Abstract
Objective:
The multipotential nature of stem or progenitor cells apparently makes them the ideal choice for any cell therapy, but this as yet remains to be proven. This study (30 subjects) was designed to compare the potential to repair articular cartilage of chondrocytes taken from different regions in osteoarthritic cartilage with that of mesenchymal stem cells prepared from bone marrow of the same subject.
Design:
Cartilage biopsies, bone marrow, and blood samples were taken from each of 30 individuals with chronic osteoarthritis (aged 62-85 years) undergoing total knee replacement. The chondrogenic potential of chondrocytes isolated from cartilage biopsies taken from different regions of osteoarthritic cartilage was compared with that of mesenchymal cells by quantitative analysis of several chondrocyte specific markers and an ex vivo cartilage differentiation assay.
Results:
Cartilage-derived articular chondrocytes are superior to bone marrow–derived cells when compared for their ex vivo chondrogenic potential. Interestingly, there was marked and significant difference in the expression of chondrocytic markers between chondrocytes derived from adjacent, visually distinct regions of the diseased cartilage. When cultured in the presence of a fibroblast growth factor 2 variant, all cell samples from both tissues showed a high degree of chondrogenic potential.
Conclusions:
Although bone marrow–derived mesenchymal cells, when supplemented with the appropriate chondrogenic factors, are a suitable source for autologous cartilage implantation, adult chondroprogenitor cells, particularly those from moderately affected regions of the osteoarthritic joints, demonstrate superior chondrogenic potential.
Keywords: autologous chondrocyte implantation, MSCs, cartilage regeneration, osteoarthritis
Articular cartilage lesions, whether of traumatic or pathological origin, do not heal spontaneously and often undergo progressive degeneration toward osteoarthritis (OA).1 Early in the disease process, the articular chondrocytes become activated, lose their differentiated phenotype of maturational arrest, start to proliferate, and become hypertrophic.2 With disease progression, there is an upregulation of metalloproteinases (MMPs), aggrecanases, vascular endothelial growth factor, and collagen types I and X, leading to the calcification and vascularization typical of afflicted cartilage.3,4
Autologous chondrocyte implantation (ACI), first described by Brittberg et al. in 19945 using primary articular chondrocytes expanded in vitro from a healthy cartilage biopsy and reintroduced into the lesion under a periosteal flap, established the principle of using cultured cells for cartilage repair. Despite successes, overall, the regeneration of physiological hyaline cartilage is not always reproducibly attained.6 Moreover, there are conflictions in the reported data as to the chondrogenic potential of cells from osteoarthritic cartilage ranging from good7 or fair8,9 to a general impression that cartilage from older and/or osteoarthritic subjects is significantly inferior in its capacity to repair cartilage.10-13 Clinical trials to study the efficacy of ACI have, therefore, classically excluded subjects with osteoarthritis under the assumption that the primary cells for ACI should be exclusively from healthy cartilage.14
An additional problem with ACI is the source material due to the paucity of cartilage that can be removed, with the concurrent danger that the invasive intervention to isolate the cartilage biopsy from a non-weightbearing region of the joint may increase the long-term risk of osteoarthritis. Under these circumstances and particularly in the absence of a good source of healthy cartilage, stem and progenitor cells have emerged as a potential alternative. Tissues such as bone marrow, adipose tissue, muscle, bone, and synovium contain multipotent cells that can be harvested using minimally invasive techniques from noncritical locations and differentiate along a chondrogenic lineage.15-21 Although relatively large amounts of these cells are available, the appropriate progenitors are present only at low density in the native tissue, necessitating expansion in vitro while retaining their uncommitted phenotype, followed by induction of chondrogenesis by exposure to selected growth and differentiation factors.15
The multipotential nature of stem or progenitor cells is widely considered to make them the ideal choice for any cell therapy, but this remains to be conclusively proved. This study was designed to systematically compare the chondrogenic potential of cells from different sources and different degrees of OA derived from the same subject. This is particularly important in view of the inherent biological variability between individuals, which necessitates the collection of data from a comprehensive population to compare cell performance between tissues of the same subject. Chondrocytes from areas afflicted to a different degree with osteoarthritis were compared to bone marrow–derived mesenchymal stem/stroma cells (MSCs) from the same individual for a variety of chondrocyte-specific markers in an assessment of their overall chondrogenic potential under in vitro culture conditions.
Materials and Methods
Cartilage, Bone Marrow, and Blood Sample Collection
Cartilage biopsies, bone marrow (BM), and blood samples were taken from 30 patients with OA (aged 62-85 years) undergoing total knee replacement. The average age of the study participants was 74.5 ± 6.4 years, and there were 22 female and 8 male subjects included in the study (Table 1). All subjects assessed had osteoarthritis at grade 3 or 4 on the Kellgren-Lawrence scale. The samples of cartilage were transported in 5-mL human articular chondrocyte medium (hAC medium; Dulbecco’s modified Eagle’s medium [DMEM]:F12 [1:1]; Gibco, Invitrogen, Carlsbad, CA) containing penicillin and streptomycin (P/S; Biological Industries, Kibbutz Beit Haemek, Israel). The samples of BM were collected from the knee during the procedure and transferred to a tube containing 5 mL BM medium (DMEM–low glucose [DMEM-LG; Gibco, Invitrogen] containing P/S), including 5 units/mL low molecular weight heparin (Celsus Laboratories, Cincinnati, OH). Blood samples were collected in serum separation tubes (SST; BD, USA) for isolation of autologous human serum. Samples of cartilage were taken from as many places on the discarded joint as was convenient but not from predefined areas. All patients had signed an informed consent approved by the Ethical Committee of the Asaf Harofe Hospital, Beer Yaakov, Israel. Commercially available healthy human bone marrow (female 20 years old; Lonza, Allendale, NJ) was used for reference.
Table 1.
Comparison in Doubling Time of Moderately or Severely Affected Osteoarthritis Chondrocytes
| Moderate | Severe | ||||
|---|---|---|---|---|---|
| Subject | Sex/Age, y | No Ligand | FGF2v1 | No Ligand | FGF2v1 |
| 1 | F/62 | ND | ND | ND | 1.8 |
| 2 | F/74 | ND | ND | ND | 2.1 |
| 3 | F/72 | ND | 1.6 | ND | 1.8 |
| 4 | F/75 | ND | ND | ND | 3.4 |
| 5 | F/71 | ND | 1.9 | ND | 2.2 |
| 6 | M/77 | ND | 1.6 | ND | 2.5 |
| 7 | F/81 | 3.8 | 2.3 | 6.8 | 3.6 |
| 8 | M/72 | ND | ND | 5.4 | 3 |
| 9 | F/77 | ND | 1.8 | ND | 4.7 |
| 10 | F/74 | 7.2 | 2.5 | 2.5 | 1.7 |
| 11 | M/84 | ND | ND | 3.3 | 2.1 |
| 12 | F/81 | 18.8 | 5 | 7.1 | 3.1 |
| 13 | F/70 | 2.6 | 1.8 | 2.6 | 2 |
| 14 | F/74 | 3.2 | 1.8 | 2.8 | 1.9 |
| 15 | F/72 | 4.1 | 2.4 | 4.8 | 2.3 |
| 16 | F/74 | 6.9 | 4.2 | 9.6 | 3.4 |
| 17 | F/81 | 10.5 | 3.3 | 9.1 | 2.7 |
| 18 | F/69 | 4.4 | 2.5 | 5.7 | 2.5 |
| 19 | M/72 | 6.2 | 3.9 | 6.2 | 2.7 |
| 20 | M/83 | 2.9 | 1.9 | 2.8 | 1.8 |
| 21 | F/69 | 6.3 | 3 | ND | ND |
| 22 | F/63 | 2.2 | 1.8 | 3 | 2 |
| 23 | M/85 | 5.9 | 2.8 | 4.3 | 2.7 |
| 24 | M/77 | 4.2 | 2.6 | 4.5 | 2.4 |
| 25 | M/66 | 5.4 | 1.8 | 4 | 1.7 |
| 26 | F/79 | 6.1 | 2.1 | 9.3 | 2.8 |
| 27 | F/79 | 4 | 2.1 | 4.8 | 2.2 |
| 28 | F/62 | 3.5 | 1.7 | 5.6 | 2.2 |
| 29 | M/84 | 3.4 | 2.2 | 3.7 | 2.5 |
| 30 | F/77 | 9.9 | 3 | 9.9 | 3.6 |
| Average | 74.5 ± 6.4 | 5.8 ± 3.7 | 2.5 ± 0.9 | 5.4 ± 2.4 | 2.5 ± 0.7 |
| P value | 0.0001 | <0.0001 | |||
Mean doubling time (in days) at P0 (7-13 days in culture) was calculated for chondrocytes isolated from moderately or severely affected cartilage and expanded ±FGF2v1. Data were analyzed with a paired t test (21 and 22 patients), and P values are shown. ND = sample was not processed.
Serum Preparation
The blood samples collected in SST were allowed to coagulate for at least 1.5 hours at 4 or 25°C and then centrifuged at 1800 g for 10 minutes. The serum from the upper layer was transferred to a new tube and kept at 4°C for further use.
Cell Isolation and Culture
Isolation of chondrocytes
Pieces of cartilage biopsies were visually graded (general appearance, color, and texture) as more or less affected by osteoarthritis. The severe OA tissue was dark yellow, gelatinous, and opaque with fissures and lacked the pearl-like translucent appearance of healthy cartilage. Moderately affected cartilage was light yellow to opaque white with a firm consistency and no fissures. The cartilage biopsies were then digested with 1000 units/mL collagenase (from Clostridium histolyticum; Worthington Biochemical Corp., Lakewood, NJ) and 2 units/mL pronase (Serva, Heidelberg, Germany) (at 37°C for 16-19 hours) to provide a homogeneous population of isolated chondrocytes. The resulting cell suspension was centrifuged at 1400 g for 10 minutes, and the cell pellet was washed with hAC medium to remove the remaining enzymes. After centrifugation, the cell pellets were resuspended in the same medium and the number and viability of the cells measured by Trypan blue exclusion using a Vi-Cell XR (Beckman Coulter, Fullerton, CA). Harvested chondrocytes were then seeded in 25-cm2 flasks (Corning, New York, NY) coated with 200 µg/mL fibrinogen (Johnson & Johnson, New Brunswick, NJ) at 2000 to 4000 cells/cm2 in hAC medium supplemented with 10% autologous human serum ± recombinant fibroblast growth factor 2 variant (FGF2v1, 10 ng/mL; ProChon, Ness Ziona, Israel). The FGF2v1 used here is a genetically engineered ligand with improved characteristics with respect to potency and stability, which has been shown to successfully preserve the chondrogenic potential of chondrocytes cultured for ACI on a fibrin/hyaluronic acid construct, BioCart II.16 All cultures were maintained in a 5% CO2 incubator at 37°C, with medium additions every 3 to 4 days. After 7 to 14 days, the primary cultured cells (P0) were trypsinized using 0.25% Trypsin-EDTA (Bio-Lab, Jerusalem, Israel). The harvested cells were either subjected to 3D micro mass culture assay or transferred to a new flask for further culture. For the proliferation assays, cells were subsequently cultured for 2 to 3 more passages in growth medium at a density of 25,000 cells per well in 24-well plates (Corning). At each passage, cells were trypsinized, counted, and then reseeded. Doubling time at P0 was calculated by the ratio of T to log2 (N/N0), where N0 and N are the numbers of cells at the beginning and the end of the expansion phase, respectively, and T is the time.17
Isolation of BM-MSC
BM aspirates 5 to 45 mL were diluted 1:2 in phosphate-buffered saline (PBS) centrifuged at 340 g for 10 minutes, and then the lipid upper layer was removed. The cell suspension was diluted to 50 mL with PBS and centrifuged at 1400 g for 10 minutes. The cell pellet was resuspended in BM medium supplemented with 10% autologous human serum and FGF2v1 (10 ng/mL) and seeded in 200-µg/mL fibrinogen-coated 25-cm2 flasks. After 1 to 2 days in culture, the nonadherent cells were washed away to leave the adherent mesenchymal stem cells. Proliferation assays were performed as described above for chondrocytes.
Chondrocyte Differentiation Assay
The chondrogenic capacity of expanded chondrocytes and MSCs (most of the samples at P0 and at the first passage [P1] with some at the second passage [P2]) was examined in an optimized 3D micro mass culture system modified from Johnstone et al.18 using a defined serum-free medium: DMEM supplemented with ITS + 1 (Sigma Aldrich, St. Louis, MO; i.e., 10 µg/mL insulin, 5.5 mg/mL transferrin, 5 ng/mL selenium, 0.5 mg/mL bovine serum albumin [BSA], 4.7 mg/mL linoleic acid [ITS]), with 0.28 mM ascorbic acid, 10−7 M dexamethasone (Sigma Aldrich), 1 mM sodium pyruvate, 40 µg/mL proline (Biological Industries), and 10 ng/mL TGF-β3 (Prospec-Tany TechnoGene, Rehovot, Israel). Aliquots of 5 × 105 cells/0.5 mL were centrifuged at 150 g for 10 minutes to form a dense cell pellet. Pellets were cultured for 2 to 3 weeks with medium changes twice per week and subsequently processed for histology or RNA analysis as described below.
Histological Analysis
Three-week-old pellets were fixed in 4% formaldehyde, embedded in paraffin, cross sectioned (4 µm thick), and stained with hematoxylin and eosin, Alcian blue (pH 1), or 0.1% (w/v) safranin O (all histological reagents from Sigma Aldrich) for the assessment of proteoglycans and sulfated glycosaminoglycan content, respectively. The diameter of the pellets (in millimeters) was measured in histology sections.
Quantitative PCR Analysis
Total RNA was extracted from 2-week-old pellets using the EZ-RNA kit (Biological Industries) and frozen at −70°C. The RNA was isolated using a homogenizer and then according to the EZ-RNA kit protocol. Then, 1 µg of RNA was used to generate cDNA with the Verso cDNA kit (Thermo Fisher Scientific, Waltham, MA). The expression pattern of 3 related cartilage genes was analyzed by real-time PCR with an ABI PRISM 5700 sequence detector and software system (Applied Biosystems, Foster City, CA). TaqMan MGB probes (FAM dye-labeled) and primers for type I collagen (Col I) (Hs00164004_m1), type IIA1 collagen (Col II) (Hs01064869_m1), type X collagen (Col X) (Hs00166657_m1), and RPLPO (Hs99999902_m1, endogenous control) were ordered from Applied Biosystems assays-on-demand. All reactions were designed to amplify fragments of 65 to 105 base pairs with 2 µL cDNA, 10 µL of Absolute Blue QPCR ROX Mix (Thermo Fisher Scientific), and 1 µL assay-on-demand. After an initial denaturation step at 95°C for 15 minutes, the cDNA products were amplified with 40 PCR cycles consisting of a denaturation step at 95°C for 15 seconds and an extension step at 60°C for 1 minute. All samples were analyzed in duplicate, and PCR was performed in optical strips (Applied Biosystems). To analyze the real-time PCR data for each cDNA sample, the threshold cycle (Ct) value of each target sequence was subtracted from the Ct value of RPLPO, to derive ΔCt. The level of expression of Col I, Col II, or Col X was calculated as 2ΔCt. The differentiation index was determined by the ratio of Col II to Col I expression.19,20 To compensate for any variation between assays, 2 samples from each run were reanalyzed together with the same 3 reference samples to normalize the Ct values.
Statistical Analysis
Differences in viability, yield of cells, pellet culture size, and relative expression of Col I, Col II, and Col X between MSCs and chondrocytes and between moderate or severe OA-derived chondrocytes cultured ± FGF2v1 were analyzed with the 1- or 2-tailed paired t test. The differentiation indices and other parameters were compared by the 2-tailed Mann Whitney test if the pairing was not significantly effective. P < 0.05 was considered significant. Statistical analyses were done with GraphPad Prism 5.
Results
Support for the visual method of assessment of OA severity was given by a number of more objective measurements. Following enzymatic digestion, the total density of viable cells was found to be very similar between the moderate or severely affected cartilage (3.5 and 3.14 × 106 cells/g tissue, respectively). In contrast, cell viability was significantly higher for cells isolated from the more moderate OA regions (63%) compared to that of cells retrieved from the more severely affected regions (55%; P = 0.0004; Fig. 1a). Moreover, the calculated differentiation index was significantly higher: 7.8-fold for the moderate compared to the severely OA affected cells in the primary culture (Fig. 1b).
Figure 1.
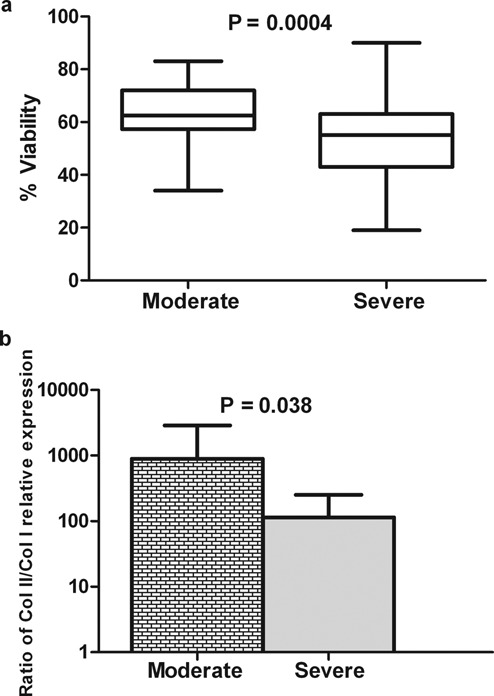
Comparable analysis of viability and the collagen II/collagen I ratio of freshly isolated chondrocytes from moderate or severely affected osteoarthritis (OA) cartilage. Chondrocytes isolated by enzymatic digestion from moderate or severely affected OA cartilage from the same patient were counted using a Vi-Cell XR, and a box-and-whiskers plot of percentage viability is shown in panel a. Panel b shows the ratio of Col II/Col I relative expression (mean and standard deviation) of the fresh cells immediately after isolation analyzed by qPCR. The viability data were analyzed with a 2-tailed paired t test (19 patients). In panel b, the changes in the ratio of Col II/Col I were analyzed with a 1-tailed paired t test (19 patients). Significant P values are shown.
The rates of proliferation of chondrocytes isolated from moderately or severely affected cartilage and of MSCs from 3 representative patients are shown in Figure 2. Although the absolute rates of proliferation were individual to the different patients, the trend for relative rates was maintained between subjects. Chondrocytes cultured with FGF2v1 proliferated faster than cells cultured without FGF2v1, whether derived from moderate or severely affected cartilage. Analysis of the doubling time for chondrocytes isolated from either moderate or severely affected OA cartilage showed no significant difference between them, although both tissue types demonstrated a highly significant decrease in doubling time when the cells were cultured with FGF2v1 (2.5 days with ligand vs 5.8 days without ligand for the moderate and 2.5 vs 5.4 days for the severely affected OA chondrocytes; Table 1).
Figure 2.
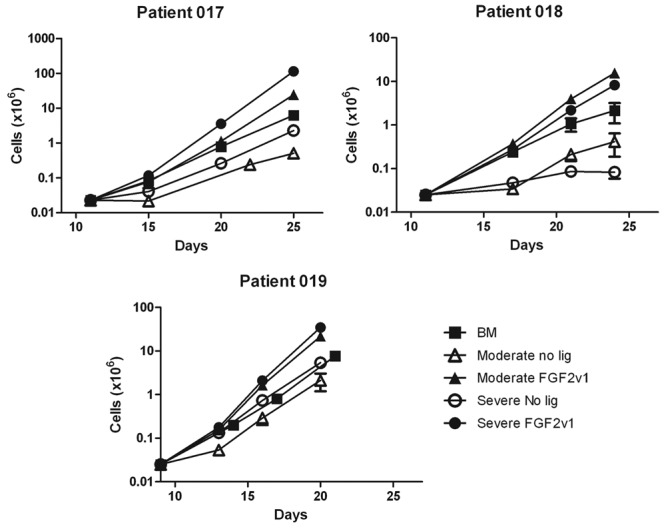
Proliferation curves of bone marrow (BM)–mesenchymal stem cell (MSC) and moderate or severely affected chondrocytes. BM-derived MSCs or chondrocytes derived from cartilage moderately or severely affected with osteoarthritis were cultured as described in the Materials and Methods section. The cell concentration and viability were determined at various time points during the culture. The results from 3 individual subjects (mean and standard deviation) are shown to illustrate the individual variability in the rate of cell proliferation.
Analysis of the chondrogenic potential of control MSCs (isolated from a healthy donor) in the optimized micro mass culture, a well-accepted method to assess the chondrogenic potential of cells,22,23 revealed that, in the absence of FGF2v1, there was little or no formation of cartilage-like matrix (Fig. 3). In contrast, when the cells were grown in the presence of the ligand, there was massive production of extracellular matrix, which stained for total and sulfated proteoglycans (Fig. 3). This difference was also confirmed by qPCR analysis where the expression of Col II in the pellets of MSCs grown without FGF2v1 was undetectable (data not shown), but there were high levels of expression of Col II in the presence of ligand. Because of this, all results with MSCs isolated from the osteoarthritic patients relate only to cells grown with FGF2v1.
Figure 3.
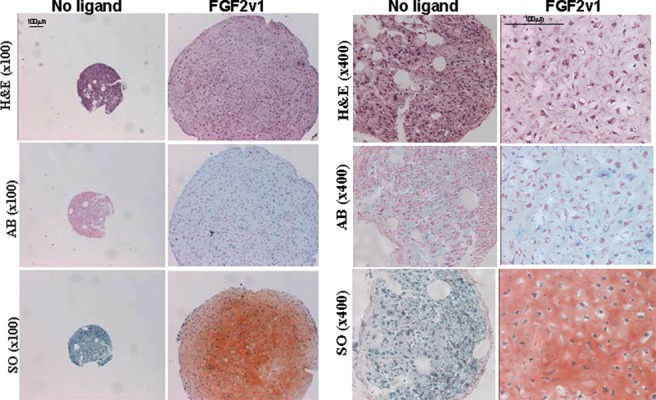
Histology sections of micro mass cultures of healthy mesenchymal stem cells (MSCs). Micro mass cultures of MSCs from healthy human bone marrow (purchased from Lonza, Allendale, NJ) expanded without or with FGF2v1 and analyzed by histology (hematoxylin and eosin [H&E] stain, Alcian blue [AB], and safranin O [SO] stains). Bars = 100 µm. In the presence of ligand, the pellets are much bigger than without ligand, and there is positive staining for both proteoglycans (Alcian blue) and sulfated glycosaminoglycans (safranin O).
A representative histological analysis of the micro mass cultures is shown in Figure 4. Larger pellets were obtained for chondrocytes cultured with FGF2v1 than for chondrocytes cultured without FGF2v1 or for MSCs, but there was no difference between the chondrogenic potential for cultures derived from more or less OA affected areas. All cultures, whether of MSCs or chondrocytes, were positively stained with Alcian blue and safranin O, indicative of the presence of sulfated proteoglycans (Fig. 4). Measurement of the diameter of the pellets from moderate or severely affected OA-derived chondrocytes demonstrated no significant difference between the pellet size (2 vs 2.1 mm, respectively; Table 2). However, smaller pellets were obtained for the BM-MSC (cultured with FGF2v1) and for the chondrocytes cultured without FGF2v1 (1.4, 1.6, or 1.6 mm for BM-MSC; moderate, no ligand; or severe, no ligand, respectively; Table 2).
Figure 4.
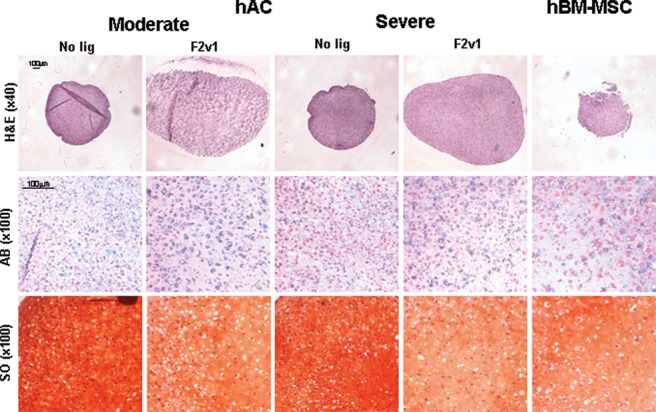
Histology sections of micro mass cultures of mesenchymal stem cells (MSCs) and moderate or severely affected chondrocytes. Micro mass cultures of MSCs (hBM-MSC) or chondrocytes (hAC) derived from moderate or severely affected osteoarthritis tissue from a representative subject were analyzed by histology (hematoxylin and eosin [H&E] stain; bars = 100 µm) with (F2v1) or without (no ligand) the presence of FGF2v1 in the culture medium used to expand the cells. The presence of proteoglycans and sulfated glycosaminoglycans was examined by Alcian blue (AB) and safranin O (SO) stains (bars = 100 µm).
Table 2.
Comparison in Pellet Size of BM-MSC and Moderately or Severely Affected Osteoarthritis Chondrocytes
| BM-MSC | Moderate | Severe | |||
|---|---|---|---|---|---|
| Subject | FGF2v1 | No Ligand | FGF2v1 | No Ligand | FGF2v1 |
| 2 | ND | ND | ND | ND | 2.28 |
| 3 | 1.04 | ND | ND | ND | 2.76 |
| 4 | 2.08 | ND | ND | ND | 1.68 |
| 5 | 1.34 | ND | 3.2 | ND | 3.36 |
| 6 | 1.7 | ND | 2.46 | ND | 1.84 |
| 7 | 1.6 | 1.92 | 2.12 | 2.88 | 2.94 |
| 8 | 0.8 | ND | ND | 1.32 | 2.46 |
| 9 | 0.94 | ND | 2.06 | ND | 1.74 |
| 10 | 1.14 | 1.06 | ND | 1.5 | 2.3 |
| 11 | 1.84 | ND | ND | 2.16 | 2.34 |
| 12 | 0.98 | 2.34 | 2.36 | 1.06 | 2.18 |
| 13 | 1.84 | 1.28 | 1.82 | 1.22 | 1.84 |
| 14 | 1.16 | 1.76 | 3.06 | 1.52 | 2.52 |
| 15 | 1.58 | 1.96 | 2.96 | 2.16 | 2.54 |
| 16 | 1.84 | 1.94 | 1.46 | 2.52 | 1.68 |
| 17 | 1.12 | 1.88 | 1.94 | 2.1 | 1.58 |
| 18 | 0.94 | 2.8 | 3.08 | 2.46 | 3.2 |
| 19 | 1.64 | 2.28 | 1.52 | 1.94 | 1.96 |
| 20 | ND | 2.64 | 3.74 | 1.98 | 3.24 |
| 21 | 1.2 | 0.98 | 1.4 | ND | ND |
| 22 | 1.2 | 1.08 | 1.94 | 1.52 | 1.26 |
| 23 | ND | 1 | 1.52 | 0.98 | 1.82 |
| 24 | 1.16 | 1 | 1.12 | ND | 1.3 |
| 25 | 1.08 | 1.2 | 2.12 | 1.44 | 2.36 |
| 26 | ND | 1.18 | 1.66 | 1.46 | 2.28 |
| 27 | 1.62 | 1.84 | 1.12 | ND | 1.28 |
| 28 | 1.48 | 1.16 | 1.2 | 0.84 | 1.36 |
| 29 | 1.54 | 1.06 | 1.52 | 0.82 | 1.56 |
| 30 | 1.44 | 1.08 | 1.52 | 0.8 | 1.34 |
| Average | 1.4 ± 0.3 | 1.6 ± 0.6 | 2.0 ± 0.7 | 1.6 ± 0.6 | 2.1 ± 0.6 |
| P value | 0.014 | 0.0009 | |||
| P value compared to MSCs | 0.0009* | <0.0001* | |||
Pellet diameter (in millimeters) was measured in histology sections. Mean values are shown for each type of pellet produced from BM-MSC and moderately or severely affected osteoarthritis cartilage cultured ±FGF2v1 from the same patient. Changes in pellet size were analyzed with a 2-tailed paired t test (20 patients) or with the Mann Whitney test (marked by *), as described in Materials and Methods, and P values are shown. BM = bone marrow; MSC = mesenchymal stem cell; ND = sample was not processed.
The expression of Col I and Col II was systematically analyzed by qPCR on RNA purified from micro mass cultures of both chondrocytes and MSCs derived from each subject (Figs. 5, 6). Addition of FGF2v1 to the chondrocyte cultures dramatically lowered the expression levels of Col I (Fig. 5a): 3.6-fold for the moderate OA-derived cells (P = 0.033) and 3-fold for the severe OA-derived cells (P = 0.036). Col II, the hallmark of hyaline cartilage (Fig. 5b), was also significantly increased in micro mass cultured chondrocytes expanded in the presence of FGF2v1 compared with those expanded without the growth factor (P = 0.0021 and P = 0.013 for moderate and severely OA affected tissue, respectively). Interestingly, there was no significant difference in Col II expression in FGF2v1-treated chondrocytes between those derived from moderately or severely affected OA tissue (Fig. 5b).
Figure 5.
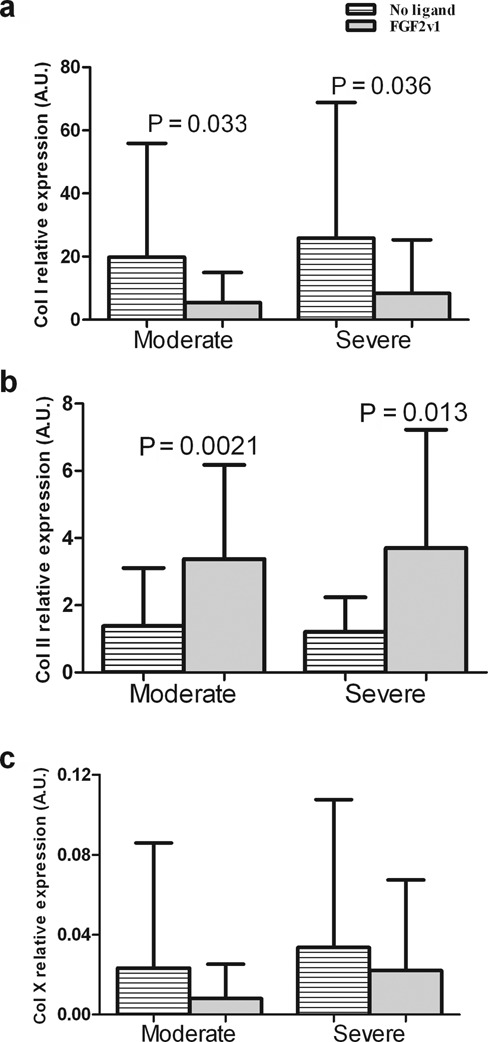
Collagen I, II, and X expression in micro mass cultures of moderate or severely affected chondrocytes. Micro mass cultures of chondrocytes derived from moderate or severely affected osteoarthritis tissue were analyzed by qPCR for the expression of (a) Col I, (b) Col II, and (c) Col X. Col I is decreased in differentiated cartilage; Col II is characteristic of articular cartilage. The presence of Col X is indicative of hypertrophic cartilage. Mean and standard deviation values are presented in each graph. Changes in Col I, Col II, and Col X relative expression between the different groups were analyzed with a 2-tailed paired t test (21-22 patients) as described in Materials and Methods, and significant P values are shown.
Figure 6.
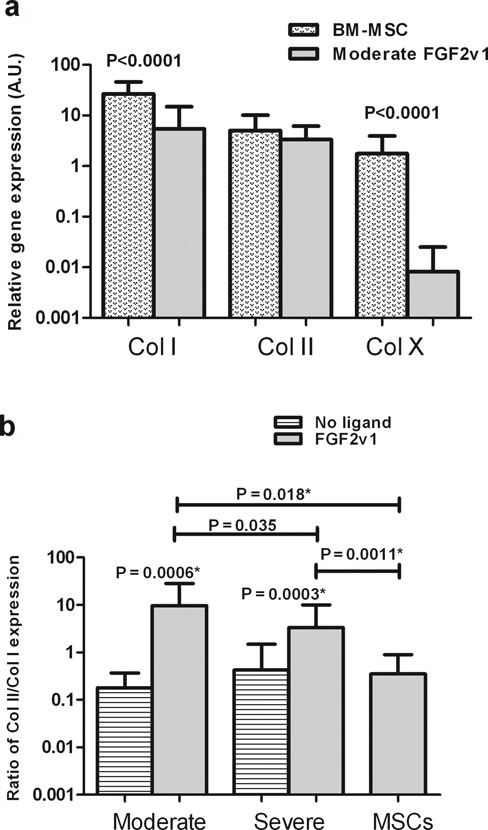
Collagen I, II, and X expression of micro mass cultures of mesenchymal stem cells (MSCs) and moderate and severely affected osteoarthritis (OA) chondrocytes. (a) Expression of Col I, Col II, and Col X of micro mass cultures of MSCs and chondrocytes from moderately affected OA tissue, grown with FGF2v1. (b) Ratio of Col II/Col I in micro mass cultures of MSCs or chondrocytes derived from moderate or severely OA tissue. Mean and standard deviation values are presented in each graph. Changes in relative expression were analyzed with a 2-tailed paired t test (21-24 patients) except when the pairing was not significantly effective, and then the 2-tailed Mann Whitney test was performed (marked with *). The statistical tests were performed as described in Materials and Methods, and significant P values are shown.
Expression of Col X, which is indicative of chondrocyte hypertrophy and is an early marker in OA, was also examined and found to be 2.75-fold higher in micro mass cultures derived from severe compared to moderately affected OA chondrocytes cultured with FGF2v1, although this difference was not significant (Fig. 5c). This is in accordance with the findings that osteoarthritic cartilage is hypertrophic and has elements in common with growth plate chondrocytes.14-16
The expression of Col I, Col II, and Col X in micro mass cultures of MSCs was compared to the values of moderately affected OA chondrocytes cultured with FGF2v1 (Fig. 6a). Overall, the Col I and Col X expression in articular chondrocytes was significantly lower than in MSCs (P < 0.0001 for both genes; Fig. 6a). However, the level of Col II expression was very similar for both cell types (Fig. 6a). Calculation of the differentiation index (Col II/Col I) demonstrated that chondrocytes cultured with FGF2v1 showed a significantly higher chondrogenic potential than cells cultured without FGF2v1 under otherwise identical culture conditions (Fig. 6b), whether the cells were derived from moderate or severely affected tissue (P = 0.0006 or P = 0.0003, respectively). It is important to note that the cultures with and without ligand were from the same individual and used the same serum, which is otherwise a source of high intersubject variation. Furthermore, the chondrogenic potential of these cells was significantly higher than that of MSCs (P = 0.018 and P = 0.0011 for moderate or severe OA; Fig. 6b). In addition, a significantly higher (2.9-fold) chondrogenic potential was observed for moderate compared to severely affected OA chondrocytes (Fig. 6b). It is apparent from Figure 6 that the major difference between the cell types was in the increased Col X expression in the MSCs.
Discussion
The great variability in chondrogenic potential of cells of different individuals presented in the study demonstrates the importance of assaying tissues within the same subject, from a large enough population, to make valid comparisons. Our study showed that chondrocytes, even from cartilage affected with OA, as well as MSCs, can, under appropriate culture conditions, express good chondrogenic potential and serve as a valid source of cells for regeneration of cartilage.
Interesting differences were found between cartilage tissue more or less affected with OA. Although the categorization of the cartilage was by gross morphological characteristics, the biological findings and markers confirmed the difference in the tissue phenotype. While the absolute yield of viable cells from moderate or severe OA-affected cartilage was very similar, the percentage of viable cells was significantly higher in samples from the more moderate-looking OA regions. This may represent either a pathologically accelerated rate of cell death in the more severely affected OA area or an intrinsically enhanced sensitivity of these cells to the conditions used during enzymatic digestion of the tissue. The calculated total density of cells in severely affected OA cartilage is therefore higher than in normal cartilage, most likely due to the cytokine-rich, inflammatory environment with an increase in proliferation as well as in apoptosis associated with osteoarthritic cartilage.7,24,25
Osteoarthritis is often a consequence of cartilage trauma and is not considered a systemic disease, although it does seem to spread within a joint or joints. This may well be because of abnormal loading on adjacent areas resulting from the original focal trauma. The progression of the disease has been described as being related to the adverse effects of “abnormal” loading on normal cartilage or “normal” loading on abnormal cartilage.21 Interestingly, under tissue culture conditions, the doubling time of the cells from the more or less severely OA-affected tissue was essentially the same for both cell types.
The limited proliferative capacity of MSCs in the absence of FGF is in agreement with previous studies suggesting that activating FGF signaling pathways may be critical for directing noncommitted stem/progenitor cells toward the chondrogenic lineage.22,23,26 The inclusion of FGF2v1 in this study was beneficial for all the chondrogenic parameters measured, the differentiation index, and the size of the micro mass cultures as previously reported,8,27-31 and this was true for all categories of OA-affected tissue. Systematic analysis of the expression of Col I and Col II by PCR showed that the increase in the differentiation index due to FGF2 was accomplished both by decreasing the expression of Col I and by increasing the expression of Col II. Interestingly, the higher differentiation index in the moderate compared to the more seriously affected OA tissue was due mainly to the lower level of Col I expression in the former, whereas both types had similar levels of Col II.
All chondrocytes showed a higher chondrogenic potential than the MSCs. This was due to significantly higher expression of Col I and Col X in the MSCs that reflects a more terminally differentiated, hypertrophic phenotype, which has been noted previously in other studies for MSCs.32 In some cases, late passage human MSCs spontaneously differentiated into osteoblasts.24 These observations most likely reflect a tendency of stem cells to progress past the mature articular cartilage stage onto hypertrophy and bone formation,19,32 which is a serious concern for MSC applications in articular cartilage tissue engineering.
It should be noted that this study was purely ex vivo and that a multitude of other factors may play a role in directing cell differentiation and fate in the intact tissue or organism, restricting the differentiation potential for the MSCs. However, until an efficient strategy for preventing terminal differentiation and hypertrophy can be achieved, chondrocyte progenitors obtained from cartilage biopsies would appear to be a more suitable cell source for cartilage repair.15,25
Footnotes
Acknowledgments and Funding: ProChon Biotech LTD would like to acknowledge the support of Bereshit, the Israeli Consortium for Cell Therapy.
Declaration of Conflicting Interests: R. Goldschmid, M. Schrift-Tzadok, R. Gal-Levy, T. Fischler, and S. Blumenstein are employed by ProChon Biotech Ltd., Ness Ziona, Israel. A. Yayon is part owner and CSO of ProChon Biotech Ltd., Ness Ziona, Israel. ProChon Biotech Ltd. owns the proprietary rights to the FGF2v1 growth factor variant used for the culture of cells in this study.
References
- 1. Moskowitz RW. The burden of osteoarthritis: clinical and quality-of-life issues. Am J Manag Care. 2009;15:S223-9. [PubMed] [Google Scholar]
- 2. Bonnet CS, Walsh DA. Osteoarthritis, angiogenesis and inflammation. Rheumatology (Oxford). 2005;44:7-16. [DOI] [PubMed] [Google Scholar]
- 3. Chun JS, Oh H, Yang S, Park M. Wnt signaling in cartilage development and degeneration. BMB Rep. 2008;41:485-94. [DOI] [PubMed] [Google Scholar]
- 4. Nagase H, Kashiwagi M. Aggrecanases and cartilage matrix degradation. Arthritis Res Ther. 2003;5:94-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331:889-95. [DOI] [PubMed] [Google Scholar]
- 6. Magnussen RA, Dunn WR, Carey JL, Spindler KP. Treatment of focal articular cartilage defects in the knee: a systematic review. Clin Orthop Relat Res. 2008;466:952-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hollander AP, Dickinson SC, Sims TJ, Brun P, Cortivo R, Kon E, et al. Maturation of tissue engineered cartilage implanted in injured and osteoarthritic human knees. Tissue Eng. 2006;12:1787-98. [DOI] [PubMed] [Google Scholar]
- 8. Dehne T, Karlsson C, Ringe J, Sittinger M, Lindahl A, Karlsson C, et al. Chondrogenic differentiation potential of osteoarthritic chondrocytes and their possible use in matrix-associated autologous chondrocyte transplantation: genome-wide expression profiling reveals new candidate genes associated with osteoarthritis. Arthritis Res Ther. 2009;11:R133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tallheden T, Bengtsson C, Brantsing C, Sjogren-Jansson E, Carlsson L, Peterson L, et al. Proliferation and differentiation potential of chondrocytes from osteoarthritic patients. Arthritis Res Ther. 2005;7:R560-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Acosta CA, Izal I, Ripalda P, Douglas-Price AL, Forriol F. Gene expression and proliferation analysis in young, aged, and osteoarthritic sheep chondrocytes effect of growth factor treatment. J Orthop Res. 2006;24:2087-94. [DOI] [PubMed] [Google Scholar]
- 11. Barbero A, Grogan S, Schafer D, Heberer M, Mainil-Varlet P, Martin I. Age related changes in human articular chondrocyte yield, proliferation and post-expansion chondrogenic capacity. Osteoarthritis Cartilage. 2004;12:476-84. [DOI] [PubMed] [Google Scholar]
- 12. Dorotka R, Bindreiter U, Vavken P, Nehrer S. Behavior of human articular chondrocytes derived from nonarthritic and osteoarthritic cartilage in a collagen matrix. Tissue Eng. 2005;11:877-86. [DOI] [PubMed] [Google Scholar]
- 13. Murphy JM, Dixon K, Beck S, Fabian D, Feldman A, Barry F. Reduced chondrogenic and adipogenic activity of mesenchymal stem cells from patients with advanced osteoarthritis. Arthritis Rheum. 2002;46:704-13. [DOI] [PubMed] [Google Scholar]
- 14. Brittberg M. Autologous chondrocyte implantation: technique and long-term follow-up. Injury. 2008;39(suppl 1):S40-9. [DOI] [PubMed] [Google Scholar]
- 15. Hwang NS, Elisseeff J. Application of stem cells for articular cartilage regeneration. J Knee Surg. 2009;22:60-71. [DOI] [PubMed] [Google Scholar]
- 16. Nehrer S, Chiari C, Domayer S, Barkay H, Yayon A. Results of chondrocyte implantation with a fibrin-hyaluronan matrix: a preliminary study. Clin Orthop Relat Res. 2008;466:1849-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jakob M, Demarteau O, Schafer D, Hintermann B, Dick W, Heberer M, et al. Specific growth factors during the expansion and redifferentiation of adult human articular chondrocytes enhance chondrogenesis and cartilaginous tissue formation in vitro. J Cell Biochem. 2001;81:368-77. [DOI] [PubMed] [Google Scholar]
- 18. Johnstone B, Hering TM, Caplan AI, Goldberg VM, Yoo JU. In vitro chondrogenesis of bone marrow–derived mesenchymal progenitor cells. Exp Cell Res. 1998;238:265-72. [DOI] [PubMed] [Google Scholar]
- 19. Martin I, Jakob M, Schafer D, Dick W, Spagnoli G, Heberer M. Quantitative analysis of gene expression in human articular cartilage from normal and osteoarthritic joints. Osteoarthritis Cartilage. 2001;9:112-8. [DOI] [PubMed] [Google Scholar]
- 20. Goldring SR, Goldring MB. The role of cytokines in cartilage matrix degeneration in osteoarthritis. Clin Orthop Relat Res. 2004;(427, suppl):S27-36. [DOI] [PubMed] [Google Scholar]
- 21. Goldring MB, Goldring SR. Articular cartilage and subchondral bone in the pathogenesis of osteoarthritis. Ann N Y Acad Sci. 2010;1192:230-7. [DOI] [PubMed] [Google Scholar]
- 22. Solchaga LA, Penick K, Porter JD, Goldberg VM, Caplan AI, Welter JF. FGF-2 enhances the mitotic and chondrogenic potentials of human adult bone marrow–derived mesenchymal stem cells. J Cell Physiol. 2005;203:398-409. [DOI] [PubMed] [Google Scholar]
- 23. Solchaga LA, Penick K, Goldberg VM, Caplan AI, Welter JF. Fibroblast growth factor-2 enhances proliferation and delays loss of chondrogenic potential in human adult bone-marrow-derived mesenchymal stem cells. Tissue Eng Part A. 2010;16:1009-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Banfi A, Bianchi G, Notaro R, Luzzatto L, Cancedda R, Quarto R. Replicative aging and gene expression in long-term cultures of human bone marrow stromal cells. Tissue Eng. 2002;8:901-10. [DOI] [PubMed] [Google Scholar]
- 25. Hwang NS, Varghese S, Lee HJ, Zhang Z, Ye Z, Bae J, et al. In vivo commitment and functional tissue regeneration using human embryonic stem cell–derived mesenchymal cells. Proc Natl Acad Sci U S A. 2008;105:20641-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Stewart AA, Byron CR, Pondenis H, Stewart MC. Effect of fibroblast growth factor-2 on equine mesenchymal stem cell monolayer expansion and chondrogenesis. Am J Vet Res. 2007;68:941-5. [DOI] [PubMed] [Google Scholar]
- 27. Aigner T, Fundel K, Saas J, Gebhard PM, Haag J, Weiss T, et al. Large-scale gene expression profiling reveals major pathogenetic pathways of cartilage degeneration in osteoarthritis. Arthritis Rheum. 2006;54:3533-44. [DOI] [PubMed] [Google Scholar]
- 28. Horton WE, Jr, Yagi R, Laverty D, Weiner S. Overview of studies comparing human normal cartilage with minimal and advanced osteoarthritic cartilage. Clin Exp Rheumatol. 2005;23:103-12. [PubMed] [Google Scholar]
- 29. Tchetina EV, Kobayashi M, Yasuda T, Meijers T, Pidoux I, Poole AR. Chondrocyte hypertrophy can be induced by a cryptic sequence of type II collagen and is accompanied by the induction of MMP-13 and collagenase activity: implications for development and arthritis. Matrix Biol. 2007;26:247-58. [DOI] [PubMed] [Google Scholar]
- 30. Wu J, Liu W, Bemis A, Wang E, Qiu Y, Morris EA, et al. Comparative proteomic characterization of articular cartilage tissue from normal donors and patients with osteoarthritis. Arthritis Rheum. 2007;56:3675-84. [DOI] [PubMed] [Google Scholar]
- 31. Yang KG, Saris DB, Geuze RE, van Rijen MH, van der Helm YJ, Verbout AJ, et al. Altered in vitro chondrogenic poperties of chondrocytes harvested from unaffected cartilage in osteoarthritic joints. Osteoarthritis Cartilage. 2006;14:561-70. [DOI] [PubMed] [Google Scholar]
- 32. Loeser RF. Molecular mechanisms of cartilage destruction: mechanics, inflammatory mediators, and aging collide. Arthritis Rheum. 2006;54:1357-60. [DOI] [PMC free article] [PubMed] [Google Scholar]


