Abstract
Sessile serrated adenomas (SSA) and traditional serrated adenomas (TSA) are considered as precursors of colorectal cancer, and are often diagnostic challenges. Their true prevalence is masked by significant inter-observer variations. To investigate the true prevalence and synchronous colorectal carcinoma (sCRC) of colorectal serrated polyps (CSP) and their associated factors, we first retrospectively identified all colorectal polyps collected at our institution between June 1995 and May 2013. After centrally reclassifying all CSP to reduce inter-observer variations, Chi-square tests and logistic regression analyses were used to analyze the potential factors. Among the included 5501 colorectal polyps, 499 CSP of 428 patients were identified and studied, including 353 hyperplastic polyps (HP, 70.7%), 80 SSA (16.0%), 61 TSA (12.2%) and 5 mixed polyp (1.0%). Diagnostic disagreements were found in 68 CSP (13.63% of CSP). SSA and TSA were more often larger than 5 mm and in proximal colon than HP. SSA were also more likely associated with older age (p=0.005), size ≥5 mm (p<0.001) and ≥3 polyps (p=0.004) than HP in distal colon, but only more likely associated with older age (p=0.006) in proximal colon. Multivariate regression analysis demonstrated that CSP with sCRC, compared with CSP without sCRC, were linked to CSP size ≥1 cm (vs <1 cm, odds ratio [OR] 4.412, 95% confidence interval [CI] 1.684-11.556, P=0.003) and a diagnosis of SSA or TSA (vs HP, OR 6.194, 95% CI 1.870-20.513, P=0.003 and OR 6.754, 95% CI 1.981-23.028, P=0.002, respectively), but not age, gender, polyp number and polyp shape. SSA and TSA are similarly often associated with sCRC (P=0.460). In conclusion, histology subtypes and polyp size may serve as markers for sCRC of CSP. SSA and TSA may warrant careful endoscopic examinations and similar follow-up intervals.
Keywords: Conventional adenoma, sessile serrated adenoma, traditional serrated adenoma, hyperplastic polyp, colon
Introduction
Colorectal cancer (CRC) is the third most common cancer and the third deadliest cancer in the United States [1]. Studies have shown that screening colonoscopy and sigmoidoscopy with polypectomy reduced colon cancer mortality [2-4]. The current U.S., European and U.K. guidelines on post-colonoscopy follow-up are primarily based on polyp size, polyp number and villous histology (not in the U.K. guidelines), despite some minor differences [5-8]. Given that approximately 30% of CRC develop through a serrated pathway [9-11], sessile serrated adenoma (SSA) and traditional serrated adenoma (TSA) have been recognized by the U.S. and European guidelines as distinct histology sub-types of colorectal serrated polyp (CSP) that warrant shorter follow-up intervals [6,7]. Specifically, the European Society of Gastrointestinal Endoscopy and the U.S. multi-society task force both consider SSA <10 mm without dysplasia as low risk group/adenoma, and SSA with dysplasia, SSA ≥10 mm, or TSA as high risk group/adenoma, respectively [6,7]. The updated U.K. guidelines published in 2010, however, have not yet specified management recommendations for CSP [8]. Before the guidelines agree on the best management of CSP much work is still needed to further define the true prevalence and biological behaviors of the various subtypes of CSP.
Several questions seem particularly critical. First, what are the factors associated with synchronous colorectal carcinoma (sCRC) of CSP? SCRC of all CSP subtypes, to our knowledge, have not been characterized, although several groups demonstrated various factors associated with synchronous advanced neoplasia (sAN) [12-16]. SCRC carries a worse prognosis than sAN. Investigation on the factors associated with sCRC of CSP therefore may help better identify CRC related to CSP. It is noteworthy that Mohammadi et al. studied sCRC of hyperplastic polyp (HP) and SSA excluding that of TSA [17], and Lash et al. studied only CRC arising in SSA excluding that of HP or TSA [18]. Another study by Hu et al. defined sCRC as presence of 2 or more primary invasive adenocarcinomas of the same patient at the time of diagnosis [19], which is different than this study and others [12-16].
Second, what is the true prevalence of CSP? Studies have shown significant diagnostic variations of CSP among both pathologists and endoscopists [20-24]. Those variations may be in part attributed to the lack of diagnostic criteria. In fact, the pathologic diagnostic criteria of SSA were not proposed till in 2010 by German Society of Pathology and in 2012 by an American expert panel [10,25]. The studies published before then may have significant inter-observer bias. Thus true prevalence of CSP remains to be examined.
Last, are the current studies on CSP of high-quality? The moderate to modest sample sizes of recent, well-designed studies have reduced their statistical power. The studies on sAN of CSP included 248 to 744 CSP, 28 to 2416 SSA and/or 1-9 TSA [12,14-18,26-29]. The time interval of the collected CSP was also often less than a year. Moreover, some of them did not specify the subtypes of CSP or only included SSA [16,18,27]. Furthermore, TSA has often been omitted or included in the CSP group, and is less characterized. Therefore, there is a need for large-scale study on sAN and sCRC of all subtypes of CSP.
To address the aforementioned questions, all CSP collected at our institution in the past 18 years were re-classified by using the latest diagnostic criteria [10,25] to reduce diagnostic variations and to define the true prevalence of the CSP subtypes. The primary outcome of this study was to explore the factors associated with sCRC of CSP. Those factors may be used to stratify patients with sCRC of CSP in clinical practice, and to study the molecular and cellular mechanisms of sCRC of CSP in the future. Identification of the factors associated with CSP prevalence and its location is the secondary outcomes.
Materials and methods
Study design
This retrospective, cross-sectional study was approved by the Ethics Committee Review of Shanghai Tongji Hospital, Tongji University School of Medicine, Shanghai, China. All cases were obtained from the Department of Pathology archives of Shanghai Tongji Hospital Affiliated to Tongji University School of Medicine, Shanghai, China. We included all colorectal polyps detected by colonoscopy between June 1995 and May 2013. They included conventional adenomas (AD) (including tubular adenomas [TA], tubulovillous adenomas [TVA]; villous adenomas [VA]), HP, SSA, TSA and other types of polyps (OP) including inflammatory polyp (IP), juvenile polyp and others. To re-classify CSP, all the pathological sections of the colorectal polyps were divided into two groups and examined by two groups of pathologists (3 in each group) who were blinded to the clinical, pathologic and demographic data. The two groups of pathologists then exchanged the pathological sections with the other group, and performed a second round of examination for re-classification. Subsequently, a consensus conference was conducted to review the discordant cases. After the consensus conference, the cases with a final diagnosis of CSP were selected and further grouped into CSP with sCRC and without sCRC depending on whether a carcinoma was detected during the same procedure or within 1 month before and after the index procedure. In addition, we randomly selected 192 AD of 146 patients from the same cohort of polyps as control AD.
Morphological reclassification
CSP were reclassified as HP, SA, TSA, and mixed polyp (MP) by using the recommendations by Rex et al. [10] and the diagnostic criteria by German Society of Pathology, the latter of which was adopted by the European Society of Gastrointestinal Endoscopy [6,25]. Briefly, HP are defined as the lesions with elongated crypts and serrated architecture in the upper half of the crypts, while their cells show small oval or slightly elongated, uniform, and basally placed nuclei. SSA are characterized by the presence of exaggerated serration, distorted crypt including crypt dilatation and horizontally extended crypt (such as “boot”, “L” or “anchor” -shaped crypts) bases, increased mucin production (both intracellular and intraluminal), and mild nuclear changes falling short of dysplasia. TSA microscopically shows prominent serration, diffuse cytoplasmic eosinophilia, cytological atypia, “pencil-like” nuclei and ectopic crypt formation. More important is that TSA must be unequivocally dysplastic or adenomatous. MP comprised non-dysplastic HP or SSA and had a dysplastic component that resembled either TSA or AD (TA, tubular adenomas; TVA, tubulovillous adenomas; VA, villous adenomas). The representative histologic images of CSP are shown in Figure 1. A sCRC was defined as a biopsy-confirmed CRC with one or more CSPs identified during the same endoscopic operation, according to the endoscopic report or the pathology requisition form. All biopsy-confirmed invasive CRCs were included regardless of depth of invasion.
Figure 1.
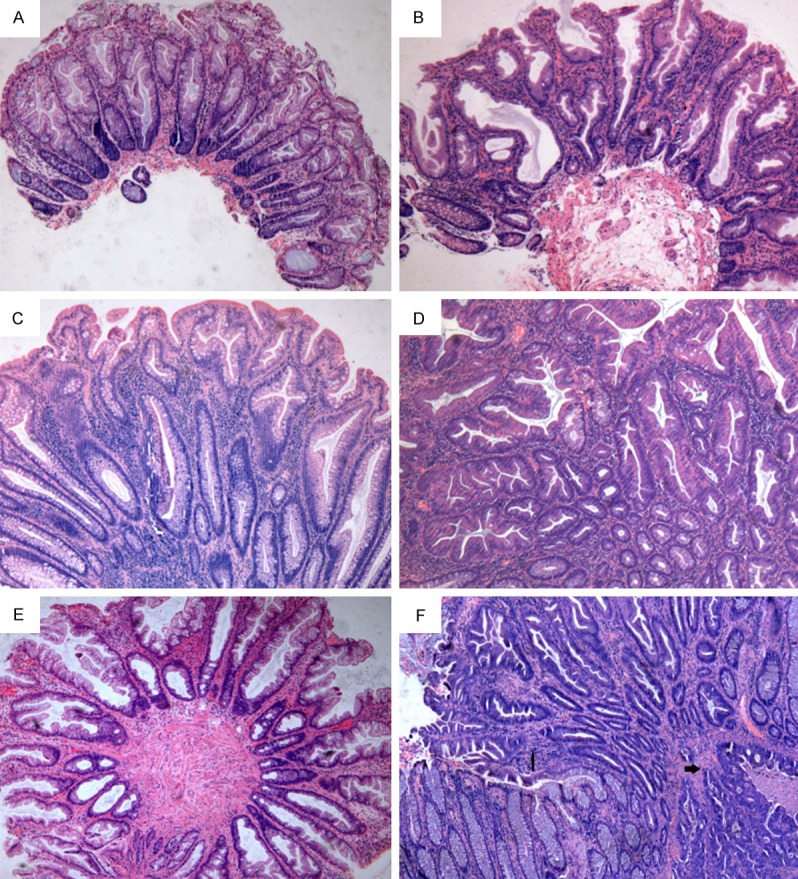
Representative photomicrographs of CSP: A. HP (×40); B. SSA (×40); C. TA and HP (mixed polyp) (×40); D. TSA (×40); E. Polyp with indeterminate features between SSA and HP (×40); F. An sCRC (bold arrow) and residual SSA with cytological dysplasia (thin arrow) (×40). CSP, colorectal serrated polyp; HP, hyperplastic polyp; SSA, sessile serrated adenoma; TSA, traditional serrated adenoma; TA, tubular adenoma; sCRC, synchronous colorectal cancer.
Demographic and clinicopathological data
The demographic and clinicopathological data of all CSP were collected, including patient age and gender, lesion size, shape and location described in the endoscopic reports, and polyp number detected in the same endoscopic procedure. The missing demographic and clinical data were obtained by searching in the medical records or by telephone follow-ups. Unfortunately, the majority of the endoscopic reports did not include CSP endoscopic findings. The CSP were also divided into two groups according to their locations: proximal (cecum, ascending colon, and transverse colon including the splenic flexure) and distal (descending colon, sigmoid colon and rectum). The size and shape of CSP were determined on the basis of endoscopic measurements, or microscopic measurements if the endoscopic measurements were not unavailable in the endoscopic report. It is noteworthy that the endoscopic and microscopic measures of TSA and SSA were found similar, but that of HP and AD were not [30]. The polyp shape was classified into pedunculated and sessile (flat) according the endoscopic reports.
Statistical analysis
All statistical analyses were performed by using SPSS 17.0 (SPSS Inc, Chicago, IL). Continuous variables were shown as mean ( standard deviation) (mean (SD)) and analyzed by Student t-test. We used χ2 test (N>5) or Fisher’s exact test (N≤5) to analyze possible associations between categorical variables. Two sided P values were calculated. The logistic regression analyses (LRA), both univariate and multivariate, were used to identify the associated factors of sCRC. The multivariate LRA was performed by using the variables with a P value <0.05 in the univariate analysis. Both odds ratio (OR) and 95% confidence interval (CI) were displayed for potential factors associated with sCRC. A P value was considered to be statistically significant when less than 0.05.
Results
Prevalence and clinicopathological characteristics of CSP
Figure 2 shows the diagnostic disagreements between the two groups of pathologists in various CSP. The prevalence of different types of colorectal polyps is shown in Figure 3. A total of 5501 colorectal polyps were identified, and included 3222 (58.6%) TA, 1078 (19.6%) TVA, 41 (0.7%) VA, 353 (6.4%) HP, 80 (1.5%) SSA, 61 (1.1%) TSA and 176 (3.2%) IP. Among them (Table 1), 499 polyps of 428 patients were classified as CSP with 340 in males (68.1%) and 159 in females (31.9%). The mean age was 57.0 (12.9) years in males and 65.2 (13.7) years in females (P<0.001). For the 499 CSP, there were 353 (70.7%) HP, 80 (16.0%) SSA, 61 (12.2%) TSA, and 5 (1.0%) MP. Because of the small number of MP (n=5), they were excluded in the LRA analysis. There were no significant differences in the gender distribution for AD, HP, SSA and TSA (Table 1). For the patients with HP, the average age was 57.0 (12.5) years and younger than patients with AD, SSA or TSA. In addition, the patients with AD were older than the patients with TSA or HP. There was no significant age difference between SSA and AD, or SSA and TSA.
Figure 2.
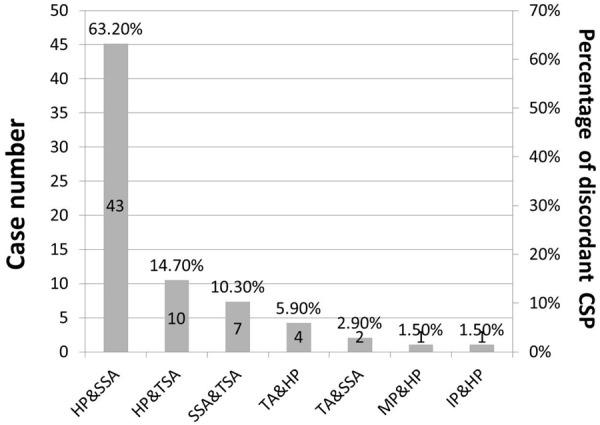
The discordant diagnoses between the two groups of pathologists. HP, hyperplastic polyp; SSA, sessile serrated adenoma; TSA, traditional serrated adenoma; TA, tubular adenoma; MP, mixed polyp; IP, inflammatory polyp.
Figure 3.
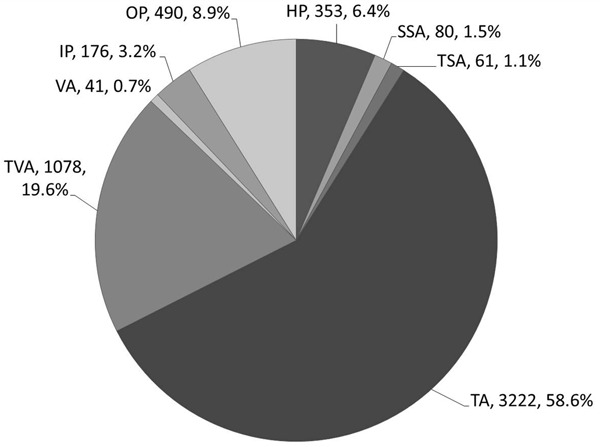
Prevalence of different colorectal polyps in our study. HP, hyperplastic polyp; SSA, sessile serrated adenoma; TSA, traditional serrated adenoma; TA, tubular adenoma; MP, mixed polyp; IP, inflammatory polyp; OP, other types of polyps including polypoid hyperplasia, adenomatoid hyperplasia, juvenile polyp, hamartomatous polyp and mixed polyp.
Table 1.
Clinicopathological characteristics of the CSP
| Variable | AD (n=192) | CSP (n=499) | P value | ||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| HP(n=353, 70.7%) | SSA(n=80, 16.0%) | TSA(n=61, 12.2%) | HP vs. SSA | HP vs. TSA | SSA vs. TSA | ||
| Gender | |||||||
| Male | 123 (64.1) | 247 (70.0) | 51 (63.8) | 38 (62.3) | |||
| Female | 69 (35.9) | 106 (30.0) | 29 (36.2) | 23 (37.7) | 0.278 | 0.232 | 0.859 |
| Age (years) | |||||||
| Mean (SD) | 65.2 (10.0) | 57.0 (12.5)b | 63.4 (13.1) | 61.7 (16.6)c | <0.001 | 0.010 | 0.482 |
| Size (mm) | |||||||
| ≤5 | 82 (42.7) | 263 (74.5) | 36 (45.0) | 11 (18.0) | |||
| >5 | 110 (57.3) | 90 (25.5)b | 44 (55.0) | 50 (82.0)b | <0.001 | <0.001 | 0.001 |
| Location | |||||||
| Distal | 122 (63.5) | 289 (81.9) | 56 (70.0) | 42 (68.9) | |||
| Proximal | 70 (36.5) | 64 (18.1)b | 24 (30.0) | 19 (31.1) | 0.017 | 0.019 | 0.883 |
| Shape | |||||||
| Pedunculated | 31 (43.7) | 11 (21.6) | 5 (22.7) | 14 (43.8) | |||
| Flat-type | 40 (56.3) | 40 (78.4)c | 17 (77.3) | 18 (56.3) | 1.000 | 0.990 | 0.112 |
| Unknowna | 121 | 302 | 58 | 29 | |||
| Number of polyps | |||||||
| 0~2 | 130 (67.7) | 305 (86.4) | 59 (73.8) | 56 (91.8) | |||
| ≥3 | 62 (32.3) | 48 (13.6)b | 21 (26.3) | 5 (8.2)b | 0.005 | 0.244 | 0.006 |
Note: All values are expressed as n (%) unless otherwise indicated;
Those polyps were excluded in the analysis due to a lack of CSP shapes data in the endoscopic report;
P value <0.001, compared with control AD;
P value <0.05, compared with control AD;
CSP, colorectal serrated polyp; HP, hyperplastic polyp; SSA, sessile serrated adenoma; TSA, traditional serrated adenoma; AD, conventional adenoma.
We also found diagnostic disagreements of CSP in 68 polyps (13.63% of all CSP) by the two groups of pathologists with 3 in each group (Figure 2). Among the 68 discordant cases, there were 43 (63.24%) cases disagreed between HP and SSA, 10 (14.71%) between HP and TSA, 7 (10.29%) between SSA and TSA, 4 (5.88%) between TA and HP, 2 (2.94%) between TA and TSA, 1 (1.47%) between MP and HP and 1 (1.47%) between IP and HP. In the consensus conference, majority of the discordant cases (52 of 68, 76.5%) involved a differential diagnosis of SSA. In particular, 30 (69.8%) of the 43 CSPs with disagreement between HP and SSA were reclassified as HP by all 6 observers, and the remaining 13 (30.2%) were reclassified as SSA by the same observers. Figure 1E shown a polyp with indeterminate features between SSA and HP.
Although SSA, TSA and AD were more likely to be located in the proximal colon than HP (P=0.017, P=0.019, P<0.001, respectively), there were 122 (63.5%) AD, 289 (81.9%) HP, 56 (70.0%) SSA and 42 (68.9%) TSA located in distal colon. Therefore, the above four types of colorectal polyps were more often in distal colon. Excluding those polyps whose shape were unknown, HP was more likely to be sessile than AD (P=0.011). There were no significant endoscopic shape differences between other types of colorectal polyps. In addition, SSA were more likely associated with ≥3 polyps than HP and TSA (P=0.005 and P=0.006, respectively). There was no significant difference in the number of polyp number ≥3 between SSA and AD (P=0.324). Compared with AD, TSA was more likely larger than 5 mm in size, while HP less likely (P<0.05 for both). Moreover, except SSA and AD (P=0.728), there were significant difference between them in the likelihood of having a polyp size >5 mm.
Factors associated with the location of CSP
Location is a factor associated with distinct CSP molecular profiles and biological behaviors [12,15,31-35]. Therefore, we examined the potential factors associated with CSP locations. As shown in Table 2, regardless of CSP location, the SSA patients were older than the HP patients. Compared with HPs, SSAs in distal colon were more likely to be larger than 5 mm (50.0% vs 19.0%, P<0.001) and to have more than 2 (≥3) polyps in one colonoscopy procedure (23.2% vs 9.7%, P=0.004), while such a difference was not found between the HPs and SSAs in proximal colon. Interestingly, the male: female ratio and endoscopic polyp shape were not associated with either HP or SSA, regardless of CSP location.
Table 2.
Factors associated with the location of HP and SSA
| Location | Any part of the colon | Proximal colon | Distal colon | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Variable | SSA(n=80) | HP(n=353) | P value | SSA(n=24) | HP(n=64) | P value | SSA(n=56) | HP(n=289) | P value |
| Gender | |||||||||
| Male | 51 (63.8) | 247 (70.0) | 14 (58.3) | 31 (48.4) | 37 (66.1) | 216 (74.7) | |||
| Female | 29 (36.2) | 106 (30.0) | 0.278 | 10 (41.7) | 33 (51.6) | 0.408 | 19 (33.9) | 73 (25.3) | 0.179 |
| Age (years) | |||||||||
| Mean (SD) | 63.4 (13.1) | 57.0 (12.5) | <0.001 | 67.5 (13.6) | 58.8 (12.4) | 0.006 | 61.7 (12.6) | 56.5 (12.5) | 0.005 |
| Size (mm) | |||||||||
| ≤5 | 36 (45.0) | 263 (74.5) | 8 (33.3) | 30 (46.9) | 28 (50.0) | 234 (81.0) | |||
| >5 | 44 (55.0) | 90 (25.5) | <0.001 | 16 (66.7) | 34 (53.1) | 0.253 | 28 (50.0) | 55 (19.0) | <0.001 |
| Shape | |||||||||
| Protruded | 14 (43.8) | 11 (21.6) | 0 (0.0) | 1 (14.3) | 5 (31.3) | 10 (22.7) | |||
| Flat-type | 18 (56.3) | 40 (78.4) | 1.000 | 6 (100.0) | 6 (85.7) | 1.000 | 11 (78.6) | 34 (77.3) | 0.736 |
| Unknowna | 29 | 302 | 18 | 57 | 40 | 245 | |||
| Number of polyps | |||||||||
| 0~2 | 56 (91.8) | 305 (86.4) | 16 (66.7) | 45 (70.3) | 43 (76.8) | 261 (90.3) | |||
| ≥3 | 5 (8.2) | 48 (13.6) | 0.005 | 8 (33.3) | 19 (29.7) | 0.741 | 13 (23.2) | 28 (9.7) | 0.004 |
Note: All values are expressed as n (%) unless otherwise indicated;
Those polyps were excluded in the analysis due to a lack of CSP shapes data in the endoscopic report. HP, hyperplastic polyp;
SSA, sessile serrated adenoma.
Factors associated with the synchronous colorectal carcinoma of CSP
Between June 1995 and May 2013, there were 414 CSP identified at our institution including 25 (6%) cases with sCRC (Table 3). Sixteen of the patients with sCRC were male and the remaining 9 were female. Five SCRC were synchronous of HP (20%), 11 sCRC of TSA (44%) and 9 sCRC of SSA (36%). Moreover, 14 (56%) sCRC were located the distal colon and 11 (44%) sCRC in the proximal colon. Fourteen CSP, including 6 SSA and 8 TSA, were adjacent to the sCRC. Figure 1F shows an SSA with cytological dysplasia and adjacent sCRC.
Table 3.
The factors associated with CSP with sCRC
| Variable | n | CSP without sCRC (n=389) | CSP with sCRC (n=25) | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| OR (95% CI) | P value | OR (95% CI) | P value | ||||
| Gender | |||||||
| Male | 280 | 264 (94.3) | 16 (5.7) | 1.00 | |||
| Female | 134 | 125 (93.3) | 9 (6.7) | 1.188 (0.511-2.763) | 0.689 | ||
| Age (years) | |||||||
| Mean (SD) | 57.8 (13.5) | 65.0 (13.7) | 1.041 (1.008-1.076) | 0.014 | 1.016 (0.985-1.048) | 0.318 | |
| Size (mm) | |||||||
| <10 | 366 | 354 (96.7) | 12 (3.3) | 1.00 | 1.00 | ||
| ≥10 | 48 | 35 (72.9) | 13 (27.1) | 10.957 (4.646-25.843) | <0.001 | 4.412 (1.684-11.556) | 0.003 |
| Location | |||||||
| Distal | 328 | 310 (94.5) | 18 (5.5) | 1.00 | |||
| Proximal | 86 | 79 (91.9) | 7 (8.1) | 1.526 (0.616-3.781) | 0.361 | ||
| Shape | |||||||
| Pedunculated | 24 | 22 (91.7) | 2 (8.3) | 1.00 | |||
| Flat-type | 64 | 57 (89.1) | 7 (10.9) | 1.351 (0.260-7.011) | 0.720 | ||
| Unknowna | 310 | 16 | |||||
| Number of polyps | |||||||
| 0~2 | 380 | 357 (93.9) | 23 (6.1) | 1.00 | |||
| ≥3 | 34 | 32 (94.1) | 2 (5.9) | 0.970 (0.219-4.302) | 0.779 | ||
| Polyp type | |||||||
| HP | 296 | 291 (98.3) | 5 (1.7) | 1.00 | 1.00 | ||
| SSA* | 62 | 53 (85.5) | 9 (14.5) | 9.883 (3.187-30.647) | <0.001 | 6.194 (1.870-20.513) | 0.003 |
| TSA* | 56 | 45 (80.4) | 11 (19.6) | 14.227 (4.723-42.857) | <0.001 | 6.754 (1.981-23.028) | 0.002 |
Note: All values are expressed as n (%) unless otherwise indicated;
Those polyps were excluded in the analysis due to a lack of CSP shape data in the endoscopic report.
CSP, colorectal serrated polyp; HP, hyperplastic polyp; SSA, sessile serrated adenoma; TSA, traditional serrated adenoma; sCRC, synchronous colorectal cancer.
SSA and TSA had a similar likelihood of correlating to a sCRC (OR 0.695, 95% CI 0.264-1.826, P=0.460).
LRA was then used to identify the potential factors associated with sCRC, including gender, age, and the size, location, shape, number and type of CSP. Our univariate LRA showed sCRC of CSP was associated with older age (OR 1.041, 95% CI 1.008-1.076, P=0.014), polyps ≥10 mm (OR 10.975, 95% CI 4.646-25.843, P<0.001), and a pathologic diagnosis of SSA (OR 9.883, 95% CI 3.187-30.647; P<0.001) or TSA (OR 14.227, 95% CI 4.723-42.857, P<0.001), but not associated with gender, polyp number (≤2 vs >3), polyp location or polyp shape. However, the multivariate analysis demonstrated that only polyp size ≥10 mm (OR 4.412, 95% CI 1.684-11.556, P=0.003), and presence of SSA (OR 6.194, 95% CI 1.870-20.513, P=0.003) or TSA (OR 6.754, 95% CI 1.981-23.028, P=0.002) were significantly associated with sCRC. Older age was no longer associated with sCRC of CSP in the multivariate LRA. In addition, SSA and TSA had a similar likelihood of correlating to a sCRC (OR 0.695, 95% CI 0.264-1.826, P=0.460).
Discussion
This study of 499 CSP, to our knowledge, for the first time showed that SSA and TSA, compared with HP, had similarly high likelihoods (OR >6) of associating with sCRC. Our MRA also found that sCRC of CSP was linked to size >10 mm, but not age, sex, polyp number or location. We also reclassified and characterized the 499 CSP identified among the 5501 colorectal polyps incurred between 1995 and 2013. The SSA and HP in our series differed in patient age, CSP size and number of polyp in the distal colon, but only in patient age in the proximal colon, suggesting different clinicopathologic features of SSAs in the proximal and distal colons.
SSA has been found associated with sAN, namely the polyps with a size larger than 1 cm, villous component, high-grade dysplasia or cancer. We for the first time revealed that SSA is more likely associated with sCRC than HP, indicating a strong association between SSA and CRC. Given such an association, thorough examination of the remaining colon is warranted if a SSA is identified. In fact, Vu et al. also showed that sCRC were exclusively associated with SSA, despite a lack of statistical significance between SSA and AD (P=0.06) [28]. Our finding, that SSA had a similar likelihood of linking to sCRC as TSA, also suggests similar follow-up intervals for SSA and TSA. However, current U.S. Multi-Society Task Force on Colorectal Cancer recommends different intervals for SSA (without atypia) and TSA, 5 years and 3 years, respectively [7]. The U.K. and European guidelines did not even specify follow-up intervals for CSPs. Our data here provide additional evidence in support of a strong link between histopathology of SSA or TSA and sCRC. The histopathology diagnosis of SSA or TSA hence may be used as one of the criteria for determining sCRC likelihood, and follow-up intervals. Future randomized, prospective studies are needed to confirm the potential use of histopathology.
The sAN of TSA is poorly characterized, in part due to its rarity. Almost all studies characterizing sAN of CSP were focused on SSA or lumped TSA, HP and SSA together as a group [12-17,26]. Little is known regarding the association between sCRC and TSA. In fact, the European and U.K. guidelines did not specify follow-up intervals for TSA [6,8]. In contrast, the U.S. Multi-Society Task Force on Colorectal Cancer recommends TSA, SSA with dysplasia and SSA ≥10 mm to be followed up within 3 years, and TSA <10 mm without dysplasia followed up within 5 years [7], while a U.S. expert panel added TSA or SSA with more than 3 polyps as an additional criterion for a followed up within 3 years [10]. Similar disagreement on the use of histopathology between the U.S. and U.K. guidelines also exists on the use of intestinal metaplasia for diagnosing Barrett’s esophagus [36-38], although the most recent British Society of Gastroenterology guidelines took presence of intestinal metaplasia into the consideration of follow-up, but still not considering it as a diagnostic criterion for Barrett’s esophagus [39]. Our data indicate TSA and SSA are more likely (similarly higher likelihood between the two) linked to sCRC than HP, implying their role as a marker for sCRC. Our data also support a potential role of CSP histology in determining follow-up intervals as the U.S. guidelines or experts recommended.
This study is the first to demonstrate CSP size >1 cm as a factor associated with sCRC of CSP. Our evidence is consistent with that of prior studies on sAN of CSP or SSA [12,15,16]. Indeed, polyps larger than 1 cm are known to associate with CRC and warrant a closer follow-up [6-8,10], further supporting our findings. Interestingly, TSA had the highest frequency of size larger than 5 mm in our series, SSA had second highest and HP had the lowest. Such a size frequency difference was no longer present in the proximal colon when the SSA and HP were sub-grouped in to the proximal and distal colon groups. This partial dependency of polyp size on CSP location may be in part caused by our perceived SSA features, which include both size >5 mm and location of proximal colon. To be clear, the German criteria include those two factors as important features of SSA, but not as diagnostic criteria [25]. Pathologists including us, however, still very much tended to use those two factors for diagnosing SSA. As our experiences showed, one would tend to give more weights to the size criterion for distal colon CSP than the proximal colon CSP, since some of the clinical features of proximal location are not valid in the distal colon. This potential bias also raises a significant question of whether the CSP size should be considered for diagnosing a SSA at all. Rex et al. instead recommend only presence of qualified crypt(s) as the criterion, without mentioning of the size or location [10]. More work is needed to validate the proposed criteria sets [10,25], while it is clear that the patients with SSA larger than 1 cm should be carefully examined for sCRC.
The polyp location may be an important factor for CSP associated sCRC. Two groups have demonstrated that sAN was associated with proximal location, in addition to larger size [12,15]. In contrast, Li at el. found no such an association [16]. In support of Li’s findings, neither did we identify an association between sCRC and CSP location. It is noteworthy that location is a part of the important clinical features or recommendations of SSA [10,25]. Therefore, polyp location could be a confounding factor in analyzing CSP. Our sub-group analysis revealed SSA and HP only differed in patient age in proximal colon, while they differed in polyps number, frequency of size >5 mm and age in distal colon, suggesting the location of CSP correlates with different clinicopathologic factors. None of those factors, however, was associated with sCRC of CSP, except that size >5 mm may have some association with size >1 cm. These factors’ clinical significance remains to be defined. In order to exclude a possibility of confounding factor, one needs to perform sub-group analysis on the polyps from the 2 studies supporting the link between polyp location and sAN [12,15]. Nonetheless, more data are needed to further clarify the association between polyp location and sCRC or sAN.
Inter-observer variation and lack of diagnostic criteria on SSA are the major challenges in investigating the true prevalence of CSP, which are significantly different among the studies and countries. The major strength of our study is our centralized reclassification of large number of colorectal polyps spanning 18 years. The prevalence of AD and TSA in our Chinese cohort was higher than that reported by two Australian studies [40,41], and consequently that of HP and SSA was lower than theirs. On the other hand, the HP prevalence of 6.4% and SSA prevalence of 1.5% in our 5501 polyps were comparable to that in Americans [20], Italians [14] and Koreans [42]. The SSA prevalence in our cohort is also similar to that in the Chinese in Australia [43] and the Chinese in China [44], but slightly lower than the 4.3% reported in the U.S. [26] and the 4.8% in the Netherlands [12]. Hence, it seems that HP and SSA are less prevalent in Asians than Australian Caucasians [43]. Race, climate and dietary customs may contribute to the prevalence difference.
Several factors were associated with inter-observer variation in diagnosing CSP. The reported inter-observer agreement, as the kappa values indicated, ranged from 0.16 to 0.66 [22,45], and were better between paired pathologists and in a recent study with fewer observers [22,26]. In addition, an international study published prior to the German and U.S. SSA diagnostic criteria showed that pathologists with GI fellowship training performed much better than pathologists without [24]. They also found reading related article improved the performance of the pathologists without GI pathology fellowship training, which was still worse than the ones with GI fellowship training [24]. Therefore, our pathologists went through several sessions of training with the GI fellowship trained pathologist (LZ), and should have an improved performance. However, our discordance rate of 13.6% among the CSP was still significantly higher than the 6% shown by Sandmeier et al [23]. The difference may be attributable to our larger study size (499 versus 102 CSP) and longer sample inclusion times (June 1995-May 2013 versus Nov. 2005-Jan. 2006). Indeed, our study to our knowledge is the first to re-classify the CSP cases of past 18 years. Recent cases may have higher agreement rates than older ones, because of better awareness of SSA among the pathologists and publication of diagnostic criteria.
The strength of this study includes the large case number of all subtypes of CSP including HP, SSA and TSA, sub-grouping analysis on the polyp locations, and centralized reclassification of CSP. This study also has several limitations. First, the nature of retrospective study may lead to selection and observer biases (i.e. observers might tend to reclassify a HP to a SSA). In addition, a retrospective study could only demonstrate the association between factors and entities. Prospective, ideally randomized, studies are needed to reduce those biases and to better define the factors associated with sCRC and SSA. Second, inter-observer variation may still exist among our group members, and between us and other pathology groups. We were aware of such a variation and attempted to reduce it by multiple consensus meetings within our group and several training sessions with the U.S. gastrointestinal fellowship trained pathologist (LZ). Last, patient survival data were not included in this study, but may be used for identifying the factors associated with the deaths caused by sCRC of CSP. Unfortunately, those data were not included in our original IRB application. One of the reasons for excluding survival analysis was that Chinese patients are often lost to follow-up. Another consideration of excluding survival analysis is a lack of standardized chemotherapy treatments in most cancer patients. Nonetheless, all-cause deaths/survival is still possible and should be carried out in the future.
In conclusion, this large-scale study showed that sCRC of CSP were linked to CSP >10 mm and a histology of TSA or SSA, as compared with CSP ≤10 mm and HP, respectively. Those two factors may be used as markers for sCRC of CSP. We also found that in the distal colon, but not in the proximal colon, SSAs were associated with >5 mm and ≥3 polyps when compared with HP. These lines of evidence suggest a careful examination of the remaining colon when the index CSP is a TSA, a SSA, located in the distal colon or larger than 10 mm. Shorter post-polypectomy follow-up intervals may also be indicated for those patients.
Acknowledgements
The study was funded in part by the Science and Technology Commission foundation of Key Medical Research of Shanghai, China (034119868, 09411951600 to Xianghua Yi), Key Medical Research Foundation of Health Bureau of Shanghai, China (20134034 to Xianghua Yi), the Department of Chemical Biology, Rutgers Earnest Mario School of Pharmacy (to Lanjing Zhang), and Center for Cancer Prevention Research at Rutgers University (to Lanjing Zhang). We thank Weizhe Qiu, Long Zhang, Yudong Zhang and Jun Gu for their invaluable help with data input. All authors approved the final version of the article, including the authorship list.
Disclosure of conflict of interest
None disclosed.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Shaukat A, Mongin SJ, Geisser MS, Lederle FA, Bond JH, Mandel JS, Church TR. Long-term mortality after screening for colorectal cancer. N Engl J Med. 2013;369:1106–1114. doi: 10.1056/NEJMoa1300720. [DOI] [PubMed] [Google Scholar]
- 3.le Clercq CM, Bouwens MW, Rondagh EJ, Bakker CM, Keulen ET, de Ridder RJ, Winkens B, Masclee AA, Sanduleanu S. Postcolonoscopy colorectal cancers are preventable: a population-based study. Gut. 2014;63:957–963. doi: 10.1136/gutjnl-2013-304880. [DOI] [PubMed] [Google Scholar]
- 4.Winawer SJ, Zauber AG, Ho MN, O’Brien MJ, Gottlieb LS, Sternberg SS, Waye JD, Schapiro M, Bond JH, Panish JF, et al. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med. 1993;329:1977–1981. doi: 10.1056/NEJM199312303292701. [DOI] [PubMed] [Google Scholar]
- 5.Lieberman DA American Gastroenterological Association. Colon polyp surveillance: clinical decision tool. Gastroenterology. 2014;146:305–306. doi: 10.1053/j.gastro.2013.11.029. [DOI] [PubMed] [Google Scholar]
- 6.Hassan C, Quintero E, Dumonceau JM, Regula J, Brandão C, Chaussade S, Dekker E, Dinis-Ribeiro M, Ferlitsch M, Gimeno-García A, Hazewinkel Y, Jover R, Kalager M, Loberg M, Pox C, Rembacken B, Lieberman D European Society of Gastrointestinal Endoscopy. Post-polypectomy colonoscopy surveillance: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2013;45:842–851. doi: 10.1055/s-0033-1344548. [DOI] [PubMed] [Google Scholar]
- 7.Lieberman DA, Rex DK, Winawer SJ, Giardiello FM, Johnson DA, Levin TR United States Multi-Society Task Force on Colorectal Cancer. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2012;143:844–857. doi: 10.1053/j.gastro.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 8.Cairns SR, Scholefield JH, Steele RJ, Dunlop MG, Thomas HJ, Evans GD, Eaden JA, Rutter MD, Atkin WP, Saunders BP, Lucassen A, Jenkins P, Fairclough PD, Woodhouse CR. Guidelines for colorectal cancer screening and surveillance in moderate and high risk groups (update from 2002) Gut. 2010;59:666–689. doi: 10.1136/gut.2009.179804. [DOI] [PubMed] [Google Scholar]
- 9.Rosty C, Hewett DG, Brown IS, Leggett BA, Whitehall VL. Serrated polyps of the large intestine: current understanding of diagnosis, pathogenesis, and clinical management. J Gastroenterol. 2013;48:287–302. doi: 10.1007/s00535-012-0720-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rex DK, Ahnen DJ, Baron JA, Batts KP, Burke CA, Burt RW, Goldblum JR, Guillem JG, Kahi CJ, Kalady MF, O’Brien MJ, Odze RD, Ogino S, Parry S, Snover DC, Torlakovic EE, Wise PE, Young J, Church J. Serrated lesions of the colorectum: review and recommendations from an expert panel. Am J Gastroenterol. 2012;107:1315–1329. doi: 10.1038/ajg.2012.161. quiz 1314, 1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Snover DC. Update on the serrated pathway to colorectal carcinoma. Hum Pathol. 2011;42:1–10. doi: 10.1016/j.humpath.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 12.Hazewinkel Y, de Wijkerslooth TR, Stoop EM, Bossuyt PM, Biermann K, van de Vijver MJ, Fockens P, van Leerdam ME, Kuipers EJ, Dekker E. Prevalence of serrated polyps and association with synchronous advanced neoplasia in screening colonoscopy. Endoscopy. 2014;46:219–224. doi: 10.1055/s-0033-1358800. [DOI] [PubMed] [Google Scholar]
- 13.Alvarez C, Andreu M, Castells A, Quintero E, Bujanda L, Cubiella J, Salas D, Lanas A, Carballo F, Morillas JD, Hernandez C, Jover R, Sarasqueta C, Enriquez-Navascues JM, Hernandez V, Estevez P, Macenlle R, Sala T, Balaguer F, Pellise M, Moreira L, Gil I, Peris A, Gonzalez-Rubio F, Ferrandez A, Poves C, Ponce M, Grau J, Serradesanferm A, Ono A, Cruzado J, Perez-Riquelme F, Alonso-Abreu I, Carrillo-Palau M, Santander C, Diaz Tasende J, Herreros A, Cacho G, Barranco LE, Bessa X. Relationship of colonoscopy-detected serrated polyps with synchronous advanced neoplasia in average-risk individuals. Gastrointest Endosc. 2013;78:333–341. e331. doi: 10.1016/j.gie.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 14.Buda A, De Bona M, Dotti I, Piselli P, Zabeo E, Barbazza R, Bellumat A, Valiante F, Nardon E, Probert CS, Pignatelli M, Stanta G, Sturniolo GC, De Boni M. Prevalence of different subtypes of serrated polyps and risk of synchronous advanced colorectal neoplasia in average-risk population undergoing first-time colonoscopy. Clin Transl Gastroenterol. 2012;3:e6. doi: 10.1038/ctg.2011.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schreiner MA, Weiss DG, Lieberman DA. Proximal and large hyperplastic and nondysplastic serrated polyps detected by colonoscopy are associated with neoplasia. Gastroenterology. 2010;139:1497–1502. doi: 10.1053/j.gastro.2010.06.074. [DOI] [PubMed] [Google Scholar]
- 16.Li D, Jin C, McCulloch C, Kakar S, Berger BM, Imperiale TF, Terdiman JP. Association of large serrated polyps with synchronous advanced colorectal neoplasia. Am J Gastroenterol. 2009;104:695–702. doi: 10.1038/ajg.2008.166. [DOI] [PubMed] [Google Scholar]
- 17.Mohammadi M, Kristensen MH, Nielsen HJ, Bonde JH, Holck S. Qualities of sessile serrated adenoma/polyp/lesion and its borderline variant in the context of synchronous colorectal carcinoma. J Clin Pathol. 2012;65:924–927. doi: 10.1136/jclinpath-2012-200803. [DOI] [PubMed] [Google Scholar]
- 18.Lash RH, Genta RM, Schuler CM. Sessile serrated adenomas: prevalence of dysplasia and carcinoma in 2139 patients. J Clin Pathol. 2010;63:681–686. doi: 10.1136/jcp.2010.075507. [DOI] [PubMed] [Google Scholar]
- 19.Hu H, Chang DT, Nikiforova MN, Kuan SF, Pai RK. Clinicopathologic features of synchronous colorectal carcinoma: A distinct subset arising from multiple sessile serrated adenomas and associated with high levels of microsatellite instability and favorable prognosis. Am J Surg Pathol. 2013;37:1660–1670. doi: 10.1097/PAS.0b013e31829623b8. [DOI] [PubMed] [Google Scholar]
- 20.Hetzel JT, Huang CS, Coukos JA, Omstead K, Cerda SR, Yang S, O’Brien MJ, Farraye FA. Variation in the detection of serrated polyps in an average risk colorectal cancer screening cohort. Am J Gastroenterol. 2010;105:2656–2664. doi: 10.1038/ajg.2010.315. [DOI] [PubMed] [Google Scholar]
- 21.Garcia-Solano J, Perez-Guillermo M, Conesa-Zamora P, Acosta-Ortega J, Trujillo-Santos J, Cerezuela-Fuentes P, Makinen MJ. Clinicopathologic study of 85 colorectal serrated adenocarcinomas: further insights into the full recognition of a new subset of colorectal carcinoma. Hum Pathol. 2010;41:1359–1368. doi: 10.1016/j.humpath.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 22.Khalid O, Radaideh S, Cummings OW, O’Brien MJ, Goldblum JR, Rex DK. Reinterpretation of histology of proximal colon polyps called hyperplastic in 2001. World J Gastroenterol. 2009;15:3767–3770. doi: 10.3748/wjg.15.3767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sandmeier D, Seelentag W, Bouzourene H. Serrated polyps of the colorectum: is sessile serrated adenoma distinguishable from hyperplastic polyp in a daily practice? Virchows Arch. 2007;450:613–618. doi: 10.1007/s00428-007-0413-8. [DOI] [PubMed] [Google Scholar]
- 24.Glatz K, Pritt B, Glatz D, Hartmann A, O’Brien MJ, Blaszyk H. A multinational, internet-based assessment of observer variability in the diagnosis of serrated colorectal polyps. Am J Clin Pathol. 2007;127:938–945. doi: 10.1309/NXDB6FMTE9X5CD6Y. [DOI] [PubMed] [Google Scholar]
- 25.Aust DE, Baretton GB. Serrated polyps of the colon and rectum (hyperplastic polyps, sessile serrated adenomas, traditional serrated adenomas, and mixed polyps)-proposal for diagnostic criteria. Virchows Arch. 2010;457:291–297. doi: 10.1007/s00428-010-0945-1. [DOI] [PubMed] [Google Scholar]
- 26.Pai RK, Hart J, Noffsinger AE. Sessile serrated adenomas strongly predispose to synchronous serrated polyps in non-syndromic patients. Histopathology. 2010;56:581–588. doi: 10.1111/j.1365-2559.2010.03520.x. [DOI] [PubMed] [Google Scholar]
- 27.Gurudu SR, Heigh RI, De Petris G, Heigh EG, Leighton JA, Pasha SF, Malagon IB, Das A. Sessile serrated adenomas: demographic, endoscopic and pathological characteristics. World J Gastroenterol. 2010;16:3402–3405. doi: 10.3748/wjg.v16.i27.3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vu HT, Lopez R, Bennett A, Burke CA. Individuals with sessile serrated polyps express an aggressive colorectal phenotype. Dis Colon Rectum. 2011;54:1216–1223. doi: 10.1097/DCR.0b013e318228f8a9. [DOI] [PubMed] [Google Scholar]
- 29.Teriaky A, Driman DK, Chande N. Outcomes of a 5-year follow-up of patients with sessile serrated adenomas. Scand J Gastroenterol. 2012;47:178–183. doi: 10.3109/00365521.2011.645499. [DOI] [PubMed] [Google Scholar]
- 30.Li F, Chen Z, Yang Y, Yi X, Yang Y, Zhang L. Characterization of the pathologic and endoscopic measurements of colorectal polyp sizes with a focus on sessile serrated adenoma and high-grade dysplasia. Int J Clin Exp Pathol. 2014;7:1635–1643. [PMC free article] [PubMed] [Google Scholar]
- 31.Sandmeier D, Benhattar J, Martin P, Bouzourene H. Serrated polyps of the large intestine: a molecular study comparing sessile serrated adenomas and hyperplastic polyps. Histopathology. 2009;55:206–213. doi: 10.1111/j.1365-2559.2009.03356.x. [DOI] [PubMed] [Google Scholar]
- 32.O’Brien MJ, Yang S, Clebanoff JL, Mulcahy E, Farraye FA, Amorosino M, Swan N. Hyperplastic (serrated) polyps of the colorectum: relationship of CpG island methylator phenotype and K-ras mutation to location and histologic subtype. Am J Surg Pathol. 2004;28:423–434. doi: 10.1097/00000478-200404000-00001. [DOI] [PubMed] [Google Scholar]
- 33.Rustagi T, Rangasamy P, Myers M, Sanders M, Vaziri H, Wu GY, Birk JW, Protiva P, Anderson JC. Sessile serrated adenomas in the proximal colon are likely to be flat, large and occur in smokers. World J Gastroenterol. 2013;19:5271–5277. doi: 10.3748/wjg.v19.i32.5271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee CK, Kim YW, Shim JJ, Jang JY. Prevalence of proximal serrated polyps and conventional adenomas in an asymptomatic average-risk screening population. Gut Liver. 2013;7:524–531. doi: 10.5009/gnl.2013.7.5.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dada M, Wang LM, Chetty R. Incidence and review of sessile serrated polyp reporting in a district general hospital in the UK. Virchows Arch. 2013;463:633–636. doi: 10.1007/s00428-013-1477-2. [DOI] [PubMed] [Google Scholar]
- 36.Wang KK, Sampliner RE. Updated guidelines 2008 for the diagnosis, surveillance and therapy of Barrett's esophagus. Am J Gastroenterol. 2008;103:788–797. doi: 10.1111/j.1572-0241.2008.01835.x. [DOI] [PubMed] [Google Scholar]
- 37.Boyer J, Laugier R, Chemali M, Arpurt JP, Boustiere C, Canard JM, Dalbies PA, Gay G, Escourrou J, Napoleon B, Palazzo L, Ponchon T, Richard-Mollard B, Sautereau D, Tucat G, Vedrenne B. French Society of Digestive Endoscopy SFED guideline: monitoring of patients with Barrett’s esophagus. Endoscopy. 2007;39:840–842. doi: 10.1055/s-2007-966653. [DOI] [PubMed] [Google Scholar]
- 38.Murphy SJ, Johnston BT, Murray LJ. British Society of Gastroenterology guidelines for the diagnosis of Barrett's oesophagus: are we casting the net too wide? Gut. 2006;55:1821–1822. [PMC free article] [PubMed] [Google Scholar]
- 39.Fitzgerald RC, di Pietro M, Ragunath K, Ang Y, Kang JY, Watson P, Trudgill N, Patel P, Kaye PV, Sanders S, O’Donovan M, Bird-Lieberman E, Bhandari P, Jankowski JA, Attwood S, Parsons SL, Loft D, Lagergren J, Moayyedi P, Lyratzopoulos G, de Caestecker J. British Society of Gastroenterology guidelines on the diagnosis and management of Barrett’s oesophagus. Gut. 2014;63:7–42. doi: 10.1136/gutjnl-2013-305372. [DOI] [PubMed] [Google Scholar]
- 40.Spring KJ, Zhao ZZ, Karamatic R, Walsh MD, Whitehall VL, Pike T, Simms LA, Young J, James M, Montgomery GW, Appleyard M, Hewett D, Togashi K, Jass JR, Leggett BA. High prevalence of sessile serrated adenomas with BRAF mutations: a prospective study of patients undergoing colonoscopy. Gastroenterology. 2006;131:1400–1407. doi: 10.1053/j.gastro.2006.08.038. [DOI] [PubMed] [Google Scholar]
- 41.Carr NJ, Mahajan H, Tan KL, Hawkins NJ, Ward RL. Serrated and non-serrated polyps of the colorectum: their prevalence in an unselected case series and correlation of BRAF mutation analysis with the diagnosis of sessile serrated adenoma. J Clin Pathol. 2009;62:516–518. doi: 10.1136/jcp.2008.061960. [DOI] [PubMed] [Google Scholar]
- 42.Kim HY, Kim SM, Seo JH, Park EH, Kim N, Lee DH. Age-specific prevalence of serrated lesions and their subtypes by screening colonoscopy: a retrospective study. BMC Gastroenterol. 2014;14:82. doi: 10.1186/1471-230X-14-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kumbhari V, Behary J, Hui JM. Prevalence of adenomas and sessile serrated adenomas in Chinese compared with Caucasians. J Gastroenterol Hepatol. 2013;28:608–612. doi: 10.1111/jgh.12100. [DOI] [PubMed] [Google Scholar]
- 44.Qiu Y, Fu X, Zhang W, Xu Y, Xiao L, Chen X, Shi L, Zhou X, Xia G, Peng Y, Deng M. Prevalence and molecular characterisation of the sessile serrated adenoma in a subset of the Chinese population. J Clin Pathol. 2014;67:491–498. doi: 10.1136/jclinpath-2013-202092. [DOI] [PubMed] [Google Scholar]
- 45.Farris AB, Misdraji J, Srivastava A, Muzikansky A, Deshpande V, Lauwers GY, Mino-Kenudson M. Sessile serrated adenoma: challenging discrimination from other serrated colonic polyps. Am J Surg Pathol. 2008;32:30–35. doi: 10.1097/PAS.0b013e318093e40a. [DOI] [PubMed] [Google Scholar]


