Abstract
Background:
New matrix-associated autologous chondrocyte transplantation (MACT) techniques may facilitate the treatment of chondral defects in talar cartilage and provide good clinical outcome in the long term. The aim of this prospective case series was to monitor the clinical outcome after autologous chondrocyte transplantation (ACT) and MACT in the ankle to gain data on the mid-term efficacy of the procedure.
Methods:
Seventeen cases of talar cartilage defects were assessed with the American Orthopaedic Foot and Ankle Score (AOFAS), a modified Cincinnati score, and a subjective ankle-hindfoot score (AHS) at a mean of 61 (24-135) months after surgery. Nine patients consented to an additional magnetic resonance imaging (MRI) exam, including T2 mapping at 3T. ACT was carried out with a periosteal flap (4 cases) or with a matrix-assisted ACT technique (Hyalograft C; 13 cases).
Results:
Significant improvement was found in all cases. The AOFAS improved from 50.0 to 87.3, the AHS from 43.8 to 84.1, and the modified Cincinnati score from 2.9 to 6.9. MRI data demonstrated good defect filling, and T2 mapping results indicated that the collagen and water content of the repair tissue was comparable to adjacent cartilage.
Discussion:
MACT and ACT in the ankle can provide good and excellent long-term outcome and resulted in repair tissue with T2 properties similar to native cartilage in the majority of cases. Matrix-assisted implantation with the hyaluronan matrix allows for a less invasive surgical procedure.
Level of evidence:
4; prospective case series study.
Keywords: ACI, autologous chondrocyte transplantation, ankle, cartilage repair, T2 mapping, 3T
Cartilage lesions in the talus frequently result from trauma1,2; arthroscopic assessment of 288 consecutive cases with ankle joint fractures revealed that 79.2% had cartilage lesions, and a retrospective magnetic resonance imaging (MRI) analysis of 430 cases of twisted ankles demonstrated that 50% were affected by osteochondral lesions associated with ligament damage.3 In contrast, approximately 7% to 15% of patients with osteochondral lesions in the talus have no previous history of trauma and mostly relate to osteochondritis dissecans (OCD).1 The overall frequency of osteochondral lesions in the ankle has undoubtedly been underestimated.4
Although defects can often be asymptomatic, continuing overuse, joint instability, and repetitive trauma can establish a chronic symptomatic cartilage defect that may eventually result in osteoarthritis. It is worth noting that no long-term study of untreated osteochondral defects demonstrating progressive deterioration is available at this time.5 However, surgical treatment of the defect is essential to relieve pain and has the goal to prevent the onset of osteoarthritis.
Surgical treatment options include refixation or removal of loose fragments, microfracture or drilling of the defect, mosaicplasty, and osteochondral grafting, including allograft implantation in severe cases of osteochondral destruction. Satisfactory results are reported in the majority of cases. Excision curettage and bone marrow stimulation are reported to yield an overall success rate of 85% in OCD.6 In mosaicplasty, good to excellent results are reported in 94%, but instable fixation, incomplete defect fill, and incongruent repair site surface in osteochondral grafting as well as immunological problems in allograft procedures remain inherent limitations of mosaicplasty7; furthermore, grafting procedures often require osteotomy of the medial malleolus and an extensive surgical approach, including donor site morbidity in the ipsilateral knee.7
Autologous chondrocyte transplantation (ACT) using a sutured periosteal flap over the defect site for providing a compartment for the transplanted cell suspension is a promising technique for the treatment of full-thickness focal chondral defects of the knee.8-13 The excellent long-term clinical outcomes obtained in the knee8 encouraged clinicians to use the ACT procedure also to treat chondral and osteochondral defects in the ankle joint. The short- and mid-term clinical and histological outcomes are promising,14-19 albeit on a limited number of cases.
The ACT procedure in the ankle often requires osteotomy of the medial malleolus1 to assess the defect and perform the periosteal patching. Furthermore, ACT is associated with intrinsic limitations, such as problems with harvesting and suturing of the periosteum, delamination of the graft, and periosteal hypertrophy.8,20
In matrix-associated autologous chondrocyte transplantation (MACT), the periosteal flap is replaced by biodegradable polymers.21-26 In this series, we used a tissue engineered graft (Hyalograft C; Fidia Advanced Biopolymers, Abano Terme, Italy), composed of autologous cells grown on a biodegradable, hyaluronan-based HYAFF scaffold.27-32 This engineered cartilage graft allows for a minimum exposure of the joint during the surgical procedure since it can be implanted in mini-invasive arthrotomy or in arthroscopy.14,31,33
Aside from defect filling, repair tissue (RT) composition influences clinical long-term outcome, and it is therefore of considerable interest to assess RT composition in the course of the clinical evaluation of cartilage repair techniques.34,35 Recent progress in MRI technology has yielded imaging techniques to directly visualize molecular tissue properties in cartilage repair.36,37 Among these, T2 mapping has been demonstrated to provide information on cartilage repair tissue collagen organization and water content.38,39
This study reports on the clinical outcome of 17 cases after ACT and after Hyalograft C; 9 patients also consented to MRI follow-up, including T2 mapping at 3T. The study also discusses technical issues of ACT and MACT in the ankle.
Patients and Methods
Patients
Seventeen patients with chronic symptomatic cartilage defects in the ankle (7 men, 10 women; mean age at the time of implantation 28 [19-44] years and mean body mass index 25.3 ± 4.2 [18.6-34.4] kg/m2) were included. Four cases had traumatic lesions, 12 cases had OCD, and 1 case had a defect resulting from a hemangioma. All lesions were singular and located on the talus (6 lateral, 11 medial). The mean size of the defects was 1.5 ± 0.7 cm2. The duration of symptoms before surgery was between 2 months and 15 years (mean 4 years). Twelve patients had previous surgery: 6 were treated by microfracture as first-line treatment, and 6 had obtained arthroscopic debridement. During the arthroscopic harvest procedure, loose bodies were found in 6 cases (for details, see Table 1).
Table 1.
Parameters for All Patients
| Presurgery | Postsurgery | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case | Cartilage Repair Type | First-Line Treatment | Age at Implantation | BMI, kg/m2 | Defect Size, cm2 | Pathology | OBR | Location | Surgery Date | Follow-up Period, mo | AOFAS | AHS | CIN | AOFAS | AHS | CIN |
| 1 | ACT | NO | 25 | 18.6 | 1 | Hemangioma | 4 | Lat | IV./96 | 135 | 26 | 10 | 3 | 81 | 60 | 4 |
| 2 | ACT | NO | 42 | 34.4 | 3 | OCD | 3 | Lat | III./99 | 100 | 16 | 25 | 0 | 96 | 90 | 6 |
| 3 | ACT | NO | 29 | 24.9 | 2 | OCD | 3 | Med | III./99 | 100 | 57 | 45 | 3 | 100 | 100 | 9 |
| 4 | ACT | NO | 25 | 21.6 | 2 | OCD | 4 | Med | V./01 | 87 | 28 | 30 | 3 | 87 | 90 | 8 |
| 5 | Hyalograft | MFX | 33 | 27.8 | 1 | Trauma | 4 | Lat | III./05 | 38 | 60 | 60 | 2 | 85 | 70 | 4 |
| 6 | Hyalograft | MFX | 30 | 29.6 | 0.8 | Trauma | 4 | Lat | IV./02 | 73 | 62 | 45 | 3 | 72 | 50 | 3 |
| 7 | Hyalograft | MFX | 19 | 26.3 | 1.5 | OCD | 4 | Med | I./07 | 29 | 61 | 55 | 4 | 75 | 90 | 8 |
| 8 | Hyalograft | NO | 19 | 26.3 | 1.5 | OCD | 4 | Med | VII./06 | 35 | 61 | 55 | 4 | 100 | 100 | 8 |
| 9 | Hyalograft | MFX | 30 | 28.4 | 1.5 | OCD | 4 | Med | VII./02 | 70 | 10 | 10 | 1 | 80 | 90 | 8 |
| 10 | Hyalograft | NO | 44 | 28.3 | 2 | OCD | 3 | Med | IV./05 | 27 | 54 | 45 | 3 | 77 | 75 | 7 |
| 11 | Hyalograft | NO | 21 | 25 | 2.5 | OCD | 3 | Lat | VI./01 | 73 | 39 | 45 | 3 | 88 | 90 | 8 |
| 12 | Hyalograft | MFX | 22 | 18.6 | 1 | OCD | 4 | Med | III./04 | 50 | 34 | 30 | 2 | 85 | 85 | 8 |
| 13 | Hyalograft | NO | 23 | 22.9 | 2 | OCD | 4 | Med | VI./04 | 37 | 86 | 85 | 5 | 100 | 95 | 8 |
| 14 | Hyalograft | MFX | 31 | 24.7 | 0.8 | OCD | 4 | Med | XII./03 | 53 | 70 | 40 | 3 | 90 | 85 | 8 |
| 15 | Hyalograft | NO | 32 | 29.4 | 1.5 | Trauma | 4 | Med | IX./04 | 44 | 60 | 65 | 3 | 90 | 90 | 8 |
| 16 | Hyalograft | NO | 33 | 21.3 | 0.5 | OCD | 4 | Med | VIII./05 | 24 | 68 | 60 | 3 | 88 | 75 | 5 |
| 17 | Hyalograft | NO | 19 | 21.3 | 1.5 | Trauma | 4 | Lat | I./04 | 65 | 58 | 40 | 4 | 90 | 95 | 8 |
| Mean | 28.1 | 25.3 | 1.5 | 61.2 | 50.0 | 43.8 | 2.9 | 87.3 | 84.1 | 6.9 | ||||||
| Standard deviation | 7.5 | 4.2 | 0.7 | 31.0 | 20.8 | 19.3 | 1.2 | 8.6 | 13.8 | 1.8 | ||||||
| Minimum | 19 | 18.6 | 0.5 | 24 | 10 | 10 | 0 | 72 | 50 | 3 | ||||||
| Maximum | 44 | 34.4 | 3 | 135 | 86 | 85 | 5 | 100 | 100 | 9 | ||||||
ACT = autologous chondrocyte transplantation; AHS = ankle-hindfoot score; AOFAS = American Orthopaedic Foot and Ankle Score; BMI = body mass index; CIN = Cincinnati Rating Scale score; MFX = microfracture; NO = no specific previous cartilage surgery; OBR = outerbridge grade; OCD = osteochondritis dissecans; The roman numerals indicate the month of surgery.
Biopsy and Defect Assessment
All patients had arthroscopy after clinical and radiological examination and were biopsied for chondrocyte transplantation from the affected ankle joint. The arthroscopy was performed through an anteromedial and anterolateral arthroscopic portal using a standard technique. The joint was inspected and the cartilage defect measured and graded to assess treatment options. Grade 3 and 4 lesions (according to the Outerbridge classification) larger than 1 cm in diameter were included. The biopsy was taken from the anterior aspect of the talus from the non-weightbearing anterior cartilage rim. The cartilage sample size was a minimum of 120 mg; in the 6 cases with loose bodies, intact cartilage was harvested, but the transplants were augmented with cells from the loose bodies.
Autologous Chondrocyte Transplantation
Chondrocytes were enzymatically isolated, expanded over a period of at least 3 weeks, and subsequently transplanted as a cell suspension under a patched periosteal flap (Carticel; Genzyme Tissue Repair, Cambridge, MA) as reported in previous studies.18
Depending on the defect location, a medial or lateral miniarthrotomy was performed and the defect debrided to achieve a clean osseous ground of the defect with perpendicular borders. The periosteal flap was harvested from the adjacent distal tibia and sutured to the adjacent cartilaginous border with 6-0 Vicryl sutures to create a waterproof compartment in the defect. Finally, the chondrocyte suspension was injected into the defect site underneath the periosteal patch and closed with the last suture (Fig. 1). It may be worth noting that osteotomy was inevitable in two cases because of the suturing of the graft rather than because of the accessibility of the defect sites.
Figure 1.
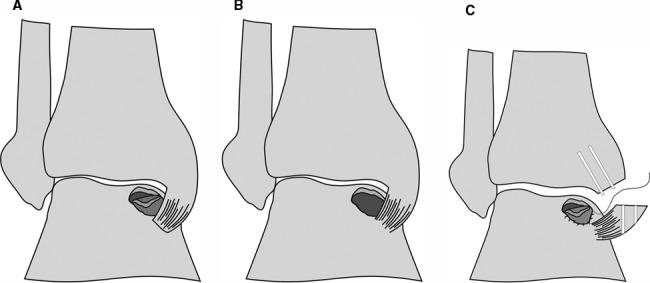
Surgical procedure of autologous chondrocyte transplantation with a periosteal flap: osteochondral defect with a (A) delaminated piece of cartilage, (B) debrided defect, and (C) malleotomy and suturing of the periosteal flap.
MACT with a Hyaluronan Matrix (Hyalograft C)
In 13 patients, the cultured cells were seeded on a hyaluronan matrix (Hyalograft C; Fidia Advanced Biopolymers). Briefly, the graft consists of a hyaluronan-based polymer that promotes cell differentiation and undergoes spontaneous hydrolysis in vivo. After 2 weeks of in vitro expansion, the chondrocytes are seeded on the scaffold. After another 2 weeks, the graft already has extracellular matrix components and is ready for implantation.26,28,32,40
The joint was opened through a medial or lateral mini-arthrotomy. Under moderate traction and plantar flexion, the defect was accessible from the anterior (Fig. 2). In 3 of 13 cases, medial malleolar osteotomy was necessary to achieve satisfactory access to the defect site. After debridement of the defect, the matrix was trimmed to fit the defect and implanted with fibrin glue fixation on the borders of the defect. The tourniquet was opened and the joint moved to verify graft stability.
Figure 2.
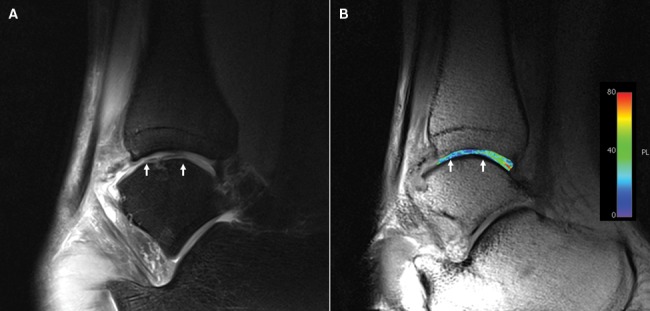
(A) Morphologic sagittal proton density-weighted image and (B) corresponding T2 map. Subchondral alterations are visible in the area of the repair site (white arrows). A homogeneous distribution of T2 values throughout is found, but values are lower at the repair site.
Six cases obtained microfracture as first-line treatment. In those cases, the cartilage sample was frozen with the option to be processed for implantation until up to 12 months after biopsy.
Postoperative Rehabilitation
Postoperatively, the patients received an immobilization plaster for 2 days to avoid early dislodgment of the grafts. After that, patients with medial malleolar osteotomy received a walker brace to allow early movement out of the brace. Non-weightbearing ambulation was allowed for 6 weeks using crutches, followed by 4 to 6 weeks of gradual weight bearing. Cycling on a stationary cycle was allowed after 6 weeks and more extensive walking after 6 months. Moderate sports such as jogging were started after 9 to 12 months, but stop-and-go activity and impact sports were not recommended until after 18 months.
Clinical Follow-up
Follow-up was carried out 61 ± 31.0 (24-135) months after implantation (from baseline to last follow-up, see Table 1). Preoperative and follow-up evaluation was performed with the American Orthopaedic Foot and Ankle Score (AOFAS; Ankle-Hindfoot Scale),41 a subjective ankle-hindfoot score,42 and the modified Cincinnati Rating Scale43 adapted for ankle patients. Statistical analysis was performed with descriptive analysis of means and standard deviation, and double-tailed, paired t tests were performed to assess statistical significance.
MRI Follow-up
Nine patients consented to additional MRI examinations at follow-up. All measurements were performed on a 3T MR unit. For morphologic imaging, an isotropic 3-dimensional (3D) Gradient Echo (True FISP) sequence with a 0.4 × 0.4 × 0.4-mm resolution was used for volumetric defect site measurements; a proton density turbo spin echo (PD-FS-TSE) sequence with an in-plane resolution of 0.3 × 0.3 mm was used to assess articular cartilage, effusion, and the subchondral bone. T2 mapping was carried out with a multiecho spin echo sequence that yielded an in-plane resolution of 0.4 × 0.4 mm with a slice thickness of 3 mm; TR was 1.000 s, and 6 different echo times (13.8 ms, 27.6 ms, 41.4 ms, 55.3 ms, 69 ms, and 82.8 ms) were used. T2 maps were calculated with a pixel-wise, monoexponential nonnegative least squares (NNLS) fit analysis (MapIt, Siemens, Munich, Germany).
An experienced musculoskeletal radiologist blinded to clinical outcome (S.T., 20 years of experience) assessed the morphologic MRI, considering defect filling volume (<25%, 25%-50%, 50%-75%, 75%-100%, 100%, and >100%), the integrity of the surface (no fissures, fissures <50%, fissures >50%, full-depth fissures), subchondral edema (minor, moderate <2 cm, severe >2 cm), and effusion.
Regarding T2 mapping, region-of-interest (ROI) analysis was carried out by assessing the full thickness of RT and reference cartilage (RC) layers in each slice. The data from all slices were then used to calculate mean RT T2 and RC T2, respectively, and relative T2 (rT2) values were calculated for the individual cases (rT2 = RT T2/RC T2).44,45
Results
Clinical Outcome
At the time of inclusion, the preoperative AOFAS was 50.0 ± 20.8 (10-86), the subjective ankle-hindfoot score was 43.8 ± 19.3 (10-85), and the modified Cincinnati Rating Scale score was 2.9 ± 1.2 (0-5). The time period between biopsy and cell implantation was 6 weeks to 6 months in regular cases; in the microfracture first-line treatment cases, the interval between biopsy and implantation was 4 to 12 months.
The follow-up period was a mean of 61 (24-135) months (4 years; range, 2-11 years) with no loss to follow-up. At the time of evaluation, all patients considered ankle joint function improved and could pursue everyday life activities without restrictions. The postoperative AOFAS was 87.3 ± 8.6 (72-100) points, the subjective ankle-hindfoot score was 84.1 ± 13.8 (50-100), and the modified Cincinnati Rating Scale score was 6.9 ± 1.8 (3-9). All clinical scores improved significantly (P < 0.001 in paired t tests; see Fig. 3).
Figure 3.
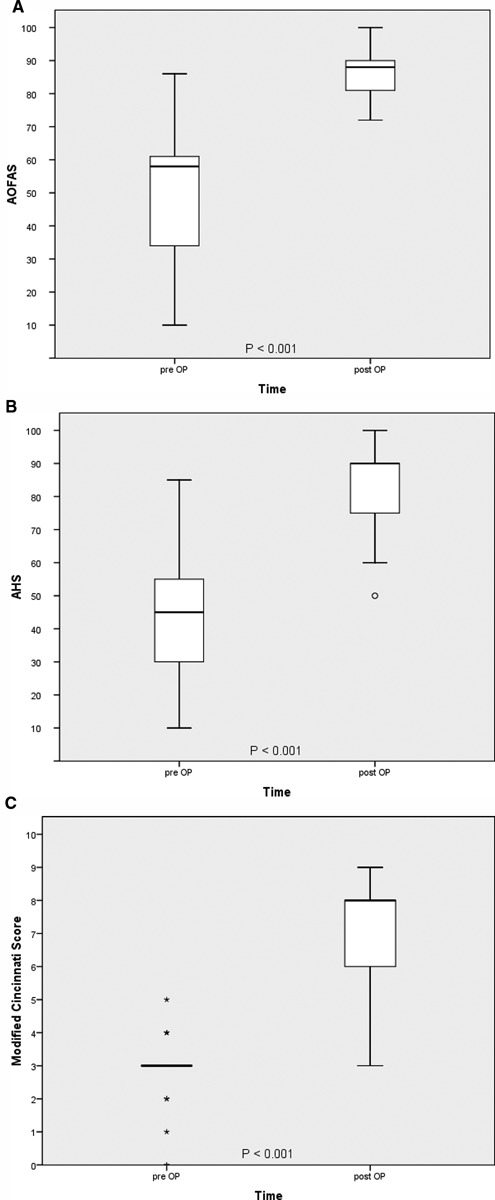
Clinical outcome in various clinical scores (A-C). Clinical improvement is highly significant in all score systems. Clinical improvement could be found in all cases.
No complications or major adverse events occurred. All osteotomies healed without complications, and 2 patients opted for screw removal. Two other patients (Hyalograft group) underwent a second-look arthroscopy. In one, a minor hypertrophy of the graft was observed, and after smoothing the graft, the patient was symptom free. The second patient required removal of a ventral osteophyte of the distal tibia to achieve a greater range of motion postoperatively.
MRI
Morphologic MRI evaluation demonstrated complete defect coverage in all cases (Table 2). Defect filling was defined as complete in 3 cases, 4 had moderate hypertrophy, and the remaining 2 cases had near-complete defect filling. All cases had an intact interface of the repair tissue and the adjacent talar cartilage. Fissures occurred in 2 cases; the remaining repair sites had intact surfaces. Interestingly, minor effusion was observed in 5 cases, and 7 cases had signs of bone marrow edema.
Table 2.
Morphologic MRI Evaluation
| Case | Age at MRI | Defect Filling (%) | Interface | Surface | Edema | Effusion | AOFAS | RT T2 | RC T2 | rT2 |
|---|---|---|---|---|---|---|---|---|---|---|
| 5 | 36 | 100 | Complete | Intact | None | No | Good | 25 | 28 | 0.89 |
| 6 | 35 | 100 | Complete | Intact | Severe | Yes | Fair | 37 | 47 | 0.78 |
| 9 | 35 | 75-100 | Complete | Intact | Minor | No | Good | 29 | 34 | 0.88 |
| 14 | 37 | 75-100 | Complete | <50% of RT depth | Moderate | No | Excellent | 25 | 35 | 0.72 |
| 15 | 36 | 100 | Complete | Intact | Moderate | Yes | Excellent | 23 | 27 | 0.87 |
| 12 | 27 | >100 | Complete | Intact | Moderate | No | Good | 33 | 41 | 0.80 |
| 7 | 21 | >100 | Complete | <50% of RT depth | None | Yes | Fair | 41 | 44 | 0.93 |
| 4 | 34 | >100 | Complete | Intact | Minor | Yes | Good | 43 | 34 | 1.26 |
| 17 | 24 | >100 | Complete | Intact | Minor | Yes | Excellent | 22 | 46 | 0.49 |
| Mean | 31.7 | 30.9 | 37.3 | 0.85 | ||||||
| Standard deviation | 6.0 | 7.9 | 7.5 | 0.21 | ||||||
| Min | 21 | 22 | 27 | 0.49 | ||||||
| Max | 37 | 43 | 47 | 1.26 |
AOFAS = American Orthopaedic Foot and Ankle Score; MRI = magnetic resonance imaging; RC = reference cartilage; RT = repair tissue; rT2 = relative T2.
The resolution of the T2 maps allowed for ROI analysis with 100 pixels minimum. RT T2 was 30.9 ± 7.9 (22-43) ms, and RC T2 was 37.3 ± 7.5 (27-47) ms. The unpaired double-tailed Student t test demonstrated no significant difference between RT and RC T2 (P = 0.095). Relative T2 (rT2) was 0.85 ± 0.21 (0.49-1.26). Please see Table 2 for details.
Discussion
This study evaluates the mid-term outcome of ACT and MACT using a hyaluronan matrix in the ankle. We are aware of the limited level of evidence associated with a nonrandomized case series study without control and of the limitations of clinical scores as a measurement of surgical treatment; however, there are few data on mid-term results after MACT in the ankle, and we felt that the inclusion of MR analysis yielded valuable information on the composition of the repair tissue.
Both ACT and MACT proved to be safe procedures and provided good to excellent outcomes in the majority of cases. No severe adverse events or graft failures occurred during follow-up.
Significant improvement was found in all clinical scores. The AOFAS predominantly assesses pain, swelling, and symptoms of instability. Both subjective and objective criteria are assessed. The outcome was excellent in 7, good in 7, and fair in 3 cases (82% good and excellent),46 and all patients reported to be satisfied with the outcome in everyday life activities. The subjective ankle-hindfoot score is similar to the AOFAS but concentrates on subjective criteria; there was a trend toward lower scores, but only 2 cases differed substantially (cases 1 and 6; see Table 1). The modified Cincinnati score was used to assess the level of activity; patients 1, 4, and 6 reported moderate limitations to daily life and avoided sports (modified Cincinnati score 3 or 4 in 17.6%). Two patients reported limitations to sports (modified Cincinnati score 5 or 6 in 11.7%), and 11 patients reported sport activity on a regular basis with almost no restrictions (modified Cincinnati score 7 or 8 in 64.7%). Case 3 reported regular sports without any restrictions (ACT; more than 8 years post-op).
Clinical results after bone marrow–stimulating techniques in the ankle have high overall success rates. Saxena et al.46 report that in athletes they achieved good to excellent outcomes after microfracture in all cases (AOFAS higher than 80 points; 26 cases, Hepple 1-4 lesions). In 20 cases with Hepple 5 defects treated with autogeneous bone grafts, they report good to excellent outcomes in 90%. The average follow-up period of their series was 32 (24-55) months.
In contrast, Becher et al.47 found good to excellent results in 79% at 5.8 ± 2.0 years in a series of 45 cases with heterogeneous age and body mass index (BMI); they report that BMI correlated with clinical outcome, whereas age did not. In the treatment of OCD, excision curettage and bone marrow stimulation are reported to yield an overall success rate of 85%.6
Studies on the clinical efficacy of ACT or Hyalograft mostly concern short- to mid-term outcome or technical aspects. Giannini et al.15 reported on 8 patients with good clinical results at 2 years, Whittaker et al.,16 found good results in 10 cases at 3 years, and Peterson et al.18 found good and excellent results in 75%. A prospective series of 46 cases treated with Hyalograft C reports good and excellent outcomes in more than 80% at 36 months,48 but long-term outcome has scarcely been reported on. In the current series, the follow-up period was 6 to 11 years in ACT and 2 to 6 years in MACT. The overall outcomes were comparable to the results reported by other investigators, suggesting stable clinical outcome. In light of the results after bone marrow–stimulating surgery in the ankle, it remains to be determined if ACT techniques will actually yield better results in the ankle in the long term.
It may be of interest to note that in contrast to ACT, the MACT technique was feasible without osteotomy. Biopsy from the same joint was successfully used for the isolation of the chondrocytes. In concordance with the findings of Giannini et al.,49 we did not observe any disadvantage or harvest site morbidity related to the biopsy.
The mini-arthrotomy was performed medial or lateral according to the defect location and allowed for good access to the defect. In cases where the defect extended to the dorsal aspect of the talus, we used a scope to facilitate exact debridement. The matrix was fitted by templating of the defect. The implantation of the soft matrix was then carried out easily; in the cases with osteochondral defects (maximum depth 5 mm), multiple layers were used to ensure complete defect filling.
The long-term outcome of cartilage repair in the knee has been found to depend on the composition of the repair tissue; high proteoglycan content is generally assumed to provide more stable tissue and thus provide better outcome in the long term.34,35,50-53 At this time, histological biopsy evaluation of Hyalograft RT in the ankle is very limited. Only 3 cases after Hyalograft C have been reported in the ankle; hyaline-like RT was reported in all cases, and the International Cartilage Repair Society repair categories were normal, nearly normal, and nearly normal.48
A T2 mapping protocol at 3T that allows for high resolutions, allowing for a separate assessment of the talar and tibial cartilage layers, has been reported recently.54,55 Briefly, T2 relaxation of cartilage is dominated by the dipolar interaction of water molecules56 and is therefore influenced both by the free water content of the tissue and by collagen anisotropy.57,58 Evaluation with polarized light microscopy has demonstrated a strong agreement of T2 variation across the cartilage layers and the zonal organization of the cartilage matrix and of cartilage repair tissue.58,59 T2 is very sensitive, however, and unspecific due to the numerous factors contributing to transverse relaxation in cartilage60; relative T2 directly compares the repair tissue to the adjacent native cartilage and has been introduced in the literature to compare repair tissue composition among different cases.44,45
In a case series of 12 patients after MACT in the ankle with a mean age of 32.8 ± 8.5 years and post-op follow-up interval of 19.8 ± 12.6 (6-54) months, the T2 values of the talar control cartilage and repair sites were 47.6 ± 9.3 ms and 50.1 ± 8.0 ms, respectively.54 Interestingly, lower values were found in the current series (Table 2). Still, rT2 values indicate that the repair tissue water and collagen are close to adjacent cartilage in most of the cases except for case 17; an rT2 of 0.49 indicates fibrous repair tissue.39
Regarding morphologic MRI outcome, defect filling, graft interface, and repair tissue surface can be considered excellent. Mild effusion was found in 5 cases. It is surprising that subchondral edema was found frequently. Minor and moderate edema (below 2 cm in diameter) apparently did not influence the clinical outcome (good or excellent outcomes in the AOFAS); in contrast, patient 6 (severe edema, fair outcome) reported avoiding sports and stress on the ankle. The current sample size is too small to permit a statistical evaluation of a possible relationship between bone marrow edema and clinical outcome. Corresponding MRI analyses after MACT in the knee demonstrate that unfavorable clinical results are indeed associated with the edema.5
In summary, ACT and MACT have the potential to provide excellent outcome at mid-term. Good to excellent outcome was found in 82% in this series. Matrix-assisted implantation allows for a less invasive procedure and can provide good defect filling as well as repair tissue with a water and collagen content similar to the adjacent cartilage. Further studies in higher numbers, however, will be required to further substantiate these results.
Footnotes
Acknowledgments and Funding: Funding of the MRI assessment was covered by a grant from the Medical Scientific Fund of the Mayor of the City of Vienna, Project Number 08011.
Declaration of Conflicting Interests: Stefan Nehrer and Ronald Dorotka have received travel support by Fidia Advanced Biomaterials, Abano Terme, Italy.
References
- 1. Mandelbaum BR, Gerhardt MB, Peterson L. Autologous chondrocyte implantation of the talus. Arthroscopy. 2003;19(suppl 1):129-37. [DOI] [PubMed] [Google Scholar]
- 2. Ronga M, Grassi FA, Montoli C, Bulgheroni P, Genovese E, Cherubino P. Treatment of deep cartilage defects of the ankle with matrix-induced autologous chondrocyte implantation (MACI). Foot Ankle Surg. 2005;11:29-33. [Google Scholar]
- 3. Toth AP, Easley ME. Ankle chondral injuries and repair. Foot Ankle Clin. 2000;5:799-840. [PubMed] [Google Scholar]
- 4. Hintermann B, Boss A, Schafer D. Arthroscopic findings in patients with chronic ankle instability. Am J Sports Med. 2002;30:402-9. [DOI] [PubMed] [Google Scholar]
- 5. Gomoll AH, Madry H, Knutsen G, van Dijk N, Seil R, Brittberg M, et al. The subchondral bone in articular cartilage repair: current problems in the surgical management. Knee Surg Sports Traumatol Arthrosc. 2010;18:434-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zengerink M, Struijs PA, Tol JL, van Dijk CN. Treatment of osteochondral lesions of the talus: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2010;18:238-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hangody L, Feczko P, Bartha L, Bodó G, Kish G. Mosaicplasty for the treatment of articular defects of the knee and ankle. Clin Orthop Relat Res. 2001;391(suppl):S328-36. [DOI] [PubMed] [Google Scholar]
- 8. Peterson L, Brittberg M, Kiviranta I, Akerlund EL, Lindahl A. Autologous chondrocyte transplantation: biomechanics and long-term durability. Am J Sports Med. 2002;30:2-12. [DOI] [PubMed] [Google Scholar]
- 9. Knutsen G, Drogset JO, Engebretsen L, Grøntvedt T, Isaksen V, Ludvigsen TC, et al. A randomized trial comparing autologous chondrocyte implantation with microfracture: findings at five years. J Bone Joint Surg Am. 2007;89:2105-12. [DOI] [PubMed] [Google Scholar]
- 10. Knutsen G, Engebretsen L, Ludvigsen TC, Drogset JO, Grøntvedt T, Solheim E, et al. Autologous chondrocyte implantation compared with microfracture in the knee: a randomized trial. J Bone Joint Surg Am. 2004;86A:455-64. [DOI] [PubMed] [Google Scholar]
- 11. Saris DB, Vanlauwe J, Victor J, Almqvist KF, Verdonk R, Bellemans J, et al. Treatment of symptomatic cartilage defects of the knee: characterized chondrocyte implantation results in better clinical outcome at 36 months in a randomized trial compared to microfracture. Am J Sports Med. 2009;37(suppl 1):10S-19S. [DOI] [PubMed] [Google Scholar]
- 12. Saris DB, Vanlauwe J, Victor J, Haspl M, Bohnsack M, Fortems Y, et al. Characterized chondrocyte implantation results in better structural repair when treating symptomatic cartilage defects of the knee in a randomized controlled trial versus microfracture. Am J Sports Med. 2008;36:235-46. [DOI] [PubMed] [Google Scholar]
- 13. Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331:889-95. [DOI] [PubMed] [Google Scholar]
- 14. Giannini S, Vannini F. Operative treatment of osteochondral lesions of the talar dome: current concepts review. Foot Ankle Int. 2004;25:168-75. [DOI] [PubMed] [Google Scholar]
- 15. Giannini S, Buda R, Grigolo B, Vannini F. Autologous chondrocyte transplantation in osteochondral lesions of the ankle joint. Foot Ankle Int. 2001;22:513-7. [DOI] [PubMed] [Google Scholar]
- 16. Whittaker JP, Smith G, Makwana N, Roberts S, Harrison PE, Laing P, et al. Early results of autologous chondrocyte implantation in the talus. J Bone Joint Surg Br. 2005;87:179-83. [DOI] [PubMed] [Google Scholar]
- 17. Giannini S, Buda R, Faldini C, Vannini F, Bevoni R, Grandi G, et al. Surgical treatment of osteochondral lesions of the talus in young active patients. J Bone Joint Surg Am. 2005;87(suppl 2):28-41. [DOI] [PubMed] [Google Scholar]
- 18. Peterson L, Brittberg M, Lindahl A. Autologous chondrocyte transplantation of the ankle. Foot Ankle Clin. 2003;8:291-303. [DOI] [PubMed] [Google Scholar]
- 19. Dorotka R, Kotz R, Trattnig S, Nehrer S. Mid-term results of autologous chondrocyte transplantation in knee and ankle: a one- to six-year follow-up study [in German]. Z Rheumatol. 2004;63:385-92. [DOI] [PubMed] [Google Scholar]
- 20. Peterson L, Minas T, Brittberg M, Nilsson A, Sjogren-Jansson E, Lindahl A. Two- to 9-year outcome after autologous chondrocyte transplantation of the knee. Clin Orthop Relat Res. 2000;374:212-34. [DOI] [PubMed] [Google Scholar]
- 21. Freed LE, Grande DA, Lingbin Z, Emmanual J, Marquis JC, Langer R. Joint resurfacing using allograft chondrocytes and synthetic biodegradable polymer scaffolds. J Biomed Mater Res. 1994;28:891-9. [DOI] [PubMed] [Google Scholar]
- 22. Kim WS, Vacanti JP, Cima L, Mooney D, Upton J, Puelacher WC, et al. Cartilage engineered in predetermined shapes employing cell transplantation on synthetic biodegradable polymers. Plast Reconstr Surg. 1994;94:233-7; discussion 238-40. [PubMed] [Google Scholar]
- 23. Robinson D, Efrat M, Mendes DG, Halperin N, Nevo Z. Implants composed of carbon fiber mesh and bone-marrow-derived, chondrocyte-enriched cultures for joint surface reconstruction. Bull Hosp Jt Dis. 1993;53:75-82. [PubMed] [Google Scholar]
- 24. Sittinger M, Reitzel D, Dauner M, Hierlemann H, Hammer C, Kastenbauer E, et al. Resorbable polyesters in cartilage engineering: affinity and biocompatibility of polymer fiber structures to chondrocytes. J Biomed Mater Res. 1996;33:57-63. [DOI] [PubMed] [Google Scholar]
- 25. Toolan BC, Frenkel SR, Pachence JM, Yalowitz L, Alexander H. Effects of growth-factor-enhanced culture on a chondrocyte-collagen implant for cartilage repair. J Biomed Mater Res. 1996;31:273-80. [DOI] [PubMed] [Google Scholar]
- 26. Campoccia D, Doherty P, Radice M, Brun P, Abatangelo G, Williams DF. Semisynthetic resorbable materials from hyaluronan esterification. Biomaterials. 1998;19:2101-27. [DOI] [PubMed] [Google Scholar]
- 27. Aigner J, Tegeler J, Hutzler P, Campoccia D, Pavesio A, Hammer C, et al. Cartilage tissue engineering with novel nonwoven structured biomaterial based on hyaluronic acid benzyl ester. J Biomed Mater Res. 1998;42:172-81. [DOI] [PubMed] [Google Scholar]
- 28. Benedetti L, Cortivo R, Berti T, Berti A, Pea F, Mazzo M, et al. Biocompatibility and biodegradation of different hyaluronan derivatives (Hyaff) implanted in rats. Biomaterials. 1993;14:1154-60. [DOI] [PubMed] [Google Scholar]
- 29. Cortivo R, Brun P, Rastrelli A, Abatangelo G. In vitro studies on biocompatibility of hyaluronic acid esters. Biomaterials. 1991;12:727-30. [DOI] [PubMed] [Google Scholar]
- 30. Brun P, Abatangelo G, Radice M, Zacchi V, Guidolin D, Daga Gordini D, et al. Chondrocyte aggregation and reorganization into three-dimensional scaffolds. J Biomed Mater Res. 1999;46:337-46. [DOI] [PubMed] [Google Scholar]
- 31. Grigolo B, Lisignoli G, Piacentini A, Fiorini M, Gobbi P, Mazzotti G, et al. Evidence for redifferentiation of human chondrocytes grown on a hyaluronan-based biomaterial (HYAff 11): molecular, immunohistochemical and ultrastructural analysis. Biomaterials. 2002;23:1187-95. [DOI] [PubMed] [Google Scholar]
- 32. Pavesio A, Abatangelo G, Borrione A, Brocchetta D, Hollander AP, Kon E, et al. Hyaluronan-based scaffolds (Hyalograft C) in the treatment of knee cartilage defects: preliminary clinical findings. Novartis Found Symp. 2003;249:203-17; discussion 229-33, 234-38, 239-41. [PubMed] [Google Scholar]
- 33. Marcacci M, Zaffagnini S, Kon E, Visani A, Iacono F, Loreti I. Arthroscopic autologous chondrocyte transplantation: technical note. Knee Surg Sports Traumatol Arthrosc. 2002;10:154-159. [DOI] [PubMed] [Google Scholar]
- 34. Henderson I, Lavigne P, Valenzuela H, Oakes B. Autologous chondrocyte implantation: superior biologic properties of hyaline cartilage repairs. Clin Orthop Relat Res. 2007;455:253-261. [DOI] [PubMed] [Google Scholar]
- 35. Nehrer S, Spector M, Minas T. Histologic analysis of tissue after failed cartilage repair procedures. Clin Orthop Relat Res. 1999;365:149-162. [DOI] [PubMed] [Google Scholar]
- 36. Domayer SE, Welsch GH, Dorotka R, Mamisch TC, Marlovits S, Szomolanyi P, et al. MRI monitoring of cartilage repair in the knee: a review. Semin Musculoskelet Radiol. 2008;12:302-17. [DOI] [PubMed] [Google Scholar]
- 37. Welsch GH, Mamisch TC, Hughes T, Domayer S, Marlovits S, Trattnig S. Advanced morphological and biochemical magnetic resonance imaging of cartilage repair procedures in the knee joint at 3 Tesla. Semin Musculoskelet Radiol. 2008;12:196-211. [DOI] [PubMed] [Google Scholar]
- 38. Trattnig S, Mamisch TC, Welsch GH, Glaser C, Szomolanyi P, Gebetsroither S, et al. Quantitative T2 mapping of matrix-associated autologous chondrocyte transplantation at 3 Tesla: an in vivo cross-sectional study. Invest Radiol. 2007;42:442-448. [DOI] [PubMed] [Google Scholar]
- 39. Welsch GH, Mamisch TC, Domayer SE, Dorotka R, Kutscha-Lissberg F, Marlovits S, et al. Cartilage T2 assessment at 3-T MR imaging: in vivo differentiation of normal hyaline cartilage from reparative tissue after two cartilage repair procedures—initial experience. Radiology. 2008;247:154-61. [DOI] [PubMed] [Google Scholar]
- 40. Girotto D, Urbani S, Brun P, Renier D, Barbucci R, Abatangelo G. Tissue-specific gene expression in chondrocytes grown on three-dimensional hyaluronic acid scaffolds. Biomaterials. 2003;24:3265-75. [DOI] [PubMed] [Google Scholar]
- 41. Kitaoka HB, Alexander IJ, Adelaar RS, Nunley JA, Myerson MS, Sanders M. Clinical rating systems for the ankle-hindfoot, midfoot, hallux, and lesser toes. Foot Ankle Int. 1994;15:349-53. [DOI] [PubMed] [Google Scholar]
- 42. Wülker N, Wirth CJ, Rudert M. Die Behandlung der fibularen Kapselband-Ruptur. Eine Multicenter-Studie. Z.Orthop 1996;134:149-154. [DOI] [PubMed] [Google Scholar]
- 43. Roos E. Rigorous statistical reliability, validity, and responsiveness testing of the Cincinnati Knee Rating System in 350 subjects with uninjured, injured, or anterior cruciate ligament-reconstructed knee. Am J Sports Med. 2000;28:436-8. [PubMed] [Google Scholar]
- 44. Domayer SE, Kutscha-Lissberg F, Welsch G, Dorotka R, Nehrer S, Gabler C, et al. T2 mapping in the knee after microfracture at 3.0 T: correlation of global T2 values and clinical outcome—preliminary results. Osteoarthritis Cartilage. 2008;16:903-8. [DOI] [PubMed] [Google Scholar]
- 45. Welsch GH, Trattnig S, Domayer S, Marlovits S, White LM, Mamisch TC. Multimodal approach in the use of clinical scoring, morphological MRI and biochemical T2-mapping and diffusion-weighted imaging in their ability to assess differences between cartilage repair tissue after microfracture therapy and matrix-associated autologous chondrocyte transplantation: a pilot study. Osteoarthritis Cartilage. 2009;17:1219-27. [DOI] [PubMed] [Google Scholar]
- 46. Saxena A, Eakin C. Articular talar injuries in athletes: results of microfracture and autogenous bone graft. Am J Sports Med. 2007;35:1680-7. [DOI] [PubMed] [Google Scholar]
- 47. Becher C, Driessen A, Hess T, Longo UG, Maffulli N, Thermann H. Microfracture for chondral defects of the talus: maintenance of early results at midterm follow-up. Knee Surg Sports Traumatol Arthrosc. 2010;18:656-63. [DOI] [PubMed] [Google Scholar]
- 48. Giannini S, Buda R, Vannini F, Di Caprio F, Grigolo B. Arthroscopic autologous chondrocyte implantation in osteochondral lesions of the talus: surgical technique and results. Am J Sports Med. 2008;36:873-80. [DOI] [PubMed] [Google Scholar]
- 49. Giannini S, Buda R, Grigolo B, Vannini F, De Franceschi L, Facchini A. The detached osteochondral fragment as a source of cells for autologous chondrocyte implantation (ACI) in the ankle joint. Osteoarthritis Cartilage. 2005;13:601-7. [DOI] [PubMed] [Google Scholar]
- 50. Kangarlu A, Gahunia HK. Magnetic resonance imaging characterization of osteochondral defect repair in a goat model at 8 T. Osteoarthritis Cartilage. 2006;14:52-62. [DOI] [PubMed] [Google Scholar]
- 51. Knutsen G, Drogset JO, Engebretsen L, Grontvedt T, Isaksen V, Ludvigsen TC, et al. A randomized trial comparing autologous chondrocyte implantation with microfracture: findings at five years. J Bone Joint Surg Am. 2007;89:2105-12. [DOI] [PubMed] [Google Scholar]
- 52. Knutsen G, Engebretsen L, Ludvigsen TC, Drogset JO, Grontvedt T, Solheim E, et al. Autologous chondrocyte implantation compared with microfracture in the knee: a randomized trial. J Bone Joint Surg Am. 2004;86A:455-64. [DOI] [PubMed] [Google Scholar]
- 53. Saris DB, Vanlauwe J, Victor J, Haspl M, Bohnsack M, Fortems Y, et al. Characterized chondrocyte implantation results in better structural repair when treating symptomatic cartilage defects of the knee in a randomized controlled trial versus microfracture. Am J Sports Med. 2008;36:235-46. [DOI] [PubMed] [Google Scholar]
- 54. Quirbach S, Trattnig S, Marlovits S, Zimmermann V, Domayer S, Dorotka R, et al. Initial results of in vivo high-resolution morphological and biochemical cartilage imaging of patients after matrix-associated autologous chondrocyte transplantation (MACT) of the ankle. Skeletal Radiol. 2009;38:751-60. [DOI] [PubMed] [Google Scholar]
- 55. Welsch GH, Mamisch TC, Weber M, Horger W, Bohndorf K, Trattnig S. High-resolution morphological and biochemical imaging of articular cartilage of the ankle joint at 3.0 T using a new dedicated phased array coil: in vivo reproducibility study. Skeletal Radiol. 2008;37:519-26. [DOI] [PubMed] [Google Scholar]
- 56. Mlynarik V, Szomolanyi P, Toffanin R, Vittur F, Trattnig S. Transverse relaxation mechanisms in articular cartilage. J Magn Reson. 2004;169:300-7. [DOI] [PubMed] [Google Scholar]
- 57. Menezes NM, Gray ML, Hartke JR, Burstein D. T2 and T1rho MRI in articular cartilage systems. Magn Reson Med. 2004;51:503-9. [DOI] [PubMed] [Google Scholar]
- 58. Nieminen MT, Rieppo J, Toyras J, Hakumaki JM, Silvennoinen J, Hyttinen MM, et al. T2 relaxation reveals spatial collagen architecture in articular cartilage: a comparative quantitative MRI and polarized light microscopic study. Magn Reson Med. 2001;46:487-93. [DOI] [PubMed] [Google Scholar]
- 59. White LM, Sussman MS, Hurtig M, Probyn L, Tomlinson G, Kandel R. Cartilage T2 assessment: differentiation of normal hyaline cartilage and reparative tissue after arthroscopic cartilage repair in equine subjects. Radiology. 2006;241:407-14. [DOI] [PubMed] [Google Scholar]
- 60. Burstein D, Gray ML. Is MRI fulfilling its promise for molecular imaging of cartilage in arthritis? Osteoarthritis Cartilage. 2006;14:1087-90. [DOI] [PubMed] [Google Scholar]


