AUTHOR CORRECTION
Volume 88, no. 11, p. 6148–6157, 2014. Page 6149, column 2, Results, line 8: “(see Fig. 2, 4, and 5)” should read “(see Fig. 2, 4, and 6).”
Page 6150, column 2, Results, line 19: “Fig. 2c” should read “Fig. 3c,” and line 21 “Fig. 2d” should read “Fig. 3d.”
Page 6152, legend to Fig. 3: The legend, with the corrections shown in boldface, should read “Fig. 3 Neutralization of VSV with nonimmune human serum depends on serum IgM antibodies. (a and b) VSV-GFP was incubated with nonimmune human serum or nonimmune human serum that was treated with anti-IgM antibody (30 min at room temperature) or, as a control, with medium only. The virus-serum or virus-medium mixture was incubated for 1 h at 37°C. Following incubation, the mixture was overlaid on a 12-well plate of overnight-plated Vero cells and micrographs were taken 24 h postinfection (a) or the mixture was diluted 10-fold and titrated on 96-well plates of overnight-plated Vero cells for a TCID50/ml determination (hatched, medium; checked, normal serum; stippled, normal serum plus anti-IgM antibody) (this figure was reproduced from Fig. 1b of Tesfay et al, J Virol 87:3752, 2013 [39]) (b). (c) VSV-GFP was incubated with nonimmune MM patient serum or nonimmune normal serum, or as a control, virus was incubated with medium for 1 h at 37°C. Following incubation, the virus-serum mixture was diluted 10-fold and titrated on 96-well plates of overnight-plated Vero cells for a TCID50/ml determination as for panel b. (d) IgM concentration of nonimmune human serum (NHS) (n = 3) and a multiple myeloma patient sample (n = 3) were analyzed, and the averages are shown. (e) VSV-GFP (1 × 104 TCID50) was incubated for 1 h at 37°C with media containing 10% guinea pig complement (the same picture from Fig. 2d left complement panel is shown here for comparison), 15 μg of purified IgM protein, or 15 μg purified IgM protein reconstituted with 10% (by volume) guinea pig complement, followed by plating the mixture on monolayers of 96-well plates of Vero cells. Micrographs were taken 24 h postinfection. All virus neutralization assay experiments were conducted in triplicate, and the error bars show standard deviations.”
Page 6155: Figure 6 and its legend, with the corrections shown in boldface, should appear as shown below.
FIG 6.
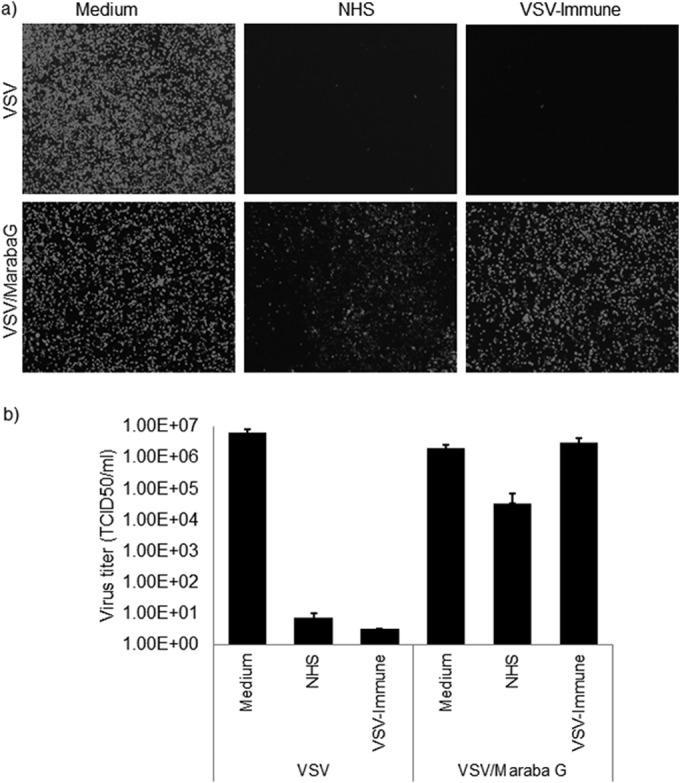
Maraba G-pseudotyped VSV escapes serum neutralization. VSV-GFP or VSV/Maraba G (5-μl volume with 1 × 108 TCID50) was incubated with 100 μl undiluted nonimmune human serum that contained 10% standard guinea pig complement (Cedarlane) or heat-inactivated VSV-immune serum at 37°C for 1 h, followed by determination of the virus titer (TCID50/ml) on Vero cells (1 × 104 cells/well). As a control, virus was incubated with medium only and plated on Vero cells. The neutralization assay result was read at 48 h after infection with virus that was treated or not with serum. Alternatively, virus treated in the presence or absence of serum was plated on 12-well plates of Vero cells (1 × 105 cells/well) and fluorescence images were taken at 24 h postinfection for each condition. VSV serum neutralization data from Fig. 4a (upper panel, VSV) and Fig. 4b (left, VSV) are included here for comparison (Fig. 6a upper panel, VSV; Fig. 6b left, VSV), as all three viruses were tested at the same time. All virus neutralization assay experiments were conducted in triplicate. The error bars represent standard deviations.
Page 6155, column 2, line 6: “(Fig. 2)” should read “(Fig. 1).”
The authors regret these errors.


