ABSTRACT
Human respiratory syncytial virus (RSV) is a major cause of morbidity and severe lower respiratory tract disease in the elderly and very young, with some infants developing bronchiolitis, recurrent wheezing, and asthma following infection. Previous studies in humans and animal models have shown that vaccination with formalin-inactivated RSV (FI-RSV) leads to prominent airway eosinophilic inflammation following RSV challenge; however, the roles of pulmonary eosinophilia in the antiviral response and in disease pathogenesis are inadequately understood. In vivo studies in mice with eotaxin and/or interleukin 5 (IL-5) deficiency showed that FI-RSV vaccination did not lead to enhanced pulmonary disease, where following challenge there were reduced pulmonary eosinophilia, inflammation, Th2-type cytokine responses, and altered chemokine (TARC and CCL17) responses. In contrast to wild-type mice, RSV was recovered at high titers from the lungs of eotaxin- and/or IL-5-deficient mice. Adoptive transfer of eosinophils to FI-RSV-immunized eotaxin- and IL-5-deficient (double-deficient) mice challenged with RSV was associated with potent viral clearance that was mediated at least partly through nitric oxide. These studies show that pulmonary eosinophilia has dual outcomes: one linked to RSV-induced airway inflammation and pulmonary pathology and one with innate features that contribute to a reduction in the viral load.
IMPORTANCE This study is critical to understanding the mechanisms attributable to RSV vaccine-enhanced disease. This study addresses the hypothesis that IL-5 and eotaxin are critical in pulmonary eosinophil response related to FI-RSV vaccine-enhanced disease. The findings suggest that in addition to mediating tissue pathology, eosinophils within a Th2 environment also have antiviral activity.
INTRODUCTION
Respiratory syncytial virus (RSV) is a serious lower respiratory tract infection in infants, the elderly, and the immunocompromised (1–4), resulting in >130,000 hospitalizations in the United States each year (5, 6). Children who experience acute RSV infection of the lower respiratory tract have an increased likelihood of developing childhood asthma (7). To date, there is no safe and effective RSV vaccine available. This is in part linked to the negative outcome of a 1960s clinical trial using a formalin-inactivated alum-precipitated RSV (FI-RSV) vaccine (8). Children immunized with the FI-RSV vaccine experienced more severe disease following subsequent natural exposure to RSV, with one study showing 69% of immunized children developing pneumonia compared to only 9% of children in the unimmunized control group (9). In a second study, 80% of FI-RSV-immunized infants were hospitalized and two died, compared with only 5% hospitalization and no death in the control group (10). FI-RSV vaccine-enhanced illness was clinically characterized as severe primary RSV infection, with bronchiolitis, hypoxemia, and pneumonia. However, in contrast to the neutrophilic inflammation observed during primary RSV infection, the more severe and fatal vaccine cases exhibited extensive mononuclear cell infiltration with concurrent pulmonary eosinophilia (9–11). Although it has been generally assumed that eosinophil infiltration contributed to clinical disease in the vaccinated individuals, this assumption has been difficult to formally test and conflicts with data that have emerged recently supporting an antiviral role for eosinophils (12).
Mouse models of FI-RSV vaccine-enhanced disease have shown that pulmonary eosinophilia is a hallmark of disease and is linked to prominent production of Th2 cytokines, including interleukin 4 (IL-4), IL-5, and IL-13 (13–16). IL-4 and IL-5 play major roles in pathogenesis in the mouse models of FI-RSV vaccine-enhanced disease, since interfering with the functions of these cytokines markedly decreases the severity of disease (14, 17). Studies involving immunization of mice with a recombinant vaccinia virus expressing the secreted form of RSV G protein followed by RSV challenge have also shown significant pulmonary eosinophilia accompanied by the production of IL-5 and eotaxin 1 (18).
The roles of eosinophils in the antiviral response and RSV disease pathogenesis were examined in this study. As IL-5 and eotaxin synergize in the induction of airway hyperresponsiveness (AHR), pulmonary eosinophilia has become the hallmark of RSV vaccine-enhanced disease (13, 18, 19). The results suggest that eotaxin and IL-5 are involved in regulating eosinophilic lung pathology. The findings also suggest that, in addition to their proinflammatory role in FI-RSV vaccine-enhanced disease, eosinophils can contribute to virus clearance. These findings contribute to a growing awareness of the potential for eosinophils to contribute to antiviral defense.
MATERIALS AND METHODS
Viruses.
The A2 strain of RSV was used in all experiments and propagated in Vero E6 cells as previously described (20). Viral titers were determined by 50% tissue culture infective dose (TCID50) assay. Briefly, 1 × 106 Hep-2 (human laryngeal epithelial) cells/ml were resuspended in Dulbecco's modified Eagle's medium (DMEM) (Gibco Invitrogen) with 10% fetal calf serum (FCS) (Gibco Invitrogen), and 100 μl of cell suspension was added to each well of a 96-well plate. The cells were incubated at 37°C/5% CO2 in air until 80% confluent. Virus was serially diluted across the 96-well plate of Hep-2 cells in DMEM supplemented with 0.5% penicillin-streptomycin antibiotics (Sigma). The cells and virus were left to incubate for 1.5 h to allow virus adsorption. Then, DMEM-10%FCS was added, and the plates were incubated at 37°C/5% CO2. After 5 days, the medium in the plate was aspirated, and the cells were fixed with acetone-methanol (Sigma), stained with 0.02% crystal violet, and scored for the presence of syncytia. The TCID50 was calculated using the Reed-Muench formula of viral titer and presented as log10 TCID50/ml.
Mice.
Eotaxin (Eot−/−) (21) and IL-5 (IL-5−/−) (22) gene single-knockout mice and IL-5-transgenic (IL-5Tg) (23, 24) mice were backcrossed to a BALB/c background for 10 generations; double-knockout (EotIL-5−/−) mice were generated by intercrossing BALB/c Eot−/− and IL-5−/− mice to the F2 generation (25). Transgenic mice were provided by the Gene Targeting Laboratory, The John Curtin School of Medical Research, Australian National University, Australia, and Laboratory Animal Services, University of Adelaide, Adelaide, Australia. BALB/c wild-type (WT) mice were purchased from the Animal Resource Centre, Perth, WA, Australia. All the mice were housed in the pathogen-free animal facility at the University of Canberra, Canberra, ACT, Australia. All procedures were approved by the University of Canberra Animal Ethics Committee, the Griffith University Animal Ethics Committee, and the Australian National University Animal Experimentation Ethics Committee and conducted according to the Animal Welfare Guidelines of the National Health and Medical Research Council of Australia.
In vivo infection.
Six- to 8-week-old female BALB/c WT, IL-5Tg, Eot−/−, IL-5−/−, and EotIL-5−/− mice were immunized intramuscularly in the hind limb with 0.1 ml of FI-RSV diluted 1:10 in phosphate-buffered saline (PBS) at day 0 and day 7. FI-RSV vaccine was prepared as described previously (26). Unimmunized WT, IL-5Tg, Eot−/−, IL-5−/−, and EotIL-5−/− mice were similarly injected with 0.1 ml of PBS on day 0 and day 7. On day 21, the mice were intratracheally challenged with 106 PFU of live RSV in 0.1 ml of Dulbecco's PBS (Gibco, Invitrogen). Two additional control groups, unimmunized/PBS challenged and FI-RSV immunized/PBS challenged, were also included. The mice were sacrificed by carbon dioxide asphyxiation on day 27, and lung tissue was removed for in vitro study.
RNI assay.
The reactive nitrogen intermediate (RNI) assay was performed as previously described (27). Briefly, nitrite was measured by the addition of 100 μl of Griess reagent [5% phosphoric acid, 1% sulfanilic acid, and 0.1% N(1-napthyl-7)ethylene diamine dihydrochloride] to 30 μl of control or RSV-treated eosinophil culture supernatants. Protein was precipitated and removed by adding 100 μl of 10% trichloroacetic acid before centrifugation. A volume of 200 μl of each supernatant was transferred to a 96-well flat-bottom plate, and the absorbance was read using a Bio-Rad microplate reader (Hercules, CA, USA).
RT-PCR.
RNA was extracted from eosinophils and lung tissues with TRIzol reagent (Invitrogen Life Technologies, Carlsbad, CA, USA), DNase treated using RNase-free DNase I (Qiagen, Clifton Hill, Victoria, Australia), and purified using an RNeasy minikit (Qiagen) according to the manufacturer's protocol. Reverse transcription (RT) for cDNA synthesis and PCR amplification of cytokine mRNA were performed as previously described (28). Primer sequences for the hypoxanthine guanine phosphoribosyltransferase (HPRT) and eosinophil-associated RNase (Ear1 and Ear2) housekeeping genes have been published previously (12, 29, 30). The cycle numbers used for amplification of each gene product were as follows: Ear1 and Ear2, 30 cycles, and HPRT, 25 cycles.
Real-time PCR.
RNA extraction was performed as outlined above, and samples were purified using RNase-free DNase I (Qiagen, Clifton Hill, Australia) and passed through RNeasy minikits (Qiagen). RNA quantification was performed with a NanoDrop ND-1000 spectrophotometer (Wilmington, DE, USA). Real-time PCR analysis was performed using a Rotor-Gene 3000 four-channel multiplexing system (Corbett Research, Sydney, Australia) for β-actin, IL-4, IL-5, gamma interferon (IFN-γ), RANTES/CCL5, and CCR3 as previously reported (31, 32). All primers were purchased from Geneworks. Primer sequences for IL-13 and TARC are as follows: IL-13, forward, 5′ CCC ATC CCA TCC CTA CAG AA 3′, and reverse, 5′ TGC CTC AGT TGC CCT GTG T 3′; TARC, forward, 5′ TTG TGT TCG CCT GTA GTG CAT A 3′, and reverse, 5′ CAG GAA GTT GGT GAG CTG GTA TA 3′.
BAL and cytospin.
Bronchoalveolar lavage (BAL) fluid collection and cytospin were performed as described previously (28). Briefly, BAL fluid was centrifuged at room temperature, and the cell pellets were resuspended in PBS. The cells were centrifuged onto glass slides using a cytospin (Rotofix 32 Hettich, Tuttlingen, Germany). The slides were fixed in methanol and stained with modified May-Grünwald and Giemsa stain. Differential cell counts were undertaken by light microscopy, and total BAL fluid cells were counted using a hemocytometer to determine absolute numbers of eosinophils, neutrophils, lymphocytes, and macrophages.
Histological analysis.
The lower lobes of the nonlavaged lung were fixed in 10% formalin (Sigma) and embedded in paraffin, and 3-mm sections were stained with hematoxylin and eosin (H&E) or periodic acid-Schiff (PAS) stain. An observer blinded to the treatment groups scored all the slides. Severity of inflammation was quantified on a scale of 0 to 3, with untreated control mice defined as 0 and 3 being the maximum inflammation score. For airway mucus occlusion, 0 signified no occlusion, 1 was small areas of luminal accumulation of mucus, 2 was partial occlusion of at least one small airway, and 3 was complete occlusion of at least one small airway. For epithelial thickness, 0 signified a thickness the same as that seen in control mice, 1 was 1 to 1.5 times the thickness seen in control mice, 2 was 1.5 to 2 times the thickness seen in control mice, and 3 was >2 times the thickness seen in control mice. For leukocytes around the bronchi and vessels, a score of 0 signified no leukocytes; 1 was occasional leukocytes; 2 was moderate numbers of leukocytes, including some adjacent to each other; and 3 was large areas of predominantly leukocytes (31, 32).
Adoptive transfer of eosinophils and macrophages.
Unimmunized WT and FI-RSV-immunized EotIL-5−/− mice challenged with RSV were anesthetized with Alfaxan by tail vein injection 3 days after infection. This was followed by intratracheal administration of 106 eosinophils in a 40-μl volume or of vehicle only (PBS-2% FCS). Three days after adoptive transfer of eosinophils, the mice were sacrificed, and lung tissues were obtained for determination of viral titers and RT-PCR. Adoptive transfer of macrophages (106 cells) was included as an additional control. Macrophages were isolated from uninfected mouse spleens by incubation of splenocytes with a biotin-conjugated anti-F4/80 antibody and anti-biotin microbeads (Miltenyi Biotech), followed by magnetic bead separation (Miltenyi Biotech).
Intratracheal instillation of eotaxin.
FI-RSV-immunized Eot−/− mice challenged with RSV were anesthetized with Alfaxan by tail vein injection 3 days after infection. Murine recombinant eotaxin (PeproTech; 3 μg dissolved in 20 μl vehicle, 0.1% bovine serum albumin [BSA]-PBS) or control vehicle (0.1% BSA-PBS) was instilled into the airways. Administration of cytokine via the intratracheal route is effective in the delivery of cytokine to the lung (33, 34). Three days later, the mice were sacrificed, and lung tissues were obtained for determination of viral titers and pulmonary eosinophil numbers and for RT-PCR.
Eosinophils in peripheral blood.
Whole-blood samples were collected by heart puncture and treated with the anticoagulant EDTA (Sigma). Five milliliters of blood was mixed with 95 ml of acetic acid-methylene blue for 10 min to lyse red blood cells (RBC), and total nucleated-cell counts were determined using a hemocytometer. Blood smears were stained with modified May-Grünwald and Giemsa stains, and a differential cell count was undertaken to determine the total number of peripheral blood eosinophils.
Cytokine assays.
Mouse IL-5, IFN-γ (from OptEIA and BD Biosciences), and IFN-β (PBL Biomedical Laboratories) cytokine concentrations were determined by enzyme-linked immunosorbent assay (ELISA) according to the manufacturer's instructions.
Determination of lung RSV titers.
On day 27 (6 days post-RSV challenge), the mice were sacrificed by carbon dioxide asphyxiation, and one lobe of the lung was taken for determining RSV titers. The lung tissues were homogenized and clarified by centrifugation before being serially diluted and assayed for the TCID50 as described above.
Nitric oxide synthase inhibition in vivo.
N-Methyl-l-arginine (l-NMA) (Sigma) or its inactive d-enantiomer (d-NMA) was administered (5 mg/200 μl PBS) via intraperitoneal (i.p.) injection on days 1 and 2 post-RSV infection.
Statistical analysis.
Data are presented as means and standard errors of the mean (SEM). The two-tailed unpaired Student's t test was used to determine statistical significance. Data were analyzed using StatView statistical software (version 5.0; Abacus Concepts, Berkeley, CA, USA). A P value of less than 0.05 was considered statistically significant.
RESULTS
Deficiency in IL-5 and eotaxin reduces immunohistopathology in lungs in a mouse model of FI-RSV vaccine-enhanced disease.
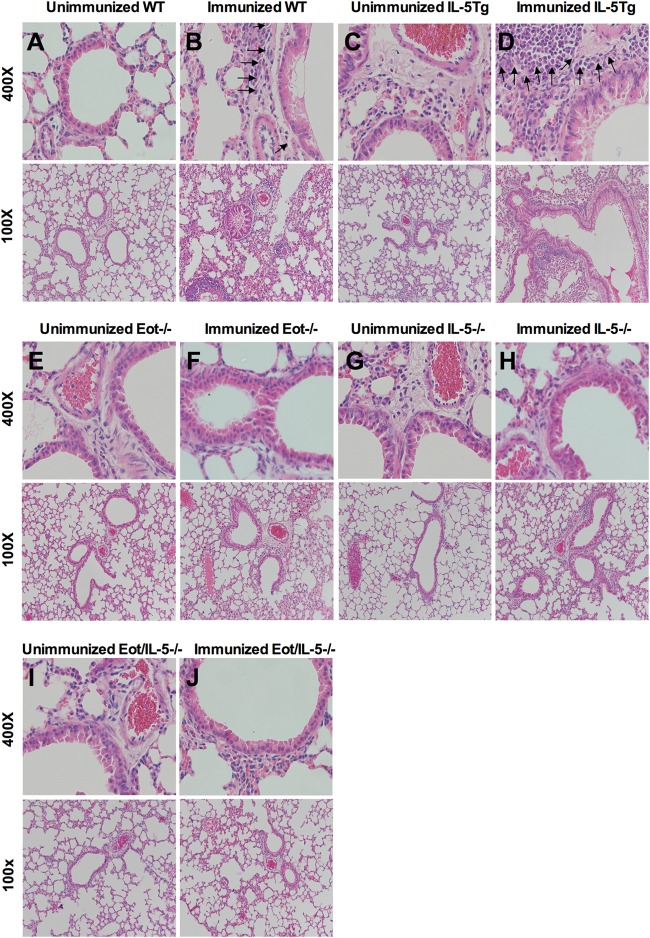
The importance of IL-5 or eotaxin in eosinophil maturation and migration was explored further using eotaxin and IL-5 knockout (Eot−/−, IL-5−/−, and EotIL-5−/−) mice and IL-5 transgenic (IL-5Tg) mice in an FI-RSV vaccine-enhanced disease model in which pulmonary eosinophilia is a hallmark of disease. FI-RSV-immunized WT and IL-5Tg mice had prominent cellular infiltration in both the perivascular and peribronchial areas of the lung at day 6 post-RSV challenge, together with considerable tissue consolidation and a reduction in air space (Fig. 1B and D). Although some cellular infiltration was observed in the lungs of Eot−/−, and IL-5−/− mice (Fig. 1F and H), the severity of inflammation was reduced compared to WT and IL-5Tg mice. EotIL-5−/− mice showed very mild airway inflammation with reduced cellular infiltration in the perivascular and peribronchial areas, as well as intact air spaces (Fig. 1J), features similar to those seen for unimmunized WT mice with subsequent RSV challenge (PBS-RSV) (Fig. 1A). The severity of inflammation was substantially reduced in unimmunized Eot−/−, IL-5−/−, and EotIL-5−/− mice with subsequent RSV challenge compared to unimmunized WT mice with subsequent RSV challenge (Fig. 1E, G, and I). Additional control groups, unimmunized and FI-RSV-immunized mice challenged with PBS, showed no inflammation (data not shown).
FIG 1.
Immunohistopathology in the lungs of WT, IL-5Tg, Eot−/−, IL-5−/−, and EotIL-5−/− mice with FI-RSV vaccine-enhanced disease. The images are representative of lungs from each of the different groups of mice. Sections were stained with H&E and are shown at ×400 and ×100 magnifications. Eosinophils are indicated by arrows. The data shown are representative of two separate experiments.
Cellular recruitment, inflammation, and mucus production are dependent on IL-5 and eotaxin.
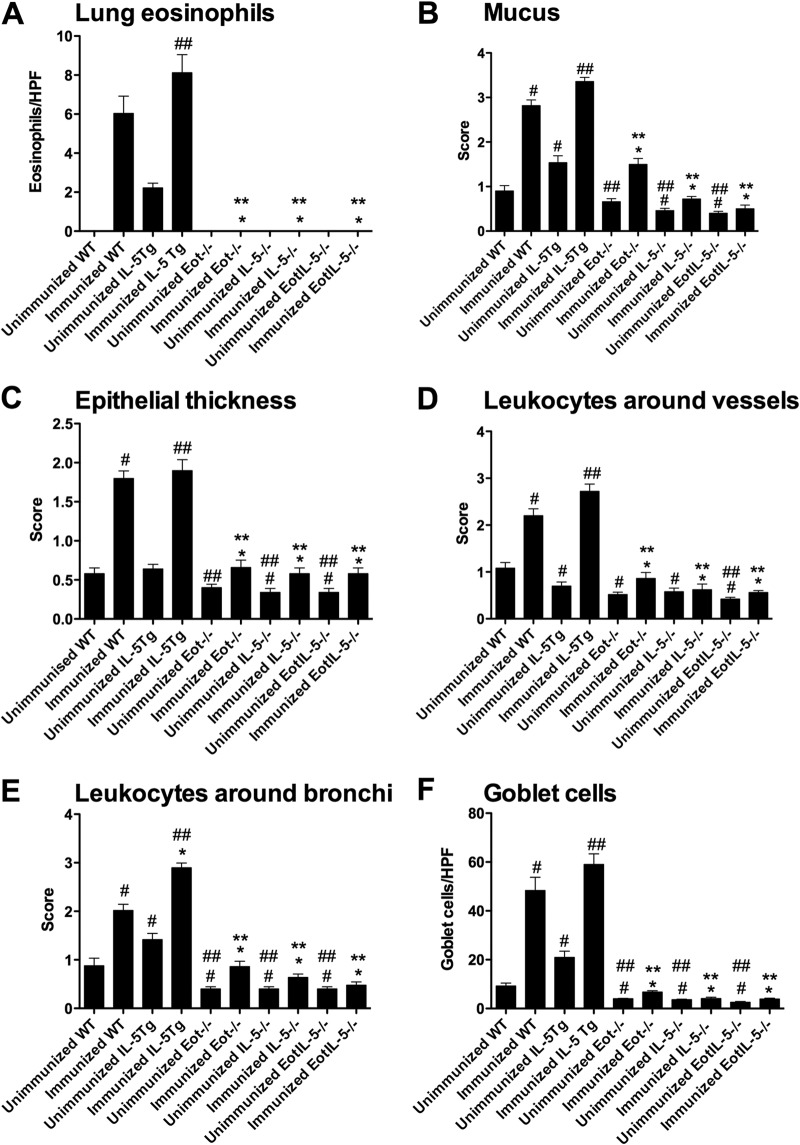
Consistent with the known roles of IL-5 and eotaxin in eosinophil recruitment (35), eosinophils were detected in the lungs of FI-RSV WT and IL-5Tg mice and unimmunized IL-5Tg mice, but not in unimmunized RSV-infected WT mice or in immunized or unimmunized Eot−/− and IL-5−/− single- and double-deficient mice (Fig. 2A).
FIG 2.
Pulmonary inflammation, mucus production, and eosinophil numbers in lungs with FI-RSV vaccine-enhanced disease. (A) Eosinophil numbers in the airway. HPF, high-power field. (B to E) Semiquantitation of histology. (F) Goblet cell numbers in the airways. Quantitation of peribronchial eosinophils and airway goblet cells was determined by the eosinophil- and PAS-positive goblet cell number per 250 mm of epithelium. Unimmunized WT, Eot−/−, IL-5−/−, EotIL-5−/−, and IL-5Tg mice challenged with RSV were included as controls. The data are shown as means and SEM; n = 6. *, P < 0.05 compared with WT FI-RSV vaccine-immunized and RSV-infected mice; **, P < 0.05 compared with IL-5Tg FI-RSV vaccine-immunized and RSV-infected mice; #, P < 0.05 compared with WT unimmunized and RSV-infected mice; ##, P < 0.05 compared with IL-5Tg unimmunized and RSV-infected mice. The data shown are representative of two separate experiments.
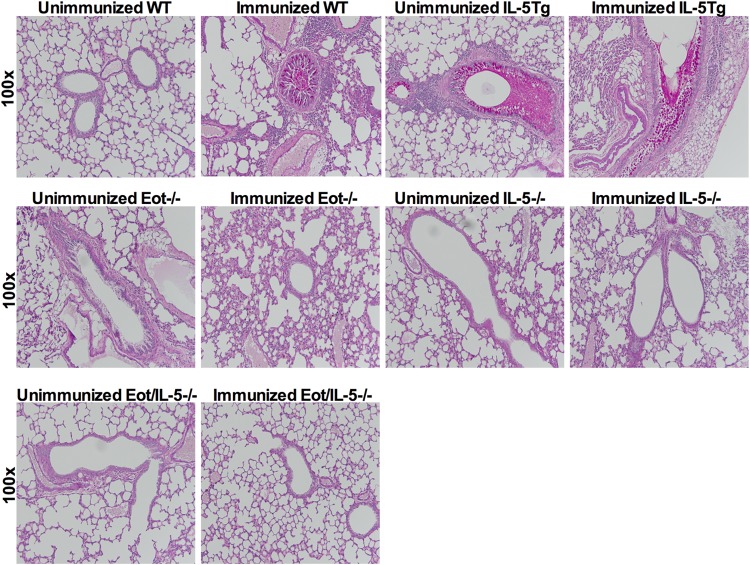
Airway mucus production, epithelial thickness, and perivascular and peribronchial leukocyte numbers were significantly reduced in Eot−/−, IL-5−/−, and EotIL-5−/− mice compared to WT and IL-5Tg mice (Fig. 2B to E). No substantial inflammation was observed in unimmunized RSV-infected WT, Eot−/−, IL-5−/−, and EotIL-5−/− mice (Fig. 2B to E). Quantification of bronchial goblet cells, as determined by counting goblet cells in six high-power fields, revealed significantly reduced numbers in the airways of FI-RSV Eot−/−, IL-5−/−, and EotIL-5−/− mice compared to FI-RSV WT and IL-5Tg mice (Fig. 2F). Examination of mucus production, determined by PAS staining, revealed substantial amounts of mucus in the airway epithelium and the airway lumen in WT and IL-5Tg mice, whereas no mucus was detected in Eot−/−, IL-5−/−, and EotIL-5−/− mice (Fig. 3). These findings suggest that IL-5 and eotaxin are important mediators of FI-RSV-induced inflammation, leukocyte recruitment, and mucus production in the lung. Unimmunized RSV-infected mice had no significant mucus levels (Fig. 3).
FIG 3.
Lung histology with PAS stain. Mucus production in the lungs of WT, IL-5Tg, Eot−/−, IL-5−/−, and EotIL-5−/− mice with FI-RSV vaccine-enhanced disease. The images are representative of lungs from each of the different groups of mice. Sections were stained with H&E and are shown at ×100 magnification. The data shown are representative of two separate experiments.
Eotaxin contributes to the migration of eosinophils from the periphery to the airway.
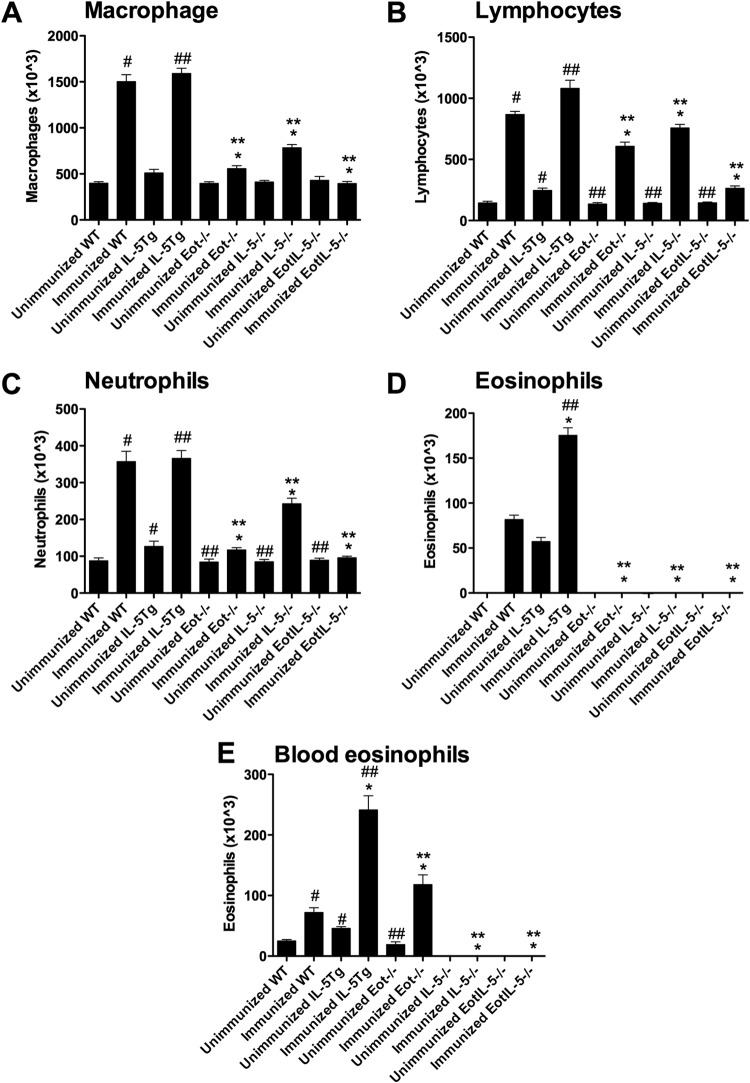
To compare the BAL cell responses in unimmunized RSV-infected mice, the BAL cellular infiltrates from FI-RSV WT, IL-5Tg, Eot−/−, IL-5−/−, and EotIL-5−/− mice were evaluated at day 6 post-RSV infection (Fig. 4A to D). The BAL fluid from WT and IL-5Tg mice predominantly consisted of macrophages (Fig. 4A) and lymphocytes (Fig. 4B), with substantial numbers of neutrophils (Fig. 4C) and eosinophils (Fig. 4D), whereas no eosinophil infiltration was observed in the lungs of Eot−/−, IL-5−/−, or EotIL-5−/− mice (Fig. 4D). The lack of eosinophil infiltration was supported by differential cell counts of the peripheral blood, in which no eosinophils were detected in the IL-5−/− or EotIL-5−/− mice (Fig. 4E). Substantially reduced numbers of macrophages, lymphocytes, and neutrophils were observed in unimmunized RSV-infected WT, Eot−/−, IL-5−/−, and EotIL-5−/− mice (Fig. 4A to C). As expected, no eosinophils were detected in the BAL fluid of unimmunized WT, Eot−/−, IL-5−/−, and EotIL-5−/− mice (Fig. 4D).
FIG 4.
Quantitation of cell subpopulations in BAL fluid and eosinophils in peripheral blood in mice with FI-RSV vaccine-enhanced disease. (A to D) Cell subpopulations in BAL fluid. (E) Eosinophil numbers in peripheral blood. Unimmunized WT, Eot−/−, IL-5−/−, EotIL-5−/−, and IL-5Tg mice challenged with RSV were included as controls. The data are shown as means and SEM; n = 6. *, P < 0.05 compared with WT FI-RSV-immunized mice challenged with RSV; **, P < 0.05 compared with IL-5Tg FI-RSV-immunized mice challenged with RSV; #, P < 0.05 compared with WT unimmunized and RSV-infected mice; ##, P < 0.05 compared with IL-5Tg unimmunized and RSV-infected mice. The data shown are representative of two separate experiments.
To determine if IL-5 and/or eotaxin expression was linked to peripheral blood eosinophilia in FI-RSV vaccine-enhanced disease, the numbers of peripheral blood eosinophils were determined in WT, IL-5Tg, Eot−/−, IL-5−/−, and EotIL-5−/− FI-RSV-immunized mice at day 6 post-RSV challenge (Fig. 4E). Approximately three times the number of eosinophils were detected in the peripheral blood of FI-RSV-immunized WT mice (7.6 × 104 cells/ml) as in unimmunized RSV-infected WT mice (2.9 × 104 cells/ml), an observation consistent with previous studies (36). No eosinophils were detected in the peripheral blood of FI-RSV-immunized IL-5−/− or EotIL-5−/− mice challenged with RSV, while substantially higher levels of circulating eosinophils were detected in FI-RSV-immunized IL-5Tg mice challenged with RSV (Fig. 4E). Interestingly, the number of eosinophils in the peripheral blood of FI-RSV-immunized Eot−/− mice challenged with RSV was significantly increased compared to FI-RSV-immunized WT mice challenged with RSV (Fig. 4E), and unimmunized Eot−/− mice challenged with RSV showed peripheral eosinophil numbers similar to those of unimmunized WT mice challenged with RSV (Fig. 4E). No eosinophils were detected in the peripheral blood of unimmunized RSV-infected IL-5−/−, and EotIL-5−/− mice (Fig. 4E). Taken together, these data reinforce the role of IL-5 as a key mediator of pulmonary eosinophilia and suggest eotaxin has a role in eosinophil migration from the blood into the airway.
Cytokine and chemokine expression is affected by eosinophil maturation and migration in FI-RSV-immunized mice challenged with RSV.
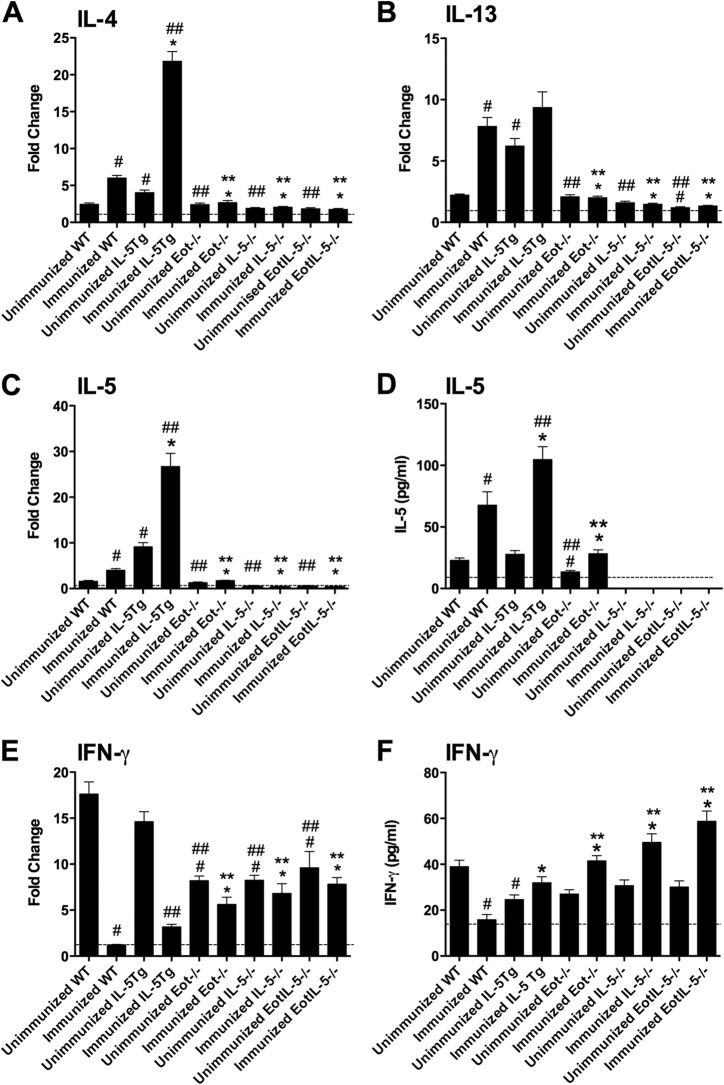
To better understand the roles of the Th2 cytokines IL-4, IL-5, and IL-13 in pulmonary eosinophilia associated with FI-RSV vaccine enhancement, mRNA expression levels were evaluated in the lung tissues of FI-RSV-immunized WT, Eot−/−, IL-5−/−, IL-5Tg, and EotIL-5−/− mice challenged with RSV and in unimmunized WT, Eot−/−, IL-5−/−, IL-5Tg, and EotIL-5−/− mice challenged with RSV only (Fig. 5). The mRNA levels of IL-4 and IL-13 were significantly reduced in FI-RSV-immunized Eot−/−, IL-5−/−, and EotIL-5−/− mice compared to FI-RSV-immunized WT and IL-5Tg mice challenged with RSV (Fig. 5A and B). No difference in IL-13 mRNA expression was detected in FI-RSV-immunized IL-5Tg mice challenged with RSV (Fig. 5B); however, expression of IL-4 mRNA was significantly increased compared to WT mice (Fig. 5A).
FIG 5.
Expression of cytokines in the lung. (A to C and E) Cytokine mRNA levels. The data are expressed as fold change, with values from control untreated (unimmunized and uninfected) WT mice defined as 1, as indicated by the dashed lines. (D and F) Cytokine protein concentrations. Cytokine mRNA levels were determined by quantitative RT-PCR, and the data were normalized with β-actin. Detection limits for the cytokines are indicated by the dashed lines. Unimmunized WT, Eot−/−, IL-5−/−, EotIL-5−/−, and IL-5Tg mice challenged with RSV were included as controls. The data are shown as means and SEM; n = 6. *, P < 0.05 compared with WT FI-RSV-immunized mice challenged with RSV; **, P < 0.05 compared with IL-5Tg FI-RSV-immunized mice challenged with RSV; #, P < 0.05 compared with WT unimmunized and RSV-infected mice; ##, P < 0.05 compared with IL-5Tg unimmunized and RSV-infected mice. The data shown are representative of two separate experiments.
The expression of IL-5 in the lung was also examined, as its expression has been correlated with the degree of pulmonary eosinophil infiltration (37). As expected, no IL-5 mRNA was detected in either the IL-5−/− or EotIL-5−/− mice, whereas a significant increase in expression was seen in immunized RSV-challenged IL-5Tg mice compared to immunized RSV-challenged WT mice (Fig. 5C). No IL-5 protein was detected in the BAL fluid of IL-5−/− or EotIL-5−/− mice, and there was a significant increase in protein in the immunized RSV-challenged IL-5Tg mice compared to the immunized RSV-challenged WT mice (Fig. 5D). IL-5 mRNA expression in the lung and IL-5 protein levels in the BAL fluid of Eot−/− mice were significantly decreased compared with WT and IL-5Tg mice (Fig. 5C and D), suggesting that IL-5 expression may also be affected by eotaxin deficiency following FI-RSV vaccine-enhanced RSV infection. IL-4 and IL-13 were detected at very low levels in unimmunized RSV-challenged Eot−/−, IL-5−/−, and EotIL-5−/− mice, while IL-5 was detected at very low levels in unimmunized RSV-challenged Eot−/− mice (Fig. 5A to D).
IFN-γ, a Th1-type cytokine that counterbalances Th2-type cytokine expression (38), was examined in FI-RSV-immunized and -challenged mouse groups. IFN-γ mRNA expression was significantly (P < 0.05) increased in the lungs of all FI-RSV-immunized knockout mice compared to FI-RSV-immunized WT and IL-5Tg mice challenged with RSV (Fig. 5E). This trend was replicated in the BAL fluid from all immunized knockout mice, showing substantially higher levels of IFN-γ protein than that of the immunized WT and IL-5Tg mice challenged with RSV (Fig. 5F). The increased IFN-γ mRNA levels correlated with a reduction in Th2-type cytokine expression in the immunized and RSV-challenged knockout mice; however, IFN-γ expression did not appear to play a substantial role in counterbalancing Th2 cytokine expression in FI-RSV-immunized IL-5Tg mice challenged with RSV. A possible explanation could be that excessive production of IL-5 in FI-RSV-immunized IL-5Tg mice challenged with RSV may induce more IFN-γ for immune homeostasis (39). At the protein level, unimmunized RSV-infected WT mice had IFN-γ levels similar to those of the unimmunized knockout mouse groups challenged with RSV (Fig. 5F).
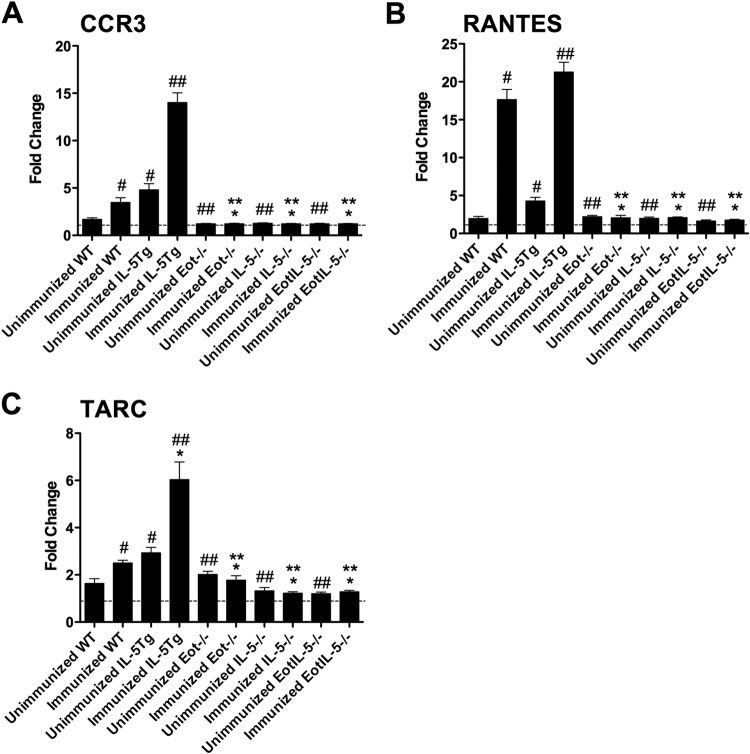
The importance of the chemokine network previously associated with FI-RSV vaccine-enhanced disease, i.e., CCR3, RANTES, and TARC (40, 41), was also examined in the context of IL-5 and/or eotaxin deficiency. CCR3 is highly expressed by eosinophils and is thought to be the major eosinophil chemokine receptor (42). The mRNA levels of CCR3 were determined in unimmunized and FI-RSV-immunized mouse groups challenged with RSV (Fig. 6A). CCR3 mRNA expression was significantly increased in FI-RSV-immunized IL-5Tg mice challenged with RSV and significantly decreased in all FI-RSV-immunized knockout mice challenged with RSV compared to FI-RSV-immunized WT mice challenged with RSV (Fig. 6A). Unimmunized RSV-challenged WT mice had levels similar to those seen in the unimmunized and FI-RSV-immunized knockout mice (Fig. 6A). The CCR3 mRNA expression profile is consistent with the prominent eosinophilia detected in the lungs and airways of FI-RSV-immunized WT and IL-5Tg mice challenged with RSV and the absence of pulmonary eosinophils in FI-RSV-immunized Eot−/−, IL-5−/−, and EotIL-5−/− mice challenged with RSV. A more substantial increase in CCR3 mRNA expression (Fig. 6A) was detected in FI-RSV-immunized IL-5Tg mice challenged with RSV than there was pulmonary eosinophilia (Fig. 4D). A substantial increase in CCR3 mRNA expression (Fig. 6A) was detected in FI-RSV-immunized IL-5Tg mice challenged with RSV, similar to the results for pulmonary eosinophilia (Fig. 4D).
FIG 6.
Expression of chemokine and chemokine receptor mRNA in the lungs of mice with FI-RSV vaccine-enhanced disease. Shown are CCR3 (A), RANTES (B), and TARC (C) mRNA levels. Cytokine mRNA levels were determined by quantitative RT-PCR, and the data were normalized with β-actin. The data are expressed as fold change, with values from control untreated (unimmunized and uninfected) WT mice defined as 1, as indicated by the dashed lines. Unimmunized WT, Eot−/−, IL-5−/−, EotIL-5−/−, and IL-5Tg mice challenged with RSV were included as controls. The data are shown as means and SEM; n = 6. *, P < 0.05 compared with WT FI-RSV-immunized mice challenged with RSV; **, P < 0.05 compared with IL-5Tg FI-RSV-immunized mice challenged with RSV; #, P < 0.05 compared with WT unimmunized and RSV-infected mice; ##, P < 0.05 compared with IL-5Tg unimmunized and RSV-infected mice. The data shown are representative of two separate experiments.
RANTES (CCL5) is a chemokine that, like eotaxin, induces eosinophil recruitment through binding and activation of CCR3 on the eosinophil surface. RANTES mRNA levels in the lung were significantly reduced in FI-RSV-immunized Eot−/−, IL-5−/−, and EotIL-5−/− mice challenged with RSV compared to FI-RSV-immunized WT and IL-5Tg mice challenged with RSV (Fig. 6B). Unimmunized RSV-infected control mice all had RANTES mRNA levels similar to those of the unimmunized and FI-RSV-immunized knockout mouse groups challenged with RSV (Fig. 6B). These results show that FI-RSV vaccine-enhanced disease is associated with the expression of RANTES through a process that requires both eotaxin and IL-5.
TARC (CCL17) regulates the recruitment of Th2 cells in allergic responses (29, 43) and can be expressed on pulmonary epithelial cells (44). In FI-RSV-immunized WT and IL-5Tg mice challenged with RSV, TARC mRNA levels in the lung were significantly higher than in FI-RSV-immunized IL-5−/−, Eot−/−, and EotIL-5−/− mice challenged with RSV (Fig. 6C). Unimmunized, RSV-infected control mice all had TARC mRNA levels similar to those of the unimmunized and FI-RSV-immunized knockout mouse groups challenged with RSV (Fig. 6C). These results suggest the possibility that CCL17/TARC plays a role in mediating Th2-driven FI-RSV vaccine-enhanced disease.
Eotaxin expression is linked to enhanced virus clearance during FI-RSV vaccine-enhanced disease.
To determine whether eosinophils contributed to virus clearance, lung RSV titers were determined in RSV-infected unimmunized and FI-RSV-immunized WT, IL-5−/−, Eot−/−, EotIL-5−/−, and IL-5Tg mice. In the unimmunized group, RSV titers in IL-5−/−, Eot−/−, and EotIL-5−/− mice were similar to those in WT mice (Fig. 7A). In addition, virus titers from RSV-challenged unimmunized IL-5Tg mice were significantly (P < 0.05) lower than those from RSV-challenged unimmunized WT mice (Fig. 7A and B). In the FI-RSV-immunized group, significantly (P < 0.05) higher lung RSV titers were detected in IL-5−/−, Eot−/−, and EotIL-5−/− mice than in WT and IL-5Tg mice (Fig. 7A). Lung virus titers in immunized RSV-challenged IL-5Tg mice, which have high eosinophil levels in the lungs, airways, and peripheral blood, were significantly lower at day 3 postinfection (Fig. 7B). At day 6 postinfection, virus titers were lower, albeit not significantly, than those of the WT mice (Fig. 7A and B). These data suggest that a reduction of eosinophils in the lungs and airways can affect viral clearance, bolstering the notion that eosinophils have an antiviral function in vaccine-enhanced RSV infection.
FIG 7.
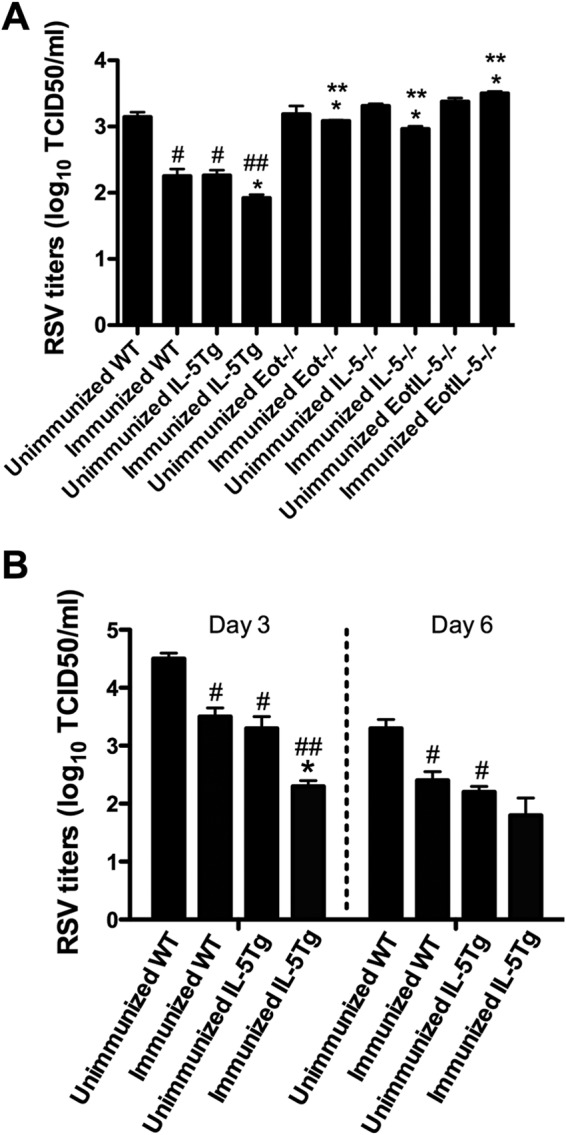
Virus titers in the lungs of mice with FI-RSV vaccine-enhanced disease. (A) RSV titers in unimmunized and immunized and RSV-infected WT, IL-5Tg, IL-5−/−, Eot−/−, and EotIL-5−/− mice at day 6 p.i. The data are shown as means and SEM; n = 3. (B) RSV titers in unimmunized and immunized RSV-infected WT and IL-5Tg mice at days 3 and 6 p.i. The data are shown as means and SEM; n = 3. *, P < 0.05 compared with WT FI-RSV vaccine-immunized and RSV-infected mice; **, P < 0.05 compared with IL-5Tg FI-RSV vaccine-immunized and RSV-infected mice; #, P < 0.05 compared with WT unimmunized and RSV-infected mice; ##, P < 0.05 compared with IL-5Tg unimmunized and RSV-infected mice. The data shown are representative of two separate experiments.
To further demonstrate the role of eosinophils in antiviral defense, we delivered recombinant eotaxin intratracheally to FI-RSV-immunized Eot−/− mice challenged with RSV. Eosinophils were detected in the lungs of immunized RSV-infected Eot−/− mice treated with recombinant eotaxin, but not in untreated, immunized RSV-infected Eot−/− mice (Fig. 8A). The presence of eosinophils was associated with lower lung virus titers (1-log-unit difference in virus titers between the two groups) and higher Ear1 and Ear2 mRNA expression levels than the untreated group (Fig. 8B and C).
FIG 8.
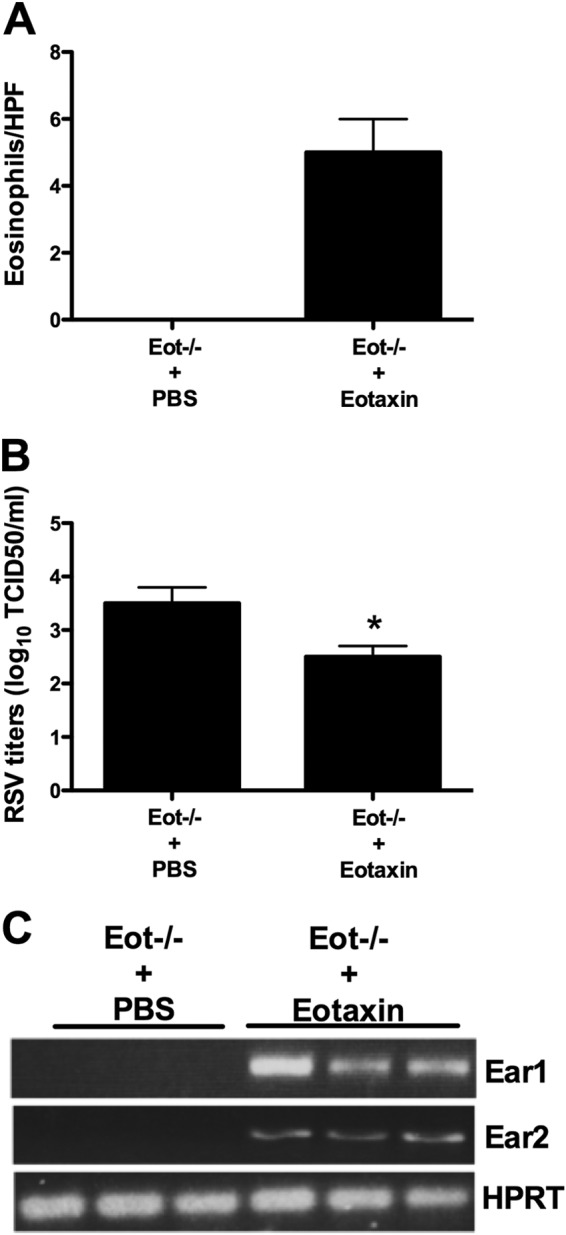
Virus titers in the lung and expression of antiviral mediators following eotaxin treatment. FI-RSV-immunized RSV-infected Eot−/− mice were intratracheally treated with recombinant eotaxin at day 3 postinfection. Control mice were treated with vehicle alone. Three days after eotaxin treatment, mice were sacrificed, and lung eosinophil numbers (A), virus titers (B), and expression of antiviral mediators (C) in the lungs were determined. The data are shown as means and SEM; n = 3. *, P < 0.05. The data shown are representative of two separate experiments.
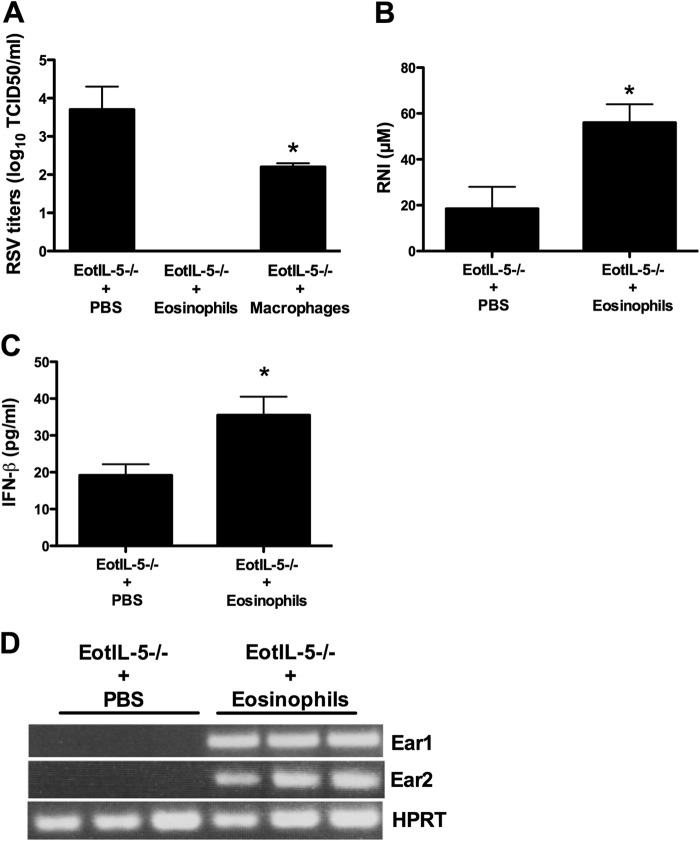
In a separate experiment, we intratracheally transferred eosinophils from IL-5Tg mice to FI-RSV-immunized RSV-infected EotIL-5−/− mice 3 days after infection. The transfer of eosinophils to EotIL-5−/− mice resulted in a reduction of lung virus titers to below the limit of detection, which contrasts with the substantial virus titers recovered from the lung in the control group (FI-RSV-immunized RSV-infected EotIL-5−/− mice without eosinophil transfer) (Fig. 9A). As an additional control, we included the transfer of macrophages in the adoptive-transfer experiments to demonstrate that the enhanced clearance is mediated by eosinophils and not due to the increase in the local concentration of immune cells. The data show that the virus titer was significantly (P < 0.05) reduced as a result of macrophage transfer compared to the control group (Fig. 9A). However, transfer of eosinophils showed complete clearance of RSV compared to the control group. In addition, expression of a number of antiviral mediators, such as RNI, IFN-β, Ear-1, and Ear-2, was markedly higher (RNI, 3-fold; IFN-β, 1.5-fold) in the lungs of immunized RSV-infected EotIL-5−/− mice that received eosinophil transfer than in immunized RSV-infected EotIL-5−/− mice without eosinophil transfer (Fig. 9B, C, and D).
FIG 9.
Virus titers in the lung and expression of antiviral mediators following eosinophil treatment. FI-RSV-immunized RSV-infected EotIL-5−/− mice were intratracheally treated with eosinophils at day 3 postinfection. Control mice were treated with medium alone. The transfer of macrophages in the adoptive-transfer experiments was included as an additional control. Three days after eosinophil treatment, the mice were sacrificed and virus titers (A) and expression of antiviral mediators (B to D) in the lungs were determined. The data are shown as means and SEM; n = 3. *, P < 0.05. The data shown are representative of two separate experiments.
Nitric oxide plays a key role in the antiviral activity of eosinophils.
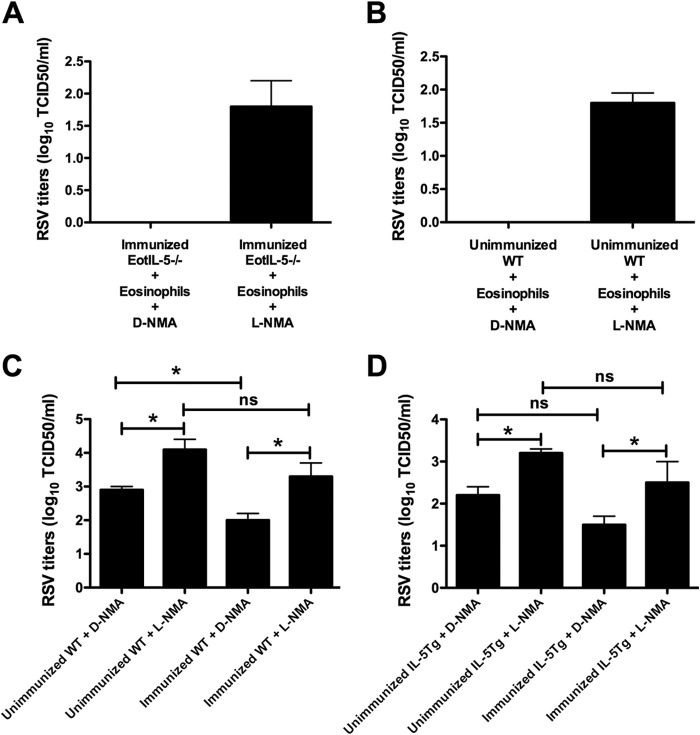
To define the potential role of eosinophil-derived NO in viral clearance observed in immunized RSV-infected EotIL-5−/− mice that received eosinophil transfer, the mice were treated with the NOS-2 inhibitor l-NMA on days 1 and 2 after eosinophil transfer. In addition, we also transferred eosinophils to unimmunized RSV-infected WT mice (an experimental group with no eosinophils) and treated them with l-NMA. Control groups were given the inactive d-enantiomer (d-NMA). l-NMA treatment substantially enhanced RSV titers in mice and partially reversed the protection conferred by eosinophil transfer (Fig. 10A and B).
FIG 10.
Role of eosinophil-derived NO in viral clearance. (A and B) Effects of treatment with l-NMA on RSV replication in immunized RSV-infected EotIL-5−/− mice treated with eosinophils (A) and unimmunized RSV-infected WT mice treated with eosinophils (B). Immunized RSV-infected EotIL-5−/− mice and unimmunized RSV-infected WT mice were intratracheally treated with eosinophils on day 3 postinfection. The mice were treated with l-NMA or d-NMA on days 1 and 2 after eosinophil treatment. Three days after eosinophil treatment, the mice were killed and virus titers in the lung were determined. (C and D) Effects of treatment with l-NMA on RSV replication in unimmunized and FI-RSV-immunized RSV-infected WT and IL-5Tg mice. The mice were treated with l-NMA or d-NMA on days 1 and 2 after infection. The data are shown as means and SEM; n = 3. *, P < 0.05; ns, not significant. The data shown are representative of two separate experiments.
In a separate experiment, we showed that RNI was detected at high levels in immunized RSV-challenged WT and IL-5Tg mice compared to unimmunized RSV-challenged mice (data not shown). To further address the role of NO in viral clearance, unimmunized and immunized RSV-infected WT mice (an experimental group exhibiting high levels of eosinophils) were treated with l-NMA or d-NMA. l-NMA treatment significantly enhanced viral titers in unimmunized and immunized RSV-infected WT and IL-5Tg mice (Fig. 10C and D). The results clearly demonstrate the importance of NO as an antiviral molecule in this model of enhanced eosinophilic disease.
DISCUSSION
FI-RSV vaccine-enhanced disease following RSV challenge has been associated with a substantial accumulation of eosinophils in the lungs of mice, and it has been proposed that this cell type is a hallmark of disease and a major contributor to disease pathology (13, 17, 45). Our research described here is highly significant because it models the disastrous early attempts at RSV vaccination that resulted in enhanced disease. In this study, we focused on the potential antiviral functions of eosinophils and the development of eosinophilic inflammation in the lung during vaccine-enhanced RSV disease. In a model of vaccine-enhanced RSV disease, the accumulation of eosinophils in the lung was markedly reduced in IL-5- and eotaxin-double-deficient mice, with a corresponding increase in the virus titer. Intratracheal treatment of Eot−/− mice with recombinant eotaxin resulted in reduced virus titers recovered from the lung. Adoptive transfer of eosinophils to IL-5- and eotaxin-double-deficient mice resulted in rapid clearance of RSV via antiviral mediators produced by the eosinophils. These results suggest dual antiviral and proinflammatory functions for eosinophils during vaccine-enhanced RSV disease.
We sought to establish a mouse model of FI-RSV vaccine-enhanced RSV disease to evaluate the antiviral activity associated with eosinophilic inflammation. In these studies, mice deficient in IL-5 and/or eotaxin were evaluated, since these molecules play critical roles in the development of eosinophilic inflammation (25, 46, 47). In addition, eotaxin and IL-5 have been previously shown to govern aspects of eosinophilic inflammation using a different model of vaccine-enhanced RSV disease involving initial sensitization with vaccinia virus expressing RSV G glycoprotein (vvG), followed by RSV challenge (18). As an additional model in which to study the importance of eosinophils, we also used IL-5Tg mice, which have high levels of circulating eosinophils and exhibit enhanced eosinophilic inflammation in some disease models (25). The prominent airway eosinophilia in vaccine-enhanced RSV disease was markedly reduced in eotaxin- and/or IL-5-deficient mice. Circulating eosinophils were largely absent in IL-5−/− mice, suggesting that the reduced eosinophilic inflammation in the lungs of these mice was due to the known effects of IL-5 on eosinophilopoiesis. In contrast, circulating eosinophil numbers were unaffected by eotaxin deficiency, and it is likely that the reduced eosinophilic inflammation in Eot−/− mice was due to impaired migration into the lung. Our results are consistent with earlier studies in mouse models of allergic airway inflammation showing that both eotaxin and IL-5 are required for eosinophil migration in the airway and periphery (28, 46, 48–50).
Th2 cytokines are increased in the FI-RSV vaccine-enhanced disease model following RSV infection (51). In this study, the expression of Th2 cytokines, IL-4, IL-5, and IL-13, were markedly reduced or absent in eotaxin- and/or IL-5-deficient mice compared to WT mice. The reduced level of Th2 cytokine expression in the lungs of eotaxin-deficient mice likely reflects impaired migration of Th2-type cells into the lung (52). Similarly, IL-4 and IL-13 were reduced in FI-RSV-vaccinated IL-5−/− mice challenged with RSV, a feature proposed to affect coordination of their regulation (53). In IL-4- and IL-5-deficient mice, abrogation of allergic airway inflammation with eosinophil infiltration and lung damage has been reported (52, 54), which is consistent with our findings. Our study reveals that IL-5 deficiency not only affects eosinophil growth and migration, but also hampers IL-4 and IL-13 production in the airway. The increased production of the Th1 cytokine IFN-γ may have an additional protective effect by counterbalancing detrimental effects of Th2 cytokines (55, 56). The chemokine RANTES is important in controlling eosinophil migration (57) and airway hyperresponsiveness in an ovalbumin-sensitized murine model (58), and the reduced RANTES expression in the lungs of eotaxin- and/or IL-5-deficient cohorts may contribute to protection from pathology in these mice. Another chemokine, TARC, has recently been reported to be highly increased in acute RSV infection (41). In our study, TARC was associated with vaccine-induced airway inflammation, since its expression was increased in IL-5Tg mice with severe eosinophilic inflammation and reduced in eotaxin- and/or IL-5-deficient mice. Similar to Th2 cytokines, RANTES is important for eosinophil migration and strongly associated with eosinophil-driven disease. Our findings and those of others suggest that blockade of RANTES may be a potential therapeutic approach for the treatment of allergy-like disease and RSV-induced airway eosinophilic inflammation (59).
Eosinophils have previously been reported to have an antiviral role during RSV infection (12, 60–62). In the vaccine-enhanced model of RSV disease, viral titers in the lung were significantly increased in IL-5−/−, Eot−/−, and EotIL-5−/− mice, all of which had markedly reduced eosinophilic inflammation in the lung. Conversely, virus titers were lower in WT and IL-5Tg mice, which featured enhanced eosinophil numbers in the lung. Transfer of eosinophils from IL-5Tg mice accelerated viral clearance in unimmunized WT and immunized EotIL-5−/− mice, which might be explained by direct production of antiviral mediators by eosinophils (e.g., RNI, ribonucleases, and IFN-β). Indeed, using l-NMA to block NO production, we demonstrated that eosinophils mediate antiviral effects in the RSV vaccine-enhanced disease model partly through NO production. A recent study showed that eotaxin 1 triggers the secretion of ribonucleases (Ears) from mouse eosinophils and their cell-free granules (63). In this regard, intratracheal delivery of recombinant eotaxin to FI-RSV-immunized Eot−/− mice challenged with RSV was associated with lower lung virus titers and higher Ear1 and Ear2 expression, and we speculate that ribonucleases might also exert antiviral activity in the vaccine-enhanced model.
Eosinophil degranulation in the lung has been documented during RSV infection (64, 65). Several studies have suggested that eosinophil degranulation can cause significant lung tissue damage (66, 67). It will be important to determine the mechanisms of eosinophil activation during RSV infection. Our own in vitro studies have indicated that eosinophils can degranulate on direct exposure to RSV virions (unpublished data), while other studies have suggested that interaction between eosinophils and infected airway epithelial cells is required for activation (68, 69).
Taken together, our findings indicate that in the vaccine-enhanced RSV disease model (a Th2-polarized inflammatory response), the influx of eosinophils and their subsequent activation in the lung have both inflammatory and antiviral activities. These findings, which have been previously unrecognized, provide mechanistic insights into the role of eosinophils in vaccine-enhanced disease. The significance of our work is further supported by a recent study by Percopo et al. showing that, while eosinophils within a Th2-polarized inflammatory response may have pathophysiologic features, they promote survival in response to a lethal infection with pneumonia virus of mice (PVM) (70). Our results support and extend these findings by showing a dual proinflammatory and antiviral role of eosinophils in a strong Th2 model of infection with the human pathogen RSV. Based on the failed early RSV vaccine clinical trials, it has been generally assumed that the eosinophilic inflammation in the lung contributed substantially to pathology. However, our studies suggest a more complex role for eosinophils in vaccine-enhanced RSV disease, with both pathological and protective components.
ACKNOWLEDGMENTS
This work was supported by Australian National Health and Medical Research Council (NHMRC) grants (399701 and 1047250) to S.M. Y.-C.S. is the recipient of an Australian NHMRC Peter Doherty Training Fellowship. S.M. is the recipient of an Australian NHMRC Senior Research Fellowship (1059167).
We thank Jürgen Schwarze (University of Edinburgh) for critical readings of the manuscript and useful discussions.
REFERENCES
- 1.Couch RB, Englund JA, Whimbey E. 1997. Respiratory viral infections in immunocompetent and immunocompromised persons. Am J Med 102:2–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ebbert JO, Limper AH. 2005. Respiratory syncytial virus pneumonitis in immunocompromised adults: clinical features and outcome. Respiration 72:263–269. doi: 10.1159/000085367. [DOI] [PubMed] [Google Scholar]
- 3.Falsey AR. 1998. Respiratory syncytial virus infection in older persons. Vaccine 16:1775–1778. doi: 10.1016/S0264-410X(98)00142-X. [DOI] [PubMed] [Google Scholar]
- 4.Falsey AR, Hennessey PA, Formica MA, Cox C, Walsh EE. 2005. Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J Med 352:1749–1759. doi: 10.1056/NEJMoa043951. [DOI] [PubMed] [Google Scholar]
- 5.Openshaw PJ. 2002. Potential therapeutic implications of new insights into respiratory syncytial virus disease. Respir Res 3(Suppl 1):S15–S20. doi: 10.1186/rr184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shay DK, Holman RC, Newman RD, Liu LL, Stout JW, Anderson LJ. 1999. Bronchiolitis-associated hospitalizations among US children, 1980–1996. JAMA 282:1440–1446. doi: 10.1001/jama.282.15.1440. [DOI] [PubMed] [Google Scholar]
- 7.Lemanske RF. 2004. Viral infections and asthma inception. J Allergy Clin Immunol 114:1023–1026. doi: 10.1016/j.jaci.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 8.Potash L, Tytell AA, Sweet BH, Machlowitz RA, Stokes J Jr, Weibel RE, Woodhour AF, Hilleman MR. 1966. Respiratory virus vaccines. I. Respiratory syncytial and parainfluenza virus vaccines. Am Rev Respir Dis 93:536–548. [DOI] [PubMed] [Google Scholar]
- 9.Kapikian AZ, Mitchell RH, Chanock RM, Shvedoff RA, Stewart CE. 1969. An epidemiologic study of altered clinical reactivity to respiratory syncytial (RS) virus infection in children previously vaccinated with an inactivated RS virus vaccine. Am J Epidemiol 89:405–421. [DOI] [PubMed] [Google Scholar]
- 10.Kim HW, Canchola JG, Brandt CD, Pyles G, Chanock RM, Jensen K, Parrott RH. 1969. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol 89:422–434. [DOI] [PubMed] [Google Scholar]
- 11.Everard ML, Swarbrick A, Wrightham M, McIntyre J, Dunkley C, James PD, Sewell HF, Milner AD. 1994. Analysis of cells obtained by bronchial lavage of infants with respiratory syncytial virus infection. Arch Dis Child 71:428–432. doi: 10.1136/adc.71.5.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Phipps S, Lam CE, Mahalingam S, Newhouse M, Ramirez R, Rosenberg HF, Foster PS, Matthaei KI. 2007. Eosinophils contribute to innate antiviral immunity and promote clearance of respiratory syncytial virus. Blood 110:1578–1586. doi: 10.1182/blood-2007-01-071340. [DOI] [PubMed] [Google Scholar]
- 13.Castilow EM, Meyerholz DK, Varga SM. 2008. IL-13 is required for eosinophil entry into the lung during respiratory syncytial virus vaccine-enhanced disease. J Immunol 180:2376–2384. doi: 10.4049/jimmunol.180.4.2376. [DOI] [PubMed] [Google Scholar]
- 14.Connors M, Giese NA, Kulkarni AB, Firestone CY, Morse HC III, Murphy BR. 1994. Enhanced pulmonary histopathology induced by respiratory syncytial virus (RSV) challenge of formalin-inactivated RSV-immunized BALB/c mice is abrogated by depletion of interleukin-4 (IL-4) and IL-10. J Virol 68:5321–5325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Connors M, Kulkarni AB, Firestone CY, Holmes KL, Morse HC III, Sotnikov AV, Murphy BR. 1992. Pulmonary histopathology induced by respiratory syncytial virus (RSV) challenge of formalin-inactivated RSV-immunized BALB/c mice is abrogated by depletion of CD4+ T cells. J Virol 66:7444–7451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Neuzil KM, Johnson JE, Tang YW, Prieels JP, Slaoui M, Gar N, Graham BS. 1997. Adjuvants influence the quantitative and qualitative immune response in BALB/c mice immunized with respiratory syncytial virus FG subunit vaccine. Vaccine 15:525–532. doi: 10.1016/S0264-410X(97)00218-1. [DOI] [PubMed] [Google Scholar]
- 17.Johnson TR, Graham BS. 1999. Secreted respiratory syncytial virus G glycoprotein induces interleukin-5 (IL-5), IL-13, and eosinophilia by an IL-4-independent mechanism. J Virol 73:8485–8495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson TR, Rothenberg ME, Graham BS. 2008. Pulmonary eosinophilia requires interleukin-5, eotaxin-1, and CD4+ T cells in mice immunized with respiratory syncytial virus G glycoprotein. J Leukoc Biol 84:748–759. doi: 10.1189/jlb.0907621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haynes LM, Jones LP, Barskey A, Anderson LJ, Tripp RA. 2003. Enhanced disease and pulmonary eosinophilia associated with formalin-inactivated respiratory syncytial virus vaccination are linked to G glycoprotein CX3C-CX3CR1 interaction and expression of substance P. J Virol 77:9831–9844. doi: 10.1128/JVI.77.18.9831-9844.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haynes LM, Tonkin J, Anderson LJ, Tripp RA. 2002. Neutralizing anti-F glycoprotein and anti-substance P antibody treatment effectively reduces infection and inflammation associated with respiratory syncytial virus infection. J Virol 76:6873–6881. doi: 10.1128/JVI.76.14.6873-6881.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rothenberg ME, MacLean JA, Pearlman E, Luster AD, Leder P. 1997. Targeted disruption of the chemokine eotaxin partially reduces antigen-induced tissue eosinophilia. J Exp Med 185:785–790. doi: 10.1084/jem.185.4.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kopf M, Brombacher F, Hodgkin PD, Ramsay AJ, Milbourne EA, Dai WJ, Ovington KS, Behm CA, Köhler G, Young IG, Matthaei KI. 1996. IL-5-deficient mice have a developmental defect in CD5+ B-1 cells and lack eosinophilia but have normal antibody and cytotoxic T cell responses. Immunity 4:15–24. doi: 10.1016/S1074-7613(00)80294-0. [DOI] [PubMed] [Google Scholar]
- 23.Dent LA. 2002. For better or worse: common determinants in immunity and inflammatory diseases, homeostasis and reproduction. J Reprod Immunol 57:255–272. doi: 10.1016/S0165-0378(02)00017-7. [DOI] [PubMed] [Google Scholar]
- 24.Dent LA, Strath M, Mellor A, Sanderson CJ. 1990. Eosinophilia in transgenic mice expressing interleukin 5. J Exp Med 172:1425–1431. doi: 10.1084/jem.172.5.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pope SM, Brandt EB, Mishra A, Hogan SP, Zimmermann N, Matthaei KI, Foster PS, Rothenberg ME. 2001. IL-13 induces eosinophil recruitment into the lung by an IL-5- and eotaxin-dependent mechanism. J Allergy Clin Immunol 108:594–601. doi: 10.1067/mai.2001.118600. [DOI] [PubMed] [Google Scholar]
- 26.Peebles RS Jr, Sheller JR, Collins RD, Jarzecka K, Mitchell DB, Graham BS. 2000. Respiratory syncytial virus (RSV)-induced airway hyperresponsiveness in allergically sensitized mice is inhibited by live RSV and exacerbated by formalin-inactivated RSV. J Infect Dis 182:671–677. doi: 10.1086/315783. [DOI] [PubMed] [Google Scholar]
- 27.Rolph MS, Mahalingam S, Cowden WB. 2004. Nonspecific antiviral immunity by formalin-fixed Coxiella burnetii is enhanced in the absence of nitric oxide. Virology 326:1–5. doi: 10.1016/j.virol.2004.04.049. [DOI] [PubMed] [Google Scholar]
- 28.Mattes J, Yang M, Mahalingam S, Kuehr J, Webb DC, Simson L, Hogan SP, Koskinen A, McKenzie AB, Dent LA, Rothenberg ME, Matthaei KI, Young IG, Foster PS. 2002. Intrinsic defect in T cell production of interleukin (IL)-13 in the absence of both IL-5 and eotaxin precludes the development of eosinophilia and airways hyperreactivity in experimental asthma. J Exp Med 195:1433–1444. doi: 10.1084/jem.20020009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Panina-Bordignon P, Papi A, Mariani M, Di Lucia P, Casoni G, Bellettato C, Buonsanti C, Miotto D, Mapp C, Villa A, Arrigoni G, Fabbri LM, Sinigaglia F. 2001. The C-C chemokine receptors CCR4 and CCR8 identify airway T cells of allergen-challenged atopic asthmatics. J Clin Invest 107:1357–1364. doi: 10.1172/JCI12655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang M, Rangasamy D, Matthaei KI, Frew AJ, Zimmermann N, Mahalingam S, Webb DC, Tremethick DJ, Thompson PJ, Hogan SP, Rothenberg ME, Cowden WB, Foster PS. 2006. Inhibition of arginase I activity by RNA interference attenuates IL-13-induced airways hyperresponsiveness. J Immunol 177:5595–5603. doi: 10.4049/jimmunol.177.8.5595. [DOI] [PubMed] [Google Scholar]
- 31.Su YC, Rolph MS, Cooley MA, Sewell WA. 2006. Cyclophosphamide augments inflammation by reducing immunosuppression in a mouse model of allergic airway disease. J Allergy Clin Immunol 117:635–641. doi: 10.1016/j.jaci.2005.10.042. [DOI] [PubMed] [Google Scholar]
- 32.Su YC, Rolph MS, Hansbro NG, Mackay CR, Sewell WA. 2008. Granulocyte-macrophage colony-stimulating factor is required for bronchial eosinophilia in a murine model of allergic airway inflammation. J Immunol 180:2600–2607. doi: 10.4049/jimmunol.180.4.2600. [DOI] [PubMed] [Google Scholar]
- 33.Yang M, Hogan SP, Mahalingam S, Pope SM, Zimmermann N, Fulkerson P, Dent LA, Young IG, Matthaei KI, Rothenberg ME, Foster PS. 2003. Eotaxin-2 and IL-5 cooperate in the lung to regulate IL-13 production and airway eosinophilia hyperreactivity. J Allergy Clin Immunol 112:935–943. doi: 10.1016/j.jaci.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 34.Sharkhuu T, Matthaei KI, Forbes E, Mahalingam S, Hogan SP, Hansbro PM, Foster PS. 2006. Mechanisms of interleukin-25 (IL-17E)-induced pulmonary inflammation and airways hyper-reactivity. Clin Exp Allergy 36:1575–1583. doi: 10.1111/j.1365-2222.2006.02595.x. [DOI] [PubMed] [Google Scholar]
- 35.Huffnagle GB, Boyd MB, Street NE, Lipscomb MF. 1998. IL-5 is required for eosinophil recruitment, crystal deposition, and mononuclear cell recruitment during a pulmonary Cryptococcus neoformans infection in genetically susceptible mice (C57BL/6). J Immunol 160:2393–2400. [PubMed] [Google Scholar]
- 36.Wang J, Palmer K, Lotvall J, Milan S, Lei XF, Matthaei KI, Gauldie J, Inman MD, Jordana M, Xing Z. 1998. Circulating, but not local lung, IL-5 is required for the development of antigen-induced airways eosinophilia. J Clin Invest 102:1132–1141. doi: 10.1172/JCI2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wardlaw AJ. 1999. Molecular basis for selective eosinophil trafficking in asthma: a multistep paradigm. J Allergy Clin Immunol 104:917–926. doi: 10.1016/S0091-6749(99)70069-2. [DOI] [PubMed] [Google Scholar]
- 38.Romagnani S. 1994. Regulation of the development of type 2 T-helper cells in allergy. Curr Opin Immunol 6:838–846. doi: 10.1016/0952-7915(94)90002-7. [DOI] [PubMed] [Google Scholar]
- 39.Kanda A, Driss V, Hornez N, Abdallah M, Roumier T, Abboud G, Legrand F, Staumont-Salle D, Queant S, Bertout J, Fleury S, Remy P, Papin JP, Julia V, Capron M, Dombrowicz D. 2009. Eosinophil-derived IFN-gamma induces airway hyperresponsiveness and lung inflammation in the absence of lymphocytes. J Allergy Clin Immunol 124:573–582. doi: 10.1016/j.jaci.2009.04.031. [DOI] [PubMed] [Google Scholar]
- 40.Culley FJ, Pennycook AM, Tregoning JS, Dodd JS, Walzl G, Wells TN, Hussell T, Openshaw PJ. 2006. Role of CCL5 (RANTES) in viral lung disease. J Virol 80:8151–8157. doi: 10.1128/JVI.00496-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Monick MM, Powers LS, Hassan I, Groskreutz D, Yarovinsky TO, Barrett CW, Castilow EM, Tifrea D, Varga SM, Hunninghake GW. 2007. Respiratory syncytial virus synergizes with Th2 cytokines to induce optimal levels of TARC/CCL17. J Immunol 179:1648–1658. doi: 10.4049/jimmunol.179.3.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ponath PD, Qin S, Post TW, Wang J, Wu L, Gerard NP, Newman W, Gerard C, Mackay CR. 1996. Molecular cloning and characterization of a human eotaxin receptor expressed selectively on eosinophils. J Exp Med 183:2437–2448. doi: 10.1084/jem.183.6.2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sekiya T, Miyamasu M, Imanishi M, Yamada H, Nakajima T, Yamaguchi M, Fujisawa T, Pawankar R, Sano Y, Ohta K, Ishii A, Morita Y, Yamamoto K, Matsushima K, Yoshie O, Hirai K. 2000. Inducible expression of a Th2-type CC chemokine thymus- and activation-regulated chemokine by human bronchial epithelial cells. J Immunol 165:2205–2213. doi: 10.4049/jimmunol.165.4.2205. [DOI] [PubMed] [Google Scholar]
- 44.Berin MC, Eckmann L, Broide DH, Kagnoff MF. 2001. Regulated production of the T helper 2-type T-cell chemoattractant TARC by human bronchial epithelial cells in vitro and in human lung xenografts. Am J Respir Cell Mol Biol 24:382–389. doi: 10.1165/ajrcmb.24.4.4360. [DOI] [PubMed] [Google Scholar]
- 45.Power UF, Huss T, Michaud V, Plotnicky-Gilquin H, Bonnefoy JY, Nguyen TN. 2001. Differential histopathology and chemokine gene expression in lung tissues following respiratory syncytial virus (RSV) challenge of formalin-inactivated RSV- or BBG2Na-immunized mice. J Virol 75:12421–12430. doi: 10.1128/JVI.75.24.12421-12430.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mould AW, Ramsay AJ, Matthaei KI, Young IG, Rothenberg ME, Foster PS. 2000. The effect of IL-5 and eotaxin expression in the lung on eosinophil trafficking and degranulation and the induction of bronchial hyperreactivity. J Immunol 164:2142–2150. doi: 10.4049/jimmunol.164.4.2142. [DOI] [PubMed] [Google Scholar]
- 47.Matthews SP, Tregoning JS, Coyle AJ, Hussell T, Openshaw PJ. 2005. Role of CCL11 in eosinophilic lung disease during respiratory syncytial virus infection. J Virol 79:2050–2057. doi: 10.1128/JVI.79.4.2050-2057.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mould AW, Matthaei KI, Young IG, Foster PS. 1997. Relationship between interleukin-5 and eotaxin in regulating blood and tissue eosinophilia in mice. J Clin Invest 99:1064–1071. doi: 10.1172/JCI119234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hogan SP, Koskinen A, Matthaei KI, Young IG, Foster PS. 1998. Interleukin-5-producing CD4+ T cells play a pivotal role in aeroallergen-induced eosinophilia, bronchial hyperreactivity, and lung damage in mice. Am J Respir Crit Care Med 157:210–218. doi: 10.1164/ajrccm.157.1.9702074. [DOI] [PubMed] [Google Scholar]
- 50.Mattes J, Foster PS. 2003. Regulation of eosinophil migration and Th2 cell function by IL-5 and eotaxin. Curr Drug Targets Inflamm Allergy 2:169–174. doi: 10.2174/1568010033484214. [DOI] [PubMed] [Google Scholar]
- 51.Johnson TR, Varga SM, Braciale TJ, Graham BS. 2004. Vbeta14(+) T cells mediate the vaccine-enhanced disease induced by immunization with respiratory syncytial virus (RSV) G glycoprotein but not with formalin-inactivated RSV. J Virol 78:8753–8760. doi: 10.1128/JVI.78.16.8753-8760.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hamelmann E, Wahn U, Gelfand EW. 1999. Role of the Th2 cytokines in the development of allergen-induced airway inflammation and hyperresponsiveness. Int Arch Allergy Immunol 118:90–94. doi: 10.1159/000024037. [DOI] [PubMed] [Google Scholar]
- 53.Kelly BL, Locksley RM. 2000. Coordinate regulation of the IL-4, IL-13, and IL-5 cytokine cluster in Th2 clones revealed by allelic expression patterns. J Immunol 165:2982–2986. doi: 10.4049/jimmunol.165.6.2982. [DOI] [PubMed] [Google Scholar]
- 54.Foster PS, Hogan SP, Ramsay AJ, Matthaei KI, Young IG. 1996. Interleukin 5 deficiency abolishes eosinophilia, airways hyperreactivity, and lung damage in a mouse asthma model. J Exp Med 183:195–201. doi: 10.1084/jem.183.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huang TJ, MacAry PA, Eynott P, Moussavi A, Daniel KC, Askenase PW, Kemeny DM, Chung KF. 2001. Allergen-specific Th1 cells counteract efferent Th2 cell-dependent bronchial hyperresponsiveness and eosinophilic inflammation partly via IFN-gamma. J Immunol 166:207–217. doi: 10.4049/jimmunol.166.1.207. [DOI] [PubMed] [Google Scholar]
- 56.Weigt H, Nassenstein C, Tschernig T, Muhlradt PF, Krug N, Braun A. 2005. Efficacy of macrophage-activating lipopeptide-2 combined with interferon-gamma in a murine asthma model. Am J Respir Crit Care Med 172:566–572. doi: 10.1164/rccm.200411-1490OC. [DOI] [PubMed] [Google Scholar]
- 57.Rot A, Krieger M, Brunner T, Bischoff SC, Schall TJ, Dahinden CA. 1992. RANTES and macrophage inflammatory protein 1 alpha induce the migration and activation of normal human eosinophil granulocytes. J Exp Med 176:1489–1495. doi: 10.1084/jem.176.6.1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Koya T, Takeda K, Kodama T, Miyahara N, Matsubara S, Balhorn A, Joetham A, Dakhama A, Gelfand EW. 2006. RANTES (CCL5) regulates airway responsiveness after repeated allergen challenge. Am J Respir Cell Mol Biol 35:147–154. doi: 10.1165/rcmb.2005-0394OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Elliott MB, Tebbey PW, Pryharski KS, Scheuer CA, Laughlin TS, Hancock GE. 2004. Inhibition of respiratory syncytial virus infection with the CC chemokine RANTES (CCL5). J Med Virol 73:300–308. doi: 10.1002/jmv.20091. [DOI] [PubMed] [Google Scholar]
- 60.Domachowske JB, Bonville CA, Dyer KD, Rosenberg HF. 1998. Evolution of antiviral activity in the ribonuclease A gene superfamily: evidence for a specific interaction between eosinophil-derived neurotoxin (EDN/RNase 2) and respiratory syncytial virus. Nucleic Acids Res 26:5327–5332. doi: 10.1093/nar/26.23.5327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Domachowske JB, Dyer KD, Bonville CA, Rosenberg HF. 1998. Recombinant human eosinophil-derived neurotoxin/RNase 2 functions as an effective antiviral agent against respiratory syncytial virus. J Infect Dis 177:1458–1464. doi: 10.1086/515322. [DOI] [PubMed] [Google Scholar]
- 62.Rosenberg HF, Dyer KD, Domachowske JB. 2009. Respiratory viruses and eosinophils: exploring the connections. Antiviral Res 83:1–9. doi: 10.1016/j.antiviral.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shamri R, Melo RC, Young KM, Bivas-Benita M, Xenakis JJ, Spencer LA, Weller PF. 2012. CCL11 elicits secretion of RNases from mouse eosinophils and their cell-free granules. FASEB J 26:2084–2093. doi: 10.1096/fj.11-200246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Garofalo R, Kimpen JL, Welliver RC, Ogra PL. 1992. Eosinophil degranulation in the respiratory tract during naturally acquired respiratory syncytial virus infection. J Pediatr 120:28–32. doi: 10.1016/S0022-3476(05)80592-X. [DOI] [PubMed] [Google Scholar]
- 65.Harrison AM, Bonville CA, Rosenberg HF, Domachowske JB. 1999. Respiratory syncytical virus-induced chemokine expression in the lower airways: eosinophil recruitment and degranulation. Am J Respir Crit Care Med 159:1918–1924. doi: 10.1164/ajrccm.159.6.9805083. [DOI] [PubMed] [Google Scholar]
- 66.Filley WV, Holley KE, Kephart GM, Gleich GJ. 1982. Identification by immunofluorescence of eosinophil granule major basic protein in lung tissues of patients with bronchial asthma. Lancet ii:11–16. [DOI] [PubMed] [Google Scholar]
- 67.Gleich GJ. 2000. Mechanisms of eosinophil-associated inflammation. J Allergy Clin Immunol 105:651–663. doi: 10.1067/mai.2000.105712. [DOI] [PubMed] [Google Scholar]
- 68.Kampen GT, Stafford S, Adachi T, Jinquan T, Quan S, Grant JA, Skov PS, Poulsen LK, Alam R. 2000. Eotaxin induces degranulation and chemotaxis of eosinophils through the activation of ERK2 and p38 mitogen-activated protein kinases. Blood 95:1911–1917. [PubMed] [Google Scholar]
- 69.Olszewska-Pazdrak B, Pazdrak K, Ogra PL, Garofalo RP. 1998. Respiratory syncytial virus-infected pulmonary epithelial cells induce eosinophil degranulation by a CD18-mediated mechanism. J Immunol 160:4889–4895. [PubMed] [Google Scholar]
- 70.Percopo CM, Dyer KD, Ochkur SI, Luo JL, Fischer ER, Lee JJ, Lee NA, Domachowske JB, Rosenberg HF. 2014. Activated mouse eosinophils protect against lethal respiratory virus infection. Blood 123:743–752. doi: 10.1182/blood-2013-05-502443. [DOI] [PMC free article] [PubMed] [Google Scholar]