ABSTRACT
To date, the response of teleost B cells to specific pathogens has been only scarcely addressed. In this work, we have demonstrated that viral hemorrhagic septicemia virus (VHSV), a fish rhabdovirus, has the capacity to infect rainbow trout spleen IgM-positive (IgM+) cells, although the infection is not productive. Consequently, we have studied the effects of VHSV on IgM+ cell functionality, comparing these effects to those elicited by a Toll-like receptor 3 (TLR3) ligand, poly(I·C). We found that poly(I·C) and VHSV significantly upregulated TLR3 and type I interferon (IFN) transcription in spleen and blood IgM+ cells. Further effects included the upregulated transcription of the CK5B chemokine. The significant inhibition of some of these effects in the presence of bafilomycin A1 (BAF), an inhibitor of endosomal acidification, suggests the involvement of an intracellular TLR in these responses. In the case of VHSV, these transcriptional effects were dependent on viral entry into B cells and the initiation of viral transcription. VHSV also provoked the activation of NF-κB and the upregulation of major histocompatibility complex class II (MHC-II) cell surface expression on IgM+ cells, which, along with the increased transcription of the costimulatory molecules CD80/86 and CD83, pointed to VHSV-induced IgM+ cell activation toward an antigen-presenting profile. Finally, despite the moderate effects of VHSV on IgM+ cell proliferation, a consistent effect on IgM+ cell survival was detected.
IMPORTANCE Innate immune responses to pathogens established through their recognition by pattern recognition receptors (PRRs) have been traditionally ascribed to innate cells. However, recent evidence in mammals has revealed that innate pathogen recognition by B lymphocytes is a crucial factor in shaping the type of immune response that is mounted. In teleosts, these immediate effects of viral encounter on B lymphocytes have not been addressed to date. In our study, we have demonstrated that VHSV infection provoked immediate transcriptional effects on B cells, at least partially mediated by intracellular PRR signaling. VHSV also activated NF-κB and increased IgM+ cell survival. Interestingly, VHSV activated B lymphocytes toward an antigen-presenting profile, suggesting an important role of IgM+ cells in VHSV presentation. Our results provide a first description of the effects provoked by fish rhabdoviruses through their early interaction with teleost B cells.
INTRODUCTION
In mammals, Toll-like receptors (TLRs) recognize highly conserved structures of viral (TLR3, -7, -8, and -9) and bacterial (TLR1, -2, -4, -5, -6, -7, -8, and -9) origins. While TLR1, -2, -4, -5, and -6, together with TLR11 and TLR12 in mice and TLR10 in humans, are mostly expressed on the cell surface, a second group of TLRs, including TLR3, -7, -8, and -9, are localized within endosomal compartments and detect foreign nucleic acids (1). Recognition of pathogen-associated molecular patterns (PAMPs) through TLRs and other pattern recognition receptors (PRRs) leads to the activation and maturation of innate immune cells such as macrophages or dendritic cells (DCs). Additionally, once the presence of several TLR receptors on distinct populations of human and murine B cells was verified, further investigations concluded that B cells have evolved to directly sense microbes and that this TLR-mediated activation of B cells contributes to the establishment of an adequate humoral response (2). However, controversy remains as to what degree TLR signaling in B cells conditions the antibody response. On one hand, early studies showed that mice lacking B cell TLR signaling failed to mount an efficient antibody response (3). However, subsequent studies suggested a slightly different model in which these receptors play a role in the regulation of antibody class switching and in sustaining antibody secretion at late times after immunization in B cells (4), contributing to the amplification of the humoral response but not being completely responsible for it (5). In support of these observations, further studies demonstrated that the primary responses of some immunoglobulin (Ig) subclasses (i.e., IgG2a or IgG2c) were absolutely dependent on signaling through the adaptor protein MyD88, used by most TLRs, whereas other Ig classes were not (IgG1 and IgG3) or were much less (IgG2b and IgA) dependent on the MyD88 signaling cascade (6, 7). Interestingly, the conditional deletion of MyD88 in either DCs or B cells revealed that the antibody response to virus-like particles required TLR signaling in B cells, while the response to a soluble antigen was dependent on TLR signaling on DCs (8). This result reveals an ability of B cells to discriminate among antigens based on their physical form.
Several studies have examined the expression of TLRs across B cell subsets in mice and in human tissues, revealing important species-specific differences in the range of TLRs expressed by each subset. In mice, evaluation of follicular B cells, marginal zone B cells, B1 cells, and Peyer's patch B cells indicated broad (except for TLR5 and TLR8) yet differential TLR expression and distinct responsiveness to TLR agonists (9). In contrast, human naive tonsil or blood B cells lack TLR3, TLR4, and TLR8 expression (10, 11), even though the expression of these three TLRs can be detected in human plasma cells (11). As a result, for example, human naive B cells do not express TLR4 and therefore do not recognize the bacterial lipopolysaccharide (LPS) typically recognized by this receptor in murine B cells (10). Despite these specificities, overall, mammalian naive B lymphocytes express relatively low levels of TLRs in comparison to activated and memory cells (2). Furthermore, although the usual consequences of TLR activation in responsive B cells are direct effects on cell proliferation and antibody secretion, some additional effects have been reported for B1 cells. In this B cell subset, TLR signaling directly induced nitric oxide (NO) via inducible NO synthase (iNOS) activation (12) and increased the response to chemokines through a rapid, specific downregulation of integrins and CD9 (13). In fact, the role of TLR signaling appears prominent in innate B cell subsets; for example, B1 cells and marginal zone B cells are known to generate natural antibodies through a T-independent mechanism that involves the recognition of microbial products through TLRs and low-affinity Ig receptors (14, 15). This rapid antibody response interferes with pathogen replication until more specific T-dependent antibody responses are mounted.
Given the propensity for delayed T-dependent responses in teleosts, TLR activation may play a more prominent role in the B cell response to viral infections. Accordingly, rainbow trout (Oncorhynchus mykiss) IgM-positive (IgM+) cells from different tissues, including kidney, spleen, blood, intestine, gills, and liver, have relatively high constitutive transcription levels of TLR1, TLR2, TLR3, TLR5, TLR7, TLR8a, TLR9, and TLR22 (16). Furthermore, in sharp contrast to mammals, where TLR3 (TLR responsible for sensing intracellular double-stranded RNA [dsRNA]) is not a predominant TLR in B cells, trout IgM+ cells appeared to be the main cell type expressing TLR3 in the spleen, according to the ratios of TLR3 transcription observed between IgM+ and IgM-negative (IgM−) populations (21 times higher in IgM+ cells than in IgM− cells) (16). This suggests a high capacity of teleost B cells for sensing viral ligands. Unfortunately, to date, studies dealing with TLR responses in B cells are limited to examinations of the proliferative and antibody-secreting responses observed after LPS stimulation (17, 18).
On the other hand, many viruses have a demonstrated capacity to infect mammalian B lymphocytes, affecting their functionality. For example, Epstein-Barr virus establishes latency in B lymphocytes, which can be reactivated, leading lymphocytes to shed virus (19). Porcine circovirus type 2 (PCV2) spliced capsid mRNA (Cap mRNA) and viral DNA were detected in B lymphocytes from pigs, although no further studies were performed to determine if the presence of these nucleic acids corresponds to a productive infection, with release of viral progeny from these cells (20). The capacity of simian foamy viruses (SFVs) to transcribe genes in human B lymphocytes was also recently described (21). Interestingly, live rabies virus vaccines have been shown to infect murine and human B lymphocytes (22).
In this context, we decided to undertake a functional study to examine the effects of viral hemorrhagic septicemia virus (VHSV) on the functionality of IgM+ cells in fish. VHSV, an enveloped negative-sense single-stranded RNA (ssRNA) rhabdovirus, is the etiological agent of a lethal disease of many cultivated fish species worldwide, including rainbow trout. We analyzed the capacity of VHSV to infect trout IgM+ cells and also studied the effects that VHSV had on different aspects of B cell functionality, comparing these effects to those provoked by a TLR3 agonist, such as poly(I·C), and a TLR9 agonist (CpG). Our results demonstrate that VHSV infects B lymphocytes, where it is able to transcribe viral genes and remain viable, even though active replication was not detected. Consequently, this early virus-B lymphocyte interaction provokes different effects in the cell, including transcriptional upregulation of TLR3 and type I interferon (IFN), NF-κB activation, increased survival, and increased major histocompatibility complex class II (MHC-II) surface expression. These effects highlight the importance of B lymphocytes in the early immune response to VHSV and reveal an important role of innate immune signals in the viral response of teleost B cells, probably conditioning posterior acquired B lymphocyte responses.
MATERIALS AND METHODS
Fish.
Healthy specimens of female rainbow trout (Oncorhynchus mykiss) weighing ∼50 to 70 g were obtained from the Centro de Acuicultura El Molino (Madrid, Spain), located in a VHSV-free area. Fish were maintained at the Centro de Investigación en Sanidad Animal (CISA-INIA) laboratory at 16°C to 18°C with a recirculating water system and a 12-h-light/12-h-dark photoperiod. Fish were fed twice a day with a commercial diet (Skretting, Spain). Prior to any experimental procedure, fish were acclimatized to laboratory conditions for 2 weeks, and during this period, no clinical signs were ever observed. All experiments described here comply with the guidelines of the European Union Council (2010/63/EU) for the use of laboratory animals and were approved by the ethics committee of the Instituto Nacional de Investigación y Tecnología Agraria y Alimentaria (INIA).
Tissue collection.
Rainbow trout were sacrificed via MS-222 (Sigma) overdose, and blood was extracted from the caudal vein with a heparinized needle and diluted 10 times with Leibovitz medium (L-15; Life Technologies) supplemented with 100 IU/ml penicillin, 100 μg/ml streptomycin, 10 units/ml heparin, and 5% fetal calf serum (FCS) (all supplements were also obtained from Life Technologies). Spleen was collected, and single-cell suspensions were generated by using 100-μm nylon cell strainers (BD Biosciences). Blood cell suspensions were placed onto 51% Percoll (GE Healthcare) cushions, whereas spleen suspensions were placed onto 30% to 51% discontinuous density gradients. All suspensions were then centrifuged at 500 × g for 30 min at 4°C. The interface cells were collected and washed twice in L-15 medium containing 5% FCS.
Cell stimulation.
Total leukocyte populations from individual fish were dispensed into 24-well plates at a density of 1 × 106 cells per ml and incubated with VHSV or the different TLR agonists. Nonstimulated controls were always included. Cells were then incubated at 20°C for different periods of time, depending on the experiments. Poly(I·C) (Sigma) was used at a concentration of 50 μg/ml. For CpG stimulation, a plasmid containing four copies of a DNA sequence encoding different immunostimulatory CpG motifs, which was previously described (23), was used at a final concentration of 5 μg/ml. VHSV, propagated in the Epithelioma papulosum cyprini (EPC) cell line and titrated as previously described (24), was used at a final concentration of 1 × 106 50% tissue culture infective doses (TCID50)/ml. For VHSV inactivation, the virus was incubated at 56°C for 30 min. Complete viral inactivation was confirmed by inoculation into EPC cells, followed by checking for cytopathic effects during 10 days. In some experiments, a 1-h preincubation of cells with 50 nM the endosomal TLR inhibitor bafilomycin A1 (BAF; Sigma) dissolved in dimethyl sulfoxide (DMSO) was performed prior to the addition of other stimuli. LPS (Sigma) was used at a final concentration of 100 μg/ml as a positive control for B cell proliferation.
Cell sorting.
After stimulation, leukocytes were collected from the wells by gentle scraping, resuspended in phosphate-buffered saline (PBS), and incubated for 30 min on ice with a specific anti-trout IgM monoclonal antibody (MAb) (1.14) coupled to phycoerythrin (PE) (25). Following two washing steps, cells were resuspended in PBS, and IgM-positive cells were isolated by fluorescence-activated cell sorting (FACS) in a BD FACSAria III instrument (BD Biosciences), first by using their forward-scatter (FSC)/side-scatter (SSC) profiles (to exclude the granulocyte gate) and then on the basis of the fluorescence emitted by the anti-trout IgM antibody (16). IgM+ and IgM− cells were then collected into different tubes for RNA isolation. The expression of IgM and the absence of T cell markers (CD3 and T cell receptor α [TCRα]) were verified by PCR for some samples to ensure effective sorting.
Real-time PCR analysis of sorted cells.
Total cellular RNA was isolated from sorted IgM+ populations from different tissues by using the Power SYBR green Cells-to-Ct kit (Life Technologies) according to the manufacturer's instructions. RNAs were treated with DNase during the process to remove genomic DNA that might interfere with the PCRs. Reverse transcription was also performed by using the Power SYBR green Cells-to-Ct kit (Invitrogen) according to the manufacturer's instructions. To evaluate the levels of transcription of the different genes, real-time PCR was performed with a LightCycler 96 System instrument (Roche), using SYBR green PCR core reagents (Applied Biosystems) and specific primers described previously (16, 26–28) and summarized in Table 1. The efficiency of amplification was determined for each primer pair by using serial 10-fold dilutions of pooled cDNA, and only primer pairs with efficiency values of between 1.95 and 2 were used. Measurements were performed in duplicate for each sample under the following conditions: 10 min at 95°C followed by 45 amplification cycles (15 s at 95°C and 1 min at 60°C) plus a dissociation cycle (30 s at 95°C, 1 min at 60°C, and 30 s at 95°C). The expression levels of individual genes were normalized to the relative expression level of trout EF-1α, and the expression levels were calculated by using the 2−ΔCT method, where ΔCT is determined by subtracting the EF-1α value from the target threshold cycle (CT) value. Negative controls with no template were included in all experiments. A melting curve for each PCR was determined by reading the fluorescence at every degree between 60°C and 95°C to ensure that only a single product had been amplified.
TABLE 1.
Primers used in this study
| Gene | Primer | Sequence (5′–3′) |
|---|---|---|
| TLR3 | TLR-3-F | AGCCCTTTGCTGCCTTACAGAG |
| TLR-3-R | GTCTTCAGGTCATTTTTGGACACG | |
| TLR7 | TLR-7-F | TACAGCTTGGTAACATGACTCTCC |
| TLR-7-R | CAACTCTCTGAGACTTGTCGGTAA | |
| TLR8a2 | TLR-8a2-F | CATCTATGTTCTCATCCAGCAACC |
| TLR-8a2-R | GGTCCCCCTAATAGACAACCTCTT | |
| TLR9 | TLR-9-F | TCTTCATAGAGCTGAAGAGGCCTCA |
| TLR-9-R | GTTCCCACTGAGGAGAAGTGTTTT | |
| TLR22 | TLR-22-F | TGGACAATGACGCTCTTTTACC |
| TLR-22-R | GAGCTGATGGTTGCAATGAGG | |
| MDA5 | MDA5-F | AGAGCCCGTCCAAAGTGAAGT |
| MDA5-R | GTTCAGCATAGTCAAAGGCAGGTA | |
| LPG2A | LPG2A-F | ACACCTGCTCTTTCCGTCAC |
| LPG2A-R | GTTGGCTGGATGTCCTTTGG | |
| MHC-II | MHC-II-F | ACACCCTTATCTGCCACGTC |
| MHC-II-R | TCTGGGGTGAAGCTCAGACT | |
| IFN-1 | IFN-1-F | AAAACTGTTTGATGGGAATATGAAA |
| IFN-1-R | CGTTTCAGTCTCCTCTCAGGTT | |
| IFN-2 | IFN-2-F | TACAATGCAGAGTTGGACGTGT |
| IFN-2-R | GTCCCAGGTGACTGACTTCCTAT | |
| IFN-3 | IFN-3-F | TGACGTCTGTCACGTGGAAC |
| IFN-3-R | TCCAGAGGATTCCCAAACAC | |
| Mx | MX-F | AGCGTCTGGCTGATCAGATT |
| MX-R | AGCTGCTCGATGTTGTCCTT | |
| IL-1β | IL-1β-F | CTGAAGCCAGACCTGTAGCC |
| IL-1β-R | GCAACCTCCTCTAGGTGCAG | |
| CK5B | CK5b-F | TTTGCTGATCGTCAGATACCC |
| CK5b-R | GTGTCTGCTCCCCAGACTTC | |
| IκBα | Iκ-Bα-F | GCAAAATGATACCTAGCCAAGAA |
| Iκ-Bα-R | GGTTCATTACTGCAGGAGTTTGA | |
| CD80/86 | CD80/86-F | GTGTTTCCTGGTTCTGGTATCTA |
| CD80/86-R | AACTTGCTGCTCCCTTTCCTC | |
| CD83 | CD83-F | GCTGTTGATAGCGGGAGGTA |
| CD83-R | TGTGGACTCAAGGCAATCTG | |
| CD40 | CD40-F | TGGACTTGAATCTTAAGAGGGGAAC |
| CD40-R | GATGGCTCTCCAAATGGGATTATAG | |
| VHSV N protein | VHSV N PROTEIN-F | GAGAGAACTGGCCCTGACTG |
| VHSV N PROTEIN-R | CCCGAGTTTCTTGGTGATGT | |
| EF-1α | EF-1α-F | GATCCAGAAGGAGGTCACCA |
| EF-1α-R | TTACGTTCGACCTTCCATCC |
Western blot analysis.
To determine if VHSV was actively replicating inside spleen IgM+ cells, splenocytes were incubated with VHSV during 24 h, and IgM+ and IgM− were cells sorted and lysed independently by using radioimmunoprecipitation assay (RIPA) buffer containing protease inhibitors (Roche). Proteins were fractionated onto a denaturing 12% SDS-PAGE gel and transferred onto a polyvinylidene difluoride (PVDF) membrane (Immobilon-P; Millipore Merck). A VHSV extract was included as a positive control. After blocking in PBS with 5% skim milk for 1 h, the membrane was incubated with a MAb against the VHSV N protein, kindly donated by Niels Lorenzen (University of Aarhus, Denmark) (IP5B11 at 1:1,000), in blocking solution at 4°C overnight. After three washing steps, the membrane was incubated for 1 h with the secondary antibody, a goat anti-mouse IgG-horseradish peroxidase (HRP) conjugate (GE Healthcare Life Sciences). The reactive bands were visualized with the ECL system (GE Healthcare Life Sciences). Beta-actin staining was used as a loading control and was visualized by using a MAb raised against zebrafish β-actin (29) and a donkey anti-rabbit IgG-HRP conjugate (GE Healthcare Life Sciences).
Analysis of VHSV replication in IgM+ cells.
To further determine whether VHSV replication was taking place inside IgM+ cells, splenocyte cultures were infected as described above. At 24 h postinfection, IgM+ and IgM− cells from control and infected cultures were sorted, washed twice with fresh medium, and resuspended in 50 μl of medium. The cells were lysed by repeated freeze-thaw cycles and used to inoculate EPC cells, which are highly permissive to VHSV infection. Confluent EPC cultures in 24-well plates were infected with the cell lysates at 1:10, 1:100, and 1:1,000 dilutions in L-15 medium supplemented with 2.5% fetal bovine serum (FBS) and maintained at 14°C for 7 days. At this point, cytopathic effects were visualized under a microscope.
NF-κB translocation to the nucleus.
Trout splenocytes were isolated and seeded in complete MGFL-15 medium (MGFL-15 medium supplemented with 100 U/ml penicillin, 100 μg/ml streptomycin, 100 μg/ml gentamicin, 10% newborn calf serum [Gibco], and 5% carp serum). Cells were treated for 24 h in the presence of poly(I·C) (50 μg/ml). Following stimulation, cells were fixed in 1% formaldehyde and washed twice in PBS with 2% calf serum and 0.1% saponin (permeabilization buffer). To determine nuclear translocation in IgM+ cells, splenocytes were stained with anti-p65 (Santa Cruz Biotechnology) and anti-trout IgM (1.14) for 30 min at 4°C, followed by staining for 20 min at room temperature. Following primary staining, cells were washed and stained with goat anti-rabbit allophycocyanin (Jackson ImmunoResearch) and rabbit anti-mouse fluorescein isothiocyanate (FITC) (Jackson ImmunoResearch). Prior to acquisition, Hoechst 33342 nuclear stain (Molecular Probes) was added according to the manufacturer's recommendations. Data were collected on an ImageStream MKII system and analyzed by using IDEAS software (Amnis), as previously described (30).
MHC-II expression.
The levels of MHC-II expression on the surface of IgM+ cells were measured via flow cytometry using a MAb against trout MHC-II (31, 32). Stimulated or unstimulated splenocytes were washed in staining buffer (PBS with 1% bovine serum albumin [BSA] and 0.02% sodium azide) and coincubated with PE-labeled anti-trout IgM and the Alexa 647-labeled anti-MHC-II antibody for 30 min at 4°C, protected from light. Finally, cells were washed twice with the same buffer and analyzed by flow cytometry (FACSCalibur; BD Biosciences). To check viability, the cells were then stained with 4 μg/ml 7-aminoactinomycin D (BD) at 4°C for 15 min and analyzed with a FACSCalibur instrument with an excitation wavelength at 488 nm and an emission wavelength at 650 nm. The dye is excluded from live cells, whereas dead cells are stained. Cell viability in our cultures was always >95%. Dead cells were excluded from the analysis.
B cell proliferation.
The BrdU Flow kit (Becton Dickinson) was used to measure the specific proliferation of IgM+ cells, according to the manufacturer's instructions. Splenocytes at a concentration of 2 × 106 cells per ml were incubated for 3 days at 20°C with different stimuli [poly(I·C), CpG, a combination of poly(I·C) and CpG, VHSV, or LPS], as described above. Bromodeoxyuridine (BrdU) (10 μM) was then added to the cultures, and cells were incubated for an additional 24 h. After that time, trout cells were collected and stained with anti-IgM-PE (1.14) antibody and then fixed and permeabilized with Cytofix/Cytoperm buffer for 15 min on ice. Afterwards, cells were incubated with Cytoperm Permeabilization Buffer Plus for 10 min on ice and refixed with Cytofix/Cytoperm buffer during 5 min at room temperature. Cells were then incubated with DNase (30 μg/106 cells) for 1 h at 37°C to expose the incorporated BrdU. Finally, the cells were stained with FITC anti-BrdU antibody for 20 min at room temperature and analyzed by flow cytometry (FACSCalibur; BD Biosciences).
Statistics.
Statistical analyses were performed by using a two-tailed paired Student t test with Welch's correction when the F test indicated that the variances of both groups differed significantly, using GraphPad Prism 4 software and GraphPad Prism 6 software, in the case of NF-κB translocation experiments. The differences between the mean values were considered significant when the P value was <0.05.
RESULTS
VHSV infects rainbow trout spleen IgM+ cells.
First, we studied whether VHSV was capable of replicating in trout splenic IgM+ cells. For this, spleen leukocytes were infected in vitro with VHSV, and after 24 h, IgM+ cells were sorted by FACS analysis, and the transcription of viral genes and viral protein expression were detected. We detected transcription of VHSV genes in sorted IgM+ cells from samples incubated with active VHSV but not in those from control or inactivated VHSV-treated samples (Fig. 1A), indicating that VHSV can actively enter IgM+ cells and start viral gene transcription. On the other hand, viral N protein was not detected by Western blotting of splenic IgM+ cells after 24 h of infection (Fig. 1B) or at later times postinfection (data not shown). Despite these results, when lysates from IgM+ cells obtained from infected cultures were inoculated into the permissive EPC cell line, a clear cytopathic effect was visible at day 7 postinoculation, demonstrating that the virus remained viable inside IgM+ cells, even though the viral titer inside the cells did not increase with time (data not shown). Nevertheless, our results demonstrate that even if VHSV has the capacity to infect trout B lymphocytes, active replication is not taking place within IgM+ cells.
FIG 1.
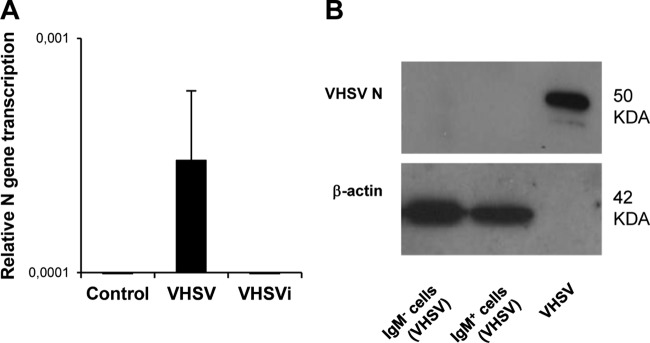
VHSV replication in trout spleen IgM+ cells. (A) Transcription of the VHSV N gene was assessed by real-time PCR in sorted IgM+ cells after 24 h of viral inoculation. Means of data from 6 independent experiments are shown. (B) VHSV N protein expression in IgM+ and IgM− sorted cells previously incubated with the virus during 24 h was evaluated by Western blotting. β-Actin was included as a loading control.
VHSV activates TLR3 transcription in spleen IgM+ cells.
Having established that spleen IgM+ cells could be infected by VHSV, we wanted to assess whether these cells were responsive to VHSV. Cells stimulated with the TLR3 agonist poly(I·C) or with CpG were included for comparative reasons, together with nonstimulated controls. After 1 h, 24 h, and 48 h, IgM+ cells were sorted by FACS analysis and used for RNA extraction and evaluation of gene transcription by real-time PCR.
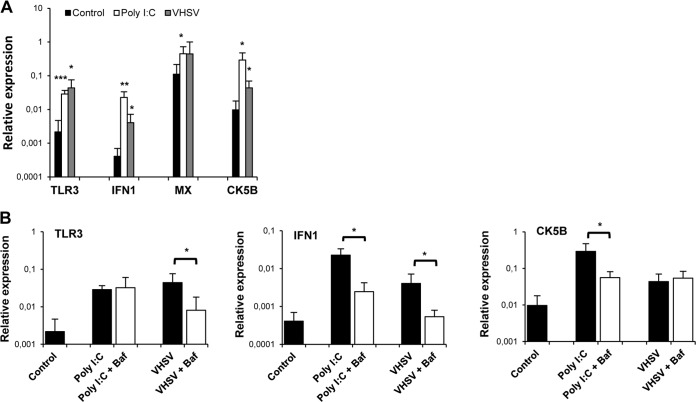
First, we analyzed the response of trout TLRs that were previously suggested to be implicated in nucleic acid recognition, namely, TLR3, -7, -8, -9, and -22 (33, 34). We observed that TLR3 was significantly upregulated in response to poly(I·C) and VHSV after 24 and 48 h of incubation (Fig. 2), whereas no significant changes in the transcription of the other TLRs analyzed were observed. Surprisingly, CpG stimulation also resulted in a significant upregulation of TLR3 transcription in IgM+ cells. Similar results were observed when IgM+ cells were sorted prior to stimulation; however, cell viability was largely decreased when the experiment was conducted according to this protocol due to the activation of cells throughout the sorting procedure by antibody engagement of the B cell receptor (BCR) (data not shown).
FIG 2.
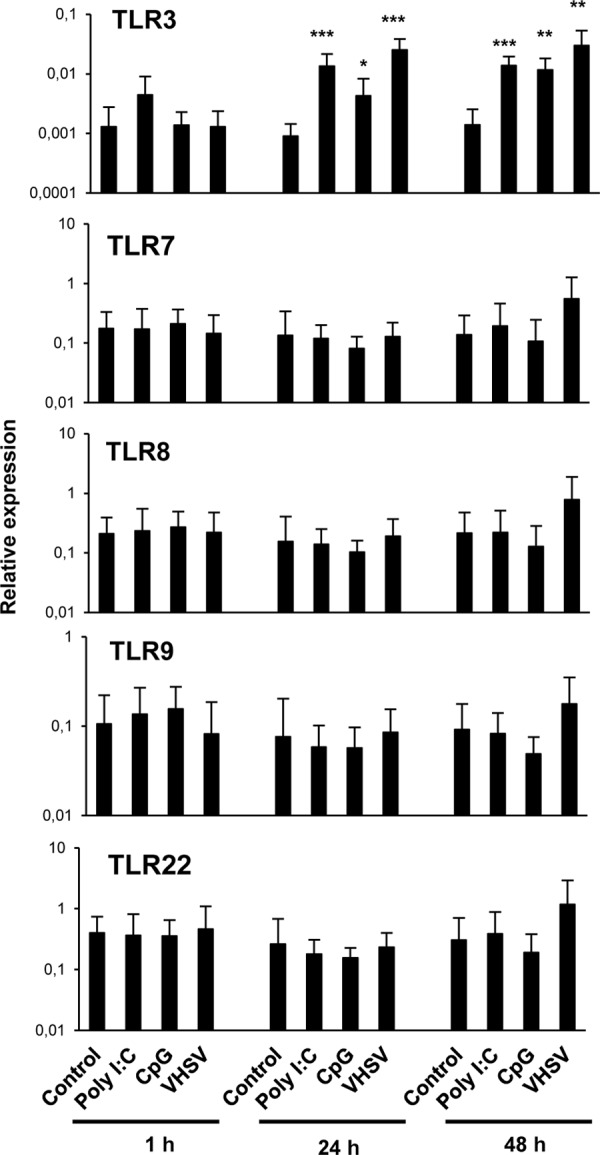
Effect of TLR agonists on the transcription of TLR genes in sorted spleen IgM+ cells. Total spleen leukocyte populations were stimulated with poly(I·C) (50 μg/ml), CpG (5 μg/ml), or VHSV (1 × 106 TCID50 ml−1) and incubated for 1 h, 24 h, or 48 h at 20°C. Nonstimulated controls were also included. After the different incubation periods, IgM+ B cells were sorted by using an anti-trout IgM MAb. RNA was extracted from sorted IgM+ cells, and the levels of transcription of TLR3, TLR7, TLR8a2, TLR9, and TLR22 were evaluated by real-time PCR in duplicate. Data from 7 independent fish are shown as mean gene expression levels relative to the expression level of an endogenous control (EF-1α) ± standard deviations. Asterisks denote significant differences between cells treated with a TLR agonist and their corresponding controls. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Finally, we also studied the effects of poly(I·C) and VHSV on the levels of transcription of two retinoic acid-inducible gene I (RIG-I)-like receptors known to sense viral RNA in the cytoplasm, namely, MDA5 (melanoma differentiation-associated protein 5) and LPG2A (Laboratory of Genetics and Physiology 2A). Only after 48 h of stimulation with poly(I·C) did we detect a significant increase in the level of transcription of MDA5 in IgM+ cells (data not shown). VHSV, on the other hand, had no effect on the transcription levels of these cytoplasmic sensors in IgM+ cells. Although TLRs and other PRRs may be implicated in viral sensing in teleost B cells without a requirement for this transcriptional modulation, our results placed particular emphasis on TLR3 as a potential regulator of viral recognition by teleost B cells.
VHSV activates IFN-1 and Mx transcription in spleen IgM+ cells.
We also studied the effect of VHSV, poly(I·C), and CpG on genes known to be regulated through the activation of TLR3 (35). We focused on IFN-1 and IFN-2, which are type I IFN molecules because of their antiviral activity (36), as well as Mx, an IFN-responsive gene (36), and the CK5B chemokine, a likely trout homologue of mammalian CCL5 (37). We focused on this chemokine because a previous proteomic analysis performed by our group showed that CK5B was one of the main proteins produced by IgM+ cells (data not shown). After 1 h, 24 h, and 48 h of incubation with the different stimuli, splenic IgM+ cells were sorted by FACS analysis, and the transcription of these genes was studied by real-time PCR. A significant upregulation of IFN-1 transcription was observed in IgM+ cells from cultures stimulated with poly(I·C) after 1 or 24 h of incubation or from cultures stimulated with VHSV at all the time points studied (Fig. 3). A significantly higher Mx transcription level was also observed in spleen IgM+ cells from cultures incubated with VHSV for 24 h than in IgM+ cells from unstimulated cultures (Fig. 3). Additionally, 48 h of incubation with either poly(I·C) or VHSV resulted in a significant upregulation of CK5B transcription levels in IgM+ B cells (Fig. 3). Finally, CpG stimulation induced the upregulation of IFN-1 at 48 h poststimulation and of CK5B transcription at both 24 h and 48 h poststimulation. These results reveal that VHSV and TLR agonists such as poly(I·C) or CpG stimulate IgM+ cells to produce type I IFN and the CK5B chemokine.
FIG 3.
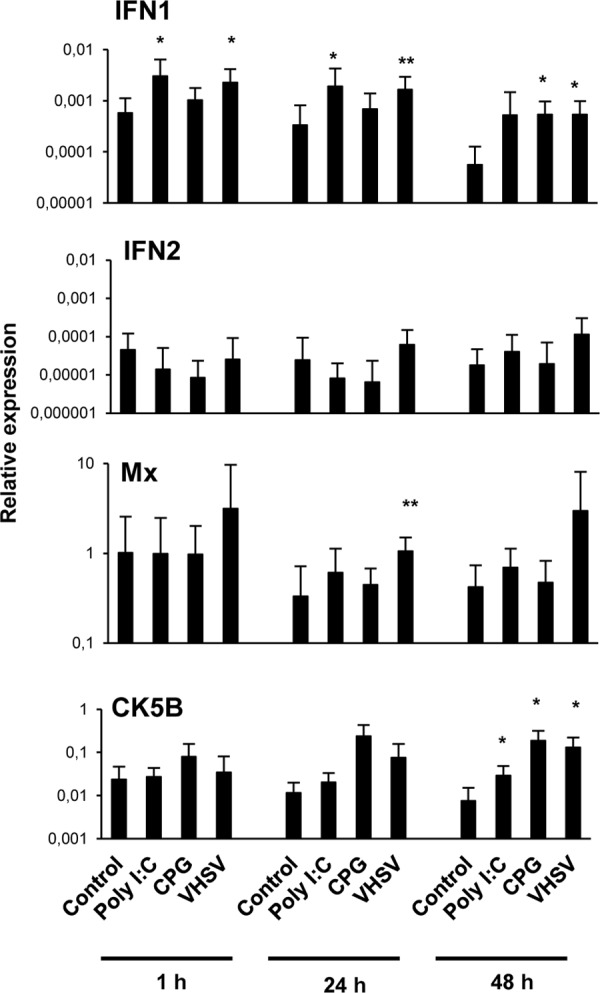
Effect of TLR agonists on the transcription of immune genes related to TLR3 activation in sorted spleen IgM+ cells. Total spleen leukocytes were treated as described in the legend of Fig. 1. RNA was extracted from sorted IgM+ cells, and the levels of transcription of IFN-1, IFN-2, Mx, and CK5B were evaluated by real-time PCR in duplicate. Data from 7 independent fish are shown as mean gene expression levels relative to the expression level of an endogenous control (EF-1α) ± standard deviations. Asterisks denote significant differences between cells treated with TLR agonists and their corresponding controls. *, P < 0.05; **, P < 0.01.
TLR3 and IFN-1 transcription in response to VHSV on IgM+ cells is mediated by an endosomal TLR.
To study whether the induction of TLR3, IFN-1, and CK5B observed in IgM+ cells in response to VHSV or poly(I·C) was in fact mediated by the activation of an intracellular TLR, we used bafilomycin A1 (BAF), a highly specific inhibitor of vacuolar-type H+-ATPase. Inhibitors of endosomal acidification abolish the signaling of all the TLRs located in endosomes, including TLR3-dependent responses (38). We found that preincubation of splenocytes with BAF significantly reduced the upregulation of both TLR3 and IFN-1 transcription observed in IgM+ cells in response to either poly(I·C) or VHSV, compared to the expression levels in cells stimulated in the absence of the inhibitor BAF (Fig. 4). These results strongly suggest that an intracellular TLR, possibly TLR3, mediates the IFN-1 response in trout splenic IgM+ cells. Nevertheless, no changes in CK5B mRNA levels were found when cells were preincubated with BAF and the appropriate stimuli, suggesting that the upregulation of CK5B observed after VHSV and poly(I·C) incubation is not a direct consequence of intracellular TLR activation.
FIG 4.
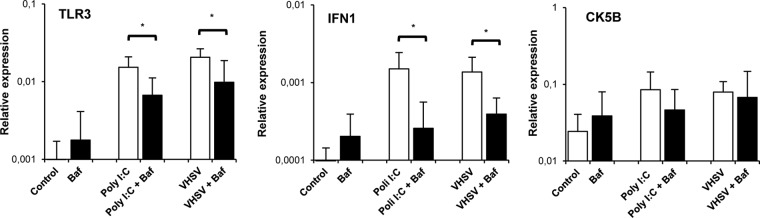
BAF downregulates poly(I·C) and VHSV-induced TLR3 and IFN-1 transcription in IgM+ cells. Spleen leukocyte suspensions were treated with BAF (50 nM) 1 h prior to the addition of poly(I·C) or VHSV. Control wells without BAF were included under all conditions. After the TLR agonists were added, the cells were incubated for 24 h (for TLR3 and IFN-1) or 48 h (for CK5B) at 20°C, and IgM+ cells were sorted. The transcription of TLR3, IFN-1, and CK5B in sorted cells was evaluated by real-time PCR. Data from 5 independent experiments are shown as mean gene expression levels relative to the expression level of an endogenous control (EF-1α) ± standard deviations. *, P < 0.05.
Blood IgM+ cells are also responsive to VHSV and poly(I·C).
Having shown that spleen IgM+ cells transcribe TLR3, IFN-1, and CK5B in response to VHSV and knowing that blood IgM+ cells also express TLR3 constitutively (16), we wondered whether blood B cells were similarly responsive. We first examined TLR3, IFN-1, Mx, and CK5B transcription in IgM+ cells from poly(I·C)- or VHSV-stimulated and control blood cultures after 24 h of incubation. We observed that blood IgM+ cells showed significantly increased TLR3, IFN-1, and CK5B mRNA levels after stimulation with either poly(I·C) or VHSV (Fig. 5A). There was also a significant upregulation of Mx in the case of poly(I·C)-treated cells.
FIG 5.
Blood IgM+ cells are also responsive to VHSV and poly(I·C). (A) Leukocyte cultures from blood were incubated with poly(I·C) (50 μg/ml) or VHSV (1 × 106 TCID50 ml−1) for 24 h at 20°C. IgM+ cells were then sorted, and the levels of expression of TLR3, IFN-1, Mx, and CK5B in sorted IgM+ cells were evaluated by real-time PCR. Data shown are mean gene expression levels relative to the expression level of an endogenous control (EF-1α) ± standard deviations (n = 4 to 8). (B) In some experiments, the TLR3 inhibitor BAF was added 1 h prior to the addition of the stimuli. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Subsequently, we assessed whether the induction of TLR3, IFN-1, and CK5B observed in blood IgM+ populations was mediated by intracellular TLR activation using BAF. The results showed that, in this case, BAF was capable of significantly downregulating only TLR3 transcription induced by VHSV and not that induced by poly(I·C) (Fig. 5B). Consistent with our observations in splenic IgM+ cells, BAF significantly reduced the IFN-1 transcription level elicited in blood IgM+ cells by both poly(I·C) and VHSV (Fig. 5B). On the other hand, unlike spleen IgM+ cells, blood IgM+ cells upregulated CK5B transcription in response to poly(I·C) in a BAF-sensitive fashion (Fig. 5B). These data indicate that although both spleen and blood IgM+ cells are highly responsive to VHSV and poly(I·C), there are some differences in poly(I·C) signaling between the two populations.
The effect of VHSV on IgM+ cells is dependent on viral transcription.
Because we have demonstrated that VHSV has the capacity to infect spleen IgM+ cells, starting viral transcription within the cells, we next studied whether this viral transcription was necessary for the activation of the TLR3, IFN-1, and CK5B transcription detected. We demonstrated that the induction of all three genes observed in response to VHSV in sorted IgM+ cells was dependent on virus viability, because when heat-inactivated VHSV was used, TLR3, IFN-1, and CK5B transcription levels were significantly lower than those obtained with live virus (Fig. 6A). These results seem to indicate that VHSV does not exert its transcriptional effects on IgM+ cells through cross-linking of the BCR. Furthermore, additional studies revealed that cross-linking of the BCR through incubation with anti-IgM antibody provoked a downregulation of TLR3 transcription in IgM+ cells and no significant effects on IFN-1 and CK5B transcription (Fig. 6B). Altogether, our results strongly suggest that the effects on the IFN and CK5B transcriptional responses elicited by VHSV in IgM+ B cells are dependent on viral transcription, since, as described above, we verified that no viral transcription was detected inside IgM+ cells treated with the inactivated virus (Fig. 1A).
FIG 6.
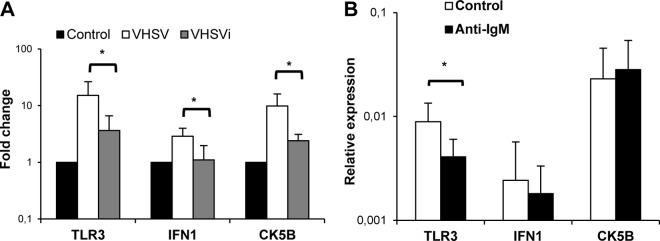
The effect of VHSV on IgM+ cells is dependent on viral transcription. (A) Spleen leukocytes were treated with VHSV, heat-inactivated VHSV (VHSVi), or medium alone, in the case of controls. After 24 h of incubation at 20°C, IgM+ cells were sorted, and the transcription of TLR3, IFN-1, and CK5B was evaluated by real-time PCR. Data from 4 to 6 independent experiments are shown as fold changes relative to the value for the unstimulated controls ± standard deviations. (B) Effect of anti-IgM on the transcription of TLR3, IFN-1, and CK5B in sorted spleen IgM+ cells after 24 h of stimulation. Data are shown as mean gene expression levels relative to the expression level of an endogenous control (EF-1α) ± standard deviations from 7 independent experiments. *, P < 0.05.
VHSV activates NF-κB in spleen IgM+ cells.
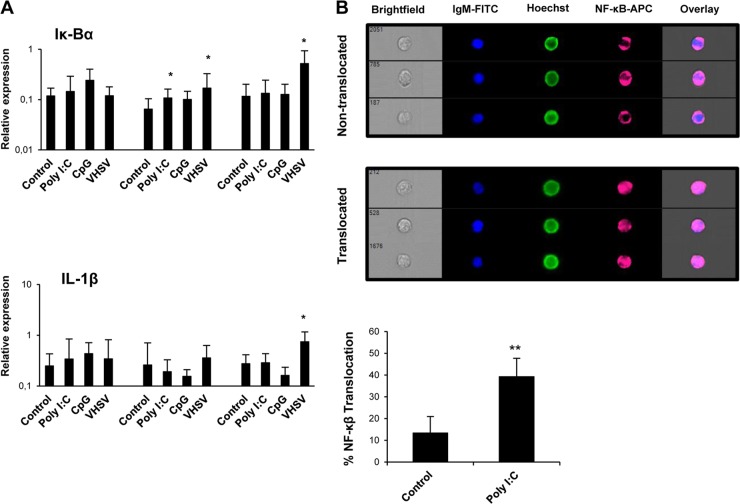
To study whether viral TLR agonists activate the transcription factor NF-κB in IgM+ B cells, we undertook two different approaches. First, we analyzed the transcription of IκBα and interleukin-1β (IL-1β) in response to different stimuli. IκBα is a member of the family of NF-κB inhibitors previously shown to be upregulated in fish when NF-κB activation occurs (39), whereas IL-1β is a proinflammatory cytokine highly responsive to NF-κB activation (40). We observed a significant upregulation of IκBα in sorted IgM+ cells from VHSV-treated cultures after 24 and 48 h of stimulation and in poly(I·C)-treated cells at 24 h (Fig. 7A). Concerning IL-1β, only VHSV was capable of significantly upregulating its transcription levels after 48 h of incubation (Fig. 7A). Additionally, we analyzed whether a dsRNA such as poly(I·C) promoted NF-κB translocation to the nucleus of IgM+ cells using an anti-NF-κB antibody and a flow cytometry approach for quantitative imaging. By the use of this methodology, translocation of NF-κB to the nucleus of B cells in response to dsRNA was confirmed (Fig. 7B). Altogether, our results strongly support the hypothesis that VHSV infection in the spleen leads to NF-κB activation in IgM+ B cells, with this being the possible mechanism through which IFN-1 production is elicited.
FIG 7.
VHSV and poly(I·C) activate NF-κB in IgM+ B cells. (A) Splenocytes were stimulated as described in the legend of Fig. 1. IgM+ B cells were then sorted, and RNA was extracted for the evaluation of IκBα and IL-1β transcription levels by real-time PCR (n = 7). Data are shown as mean gene expression levels relative to the expression level of an endogenous control (EF-1α) ± standard deviations. *, P < 0.05. (B) Translocation of NF-κB to the nucleus in trout IgM+ splenocytes following poly(I·C) stimulation. Trout splenocytes were stimulated with poly(I·C) or with medium alone for 24 h at 20°C. NF-κB translocation in IgM+ cells was determined following staining with anti-p65 and anti-trout IgM MAbs. Hoechst stain was used to visualize cell nuclei. Percentages of NF-κB translocation in trout IgM+ cells are represented together with representative images of positive nuclear translocation (Translocated) and negative translocation (Nontranslocated) using an ImageStream MKII imaging flow cytometer (Amnis). Data were collected from five different trout (n = 5) over three independent experiments. **, P < 0.05 (based on comparisons with medium-alone controls).
VHSV activates IgM+ cells to an APC profile.
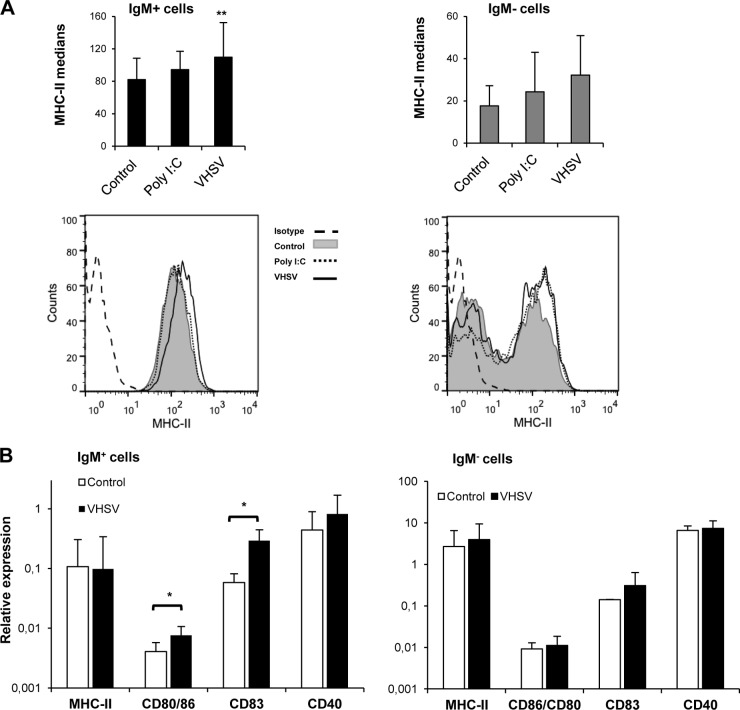
In relation to their role as antigen-presenting cells (APCs), it was previously shown that different TLR agonists, including poly(I·C), were capable of upregulating MHC-II expression in murine B cells (41). Thus, we examined whether the same was true for teleost B cells infected with VHSV. We evaluated MHC-II surface expression by flow cytometry using anti-trout MHC-II. For this, splenocyte cultures treated for 48 h with poly(I·C) or VHSV or untreated controls were costained with anti-trout MHC-II and anti-trout IgM MAbs. We observed a significant increase of MHC-II cell surface expression in VHSV-treated IgM+ cells compared to control cells but not in poly(I·C)-treated cultures (Fig. 8A). This upregulation was not detected in IgM− cells. Additionally, we evaluated the effect of VHSV on the levels of transcription of MHC-II together with those of CD80/86, CD83, and CD40, costimulatory molecules involved in antigen presentation. After 24 h of infection, VHSV provoked a significant upregulation of CD80/86 and CD83 mRNA levels in IgM+ B lymphocytes that was not visible in the IgM− cell fraction (Fig. 8B). These results strongly suggest that spleen IgM+ cells act as APCs during the course of a VHSV infection.
FIG 8.
VHSV activates IgM+ cells to an antigen-presenting phenotype. (A) MHC-II cell surface expression on IgM+ and IgM− cells after 48 h of incubation with poly(I·C) or VHSV. Data shown are mean fluorescence intensities ± standard deviations from 5 independent experiments. Representative histograms for both IgM+ and IgM− cells are shown at the bottom. (B) Splenocytes were stimulated as described in the legend of Fig. 1. IgM+ and IgM− cells were sorted after 24 h of incubation, and RNA was extracted for the evaluation of MHC-II, CD80/86, CD83, and CD40 transcription levels by real-time PCR (n = 5 to 7). Data shown are mean gene expression levels relative to the expression level of an endogenous control (EF-1α) ± standard deviations. *, P < 0.05.
Effect of VHSV on IgM+ cell proliferation.
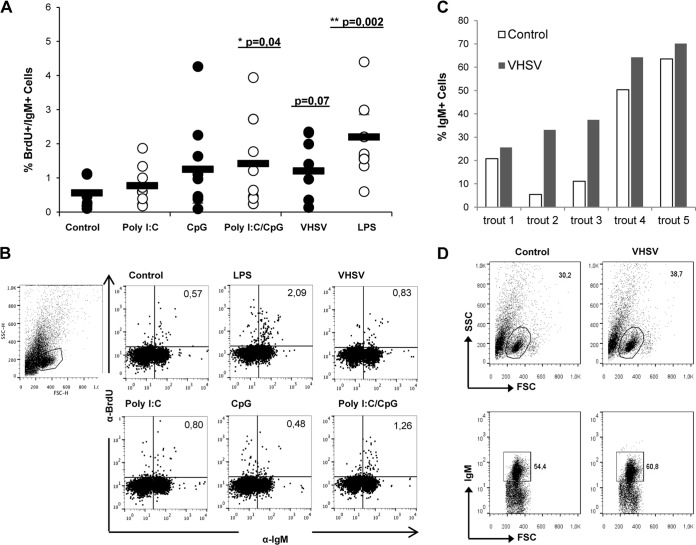
It is well known that LPS stimulation can induce B cell proliferation in fish (17); thus, we assessed whether VHSV or the different TLR agonists could also induce IgM+ proliferation on their own. For this, we incubated splenocytes with poly(I·C), CpG, poly(I·C) in combination with CpG, VHSV, or LPS (as a positive control) for 4 days, with the addition of BrdU during the last 24 h of culture. We then measured the proliferative response of IgM+ cells by costaining with anti-IgM and anti-BrdU MAbs in order to detect cell proliferation specifically in IgM+ populations. The results showed that IgM+ cells significantly proliferated in response to LPS and also in response to the combination of poly(I·C) and CpG but not when the stimuli were added independently (Fig. 9A and B), suggesting that in some cases, two independent signals are necessary for a direct effect on the proliferation of unprimed trout IgM+ cells. On the other hand, some degree of IgM+ proliferation in response to VHSV was observed in some individuals, with overall differences not being statistically significant (P = 0.08). Despite these moderate results for the number of proliferating cells, we consistently detected an increase in the total number of IgM+ cells in VHSV-infected cultures after 24 h of incubation compared to control cultures, indicating that the virus provoked increased survival of IgM+ cells (Fig. 9C and D).
FIG 9.
Effect of VHSV on IgM+ cell proliferation and survival. Spleen leukocytes were incubated with poly(I·C) (50 μg/ml), CpG (5 μg/ml), poly(I·C) combined with CpG, VHSV (1 × 106 TCID50 ml−1), or LPS (100 μg/ml) for 3 days at 20°C. After this time, cells were labeled with BrdU and incubated for a further 24 h. The percentage of proliferating (BrdU-positive) IgM+ cells was then determined as described in Materials and Methods. (A) Percentage of proliferating IgM+ cells out of all IgM+ cells. Circles indicate percentages observed in individual fish, while black bars represent mean values for each group. Significant differences between cells treated with a TLR agonist and control cells are indicated (*, P < 0.05; **, P < 0.01). (B) Dot plots from a representative fish under each condition. (C and D) Spleen leukocytes were infected in vitro with VHSV (1 × 106 TCID50 ml−1), and after 48 h, the number of IgM+ cells in the cultures was determined by flow cytometry. Data shown are percentages of IgM+ cells in control and infected cultures from 5 individual trout, along with dot plots from a representative trout.
DISCUSSION
Despite recent evidence of the important contribution of B cell TLR signaling to antibody production and antigen presentation in mammals (2, 41), there is limited information available on TLR signaling in teleost B lymphocytes. Our data indicate that VHSV, a natural pathogen of rainbow trout, can enter B lymphocytes, where it provokes multiple immediate effects on these cells, conditioning B cell functionality. VHSV was able to effectively transcribe viral genes inside IgM+ B cells, but viral translation seemed interrupted, as we did not detect viral proteins inside the cells by Western blot analysis, despite the fact that the presence of infective viral particles inside IgM+ B cells was confirmed. Live rabies virus vaccines have also been shown to infect murine and human B lymphocytes (22). In this case, even though viral proteins were detected inside B lymphocytes, the infection also turned out to be nonproductive, and no viral particles were released into the supernatants. Similarly, murine dendritic cells are also infected by rabies virus, but the infection is suppressed inside these cells, rendering it nonproductive (42). Therefore, it seems that rhabdoviruses have the capacity to infect several immune cells types, certainly affecting their functionality.
To date, eight TLRs have been identified in rainbow trout, namely, TLR1, -2, -3, -5, -7, -8, -9, and -22 (33), among which TLR3, -7, -8, and -22 have been suggested to be virus-sensing TLRs (34). TLR3 recognizes dsRNA and does not signal through the adaptor molecule MyD88, which is commonly used by other TLRs, but signals through the alternative adaptor molecule TRIF (Toll/IL-1 receptor [IL-1R] domain-containing adaptor inducing IFN-β) (43). While TLR3 is mostly intracellular, TLR22, a fish-specific TLR identified in several species (33, 34, 44), is expressed on the cell surface, where it also senses dsRNA. As for TLR3, the virus-sensing capacities of TLR22 were indirectly demonstrated by transcriptional studies (44–46), and direct evidence of its capacity to recognize long dsRNAs from the cell surface was also obtained in fugu (47). In mammals, TLR7 and TLR8 are activated by synthetic antiviral imidazoquinoline compounds and are implicated in the recognition of ssRNA, but for fish, no direct evidence for ligand specificity has been provided to date, and some contradictory results have been obtained regarding their transcriptional regulation in response to viruses and imidazoquinoline compounds (34). In our studies, only TLR3 transcription was affected by VHSV infection in teleost IgM+ cells, as it was in response to poly(I·C), a well-known TLR3 agonist. Despite the fact that none of these other TLRs were transcriptionally regulated in IgM+ B cells in response to either VHSV or any of the other TLR agonists used, we cannot rule out their implication in virus sensing in teleost B lymphocytes, exclusively on the basis of undetected transcriptional regulation. However, our results do suggest that, in contrast to what occurs in mammalian B cells, TLR3 is an important mediator of virus sensing in teleost B cells. In mammals, two independent studies determined that whereas murine follicular B cells, B1 cells, and Peyer's patch B cells showed very low levels of TLR3, marginal zone B cells transcribed TLR3 at very high levels (9, 48). In humans, TLR3 is present in CD138+ plasma cells (11) but not in naive or memory B cells (10, 11, 49). Thus, either human B cells sense dsRNA through an alternative mechanism or the engagement of TLR3 in DCs is enough to compensate for the lack of this receptor in B cells. Interestingly, a specific subset of mucosal B cells in the upper respiratory tract expressing TLR3 and responding to viral dsRNA was identified in humans (50). It was speculated that this B cell population contributed to innate resistance against certain respiratory viruses.
In this work, we have also demonstrated that trout spleen and blood IgM+ cells respond to poly(I·C) and VHSV by increasing the transcription of type I IFN. In mammals, there are only a few reports of IFN production by B cells, since previous studies pointed to DCs as the main IFN-producing leukocytes (51, 52). In both spleen and blood IgM+ cells, this upregulation of IFN-1 transcription was significantly reduced by BAF, revealing the implication of a receptor located in the endosomal compartment. The fact that mammalian endosomal TLR3 is known to induce the production of type I IFNs (53) and that it was transcriptionally regulated in our studies strongly suggest that IFN induction in trout IgM+ cells is a consequence of TLR3 signaling. However, we cannot rule out the implication of other endosomal TLRs, such as TLR7 and TLR8, in VHSV detection (54), because studies performed with mammalian rhabdoviruses such as vesicular stomatitis virus (VSV) have pointed to either TLR7 (55) or TLR13 (56) as the receptor responsible for sensing the virus. Interestingly, how BAF affected the transcriptional regulation of TLR3 and CK5B in response to poly(I·C) differed in blood and spleen IgM+ populations, revealing slight differences in how these two populations sense dsRNA. These differences might be a consequence of different stages of activation/differentiation between IgM+ cells in blood and those in spleen.
Once this type I IFN has been produced by spleen IgM+ cells, in addition to its demonstrated direct antiviral activity (28), it will surely have important consequences for both the innate and acquired regulation of other cell types and for IgM+ cells themselves, such as those previously reported in mammalian models (57). For example, in mammals, IFN drives isotype switching to the IgG2 isotype (58), the antibody isotype predominantly elicited by viral infections (59), demonstrating that PAMPs polarize the antibody response to the one best suited to deal with a specific pathogen (60). Additionally, IFN-α/β has been shown to directly upregulate CD69, CD86, and CD25 molecules in B cells (61, 62); increase their survival; and lower their threshold for further activation through the BCR, with effects on calcium fluxes, IgM internalization, induction of activation markers, and proliferation (61). Therefore, it is possible that some of the effects of VHSV on B cells, such as increased survival and the activation of costimulatory molecules, are a consequence of the type I IFN elicited. Furthermore, type I IFN has been shown to precipitate autoantibody production through the polyclonal activation of autoreactive B cells (63), such as that observed in patients with systemic lupus erythematosus (64). On the other hand, it seems that the IFN-1 secreted by trout IgM+ cells does not trigger a strong antiviral state in IgM+ cells, because Mx was poorly induced in comparison to IFN-1; thus, it might be possible that the effects that IFN-1 has on a nonimmune cell are directed toward antiviral activation, whereas the effects on B cells are directed toward the activation of specific B cell functions.
Interestingly, TLR3 and IFN-1 upregulation was also detected when trout IgM+ cells were treated with CpG, even though CpG is thought to be an agonist for TLR9, and its detection by TLR3 has not been reported for any species. Since TLR3 transcription is enhanced by LPS-induced autocrine IFN-β in murine macrophages (65), it is possible that it is the IFN produced after CpG stimulation (significantly increased levels at 48 h) by IgM+ cells and/or by other immune cells from the spleen responsible for the upregulation of TLR3. Type I IFN production in response to CpG was previously demonstrated in murine B cells through an IFN regulatory factor 3 (IRF3)-dependent mechanism (52).
The CK5B chemokine was also induced in spleen and blood IgM+ cells in response to poly(I·C) or VHSV infection. Despite the difficulty in ascribing true orthologues among fish CC chemokines and their mammalian counterparts, a recent CC chemokine classification grouped rainbow trout CK5B with mammalian CCL5/RANTES genes (66). Although the specific cell targets for CK5B have not yet been described, interestingly, mammalian CCL5 is associated with the recruitment of T helper cells (67). Therefore, it seems probable that trout IgM+ cells produce this chemokine in response to viral infection to recruit T helper cells and present the virus to the recruited cells. This hypothesis also correlates with the observed activation of B cells toward an APC profile in response to VHSV infection. We demonstrated that VHSV upregulated MHC-II surface expression in IgM+ cells, even though transcriptional regulation was not detected. Even though these results seemed surprising, it was shown previously that B cells regulate MHC-II at the translational level in mammals (68). Additionally, VHSV upregulated the levels of transcription of two costimulatory molecules, CD83 and CD80/86. The latter molecule, with similar homologies to mammalian CD80 and CD86, was consequently designated CD80/86 and was shown to increase IL-2 expression levels (69). All these results point to an important role of trout B cells in VHSV presentation. The recent discovery of the phagocytic capacity of B cells led to the identification of an important role in antigen presentation of particulate antigens by teleost B lymphocytes (70, 71). Recently, an additional role of teleost B lymphocytes in the presentation of soluble antigens was revealed in zebrafish (71) and is now supported by our results.
On the other hand, as occurs in mammalian TLR3-bearing B cell populations (9, 48), the viral TLR agonists were not capable of significantly inducing B-cell-specific proliferation on their own. In the human population of TLR3-expressing B cells found in the upper respiratory tract, poly(I·C) alone induced very little proliferation, whereas in combination with B-cell-activating factor (BAFF), significant proliferation was achieved (50). Similarly, poly(I·C) had a synergistic effect on CpG-induced proliferation, suggesting that TLR3 signaling renders the cells more sensitive to further stimulation. In our experiments, VHSV upregulated IgM+ cell proliferation in some animals, but this was not consistent, and the differences were only marginally significant. However, a consistent effect on IgM+ cell survival was observed, perhaps as an indirect consequence of IFN production.
In conclusion, we have demonstrated that trout splenic IgM+ cells can be infected by VHSV, even though the infection is not productive. This early interaction of the virus with B lymphocytes activates TLR3 and IFN-1 transcription, the latter through a mechanism residing on an endosomal receptor, probably TLR3. Additionally, VHSV activated NF-κB and upregulated the transcription of CK5B, a potential homologue of CCL5, a mammalian chemokine associated with the recruitment of T helper cells. Accordingly, VHSV activated IgM+ cells toward an APC profile, increasing MHC-II expression on the cell surface and the transcription of costimulatory molecules. Our findings provide new evidence that B cells are highly responsive to viral stimuli, suggesting an important role of teleost B cell antigen presentation in rhabdovirus infections.
ACKNOWLEDGMENTS
This work was supported by the European Research Council (ERC starting grant 2011 280469). The work was also partially supported by the Natural Sciences and Engineering Council of Canada (NSERC) through a discovery grant to D.R.B. and an NSERC CGS-M scholarship to J.J.H.
We thank Niels Lorenzen (University of Aarhus, Denmark) for providing the monoclonal antibody against the VHSV N protein.
REFERENCES
- 1.Blasius AL, Beutler B. 2010. Intracellular Toll-like receptors. Immunity 32:305–315. doi: 10.1016/j.immuni.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 2.Browne EP. 2012. Regulation of B-cell responses by Toll-like receptors. Immunology 136:370–379. doi: 10.1111/j.1365-2567.2012.03587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pasare C, Medzhitov R. 2005. Control of B-cell responses by Toll-like receptors. Nature 438:364–368. doi: 10.1038/nature04267. [DOI] [PubMed] [Google Scholar]
- 4.Gavin AL, Hoebe K, Duong B, Ota T, Martin C, Beutler B, Nemazee D. 2006. Adjuvant-enhanced antibody responses in the absence of Toll-like receptor signaling. Science 314:1936–1938. doi: 10.1126/science.1135299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meyer-Bahlburg A, Khim S, Rawlings DJ. 2007. B cell intrinsic TLR signals amplify but are not required for humoral immunity. J Exp Med 204:3095–3101. doi: 10.1084/jem.20071250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jegerlehner A, Maurer P, Bessa J, Hinton HJ, Kopf M, Bachmann MF. 2007. TLR9 signaling in B cells determines class switch recombination to IgG2a. J Immunol 178:2415–2420. doi: 10.4049/jimmunol.178.4.2415. [DOI] [PubMed] [Google Scholar]
- 7.Barr TA, Brown S, Mastroeni P, Gray D. 2009. B cell intrinsic MyD88 signals drive IFN-gamma production from T cells and control switching to IgG2c. J Immunol 183:1005–1012. doi: 10.4049/jimmunol.0803706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hou B, Saudan P, Ott G, Wheeler ML, Ji M, Kuzmich L, Lee LM, Coffman RL, Bachmann MF, DeFranco AL. 2011. Selective utilization of Toll-like receptor and MyD88 signaling in B cells for enhancement of the antiviral germinal center response. Immunity 34:375–384. doi: 10.1016/j.immuni.2011.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gururajan M, Jacob J, Pulendran B. 2007. Toll-like receptor expression and responsiveness of distinct murine splenic and mucosal B-cell subsets. PLoS One 2:e863. doi: 10.1371/journal.pone.0000863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hornung V, Rothenfusser S, Britsch S, Krug A, Jahrsdorfer B, Giese T, Endres S, Hartmann G. 2002. Quantitative expression of Toll-like receptor 1-10 mRNA in cellular subsets of human peripheral blood mononuclear cells and sensitivity to CpG oligodeoxynucleotides. J Immunol 168:4531–4537. doi: 10.4049/jimmunol.168.9.4531. [DOI] [PubMed] [Google Scholar]
- 11.Dorner M, Brandt S, Tinguely M, Zucol F, Bourquin JP, Zauner L, Berger C, Bernasconi M, Speck RF, Nadal D. 2009. Plasma cell Toll-like receptor (TLR) expression differs from that of B cells, and plasma cell TLR triggering enhances immunoglobulin production. Immunology 128:573–579. doi: 10.1111/j.1365-2567.2009.03143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tumurkhuu G, Koide N, Dagvadorj J, Noman AS, Khuda II, Naiki Y, Komatsu T, Yoshida T, Yokochi T. 2010. B1 cells produce nitric oxide in response to a series of Toll-like receptor ligands. Cell Immunol 261:122–127. doi: 10.1016/j.cellimm.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 13.Ha SA, Tsuji M, Suzuki K, Meek B, Yasuda N, Kaisho T, Fagarasan S. 2006. Regulation of B1 cell migration by signals through Toll-like receptors. J Exp Med 203:2541–2550. doi: 10.1084/jem.20061041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fagarasan S, Honjo T. 2000. T-independent immune response: new aspects of B cell biology. Science 290:89–92. doi: 10.1126/science.290.5489.89. [DOI] [PubMed] [Google Scholar]
- 15.Cerutti A, Puga I, Cols M. 2011. Innate control of B cell responses. Trends Immunol 32:202–211. doi: 10.1016/j.it.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abos B, Castro R, Pignatelli J, Luque A, Gonzalez L, Tafalla C. 2013. Transcriptional heterogeneity of IgM(+) cells in rainbow trout (Oncorhynchus mykiss) tissues. PLoS One 8:e82737. doi: 10.1371/journal.pone.0082737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zwollo P, Cole S, Bromage E, Kaattari S. 2005. B cell heterogeneity in the teleost kidney: evidence for a maturation gradient from anterior to posterior kidney. J Immunol 174:6608–6616. doi: 10.4049/jimmunol.174.11.6608. [DOI] [PubMed] [Google Scholar]
- 18.Hu YL, Xiang LX, Shao JZ. 2010. Identification and characterization of a novel immunoglobulin Z isotype in zebrafish: implications for a distinct B cell receptor in lower vertebrates. Mol Immunol 47:738–746. doi: 10.1016/j.molimm.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 19.Borza CM, Hutt-Fletcher LM. 2002. Alternate replication in B cells and epithelial cells switches tropism of Epstein-Barr virus. Nat Med 8:594–599. doi: 10.1038/nm0602-594. [DOI] [PubMed] [Google Scholar]
- 20.Yu S, Opriessnig T, Kitikoon P, Nilubol D, Halbur PG, Thacker E. 2007. Porcine circovirus type 2 (PCV2) distribution and replication in tissues and immune cells in early infected pigs. Vet Immunol Immunopathol 115:261–272. doi: 10.1016/j.vetimm.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 21.Rua R, Betsem E, Montange T, Buseyne F, Gessain A. 2014. In vivo cellular tropism of gorilla simian foamy virus in blood of infected humans. J Virol 88:13429–13435. doi: 10.1128/JVI.01801-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lytle AG, Norton JE Jr, Dorfmeier CL, Shen S, McGettigan JP. 2013. B cell infection and activation by rabies virus-based vaccines. J Virol 87:9097–9110. doi: 10.1128/JVI.00800-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martinez-Alonso S, Martinez-Lopez A, Estepa A, Cuesta A, Tafalla C. 2011. The introduction of multi-copy CpG motifs into an antiviral DNA vaccine strongly up-regulates its immunogenicity in fish. Vaccine 29:1289–1296. doi: 10.1016/j.vaccine.2010.11.073. [DOI] [PubMed] [Google Scholar]
- 24.Montero J, Garcia J, Ordas MC, Casanova I, Gonzalez A, Villena A, Coll J, Tafalla C. 2011. Specific regulation of the chemokine response to viral hemorrhagic septicemia virus at the entry site. J Virol 85:4046–4056. doi: 10.1128/JVI.02519-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DeLuca D, Wilson M, Warr GW. 1983. Lymphocyte heterogeneity in the trout, Salmo gairdneri, defined with monoclonal antibodies to IgM. Eur J Immunol 13:546–551. doi: 10.1002/eji.1830130706. [DOI] [PubMed] [Google Scholar]
- 26.Zou J, Cunningham C, Secombes CJ. 1999. The rainbow trout Oncorhynchus mykiss interleukin-1 beta gene has a differ organization to mammals and undergoes incomplete splicing. Eur J Biochem 259:901–908. [DOI] [PubMed] [Google Scholar]
- 27.Aquilino C, Castro R, Fischer U, Tafalla C. 2014. Transcriptomic responses in rainbow trout gills upon infection with viral hemorrhagic septicemia virus (VHSV). Dev Comp Immunol 44:12–20. doi: 10.1016/j.dci.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 28.Zou J, Carrington A, Collet B, Dijkstra JM, Yoshiura Y, Bols N, Secombes C. 2005. Identification and bioactivities of IFN-gamma in rainbow trout Oncorhynchus mykiss: the first Th1-type cytokine characterized functionally in fish. J Immunol 175:2484–2494. doi: 10.4049/jimmunol.175.4.2484. [DOI] [PubMed] [Google Scholar]
- 29.Lambrechts A, Van Troys M, Ampe C. 2004. The actin cytoskeleton in normal and pathological cell motility. Int J Biochem Cell Biol 36:1890–1909. doi: 10.1016/j.biocel.2004.01.024. [DOI] [PubMed] [Google Scholar]
- 30.Rieger AM, Hall BE, Barreda DR. 2010. Macrophage activation differentially modulates particle binding, phagocytosis and downstream antimicrobial mechanisms. Dev Comp Immunol 34:1144–1159. doi: 10.1016/j.dci.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 31.Castro R, Abós B, Pignatelli J, von Gersdorff Jørgensen L, González Granja A, Buchmann K, Tafalla C. 2014. Early immune responses in rainbow trout liver upon viral hemorrhagic septicemia virus (VHSV) infection. PLoS One 9:e111084. doi: 10.1371/journal.pone.0111084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luque A, Gonzalez Granja A, Gonzalez L, Tafalla C. 2014. Establishment and characterization of a rainbow trout heart endothelial cell line with susceptibility to viral hemorrhagic septicemia virus (VHSV). Fish Shellfish Immunol 38:255–264. doi: 10.1016/j.fsi.2014.03.027. [DOI] [PubMed] [Google Scholar]
- 33.Palti Y. 2011. Toll-like receptors in bony fish: from genomics to function. Dev Comp Immunol 35:1263–1272. doi: 10.1016/j.dci.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 34.Pietretti D, Wiegertjes GF. 2014. Ligand specificities of Toll-like receptors in fish: indications from infection studies. Dev Comp Immunol 43:205–222. doi: 10.1016/j.dci.2013.08.010. [DOI] [PubMed] [Google Scholar]
- 35.Matsumoto M, Seya T. 2008. TLR3: interferon induction by double-stranded RNA including poly(I:C). Adv Drug Deliv Rev 60:805–812. doi: 10.1016/j.addr.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 36.Zou J, Secombes CJ. 2011. Teleost fish interferons and their role in immunity. Dev Comp Immunol 35:1376–1387. doi: 10.1016/j.dci.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 37.Laing KJ, Secombes CJ. 2004. Trout CC chemokines: comparison of their sequences and expression patterns. Mol Immunol 41:793–808. doi: 10.1016/j.molimm.2004.03.038. [DOI] [PubMed] [Google Scholar]
- 38.Matsumoto M, Funami K, Tanabe M, Oshiumi H, Shingai M, Seto Y, Yamamoto A, Seya T. 2003. Subcellular localization of Toll-like receptor 3 in human dendritic cells. J Immunol 171:3154–3162. doi: 10.4049/jimmunol.171.6.3154. [DOI] [PubMed] [Google Scholar]
- 39.Sangrador-Vegas A, Smith TJ, Cairns MT. 2005. Cloning and characterization of a homologue of the alpha inhibitor of NF-kappaB in rainbow trout (Oncorhynchus mykiss). Vet Immunol Immunopathol 103:1–7. doi: 10.1016/j.vetimm.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 40.Cogswell JP, Godlevski MM, Wisely GB, Clay WC, Leesnitzer LM, Ways JP, Gray JG. 1994. NF-kappa B regulates IL-1 beta transcription through a consensus NF-kappa B binding site and a nonconsensus CRE-like site. J Immunol 153:712–723. [PubMed] [Google Scholar]
- 41.Barr TA, Brown S, Ryan G, Zhao J, Gray D. 2007. TLR-mediated stimulation of APC: distinct cytokine responses of B cells and dendritic cells. Eur J Immunol 37:3040–3053. doi: 10.1002/eji.200636483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Faul EJ, Wanjalla CN, Suthar MS, Gale M, Wirblich C, Schnell MJ. 2010. Rabies virus infection induces type I interferon production in an IPS-1 dependent manner while dendritic cell activation relies on IFNAR signaling. PLoS Pathog 6:e1001016. doi: 10.1371/journal.ppat.1001016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yamamoto M, Sato S, Hemmi H, Hoshino K, Kaisho T, Sanjo H, Takeuchi O, Sugiyama M, Okabe M, Takeda K, Akira S. 2003. Role of adaptor TRIF in the MyD88-independent Toll-like receptor signaling pathway. Science 301:640–643. doi: 10.1126/science.1087262. [DOI] [PubMed] [Google Scholar]
- 44.Munoz I, Sepulcre MP, Meseguer J, Mulero V. 2014. Toll-like receptor 22 of gilthead seabream, Sparus aurata: molecular cloning, expression profiles and post-transcriptional regulation. Dev Comp Immunol 44:173–179. doi: 10.1016/j.dci.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 45.Ding X, Lu DQ, Hou QH, Li SS, Liu XC, Zhang Y, Lin HR. 2012. Orange-spotted grouper (Epinephelus coioides) Toll-like receptor 22: molecular characterization, expression pattern and pertinent signaling pathways. Fish Shellfish Immunol 33:494–503. doi: 10.1016/j.fsi.2012.05.034. [DOI] [PubMed] [Google Scholar]
- 46.Su J, Heng J, Huang T, Peng L, Yang C, Li Q. 2012. Identification, mRNA expression and genomic structure of TLR22 and its association with GCRV susceptibility/resistance in grass carp (Ctenopharyngodon idella). Dev Comp Immunol 36:450–462. doi: 10.1016/j.dci.2011.08.015. [DOI] [PubMed] [Google Scholar]
- 47.Matsuo A, Oshiumi H, Tsujita T, Mitani H, Kasai H, Yoshimizu M, Matsumoto M, Seya T. 2008. Teleost TLR22 recognizes RNA duplex to induce IFN and protect cells from birnaviruses. J Immunol 181:3474–3485. doi: 10.4049/jimmunol.181.5.3474. [DOI] [PubMed] [Google Scholar]
- 48.Genestier L, Taillardet M, Mondiere P, Gheit H, Bella C, Defrance T. 2007. TLR agonists selectively promote terminal plasma cell differentiation of B cell subsets specialized in thymus-independent responses. J Immunol 178:7779–7786. doi: 10.4049/jimmunol.178.12.7779. [DOI] [PubMed] [Google Scholar]
- 49.Bernasconi NL, Onai N, Lanzavecchia A. 2003. A role for Toll-like receptors in acquired immunity: up-regulation of TLR9 by BCR triggering in naive B cells and constitutive expression in memory B cells. Blood 101:4500–4504. doi: 10.1182/blood-2002-11-3569. [DOI] [PubMed] [Google Scholar]
- 50.Xu W, Santini PA, Matthews AJ, Chiu A, Plebani A, He B, Chen K, Cerutti A. 2008. Viral double-stranded RNA triggers Ig class switching by activating upper respiratory mucosa B cells through an innate TLR3 pathway involving BAFF. J Immunol 181:276–287. doi: 10.4049/jimmunol.181.1.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ronnblom L, Alm GV. 2002. The natural interferon-alpha producing cells in systemic lupus erythematosus. Hum Immunol 63:1181–1193. doi: 10.1016/S0198-8859(02)00757-7. [DOI] [PubMed] [Google Scholar]
- 52.Oganesyan G, Saha SK, Pietras EM, Guo B, Miyahira AK, Zarnegar B, Cheng G. 2008. IRF3-dependent type I interferon response in B cells regulates CpG-mediated antibody production. J Biol Chem 283:802–808. doi: 10.1074/jbc.M704755200. [DOI] [PubMed] [Google Scholar]
- 53.Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. 2001. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature 413:732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 54.Kuznik A, Bencina M, Svajger U, Jeras M, Rozman B, Jerala R. 2011. Mechanism of endosomal TLR inhibition by antimalarial drugs and imidazoquinolines. J Immunol 186:4794–4804. doi: 10.4049/jimmunol.1000702. [DOI] [PubMed] [Google Scholar]
- 55.Ahmed M, Mitchell LM, Puckett S, Brzoza-Lewis KL, Lyles DS, Hiltbold EM. 2009. Vesicular stomatitis virus M protein mutant stimulates maturation of Toll-like receptor 7 (TLR7)-positive dendritic cells through TLR-dependent and -independent mechanisms. J Virol 83:2962–2975. doi: 10.1128/JVI.02030-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shi Z, Cai Z, Sanchez A, Zhang T, Wen S, Wang J, Yang J, Fu S, Zhang D. 2011. A novel Toll-like receptor that recognizes vesicular stomatitis virus. J Biol Chem 286:4517–4524. doi: 10.1074/jbc.M110.159590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rizza P, Moretti F, Belardelli F. 2010. Recent advances on the immunomodulatory effects of IFN-alpha: implications for cancer immunotherapy and autoimmunity. Autoimmunity 43:204–209. doi: 10.3109/08916930903510880. [DOI] [PubMed] [Google Scholar]
- 58.Finkelman FD, Svetic A, Gresser I, Snapper C, Holmes J, Trotta PP, Katona IM, Gause WC. 1991. Regulation by interferon alpha of immunoglobulin isotype selection and lymphokine production in mice. J Exp Med 174:1179–1188. doi: 10.1084/jem.174.5.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Coutelier JP, van der Logt JT, Heessen FW, Warnier G, Van Snick J. 1987. IgG2a restriction of murine antibodies elicited by viral infections. J Exp Med 165:64–69. doi: 10.1084/jem.165.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Janeway CA., Jr 1989. Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harbor Symp Quant Biol 54(Part 1):1–13. [DOI] [PubMed] [Google Scholar]
- 61.Braun D, Caramalho I, Demengeot J. 2002. IFN-alpha/beta enhances BCR-dependent B cell responses. Int Immunol 14:411–419. doi: 10.1093/intimm/14.4.411. [DOI] [PubMed] [Google Scholar]
- 62.Coro ES, Chang WL, Baumgarth N. 2006. Type I IFN receptor signals directly stimulate local B cells early following influenza virus infection. J Immunol 176:4343–4351. doi: 10.4049/jimmunol.176.7.4343. [DOI] [PubMed] [Google Scholar]
- 63.Ronnblom LE, Alm GV, Oberg KE. 1991. Autoimmunity after alpha-interferon therapy for malignant carcinoid tumors. Ann Intern Med 115:178–183. doi: 10.7326/0003-4819-115-3-178. [DOI] [PubMed] [Google Scholar]
- 64.Ronnblom L, Alm GV, Eloranta ML. 2011. The type I interferon system in the development of lupus. Semin Immunol 23:113–121. doi: 10.1016/j.smim.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 65.Heinz S, Haehnel V, Karaghiosoff M, Schwarzfischer L, Muller M, Krause SW, Rehli M. 2003. Species-specific regulation of Toll-like receptor 3 genes in men and mice. J Biol Chem 278:21502–21509. doi: 10.1074/jbc.M301476200. [DOI] [PubMed] [Google Scholar]
- 66.Peatman E, Liu Z. 2007. Evolution of CC chemokines in teleost fish: a case study in gene duplication and implications for immune diversity. Immunogenetics 59:613–623. doi: 10.1007/s00251-007-0228-4. [DOI] [PubMed] [Google Scholar]
- 67.Sallusto F, Kremmer E, Palermo B, Hoy A, Ponath P, Qin S, Forster R, Lipp M, Lanzavecchia A. 1999. Switch in chemokine receptor expression upon TCR stimulation reveals novel homing potential for recently activated T cells. Eur J Immunol 29:2037–2045. [DOI] [PubMed] [Google Scholar]
- 68.Matsuki Y, Ohmura-Hoshino M, Goto E, Aoki M, Mito-Yoshida M, Uematsu M, Hasegawa T, Koseki H, Ohara O, Nakayama M, Toyooka K, Matsuoka K, Hotta H, Yamamoto A, Ishido S. 2007. Novel regulation of MHC class II function in B cells. EMBO J 26:846–854. doi: 10.1038/sj.emboj.7601556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang YA, Hikima J, Li J, LaPatra SE, Luo YP, Sunyer JO. 2009. Conservation of structural and functional features in a primordial CD80/86 molecule from rainbow trout (Oncorhynchus mykiss), a primitive teleost fish. J Immunol 183:83–96. doi: 10.4049/jimmunol.0900605. [DOI] [PubMed] [Google Scholar]
- 70.Li J, Barreda DR, Zhang YA, Boshra H, Gelman AE, Lapatra S, Tort L, Sunyer JO. 2006. B lymphocytes from early vertebrates have potent phagocytic and microbicidal abilities. Nat Immunol 7:1116–1124. doi: 10.1038/ni1389. [DOI] [PubMed] [Google Scholar]
- 71.Zhu LY, Lin AF, Shao T, Nie L, Dong WR, Xiang LX, Shao JZ. 2014. B cells in teleost fish act as pivotal initiating APCs in priming adaptive immunity: an evolutionary perspective on the origin of the B-1 cell subset and B7 molecules. J Immunol 192:2699–2714. doi: 10.4049/jimmunol.1301312. [DOI] [PubMed] [Google Scholar]