Abstract
Objective:
To assess the safety and efficacy of accelerated compared with traditional postoperative weightbearing (WB) rehabilitation following matrix-induced autologous chondrocyte implantation (MACI) of the knee, using MRI.
Methods:
A randomized controlled study design was used to assess MRI-based outcomes of MACI grafts in 70 patients (45 men, 25 women) who underwent MACI to the medial or lateral femoral condyle, in combination with either traditional or accelerated approaches to postoperative WB rehabilitation. High-resolution MRI was undertaken and assessed 8 previously defined pertinent parameters of graft repair, as well as a combined MRI composite score at 3, 12, and 24 months postsurgery. The association between clinical and MRI-based outcomes, patient demographics, chondral defect parameters, and injury/surgery history was investigated.
Results:
Both groups significantly improved (P < 0.05) in the MRI composite score and pertinent descriptors of graft repair throughout the postoperative period until 24 months postsurgery. There were no differences (P > 0.05) observed between the 2 groups. Patient age, body mass index, chondral defect size, and duration of preoperative symptoms were significantly correlated (P < 0.05) with several MRI-based outcomes at 24 months, whereas there were no significant pertinent correlations (P > 0.05) observed between clinical and MRI-based outcomes.
Conclusion:
The accelerated WB approach was not detrimental to graft development at any stage throughout the postoperative assessment timeline from baseline to 24 months postsurgery and may potentially accelerate patient return to normal function, while reducing postoperative muscle loss, intra-articular adhesions, and associated gait abnormalities.
Keywords: matrix-induced autologous chondrocyte implantation (MACI), magnetic resonance imaging (MRI), partial weight bearing (PWB), rehabilitation, gait
Introduction
Matrix-induced autologous chondrocyte implantation (MACI) is an established technique for the repair of full-thickness chondral defects in the knee.1-3 Four primary factors4 have been proposed that influence short- and long-term patient and graft outcome following MACI, including 1) successful cell harvest and culturing procedures, 2) efficiency of the surgical procedure, 3) patient cooperation in all aspects of the preoperative and postoperative program, and 4) timely progression of weight bearing (WB) and postoperative rehabilitation. The postoperative mechanical environment is imperative in allowing optimal chondrocyte differentiation and development5 and potentially the optimal regeneration of hyaline-like tissue. Research has supported the need for dynamic compression6 and shear loading,7 similar to that experienced in normal daily activity, whereas static compression8 and immobilization9 seem detrimental to cell proliferation and matrix synthesis. To achieve this, an appropriately structured and well-monitored rehabilitation program following MACI is recommended.3,10,11 However, a lack of consensus remains regarding the optimal gradient and time to full WB that is both safe and stimulatory to the tissue repair process.
Postoperative WB rehabilitation programs traditionally used following ACI have been conservative.4,12 However, this may also be associated with a delayed return to normal function, postoperative muscle loss, intra-articular adhesions, and gait abnormalities, which may contribute to a poorer patient outcome. Accelerated postoperative WB protocols following MACI have emerged3,10 and have demonstrated comparable, if not superior, outcomes to conservative methods, with a faster return to normal physical function while showing no early detrimental side effects to the developing graft.
Although optimizing postoperative rehabilitation may act to improve patient and graft outcomes, there is an increasing need for an accurate and reproducible method for the evaluation of tissue repair. MRI has emerged as a noninvasive and increasingly effective method for evaluating the morphological status of the repair tissue produced throughout the postoperative period following ACI.3,6-9 Furthermore, it is important to investigate whether graft status, as assessed by MRI, correlates with patient functional outcomes. Correlation of MRI with clinical outcomes has been attempted, although with mixed results.3,6,13-15
We have investigated the safety and efficacy of accelerated WB following MACI using an established MRI evaluation system. We also investigated the controversial relationship between clinical and MRI-based outcomes. First, we hypothesized that there would be a significant improvement in pertinent morphological outcomes of graft repair and a combined MRI composite score throughout the postoperative period up until 24 months. Second, we hypothesized that there would be no significant difference in these MRI-based outcomes between accelerated and traditional approaches to postoperative rehabilitation. Third, we hypothesized that patient demographics, cartilage defect parameters, and injury/surgery history would be associated with graft outcome, as assessed by MRI. Finally, we hypothesized that there would be significant correlations between clinical and MRI-based outcomes at 24 months postsurgery.
Methods
Participants
Patients enrolled in this trial were recruited at the Hollywood Functional Rehabilitation Clinic, in association with the University of Western Australia. A block randomization procedure (gender; age <40 years or >40 years) was used to allocate 70 patients (47 men, 23 women) to either traditional or accelerated rehabilitation pathways (Fig. 1), and all but 1 patient was retained up until 24 months postsurgery (motor vehicle accident resulting in death at 7 months postsurgery and subsequent exclusion from the study analysis). Only patients who underwent MACI to localized, full-thickness medial or lateral femoral condylar defects to the knee participated in this study. Further recruitment criteria included patients 15 to 65 years of age and deemed able to follow the rehabilitation program. Patients were excluded if they had a body mass index (BMI) >35 kg/m2, had ligamentous instability, had varus/valgus abnormalities (>5° tibiofemoral anatomic angle),14,16 had undergone a prior extensive meniscectomy, or had ongoing progressive inflammatory arthritis. The sample sizes used were based on an a priori power calculation that showed at least 22 subjects were required in each of the 2 groups to reveal differences at the 5% significance level, with 80% power. Patients provided their written informed consent prior to study participation, and ethics approval was obtained from the relevant university and hospital ethics committees.
Figure 1.
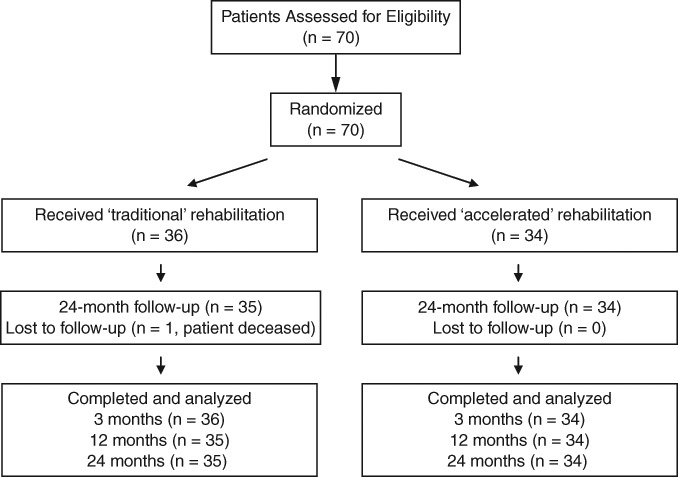
Patient randomization and assessment throughout the trial.
MACI Technique
The surgical technique used in this study has been previously described.10 Briefly, arthroscopic surgery was first performed to harvest a sample of normal articular cartilage from a non-WB area of the knee. Following harvest, healthy chondrocytes were isolated and cultured (Genzyme, Perth, Western Australia) and then seeded onto a type I/III collagen membrane (ACI-Maix Matricel GmbH, Germany) ex vivo over a 6- to 8-week period. The second stage of surgery involved implantation of the collagen membrane with seeded cells through an open arthrotomy. The chondral defect was prepared by removing all damaged cartilage down to, but not through, the subchondral plate. The resultant defect was then measured and used to shape the membrane, pressed into the defect, and secured using a thin layer of fibrin glue spread over the entirety of the subchondral bed. Following assessment of graft stability, the wound was closed.
Traditional and Accelerated Rehabilitation Protocols
Patients in both groups received the same inpatient education and rehabilitation. This consisted of continuous passive motion (CPM) set at 0° to 30° within 12 to 24 hours after surgery, for a minimum of 1 hour daily, to reduce the chance of intra-articular adhesions16; cryotherapy to control edema; active dorsiflexion and plantar flexion of the ankle to encourage lower extremity circulation; isometric contraction of the quadriceps, hamstrings, and gluteal musculature to maintain muscle tone and minimize muscle loss17-19; and teaching of proficient toe-touch WB at ≤20% body weight (BW) through the affected limb. A range-of-motion control brace was worn at all times in the early inpatient setting, unless the patient was undertaking CPM.
Following hospital discharge, patients attended the outpatient rehabilitation clinic on 2 occasions per week, for a period of 12 weeks. Patients were randomized into either a traditional (conservative) or accelerated load-bearing rehabilitation protocol (Table 1). The accelerated protocol consisted of a 2-week period of WB at 20% BW for early graft protection, with a progressive increase to full WB at 8 weeks postsurgery. The traditional protocol consisted of a 5-week period of WB at 20% BW, followed by a progressive increase to full WB at 11 weeks postsurgery. Throughout the progressive WB program, 1 or 2 forearm crutches were used dependent on the WB restrictions employed throughout the rehabilitation timeline (Table 1).
Table 1.
The Load-Bearing Gradients followed by Matrix-Induced Autologous Chondrocyte Implantation Patients in the Traditional and Accelerated Rehabilitation Groups
| Traditional Group | |||||||||||
| Weeks Postsurgery | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
| Weightbearing (% body weight) | ≤20 | 50 | 60 | 70 | 80 | 90 | 100 | ||||
| Crutches | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | |||
| Accelerated group | |||||||||||
| Weeks postsurgery | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
| Weightbearing (% body weight) | ≤20 | 30 | 40 | 50 | 60 | 80 | 100 | ||||
| Crutches | 2 | 2 | 2 | 2 | 1 | 1 | 1 | 0 |
The bathroom scale method was used to teach patients the WB restriction,11,16 whereas WB replication training was an important component of each and every session up until the time the patient returned to full WB (Table 1). Mixed results surround the evaluation of the bathroom scale method as an effective tool for teaching WB restrictions, whereby both good20,21 and poor22,24 replication ability has been reported, particularly at low levels of WB. Nevertheless, this method remains the most practical and widely used modality for teaching WB restrictions,11,16 and although some error may potentially exist in the ability of patients to replicate the desired loads, this error would be expected in both rehabilitation groups. Furthermore, this study looked at the time to full WB in addition to the postoperative WB gradient, whereby full WB was ensured in the traditional group at 11 weeks and at 8 weeks in the accelerated group, once use of crutches was ceased.
A range-of-motion control brace was also worn at all times following hospital discharge, unless the patient was undertaking plinth-based rehabilitation exercises in the supervised clinical setting, undertaking hydrotherapy work in the supervised clinical setting, or when showering, providing the patient was seated in the shower. Apart from the difference in the gradient and time to full WB, all rehabilitation exercises and exercise modalities were identical between the 2 groups (Table 2).
Table 2.
Overview of the Progression of Knee Range of Motion (ROM) Status, Knee Bracing, and Exercise Rehabilitation Undertaken by Both the Accelerated and Traditional Rehabilitation Groups
| Postoperative Timeline | Rehabilitation Overview |
|---|---|
| Week 1 | Knee ROM: passive and active ROM from 0°-30° Knee bracing: 0°-30° Treatment/rehabilitation: isometric contractions and circulation exercises, CPM (0°-30°) and cryotherapy |
| Weeks 2-3 | Knee ROM: active ROM from 0°-30° (week 2) to 0°-60° (week 3) Knee bracing: 0°-30° (week 1-2) to 0°-60° (week 3) Treatment/rehabilitation: isometric and straight leg exercises, passive and active knee flexion exercises, remedial massage, soft tissue and patella mobilization, CPM (0°-90°), cryotherapy, and hydrotherapy |
| Weeks 4-6 | Knee ROM: active ROM from 0°-90° (week 4) to 0°-130° (week 6) Knee bracing: 0°-90° (week 4) to full range (week 5) Treatment/rehabilitation: introduction of calf raises, weighted hip adduction and abduction, trunk strengthening, recumbent cycling |
| Weeks 7-12 | Knee ROM: active ROM from 0°-130° (week 6) to full range (week 7) Knee bracing: full knee flexion (brace worn until 12 weeks postsurgery) Treatment/rehabilitation: introduction of proprioceptive/balance activities, cycling, walking, resistance, and CKC activities |
Note: ROM = range of motion; BW = body weight; CPM = continuous passive motion; CKC = closed kinetic chain; OKC = open kinetic chain.
Clinical Assessment
Defect size (cm2) was calculated based on the dimensions of the chondral graft at the time of second-stage implantation, whereas the number of prior cartilage repair procedures and the duration of preoperative symptoms (y) were obtained via a thorough patient history. For the correlation of clinical and MRI-based outcomes at 24 months postsurgery, 4 subjective and functional scores were undertaken: 1) the Knee Injury and Osteoarthritis Outcome Score (KOOS)25 to assess knee pain, symptoms, activities of daily living (ADL), sport and recreation, and knee-related quality of life; 2) the Short Form Health Survey (SF-36), which evaluated the general health of the patient, producing a mental (MCS) and physical component score (PCS)26; 3) the 6-minute walk test,10,14 to assess the maximum comfortable distance the patient could walk in a 6-minute time period; and 4) activity level using a validated activity monitor (Actigraph, MTI Health Services, Ft. Walton Beach, FL)27 to assess the total number of steps taken over a 7-day period. Although the KOOS, SF-36, and 6-minute walk test were undertaken in all 70 patients (less the 1 deceased patient) at 24 months postsurgery, activity level assessment was undertaken in 60 patients (31 accelerated, 29 traditional) at 3, 6, and 12 months postsurgery only. Therefore, an average of the 3-, 6-, and 12-month activity evaluations was calculated for each patient, creating a combined activity score representing a snapshot of the first 12 postoperative months.
MRI Assessment
MRI was conducted at 3, 12, and 24 months postsurgery using a Siemens Symphony 1.5 T scanner (Siemens, Erlangen, Germany). Standardized proton density and T2-weighted fat-saturated images were obtained in coronal and sagittal planes (slice thickness 3 mm, field of view 14-15 cm, 512 matrix in at least one axis for proton density images with a minimum 256 matrix in one axis for T2-weighted images). Additional axial proton density fat-saturated images were also obtained (slice thickness 3-4 mm, field of view 14-15 cm, minimum 224 matrix in at least one axis).
MRI evaluation employed in this study assessed 8 pertinent parameters of graft repair (Table 3) that have been previously outlined7 and closely followed MRI scoring systems previously reported for ACI assessment.9,14 Some modification was required to allow for discrepancies in MRI equipment and sequence protocols. MRI parameters (signal intensity, graft infill, border integration, surface contour, structure, subchondral lamina, subchondral bone, and effusion) were selected to best describe the morphology and signal intensity of the repair tissue. These parameters were scored individually from 1 to 4 (1 = poor, 2 = fair, 3 = good, 4 = excellent) in comparison to the adjacent native cartilage. For the scoring parameter “graft infill,” an additional score of 3.5 was awarded for a fifth level (very good), corresponding with “graft hypertrophy,” as indicated by previous work.7,9 In addition to individual parameter scoring, a combined MRI composite score was calculated by multiplying each individual score by a weighting factor14 and adding the scores together (Table 3). This composite score was therefore also scored from 1 to 4 (1 = poor, 2 = fair, 3 = good, 4 = excellent). MRI evaluation was performed by an independent, experienced musculoskeletal radiologist, blinded to the clinical details and clinical outcome assessment.
Table 3.
Postoperative Magnetic Resonance Imaging (MRI) Assessment of Grafts: Scoring of Parameters and Calculation of the MRI Composite Score
| Scoring Parameter | Score | Description | Weighting Factor |
|---|---|---|---|
| 1. Signal intensity | 1 = poor | Fluid signal/hyperintense diffuse | 0.3 |
| 2 = fair | Hyperintense basal layer >50%/<50% | ||
| 3 = good | Hypointense | ||
| 4 = excellent | Isointense | ||
| 2. Graft infill | 1 = poor | Subchondral bone exposed | 0.2 |
| 2 = fair | <50% height of adjacent cartilage | ||
| 3 = good | >50% height of adjacent cartilage | ||
| 3.5 = very good | Hypertrophy | ||
| 4 = excellent | Complete infill | ||
| 3. Border integration | 1 = poor | Incomplete border, visible defect | 0.15 |
| 2 = fair | Incomplete border, split visible | ||
| 3 = good | Complete border, minor split | ||
| 4 = excellent | Complete integration | ||
| 4. Surface contour | 1 = poor | Ulceration, delamination, full thickness | 0.1 |
| 2 = fair | <50% surface fibrillation | ||
| 3 = good | Focal changes only | ||
| 4 = excellent | Smooth surface | ||
| 5. Structure | 1 = poor | Heterogenous, clefts | 0.1 |
| 2 = fair | Heterogenous, no clefts | ||
| 3 = good | >50% homogenous | ||
| 4 = excellent | >75% homogenous | ||
| 6. Subchondral Lamina | 1 = poor | No visible lamina | 0.05 |
| 2 = fair | <25% intact | ||
| 3 = good | >50% intact | ||
| 4 = excellent | Fully reconstituted | ||
| 7. Subchondral bone | 1 = poor | Cysts, sclerosis, edema | 0.05 |
| 2 = fair | Edema >1 cm from lamina | ||
| 3 = good | Edema <1 cm from lamina | ||
| 4 = excellent | Intact, no significant edema | ||
| 8. Effusion | 1 = poor | Severe | 0.05 |
| 2 = fair | Moderate | ||
| 3 = good | Mild | ||
| 4 = excellent | None |
Statistical Analysis
Initially, an intraobserver reliability assessment using the Spearman’s rank order correlation was undertaken for the 8 pertinent MRI scores and the MRI composite score, using 20 randomly selected images filtered through a second time to the radiologist. Repeated-measures analysis of variance was used to investigate the progression of the MRI composite and individual MRI parameter scores over time, between the accelerated and traditional patient groups. Independent t tests were used to evaluate the difference in clinical outcomes between the 2 groups at 24 months postsurgery, for correlation with MRI-based parameters. Correlation of the MRI composite score and the 8 pertinent parameters of tissue repair with patient demographics (age, BMI), chondral defect parameters (defect size), injury/surgery history (prior cartilage repair procedures, duration of symptoms), and clinical scores (KOOS, SF-36, 6-minute walk distance, activity level) was undertaken using the Spearman’s correlation coefficient, to determine any association between MRI outcomes and patient descriptive and clinical outcomes. Statistical analysis was performed using SPSS software (SPSS, version 17.0, SPSS Inc., Chicago, IL), whereas statistical significance was determined at P < 0.05.
Results
There were no significant differences (P > 0.05) observed between the accelerated and traditional rehabilitation groups in any of the patient, chondral defect, injury, or surgical historical descriptive parameters (Table 4).
Table 4.
Descriptive Parameters for the Accelerated and Traditional Rehabilitation Groups
| Accelerated | Traditional | P Value | |
|---|---|---|---|
| Number of patients | 34 | 35 | NA |
| Gender (M/W) | 22/12 | 22/13 | NA |
| Body weight (kg) | 79.0 (56.0-104.0) | 83.8 (59.3-105.0) | 0.120 |
| Body mass index (kg/m2) | 25.6 (19.4-31.6) | 27.6 (21.1-32.4) | 0.560 |
| Age (years) | 36.6 (21-62) | 39.8 (16-63) | 0.304 |
| 10-19 | 0 | 2 | NA |
| 20-29 | 9 | 7 | NA |
| 30-39 | 13 | 12 | NA |
| 40-49 | 8 | 8 | NA |
| 50-59 | 3 | 4 | NA |
| 60-69 | 1 | 2 | NA |
| Defect location (MFC/LFC) | 26/8 | 26/9 | NA |
| Defect size (cm2) | 3.22 (0.65-10.00) | 3.31 (0.75-10.00) | 0.891 |
| ≤1.0 | 3 | 5 | N/A |
| 1.1-2.0 | 9 | 9 | NA |
| 2.1-3.0 | 2 | 3 | NA |
| 3.1-4.0 | 1 | 4 | NA |
| 4.1-5.0 | 4 | 2 | NA |
| ≥5.1 | 15 | 12 | NA |
| Prior procedures | 1.2 (0-3) | 1.4 (0-4) | 0.673 |
| Duration of symptoms (years) | 7.4 (1.0-18.0) | 7.9 (0.2-19.0) | 0.445 |
Note: Shown are means (range). M = men; W = women; MFC = medial femoral condyle; LFC = lateral femoral condyle; NA = not applicable.
Clinical Assessment
Clinical scores were calculated at 24 months postsurgery for the purpose of correlation with MRI-based parameters. For the outcomes used, the accelerated group performed significantly better (P < 0.05) than the traditional group in the 6-minute walk test at 24 months postsurgery (Table 5). All other KOOS subscales and both subscales of the SF-36 (PCS and MCS) revealed no differences (P > 0.05) at 24 months, while there was no difference in the combined activity score (P > 0.05; Table 5).
Table 5.
Summary of Mean (SE) 24-Month Postoperative Clinical Results for the Accelerated and Traditional Groups
| Variable | Accelerated | Traditional | P Value |
|---|---|---|---|
| KOOS (pain) | 86.15 (2.82) | 82.5 (2.82) | 0.422 |
| KOOS (symptoms) | 88.04 (2.59) | 82.86 (2.59) | 0.197 |
| KOOS (ADL) | 92.79 (2.36) | 90.32 (2.36) | 0.398 |
| KOOS (sport) | 61.17 (5.80) | 55.00 (5.80) | 0.519 |
| KOOS (QOL) | 59.59 (4.33) | 58.75 (4.33) | 0.960 |
| SF-36 (PCS) | 49.79 (1.80) | 47.02 (1.80) | 0.289 |
| SF-36 (MCS) | 55.95 (1.07) | 56.11 (1.07) | 0.669 |
| 6-minute walk test (m) | 661.5 (20.1) | 580.7 (20.1) | 0.016 |
| Activity score (steps) | 11819 (658) | 10279 (620) | 0.054 |
Note: KOOS = Knee Injury and Osteoarthritis Outcome Score; ADL = activities of daily living; QOL = quality of life; PCS = physical component score; MCS = mental component score.
MRI Assessment
Evaluation of intraobserver reliability for the defined MRI scoring method indicated a significant correlation (P < 0.01) between MRI-based scores within each of the 8 pertinent MRI scoring variables (signal intensity rho = 1.00, graft infill rho = 0.949, border integration rho = 0.982, surface contour rho = 1.00, structure rho = 0.840, subchondral lamina rho = 1.00, subchondral bone rho = 0.920, and effusion rho = 0.993) and the MRI composite score (rho = 0.811) for the 20 randomly selected image pairs.
Both rehabilitation groups demonstrated an increased MRI composite score over time that significantly improved (P < 0.0001) from 3 to 24 months postsurgery (Figs. 2 and 3; Table 6). Although a minor fall (not significant) in the MRI composite score was observed between 12 and 24 months, the 24-month outcome still rated significantly better (P < 0.05) than 3 months postsurgery (Fig. 2). There was no significant group or interaction effect (P > 0.05) for the MRI composite score over time. With regard to the 8 individual MRI scoring parameters, a significant time effect (P < 0.05) was observed for signal intensity, graft infill, subchondral lamina, subchondral bone, and effusion, although there were no significant group or interaction effects (P > 0.05) for all variables (Table 6).
Figure 2.
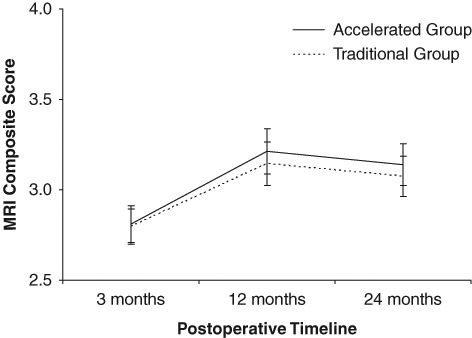
Change in the MRI composite score over time for the accelerated and traditional patient groups, throughout the postoperative timeline.
Figure 3.
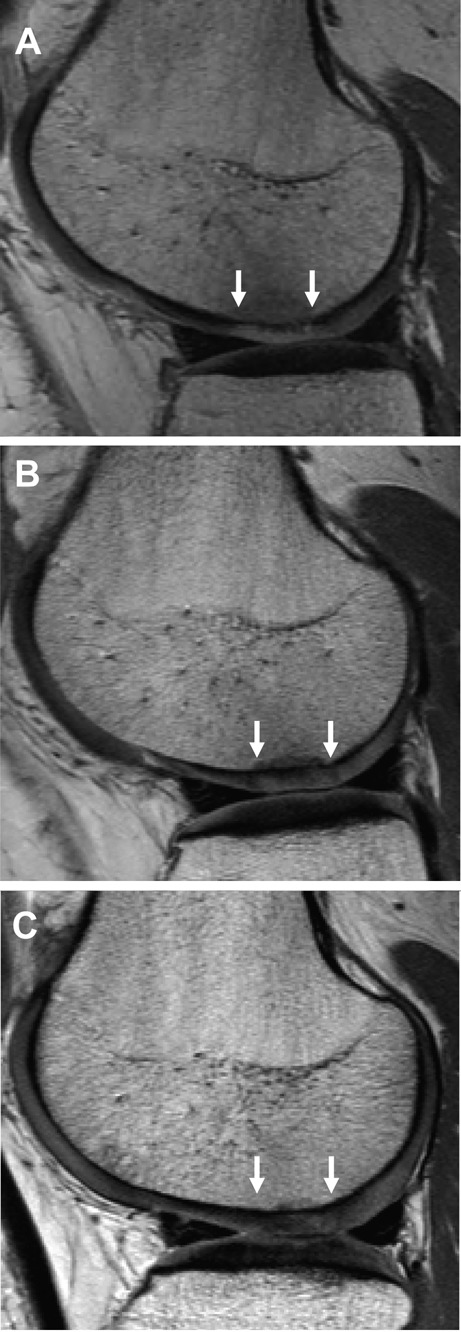
Sagittal proton density fast spin echo MRI of a matrix-induced autologous chondrocyte implantation graft (between white arrows) to the lateral femoral condyle. Images are of the same patient and representative of (A) 3 months postsurgery with reduced thickness compared with the adjacent native cartilage and a hyperintense signal; (B) 12 months postsurgery with similar thickness to the adjacent native cartilage and an isointense signal, although with some subchondral bone abnormality; and (C) 24 months postsurgery with equivalent signal and thickness characteristics to the adjacent native cartilage, resolution of subchondral bone abnormality, good border zone integration, and good surface contour.
Table 6.
Postoperative MRI Assessment of Grafts for the Accelerated (Acc) and Traditional (Trad) Rehabilitation Groups
| Variable | MRI Composite Score | Signal Intensity | Graft Infill | Border Integration | Surface Contour | Structure | Subchondral Lamina | Subchondral Bone | Effusion |
|---|---|---|---|---|---|---|---|---|---|
| Acc (3 mo) | 2.81 (0.10) | 1.90 (0.11) | 2.97 (0.16) | 2.77 (0.19) | 2.90 (0.15) | 3.40 (0.18) | 3.20 (0.14) | 2.97 (0.14) | 3.73 (0.10) |
| Trad (3 mo) | 2.80 (0.10) | 2.22 (0.10) | 2.80 (0.16) | 2.66 (0.18) | 2.81 (0.15) | 3.53 (0.17) | 3.34 (0.14) | 2.75 (0.14) | 3.50 (0.10) |
| Acc (12 mo) | 3.21 (0.13) | 2.93 (0.17) | 3.48 (0.16) | 2.90 (0.19) | 2.80 (0.18) | 3.33 (0.19) | 3.80 (0.07) | 2.97 (0.15) | 3.87 (0.08) |
| Trad (12 mo) | 3.15 (0.12) | 2.94 (0.17) | 3.30 (0.16) | 2.84 (0.19) | 2.81 (0.17) | 3.41 (0.18) | 3.81 (0.07) | 2.91 (0.14) | 3.69 (0.08) |
| Acc (24 mo) | 3.14 (0.12) | 2.76 (0.14) | 3.40 (0.17) | 2.93 (0.21) | 2.97 (0.20) | 3.30 (0.19) | 3.90 (0.05) | 2.43 (0.19) | 3.67 (0.10) |
| Trad (24 mo) | 3.07 (0.11) | 2.81 (0.13) | 3.30 (0.16) | 2.75 (0.20) | 2.72 (0.19) | 3.22 (0.18) | 3.97 (0.05) | 2.75 (0.18) | 3.56 (0.09) |
| Time effect (P value) | <0.0001 | <0.0001 | <0.0001 | 0.113 | 0.902 | 0.095 | <0.0001 | 0.018 | 0.035 |
| Group effect (P value) | 0.740 | 0.435 | 0.462 | 0.659 | 0.610 | 0.861 | 0.423 | 0.937 | 0.102 |
| Interaction Effect (P value) | 0.796 | 0.279 | 0.878 | 0.733 | 0.515 | 0.508 | 0.728 | 0.096 | 0.661 |
Note: Scoring of the 8 individual MRI parameters and calculation of the MRI composite score (1 = poor, 2 = fair, 3 = good, 4 = excellent), in comparison with the adjacent native cartilage. Shown are means (SE).
A detailed analysis of the progression of the 8 graft descriptive parameters at 3, 12, and 24 months postsurgery within the accelerated and traditional patient groups is presented in Table 7.
Table 7.
Percentage of Patients within the Accelerated (Acc) and Traditional (Trad) Rehabilitation Groups Who Scored Good to Excellent (Good-Excellent) and Poor to Fair (Poor-Fair) for Each of the 8 Individual MRI Scoring Parameters, at the 3-, 12-, and 24-Month Assessment Time Points
| Group | Rating | Graft Infill | Signal Intensity | Border Integration | Surface Contour | Structure | Subchondral Lamina | Subchondral Bone | Effusion |
|---|---|---|---|---|---|---|---|---|---|
| Acc (3 months) | Good-excellent | 74% (n = 25) | 9% (n = 3) | 68% (n = 23) | 82% (n = 28) | 79% (n = 27) | 71% (n = 24) | 71% (n = 24) | 97% (n = 33) |
| Poor-fair | 26% (n = 9) | 91% (n = 31) | 32% (n = 11) | 18% (n = 6) | 21% (n = 7) | 29% (n = 10) | 29% (n = 10) | 3% (n = 1) | |
| Trad (3 months) | Good-excellent | 62% (n = 21) | 18% (n = 6) | 68% (n = 23) | 79% (n = 27) | 85% (n = 29) | 91% (n = 31) | 59% (n = 20) | 97% (n = 34) |
| Poor-fair | 38% (n = 14) | 82% (n = 29) | 32% (n = 12) | 21% (n = 8) | 15% (n = 6) | 9% (n = 4) | 41% (n = 15) | 3% (n = 1) | |
| Acc (12 months) | Good-excellent | 88% (n = 30) | 62% (n = 21) | 71% (n = 24) | 68% (n = 23) | 85% (n = 29) | 100% (n = 34) | 74% (n = 25) | 100% (n = 34) |
| Poor-fair | 12% (n = 4) | 38% (n = 13) | 29% (n = 10) | 32% (n = 11) | 15% (n = 5) | 0% (n = 0) | 26% (n = 9) | 0% (n = 0) | |
| Trad (12 months) | Good-excellent | 88% (n = 30) | 68% (n = 23) | 71% (n = 24) | 74% (n = 25) | 85% (n = 29) | 100% (n = 35) | 68% (n = 23) | 100% (n = 35) |
| Poor-fair | 12% (n = 5) | 32% (n = 12) | 29% (n = 11) | 26% (n = 10) | 15% (n = 6) | 0% (n = 0) | 32% (n = 12) | 0% (n = 0) | |
| Acc (24 months) | Good-excellent | 91% (n = 31) | 71% (n = 24) | 76% (n = 6) | 76% (n = 26) | 85% (n = 29) | 100% (n = 34) | 74% (n = 25) | 97% (n = 33) |
| Poor-fair | 9% (n = 3) | 29% (n = 10) | 24% (n = 8) | 24% (n = 8) | 15% (n = 6) | 0% (n = 0) | 26% (n = 9) | 3% (n = 1) | |
| Trad (24 mo) | Good-excellent | 85% (n = 29) | 71% (n = 24) | 68% (n = 23) | 74% (n = 25) | 88% (n = 30) | 100% (n = 35) | 71% (n = 24) | 100% (n = 35) |
| Poor-fair | 15% (n = 6) | 29% (n = 11) | 32% (n = 12) | 26% (n = 10) | 12% (n = 5) | 0% (n = 0) | 29% (n = 11) | 0% (n = 0) |
For the MRI evaluations at 3, 12, and 24 months postsurgery, a descriptive analysis of the 8 pertinent parameters of graft repair was obtained, comparing the 2 rehabilitation groups. At 3 months postsurgery, 74% (n = 25) and 62% (n = 21) of patients in the accelerated and traditional groups, respectively, demonstrated good to excellent filling of the chondral defect. Signal intensity was described as good to excellent in only 9% (n = 3) of the accelerated group, compared with 18% (n = 6) of the traditional group, whereas 68% (n = 23) of patients in both groups demonstrated good to excellent border integration with the adjacent native cartilage. The surface contour of repair tissue was rated as good to excellent in 82% (n = 28) and 79% (n = 27) of accelerated and traditional patients, respectively, whereas the structure of reparative tissue was rated as good to excellent in 79% (n = 27) and 85% (n = 29), respectively. The subchondral lamina was rated as good to excellent in 71% (n = 24) of patients in the accelerated group and 91% (n = 31) of traditional patients, whereas good to excellent resolution of subchondral bone abnormality was demonstrated in 71% (n = 24) and 59% (n = 20) of the accelerated and traditional group, respectively. Finally, mild to no knee joint effusion was evident in 97% (accelerated, n = 33; traditional, n = 34), of patients in both rehabilitation groups.
At 12 months’ postsurgery, the percentage of patients with good to excellent infill had increased to 88% (n = 30) in both groups, whereas the rating of good to excellent signal intensity had improved to 62% (n = 21) and 68% (n = 23) of patients in the accelerated and traditional groups, respectively. Although the percentage of patients with good to excellent subchondral bone improved to 74% (n = 25) and 68% (n = 23) of patients in the accelerated and traditional groups, respectively, a rating of good to excellent for surface contour decreased to only 68% (n = 23) and 74% (n = 25) of patients within the accelerated and traditional groups, respectively. From 3 to 12 months, both groups improved in the remaining scoring measures, with 71% (n = 24), 85% (n = 29), 100% (accelerated, n = 34; traditional, n = 35), and 100% (accelerated, n = 34; traditional, n = 35) of patients demonstrating a good to excellent rating for border integration, structure, subchondral lamina, and effusion, respectively.
By 24 months postsurgery, further improvement was demonstrated within the accelerated group, with an increased percentage of patients rated as good to excellent for signal intensity (71%, n = 24), border integration (76%, n = 26), and surface contour (76%, n = 26). A good to excellent rating was observed for graft infill in 91% (n = 31) of accelerated patients, whereas 85% (n = 29) of patients demonstrated complete infill (or graft hypertrophy) in comparison with the adjacent native cartilage. Furthermore, a good to excellent rating was demonstrated in the same percentage of patients from 12 to 24 months in the scoring measures of structure (85%, n = 29), subchondral lamina (100%, n = 34), and subchondral bone (74%, n = 25). Joint effusion deteriorated from 12 to 24 months in the accelerated group, with 1 patient demonstrating a poor to fair rating. For the traditional group at 24 months postsurgery, further improvement was demonstrated via an increased percentage of patients rating as good to excellent in the scoring variables of signal intensity (71%, n = 24), structure (88%, n = 30), and subchondral bone (71%, n = 30). A good to excellent rating was maintained in the same percentage of patients within the traditional group from 12 to 24 months for surface contour (74%, n = 25), subchondral lamina (100%, n = 35), and joint effusion (100%, n = 35). Deterioration in the percentage of patients in the traditional group rating as good to excellent was observed for border integration (68%, n = 23) and graft infill (85%, n = 29). For the traditional group, 82% (n = 28) of patients demonstrated complete infill (or graft hypertrophy).
Complications
The incidence of graft hypertrophy was reported in 4% of patients (accelerated, n = 2; traditional, n = 1) at 3 months postsurgery, in 16% of patients (accelerated, n = 6; traditional, n = 5) at 12 months, and in 27% of patients (accelerated, n = 8; traditional, n = 11) at 24 months postsurgery. At 24 months postsurgery, all patients with hypertrophic grafts remained nonsymptomatic. There was 1 incidence of graft loss between 6 and 9 months postsurgery in a patient who underwent the accelerated rehabilitation pathway, although there was no further incidence of graft delamination, either partially or in its entirety, to the 24-month time point. There were a further 2 patients (1 accelerated and 1 traditional) at 24 months postsurgery who demonstrated a subchondral bed devoid of any significant repair tissue at 3, 12, and 24 months, indicating graft failure.
Correlation of MRI-Based Outcomes with Patient Demographics, Defect Parameters, and Preoperative Injury/Surgery History
At 24 months postsurgery, patient age was significantly correlated (P < 0.05) with the MRI composite score (Fig. 4), graft infill, border integration, and surface contour, whereas BMI was significantly correlated (P < 0.05) with the MRI composite score (Fig. 5), graft infill, and knee joint effusion (Table 8). Defect size was significantly correlated (P < 0.05) with the MRI composite score and all pertinent MRI descriptive parameters, besides graft infill and subchondral bone, whereas the duration of symptoms was significantly correlated (P < 0.05) with the MRI composite score, graft infill, surface contour, and structure. The amount of previous cartilage repair procedures undertaken was significantly correlated (P < 0.05) only with surface contour (Table 8).
Figure 4.
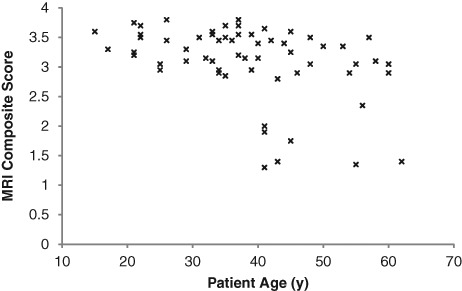
The correlation of patient age with the MRI composite score at 24 months postsurgery.
Figure 5.
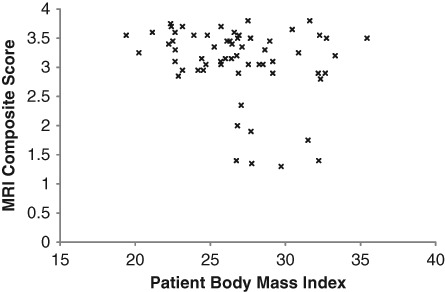
The correlation of patient body mass index (BMI) with the MRI composite score at 24 months postsurgery.
Table 8.
Spearman Correlation Coefficients of the MRI Composite Score and the 8 Pertinent Parameters of Tissue Repair with Patient Demographics (Age, Body Mass Index), Chondral Defect Parameters (Defect Size), Injury/Surgery History (Prior Cartilage Repair Procedures, Duration of Symptoms), and Clinical Scores (KOOS, SF-36, 6-Minute Walk Distance, Activity Level) at 24 Months Postsurgery
| Variable | MRI Composite Score | Graft Infill | Signal Intensity | Border Integration | Surface Contour | Structure | Subchondral Lamina | Subchondral Bone | Effusion |
|---|---|---|---|---|---|---|---|---|---|
| Age | −0.40** | −0.28* | −0.09 | −0.30* | −0.50** | −0.16 | −0.02 | 0.05 | −0.24 |
| Body mass index | −0.35** | −0.30* | −0.14 | −0.10 | −0.22 | −0.20 | −0.02 | 0.15 | −0.32** |
| Defect size | −0.39** | −0.19 | −0.29* | −0.26* | −0.37** | −0.26* | −0.23* | 0.06 | −0.34** |
| Prior procedures | −0.19 | −0.16 | −0.19 | −0.06 | −0.28* | −0.14 | −0.09 | −0.12 | −0.20 |
| Duration of symptoms | −0.36** | −0.30** | −0.07 | −0.13 | −0.29* | −0.32** | −0.22 | −0.10 | −0.20 |
| KOOS (pain) | 0.12 | 0.06 | 0.09 | 0.12 | −0.04 | −0.04 | −0.18 | 0.06 | 0.11 |
| KOOS (symptoms) | −0.06 | −0.12 | 0.13 | −0.06 | −0.22 | −0.14 | −0.23 | 0.08 | 0.08 |
| KOOS (ADL) | 0.03 | 0.11 | −0.01 | 0.03 | −0.08 | −0.06 | −0.17 | −0.03 | 0.25* |
| KOOS (sport) | −0.02 | −0.11 | −0.07 | 0.09 | −0.10 | −0.09 | 0.06 | 0.01 | 0.02 |
| KOOS (QOL) | 0.03 | 0.04 | −0.03 | 0.11 | −0.09 | −0.09 | 0.02 | 0.05 | 0.20 |
| SF-36 (MCS) | 0.02 | 0.1 | −0.17 | 0.1 | 0.02 | −0.14 | −0.05 | 0.37** | 0.13 |
| SF-36 (PCS) | 0.01 | 0.03 | 0.08 | −0.01 | −0.10 | −0.05 | −0.15 | −0.01 | 0.14 |
| 6-minute walk test | 0.06 | 0.12 | −0.05 | 0.13 | 0.07 | 0.11 | 0.09 | −0.32** | 0.04 |
| Activity score | −0.16 | 0.13 | −0.10 | −0.24 | −0.02 | 0.01 | −0.21 | −0.10 | 0.11 |
Note: KOOS = Knee Injury and Osteoarthritis Outcome Score; ADL = activities of daily living; QOL = quality of life; MCS = mental component score; PCS = physical component score.
P < 0.05. **P < 0.01.
Correlation of MRI-Based Outcomes and Clinical Scores
The 5 KOOS subscales exhibited no significant correlations (P > 0.05) with any of the descriptive MRI variables, apart from that observed between ADL and knee joint effusion (Table 8). There were also no significant correlations (P > 0.05) between the MRI parameters and the 2 SF-36 subscales, besides that seen between the PCS and subchondral bone. However, the 6-minute walk test exhibited a significant correlation (P < 0.05) with subchondral bone quality. There were no further significant correlations (P > 0.05) between the 6-minute walk test and activity level with the MRI parameters (Table 8).
Discussion
Current postoperative WB rehabilitation protocols remain relatively conservative following MACI, whereas surgical and cell-culturing methods have evolved significantly since the inception of the general ACI procedure. Accelerated WB protocols following MACI have recently emerged with encouraging outcomes3,10 designed to enhance the graft developmental process, while accelerating patient return to normal function and reducing postoperative muscle loss, intra-articular adhesions, and associated gait abnormalities. This article reports on the safety and efficacy of an accelerated return to full WB following MACI, as well as the factors associated with a good outcome at 24 months postsurgery, as assessed by MRI.
The MRI composite score significantly improved (P < 0.05) over time for both rehabilitation groups (Fig. 2; Table 6). This composite score fell marginally (accelerated, 3.2 to 3.1; traditional, 3.1 to 3.0) between 12 and 24 months, although this was not significantly different (P > 0.05) between the 2 time points, whereas the 24-month outcome still rated significantly better (P < 0.05) than 3 months postsurgery (Fig. 2). This indicates that the tissue produced maintained its structure and maturity to 24 months, regardless of the rehabilitation intervention. Although signal intensity, graft infill, subchondral lamina, subchondral bone, and effusion all significantly improved over time for both groups (Table 6), there were no differences between the 2 rehabilitation groups in the MRI composite score or each of the 8 individual MRI descriptive variables (Table 6). It is not appropriate to compare these results with other MRI scoring systems or between varying assessors. However, this method closely followed that presented by Robertson and others,14 and the change in the MRI composite score over time for both MACI groups in this study is comparable to their results for collagen-covered ACI at 24 months, although better at 3 and 12 months.
As represented by the MRI scoring measures at 3 months postsurgery, this time point coincided with a developing and immature graft. At 3 months, tissue infill above 50% of the adjacent native cartilage was observed in the majority of patients in both groups (74% of the accelerated group, 66% of the traditional group), as was a uniformly hyperintense tissue signal relative to the native cartilage. Signal hyperintensity at the graft/native cartilage interface was common at 3 months postsurgery. In most cases (71% of the accelerated group, 91% of the traditional group), good reconstitution of the subchondral lamina was observed, indicating it was probably intact in the majority of patients at the time of MACI grafting. A good to excellent rating was indicated for subchondral bone marrow edema in 71% (n = 24) and 59% (n = 20) of patients in the accelerated and traditional groups, respectively, whereas a moderate to severe knee effusion was evident in only 3% of patients in both groups. Both bone edema and joint effusion may be seen as signs of loading and, subsequently, an inability of the early regenerative tissue to appropriately transmit forces acting across the tissue.3 Patients in the accelerated group demonstrated scores for these 2 parameters that were no worse than the traditional group at 3 months postsurgery. This suggests that the earlier return to full WB did not seem to compromise the MRI outcome at 24 months, as indicated by these potential signs of overloading.
With the follow-up MRI assessments at 12 and 24 months postsurgery, several changes were observed within both groups. First, further tissue infill was observed, with 91% (n = 31) of patients in the accelerated group demonstrating infill above 50% of the adjacent native cartilage and 85% (n = 29) of patients demonstrating complete infill (or graft hypertrophy) in comparison to the adjacent native cartilage. For the traditional group, 85% (n = 29) of patients demonstrated infill above 50% of the adjacent native cartilage, and 82% (n = 28) of patients demonstrated complete infill (or graft hypertrophy) in comparison with the adjacent native cartilage. The percentage of patients in both groups at 24 months with complete infill in this study is similar, if not marginally better, than those previously reported for ACI13 and MACI.6,9 Signal intensity generally decreased to become isointense or hypointense compared with 3-month images, whereas signal hyperintensity at the graft/subchondral bone plate and graft/native cartilage interfaces resolved. A homogenous structure of repair tissue was observed, while there was full reconstitution of the subchondral lamina evident in all patients at 24 months. These generalized MRI findings throughout the postoperative timeline suggest that a 3-month MRI may be beneficial in assessing the status of the early postoperative graft; however, it is by no means a definitive result due to immaturity of the graft. This has been further noted by Henderson and others,13 who recommended 12 months postsurgery as a reasonable time for assessing graft maturation via MRI.
There was 1 incidence of complete graft loss in this patient cohort that occurred between 6 and 9 months postsurgery. Interestingly, graft infill at 3 months postsurgery for this patient, as assessed by MRI, was rated as good (>50% height of adjacent cartilage), almost approximating the height of the adjacent native cartilage. At this 3-month time point, this patient’s BMI was 29.9, and at the time of graft delamination (approximately 8 months postsurgery), the patient’s BMI was 33.1, indicative of a 10.3-kg increase in BW over the period. The cause of graft loss remains unknown, although it may have been contributed to by this patient’s intensive work practices and increase in BMI throughout this period. Graft delamination generally presents within the first 6 months and is reported in approximately 5% of patients.12 This graft loss highlights the potential importance of maintaining a healthy BW prior to graft maturation, due to the exponential increase in knee articular loading that accompanies each unit increase of BW.28 There were an additional 2 documented graft failures, whereby there was no discernable tissue infill at 3, 12, or 24 months. These consisted of 1 patient in the accelerated group who was 62 years old with a BMI of 26.7 at the time of surgery and 1 patient in the traditional group who was 45 years of age with a BMI of 31.5. The graft delamination, in addition to the other 2 documented graft failures, indicates a 4.3% failure rate of the cohort (5.9% of the accelerated group and 2.6% of the traditional group), comparable with previous studies.1,2,29
Another important finding in this study was the rate of graft hypertrophy, somewhat higher at 24 months postsurgery (27% of patients) than rates published previously for MACI9 and collagen-covered ACI,14,30 and similar to those presented for periosteal-covered ACI.30,31 Throughout the MRI evaluation timeline, there were similar hypertrophy rates for both the accelerated and traditional groups, and the reason for this high rate is unknown. However, all patients with hypertrophic grafts were nonsymptomatic at 24 months, although they will be closely monitored from this time to ascertain whether symptoms relating to graft overfill emerge.
A series of correlations were undertaken on the data collected at 24 months postsurgery to investigate 1) the association between patient demographics, chondral defect parameters, and preoperative injury/surgery characteristics on MRI-based graft outcomes and 2) the association between MRI-based graft outcomes and clinical scores. Age and BW restrictions are generally indicated for patients undergoing ACI,32,33 and although age and gender have not been shown to significantly affect postoperative clinical outcome,34 it is well known that as one ages, one’s associated regenerative capacity is reduced. Furthermore, it is also well known that excessive BW results in a significantly greater increase in the loads transmitted through the knee during WB activities.28 It is currently unknown how these additional loads may affect the tissue regeneration process. In this study, BMI was significantly correlated (P < 0.05) with the MRI composite score, graft infill, and knee joint effusion, while patient age was significantly correlated with the MRI composite score (P < 0.01), surface contour (P < 0.01), graft infill (P < 0.05), and border integration (P < 0.05). Interestingly, the current study revealed that all patients with an MRI composite score less than 2.5 at 24 months postsurgery were older than 40 years (Fig. 4) and/or demonstrated a BMI greater than 27 (Fig. 5). Furthermore, the only 4 patients with an MRI composite less than 1.5 were older than 40 years combined with a BMI greater than 27. This highlights the widely accepted notion that excessive BW and age (primarily an older cohort) may be critical factors in tissue regeneration.
With regard to preoperative injury/surgery characteristics, it is well known that a short (acute) history of trauma, pain, and symptoms leading up to the MACI procedure may be decisive factors in a good clinical outcome when compared with long-standing trauma or patients suffering from degenerative cartilage defects.34 However, MACI is often indicated as a secondary treatment following alternative failed cartilage repair procedures and therefore can be associated with a long duration of symptoms.11 Although the number of prior cartilage repair procedures had no significant correlation with MRI variables, apart from surface contour (P < 0.05), the defect size and the duration of preoperative symptoms were significantly correlated (P < 0.05) with the MRI composite score and a number of additional descriptive MRI parameters (Table 8). The pathophysiology underlining these correlations is undetermined, although with further research, this may prove important in predicting long-term graft outcome.
Our results revealed no correlations between the MRI composite score and any of the subjective and functional clinical measures. This lack of correlation may be the result of a small sample size or subjective measures that are not specific enough to detect changes and/or improvements resulting from ACI. Although the subjective tools selected (KOOS, SF-36) have been used routinely for ACI,6,10,14,26,35 a recent report stated that there are currently no cartilage repair–specific outcome measures.36 With the development of more specific tools to assess patients following ACI, a higher association between clinical and MRI-based results may emerge. These findings do indicate that, at present, both MRI-based assessment and clinically patient-reported outcomes are important and combine to assess patient and graft outcome. An important aim of ACI is to reduce pain and symptoms while returning the patient to a normally active lifestyle, variables that can be reported only verbally (or through questionnaires) by the patient. However, the ability of ACI to produce a hyaline-like regenerative tissue that may potentially withstand the high loading demands placed upon it, and prevent or delay the onset of osteoarthritis associated with articular cartilage pathology, can be assessed only by methods such as noninvasive MRI.
This study demonstrated that graft status following MACI, as assessed via MRI, significantly improved to 24 months postsurgery, although there were no differences observed between an accelerated or traditional postoperative return to full WB, supporting our first 2 hypotheses. Our third hypothesis was generally supported, whereby patient age, BMI, chondral defect size, and preoperative duration of symptoms were significantly associated with MRI-based outcomes. This study confirmed the generally accepted notion that factors such as age and BMI may be relevant in patients with cartilage problems and associated cartilage-repair procedures.3 Our final hypothesis was not supported, whereby no significant correlations existed between clinical and MRI-based outcome measures at 24 months. This requires further investigation and remains to be determined whether a hyaline-like repair tissue actually needs to be restored to provide durable long-term results.
The accelerated postoperative WB protocol presented appears to be safe and has no detrimental effect on the graft at 24 months postsurgery, as represented by the defined MRI evaluation protocol. It is well known that the postoperative mechanical environment is imperative in allowing optimal chondrocyte differentiation and development.5 However, in providing this mechanical stimulus, an excessively aggressive approach may risk graft delamination, whereas a too conservative approach may not provide adequate biomechanical graft stimulus, while delaying the patient’s return to normal function and promoting postoperative muscle loss, the development of intra-articular adhesions, and associated gait abnormalities, contributing to a poorer outcome. This article presents a relatively short-term follow-up of MRI-based assessment in patients randomly assigned to accelerated and traditional approaches to postoperative rehabilitation following MACI. Long-term follow-up is required to determine if there are any detrimental effects that may later emerge as a result of the accelerated return to full WB.
Footnotes
Acknowledgments and Funding: This research has received funding from the Hollywood Private Hospital Research Foundation (RF16 and RF31) and the National Health and Medical Research Council (ID254622). Ethics approval was obtained by the University of Western Australia and Hollywood Private Hospital.
Declaration of Conflicting Interests: A support grant of $6,000 AUS was provided to our group by Genzyme to assist in the data analysis and writing of the article. Genzyme had no influence in the manuscript or outcomes. Clinical Trial Registration Number: ACTRN126090007.
References
- 1. Bartlett W, Skinner JA, Gooding CR, Carrington RW, Flanagan AM, Briggs TW, et al. Autologous chondrocyte implantation versus matrix-induced autologous chondrocyte implantation for osteochondral defects of the knee: a prospective, randomised study. J Bone Joint Surg Br. 2005;87(5):640-5. [DOI] [PubMed] [Google Scholar]
- 2. Behrens P, Bitter T, Kurz B, Russlies M. Matrix-associated autologous chondrocyte transplantation/implantation (MACT/MACI)—5-year follow-up. Knee. 2006;13(3):194-202. [DOI] [PubMed] [Google Scholar]
- 3. Wondrasch B, Zak L, Welsch GH, Marlovits S. Effect of accelerated weightbearing after matrix-associated autologous chondrocyte implantation on the femoral condyle on radiographic and clinical outcome after 2 years: a prospective, randomized controlled pilot study. Am J Sports Med. 2009;37(Suppl 1):88S-96S. [DOI] [PubMed] [Google Scholar]
- 4. Robertson WB, Gilbey H, Ackland T, editors. Standard practice exercise rehabilitation protocols for matrix induced autologous chondrocyte implantation femoral condyles. Perth, Western Australia: Hollywood Functional Rehabilitation Clinic; 2004. [Google Scholar]
- 5. Hunter CJ, Imler SM, Malaviya P, Nerem RM, Levenston ME. Mechanical compression alters gene expression and extracellular matrix synthesis by chondrocytes cultured in collagen I gels. Biomaterials. 2002;23(4):1249-59. [DOI] [PubMed] [Google Scholar]
- 6. Marlovits S, Singer P, Zeller P, Mandl I, Haller J, Trattnig S. Magnetic resonance observation of cartilage repair tissue (MOCART) for the evaluation of autologous chondrocyte transplantation: determination of interobserver variability and correlation to clinical outcome after 2 years. Eur J Radiol. 2006;57(1):16-23. [DOI] [PubMed] [Google Scholar]
- 7. Marlovits S, Striessnig G, Resinger CT, Aldrian SM, Vecsei V, Imhof H, et al. Definition of pertinent parameters for the evaluation of articular cartilage repair tissue with high-resolution magnetic resonance imaging. Eur J Radiol. 2004;52(3):310-19. [DOI] [PubMed] [Google Scholar]
- 8. Trattnig S, Ba-Ssalamah A, Pinker K, Plank C, Vecsei V, Marlovits S. Matrix-based autologous chondrocyte implantation for cartilage repair: noninvasive monitoring by high-resolution magnetic resonance imaging. Magn Reson Imaging. 2005;23(7):779-87. [DOI] [PubMed] [Google Scholar]
- 9. Trattnig S, Pinker K, Krestan C, Plank C, Millington S, Marlovits S. Matrix-based autologous chondrocyte implantation for cartilage repair with Hyalograft((R))C: two-year follow-up by magnetic resonance imaging. Eur J Radiol. 2006;57(1):9-15. [DOI] [PubMed] [Google Scholar]
- 10. Ebert JR, Robertson WB, Lloyd DG, Zheng MH, Wood DJ, Ackland T. Traditional vs accelerated approaches to post-operative rehabilitation following matrix-induced autologous chondrocyte implantation (MACI): comparison of clinical, biomechanical and radiographic outcomes. Osteoarthr Cart. 2008;16:1131-40. [DOI] [PubMed] [Google Scholar]
- 11. Hambly K, Bobic V, Wondrasch B, Van Assche D, Marlovits S. Autologous chondrocyte implantation postoperative care and rehabilitation: science and practice. Am J Sports Med. 2006;34:1-19. [DOI] [PubMed] [Google Scholar]
- 12. Minas T, Peterson L. Advanced techniques in autologous chondrocyte transplantation. Clin Sport Med. 1999;18(1):13-44. [DOI] [PubMed] [Google Scholar]
- 13. Henderson IJ, Tuy B, Connell D, Oakes B, Hettwer WH. Prospective clinical study of autologous chondrocyte implantation and correlation with MRI at three and 12 months. J Bone Joint Surg Br. 2003;85(7):1060-6. [DOI] [PubMed] [Google Scholar]
- 14. Robertson WB, Fick D, Wood DJ, Linklater JM, Zheng MH, Ackland TR. MRI and clinical evaluation of collagen-covered autologous chondrocyte implantation (CACI) at two years. Knee. 2007;14(2):117-27. [DOI] [PubMed] [Google Scholar]
- 15. Takahashi T, Tins B, McCall IW, Richardson JB, Takagi K, Ashton K. MR appearance of autologous chondrocyte implantation in the knee: correlation with the knee features and clinical outcome. Skeletal Radiol. 2006;35(1):16-26. [DOI] [PubMed] [Google Scholar]
- 16. Ebert JR, Ackland TR, Lloyd DG, Wood DJ. Accuracy of partial weight bearing after autologous chondrocyte implantation. Arch Phys Med Rehabil. 2008;89(8):1528-34. [DOI] [PubMed] [Google Scholar]
- 17. Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331(14):889-95. [DOI] [PubMed] [Google Scholar]
- 18. Minas T, Chiu R. Autologous chondrocyte implantation. Am J Knee Surg. 2000;13(1):41-50. [PubMed] [Google Scholar]
- 19. Minas T, Peterson L. Autologous chondrocyte implantation. Op Tech Orth. 1997;7(4):323-33. [Google Scholar]
- 20. Malviya A, Richards J, Jones RK, Udwadia A, Doyle J. Reproducibility of partial weight bearing. Injury. 2005;36(4):556-9. [DOI] [PubMed] [Google Scholar]
- 21. Youdas JW, Kotajarvi BJ, Padgett DJ, Kaufman KR. Partial weight-bearing gait using conventional assistive devices. Arch Phys Med Rehabil. 2005;86(3):394-8. [DOI] [PubMed] [Google Scholar]
- 22. Dabke HV, Gupta SK, Holt CA, O’Callaghan P, Dent CM. How accurate is partial weightbearing? Clin Orthop Relat Res. 2004;(421):282-6. [DOI] [PubMed] [Google Scholar]
- 23. Gray F, Gray C, McClanahan J. Assessing the accuracy of partial weight-bearing instruction. Am J Orthop. 1998;27(8):558-60. [PubMed] [Google Scholar]
- 24. Chow DHK, Cheng CTK. Quantitative analysis of the effects of audio biofeedback on weight-bearing characteristics of persons with transtibial amputation during early prosthetic ambulation. J Rehab Res Dev. 2000;37:255-60. [PubMed] [Google Scholar]
- 25. Roos EM, Roos HP, Lohmander LS, Ekdahl C, Beynnon BD. Knee Injury and Osteoarthritis Outcome Score (KOOS)—development of a self-administered outcome measure. J Orthop Sport Phys Ther. 1998;28(2):88-96. [DOI] [PubMed] [Google Scholar]
- 26. Bartlett W, Gooding CR, Carrington RW, Briggs TW, Skinner JA, Bentley G. The role of the Short Form 36 Health Survey in autologous chondrocyte implantation. Knee. 2005;12:281-5. [DOI] [PubMed] [Google Scholar]
- 27. Plasqui G, Westerterp KR. Physical activity assessment with accelerometers: an evaluation against doubly labeled water. Obesity. 2007;15(10):2371-9. [DOI] [PubMed] [Google Scholar]
- 28. Messier SP, Gutekunst DJ, Davis C, DeVita P. Weight loss reduces knee-joint loads in overweight and obese older adults with knee osteoarthritis. Arthritis Rheum. 2005;52(7):2026-32. [DOI] [PubMed] [Google Scholar]
- 29. Cherubino P, Grassi FA, Bulgheroni P, Ronga M. Autologous chondrocyte implantation using a bilayer collagen membrane: a preliminary report. J Orthop Surg. 2003;11(1):10-15. [DOI] [PubMed] [Google Scholar]
- 30. Gooding CR, Bartlett W, Bentley G, Skinner JA, Carrington R, Flanagan A. A prospective, randomised study comparing two techniques of autologous chondrocyte implantation for osteochondral defects in the knee: periosteum covered versus type I/III collagen covered. Knee. 2006;13(3):203-10. [DOI] [PubMed] [Google Scholar]
- 31. Minas T. Autologous chondrocyte implantation for focal chondral defects of the knee. Clin Orthop Relat Res. 2001(391 Suppl):S349-61. [DOI] [PubMed] [Google Scholar]
- 32. Jones DG, Peterson L. Autologous chondrocyte implantation. J Bone Joint Surg Am. 2006;88(11):2502-20. [DOI] [PubMed] [Google Scholar]
- 33. Peterson L. Chondrocyte transplantation. In: Jackson DWJ, editor. Master techniques in orthopaedic surgery reconstructive knee surgery. 2nd ed. Philadelphia: Lippincott, Williams, and Wilkins; 2003. p 353-74. [Google Scholar]
- 34. Pietschmann MF, Horng A, Niethammer T, Pagenstert I, Sievers B, Jansson V, et al. Cell quality affects clinical outcome after MACI procedure for cartilage injury of the knee. Knee Surg Sports Traumatol Arthrosc. 2009;17(11):1305-11. [DOI] [PubMed] [Google Scholar]
- 35. Ossendorf C, Kaps C, Kreuz PC, Burmester GR, Sittinger M, Erggelet C. Treatment of posttraumatic and focal osteoarthritic cartilage defects of the knee with autologous polymer-based three-dimensional chondrocyte grafts: 2-year clinical results. Arthritis Res Ther. 2007;9(2):R41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hambly K, Griva K. IKDC or KOOS? Which measures symptoms and disabilities most important to postoperative articular cartilage repair patients? Am J Sports Med. 2008;36(9):1695-704. [DOI] [PubMed] [Google Scholar]


