ABSTRACT
Varicella-zoster virus (VZV) is a human neurotropic alphaherpesvirus and the etiological agent of varicella (chickenpox) and herpes zoster (HZ, shingles). Previously, inoculation of monkeys via the subcutaneous, intratracheal, intravenous, or oral-nasal-conjunctival routes did not recapitulate all the hallmarks of VZV infection, including varicella, immunity, latency, and reactivation. Intrabronchial inoculation of rhesus macaques (RMs) with simian varicella virus (SVV), a homolog of VZV, recapitulates virologic and immunologic hallmarks of VZV infection in humans. Given that VZV is acquired primarily via the respiratory route, we investigated whether intrabronchial inoculation of RMs with VZV would result in a robust model. Despite the lack of varicella and viral replication in either the lungs or whole blood, all four RMs generated an immune response characterized by the generation of VZV-specific antibodies and T cells. Two of 4 VZV-inoculated RMs were challenged with SVV to determine cross-protection. VZV-immune RMs displayed no varicella rash and had lower SVV viral loads and earlier and stronger humoral and cellular immune responses than controls. In contrast to the results for SVV DNA, no VZV DNA was detected in sensory ganglia at necropsy. In summary, following an abortive VZV infection, RMs developed an adaptive immune response that conferred partial protection against SVV challenge. These data suggest that a replication-incompetent VZV vaccine that does not establish latency may provide sufficient protection against VZV disease and that VZV vaccination of RMs followed by SVV challenge provides a model to evaluate new vaccines and therapeutics against VZV.
IMPORTANCE Although VZV vaccine strain Oka is attenuated, it can cause mild varicella, establish latency, and in rare cases, reactivate to cause herpes zoster (HZ). Moreover, studies suggest that the HZ vaccine (Zostavax) only confers short-lived immunity. The development of more efficacious vaccines would be facilitated by a robust animal model of VZV infection. The data presented in this report show that intrabronchial inoculation of rhesus macaques (RMs) with VZV resulted in an abortive VZV infection. Nevertheless, all animals generated a humoral and cellular immune response that conferred partial cross-protection against simian varicella virus (SVV) challenge. Additionally, VZV DNA was not detected in the sensory ganglia, suggesting that viremia might be required for the establishment of latency. Therefore, VZV vaccination of RMs followed by SVV challenge is a model that will support the development of vaccines that boost protective T cell responses against VZV.
INTRODUCTION
Varicella-zoster virus (VZV), a neurotropic alphaherpesvirus, is the etiologic agent of varicella (chickenpox) and herpes zoster (HZ, shingles). Primary VZV infection likely occurs through inhalation of virus either in respiratory droplets (1, 2) or from shedding varicella lesions (3) or through direct contact with infectious vesicular fluid (4). VZV establishes latency in sensory ganglia during primary infection, and reactivation can cause HZ, which is typically a disease of the aged and immunocompromised (5, 6). While rarely life threatening, HZ can result in several morbidities, including postherpetic neuralgia (PHN), a debilitating pain that can persist for months to years after the resolution of rash (7), chronic ocular inflammation with permanent blindness in severe cases (8), vertigo and hearing loss (9), and myelitis and focal vasculopathies (10).
Clinical observations highlight the importance of cell-mediated immune responses in controlling VZV infection and reactivation. Specifically, a lack of immunoglobulin production due to agammaglobulinemia does not complicate the outcome of varicella in children (11). In contrast, T cell deficiencies, including congenital deficiencies or those induced by HIV infection or immune suppression, lead to severe and disseminated varicella (12–16). Moreover, the administration of immunoglobulins with high titers of IgG antibodies to VZV is only protective when treatment occurs within 72 h of exposure (17–19). Similarly, a higher incidence of herpes zoster in aged patients is associated with diminished T cell proliferation to VZV antigens in vitro, while antibody titers remain stable (20). Finally, several studies investigating the correlates of protection against herpes zoster have showed an inverse correlation between cellular but not humoral immunity and disease severity (21–24). Thus, the development of a VZV vaccine that does not establish latency but elicits a protective T cell response remains a significant objective.
There are currently two FDA-approved VZV vaccines, both of which contain the live-attenuated Oka strain of VZV. Although vaccine Oka is clinically attenuated, it can cause mild varicella, establish latency, and in extremely rare cases, reactivate to cause herpes zoster (25–27). Vaccination with one dose of Varivax resulted in approximately 80% reduction of varicella incidence; however, breakthrough varicella in vaccinated children prompted the addition of a second dose to the vaccination protocol in 2006 (28–30). Since then, the incidence of chickenpox and annual varicella-related hospitalizations and deaths in the United States has decreased by 97% (31). Vaccination with Zostavax reduces the incidence of HZ by 51% and the burden of disease (including postherpetic neuralgia) by 61% (32, 33). Unfortunately, immunological analyses show that VZV-specific T cells and IgG titers decline within the first year, and reports indicate that vaccine efficacy is uncertain beyond 5 years postvaccination (34, 35). There are currently four independently manufactured commercial preparations of the Oka vaccine used by different countries. Additionally, within vaccine Oka, there is a mixture of distinct genetic haplotypes (36), and there is evidence to suggest that such differences could affect the immunogenicity of the different formulations (37–39). The assessment of opportunities for improving vaccine efficacy requires a better understanding of host-pathogen interactions during primary VZV infection, latency, and reactivation, as well as the definition of humoral and cellular correlates of protection. The availability of a robust animal model would facilitate these studies; however, these efforts have been hampered by the inability of VZV to cause varicella or HZ disease in laboratory animals in studies to date.
Studies utilizing guinea pigs have provided experimental support for VZV transmission between animals via aerosolized droplets (40, 41), the administration of varicella zoster immunoglobulin (VZIG) as a prophylactic for VZV infection (42), and the development of the varicella zoster skin test (43). A rat model has been developed in order to gain a better understanding of PHN, and the results of studies using this model suggest that viable VZV is necessary to infect axons, while viral replication is not necessary for VZV-induced pain (44, 45). The use of the SCID-hu mouse model has facilitated the evaluation of the role of several VZV genes in vivo, revealed that both CD4 and CD8 T cells support VZV replication and may play a critical role in trafficking VZV to the skin, and provided insight into VZV neurotropism (46–51). However, these animal models do not develop clinical symptoms that closely mirror VZV disease seen in humans, nor do they develop both an innate and adaptive immune response to VZV, and therefore, they do not provide a functional model to evaluate new VZV vaccines.
Several reports have documented naturally acquired varicella in apes (52–54). Moreover, intradermal inoculation of chimpanzees resulted in viremia, but the rash was limited to near the site of inoculation (55). In contrast, experimental inoculation of both New and Old World monkeys with VZV did not result in clinical disease. Specifically, VZV inoculation of marmosets (Callithrix jacchus) via the combined oral-nasal-conjunctival route resulted in seroconversion and isolation of VZV from the lungs but no varicella rash (56). Similarly, seroconversion in the absence of rash was detected in patas monkeys (Erythrocebus patas) inoculated intratracheally with VZV (57). Lastly, cynomolgus monkeys inoculated intratracheally generated a T cell and antibody response to VZV, but VZV DNA was detected in bronchoalveolar lavage (BAL) samples 4 days postinfection only, which most likely reflected the initial inoculum (58). Substantial evidence pertaining to the establishment of VZV latency has not been obtained in studies utilizing apes or nonhuman primates. Therefore, a gap exists in identifying an ideal animal model to study VZV.
Simian varicella virus (SVV), a primate alphaherpesvirus, is genetically similar to VZV (59–62). The SVV and VZV genomes share 70 to 75% DNA similarity (63) and are colinear with respect to genome organization (61). Numerous studies have confirmed antigenic relatedness between SVV and VZV (59, 64–67). We have shown that intrabronchial inoculation of Indian rhesus macaques (RMs; Macaca mulatta) with SVV reproduces the hallmarks of acute VZV infection in humans, including (i) viremia, (ii) generalized varicella, (iii) T and B cell responses, (iv) resolution of viremia and varicella, and (v) establishment of latency in only ganglionic neurons (68). Therefore, in this study, we inoculated rhesus macaques with VZV intrabronchially in order to determine whether this inoculation route would result in a model that more faithfully recapitulates the cardinal features of human infection with VZV and provide protection against challenge with SVV. Of importance, our findings demonstrate cross-protection through abortive infection.
MATERIALS AND METHODS
Animals and sample collection.
This study was carried out in strict accordance with the recommendations described in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health, the Office of Animal Welfare, and the United States Department of Agriculture. All animal work was approved by the Oregon National Primate Research Center Institutional Animal Care and Use Committee. All procedures were carried out under ketamine anesthesia in the presence of veterinary staff, and all efforts were made to minimize animal suffering. A total of 4 colony-bred Rhesus macaques of Indian origin (age 4 to 5 years) were inoculated intrabronchially with 1 × 106 PFU wild-type (WT) varicella-zoster virus (VZV) strain KMcC as previously described (68). All animals were seronegative to both VZV and SVV prior to inoculation. Whole-blood (WB) and bronchial alveolar lavage (BAL) samples were collected at intervals. Two animals were euthanized 84 days postinfection (p.i.) to evaluate the establishment of VZV latency. The two remaining animals (RM 26244 and RM 26362) were challenged intrabronchially with 4 × 105 PFU wild-type simian varicella virus (SVV) 189 days post-VZV infection, along with four additional VZV-naive control animals, and were euthanized 56 days post-SVV challenge. Cells from BAL fluid were pelleted and resuspended in RPMI supplemented with 10% fetal bovine serum (FBS) and streptomycin-penicillin and l-glutamine (PSG). Peripheral blood mononuclear cells (PBMC) were isolated by centrifugation over a density gradient cell separation medium (Corning, Manassas, VA) according to the manufacturer's recommendation. Cells were stored in FetalPlex (Gemini Bio Products, West Sacramento, CA) supplemented with 10% dimethyl sulfoxide (DMSO) and cryopreserved in liquid nitrogen. Intact sensory ganglia, including trigeminal ganglia (TG) and cervical, thoracic, and lumbar-sacral dorsal root ganglia (DRG-C, DRG-T, and DRG-L/S, respectively), were divided, flash frozen, and stored at −80°C until analysis.
Cells and virus.
Wild-type VZV strain KMcC (passage 8 in WI-38 and MRC-5 cells) (69) was propagated in MRC-5 cells at 35°C in minimal essential medium (MEM) supplemented with 10% FBS and PSG. VZV-infected MRC-5 cells were frozen in FBS with 10% DMSO, stored in liquid nitrogen (LN2), and assayed by plaque assay in MRC-5 cells. Wild-type SVV was propagated in primary rhesus fibroblasts (RF) at 37°C in Dulbecco modified Eagle medium (DMEM) supplemented with 10% FBS and PSG. SVV-infected RF were frozen in FetalPlex with 10% DMSO, stored in liquid nitrogen (LN2), and assayed by plaque assay in RFs.
DNA extraction and quantitative PCR.
DNA was extracted from whole blood, BAL cells, and sensory ganglia using the ArchivePure DNA cell/tissue kit (5 Prime, Gaithersburg, MD). VZV and SVV DNA viral loads were determined by real-time PCR using Maxima probe/ROX qPCR master mix (2×) (Fermentas, Glen Burnie, MD) and primers/TaqMan probe specific for VZV open reading frame (ORF) 21 and SVV ORF 21, respectively. Following an initial step of 10 min at 95°C, 40 cycles of 15 s at 95°C and 1 min at 60°C were completed, using a StepOnePlus instrument (Life Technologies, Carlsbad, CA). VZV (70) and SVV (71) bacterial artificial chromosome (BAC) DNAs were used as quantification standards.
Lymphocyte proliferation.
PBMC and cells from BAL fluid were surface stained with antibodies against CD8β (Beckman Coulter, Brea, CA), CD4 (eBioscience, San Diego, CA), and CD28 and CD95 (BioLegend, San Diego, CA) to delineate the naive (CD28+ CD95−), central memory (CM) (CD28+ CD95+), and effector memory (EM) (CD28− CD95+) T cell subsets and with antibodies against CD20 (Beckman Coulter), IgD (Southern Biotech, Birmingham, AL), and CD27 (BioLegend) to delineate naive (IgD+ CD27−), marginal-zone-like (MZ-like) (IgD+ CD27+), memory (IgD− CD27+), and double-negative (DN) (IgD− CD27−) B cell subsets. Cells were then fixed and permeabilized (BioLegend) according to manufacturer recommendations before the addition of a Ki67-specific antibody (BD Pharmingen, San Jose, CA). Samples were analyzed using the LSRII instrument (Becton, Dickinson, and Company, San Jose, CA) and FlowJo software (TreeStar, Ashland, OR).
Intracellular cytokine staining.
PBMC and cells from BAL fluid (1 × 106 to 2 × 106 cells per well) were stimulated with either VZV (ORFs 4, 31, 62, 63, and 67) or SVV (ORFs 4, 11, 16, 31, and 37) overlapping peptide libraries (1 μg per well), VZV or SVV viral lysate (1 μg per well), phorbol myristate acetate (PMA)-ionomycin (1 μg/ml), or DMSO (1 μg/ml) for 1 h, followed by the addition of brefeldin A (Sigma) for an additional 14 h. Peptides 18 amino acids in length and overlapped by 10 amino acids were provided with a guaranteed purity range of between 65 and 75% (Sigma-Aldrich, St. Louis, MO). Lyophilized peptides were reconstituted at a concentration of 10 mg/ml in sterile endotoxin-free DMSO and stored at −80°C. Peptide pools for each ORF were prepared for VZV and SVV by combining all peptides at 1 μg/ml final concentration. VZV and SVV cell lysates were obtained by centrifugation and sonication of infected cells. After incubation, cells were stained with antibodies against CD8β and CD4, fixed, and permeabilized as described above before the addition of antibodies directed against gamma interferon (IFN-γ) (eBioscience). Samples were analyzed using the LSRII instrument and FlowJo software.
ELISA.
Enzyme-linked immunosorbent assay (ELISA) plates were coated with VZV or SVV cell lysate overnight at 4°C, washed, and incubated with heat-inactivated (56°C for 30 min) plasma samples in 3-fold dilutions in triplicate for 1 h. For antibody titers against VZV glycoprotein E (gE), nickel plates were coated with His tag-purified gE (generous gift from Merck Sharp and Dohme Corp.). After washing, horseradish peroxidase (HRP)-conjugated anti-rhesus IgG (Fitzgerald Industries, Acton, MA) was added for 1 h, the plates were washed, and o-phenylenediamine (OPD) substrate (Sigma) was added. The reaction was stopped with the addition of 1 M HCl after 20 min, and the plates were read on an Infinite F50 plate reader (Tecan, San Jose, CA) at 490 nm. All washes were done in triplicate with 0.05% Tween–phosphate-buffered saline (PBS). IgG endpoint titers were calculated using log-log transformation of the linear portion of the curve and 0.1 optical density (OD) units as the cutoff. The IgG titers were standardized using a positive-control sample that was included on every ELISA plate.
Plaque reduction assay.
Serial 2-fold dilutions of heat-inactivated RM plasma collected prior to VZV inoculation and at 84 days p.i. were incubated with approximately 100 PFU of VZV strain KMcC for 30 min at 37°C. Virus-plasma samples were then added in triplicate to MRC-5 cell monolayers and incubated for 4 days at 37°C. Monolayers were fixed with methanol and stained with crystal violet to visualize the plaques. The neutralization percentage was calculated based on the reduction in the number of plaques compared to the number in wells that did not receive plasma.
Cytokine analysis.
Plasma and BAL specimen supernatant stored at −80°C were thawed and diluted 1:2 in serum matrix for analysis in duplicate with the cytokine monkey magnetic 28-plex panel for the Luminex platform according to the manufacturer's instructions (Life Technologies). The concentrations of interleukin-1RA (IL-1RA), I-TAC, basic fibroblast growth factor (FGF-basic), granulocyte colony-stimulating factor (G-CSF), IL-15, eotaxin, monokine induced by IFN-γ (MIG), IL-6, IL-10, IL-12, macrophage inflammatory protein 1α (MIP-1α), IL-17, IL-8, epidermal growth factor (EGF), hepatocyte growth factor (HGF), vascular endothelial growth factor (VEGF), granulocyte-macrophage colony-stimulating factor (GM-CSF), tumor necrosis factor alpha (TNF-α), IL-1β, IL-2, IL-4, IL-5, RANTES (regulated upon activation normal T cell expressed and presumably secreted), MIP-1β, monocyte chemoattractant protein 1 (MCP-1), IFN-γ, macrophage-derived chemokine (MDC), and migration inhibition factor (MIF) were measured on a Magpix instrument (Luminex, Austin, TX).
Statistical analysis.
Statistical analysis and graphing were conducted with GraphPad Prism software (GraphPad Software, Inc., La Jolla, CA). Significance values for the results shown in Fig. 1 to 3 utilized one-way repeated-measures analysis of variance (ANOVA) with Dunnett's multiple comparison posttest to explore differences between values at different days postinfection and baseline (day 0). The significance values for the results shown in Fig. 4 to 7 utilized two-way repeated-measures ANOVA with Bonferroni posttest to evaluate differences between results for VZV-exposed and VZV-naive rhesus macaques. Significance for the area under the curve results shown in Fig. 4 was determined by Student's t-test with two-sample equal-variance two-tailed distribution.
FIG 1.
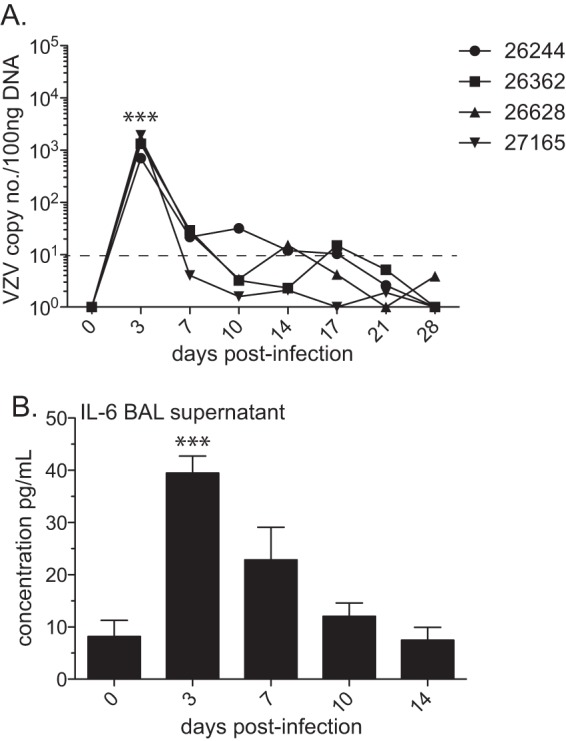
Intrabronchial infection results in a lack of VZV replication and limited inflammatory cytokine production in the lungs of rhesus macaques. (A) VZV genomic DNA in cells from bronchoalveolar lavage (BAL) fluid was measured by quantitative PCR using primers and probe specific for VZV ORF 21. Dashed line indicates limit of detection. (B) Measurement of cytokine levels in BAL specimen supernatant was performed with a multiplex Luminex assay. The results shown are the average values ± standard errors of the means (SEM). Statistical analysis was performed using one-way repeated-measures ANOVA with Dunnett's posttest to assess differences between preinfection and postinfection values (***, P < 0.001).
FIG 3.
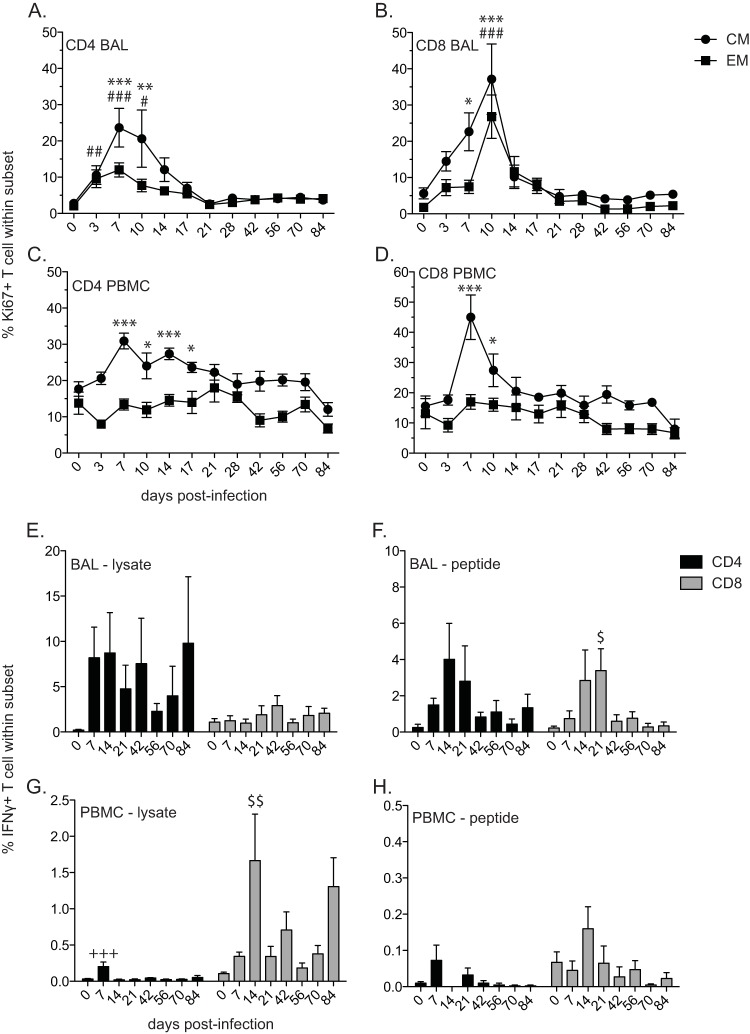
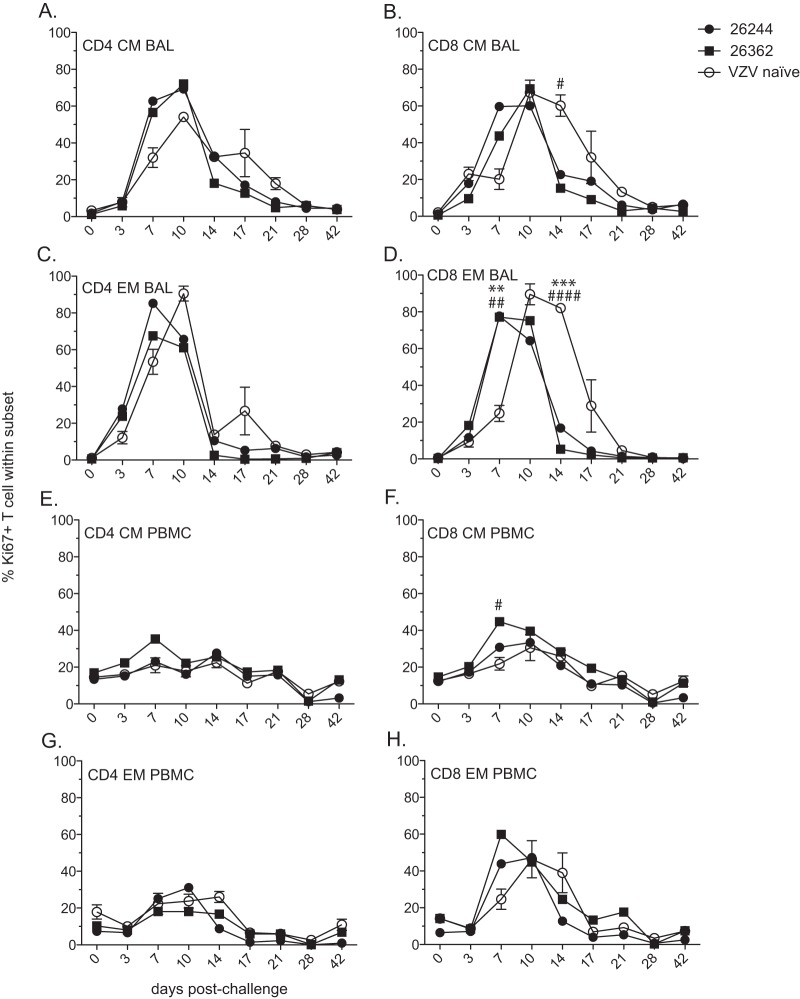
VZV-infected rhesus macaques generate a T cell response. The frequencies of proliferating central memory (CM) and effector memory (EM) CD4 (A, C) and CD8 (B, D) T cell subsets were measured by flow cytometry based on the expression of Ki67 in BAL samples (A, B) and PBMC (C, D). The frequencies of VZV-specific CD4 and CD8 T cells producing IFN-γ in BAL samples (E, F) and PBMC (G, H) were measured by intracellular cytokine staining following stimulation with VZV lysate (E, G) or VZV peptides (F, H). The results shown are the average values ± SEM. Statistical analysis was performed using one-way repeated-measures ANOVA with Dunnett's posttest to assess differences between preinfection and postinfection values (P < 0.05: *, CM; #, EM; $, CD8; P < 0.01: **, CM; ##, EM; $$, CD8; P < 0.001, ***, CM; ###, EM; +++, CD4).
FIG 4.
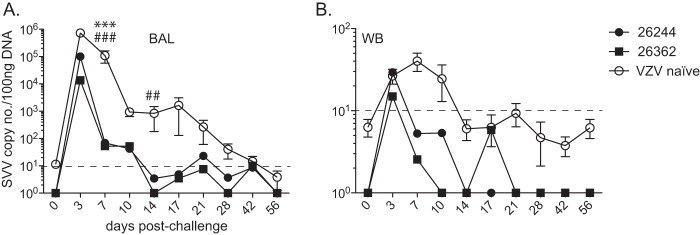
Previous exposure to VZV provides protection against SVV challenge. SVV genomic DNA in bronchoalveolar lavage (BAL) cells (A) and whole blood (WB) (B) from RM 26244, RM 26362, and VZV-naive RMs was measured by quantitative PCR using primers and probe specific for SVV ORF 21. Results for VZV-naive RMs are the average values ± SEM. Dashed line indicates limit of detection. Statistical analysis was performed using two-way repeated-measures ANOVA with Bonferroni posttest to assess differences between VZV-immune RMs and VZV-naive RMs (P < 0.01: ##, RM 26362; P < 0.001: ***, RM 26244; ###, RM 26362).
FIG 7.
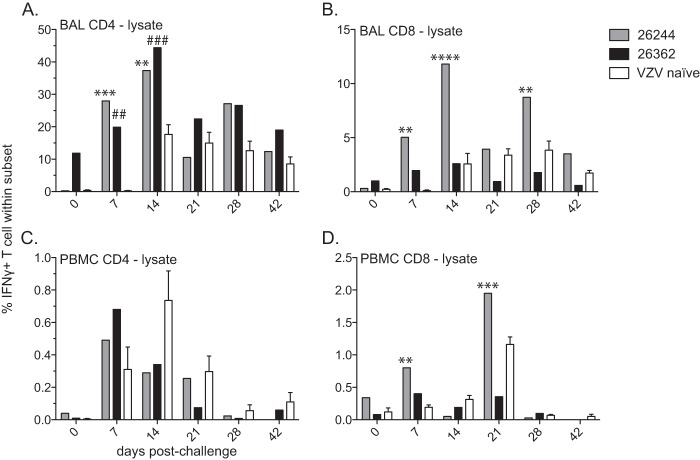
Exposure to VZV prior to SVV challenge increases the frequency of SVV-specific T cells in the lungs. The frequencies of SVV-specific CD4 and CD8 T cells producing IFN-γ in BAL samples (A, B) and PBMC (C, D) from RM 26244, RM 26362, and VZV-naive RMs were measured by intracellular cytokine staining following stimulation with SVV lysate. Results for VZV-naive RMs are the average values ± SEM. Statistical analysis was performed using two-way repeated-measures ANOVA with Bonferroni posttest to assess differences between VZV-immune RMs and VZV-naive RMs (P < 0.01: **, RM 26244; ##, RM 26362; P < 0.001: ***, RM 26244; ###, RM 26362; P < 0.0001: ****, RM 26244).
RESULTS
Intrabronchial inoculation of rhesus macaques (RMs) with VZV does not result in viral replication and engenders minimal cytokine production.
In order to establish a robust animal model of varicella-zoster virus (VZV) infection, we chose the previously characterized wild-type (WT) strain KMcC at a low passage number (56, 69, 72, 73). Four male rhesus macaques 4 to 5 years of age were infected intrabronchially with 1 × 106 PFU of cell-associated WT VZV KMcC. VZV viral loads were determined in whole blood (WB) and cells from bronchoalveolar lavage (BAL) fluid by quantitative real-time PCR (qPCR) using primers and probe specific for VZV ORF 21. Varicella rash was not observed, nor did we detect VZV DNA in WB (data not shown). In BAL cells, we were able to detect between 7 × 102 and 2 × 103 copies of VZV DNA per 100 nanograms of DNA at 3 days postinfection (p.i.), most likely a reflection of the input inoculum (Fig. 1A).
In order to characterize the host response to VZV infection in rhesus macaques, we measured multiple cytokines, chemokines, and growth factors in BAL specimen supernatant and in plasma. In BAL specimen supernatant, only the proinflammatory cytokine IL-6, secreted by T cells and macrophages to stimulate an immune response during infection (reviewed in reference 74), showed a significant increase over the baseline level (day 0) on day 3 postinfection (P < 0.001) before returning to baseline levels at 14 days p.i. (Fig. 1B). In plasma, we did not find significant changes in the concentrations of cytokines/chemokines/growth factors over baseline levels following VZV infection (data not shown).
VZV inoculation induces a B cell response in both the lungs and peripheral blood.
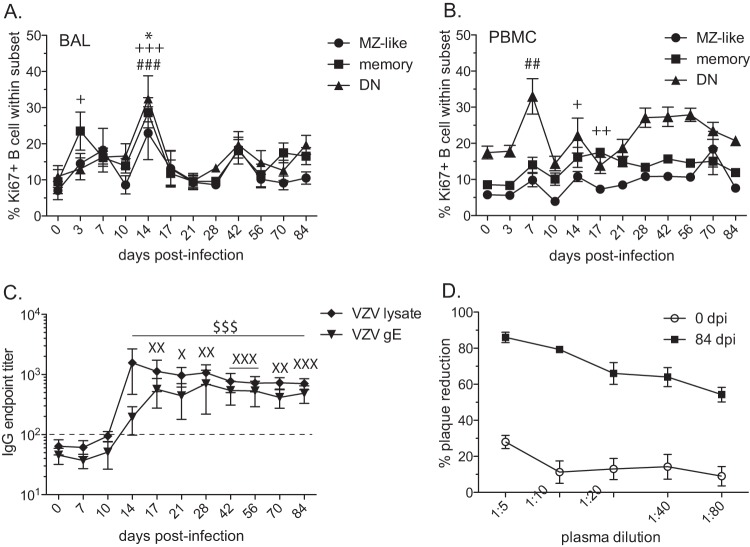
To continue our characterization of the immune response in RMs infected intrabronchially with VZV, we determined the kinetics and magnitude of B cell proliferation in response to infection using flow cytometry. Specifically, we measured the expression of Ki67, a nuclear protein involved in DNA replication (75) in marginal zone-like (MZ-like) (IgD+ CD27+), memory (IgD− CD27+), and double-negative (DN) (IgD− CD27−) B cell subsets (76) in PBMC and cells from BAL fluid. To mediate protective immunity and develop immunological memory, the proliferation and clonal expansion of antigen-specific lymphocytes are critical. Although certain Ki67-positive cells may not be antigen-specific but undergo bystander activation/proliferation, determining changes in the kinetics and magnitude of Ki67 expression provides insight into the quality and quantity of the immune response to infection (77), vaccination (78), and cancer (79). The proliferation of all B cell subsets in BAL samples peaked on day 14 postinfection (MZ-like, P < 0.05; memory, P < 0.001; and DN, P < 0.001) (Fig. 2A). The proliferation of DN B cells in PBMC peaked at 7 days p.i. (P < 0.01), while the peak proliferation of memory B cells occurred at 17 days p.i. (P < 0.01) (Fig. 2B). No significant proliferation of MZ-like B cells was measured in PBMC of RMs infected with VZV.
FIG 2.
VZV-infected rhesus macaques develop VZV-specific B cell responses. (A, B) Percentages of proliferating marginal zone-like (MZ-like), memory (M), and double-negative (DN) B cells in BAL samples (A) and PBMC (B) were measured by flow cytometry based on the expression of Ki67. Ki67+, Ki76 positive. (C) Endpoint titers of VZV-specific IgG antibody to VZV lysate or VZV gE were measured by standard ELISA. (D) Neutralizing potentials of anti-VZV antibodies were assessed using plaque reduction assay on days 0 and 84 postinfection. Dashed line indicates background level. The results shown are the average values ± SEM. Statistical analysis was performed using one-way repeated-measures ANOVA with Dunnett's posttest to assess differences between preinfection and postinfection values (P < 0.05: *, MZ-like; +, memory; X, VZV gE; P < 0.01: ++, memory; ##, DN; XX, VZV gE; P < 0.001: +++, memory; ###, DN; XXX, VZV gE; $$$, VZV lysate).
We measured the endpoint titers in plasma of VZV-specific IgG antibody directed against VZV-infected-cell lysate and against VZV glycoprotein E (gE) using standard ELISA (Fig. 2C). Antibody responses to VZV lysate peaked at 14 days p.i., while the responses to VZV gE peaked at 17 days p.i. The antibody titers to both viral lysate and gE remained significantly increased over the baseline levels through the end of the study. We also assessed neutralizing antibody titers following the inoculation of RMs with VZV using plaque reduction assays (Fig. 2D). Despite the lack of VZV replication, VZV inoculation of RMs resulted in the generation of a neutralizing antibody response at 84 days p.i.
VZV inoculation induces a T cell response both in the lungs and in peripheral blood.
We also determined the kinetics and magnitude of T cell proliferation in response to VZV infection by measuring the frequency of Ki67-positive central memory (CM) (CD28+ CD95+) and effector memory (EM) (CD28− CD95+) CD4 and CD8 T cell subsets both in cells from BAL fluid (Fig. 3A and B) and PBMC (Fig. 3C and D). In cells from BAL fluid, CD4 CM and EM T cells underwent a significant proliferative burst following VZV infection that peaked at 7 days p.i. (P < 0.001), while CD8 CM and EM T cell proliferation peaked at 10 days p.i. (P < 0.001). In PBMC, both CD4 and CD8 CM T cells proliferated significantly following VZV infection, peaking at 7 days p.i. (P < 0.001), in contrast to CD4 or CD8 EM T cells, which did not proliferate.
Additionally, we determined the frequency of VZV-specific T cells within CD4 and CD8 T cell populations by measuring the frequency of IFN-γ-producing cells following stimulation with either VZV lysate or VZV overlapping peptide pools covering ORFs 4, 31, 62, 63, and 67 using intracellular cytokine staining. VZV-specific T cell responses were detected both in cells from BAL fluid (Fig. 3E and F) and PBMC (Fig. 3G and H) isolated from infected RMs. In response to VZV lysate stimulation, VZV-specific CD4 T cells were detectable in BAL samples from 7 days p.i. through the end of the study, whereas minimal IFN-γ production from CD8 T cells was measured. In response to stimulation with VZV peptides, the frequency of VZV-specific CD4 and CD8 T cells in BAL samples peaked between 14 and 21 days p.i., after which the frequency declined. In PBMC, VZV-specific CD4 T cells were significantly increased at 7 days p.i. (P < 0.001) and VZV-specific CD8 T cells were significantly increased at 14 days p.i. (P < 0.01) in response to lysate. Similarly, in response to VZV peptides, the frequency of VZV-specific CD4 T cells from PBMC peaked at 7 days p.i., while the CD8 T cell response peaked at 14 days p.i. Overall, the T cell responses in cells from BAL fluid were larger than those observed in PBMC, and the T cell responses to lysate were larger than those to peptide pools.
Previous exposure to VZV results in earlier clearance of SVV viral load in lungs and blood.
To assess the extent of cross-protection conferred by VZV infection against SVV, we challenged VZV-immune RMs 26244 and 26362 with 4 × 105 PFU SVV intrabronchially 189 days post-VZV inoculation, along with VZV-/SVV-naive control RMs (n = 4). Varicella rash was observed in all VZV-naive RMs, while no rash was detected in VZV-immune RMs infected with SVV (data not shown). SVV viral loads were determined in WB and cells from BAL fluid by qPCR using primers and probe specific for SVV ORF 21 as previously described (68). Compared to the viral loads in VZV-naive RMs, the SVV viral loads in BAL samples from both RM 26244 and RM 26362 were significantly lower at 7 days p.i. (P < 0.001) and were below our limit of detection by 14 days p.i. (Fig. 4A), resulting in an overall lower viral burden (area under the curve, P = 0.05). Within whole blood, SVV DNA was detected at 3, 7, and 10 days p.i. for VZV-naive animals but was only detectable at 3 days p.i. for VZV-exposed RMs (Fig. 4B).
Previous exposure to VZV results in earlier antibody production in response to SVV infection.
Overall, the kinetics of B cell proliferation in response to SVV infection were similar in VZV-immune and VZV-naive RMs (Fig. 5). Within cells from BAL fluid, the peak of proliferation (14 days p.i.) in all B cell subsets was greater in VZV-naive RMs (Fig. 5A to C). The proliferation of MZ-like and DN B cell subsets in RM 26362 was significantly reduced at 14 days p.i. (P < 0.01 and P < 0.05, respectively) compared to their proliferation in VZV-naive RMs. However, in PBMC, the proliferation of MZ-like, memory, and DN B cell subsets from RM 26244 occurred earlier or attained higher peaks than observed in VZV-naive animals. The proliferation of memory B cells in RM 26362 at 10 days p.i. was also higher than in VZV-naive animals.
FIG 5.
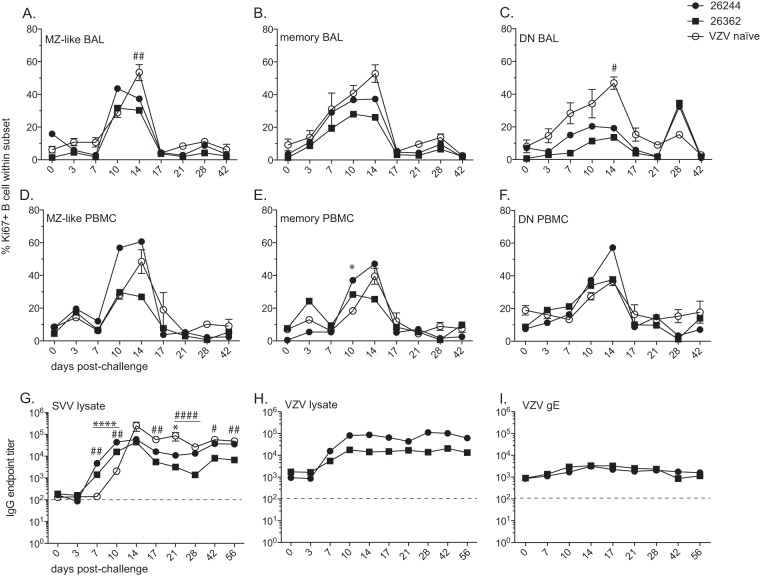
Prior VZV infection results in earlier appearance of SVV-specific antibodies following SVV challenge. Percentages of proliferating marginal zone-like (MZ-like), memory, and other B cells in BAL samples (A to C) and PBMC (D to F) from RM 26244, RM 26362, and VZV-naive RMs were measured by flow cytometry based on the expression of Ki67. SVV-specific (G) and VZV-specific (H, I) IgG antibody endpoint titers were measured by standard ELISA. Dashed line indicates background level. Results for VZV-naive RMs are the average values ± SEM. Statistical analysis was performed using two-way repeated-measures ANOVA with Bonferroni posttest to assess differences between VZV-immune RMs and VZV-naive RMs (P < 0.05: *, RM 26244; #, RM 26362; P < 0.01: **, RM 26244; ##, RM 26362; P < 0.0001: ####, RM 26362).
Interestingly, on the day of SVV challenge, the SVV-specific antibody titers were not significantly higher than the background levels in RM 26244 or RM 26362, suggesting that there were no SVV cross-reactive antibodies in VZV-immune animals. Nevertheless, VZV-immune animals developed significantly higher titers of SVV-specific antibodies at 7 and 10 days p.i. (RM 26244, P < 0.0001, and RM 26362, P < 0.01) than did the VZV-naive animals (Fig. 5G). Despite the earlier development of an SVV-specific IgG response, RM 26362 established a significantly lower plateau SVV IgG titer at 56 days p.i. (P < 0.01) than did the VZV-naive RMs. The titer of VZV-specific antibodies to viral lysate also increased after SVV infection (Fig. 5H), but the titer of antibodies specific to VZV gE did not increase (Fig. 5I).
Previous exposure to VZV results in earlier T cell proliferation and increased IFN-γ response to SVV infection in BAL fluid.
We measured the proliferation of CD4 and CD8 CM and EM T cells in BAL samples (Fig. 6A to D) and in PBMC (Fig. 6E to H) following SVV challenge. In BAL samples, T cell memory subsets from VZV-immune RMs proliferated earlier in response to SVV infection than did those from VZV-naive RMs. Specifically, at 7 days p.i., the CD8 EM T cell proliferation was greater in RM 26244 and RM 26362 than in VZV-naive RMs (P < 0.001) (Fig. 6D), and a trend toward earlier CD4 and CD8 CM proliferation was also detected (Fig. 6A and B). Moreover, memory T cell proliferation ceased earlier in VZV-immune RMs, especially within CD8 T cells, than it did in VZV-naive RMs (RM 26362 CD8 CM 14 days p.i., P < 0.05; RM 26362 CD8 EM 14 days p.i., P < 0.0001; and RM 26244 CD8 EM 14 days p.i., P < 0.001). In PBMC, earlier proliferation in VZV-immune RMs is also seen in CD8 CM (RM 26362, P < 0.05) and EM T cells at 7 days p.i., while the proliferation of CD4 T cells is comparable with that in VZV-naive RMs.
FIG 6.
Prior VZV infection results in increased earlier but shorter duration memory T cell proliferation in the lungs following SVV challenge. The frequencies of proliferating central memory (CM) and effector memory (EM) CD4 and CD8 T cells in BAL samples (A to D) and PBMC (E to H) from RM 26244, RM 26362, and VZV-naive RMs were measured by flow cytometry based on the expression of Ki67. Results for VZV-naive RMs are the average values ± SEM. Statistical analysis was performed using two-way repeated-measures ANOVA with Bonferroni posttest to assess differences between VZV-immune RMs and VZV-naive RMs (P < 0.05: #, RM 26362; P < 0.01: **, RM 26244; ##, RM 26362; P < 0.001: ***, RM 26244; P < 0.0001: ####, RM 26362).
Lastly, we determined the frequencies of SVV-specific CD4 and CD8 T cells by measuring the frequency of IFN-γ-producing cells following stimulation with either SVV lysate or overlapping peptide pools covering SVV ORFs 4, 11, 16, 31, and 37 using intracellular cytokine staining. The percentages of IFN-γ-positive cells in PBMC and BAL samples responding to the peptide pool from VZV-immune animals were too low to be informative (data not shown). In response to SVV lysate, CD4 and CD8 T cell responses both from cells from BAL fluid and from PBMC isolated from VZV-immune RMs appeared earlier and reached a higher peak (Fig. 7). In cells from BAL fluid, the percentages of responding CD4 T cells from RM 26244 and RM 26362 were significantly increased over the percentages from VZV-naive RMs on days 7 (P < 0.001 and P < 0.01, respectively) and 14 (P < 0.01 and P < 0.001, respectively) postinfection (Fig. 7A). Interestingly, animal 26362 had detectable SVV-specific CD4 T cells on the day of SVV challenge, suggesting the presence of cross-reactive CD4 T cells. The frequencies of SVV-specific CD8 T cells within BAL samples from RM 26244 on days 7, 14 and 28 postinfection were greater than those observed in VZV-naive RMs (P < 0.01, P < 0.0001, and P < 0.01, respectively) (Fig. 7B). Within PBMC, the SVV-specific CD4 T cell responses were initially (7 days p.i.) higher in VZV-immune animals, but subsequently, the CD4 T cell responses were either lower in VZV-immune animals or comparable between the two groups. The frequencies of SVV-specific CD8 T cells from RM 26244 were significantly higher at 7 days p.i. (P < 0.01) and 21 days p.i. (P < 0.001) than they were in VZV-naive RMs (Fig. 7C and D).
SVV but not VZV establishes latency in sensory ganglia.
VZV viral DNA loads were measured in the ganglia from the two VZV-infected RMs that were euthanized at 84 days p.i., and VZV and SVV viral DNA loads were measured in ganglia from RM 26244 and RM 26362, which were euthanized 56 days post-SVV challenge. Table 1 displays the SVV DNA viral loads measured in the sensory ganglia by qPCR. We were unable to detect VZV DNA by qPCR in the sensory ganglia, including the trigeminal and cervical, thoracic, and lumbar/sacral dorsal root ganglia in all four animals (data not shown). In contrast, we were able to detect SVV DNA in the trigeminal and cervical dorsal root ganglia from RM 26244 and in the cervical dorsal root ganglia and thoracic dorsal root ganglia from RM 26362.
TABLE 1.
SVV viral load in sensory ganglia
| VZV-immune animal | Sample typea | Avg copy no./μg |
|---|---|---|
| RM 26244 | TG | 339 |
| DRG-C | 160 | |
| DRG-L/S | ND | |
| RM 26362 | DRG-C | 126 |
| DRG-T | 81 | |
| DRG-L/S | ND |
TG, trigeminal ganglia; DRG-C, cervical dorsal root ganglia; DRG-T, thoracic dorsal root ganglia; DRG-L/S, lumbar/sacral dorsal root ganglia; ND, not detected.
DISCUSSION
The investigation of features of varicella-zoster virus biology and pathogenesis has been hampered by the species specificity of this virus. Previously, we have successfully reproduced cardinal features of VZV infection in humans in a rhesus macaque animal model using intrabronchial infection with SVV. Our current study sought to characterize disease progression, viral replication, immune response, and the establishment of latency in rhesus macaques following intrabronchial infection with wild-type VZV and subsequent challenge with SVV. In this initial study, we used the well-characterized wild-type VZV KMcC strain (56, 69, 72) in order to establish virological and immunological baseline values in our rhesus macaque model. The current study sets the groundwork to evaluate novel vaccine strains and to assess the impact of alterations in the manufacture of vaccines on immunogenicity in future experiments.
We were unable to measure amplification of VZV DNA at the site of inoculation, the lungs (via BAL sampling), or in the blood of rhesus macaques intrabronchially infected with VZV KMcC. Our results are similar to those of previous studies using (i) primary intratracheal and secondary intravenous inoculation of cynomolgus macaques with parental Oka strain (58), (ii) subcutaneous inoculation of rhesus macaques in both thighs with VZV OKA or KMcC (72), and (iii) intratracheal and subcutaneous inoculation with the CaQu or SPu strain of VZV in patas monkeys (57). Alternatively, following infection of common marmosets with VZV KMcC via a combined oral-nasal-conjunctival route, virus was detected in lung tissue by coculturing lung tissue from 6 to 7 days p.i. with MRC-5 cells, indicating the likelihood of low-level VZV replication (56). Collectively, these data indicate that VZV is strictly a human pathogen in terms of significant virus replication and disease induction.
We were also unable to document latent VZV in our rhesus macaque model, and no previous studies that we know of have recovered VZV DNA from sensory ganglia of infected monkeys. These observations are markedly different from those made in rodent studies, where VZV DNA was detected in the dorsal root ganglia (DRG) of rats after subcutaneous injection of VZV-infected cells along the spine (80) or after unilateral inoculation in footpads or the paraspinal region with cell-free VZV, despite the absence of viremia (81). The reasons for the differences in virus trafficking to the ganglia between the rodent and nonhuman primate studies could be the site of inoculation (subcutaneous inoculation of footpad or along spinal cord may provide easier access to the neuronal axons), altered viral protein function in different species, lack of cellular receptors, or a combination of these. Our intrabronchial infection model requires VZV to traffic from the lungs to the neurons either via the hematogenous route to sensory ganglia (49) or via retrograde transport from skin lesions (50, 82). Both of these routes require viral replication and infection of T cells, which were not observed in this study. A possible improvement of the nonhuman primate model could be to inoculate rhesus macaques with VZV-infected T cells, providing a vehicle of transport to both the ganglia and the skin. Another alternative is to derive a rhesus-adapted VZV strain to increase the virus tropism in rhesus macaques.
Overall, cytokine production in the lungs and plasma of rhesus macaques following inoculation with VZV was limited and correlated with the lack of viral replication we observed. IL-6, which was the only significantly increased cytokine in the lungs, is secreted by T cells and macrophages in response to tissue damage or infection (74), and thus, the increased expression of this proinflammatory cytokine is likely due to the activation of these cells present in the lungs at the time of inoculation. Despite the limited antigen availability and small B cell proliferative burst, VZV-specific antibodies (both binding and neutralizing) were detected in all animals. Measurable binding antibody responses were also detected in previous studies investigating VZV infection of monkeys (56–58, 72). The peak VZV-specific IgG titers, however, were 1 to 2 log lower than the IgG titers we observed in rhesus macaques infected with SVV intrabronchially (68), most likely due to reduced antigen load in the absence of viral replication.
Similarly, the kinetics of T cell proliferation in both BAL and PBMC samples in response to VZV infection are similar to those observed following SVV infection, but the magnitude of proliferation is substantially decreased, which is most likely a reflection of the lack of VZV replication. In a previous study where cynomolgus macaques were infected with the parental Oka strain first intratracheally and then intravenously 12 weeks later, CD4 T cell proliferation peaked at 2 weeks postinoculation (58). However, T cell proliferation was also observed in control animals inoculated intratracheally with uninfected cells, which suggests that the proliferative burst was not driven by viral replication. Moreover, peak T cell responses in the periphery were not detected until approximately 10 weeks postinfection (58). In contrast, we detected peak T cell proliferation, as well as VZV-specific CD4 and CD8 T cells, 7 days postinfection. The differences in the kinetics of T cell proliferation and cytokine production between the study of Willer et al. (58) and ours could result from the inoculation route, the virus dose, the strain of VZV used, or the macaque species.
Because we observed no evidence of VZV replication or latency after VZV inoculation in rhesus macaques, we were presented with an opportunity to test whether a replication-deficient vaccine could protect against a live-virus challenge. Therefore, we challenged two of the VZV-inoculated animals intrabronchially with a dose of live SVV that consistently results in clinical disease and viremia in rhesus macaques (68, 83, 84). Our data demonstrate that a replication-deficient-virus vaccination provides complete protection against clinical symptoms and partial protection against viral replication following a rigorous live-virus challenge and, therefore, merits further investigation.
Our observations are consistent with those of previous studies demonstrating that VZV inoculation of nonhuman primates provides partial protection against SVV challenge (57, 85). In one study, patas monkeys (Erythrocebus patas) were inoculated intratracheally and subcutaneously with either the CaQu or SPu strain of VZV (57). In addition, the animals inoculated with SPu were hyperimmunized with 5 weekly subcutaneous injections starting 22 days after the initial inoculation. Neither VZV immunization strategy resulted in clinical symptoms (rash). Similar to our observations, VZV was not recovered from lymphocyte or throat samples, although both strategies induced VZV-specific antibody titers in all animals. The animals were challenged via an intratracheal-subcutaneous route with the 592S strain of SVV more than 35 days after the last VZV immunization. Similar to our results described here, VZV-immunized animals did not develop rash after SVV challenge, while control nonimmunized animals developed overt disease. SVV was isolated from only two lymphocyte samples on days 3 and 5 postchallenge from two VZV-immunized animals, whereas SVV was isolated from lymphocytes, throat samples, or both from multiple days postinfection in all control non-VZV-immunized animals. These data are analogous to our viral load data, where we measured decreased SVV genome copy numbers in VZV-immunized animals early postchallenge compared to the SVV genome copy numbers in controls.
In another study, African green monkeys were immunized intramuscularly with VZV Oka alum-adsorbed gE, gB, or gH antigen or total VZV glycoproteins at 0, 4, and 8 weeks (85). The animals were challenged with SVV by intratracheal and subcutaneous abdominal infection 2 weeks after the third immunization. Animals immunized with VZV gB or gH displayed partial protection from SVV challenge, as indicated by reduced severity of viremia and rash, while immunizing with VZV gE was the least effective regimen. Likewise, we measured a boost in IgG antibody titers in response to VZV lysate at 7 days post-SVV challenge, while VZV gE-specific IgG titers remained unchanged after SVV challenge. These observations suggest that memory B cells specific for VZV gE are not capable of cross-recognizing SVV gE. Alignment studies indicate that gE has the lowest amino acid identity and gB the highest between VZV and SVV, which could account for why SVV did not boost anti-VZV gE antibodies in our study and why it was the least protective immunization strategy in the earlier study (86).
Taken together, our studies demonstrate that despite the lack of VZV replication, VZV-immune RMs compared to VZV-naive RMs after SVV challenge displayed (i) no clinical symptoms, (ii) decreased peak SVV DNA viral load in lungs and earlier clearance in lungs and whole blood, (iii) decreased peak proliferation of B cells in the lungs, (iv) earlier production of SVV-specific IgG, (v) earlier and shorter duration of memory T cell proliferation in the lungs, and (vi) earlier and increased frequency of IFN-γ-producing SVV-specific T cells in the lungs. Our studies show that prior VZV infection is not capable of completely preventing infection by SVV, although immunization significantly reduces the burden of disease, likely due to the earlier and increased induction of virus-specific antibodies and T cells. SVV-specific antibodies were absent on the day of SVV challenge, whereas low numbers of cross-reactive T cells were detected, which suggests that T cells could play a more crucial role in protection from SVV challenge. The presence of cross-reactive CD4 T cells was particularly notable in VZV-immune RM 26362 (Fig. 7). These data, although preliminary, are in agreement with our previous data that CD4 T cell responses are more critical than CD8 T cells or antibodies in the resolution of SVV infection (83). Replication-defective herpesvirus vaccine candidates have been reported for herpes simplex virus 2 (87) and cytomegalovirus (88); our data suggest that this approach may also be feasible for varicella-zoster virus.
ACKNOWLEDGMENTS
We thank the Division of Animal Resources at the Oregon National Primate Research Center for expert animal care, especially Alfred Legasse, Miranda Fischer, and Shannon Planer for collection of blood and BAL samples, and we thank Anne Lewis and Lois Colgin for conducting the necropsies and collecting tissues.
This work was supported by a sponsored research agreement with Merck Sharp & Dohme Corporation (SRA-11-079), NIH R01AG037042, 2T32AI007472-16, and NIH 8P51 OD011092-53.
REFERENCES
- 1.Leclair JM, Zaia JA, Levin MJ, Congdon RG, Goldmann DA. 1980. Airborne transmission of chickenpox in a hospital. N Engl J Med 302:450–453. doi: 10.1056/NEJM198002213020807. [DOI] [PubMed] [Google Scholar]
- 2.Sawyer MH, Chamberlin CJ, Wu YN, Aintablian N, Wallace MR. 1994. Detection of varicella-zoster virus DNA in air samples from hospital rooms. J Infect Dis 169:91–94. doi: 10.1093/infdis/169.1.91. [DOI] [PubMed] [Google Scholar]
- 3.Suzuki K, Yoshikawa T, Tomitaka A, Matsunaga K, Asano Y. 2004. Detection of aerosolized varicella-zoster virus DNA in patients with localized herpes zoster. J Infect Dis 189:1009–1012. doi: 10.1086/382029. [DOI] [PubMed] [Google Scholar]
- 4.Grose C. 1981. Variation on a theme by Fenner: the pathogenesis of chickenpox. Pediatrics 68:735–737. [PubMed] [Google Scholar]
- 5.Cohen JI, Straus SE, Arvin AM. 2007. Varicella-zoster virus replication, pathogenesis, and management, p 2773–2818 InKnipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, Straus SE (ed), Fields virology, 5th ed, vol 2 Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 6.Gershon AA, Gershon MD, Breuer J, Levin MJ, Oaklander AL, Griffiths PD. 2010. Advances in the understanding of the pathogenesis and epidemiology of herpes zoster. J Clin Virol 48(Suppl 1):S2–S7. doi: 10.1016/S1386-6532(10)70002-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Helgason S, Petursson G, Gudmundsson S, Sigurdsson JA. 2000. Prevalence of postherpetic neuralgia after a first episode of herpes zoster: prospective study with long term follow up. BMJ 321:794–796. doi: 10.1136/bmj.321.7264.794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liesegang TJ. 2008. Herpes zoster ophthalmicus natural history, risk factors, clinical presentation, and morbidity. Ophthalmology 115:S3–S12. doi: 10.1016/j.ophtha.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 9.Yeo SW, Lee DH, Jun BC, Chang KH, Park YS. 2007. Analysis of prognostic factors in Bell's palsy and Ramsay Hunt syndrome. Auris Nasus Larynx 34:159–164. doi: 10.1016/j.anl.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 10.Gilden DH, Cohrs RJ, Hayward AR, Wellish M, Mahalingam R. 2003. Chronic varicella-zoster virus ganglionitis—a possible cause of postherpetic neuralgia. J Neurovirol 9:404–407. doi: 10.1080/13550280390201722. [DOI] [PubMed] [Google Scholar]
- 11.Good RA, Zak SJ. 1956. Disturbances in gamma globulin synthesis as experiments of nature. Pediatrics 18:109–149. [PubMed] [Google Scholar]
- 12.Arvin AM, Pollard RB, Rasmussen LE, Merigan TC. 1978. Selective impairment of lymphocyte reactivity to varicella-zoster virus antigen among untreated patients with lymphoma. J Infect Dis 137:531–540. doi: 10.1093/infdis/137.5.531. [DOI] [PubMed] [Google Scholar]
- 13.Zerboni L, Nader S, Aoki K, Arvin AM. 1998. Analysis of the persistence of humoral and cellular immunity in children and adults immunized with varicella vaccine. J Infect Dis 177:1701–1704. doi: 10.1086/517426. [DOI] [PubMed] [Google Scholar]
- 14.Nader S, Bergen R, Sharp M, Arvin AM. 1995. Age-related differences in cell-mediated immunity to varicella-zoster virus among children and adults immunized with live attenuated varicella vaccine. J Infect Dis 171:13–17. doi: 10.1093/infdis/171.1.13. [DOI] [PubMed] [Google Scholar]
- 15.Redman RL, Nader S, Zerboni L, Liu C, Wong RM, Brown BW, Arvin AM. 1997. Early reconstitution of immunity and decreased severity of herpes zoster in bone marrow transplant recipients immunized with inactivated varicella vaccine. J Infect Dis 176:578–585. doi: 10.1086/514077. [DOI] [PubMed] [Google Scholar]
- 16.Wilson A, Sharp M, Koropchak CM, Ting SF, Arvin AM. 1992. Subclinical varicella-zoster virus viremia, herpes zoster, and T lymphocyte immunity to varicella-zoster viral antigens after bone marrow transplantation. J Infect Dis 165:119–126. doi: 10.1093/infdis/165.1.119. [DOI] [PubMed] [Google Scholar]
- 17.Brunell PA, Ross A, Miller LH, Kuo B. 1969. Prevention of varicella by zoster immune globulin. N Engl J Med 280:1191–1194. doi: 10.1056/NEJM196905292802201. [DOI] [PubMed] [Google Scholar]
- 18.Orenstein WA, Heymann DL, Ellis RJ, Rosenberg RL, Nakano J, Halsey NA, Overturf GD, Hayden GF, Witte JJ. 1981. Prophylaxis of varicella in high-risk children: dose-response effect of zoster immune globulin. J Pediatr 98:368–373. doi: 10.1016/S0022-3476(81)80697-X. [DOI] [PubMed] [Google Scholar]
- 19.Paryani SG, Arvin AM, Koropchak CM, Dobkin MB, Wittek AE, Amylon MD, Budinger MD. 1984. Comparison of varicella zoster antibody titers in patients given intravenous immune serum globulin or varicella zoster immune globulin. J Pediatr 105:200–205. doi: 10.1016/S0022-3476(84)80113-4. [DOI] [PubMed] [Google Scholar]
- 20.Levin MJ, Smith JG, Kaufhold RM, Barber D, Hayward AR, Chan CY, Chan IS, Li DJ, Wang W, Keller PM, Shaw A, Silber JL, Schlienger K, Chalikonda I, Vessey SJ, Caulfield MJ. 2003. Decline in varicella-zoster virus (VZV)-specific cell-mediated immunity with increasing age and boosting with a high-dose VZV vaccine. J Infect Dis 188:1336–1344. doi: 10.1086/379048. [DOI] [PubMed] [Google Scholar]
- 21.Weinberg A, Zhang JH, Oxman MN, Johnson GR, Hayward AR, Caulfield MJ, Irwin MR, Clair J, Smith JG, Stanley H, Marchese RD, Harbecke R, Williams HM, Chan IS, Arbeit RD, Gershon AA, Schodel F, Morrison VA, Kauffman CA, Straus SE, Schmader KE, Davis LE, Levin MJ. 2009. Varicella-zoster virus-specific immune responses to herpes zoster in elderly participants in a trial of a clinically effective zoster vaccine. J Infect Dis 200:1068–1077. doi: 10.1086/605611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oxman MN. 2010. Zoster vaccine: current status and future prospects. Clin Infect Dis 51:197–213. doi: 10.1086/653605. [DOI] [PubMed] [Google Scholar]
- 23.Weinberg A, Lazar AA, Zerbe GO, Hayward AR, Chan IS, Vessey R, Silber JL, MacGregor RR, Chan K, Gershon AA, Levin MJ. 2010. Influence of age and nature of primary infection on varicella-zoster virus-specific cell-mediated immune responses. J Infect Dis 201:1024–1030. doi: 10.1086/651199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Asada H, Nagayama K, Okazaki A, Mori Y, Okuno Y, Takao Y, Miyazaki Y, Onishi F, Okeda M, Yano S, Kumihashi H, Gomi Y, Maeda K, Ishikawa T, Iso H, Yamanishi K. 2013. An inverse correlation of VZV skin-test reaction, but not antibody, with severity of herpes zoster skin symptoms and zoster-associated pain. J Dermatol Sci 69:243–249. doi: 10.1016/j.jdermsci.2012.10.015. [DOI] [PubMed] [Google Scholar]
- 25.Gershon AA, Chen J, Davis L, Krinsky C, Cowles R, Reichard R, Gershon M. 2012. Latency of varicella zoster virus in dorsal root, cranial, and enteric ganglia in vaccinated children. Trans Am Clin Climatol Assoc 123:17–35 http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3540599/. [PMC free article] [PubMed] [Google Scholar]
- 26.Quinlivan M, Breuer J. 2014. Clinical and molecular aspects of the live attenuated Oka varicella vaccine. Rev Med Virol 24:254–273. doi: 10.1002/rmv.1789. [DOI] [PubMed] [Google Scholar]
- 27.Tseng HF, Schmid DS, Harpaz R, LaRussa P, Jensen NJ, Rivailler P, Radford K, Folster J, Jacobsen SJ. 2014. Herpes zoster caused by vaccine-strain varicella zoster virus in an immunocompetent recipient of zoster vaccine. Clin Infect Dis 58:1125–1128. doi: 10.1093/cid/ciu058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dworkin MS, Jennings CE, Roth-Thomas J, Lang JE, Stukenberg C, Lumpkin JR. 2002. An outbreak of varicella among children attending preschool and elementary school in Illinois. Clin Infect Dis 35:102–104. doi: 10.1086/340868. [DOI] [PubMed] [Google Scholar]
- 29.Galil K, Lee B, Strine T, Carraher C, Baughman AL, Eaton M, Montero J, Seward J. 2002. Outbreak of varicella at a day-care center despite vaccination. N Engl J Med 347:1909–1915. doi: 10.1056/NEJMoa021662. [DOI] [PubMed] [Google Scholar]
- 30.Vazquez M. 2004. Varicella zoster virus infections in children after the introduction of live attenuated varicella vaccine. Curr Opin Pediatr 16:80–84. doi: 10.1097/00008480-200402000-00015. [DOI] [PubMed] [Google Scholar]
- 31.Marin M, Zhang JX, Seward JF. 2011. Near elimination of varicella deaths in the US after implementation of the vaccination program. Pediatrics 128:214–220. doi: 10.1542/peds.2010-3385. [DOI] [PubMed] [Google Scholar]
- 32.Oxman MN, Levin MJ, Johnson GR, Schmader KE, Straus SE, Gelb LD, Arbeit RD, Simberkoff MS, Gershon AA, Davis LE, Weinberg A, Boardman KD, Williams HM, Zhang JH, Peduzzi PN, Beisel CE, Morrison VA, Guatelli JC, Brooks PA, Kauffman CA, Pachucki CT, Neuzil KM, Betts RF, Wright PF, Griffin MR, Brunell P, Soto NE, Marques AR, Keay SK, Goodman RP, Cotton DJ, Gnann JW Jr, Loutit J, Holodniy M, Keitel WA, Crawford GE, Yeh SS, Lobo Z, Toney JF, Greenberg RN, Keller PM, Harbecke R, Hayward AR, Irwin MR, Kyriakides TC, Chan CY, Chan IS, Wang WW, Annunziato PW, Silber JL. 2005. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med 352:2271–2284. doi: 10.1056/NEJMoa051016. [DOI] [PubMed] [Google Scholar]
- 33.Oxman MN, Levin MJ. 2008. Vaccination against herpes zoster and postherpetic neuralgia. J Infect Dis 197(Suppl 2):S228–S236. doi: 10.1086/522159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Levin MJ, Oxman MN, Zhang JH, Johnson GR, Stanley H, Hayward AR, Caulfield MJ, Irwin MR, Smith JG, Clair J, Chan IS, Williams H, Harbecke R, Marchese R, Straus SE, Gershon A, Weinberg A. 2008. Varicella-zoster virus-specific immune responses in elderly recipients of a herpes zoster vaccine. J Infect Dis 197:825–835. doi: 10.1086/528696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schmader KE, Oxman MN, Levin MJ, Johnson G, Zhang JH, Betts R, Morrison VA, Gelb L, Guatelli JC, Harbecke R, Pachucki C, Keay S, Menzies B, Griffin MR, Kauffman C, Marques A, Toney J, Keller PM, Li X, Chan IS, Annunziato P. 2012. Persistence of the efficacy of zoster vaccine in the shingles prevention study and the short-term persistence substudy. Clin Infect Dis 55:1320–1328. doi: 10.1093/cid/cis638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gomi Y, Sunamachi H, Mori Y, Nagaike K, Takahashi M, Yamanishi K. 2002. Comparison of the complete DNA sequences of the Oka varicella vaccine and its parental virus. J Virol 76:11447–11459. doi: 10.1128/JVI.76.22.11447-11459.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blatter MM, Klein NP, Shepard JS, Leonardi M, Shapiro S, Schear M, Mufson MA, Martin JM, Varman M, Grogg S, London A, Cambron P, Douha M, Nicholson O, da Costa C, Innis BL. 2012. Immunogenicity and safety of two tetravalent (measles, mumps, rubella, varicella) vaccines coadministered with hepatitis A and pneumococcal conjugate vaccines to children twelve to fourteen months of age. Pediatr Infect Dis J 31:e133–e140. doi: 10.1097/INF.0b013e318259fc8a. [DOI] [PubMed] [Google Scholar]
- 38.Spackova M, Wiese-Posselt M, Dehnert M, Matysiak-Klose D, Heininger U, Siedler A. 2010. Comparative varicella vaccine effectiveness during outbreaks in day-care centres. Vaccine 28:686–691. doi: 10.1016/j.vaccine.2009.10.086. [DOI] [PubMed] [Google Scholar]
- 39.Lau YL, Vessey SJ, Chan IS, Lee TL, Huang LM, Lee CY, Lin TY, Lee BW, Kwan K, Kasim SM, Chan CY, Kaplan KM, Distefano DJ, Harmon AL, Golie A, Hartzel J, Xu J, Li S, Matthews H, Sadoff JC, Shaw A. 2002. A comparison of safety, tolerability and immunogenicity of Oka/Merck varicella vaccine and VARILRIX in healthy children. Vaccine 20:2942–2949. doi: 10.1016/S0264-410X(02)00245-1. [DOI] [PubMed] [Google Scholar]
- 40.Matsunaga Y, Yamanishi K, Takahashi M. 1982. Experimental infection and immune response of guinea pigs with varicella-zoster virus. Infect Immun 37:407–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Myers MG, Duer HL, Hausler CK. 1980. Experimental infection of guinea pigs with varicella-zoster virus. J Infect Dis 142:414–420. doi: 10.1093/infdis/142.3.414. [DOI] [PubMed] [Google Scholar]
- 42.Myers MG, Connelly BL, Stanberry LR. 1991. Varicella in hairless guinea pigs. J Infect Dis 163:746–751. doi: 10.1093/infdis/163.4.746. [DOI] [PubMed] [Google Scholar]
- 43.Shiraki K, Yamanishi K, Takahashi M. 1984. Biologic and immunologic characterization of the soluble skin-test antigen of varicella-zoster virus. J Infect Dis 149:501–504. doi: 10.1093/infdis/149.4.501. [DOI] [PubMed] [Google Scholar]
- 44.Kinchington PR, Goins WF. 2011. Varicella zoster virus-induced pain and postherpetic neuralgia in the human host and in rodent animal models. J Neurovirol 17:590–599. doi: 10.1007/s13365-011-0069-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dalziel RG, Bingham S, Sutton D, Grant D, Champion JM, Dennis SA, Quinn JP, Bountra C, Mark MA. 2004. Allodynia in rats infected with varicella zoster virus—a small animal model for post-herpetic neuralgia. Brain Res Brain Res Rev 46:234–242. doi: 10.1016/j.brainresrev.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 46.Moffat JF, Stein MD, Kaneshima H, Arvin AM. 1995. Tropism of varicella-zoster virus for human CD4+ and CD8+ T lymphocytes and epidermal cells in SCID-hu mice. J Virol 69:5236–5242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moffat JF, Zerboni L, Kinchington PR, Grose C, Kaneshima H, Arvin AM. 1998. Attenuation of the vaccine Oka strain of varicella-zoster virus and role of glycoprotein C in alphaherpesvirus virulence demonstrated in the SCID-hu mouse. J Virol 72:965–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moffat JF, Zerboni L, Sommer MH, Heineman TC, Cohen JI, Kaneshima H, Arvin AM. 1998. The ORF47 and ORF66 putative protein kinases of varicella-zoster virus determine tropism for human T cells and skin in the SCID-hu mouse. Proc Natl Acad Sci U S A 95:11969–11974. doi: 10.1073/pnas.95.20.11969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zerboni L, Ku C-C, Jones CD, Zehnder JL, Arvin AM. 2005. Varicella-zoster virus infection of human dorsal root ganglia in vivo. Proc Natl Acad Sci U S A 102:6490–6495. doi: 10.1073/pnas.0501045102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ku C-C, Zerboni L, Ito H, Graham BS, Wallace M, Arvin AM. 2004. Varicella-zoster virus transfer to skin by T cells and modulation of viral replication by epidermal cell interferon-alpha. J Exp Med 200:917–925. doi: 10.1084/jem.20040634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zerboni L, Sen N, Oliver SL, Arvin AM. 2014. Molecular mechanisms of varicella zoster virus pathogenesis. Nat Rev Microbiol 12:197–210. doi: 10.1038/nrmicro3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Heuschele WP. 1960. Varicella (chicken pox) in three young anthropoid apes. J Am Vet Med Assoc 136:256–257. [PubMed] [Google Scholar]
- 53.White RJ, Simmons L, Wilson RB. 1972. Chickenpox in young anthropoid apes: clinical and laboratory findings. J Am Vet Med Assoc 161:690–692. [PubMed] [Google Scholar]
- 54.Myers MG, Kramer LW, Stanberry LR. 1987. Varicella in a gorilla. J Med Virol 23:317–322. doi: 10.1002/jmv.1890230403. [DOI] [PubMed] [Google Scholar]
- 55.Cohen JI, Moskal T, Shapiro M, Purcell RH. 1996. Varicella in chimpanzees. J Med Virol 50:289–292. doi:. [DOI] [PubMed] [Google Scholar]
- 56.Provost PJ, Keller PM, Banker FS, Keech BJ, Klein HJ, Lowe RS, Morton DH, Phelps AH, McAleer WJ, Ellis RW. 1987. Successful infection of the common marmoset (Callithrix jacchus) with human varicella-zoster virus. J Virol 61:2951–2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Felsenfeld AD, Schmidt NJ. 1979. Varicella-zoster virus immunizes patas monkeys against simian varicella-like disease. J Gen Virol 42:171–178. doi: 10.1099/0022-1317-42-1-171. [DOI] [PubMed] [Google Scholar]
- 58.Willer DO, Ambagala AP, Pilon R, Chan JK, Fournier J, Brooks J, Sandstrom P, Macdonald KS. 2012. Experimental infection of cynomolgus macaques (Macaca fascicularis) with human varicella-zoster virus. J Virol 86:3626–3634. doi: 10.1128/JVI.06264-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fletcher TM III, Gray WL. 1992. Simian varicella virus: characterization of virion and infected cell polypeptides and the antigenic cross-reactivity with varicella-zoster virus. J Gen Virol 73(Pt 5):1209–1215. doi: 10.1099/0022-1317-73-5-1209. [DOI] [PubMed] [Google Scholar]
- 60.Gray WL, Pumphrey CY, Ruyechan WT, Fletcher TM. 1992. The simian varicella virus and varicella zoster virus genomes are similar in size and structure. Virology 186:562–572. doi: 10.1016/0042-6822(92)90022-H. [DOI] [PubMed] [Google Scholar]
- 61.Pumphrey CY, Gray WL. 1992. The genomes of simian varicella virus and varicella zoster virus are colinear. Virus Res 26:255–266. doi: 10.1016/0168-1702(92)90017-4. [DOI] [PubMed] [Google Scholar]
- 62.Gray WL, Starnes B, White MW, Mahalingam R. 2001. The DNA sequence of the simian varicella virus genome. Virology 284:123–130. doi: 10.1006/viro.2001.0912. [DOI] [PubMed] [Google Scholar]
- 63.Gray WL, Oakes JE. 1984. Simian varicella virus DNA shares homology with human varicella-zoster virus DNA. Virology 136:241–246. doi: 10.1016/0042-6822(84)90263-0. [DOI] [PubMed] [Google Scholar]
- 64.Blakely GA, Lourie B, Morton WG, Evans HH, Kaufmann AF. 1973. A varicella-like disease in macaque monkeys. J Infect Dis 127:617–625. doi: 10.1093/infdis/127.6.617. [DOI] [PubMed] [Google Scholar]
- 65.Felsenfeld AD, Schmidt NJ. 1977. Antigenic relationships among several simian varicella-like viruses and varicella-zoster virus. Infect Immun 15:807–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Felsenfeld AD, Schmidt NJ. 1975. Immunological relationship between delta herpesvirus of patas monkeys and varicella-zoster virus of humans. Infect Immun 12:261–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lenac Rovis T, Bailer SM, Pothineni VR, Ouwendijk WJ, Simic H, Babic M, Miklic K, Malic S, Verweij MC, Baiker A, Gonzalez O, von Brunn A, Zimmer R, Fruh K, Verjans GM, Jonjic S, Haas J. 2013. Comprehensive analysis of varicella-zoster virus proteins using a new monoclonal antibody collection. J Virol 87:6943–6954. doi: 10.1128/JVI.00407-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Messaoudi I, Barron A, Wellish M, Engelmann F, Legasse A, Planer S, Gilden D, Nikolich-Zugich J, Mahalingam R. 2009. Simian varicella virus infection of rhesus macaques recapitulates essential features of varicella zoster virus infection in humans. PLoS Pathog 5:e1000657. doi: 10.1371/journal.ppat.1000657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Neff BJ, Weibel RE, Villarejos VM, Buynak EB, McLean AA, Morton DH, Wolanski BS, Hilleman MR. 1981. Clinical and laboratory studies of KMcC strain live attenuated varicella virus. Proc Soc Exp Biol Med 166:339–347. doi: 10.3181/00379727-166-41071. [DOI] [PubMed] [Google Scholar]
- 70.Tischer BK, Kaufer BB, Sommer M, Wussow F, Arvin AM, Osterrieder N. 2007. A self-excisable infectious bacterial artificial chromosome clone of varicella-zoster virus allows analysis of the essential tegument protein encoded by ORF9. J Virol 81:13200–13208. doi: 10.1128/JVI.01148-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gray WL, Zhou F, Noffke J, Tischer BK. 2011. Cloning the simian varicella virus genome in E. coli as an infectious bacterial artificial chromosome. Arch Virol 156:739–746. doi: 10.1007/s00705-010-0889-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Asano Y, Albrecht P, Behr DE, Neff BJ, Vickers JH, Rastogi SC. 1984. Immunogenicity of wild and attenuated varicella-zoster virus strains in rhesus monkeys. J Med Virol 14:305–312. doi: 10.1002/jmv.1890140403. [DOI] [PubMed] [Google Scholar]
- 73.Zweerink HJ, Morton DH, Stanton LW, Neff BJ. 1981. Restriction endonuclease analysis of the DNA from varicella-zoster virus: stability of the DNA after passage in vitro. J Gen Virol 55:207–211. doi: 10.1099/0022-1317-55-1-207. [DOI] [PubMed] [Google Scholar]
- 74.Hirano T. 1998. Interleukin 6 and its receptor: ten years later. Int Rev Immunol 16:249–284. doi: 10.3109/08830189809042997. [DOI] [PubMed] [Google Scholar]
- 75.Pitcher CJ, Hagen SI, Walker JM, Lum R, Mitchell BL, Maino VC, Axthelm MK, Picker LJ. 2002. Development and homeostasis of T cell memory in rhesus macaque. J Immunol 168:29–43. doi: 10.4049/jimmunol.168.1.29. [DOI] [PubMed] [Google Scholar]
- 76.Kaminski DA, Wei C, Qian Y, Rosenberg AF, Sanz I. 2012. Advances in human B cell phenotypic profiling. Front Immunol 3:302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kaur A, Hale CL, Ramanujan S, Jain RK, Johnson RP. 2000. Differential dynamics of CD4(+) and CD8(+) T-lymphocyte proliferation and activation in acute simian immunodeficiency virus infection. J Virol 74:8413–8424. doi: 10.1128/JVI.74.18.8413-8424.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li X, Miao H, Henn A, Topham DJ, Wu H, Zand MS, Mosmann TR. 2012. Ki-67 expression reveals strong, transient influenza specific CD4 T cell responses after adult vaccination. Vaccine 30:4581–4584. doi: 10.1016/j.vaccine.2012.04.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Brown JR, DiGiovanna MP, Killelea B, Lannin DR, Rimm DL. 2014. Quantitative assessment Ki-67 score for prediction of response to neoadjuvant chemotherapy in breast cancer. Lab Invest 94:98–106. doi: 10.1038/labinvest.2013.128. [DOI] [PubMed] [Google Scholar]
- 80.Sadzot-Delvaux C, Merville-Louis MP, Delree P, Marc P, Piette J, Moonen G, Rentier B. 1990. An in vivo model of varicella-zoster virus latent infection of dorsal root ganglia. J Neurosci Res 26:83–89. doi: 10.1002/jnr.490260110. [DOI] [PubMed] [Google Scholar]
- 81.Annunziato P, LaRussa P, Lee P, Steinberg S, Lungu O, Gershon AA, Silverstein S. 1998. Evidence of latent varicella-zoster virus in rat dorsal root ganglia. J Infect Dis 178(Suppl 1):S48–S51. doi: 10.1086/514261. [DOI] [PubMed] [Google Scholar]
- 82.Ku CC, Padilla JA, Grose C, Butcher EC, Arvin AM. 2002. Tropism of varicella-zoster virus for human tonsillar CD4(+) T lymphocytes that express activation, memory, and skin homing markers. J Virol 76:11425–11433. doi: 10.1128/JVI.76.22.11425-11433.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Haberthur K, Engelmann F, Park B, Barron A, Legasse A, Dewane J, Fischer M, Kerns A, Brown M, Messaoudi I. 2011. CD4 T cell immunity is critical for the control of simian varicella virus infection in a nonhuman primate model of VZV infection. PLoS Pathog 7:e1002367. doi: 10.1371/journal.ppat.1002367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Meyer C, Dewane J, Haberthur K, Engelmann F, Arnold N, Gray W, Messaoudi I. 2013. Bacterial artificial chromosome derived simian varicella virus is pathogenic in vivo. Virol J 10:278. doi: 10.1186/1743-422X-10-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Soike KF, Keller PM, Ellis RW. 1987. Immunization of monkeys with varicella-zoster virus glycoprotein antigens and their response to challenge with simian varicella virus. J Med Virol 22:307–313. doi: 10.1002/jmv.1890220403. [DOI] [PubMed] [Google Scholar]
- 86.Gray WL. 2010. Simian varicella virus: molecular virology. Curr Top Microbiol Immunol 342:291–308. doi: 10.1007/82_2010_27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Azizi A, Tang M, Gisonni-Lex L, Mallet L. 2013. Evaluation of infectious titer in a candidate HSV type 2 vaccine by a quantitative molecular approach. BMC Microbiol 13:284. doi: 10.1186/1471-2180-13-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fu TM, An Z, Wang D. 2014. Progress on pursuit of human cytomegalovirus vaccines for prevention of congenital infection and disease. Vaccine 32:2525–2533. doi: 10.1016/j.vaccine.2014.03.057. [DOI] [PubMed] [Google Scholar]