study, 65.63% of elderly LCa patients died during the follow-up period, and the MST of all the patients was 13.0 months. In Cox regression analysis, infection was the most important prognostic factor with an HR of 3.284 (P < 0.001). On comparing 1-, 2- and 3-year OS rates of Infection (+) patients with all the LCa patients cases, significantly worse OS rates were observed in the former group. It is noteworthy that the MST in patients with polymicrobial infections was significantly shorter than patients with simple infection (8.0 vs 15.0 months) and in patients without infection (8.0 vs 16.0 months, P < 0.001). Polymicrobial infections severely affect the quality of life and lead to significant morbidity; this phenomenon results from refractory polymicrobial infections because of increasing multi-resistant pathogens and many kinds of bacterial toxins. In addition, we found that patients with infection that received effective antimicrobial treatment would indeed benefit from the management of antibiotics.
The choice of empiric antimicrobial therapy is crucial when presenting with fever and cough with a suspicion of lower respiratory tract infection because these infections are associated with potentially life-threatening complications, particularly in LCa patients with a co-morbidity of COPD. Empiric antimicrobial therapy must adequately cover Gram-negative bacteria as the most common documented pathogens in lower respiratory tract infection. In our study, the majority of Klebsiella pneumoniae and Pseudomonas aeruginosa were susceptible to a combination of amikacin and cefoperazone-sulbactam. However, suspicion of Staphylococcus infection should not be forgotten in febrile patients with LCa because these infections are occasionally rapidly fatal. We detected that half of Staphylococcus were resistant to penicillin and erythromycin, but 100%, 88.2%, and 82.3% of the strains were susceptible to vancomycin, linezolid, and rifampicin, respectively. If polymicrobial infections are suspected, empiric antibiotic therapy must be frequently administered. Fungi have been documented in polymicrobial infections in up to 76.1% of cases, and the majority was Candida albicans, which were susceptible to fluconazole, ketoconazole, and 5-fluorocytosine. We considered that the frequent occurrence of fungi might be associated with catheter use and excessive use of antibiotics during previous infections. As discussed above, a combination of broad-spectrum amikacin with antifungal drug could be used successfully for lower respiratory tract infection in LCa patients.
Antibiotic prophylaxis is not recommended because side effects, susceptibility to enteric infections, and emergence of resistant endogenous organisms are of concern [39]. Nevertheless, the reduction in mortality and infection rates outweighs the detriments associated with antibiotic administration [40], prompt initiation of empiric treatment is recommended in all LCa patients, and antibiotics should be rationally used in clinical application based on the results of susceptibility test. In order to define the precise association between infection and LCa, further studies with a larger number of cases are necessary.
This study had some limitations. First, it was a retrospective study with a small sample size. Second, pathologic diagnosis could not be confirmed in all LCa patients. Finally, variable antibiotics were used because LCa patients repeatedly suffered lower respiratory tract infections; there was a lack of consistency in antimicrobial therapy of polymicrobial infections.
In conclusion, our study demonstrated that elderly LCa patients had higher infection rate and that polymicrobial infections were more common than COPD and OMD. Gram-negative bacteria accounted for the majority of documented pathogens in elderly LCa patients; the principal pathogens were Candida albicans, Klebsiella pneumoniae, Pseudomonas aeruginosa, and Staphylococcus aureus. Infection was the most important prognostic factor for elderly LCa patients. Thus, effective antimicrobial treatment is critical to prolonging the survival time.
Acknowledgements
We acknowledge the study was supported by the National Natural Science Foundation of China [grant number 81201628].
Disclosure of conflict of interest
None.
References
- 1.Soares M, Darmon M, Salluh JI, Ferreira CG, Thiéry G, Schlemmer B, Spector N, Azoulay E. Prognosis of lung cancer patients with life-threatening complications. Chest. 2007;131:840–846. doi: 10.1378/chest.06-2244. [DOI] [PubMed] [Google Scholar]
- 2.Booton R, Jones M, Thatcher N. Lung cancer 7: Management of lung cancer in elderly patients. Thorax. 2003;58:711–720. doi: 10.1136/thorax.58.8.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stephens RJ, Johnson DH. Treatment and outcomes for elderly patients with small cell lung cancer. Drugs Aging. 2000;17:229–247. doi: 10.2165/00002512-200017030-00006. [DOI] [PubMed] [Google Scholar]
- 4.Deppermann KM. Influence of age and comorbidities on the chemotherapeutic management of lung cancer. Lung Cancer. 2001;33:115–120. doi: 10.1016/s0169-5002(01)00311-7. [DOI] [PubMed] [Google Scholar]
- 5.Brown JS, Eraut D, Trask C, Davison AG. Age and the treatment of lung cancer. Thorax. 1996;51:564–568. doi: 10.1136/thx.51.6.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Connolly CK, Crawford SM, Rider PL, Smith AD, Johnston CF, Muers MF. Carcinoma of the bronchus in the Yorkshire region of England 1976-1990: trends since 1984. Eur Respir J. 1997;10:397–403. doi: 10.1183/09031936.97.10020397. [DOI] [PubMed] [Google Scholar]
- 7.Janssen-Heijnen ML, Schipper RM, Razenberg PP, Crommelin MA, Coebergh JW. Prevalence of co-morbidity in lung cancer patients and its relationship with treatment: a population-based study. Lung Cancer. 1998;21:105–113. doi: 10.1016/s0169-5002(98)00039-7. [DOI] [PubMed] [Google Scholar]
- 8.Stone P, Hardy J, Broadley K, Tookman AJ, Kurowska A, A’Hern R. Fatigue in advanced cancer: a prospective controlled cross-sectional study. Br J Cancer. 1999;79:1479–1486. doi: 10.1038/sj.bjc.6690236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Driscoll M, Corner J, Bailey C. The experience of breathlessness in lung cancer. Eur J Cancer Care (Engl) 1999;8:37–43. doi: 10.1046/j.1365-2354.1999.00129.x. [DOI] [PubMed] [Google Scholar]
- 10.Coebergh JW, Janssen-Heijnen ML, Post PN, Razenberg PP. Serious co-morbidity among unselected cancer patients newly diagnosed in the southeastern part of The Netherlands in 1993-1996. J Clin Epidemiol. 1999;52:1131–1136. doi: 10.1016/s0895-4356(99)00098-0. [DOI] [PubMed] [Google Scholar]
- 11.Janssen-Heijnen ML, Schipper RM, Razenberg PP, Crommelin MA, Coebergh JW. Prevalence of co-morbidity in lung cancer patients and its relationship with treatment: a population-based study. Lung Cancer. 1998;21:105–113. doi: 10.1016/s0169-5002(98)00039-7. [DOI] [PubMed] [Google Scholar]
- 12.Riquelme R, Torres A, El-Ebiary M, de la Bellacasa JP, Estruch R, Mensa J, Fernández-Solá J, Hernández C, Rodriguez-Roisin R. Community-acquired pneumonia in the elderly: a multivariate analysis of risk and prognostic factors. Am J Respir Crit Care Med. 1996;154:1450–1455. doi: 10.1164/ajrccm.154.5.8912763. [DOI] [PubMed] [Google Scholar]
- 13.Ruiz M, Ewig S, Marcos MA, Martinez JA, Arancibia F, Mensa J, Torres A. Etiology of community-acquired pneumonia: impact of age, comorbidity, and severity. Am J Respir Crit Care Med. 1999;160:397–405. doi: 10.1164/ajrccm.160.2.9808045. [DOI] [PubMed] [Google Scholar]
- 14.Baik I, Curhan GC, Rimm EB, Bendich A, Willett WC, Fawzi WW. A prospective study of age and lifestyle factors in relation to community-acquired pneumonia in US men and women. Arch Intern Med. 2000;160:3082–3088. doi: 10.1001/archinte.160.20.3082. [DOI] [PubMed] [Google Scholar]
- 15.Perlin E, Bang KM, Shah A, Hursey PD, Whittingham WL, Hashmi K, Campbell L, Kassim OO. The impact of pulmonary infections on the survival of lung cancer patients. Cancer. 1990;66:593–596. doi: 10.1002/1097-0142(19900801)66:3<593::aid-cncr2820660331>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 16.Kohno S, Koga H, Oka M, Kadota J, Kaku M, Soda H, Tomono K, Hara K. The pattern of respiratory infection in patients with lung cancer. Tohoku J Exp Med. 1994;173:405–411. doi: 10.1620/tjem.173.405. [DOI] [PubMed] [Google Scholar]
- 17.Remiszewski P, Słodkowska J, Wiatr E, Zych J, Radomski P, Rowińska-Zakrzewska E. Fatal infection in patients treated for small cell lung cancer in the Institute of Tuberculosis and Chest Diseases in the years 1980-1994. Lung Cancer. 2001;31:101–110. doi: 10.1016/s0169-5002(00)00185-9. [DOI] [PubMed] [Google Scholar]
- 18.Rosolem MM, Rabello LS, Lisboa T, Caruso P, Costa RT, Leal JV, Salluh JI, Soares M. Critically ill patients with cancer and sepsis: Clinical course and prognostic factors. J Crit Care. 2012;27:301–307. doi: 10.1016/j.jcrc.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 19.Akinosoglou KS, Karkoulias K, Marangos M. Infectious complications in patients with lung cancer. Eur Rev Med Pharmacol Sci. 2013;17:8–18. [PubMed] [Google Scholar]
- 20.Johannessen A, Lehmann S, Omenaas ER, Eide GE, Bakke PS, Gulsvik A. Post-bronchodilator spirometry reference values in adults and implications for disease management. Am J Respir Crit Care Med. 2006;173:1316–1325. doi: 10.1164/rccm.200601-023OC. [DOI] [PubMed] [Google Scholar]
- 21.Qaseem A, Wilt TJ, Weinberger SE, Hanania NA, Criner G, van der Molen T, Marciniuk DD, Denberg T, Schünemann H, Wedzicha W, MacDonald R, Shekelle P American College of Physicians; American College of Chest Physicians; American Thoracic Society; European Respiratory Society. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med. 2011;155:179–191. doi: 10.7326/0003-4819-155-3-201108020-00008. [DOI] [PubMed] [Google Scholar]
- 22.Berghmans T, Sculier JP, Klastersky J. A Prospective Study of Infections in Lung Cancer Patients Admitted to the Hospital. Chest. 2003;124:114–120. doi: 10.1378/chest.124.1.114. [DOI] [PubMed] [Google Scholar]
- 23.Putinati S, Trevisani L, Gualandi M, Guerra G, Rossi MR, Sartori S, Potena A. Pulmonary infections in lung cancer patients at diagnosis. Lung Cancer. 1994;11:243–249. doi: 10.1016/0169-5002(94)90544-4. [DOI] [PubMed] [Google Scholar]
- 24.Brambilla C, Romand P, Vanderkerckhove C, Moro D. Respiratory infection and bronchial cancer. Rev Mal Respir. 1992;9:49–52. [PubMed] [Google Scholar]
- 25.Meyer KC. Lung immunology and host defense. In: Bittar EE, editor. Pulmonary Biology in Health and Disease. New York: Springer; 2002. [Google Scholar]
- 26.Starczewski AR, Allen SC, Vargas E, Lye M. Clinical prognostic indices of fatality in elderly patients admitted to hospital with acute pneumonia. Age Ageing. 1988;17:181–6. doi: 10.1093/ageing/17.3.181. [DOI] [PubMed] [Google Scholar]
- 27.Loeb M, McGeer A, McArthur M, Walter S, Simor AE. Risk factors for pneumonia and other lower respiratory tract infections in elderly residents of long-term care facilities. Arch Intern Med. 1999;159:2058–2064. doi: 10.1001/archinte.159.17.2058. [DOI] [PubMed] [Google Scholar]
- 28.Bodey GP. Infection in cancer patients. A continuing association. Am J Med. 1986;81:11–26. doi: 10.1016/0002-9343(86)90510-3. [DOI] [PubMed] [Google Scholar]
- 29.Pizzo PA, Meyers J, Freifeld AG, Walsh T. Infections in the cancer patient. In: De Vita VT, Hellman S, Rosenberg SA, editors. Cancer, principles and practice of oncology. Philadelphia: JB Lippincott Company; 1993. pp. 2292–2337. [Google Scholar]
- 30.Albain KS, Crowley JJ, LeBlanc M, Livingston RB. Determinants of improved outcome in small-cell lung cancer: an analysis of the 2580-patient Southwest Oncology Group data base. J. Clin. Oncol. 1990;8:1563–1574. doi: 10.1200/JCO.1990.8.9.1563. [DOI] [PubMed] [Google Scholar]
- 31.Rawson NS, Peto J. An overview of prognostic factors in small cell lung cancer. A report from the Subcommittee for the Management of Lung Cancer of the United Kingdom Coordinating Committee on Cancer Res. Br J Cancer. 1990;61:597–604. doi: 10.1038/bjc.1990.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sculier JP, Chansky K, Crowley JJ, Van Meerbeeck J, Goldstraw P. The impact of additional prognostic factors on survival and their relationship with the anatomical extent of disease expressed by the 6th edition of the TNM Classification of Malignant Tumors and the proposals for the 7th edition. J Thorac Oncol. 2008;3:457–466. doi: 10.1097/JTO.0b013e31816de2b8. [DOI] [PubMed] [Google Scholar]
- 33.Foster NR, Mandrekar SJ, Schild SE, Nelson GD, Rowland KM Jr, Deming RL, Kozelsky TF, Marks RS, Jett JR, Adjei AA. Prognostic factors differ by tumor stage for small cell lung cancer: a pooled analysis of North Central Cancer Treatment Group trials. Cancer. 2009;115:2721–31. doi: 10.1002/cncr.24314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pagni S, McKelvey A, Riordan C, Federico JA, Ponn RB. Pulmonary resection for malignancy in the elderly: is age still a risk factor? Eur J Cardiothorac Surg. 1998;14:40–44. doi: 10.1016/s1010-7940(98)00145-6. [DOI] [PubMed] [Google Scholar]
- 35.Osaki T, Oyama T, Takenoyama M, So T, Yamashita T, Aikawa M, Ono K, Yasumoto K. Results of surgical treatment for primary lung cancer; time trends of survival and clinicopathologic features (in Japanese) J Uoeh. 2001;23:277–283. doi: 10.7888/juoeh.23.277. [DOI] [PubMed] [Google Scholar]
- 36.Hayakawa K, Mitsuhashi N, Katano S, Saito Y, Nakayama Y, Sakurai H, Akimoto T, Hasegawa M, Yamakawa M, Niibe H. High-dose radiation therapy for elderly patients with inoperable or unresectable non-small cell lung cancer. Lung Cancer. 2001;32:81–88. doi: 10.1016/s0169-5002(00)00219-1. [DOI] [PubMed] [Google Scholar]
- 37.Langendijk JA, Aaronson NK, de Jong JM, ten Velde GP, Muller MJ, Lamers RJ, Slotman BJ, Wouters EF. Prospective study on quality of life before and after radical radiotherapy in non-small-cell lung cancer. J. Clin. Oncol. 2001;19:2123–2133. doi: 10.1200/JCO.2001.19.8.2123. [DOI] [PubMed] [Google Scholar]
- 38.Earle CC, Tsai JS, Gelber RD, Weinstein MC, Neumann PJ, Weeks JC. Effectiveness of chemotherapy for advanced lung cancer in the elderly: instrumental variable and propensity analysis. J. Clin. Oncol. 2001;19:1064–1070. doi: 10.1200/JCO.2001.19.4.1064. [DOI] [PubMed] [Google Scholar]
- 39.Hughes WT, Armstrong D, Bodey GP, Bow EJ, Brown AE, Calandra T, Feld R, Pizzo PA, Rolston KV, Shenep JL, Young LS. 2002 guidelines for the use of antimicrobial agents in neutropenic patients with cancer. Clin Infect Dis. 2002;34:730–751. doi: 10.1086/339215. [DOI] [PubMed] [Google Scholar]
- 40.Gafter-Gvili A, Fraser A, Paul M, Leibovici L. Meta analysis: antibiotic prophylaxis reduces mortality in neutropenic patients. Ann Intern Med. 2005;142:979–995. doi: 10.7326/0003-4819-142-12_part_1-200506210-00008. [DOI] [PubMed] [Google Scholar]


