Abstract
Background:
Microfracture (MFX) is frequently used to treat deep cartilage defects in the ankle; however, the data on repair tissue (RT) quality after MFX are very limited at this time. T2-mapping at 3 T has been optimized for the ankle and can be used to noninvasively evaluate cartilage collagen and water content. The aim of this study was to determine if the RT after MFX in the ankle had T2 properties similar to the adjacent reference cartilage (RC).
Methods:
Fourteen cases after MFX in the ankle were assessed with morphological MRI and T2-mapping at 3 T. The American Orthopaedic Foot and Ankle Society (AOFAS) score and a modified Cinicinnati Knee Rating System rating were used to evaluate the clinical outcome. The MRI protocol included a 3-dimensional sequence and a proton-density sequence for morphological evaluation and a multiecho spin echo sequence for T2-mapping. Region of interest analyses were carried out in accordance with the morphological images to ensure complete coverage of the defect site.
Results:
Both clinical scores demonstrated significant improvement at the time of the MR examination (P < 0.001). RT T2 was 49.3 ± 10.1 (range, 35.7-69.3) milliseconds, and RC T2 was 49.9 ± 8.2 (range, 38.4-63.7) milliseconds (P = 0.838). Relative T2 (rT2) was 1.00 ± 0.20 (range, 0.72-1.36).
Conclusion:
MFX in the ankle can provide RT with T2 properties similar to adjacent cartilage.
Keywords: T2-mapping, microfracture, ankle, 3 T
Introduction
A chronic cartilage defect will lead to early degeneration and osteoarthritis of the joint.1 Surgical cartilage repair techniques aim to provide a permanent filling of the defect in order to stabilize the adjacent cartilage and prevent further degeneration. Bone marrow–stimulating techniques (abrasion, drilling, microfracture), autologous chondrocyte transplantation (ACI), matrix-associated autologous chondrocyte implantation (MACI), and ostechondral autografts (mosaicplasty) are the most frequently used approaches to provide for repair tissue.2 There is evidence that the clinical outcome after cartilage repair in the knee is influenced by defect filling and repair tissue (RT) composition; cases with hyaline-like RT are more likely to profit in the long term.3-8
The microfracture (MFX) technique is based on the principle that the introduction of pluripotent mesenchymal cells from the bone marrow will lead to a blood clot that subsequently forms cartilage RT.9 Clinical studies on MFX in the knee have demonstrated that the technique predominantly results in fibrous RT and that knee function deteriorates at midterm.3,5,6,10 In the ankle, MFX is frequently used to treat deep defects (Hepple 3 and 4) and has been reported to yield good-to-excellent results in up to 96% after 24 to 55 months.10 At this time, there are no comparable data available to determine if RT quality plays the same role in the ankle as found in the knee; however, there is clinical evidence that MFX can provide stable results at midterm.11
Both ACI and MACI can provide excellent results in the ankle at short term, midterm, and long term12-15; in a recent study, Giannini et al.16 report 9 of 10 cases had good-to-excellent outcome after more than 9 years. ACI techniques, however, have considerably higher morbidity and costs than MFX, particularly due to the fact that malleolar osteotomy will often be necessary to access the defect.15-17 It is therefore of interest if the RT composition after MFX is comparable to native cartilage or, as found in the knee, ACI techniques will more frequently provide superior RT quality.4-6,8
Recent advances in MRI technology have yielded techniques to directly visualize the molecular composition of articular cartilage and of cartilage RT.18-26 T2-mapping has been demonstrated to be sensitive for tissue collagen orientation and water content.9,10 The technique does not require the use of contrast agent, which adds to its attractiveness for clinical use. Histological validation in an animal model demonstrated a high sensitivity and specificity for MFX RT.27 In vivo studies that used T2-mapping at 3 T to assess MFX in the knee found a lack of zonal organization in MFX tissue and that global T2 was significantly lower than in control cartilage as well as in RT after MACI.28,29 The application of T2-mapping in the ankle remains challenging due to the thin cartilage layers of the ankle; however, the technique has been recently optimized and evaluated for the assessment of cartilage RT in the ankle at 3 T.29,30
The aim of this study was to obtain first data on the T2 properties of MFX RT in the ankle using an optimized T2-mapping protocol at 3 T. Clinical scores and morphological MRI were assessed as additional outcome measures.
Materials and Methods
Patients and Clinical Evaluation
Between 1997 and 2006, 31 cases with symptomatic osteochondral defects of the talar dome were treated with MFX. Fourteen cases (6 female, 8 male) consented to the MRI study protocol. The follow-up period was (mean ± standard deviation) 55 ± 28 (range, 11-98) months after surgical treatment. At the time of the MR scan, the age was 41.9 ± 13.8 years, and the body mass index (BMI) was 26.9 ± 4.7 kg/m2. All defects were singular and located on the talus (2 on the lateral, 12 on the medial aspect). Six cases had a traumatic and 8 a progredient symptom onset. The defect size was 1.4 ± 0.9 cm2. The study protocol (blinded) was approved by the institutional review board, and informed consent was obtained from all participants.
The evaluation of clinical outcome was carried out with the American Orthopaedic Foot and Ankle Society (AOFAS) score10 and with a modified Cincinnati Knee Rating System17 adapted for ankle patients before surgery and at the time of the MRI examination.
In the AOFAS score, a maximum of 100 points can be attained (50 function, 40 pain, 10 alignment): 100 to 90 was considered excellent, 89 to 80 good, 79 to 60 fair, and below 59 poor.16 The modified Cincinnati rating ranged from 0 to 10 points: 0 to 2, severe limitations to daily life; 3 to 4, moderate limitations to daily life, no sports possible; 5 to 6, minimal limitations to daily life, sports possible with compensation; 7 to 8, minimal limitations to sports activities; and 9 to 10, no limitations to sports. The AOFAS score was 39.9 ± 17.3, and the modified Cinicinnati rating was 2.7 ± 1.1 before surgery, respectively (Table 1).
Table 1.
Single-Case Data
| Case # | Age, y | Follow-up, mo | BMI, kg/m2 | Defect Size, cm2 | Modified Preoperative Cincinnati | Modified Postoperative Cincinnati | Preoperative AOFAS | Postoperative AOFAS | RT T2 | RC T2 | rT2 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 56 | 68 | 32.2 | 1.5 | 3 | 6 | 14 | 68 | 55.8 | 60.5 | 0.92 |
| 2 | 21 | 84 | 20.6 | 1.7 | 4 | 9 | 70 | 96 | 50.1 | 46.7 | 1.07 |
| 3 | 56 | 95 | 23.8 | 0.6 | 4 | 8 | 54 | 97 | 69.3 | 56.9 | 1.22 |
| 4 | 40 | 33 | 24.2 | 0.5 | 3 | 1 | 52 | 51 | 56.1 | 63.4 | 0.88 |
| 5 | 33 | 28 | 22.1 | 1.6 | 3 | 6 | 50 | 90 | 46.0 | 63.7 | 0.72 |
| 6 | 49 | 59 | 29.8 | 0.6 | 1 | 9 | 27 | 85 | 43.5 | 45.7 | 0.95 |
| 7 | 23 | 60 | 25.4 | 1 | 1 | 7 | 15 | 82 | 35.7 | 42.8 | 0.84 |
| 8 | 61 | 63 | 30.5 | 1.3 | 2 | 8 | 21 | 95 | 51.3 | 51.7 | 0.99 |
| 9 | 54 | 17 | 27 | 1.8 | 3 | 9 | 48 | 95 | 62.5 | 45.9 | 1.36 |
| 10 | 53 | 26 | 30 | 1.9 | 2 | 1 | 37 | 17 | 57.2 | 45.9 | 1.25 |
| 11 | 47 | 11 | 31.9 | 0.5 | 2 | 8 | 32 | 85 | 37.1 | 49.1 | 0.76 |
| 12 | 26 | 73 | 19.4 | 1.2 | 4 | 8 | 58 | 85 | 38.6 | 46.8 | 0.83 |
| 13 | 26 | 58 | 34.9 | 1.5 | 2 | 6 | 29 | 71 | 48.4 | 38.4 | 1.26 |
| 14 | 42 | 98 | 24.6 | 4 | 4 | 6 | 52 | 80 | 38.5 | 40.6 | 0.95 |
| Mean | 41.9 | 55.2 | 26.9 | 1.4 | 2.7 | 6.6 | 39.9 | 78.4 | 49.3 | 49.9 | 1.00 |
| Standard deviation | 13.8 | 28.2 | 4.7 | 0.9 | 1.1 | 2.6 | 17.3 | 21.8 | 10.1 | 8.2 | 0.20 |
| Minimum | 21 | 11 | 19 | 1 | 1 | 1 | 14 | 17 | 35.7 | 38.4 | 0.72 |
| Maximum | 61 | 98 | 35 | 4 | 4 | 9 | 70 | 97 | 69.3 | 63.7 | 1.36 |
Note: BMI = body mass index; AOFAS = American Orthopaedic Foot and Ankle Society; RT = repair tissue; RC = reference cartilage; rT2 = relative T2 (RT/RC).
Surgical Technique and Rehabilitation Protocol
Symptomatic deep chondral (Outerbridge grade 3 or 4) or osteochondral defects (Hepple 3 and 4) with stable adjacent cartilage were eligible for MFX; cases with rheumatoid arthritis, progressed osteoarthritis, kissing lesions, malalignment, or instability of the joint were excluded. The surgical procedure was carried out as described by Steadman et al.31 Briefly, an anteromedial and anterolateral portal was used to assess the defect. If present, loose bodies were removed, and subsequently, the defect was debrided to ensure the adjacent cartilage was stable. Either 70° or 30° angled awls were used to set the microholes with a minimum distance of 3 mm; penetration of the subchondral bone was considered sufficient when marrow fat became visible. After release of the tourniquet, bleeding from the perforations was verified.
All patients had a dorsal splint for 2 days after surgery. Afterwards, a walker brace was given, and passive movement was started under the instruction of a physical therapist. Nonweightbearing mobilization with crutches was prescribed for 6 weeks, followed by 4 to 6 weeks of gradual weightbearing according to pain and effusion. Exercise on a stationary cycle was started 6 weeks after surgery; however, it was recommended not to start sports with impact such as jogging before 6 months after treatment.
MRI Technique
All examinations were performed on a 3-T MR unit (Magnetom TIM Trio, Siemens, Erlangen, Germany) with a maximum gradient strength of 40 mT/m using an 8-channel (phased array) flexible multipurpose coil (Noras, Würzburg, Germany). For volumetric defect site measurements and for the field-of-view (FOV) planning of the morphological and T2-mapping sequences, we used an isotropic 3-dimensional (3-D) gradient echo (true FISP) sequence (Fig. 1). A 160-mm FOV and 5122 matrix resulted in 0.4 × 0.4 × 0.4-mm isotropic resolution; TR and TE were 8.86 and 1.95 milliseconds, respectively; the flip angle was 28°. Two averages were measured, and the bandwidth was 200 Hz/pixel. With the use of generalized autocalibrating partially parallel acquisition (GRAPPA), the acceleration factor was 3, resulting in an acquisition time of 9 minutes 33 seconds.
Figure 1.

Three-dimensional defect assessment and field-of-view (FOV) planning with the true FISP sequence in case 7: (A) sagittal, (B) coronal, and (C) axial plane. The sequence was used to ensure the morphological images, and the T2-maps were placed accurately over the defect. The sagittal plane (A) was used to center the FOV (Fig. 2). White arrows indicate the repair site that is accurately delineated. The defect is completely covered; however, there is synovial fluid between the tibial and talar cartilages (hyperintense band over the defect in A and B). The repair tissue is hypointense compared to the adjacent native cartilage. Further morphological analyses were based on the 2-dimensional high-resolution sequences.
A proton density fat-suppressed turbo spin echo (PD-FS-TSE) sequence and a T1-weighted spin echo sequence to assess articular cartilage, effusion, and subchondral bone were used as the morphological gold standard (Fig. 2A). The in-plane resolution of the PD sequence was 0.3 × 0.3 mm with a slice thickness of 3 mm, TR/TE was 2100/26 milliseconds, the flip angle was 16°, one average was used, the bandwidth was 244 Hz/pixel, and 16 slices were acquired, resulting in a total scan time of 6 minutes 30 seconds. The T1-weighted sequence had an in-plane resolution of 0.6 × 0.5 mm with a slice thickness of 3 mm. TR/TE was 548/16 milliseconds, the bandwidth was 205 Hz/pixel, and 19 slices were acquired, resulting in a scan time of 2 minutes 37 seconds.
Figure 2.
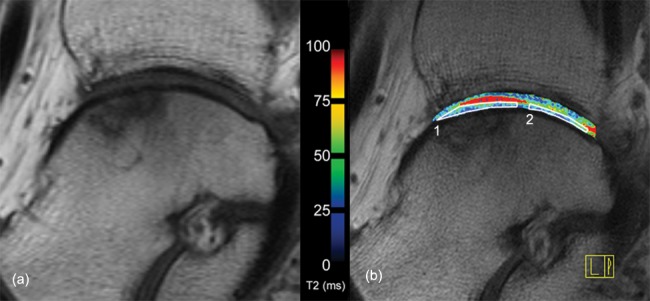
Morphological T1-weighted spin echo image (A) and corresponding T2-map (B) of case 7. Subchondral alterations are visible in the area of the repair site. Synovial fluid is seen between talar and tibial cartilage in the T2-map (red area, corresponding to Fig. 1); however, the repair site shows a complete covering of the defect and a homogeneous distribution of T2 values throughout the cartilage layers. The white boxes indicate how the regions of interest (1, repair tissue; 2, reference cartilage) were placed for T2 assessment.
A multiecho spin echo sequence was used to obtain the T2 relaxation times for T2-mapping (Fig. 2B). A 160 × 160-mm FOV, 320 × 320-pixel matrix yielded an in-plane resolution of 0.4 × 0.4 mm with a slice thickness of 3 mm; TR was 1.000 second, and 6 different echo times (13.8 milliseconds, 27.6 milliseconds, 41.4 milliseconds, 55.3 milliseconds, 69 milliseconds, and 82.8 milliseconds) were used. The bandwidth was 240 Hz/pixel, the number of averages was 1, and 16 slices were measured, resulting in a total scan time of 6 minutes 52 seconds. T2-maps were calculated with a pixel-wise, monoexponential nonnegative least squares (NNLS) fit analysis (MapIt, Siemens).
The senior author (S.T.) assessed the morphological MRI based on a modified magnetic resonance observation of cartilage repair tissue (MOCART) scoring system32: 1 = filling of the defect, 2 = cartilage interface, 3 = surface of the RT, 4 = structure, 5 = adjacent bone marrow, 6 = signal intensity, and 7 = effusion. Filling of the defect and tissue signal intensity were assessed both with the 3-D sequence and with the morphological 2-D sequences. All other categories were assessed using the morphological 2-D sequences.
Region-of-Interest Analysis
In consensus with the morphological images (Fig. 2), 2 contiguous slices covering the cartilage RT were selected, and region-of-interest (ROI) analysis was carried out. ROIs covered the full thickness of RT and reference cartilage (RC) layers in each slice; however, the readings covered the talar cartilage layer only (Fig. 2B). The resolution of the T2-maps allowed for ROI analysis with a minimum of 100 pixels. Subchondral bone and synovial fluid were carefully excluded. The data from all slices were then used to calculate mean RT T2 and RC T2. Relative T2 (rT2) of the repair sites and of intact cartilage was then calculated in order to compare the cases (rT2 = RT T2 / RC T2).28
Statistical Analyses
Statistical analyses were carried out with SPSS 14.0 (SPSS Institute, Chicago, IL) and in Microsoft Excel on a Windows XP platform (Microsoft, Redmond, WA). Metric clinical outcome values were compared with 2-sided, paired t tests. Frequency analyses were used to assess the outcome of the MOCART evaluation, and 2-sided, independent Student t tests were used to compare the ROI T2 values.
Results
The mean AOFAS score at follow-up was 78.4 ± 21.8, and the modified Cincinnati rating was 6.6 ± 2.6; the improvement from the preoperative values was highly significant in both scores (P < 0.001 in the paired, 2-sided t test). The AOFAS score was excellent in 5 (35.7%), good in 5 (35.7%), fair in 2 (14.3%), and poor in 2 cases (14.3%), respectively. Three patients had no limitations to sports, 5 patients could perform sports with minimal limitations, and 4 had minimal limitations to daily life and could perform sports with compensation. Two patients felt they were severely limited in daily life but did not wish for further surgical treatment.
Morphological MRI evaluation demonstrated complete defect coverage in all cases. Volumetric defect filling was found to be complete (100% filling) in 9 cases (64.3%), near complete (75%-100% filling) in 3 cases (21.4%), and 50% to 75% in 1 case (7.1%). All defects showed complete integration with the adjacent native cartilage. RT surface was intact in 11 cases (78.6%), superficial fissures occurred in 1 case (7.1%), and the remaining 2 (14.3%) had fissures <50% of total RT depth. The RT structure appeared homogeneous in 10 (71.4%) and inhomogeneous in 4 (28.6%) cases. Moderate bone marrow edema was present in 8 (57.1%) cases, and effusion was found in 2 cases (14.3%). See Table 2 for details.
Table 2.
Magnetic Resonance Observation of Cartilage Repair Tissue (MOCART) Score Results
| Absolute | Percentage | |
|---|---|---|
| 1. Filling of the defect | ||
| Complete | 11 | 78.6 |
| Hypertrophy | 0 | 0.0 |
| Incomplete, % | ||
| >50 | 3 | 21.4 |
| <50 | 0 | 0.0 |
| 0 | 0 | 0.0 |
| Volume fill, % | ||
| 0 | 0 | 0.0 |
| 0-25 | 0 | 0.0 |
| 25-50 | 0 | 0.0 |
| 50-75 | 1 | 7.1 |
| 75-100 | 3 | 21.4 |
| 100 | 9 | 64.3 |
| >100 | 0 | 0.0 |
| 2. Cartilage interface lengths (filling parallel to cartilage surface) | ||
| Complete (integration with surrounding cartilage) | 14 | 100,0 |
| Incomplete (integration with surrounding cartilage) | 0 | 0,0 |
| Demarcation border visible (split like) | 0 | 0.0 |
| Defect visible | ||
| <50% of length of the RT | 0 | 0.0 |
| >50% of length of the RT | 0 | 0.0 |
| 3. Surface of the repair tissue | ||
| Surface intact | 11 | 78.6 |
| Surface damaged | ||
| Fibrillations/fissures/ulcerations | 1 | 7.1 |
| <50% of RT depth | 2 | 14.3 |
| >50% of RT depth/total degeneration | 0 | 0.0 |
| 4. Structure | ||
| Homogeneous | 10 | 71.4 |
| Inhomogeneous | 4 | 28.6 |
| 5. Adjacent bone marrow | ||
| Normal | 6 | 42.9 |
| Edema | ||
| Minor | 3 | 21.4 |
| Moderate (<2 cm) | 4 | 28.6 |
| Severe (>2 cm) | 1 | 7.1 |
| Cyst | 0 | 0.0 |
| Granulation tissue | 0 | 0.0 |
| Cyst | 0 | 0.0 |
| Sclerosis | 0 | 0.0 |
| 6. Signal intensity | ||
| True FISP 3-dimensional | ||
| Isointense | 11 | 78.6 |
| Hyperintense | 0 | 0.0 |
| Hypointense | 3 | 21.4 |
| PD-TSE | ||
| Isointense | 8 | 57.1 |
| Hyperintense | 1 | 7.1 |
| Hypointense | 5 | 35.7 |
| 7. Effusion | ||
| No | 12 | 85.7 |
| Yes | 2 | 14.3 |
Note: RT = repair tissue; true FISP = true fast imaging with steady-state precession; PD-TSE = proton density turbo spin echo.
RT T2 was 49.3 ± 10.1 (range, 35.7-69.3) milliseconds, and RC T2 was 49.9 ± 8.2 (range, 38.4-63.7) milliseconds. No significant difference was found between RT and RC T2 (P = 0.838). Relative T2 (rT2) was 1.00 ± 0.20 (range, 0.72-1.36). See Table 1 for details.
Discussion
This study reports on the T2 properties of cartilage RT after MFX in the ankle. We found that the RT had T2 properties comparable to the adjacent articular cartilage. There were 71.4% (10/14) of cases that had good-to-excellent outcome in the AOFAS score.
Becher et al.11 found good-to-excellent results in 79% at 5.8 ± 2.0 years; the mean age in their series was 40 ± 14 years, and 18 cases had a BMI below and 27 had a BMI greater than 25 kg/m2, respectively. Whereas patients older and younger than 50 years had comparable outcome, there was significantly better outcome in those with a BMI below 25 kg/m2. In the current series, the mean age at follow-up was comparable; however, 8 of 14 cases had a BMI >25 kg/m2. In contrast, Saxena et al.10 report that the mean AOFAS score after MFX was 94.4 ± 6.2 at 24 to 55 months (96% good-to-excellent results) in athletes. The number of cases was too small for conclusive correlation analyses regarding the influence of BMI on clinical outcome.
Morphological MRI of the defects yielded good results in the majority of the cases (Table 2). In contrast to the series reported by Becher et al.,11 no cases with hypertrophic RT occurred. It is worth noting that case 10 had severe tendovaginitis in the area of the flexor retinaculum at the time of the study; this may explain the poor clinical outcome despite fair outcome in the evaluation of the defect.
The T2 values had a relatively wide range both in the RT and in the adjacent RC. This agrees with results of other series evaluated with T2-mapping at 3 T19,25,28,29,33; mean T2 was comparable to previous series in the ankle.30,34
T2 relaxation of cartilage is dominated by the dipolar interaction of water molecules.35 Free water is considered to influence T2 independently from the orientation to the static magnetic field, whereas the relaxation component of macromolecule-associated water is orientation dependent due to the collagen anisotropy.36 The orientation-dependent component of T2 relaxation leads to a sensitivity to the arrangement of the collagen fibrils37; direct comparison with polarized light microscopy demonstrates a strong agreement of T2 variation across the cartilage layers and the zonal organization of the cartilage matrix and of cartilage RT after MFX.27,37 In intact hyaline cartilage, T2 shows an increase from the deep toward the superficial layers. A similar variation has been found in MACI RT but not in MFX RT in the knee.19,22,29,33
Clinical studies on cartilage repair in the knee have demonstrated that ACI results in hyaline-like RT more frequently than MFX does.5,8 Consistent with these results, a preliminary cross-sectional study comparing T2 after MFX and MACI demonstrated lower T2 in MFX RT.29
In the ankle, considerably less data on RT ultrastructure are available. The cartilage layer of the talus has been found to have increased regenerative potential in comparison to the knee, and the biomechanical properties of the ankle favor the formation of RT after cartilage repair.38
T2-mapping analysis using the same protocol in healthy volunteers yielded a mean T2 of 51.1 ± 4.6 milliseconds in talar cartilage.34 In a cases series of 12 patients after matrix-associated autologous chondrocyte transplantation (MACT) in the ankle with a mean age of 32.8 ± 8.5 years and postoperative follow-up interval of 19.8 ± 12.6 (range, 6-54) months, the T2 values of the talar control cartilage and repair sites were 47.6 ± 9.3 milliseconds and 50.1 ± 8.0 milliseconds, respectively.30 The T2 values in the current series indicate that the water and collagen content of control cartilage was comparable to that of volunteers and of cases after MACI, respectively. T2 of the MFX RT was comparable to both adjacent RC and to MACI RT.
It is worth noting that T2 of cartilage has a relatively high individual variability and is subjected to methodological limitations regarding different T2-mapping techniques; Pai et al.39 demonstrated in phantoms that different T2 techniques yielded different T2 values with errors ranging from −21.0% to 20.9%. A direct comparison of absolute T2 values obtained with different techniques is therefore problematic. If the RT is described with regard to the adjacent articular cartilage, the variation of values deriving from different mapping techniques is less substantial, and individual variations in T2 can be taken into account; rT2 is therefore more suited to compare the RT composition among different cases and techniques.28
In the knee, a mean rT2 of 0.85 ± 0.10 (range, 0.61-1.02) was found in a series of 24 cases and 0.89 ± 0.12 (range, 0.78-1.03) in a series of 10 cases.28,33 The mean rT2 of 1.00 ± 0.20 (range, 0.72-1.36) found in the current series indicates that RT after MFX in the ankle has T2 properties more similar to those of native adjacent cartilage than found in the knee (Fig. 3).
Figure 3.

Examples for T2-maps of cases 3 (A) and 4 (B). Despite obvious alterations of the subchondral bone, the T2 values of the repair tissue are similar to the adjacent native cartilage; in case 3 (A), a zonal organization of the T2 values can be seen; T2 is lower near the subchondral plate (blue) and increases toward the surface (green) both in the repair tissue and in the adjacent reference cartilage.
Limitations to this study are the nonrandomization of the case series, heterogeneous follow-up intervals, and the lack of histological controls. Furthermore, bilaminar T2 ROI analysis as used in the knee was not feasible due to the limitations with regard to in-plane resolution29; therefore, the zonal organization of the MFX RT could not be assessed quantitatively.
Still, these first results indicate that in contrast to the knee, MFX in the ankle is more likely to result in RT with water and collagen content comparable to MACI. In light of the considerably lower morbidity and costs of MFX, further clinical studies to confirm these findings seem of interest.
Footnotes
Acknowledgments and Funding: Project funding was covered by a grant from the Medical Scientific Fund of the Mayor of the City of Vienna, Project Number 08011.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- 1. Buckwalter JA, Mankin HJ. Articular cartilage: degeneration and osteoarthritis, repair, regeneration, and transplantation. Instr Course Lect. 1998;47:487-504. [PubMed] [Google Scholar]
- 2. Bedi A, Feeley BT, Williams RJ., 3rd Management of articular cartilage defects of the knee. J Bone Joint Surg Am. 2010;92(4):994-1009. [DOI] [PubMed] [Google Scholar]
- 3. Kreuz PC, Steinwachs MR, Erggelet C, Krause SJ, Konrad G, Uhl M, et al. Results after microfracture of full-thickness chondral defects in different compartments in the knee. Osteoarthritis Cartilage. 2006;14(11):1119-25. [DOI] [PubMed] [Google Scholar]
- 4. Saris DB, Vanlauwe J, Victor J, Almqvist KF, Verdonk R, Bellemans J, et al. Treatment of symptomatic cartilage defects of the knee: characterized chondrocyte implantation results in better clinical outcome at 36 months in a randomized trial compared to microfracture. Am J Sports Med. 2009;37 Suppl 1:S10-9. [DOI] [PubMed] [Google Scholar]
- 5. Knutsen G, Engebretsen L, Ludvigsen TC, Drogset JO, Grontvedt T, Solheim E, et al. Autologous chondrocyte implantation compared with microfracture in the knee: a randomized trial. J Bone Joint Surg Am. 2004;86-A(3):455-64. [DOI] [PubMed] [Google Scholar]
- 6. Knutsen G, Drogset JO, Engebretsen L, Grontvedt T, Isaksen V, Ludvigsen TC, et al. A randomized trial comparing autologous chondrocyte implantation with microfracture: findings at five years. J Bone Joint Surg Am. 2007;89(10):2105-12. [DOI] [PubMed] [Google Scholar]
- 7. Henderson I, Lavigne P, Valenzuela H, Oakes B. Autologous chondrocyte implantation: superior biologic properties of hyaline cartilage repairs. Clin Orthop Relat Res. 2007;455:253-61. [DOI] [PubMed] [Google Scholar]
- 8. Saris DB, Vanlauwe J, Victor J, Haspl M, Bohnsack M, Fortems Y, et al. Characterized chondrocyte implantation results in better structural repair when treating symptomatic cartilage defects of the knee in a randomized controlled trial versus microfracture. Am J Sports Med. 2008;36(2):235-46. [DOI] [PubMed] [Google Scholar]
- 9. Mithoefer K, McAdams T, Williams RJ, Kreuz PC, Mandelbaum BR. Clinical efficacy of the microfracture technique for articular cartilage repair in the knee: an evidence-based systematic analysis. Am J Sports Med. 2009;37(10):2053-63. [DOI] [PubMed] [Google Scholar]
- 10. Saxena A, Eakin C. Articular talar injuries in athletes: results of microfracture and autogenous bone graft. Am J Sports Med. 2007;35(10):1680-7. [DOI] [PubMed] [Google Scholar]
- 11. Becher C, Driessen A, Hess T, Longo UG, Maffulli N, Thermann H. Microfracture for chondral defects of the talus: maintenance of early results at midterm follow-up. Knee Surg Sports Traumatol Arthrosc. 2010;18(5):656-63. [DOI] [PubMed] [Google Scholar]
- 12. Whittaker JP, Smith G, Makwana N, Roberts S, Harrison PE, Laing P, et al. Early results of autologous chondrocyte implantation in the talus. J Bone Joint Surg Br. 2005;87(2):179-83. [DOI] [PubMed] [Google Scholar]
- 13. Giannini S, Buda R, Grigolo B, Vannini F. Autologous chondrocyte transplantation in osteochondral lesions of the ankle joint. Foot Ankle Int. 2001;22(6):513-7. [DOI] [PubMed] [Google Scholar]
- 14. Giannini S, Buda R, Grigolo B, Vannini F, De Franceschi L, Facchini A. The detached osteochondral fragment as a source of cells for autologous chondrocyte implantation (ACI) in the ankle joint. Osteoarthritis Cartilage. 2005;13(7):601-7. [DOI] [PubMed] [Google Scholar]
- 15. Petersen L, Brittberg M, Lindahl A. Autologous chondrocyte transplantation of the ankle. Foot Ankle Clin. 2003;8(2):291-303. [DOI] [PubMed] [Google Scholar]
- 16. Giannini S, Battaglia M, Buda R, Cavallo M, Ruffilli A, Vannini F. Surgical treatment of osteochondral lesions of the talus by open-field autologous chondrocyte implantation: a 10-year follow-up clinical and magnetic resonance imaging T2-mapping evaluation. Am J Sports Med. 2009;37 Suppl 1:S112-8. [DOI] [PubMed] [Google Scholar]
- 17. Dorotka R, Kotz R, Trattnig S, Nehrer S. [Mid-term results of autologous chondrocyte transplantation in knee and ankle: a one- to six-year follow-up study]. Z Rheumatol. 2004;63(5):385-92. [DOI] [PubMed] [Google Scholar]
- 18. Trattnig S, Domayer S, Welsch GW, Mosher T, Eckstein F. MR imaging of cartilage and its repair in the knee: a review. Eur Radiol. 2009;19(7):1582-94. [DOI] [PubMed] [Google Scholar]
- 19. Domayer SE, Welsch GH, Nehrer S, Chiari C, Dorotka R, Szomolanyi P, et al. T2 mapping and dGEMRIC after autologous chondrocyte implantation with a fibrin-based scaffold in the knee: preliminary results. Eur J Radiol. 2010;73(3):636-42. [DOI] [PubMed] [Google Scholar]
- 20. Trattnig S, Mamisch TC, Pinker K, Domayer S, Szomolanyi P, Marlovits S, et al. Differentiating normal hyaline cartilage from post-surgical repair tissue using fast gradient echo imaging in delayed gadolinium-enhanced MRI (dGEMRIC) at 3 Tesla. Eur Radiol. 2008;18(6):1251-9. [DOI] [PubMed] [Google Scholar]
- 21. Trattnig S, Marlovits S, Gebetsroither S, Szomolanyi P, Welsch GH, Salomonowitz E, et al. Three-dimensional delayed gadolinium-enhanced MRI of cartilage (dGEMRIC) for in vivo evaluation of reparative cartilage after matrix-associated autologous chondrocyte transplantation at 3.0T: preliminary results. J Magn Reson Imaging. 2007;26(4):974-82. [DOI] [PubMed] [Google Scholar]
- 22. Trattnig S, Mamisch TC, Welsch GH, Glaser C, Szomolanyi P, Gebetsroither S, et al. Quantitative T2 mapping of matrix-associated autologous chondrocyte transplantation at 3 Tesla: an in vivo cross-sectional study. Invest Radiol. 2007;42(6):442-8. [DOI] [PubMed] [Google Scholar]
- 23. Domayer SE, Welsch GH, Dorotka R, Mamisch TC, Marlovits S, Szomolanyi P, et al. MRI monitoring of cartilage repair in the knee: a review. Semin Musculoskelet Radiol. 2008;12(4):302-17. [DOI] [PubMed] [Google Scholar]
- 24. Domayer SE, Trattnig S, Stelzeneder D, Hirschfeld C, Quirbach S, Dorotka R, et al. Delayed gadolinium-enhanced MRI of cartilage in the ankle at 3 T: feasibility and preliminary results after matrix-associated autologous chondrocyte implantation. J Magn Reson Imaging. 2010;31(3):732-9. [DOI] [PubMed] [Google Scholar]
- 25. Welsch GH, Trattnig S, Hughes T, Quirbach S, Olk A, Blanke M, et al. T2 and T2* mapping in patients after matrix-associated autologous chondrocyte transplantation: initial results on clinical use with 3.0-Tesla MRI. Eur Radiol. 2010;20(6):1515-23. [DOI] [PubMed] [Google Scholar]
- 26. Friedrich KM, Mamisch TC, Plank C, Langs G, Marlovits S, Salomonowitz E, et al. Diffusion-weighted imaging for the follow-up of patients after matrix-associated autologous chondrocyte transplantation. Eur J Radiol. 2010;73(3):622-8. [DOI] [PubMed] [Google Scholar]
- 27. White LM, Sussman MS, Hurtig M, Probyn L, Tomlinson G, Kandel R. Cartilage T2 assessment: differentiation of normal hyaline cartilage and reparative tissue after arthroscopic cartilage repair in equine subjects. Radiology. 2006;241(2):407-14. [DOI] [PubMed] [Google Scholar]
- 28. Domayer SE, Kutscha-Lissberg F, Welsch G, Dorotka R, Nehrer S, Gabler C, et al. T2 mapping in the knee after microfracture at 3.0 T: correlation of global T2 values and clinical outcome. Preliminary results. Osteoarthritis Cartilage. 2008;16(8):903-8. [DOI] [PubMed] [Google Scholar]
- 29. Welsch GH, Mamisch TC, Domayer SE, Dorotka R, Kutscha-Lissberg F, Marlovits S, et al. Cartilage T2 assessment at 3-T MR imaging: in vivo differentiation of normal hyaline cartilage from reparative tissue after two cartilage repair procedures. Initial experience. Radiology. 2008;247(1):154-61. [DOI] [PubMed] [Google Scholar]
- 30. Quirbach S, Trattnig S, Marlovits S, Zimmermann V, Domayer S, Dorotka R, et al. Initial results of in vivo high-resolution morphological and biochemical cartilage imaging of patients after matrix-associated autologous chondrocyte transplantation (MACT) of the ankle. Skeletal Radiol. 2009;38(8):751-60. [DOI] [PubMed] [Google Scholar]
- 31. Steadman JR, Rodkey WG, Rodrigo JJ. Microfracture: surgical technique and rehabilitation to treat chondral defects. Clin Orthop Relat Res. 2001;391 Suppl:S362-9. [DOI] [PubMed] [Google Scholar]
- 32. Marlovits S, Singer P, Zeller P, Mandl I, Haller J, Trattnig S. Magnetic resonance observation of cartilage repair tissue (MOCART) for the evaluation of autologous chondrocyte transplantation: determination of interobserver variability and correlation to clinical outcome after 2 years. Eur J Radiol. 2006;57(1):16-23. [DOI] [PubMed] [Google Scholar]
- 33. Welsch GH, Trattnig S, Domayer S, Marlovits S, White LM, Mamisch TC. Multimodal approach in the use of clinical scoring, morphological MRI and biochemical T2-mapping and diffusion-weighted imaging in their ability to assess differences between cartilage repair tissue after microfracture therapy and matrix-associated autologous chondrocyte transplantation: a pilot study. Osteoarthritis Cartilage. 2009;17(9):1219-27. [DOI] [PubMed] [Google Scholar]
- 34. Welsch GH, Mamisch TC, Weber M, Horger W, Bohndorf K, Trattnig S. High-resolution morphological and biochemical imaging of articular cartilage of the ankle joint at 3.0 T using a new dedicated phased array coil: in vivo reproducibility study. Skeletal Radiol. 2008;37(6):519-26. [DOI] [PubMed] [Google Scholar]
- 35. Mlynarik V, Szomolanyi P, Toffanin R, Vittur F, Trattnig S. Transverse relaxation mechanisms in articular cartilage. J Magn Reson. 2004;169(2):300-7. [DOI] [PubMed] [Google Scholar]
- 36. Menezes NM, Gray ML, Hartke JR, Burstein D. T2 and T1rho MRI in articular cartilage systems. Magn Reson Med. 2004;51(3):503-9. [DOI] [PubMed] [Google Scholar]
- 37. Nieminen MT, Rieppo J, Toyras J, Hakumaki JM, Silvennoinen J, Hyttinen MM, et al. T2 relaxation reveals spatial collagen architecture in articular cartilage: a comparative quantitative MRI and polarized light microscopic study. Magn Reson Med. 2001;46(3):487-93. [DOI] [PubMed] [Google Scholar]
- 38. Kuettner KE, Cole AA. Cartilage degeneration in different human joints. Osteoarthritis Cartilage. 2005;13(2):93-103. [DOI] [PubMed] [Google Scholar]
- 39. Pai A, Li X, Majumdar S. A comparative study at 3 T of sequence dependence of T2 quantitation in the knee. Magn Reson Imaging. 2008;26(9):1215-20. [DOI] [PMC free article] [PubMed] [Google Scholar]


