Abstract
Canine parvovirus type 2 (CPV-2) emerged in 1978 and spread worldwide within 2 years. Subsequently, CPV-2 was completely replaced by the variant CPV-2a, which is characterized by four specific capsid (VP2) mutations. The X-ray crystal structure of the CPV-2a capsid shows that each mutation confers small local changes. The loss of a hydrogen bond and introduction of a glycine residue likely introduce flexibility to sites that control interactions with the host receptor, antibodies, and sialic acids.
TEXT
Canine parvovirus (CPV) is closely related to feline panleukopenia virus (FPV), a parvovirus that infects domestic cats and a variety of nondomestic carnivores but does not infect dogs (1, 2). In the 1970s, canine parvovirus type 2 (CPV-2) emerged as a new pathogen of dogs, and the virus subsequently spread around the world in 1978 (3). However, by the end of 1980, CPV-2 was completely replaced globally in dogs by a genetic and antigenic variant termed CPV type 2a (CPV-2a) (4). We have previously shown that four amino acid changes at VP2 residues 87, 101, 300, and 305 characterize the CPV-2a variant. These four mutations map to or near the capsid surface and influence infection by altering binding to the carnivore transferrin receptor (TfR), the host cell attachment protein for these viruses (5). Additionally, these four mutations have been shown to alter antibody binding, as they cluster to a position on the capsid surface where an antigenic site overlaps with the receptor-binding site (5–8). Besides these four mutations, there are other changes seen between CPV-2 and later isolates that became globally distributed. VP2 residue 375, which was Asp in both FPV and CPV-2a, is Asn in most CPV-2 isolates. VP2 residue 426, changed from Asn to Asp and then from Asp to Glu in the so-called CPV-2b and -2c antigenic variant strains, respectively (9, 10). However, as the CPV-2b and -2c antigenic strains differ from CPV-2a at only one position (VP2 residue 426), they are now considered to be variants of CPV-2a rather than distinct subtypes, as are all of the CPVs circulating worldwide today.
One of the major biological differences between CPV-2 and CPV-2a is the ability of the latter to infect cats in vivo. This extended feline host tropism of CPV-2a was shown to be due to changes within VP2, demonstrating that subtle alterations in the capsid region could influence the tropism and host range of the virus (11, 12). Overall, the amino acid changes observed between the capsids of CPV-2 and CPV-2a altered their phenotypes by specifically conferring changes in the binding of the virus to the carnivore TfR, the antigenicity of the virus, and the pH-dependent binding of sialic acid (3, 7–11). The biological effects conferred by residues 87, 101, 300, and 305 individually, and in all combinations, have been reported in detail by Stucker et al. (5), which showed that these residues work in concert to determine the specific antigenic properties found in the CPV-2 and CPV-2a capsids and the differences in binding to the domestic dog and cat TfRs.
The rapid and global replacement of the CPV-2 strain by CPV-2a indicated a strong selective advantage in replication and/or transmissibility among dogs, indicating that the genetic/antigenic changes in CPV-2a had far-reaching biological consequences (13). The viruses that have descended from CPV-2a have broad host ranges and are ubiquitous pathogens of both domestic and wild carnivores, demonstrating that CPV-2a has become very widespread in many hosts since its emergence (12). CPV-2a may also possibly be displacing FPV-like viruses in many wild carnivore hosts, as CPV-2a was recently demonstrated to be dominant over FPV in sylvatic cycles in the United States (14).
To understand how the structural changes in the capsid have conferred an advantage, which allowed CPV-2a to outcompete completely its predecessor virus in dogs and other hosts, we solved the X-ray crystal structure of CPV-2a. Parvovirus capsids were purified by sucrose gradient ultracentrifugation as described previously (13). CPV-2a crystals were obtained at room temperature using the sitting-drop vapor diffusion method by mixing 10 mg ml−1 of the virus in a 1:1 ratio with a mother liquor solution containing 0.2 M magnesium acetate tetrahydrate, 0.1 M sodium cacodylate trihydrate (pH 6.5), and 20% (vol/vol) polyethylene glycol 8000 (PEG 8000). Crystals appeared in 12 days and were soaked for 30 to 90 s in mother liquor containing 10% PEG 400 and 20% glycerol and then flash frozen in liquid nitrogen. Data were collected at 100 K on the ADSC Quantum4 charge-coupled device (CCD) detector at beam line F1 at the Cornell High Energy Synchrotron Source (CHESS) using an oscillation range of 0.2° and a detector distance of 375 nm. The crystal diffracted to a resolution of 3.1 Å (Table 1).
TABLE 1.
Data collection and refinement statistics for the CPV-2a crystal structure
| Parameter | Value(s) for CPV-2a |
|---|---|
| Data collection statistics | |
| Space group | P42 21 2 |
| a, b, c (Å) | 453.10, 453.10, 319.02 |
| α, β, γ | 90.0, 90.0, 90.0 |
| Resolution range (Å) | 50–3.5 (3.56–3.50) |
| Rsym (%) | 21.9 (44.3) |
| I/σ (I) | 8.0 (4.4) |
| Completeness (%) | 99.9 (100.0) |
| Multiplicity | 6.7 (6.7) |
| Refinement statistics | |
| Resolution range (Å) | 50–3.5 |
| No. of reflections | 410,301 |
| Rwork/Rfree | 18.0/22.1 |
| No. of atoms | |
| Protein | 130,560 |
| Mg | 30 |
| Water | |
| B-factors (Å2) | |
| Protein | 23.6 |
| Wilson B | 43.96 |
| RMSD | |
| Bond length (Å) | 0.013 |
| Bond angle (°) | 1.54 |
| PDB code | 4QYK |
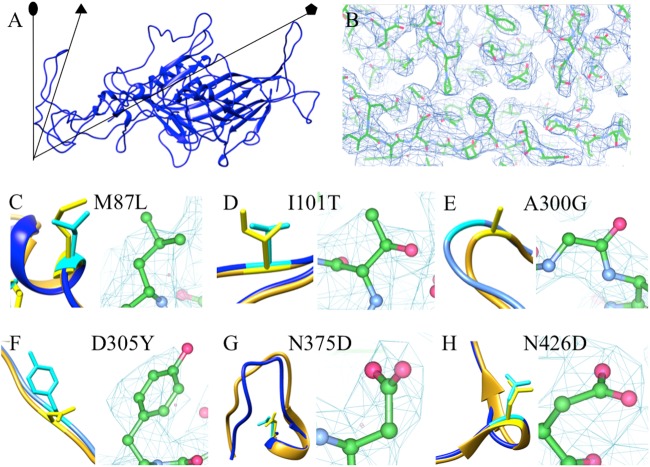
For the structure solution and refinement of the CPV-2a variant, diffraction images were indexed and integrated using MOSFLM (15). The integrated diffraction images were scaled with AIMLESS (16), and the structure of the CPV variant was solved by molecular replacement with Phaser (17), using the molecular coordinates of a previously reported CPV structure (Protein Data Bank [PDB] code 4DPV) (18) that had been mutated to the sequence identity of CPV-2a. The partial structure solution from Phaser was then subjected to iterative cycles of manual model building with Coot (19). The final structure refinement was carried out in PHENIX (20) using 6-fold noncrystallographic symmetry (NCS) averaging over the 30-capsid protein monomers, each of which is composed of 584 amino acids, covering the asymmetric unit. Final structure validation was performed manually in Coot (Fig. 1).
FIG 1.
(A) The crystal structure of a single capsid protein of CPV-2a shown as a ribbon diagram. The CPV-2a and CPV-2 (PDB code 1C8D) (25) carbon alphas superimposed with a root mean square deviation (RMSD) of 0.546 Å. Symmetry axes are indicated by black lines and symbols. (B) A representative region of the 2mFo-dFc electron density map of CPV-2a rendered at 1.0 σ shows the quality of the map with the crystal structure (green and red). (C to H) The local structure of each altered residue (cyan and yellow for CPV-2a and CPV-2, respectively) is shown with the superimposed structures of CPV-2a and CPV-2 (blue and gold, respectively) (PDB code 1C8D) (25) within the electron density to show side chain density.
The structures of CPV-2a and CPV-2 superimposed with a root mean square deviation (RMSD) of 0.55 Å, indicating high structural similarity. In addition to the four mutations in the capsid protein previously characterized, M87L, I101T, A300G, and D305Y, we also examined N375D and N426D, which were in the structure of the CPV-2a-derived strain. Each change resulted in local alterations between the capsid structures of CPV-2 and CPV-2a (Fig. 1). The most significant structural difference was seen at the Ala-to-Gly replacement of residue 300 in the GH loop, which resulted in a 3-Å movement of the polypeptide chain and the loss of a stabilizing hydrogen bond (Fig. 2). Adjacent to Gly 300 is another glycine (Gly 299), which suggests that this replacement with its lack of side chains and resultant fewer hydrogen bonds increased the flexibility of a significant surface loop of the capsid. This enhanced flexibility is reflected in the higher temperature factor seen for the CPV-2a GH loop. Single point mutations can enhance binding by changing the thermodynamic properties between two molecules without causing a detectable change in structure (21). Thus, the change at position 300 introduced increased entropy, which can influence the binding between the capsid and its major host cell receptor.
FIG 2.
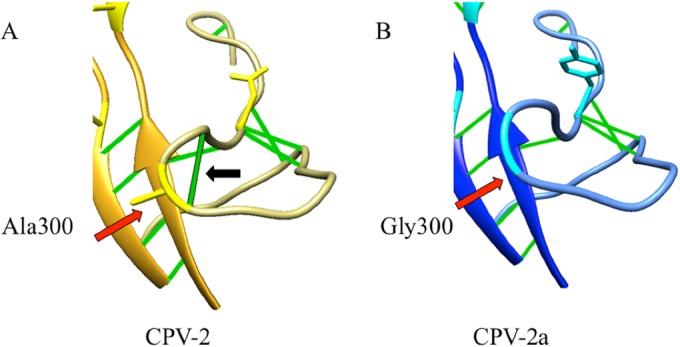
Zoomed-in view of the GH loop in the structures of CPV-2 (gold) compared to CPV-2a (blue) to show the substitution at position 300 (red arrows) from Ala (yellow in CPV-2) to Gly (cyan in CPV-2a). The ribbon diagram displays hydrogen bonds (green) and shows that the A300G change in CPV-2a results in the loss of a hydrogen bond (black arrow). The correspondingly higher B-factor in the GH loop of CPV-2a suggests enhanced flexibility.
In addition, Gly 300 is within one of the major antigenic sites (site B) on the capsid (7, 8), along with residue 305, and it is also within a region that controls host tropism through binding to the domestic dog and cat TfRs (6, 22–24). Previous studies have shown that the VP2 residue Gly 300, along with Tyr 305, make up the CPV-2a-specific antigenic epitope recognized by a number of CPV-2a-specific monoclonal antibodies, while residues Leu 87 and Thr 101 control the 5- to 20-fold-reduced level of binding of CPV-2a to domestic dog and cat cells (5).
The Asp replacement of Asn at VP2 residue 375 may have caused a slight structural alteration (Fig. 1), since there is a conformational change within the adjacent 359-375 loop, which has been described as a “flexible” loop (25). This loop is a pH-sensitive structure that directs binding to divalent ions (most likely calcium) in FPV and CPV-2 (25). In FPV, the ion density is adjacent to the flexible loop and coordinated by Asp 373 and Asp 375, as well as by the carbonyl oxygen atoms of Arg 361 and Gly 362, as previously described for the FPV structure at pH 7.5 (25). Densities corresponding to an ion, in this case presumably Mg2+ due to its presence in the crystallization solution, were observed in the density map of CPV-2a. The coordination of a Mg2+ ion likely contributed to the conformational differences between the flexible loops of CPV-2 and the CPV-2a variant.
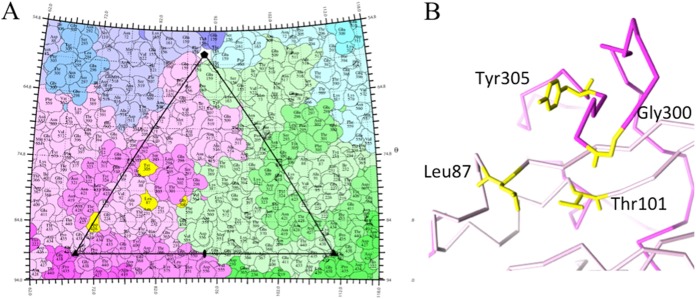
The change from Asn to Asp at residue 426 altered the characteristics of antigenic site A (in the Asp-containing CPV-2a mutants formerly referred to as CPV-2b), which prevented the binding of several monoclonal antibodies (7, 8). Although the site at position 426 conferred only insignificant main chain movement (Fig. 1), Asp introduced a negative charge that likely blocked the interaction of some of the A-site antibodies (8). Of the four surface-exposed residues that are different between CPV-2 and CPV-2a (VP2 positions 87, 300, 305, and 426; VP2 positions 101 and 375 are buried), three map to the interface between capsid proteins (Fig. 3), suggesting a contribution to capsid stability or metastability. The interlinked nature of the CPV capsid proteins allows Gly 300 and Tyr 305 of one VP2 protein to stack with Leu 87 and Thr 101 of a neighboring protein (Fig. 3), explaining how they may act in concert to alter viral properties (5).
FIG 3.
(A) Capsid protein mutations that differentiate CPV-2 and CPV-2a, including VP2 residues 87, 300, and 305 (highlighted in yellow), map to the surface of virus, which is displayed as a stereographic projection with the surface residues represented as a quilt of amino acids with one icosahedral asymmetric unit outlined by the triangle (26). The site of the VP2 426 mutation that distinguishes different variants of CPV-2a (i.e., CPV-2b and -2c) is also highlighted in yellow and is located on the top of the 3-fold spike. VP2 residue 101 is buried just beneath the surface. Individual capsid proteins are differentiated by shades of blue, magenta, and green to show the close-knit interactions that make up the capsid. (B) The stacking interaction of residues 87 and 101 (pink and yellow) with residues 300 and 305 (magenta and yellow) on a neighboring VP2 molecule is possible because the structural proteins comprising the icosahedral capsid intertwine.
In conclusion, the profound impact of the CPV-2a-associated mutations on the success of the virus in nature and its ability to globally replace CPV-2 have been conferred by minor rearrangements and subtle conformational changes. However, these small structural changes led to enhanced flexibility of the CPV-2a capsid, which influenced the host tropism, specific receptor and sialic acid binding, and antigenicity of the virus (5), and collectively resulted in the pandemic spread of CPV-2a and its ability to outcompete CPV-2 worldwide.
Protein structure accession number.
The PDB code for CPV-2a is 4QYK.
ACKNOWLEDGMENTS
Research presented here was supported by National Institutes of Health (NIH) grant R01 AI092571 to C.R.P. and S.H. This work is based upon research conducted at the Cornell High Energy Synchrotron Source (CHESS), which is supported by the National Science Foundation (NSF) and the NIH/National Institute of General Medical Sciences (NIGMS) under NSF awards DMR-1332208 and DMR-0936384, using the Macromolecular Diffraction at CHESS (MacCHESS) facility, which is supported by award GM-103485 from NIGMS, NIH. This project is funded, in part, by a grant from the Pennsylvania Department of Health using Tobacco CURE Funds. We thank the Microscopy Imaging Shared Core Facility at the Pennsylvania State University College of Medicine. A.B.A. is supported by an NRSA Fellowship (F32AI100545) from the National Institute of Allergy and Infectious Diseases, NIH. This work was made possible by an NIH SIG 1S10RR031780-01A1 award to S.H.
We thank Neela Yennawar, Katsuhiko Murakami, and Tracy Nixon for helpful scientific discussions.
REFERENCES
- 1.Barker IK, Parrish CR. 2001. Parvovirus infections, p 131–146 InWilliams ES, Barker IK (ed), Infectious diseases of wild mammals, 3rd ed Iowa State University Press, Ames, IA. [Google Scholar]
- 2.Steinel A, Parrish CR, Bloom ME, Truyen U. 2001. Parvovirus infections in wild carnivores. J Wildl Dis 37:594–607. doi: 10.7589/0090-3558-37.3.594. [DOI] [PubMed] [Google Scholar]
- 3.Parrish CR, O'Connell PH, Evermann JF, Carmichael LE. 1985. Natural variation of canine parvovirus. Science 230:1046–1048. doi: 10.1126/science.4059921. [DOI] [PubMed] [Google Scholar]
- 4.Parrish CR, Aquadro CF, Carmichael LE. 1988. Canine host range and a specific epitope map along with variant sequences in the capsid protein gene of canine parvovirus and related feline, mink, and raccoon parvoviruses. Virology 166:293–307. doi: 10.1016/0042-6822(88)90500-4. [DOI] [PubMed] [Google Scholar]
- 5.Stucker KM, Pagan I, Cifuente JO, Kaelber JT, Lillie TD, Hafenstein S, Holmes EC, Parrish CR. 2012. The role of evolutionary intermediates in the host adaptation of canine parvovirus. J Virol 86:1514–1521. doi: 10.1128/JVI.06222-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hafenstein S, Palermo LM, Kostyuchenko VA, Xiao C, Morais MC, Nelson CDS, Bowman VD, Battisti AJ, Chipman PR, Parrish CR, Rossmann MG. 2007. Asymmetric binding of transferrin receptor to parvovirus capsids. Proc Natl Acad Sci U S A 104:6585–6589. doi: 10.1073/pnas.0701574104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Strassheim ML, Gruenberg A, Veijalainen P, Sgro JY, Parrish CR. 1994. Two dominant neutralizing antigenic determinants of canine parvovirus are found on the threefold spike of the virus capsid. Virology 198:175–184. doi: 10.1006/viro.1994.1020. [DOI] [PubMed] [Google Scholar]
- 8.Hafenstein S, Bowman VD, Sun T, Nelson CD, Palermo LM, Chipman PR, Battisti AJ, Parrish CR, Rossmann MG. 2009. Structural comparison of different antibodies interacting with parvovirus capsids. J Virol 83:5556–5566. doi: 10.1128/JVI.02532-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Decaro N, Desario C, Parisi A, Martella V, Lorusso A, Miccolupo A, Mari V, Colaianni ML, Cavalli A, Di Trani L, Buonavoglia C. 2009. Genetic analysis of canine parvovirus type 2c. Virology 385:5–10. doi: 10.1016/j.virol.2008.12.016. [DOI] [PubMed] [Google Scholar]
- 10.Parrish CR. 1991. Mapping specific functions in the capsid structure of canine parvovirus and feline panleukopenia virus using infectious plasmid clones. Virology 183:195–205. doi: 10.1016/0042-6822(91)90132-U. [DOI] [PubMed] [Google Scholar]
- 11.Truyen U, Agbandje M, Parrish CR. 1994. Characterization of the feline host range and a specific epitope of feline panleukopenia virus. Virology 200:494–503. doi: 10.1006/viro.1994.1212. [DOI] [PubMed] [Google Scholar]
- 12.Truyen U, Parrish CR. 1992. Canine and feline host ranges of canine parvovirus and feline panleukopenia virus: distinct host cell tropisms of each virus in vitro and in vivo. J Virol 66:5399–5408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hueffer K, Parrish CR. 2003. Parvovirus host range, cell tropism and evolution. Curr Opin Microbiol 6:392–398. doi: 10.1016/S1369-5274(03)00083-3. [DOI] [PubMed] [Google Scholar]
- 14.Allison AB, Kohler DJ, Ortega A, Hoover EA, Grove DM, Holmes EC, Parrish CR. 2014. Host-specific parvovirus evolution in nature is recapitulated by in vitro adaptation to different carnivore species. PLoS Pathog 10:e1004475. doi: 10.1371/journal.ppat.1004475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leslie AGW, Powell HR. 2007. Processing diffraction data with MOSFLM, p 41–51 InRead RJ, Sussman JL (ed), Evolving methods for macromolecular crystallography. NATO Science Series II, vol 245 Springer, Dordrecht, The Netherlands. [Google Scholar]
- 16.Evans PR. 2011. An introduction to data reduction: space-group determination, scaling and intensity statistics. Acta Crystallogr D Biol Crystallogr 67:282–292. doi: 10.1107/S090744491003982X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCoy AJ, Grosse-Kunstleve RW, Adams PD, Winn MD, Storoni LC, Read RJ. 2007. Phaser crystallographic software. J Appl Crystallogr 40:658–674. doi: 10.1107/S0021889807021206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xie Q, Chapman MS. 1996. Canine parvovirus capsid structure, analyzed at 2.9 Å resolution. J Mol Biol 264:497–520. doi: 10.1006/jmbi.1996.0657. [DOI] [PubMed] [Google Scholar]
- 19.Emsley P, Lohkamp B, Scott WG, Cowtan K. 2010. Features and development of Coot. Acta Crystallogr D Biol Crystallogr 66:486–501. doi: 10.1107/S0907444910007493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adams PD, Afonine PV, Bunkoczi G, Chen VB, Davis IW, Echols N, Headd JJ, Hung LW, Kapral GJ, Grosse-Kunstleve RW, McCoy AJ, Moriarty NW, Oeffner R, Read RJ, Richardson DC, Richardson JS, Terwilliger TC, Zwart PH. 2010. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr D Biol Crystallogr 66:213–221. doi: 10.1107/S0907444909052925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tzeng SR, Kalodimos CG. 2009. Dynamic activation of an allosteric regulatory protein. Nature 462:368–372. doi: 10.1038/nature08560. [DOI] [PubMed] [Google Scholar]
- 22.Llamas-Saiz AL, Agbandje-McKenna M, Parker JSL, Wahid ATM, Parrish CR, Rossmann MG. 1996. Structural analysis of a mutation in canine parvovirus which controls antigenicity and host range. Virology 225:65–71. doi: 10.1006/viro.1996.0575. [DOI] [PubMed] [Google Scholar]
- 23.Palermo LM, Hafenstein SL, Parrish CR. 2006. Purified feline and canine transferrin receptors reveal complex interactions with the capsids of canine and feline parvoviruses that correspond to their host ranges. J Virol 80:8482–8492. doi: 10.1128/JVI.00683-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parker JSL, Parrish CR. 1997. Canine parvovirus host range is determined by the specific conformation of an additional region of the capsid. J Virol 71:9214–9222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simpson AA, Chandrasekar V, Hébert B, Sullivan GM, Rossmann MG, Parrish CR. 2000. Host range and variability of calcium binding by surface loops in the capsids of canine and feline parvoviruses. J Mol Biol 300:597–610. doi: 10.1006/jmbi.2000.3868. [DOI] [PubMed] [Google Scholar]
- 26.Xiao C, Rossmann MG. 2007. Interpretation of electron density with stereographic roadmap projections. J Struct Biol 158:182–187. doi: 10.1016/j.jsb.2006.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]