Abstract
The concept of using gene transfer strategies for cartilage repair originates from the idea of transferring genes encoding therapeutic factors into the repair tissue, resulting in a temporarily and spatially defined delivery of therapeutic molecules to sites of cartilage damage. This review focuses on the potential benefits of using gene therapy approaches for the repair of articular cartilage and meniscal fibrocartilage, including articular cartilage defects resulting from acute trauma, osteochondritis dissecans, osteonecrosis, and osteoarthritis. Possible applications for meniscal repair comprise meniscal lesions, meniscal sutures, and meniscal transplantation. Recent studies in both small and large animal models have demonstrated the applicability of gene-based approaches for cartilage repair. Chondrogenic pathways were stimulated in the repair tissue and in osteoarthritic cartilage using genes for polypeptide growth factors and transcription factors. Although encouraging data have been generated, a successful translation of gene therapy for cartilage repair will require an ongoing combined effort of orthopedic surgeons and of basic scientists.
Keywords: gene therapy, cartilage repair, osteoarthritis, meniscal lesions, clinical trials
Introduction
Articular cartilage defects and meniscal lesions have a reduced capacity for regeneration. The concept of using gene transfer strategies for cartilage repair originates from the idea of transferring genes encoding therapeutic factors into the repair tissue, resulting in a temporarily and spatially defined delivery of the therapeutic molecule. In this review, we will focus on gene therapy approaches for the repair of articular cartilage and meniscal fibrocartilage, including articular cartilage defects resulting from acute trauma, osteochondritis dissecans, osteonecrosis, and osteoarthritis. Possible applications for meniscal repair will be described for meniscal lesions, meniscal sutures, and meniscal transplantation. As a discussion of cartilage damage resulting from rheumatoid arthritis is beyond the scope of this review, we refer to the many reviews already published on this subject.1-9
Principles of Gene Therapy
Gene transfer is the introduction of foreign genes or gene sequences into different types of cells. Gene therapy is the treatment of diseases using gene transfer techniques. Gene transfer via nonviral vectors is named transfection; gene transfer using viral vectors is termed transduction. The foreign genetic material enters the cell and is next transferred towards the nucleus, where it either integrates into the host genome or remains extrachromosomal as an episome that generally allows only for transient transgene expression. For therapeutic applications, gene transfer into a sufficiently high number of target cells is essential for the secretion of relevant concentrations of the transgene product. Current vectors available for use in gene therapy include nonviral approaches (naked DNA, physical and chemical methods) and various viral (adenoviral, HSV, retroviral, lentiviral, rAAV) vehicles (Table 1).
Table 1.
Nonviral and Viral Gene Vectors Suitable for Gene Transfer to Cartilage Defects
| Nonviral Systems | Viral Systems | |||||
|---|---|---|---|---|---|---|
| Liposomes | Others (Chemical, Electrical, and Mechanical Methods) | Adenovirus | Retrovirus | Herpes Simplex Virus (HSV) | Adeno-Associated Virus (AAV) | |
| Advantages | Independent from cell cycle Noninfectious; repeatedly applicable Low toxicity Large capacity Easy to manufacture |
Very high efficiency Independent from cell cycle Approved for clinical trials |
High efficiency Prolonged transgene expression |
High efficiency Independent from cell cycle Large capacity |
Very high efficiency Prolonged transgene expression Independent from cell cycle Noninfectious; repeatedly applicable |
|
| Shortcomings | Cell-specific efficiency Short-term transgene expression |
Infectious with induction of immune response; single application only Cytotoxicity Risk of replication competence Short-term transgene expression |
Insertional mutagenesis Dependent from cell cycle Risk of replication competence Restricted host range |
Short-term transgene expression Cytotoxicity (first-generation HSV) |
Difficult to manufacture | |
| Integration in host genome | No | No | Yes | No | No | |
Note: Properties of nonviral and viral gene vectors currently in clinical and experimental use for gene therapy approaches to cartilage defects.
Among the nonviral systems, chemical methods of complexing DNA to various macromolecules include cationic lipids and liposomes,10-12 polymers,13 polyamines and polyethylenimines,14,15 and nanoparticles,16 but also calcium phosphate coprecipitates17 are mainly used. Nonviral systems avoid the risk of acquiring replication competence inherent to viral vectors, can be repeatedly administered, have the capacity to carry large therapeutic genes, are relatively easy to produce on a large scale, and do not elicit a detectable immune response. Nevertheless, their efficacy is often inferior to those of viral vectors. Moreover, the fact that they stay as episomal forms in the target cells often results in short-term transgene expression. To avoid low gene transfer efficacy in vivo, nonviral gene transfer strategies are often based on the transplantation of ex vivo–modified cells to cartilage defects.
Viral vectors utilize natural entry pathways in human cells. Adenoviral vectors have been among the most employed gene vehicles for cartilage repair in the past.18-22 They allow for high transduction efficiencies and transgene expression in a variety of cells, enabling direct approaches in vivo. However, serious concerns about their clinical safety were raised after the death of Jesse Gelsinger, a patient included in a gene therapy trial employing adenoviral vectors. Moreover, transgene expression via adenoviral delivery is limited for about 1 to 2 weeks as the transgenes remain episomal and due to the development of host immune responses against transduction with most of the constructs derived from these viruses.
An advantage of retroviruses is their ability to integrate in the genome of the target, allowing for the replication and maintenance of the transgene over extended periods of time. Yet, this might lead to insertional mutagenesis, with the potential for activating tumor genes. Also, retroviral vectors do not transduce nondividing cells and have a restricted host range. As for nonviral systems, ex vivo approaches with selection of transduced cells are usually required with retroviral vectors23-27 because they are produced only at relatively medium titers and do not exhibit very high efficiencies. Instead, lentiviral vectors, a subclass of retroviruses derived from the human immunodeficiency virus (HIV), can integrate in the genome of nondividing cells.28 Therefore, such vectors might be good alternatives to the use of retroviruses, as they show also higher levels of transduction in vivo and avoid the need for cell division.29,30 Yet, there are common concerns associated with their application, including the potential for insertional mutagenesis and the psychological problem of introducing genetic material carrying HIV sequences.
Herpes simplex virus (HSV)–derived vectors are large vehicles that can deliver long transgenes to almost all known cell types, including nondividing cells. Although first-generation vectors induced high levels of cytoxicity, recent work has demonstrated that second-generation HSV were less deleterious, in particular for cartilage repair.31 One problem remains the transient nature of transgene expression mediated by this family of vectors.
In any case, the direct application of viral vectors raises legitimate safety concerns, as potentially infectious agents or sequences (especially lentiviral vectors) might be introduced per se in the body. This is of particular importance for the treatment of cartilage and meniscal lesions that are not life-threatening disorders. In this regard, adeno-associated viral vectors (AAV), which are based on the nonpathogenic, replication-defective human parvovirus AAV,32 might prove more adequate in direct gene therapy settings. Vectors based on AAV (rAAV) are produced by complete removal of the viral gene coding sequences, making them less immunogenic than adenoviral vectors and less toxic than HSV. Also, the latter vectors generally mediate only short-term expression of the transgenes they carry, whereas rAAV can be transcribed for months to years due to the stabilization of the episomal transgene cassettes by concatemer formation.33-36 Cell division and integration are not required for expression of the foreign material delivered, in marked contrast with retroviral vectors.37 Redosing of vectors is practicable with rAAV, based on the manipulation of various available serotypes of the virus. For these reasons, rAAV became a preferred gene transfer method for experimental settings in vivo and for clinical applications.35,36,38,39
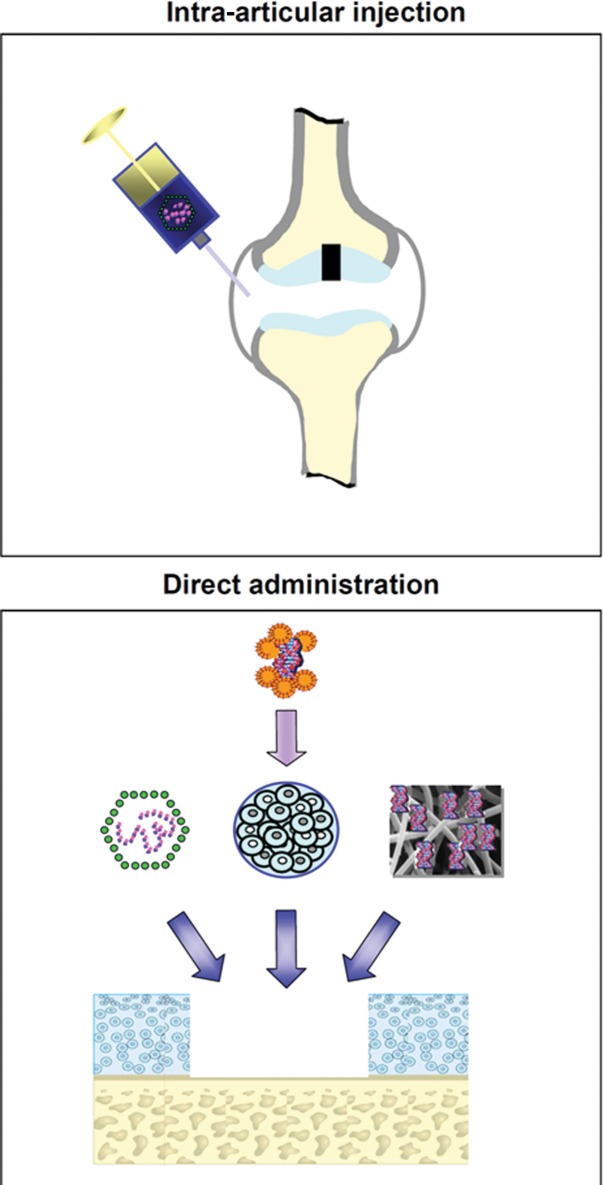
The greatest obstacle to develop efficient gene transfer protocols targeting sites of articular cartilage and meniscal fibrocartilage damage so far has been the restrained accessibility of the lesions to a treatment. Therefore, the following experimental approaches are currently employed to transfer genes to sites of interest in vivo (Fig. 1):
Figure 1.
Therapeutic genes may be transferred to sites of articular cartilage damage or to meniscal lesions in vivo via intra-articular injection or by direct application into the lesion. Intra-articular injection (upper panel) of the therapeutic formulation (most often a viral vector) results in a nonselective transduction of many intra-articular tissues. Direct administration of the therapeutic formulation (lower panel) to the target lesion (e.g., an articular cartilage defect) can be achieved by directly applying a gene vector to the repair tissue in the defect (left), by matrix-supported application (e.g., alginate) of target cells (e.g., articular chondrocytes, meniscal fibrochondrocytes, progenitor cells) that were previously genetically modified ex vivo (middle), or by application of a gene vector attached to a biomaterial (right). In vivo, it often includes an arthrotomy.
1. intra-articular injection of the therapeutic formulation, and
2. administration of the therapeutic formulation to the defect via arthrotomy:
2.1. direct application of a gene vector to the repair tissue,
2.2. application of biomaterials carrying a gene vector, and
2.3. matrix-supported application of ex vivo genetically modified cells.
The target cells in which genes may be transferred include the following:
1. progenitor cells (e.g., resulting from marrow-stimulating techniques or transplanted cells),
2. isolated articular chondrocytes or meniscal fibrochondrocytes that are transplanted into the defect, and
3. cells of the tissues adjacent to the defect:
3.1. articular cartilage: articular chondrocytes from the adjacent cartilage, osteoblasts, and osteocytes from the subchondral bone; and
3.2. meniscal tissue: meniscal fibrochondrocytes, synoviocytes from the synovial lining, and fibroblasts from the joint capsule.
Articular Cartilage
Introduction
Anatomy, Function, and Pathophysiology
Adult hyaline articular cartilage is avascular tand aneural and does not possess a lymphatic drainage.40 Its major function is to allow for a smooth gliding of the articulating surfaces of a joint and to protect the subchondral bone from mechanical stress. Hyaline articular cartilage is structured in several laminar zones and formed by chondrocytes that are surrounded by an intricate network of extracellular matrix. This cartilaginous matrix is rich in proteoglycans and collagen fibrils composed of type II collagen but also contains types VI, IX, XI, and XIV collagens and a number of additional macromolecules.41 Normal hyaline articular cartilage contains about 70% to 80% water, which is mainly bound to proteoglycans. Articular chondrocytes synthesize and degrade the extracellular matrix, thereby regulating the structural and functional properties according to the applied loads.
The integrity of articular cartilage can be disrupted as a result of mainly 4 different etiologies.42 These include focal articular cartilage defects resulting from an acute trauma, osteoarthritis, osteonecrosis, and osteochondritis dissecans.43 The resulting articular cartilage defect is characterized as being either chondral, involving only the cartilaginous zones, or osteochondral, reaching further into the subchondral bone.44 Although a chondral defect may be in part repopulated by cells from the synovial membrane,45,46 it usually remains and may expand over time. An osteochondral defect is filled with a blood clot that forms if the bone marrow communicates with the defect.47,48 The pluripotent, undifferentiated mesenchymal cells of the blood clot differentiate into chondrocytes and osteoblasts that later form the cartilaginous repair tissue and the new subchondral bone. However, over time, this repair tissue increasingly exhibits characteristics of fibrocartilage, such as an increased type I and a decreased type II collagen content and may degenerate after several years.48 If left untreated, secondary osteoarthritis of the joint may result.
Chondrogenic Therapeutic Factors
Strategies for enhancing chondrogenesis in an articular cartilage defect aim at improving the differentiation of mesenchymal cells into chondrocytes for cartilage repair and osteoblasts for the repair of the subchondral bone, the production and maintenance of a new cartilaginous matrix rich in type II collagen and proteoglycans, at increasing the cellularity of the repair tissue to prevent the hypertrophic differentiation of chondrocytes, and at inhibiting articular cartilage degeneration.
Growth and transcription factors are good candidates for these approaches. The therapeutic efficacy of polypeptide growth factors is, however, diminished by their short half-lives.49-51 For example, the fibroblast growth factor-2 polypeptide has a plasma half-life of less than 1 hour and is cleared in some hours after intra-articular administration.50 To overcome this problem, the idea of applying the gene encoding for a particular therapeutic protein has gained attraction.
Candidate factors to support chondrogenesis include members of the transforming growth factor beta (TGF-β) superfamily such as TGF-β1 and TGF-β2,27,52,53 bone morphogenetic protein 2 (BMP-2),51,53 BMP-7,54,55 members of the fibroblast growth factor family such as the basic fibroblast growth factor (FGF-2),56 growth/differentiation factor 5 (GDF-5),57 and the parathyroid hormone–related protein (PTHrP).58,59 Cell proliferation is promoted, among others, by FGF-260,61 and the insulin-like growth factor I (IGF-I).62 Particularly potent candidates to stimulate matrix synthesis include IGF-I,63,64 BMP-2 and BMP-7, and the cartilage-derived morphogenetic proteins (CDMP).65,66
Transcription factors directly modulate the expression of genes involved in chondrogenesis, such as type II collagen or aggrecan. Experimental models have demonstrated the chondrogenic properties of transcription factors, such as SOX9,67 Cbfa-1/Runx-2,68 Cart-1,69 the Ets family members,70 and various signaling molecules as well as extracellular matrix glycoproteins themselves.71,72 Another attractive approach is to inhibit degenerative pathways within the repair tissue. Potential targets include cytokines that mediate catabolic events, in particular the members of the interleukin-1 (IL-1),73 IL-17,74 and tumor necrosis factor (TNF)75 families. These strategies are based on the inhibition of the production of matrix-degrading enzymes,76 proinflammatory mediators,75 as well as apoptotic mechanisms.77
Traumatic Articular Cartilage Defects
Intra-articular Injection
Intra-articular injection is a convenient way to target the joint space and has been studied using naked DNA78 or adenoviral,79,80 retroviral,81,82 HSV,79 lentivirus,29 rAAV,81,83,84 and nonviral vectors.79,85 In 1998, Ikeda et al.80 injected adenoviral vectors encoding for the TGF-β1 gene into the joints of guinea pigs and reported elevated TGF-β1 levels in the synovial fluid for 2 weeks following gene delivery. The effectiveness of a direct intra-articular gene therapy approach in combination with a marrow stimulation technique has been shown by Morisset et al.86 Full-thickness chondral defects in equine stifle and knee joints were treated by microfracturing, followed by intra-articular application of adenoviral vectors carrying the genes for interleukin-1 receptor antagonist protein (IL-1Ra) and IGF-I. Sixteen weeks postoperatively, articular cartilage defects treated with IL-1Ra and IGF-I showed increased proteoglycan content and type II collagen expression compared with defects treated using a marrow-stimulating technique alone. Yet, articular cartilage defects cannot be specifically targeted with this approach since the transgene is expressed mainly in cells of the synovial membrane and gene transfer into articular cartilage defect is a very rare event. Therefore, many of the gene-based approaches have focused on direct gene vector delivery into a defect exposed by arthrotomy (Table 2).
Table 2.
Therapeutic Gene Transfer Studies to Articular Cartilage Defects In Vivo
| Period of Evaluation | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gene | Route | Vector | Cells | Support | Defect | Size (mm) | AnimalModel | Joint | Location | Min (wk) |
Max (wk) |
Ref. |
| BMP-2 | Ex vivo | Retroviral | Chondrocytes | Fibrin | Osteochondral | 3.6 Ø | Rabbit | Knee | Patellar groove | 4 | 12 | 120 |
| BMP-2 | Ex vivo | Adenoviral | Fat/muscle grafts | −/− | Osteochondral | 3.0 Ø | Rabbit | Knee | Patellar groove, medial femoral condyle |
6 | 6 | 124 |
| BMP-2, IGF-I | Ex vivo | Adenoviral | Perichondrial cells | Fibrin | Chondral | <1.0 Ø | Rat | 3 | 8 | 19 | ||
| BMP-7 | Ex vivo | Retroviral | Periosteal cells | PGA | Osteochondral | 3.0 Ø | Rabbit | Knee | Patellar groove | 4 | 12 | 25 |
| BMP-7 | Ex vivo | Adenoviral | Chondrocytes | Fibrin | Osteochondral | 15.0 Ø | Horse | Knee | Lateral trochlear ridge | 4 | 36 | 21 |
| IGF-I | Ex vivo | FuGENE 6 | Chondrocytes | Alginate | Osteochondral | 3.2 Ø | Rabbit | Knee | Patellar groove | 3 | 14 | 91 |
| IGF-I | Ex vivo | Adenoviral | Chondrocytes | Fibrin | Chondral | 15.0 Ø | Horse | Knee | Lateral trochlear ridge | 4 | 32 | 121 |
| IL-1RA + IGF-I | Intra-articularly | Adenoviral | −/− | −/− | Chondral (with microfracture) |
10.0 × 10.0 □ | Horse | Knee, stifle | Distal radial carpal bone, medial femoral condyle | 16 | 16 | 86 |
| IGF-I + FGF-2 | Ex vivo | FuGENE 6 | NIH 3T3 | Alginate | Osteochondral | 3.2 Ø | Rabbit | Knee | Patellar groove | 3 | 3 | 114 |
| FGF-2 | Ex vivo | FuGENE 6 | Chondrocytes | Alginate | Osteochondral | 3.2 Ø | Rabbit | Knee | Patellar groove | 3 | 14 | 90 |
| FGF-2 | In vivo | rAAV | −/− | −/− | Osteochondral | 3.2 Ø | Rabbit | Knee | Patellar groove | 1 | 18 | 36 |
| FGF-2 | Ex vivo | rAAV | Chondrocytes | Type I collagen, periosteal flap | Osteochondral | 5.0 Ø | Rabbit | Knee | Patellar groove | 4 | 12 | 99 |
| FGF-2 | In vivo | rAAV | −/− | −/− | Osteochondral | 5.0 Ø | Rabbit | Knee | Patellar groove | 4 | 12 | 163 |
| TGF-β | Ex vivo | Retroviral | NIH3T3 | −/− | Chondral | 3.0 × 6.0 □ | Rabbit | Knee | 1 | 6 | 27 | |
| TGF-β | Ex vivo | rAAV | hMSC | −/− | Osteochondral | 1.5 Ø | Rat (athymic) | Knee | Patellar groove | 4 | 12 | 38 |
| TGF-β1 | Ex vivo | Adenoviral | Bone marrow aspirate | −/− | Chondral | 6.2 Ø | Sheep | Knee | Medial femoral condyle | 26 | 26 | 125 |
| CDMP1 (GDF-5) | Ex vivo | FuGENE 6 | MSC | Type I collagen | Osteochondral | 4.0 Ø | Rabbit | Knee | Patellar groove | 2 | 8 | 66 |
Note: PGA = polyglycolic acid; MSC = mesenchymal stem cells; Ø = cylindrical defect; □ = rectangular defect.
Arthrotomy
Direct application of a gene vector in vivo
The direct delivery of therapeutic genes into cartilage defects in depth has long been arduous due to the reduced capability of nonviral and various viral vectors to penetrate the dense extracellular cartilaginous matrix. Following arthrotomy and gene vector application to cartilage defects, limited transgene expression was observed only in the superficial cartilage layers.80 With the implementation of rAAV vectors, direct gene transfer to cells within defects and adjacent cartilage has met success. Reporter gene studies demonstrated efficient transgene expression in normal and osteoarthritic human articular chondrocytes within their native matrix in situ to depths relevant for clinical applications.87 Moreover, transgene expression was also present in chondral and osteochondral articular cartilage defects in vivo for at least 4 months.87 rAAV vectors have been manipulated recently to deliver therapeutic genes such as FGF-2 directly into osteochondral cartilage defects.36 Cartilage repair was significantly enhanced 4 months after vector application.36
Application of biomaterials carrying a gene vector into defects
In order to avoid a dilution of the therapeutic agents, gene vectors or modified cells can be delivered in conjunction with biomaterials such as fibrin, collagen, gelatin, carbohydrate-based polymers (polyactic acid/polyglycolic acid, hyaluronan, agarose, alginate, chitosan), and artificial polymers (dacron, teflon, carbon fibers, polyestherurethane, polybutyric acid, polyethylmethacrylate, hydroxyapatite).45,88 When preparations of adenoviral vectors carrying a marker gene were adsorbed onto type II collagen-glycosaminoglycan matrices and implanted into osteochondral defects, transgene expression was present until day 21.89
Application of ex vivo genetically modified cells
The direct transplantation of cells genetically modified ex vivo involves their isolation, genetic modification, and reimplantation into articular cartilage defects. These modified cells can be applied without (e.g., as coagulated bone marrow aspirate) or with supportive matrices. Such components include alginate,90-92 agarose,93,94 fibrin or type I collagen gels without95-97 or with a periosteal flap,98,99 and synthetic biodegradable scaffolds.100-102 Kang et al. were the first to transplant genetically modified cells into an articular cartilage defect in vivo.103 In this study, chondrocytes were transduced with a retroviral vector. Other studies used nonviral,104-106 adenoviral,89,96,107 retroviral,103,108-111 and rAAV vectors112 to deliver marker genes in defects via ex vivo–modified cells. Although engineered chondrocytes are generally transplanted,21,96,103,104,106,111,113 fibroblasts,27,114 perichondrial,105 periosteal,108,112 or muscle-derived cells109 have been also applied. The data from these studies showed that transgenes can be expressed in cartilage defects via ex vivo strategies, remaining active for about 1 month. This is significantly longer compared with the application of recombinant proteins (Table 2). Figure 2 depicts improvements in the repair of osteochondral defects following combined gene transfer of IGF-I and FGF-2 compared with the application of a marker gene (lacZ) to NIH 3T3 fibroblasts.114
Figure 2.
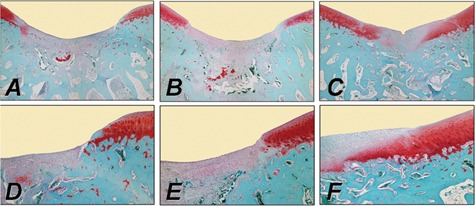
Improvement of cartilage repair in a rabbit osteochondral defect model in the trochlear groove by combined ex vivo gene transfer of human insulin-like growth factor I (hIGF-I) and fibroblast growth factor-2 (hFGF-2) in NIH 3T3 fibroblasts that were then embedded in alginate spheres and transplanted into the defects. Histological appearance of osteochondral defects following treatment with a lacZ implant (left column: A, D), an IGF-I implant (middle column: B, E), and an IGF-I/FGF-2 implant (right column: C, F) stained with safranin O. Images (D-F; 40x) are magnified views of A through C (20x), illustrating the area of integration between the repair tissue (on the left side of D-F) with the adjacent normal articular cartilage (on the right side of D-F). Implants remained in a subchondral location and are visible at the bottom of images (A, B). Transplantation of the cotransfected IGF-I/FGF-2 implants accelerated the formation of the subchondral bone and improved articular cartilage repair in a magnitude that was larger than with IGF-I alone or when compared to lacZ implants after 3 weeks in vivo.
Figure 3.
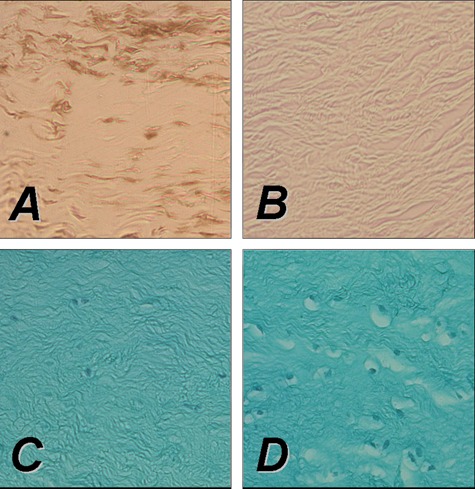
Direct rAAV-mediated gene transfer to rabbit meniscus explants in vitro using an rAAV-lacZ (left panel) or rAAV-hFGF-2 vector (right panel) (50 mL each vector). Persistent transgene expression after 10 days in vitro in meniscal explants following immunohistochemical detection of lacZ (A), while no signal is present in the control (B). Direct transduction of a rabbit meniscal explant with rAAV-hFGF-2 results in an increased cell density (D) compared with the control (C), indicative of the mitogenic effect of FGF-2 on meniscal fibrochondrocytes. (C, D) hematoxylin and eosin/fast green. All magnifications, 20x.
Periosteal cells transduced by a BMP-7 retroviral vector and attached to a polyglycolic acid scaffold improved cartilage repair at 8 and 12 weeks in vivo. Interestingly, this was the first study in which a growth factor gene was transferred into a focal defect.25 Since, many reports described the use of a variety of therapeutic genes like BMP-2, BMP-7, IGF-I, FGF-2, and TGF-β.22,90,91,114-120 Significant improvement in articular cartilage repair was noted in these reports (Table 2). Although most of the evaluations were carried out in small animal models, Hidaka et al.21 and, more recently, Goodrich et al.121 performed arthroscopic implantation of chondrocytes genetically engineered by adenoviral transduction with the BMP-721 or IGF-I121 gene in horses.
On the basis of such encouraging data, cartilage repair was addressed by matrix-supported implantation of genetically engineered mesenchymal stem cells (MSC). Kuroda et al.122 implanted BMP-4–transduced MSCs using fibrin glue in full-thickness cartilage defects in the trochlear groove of rabbit femurs. After 24 weeks, histological scoring of the defects revealed significantly better cartilage repair in the BMP-4 treatment group compared with defects receiving lacZ-transduced MSCs. Guo et al.123 seeded TGF-β1–engineered MSCs onto poly-L-lysine–coated polylactide scaffolds in vitro and allografted them into full-thickness defects in rabbits. This resulted in improved joint repair with regard to extracellular matrix formation, reconstitution of the subchondral bone, and inhibition of inflammatory immune responses. Repair of osteochondral defects was also enhanced by transplantation of MSCs transfected with the CDMP1 gene, applying a lipofection method.66
A novel method of gene therapy for the repair of osteochondral defects has recently been published by Evans et al.124 Rather than genetically modifying isolated cells, this technique describes gene transfer to biopsies of muscle and fat. An adenovirus vector carrying cDNA encoding human BMP-2 was used for genetic engineering of tissues. These gene-activated muscle or fad pads were transplanted into osteochondral defects in rabbits. Histological analysis after 6 weeks revealed the formation of a proteoglycan-rich articular surface with subchondral bone beneath and good union with the adjacent cartilage.
Ivkovic et al.125 used autologous bone marrow, transduced ex vivo, with adenoviral vectors containing the cDNA for TGF-β1. Implantation of the marrow clot improved the histological, biochemical, and biomechanical parameters of partial-thickness chondral defects in sheep at 6 months.
Osteoarthritis
Osteoarthritis (OA) is the leading, most disabling human condition and prevalent form of arthritis (80%), impairing the quality of life of millions of people worldwide. OA is a chronic disorder of diarthrodial joints, mainly characterized by a slow, gradual deterioration of the articular cartilage that remains without effective treatment to date. OA not only affects the cartilage but also the subchondral bone and, to a minor degree, the synovial lining, ligaments, tendons, and muscles. Current options to manage OA, such as pharmacological therapy and reconstructive surgical interventions, do not allow for the restoration of a native cartilage. OA is a complex disorder characterized by an activation of inflammatory cascades at the molecular level, leading ultimately to cartilage breakdown, associated with alterations of the phenotype of chondrocytes and a loss of the major components of the cartilage matrix. Under mechanical or biochemical stress (presence of IL-1 and TNF-α, NO, prostaglandins, matrix degradation products), the chondrocytes undergo pathological changes in their gene expression patterns that lead to an impairment of the overall homeostasis, with diminished production of normal cartilage matrix molecules (proteoglycans, type II collagen), enhanced production of matrix-degrading enzymes (MMPs and adamalysins, including ADAMs and ADAMTs), and decreased responsiveness to reparative stimuli, ultimately leading to the degradation of the matrix and cell senescence and apoptosis (NO, Fas/FasL signaling) by alteration of cell viability.
Gene Transfer In Vitro
Target cells in the joint include cells of the synovial lining, chondrocytes, chondroprogenitor cells, and surrounding tissues (bone, muscle, tendons, ligaments, meniscus). Application of nonviral,12,79,90,91,126-135 adenoviral,79,126,136-154 or retroviral vectors25,79,126,136,140,155-159 has been achieved in these cell types with more or less success. Instead, RAAV vectors are potent alternatives as they can efficiently and durably transduce synoviocytes,160-164 chondrocytes,36,87,99,165-168 MSCs,36,38,169,170 and cells of surrounding tissues.126,171-177
Regeneration of a normal structural and functional cartilage might be achieved by the following:
inhibiting inflammatory and catabolic pathways,
stimulating anabolic pathways to rebuild the matrix,
impeding cell senescence,
avoiding the pathological formation of osteophytes,
prevention of apoptosis, and
influencing several of these processes.
Inhibition of catabolic pathways has been achieved in vitro by expressing inhibitors of matrix-degrading enzymes (tissue inhibitor of metalloproteinases, i.e., TIMP),178,179 inhibitors of proinflammatory cytokines (IL-1Ra, the soluble receptors sIL-1R or Soluble Tumor Necrosis Factor Receptor),107,137,150,155,162 and chondroprotective cytokines (IL-4, IL-10).160,180,181 Activation of anabolic processes in vitro has been noted by single or combined administration of components of the cartilage matrix or of the enzymes that synthesize them,182,183 of growth factors and receptors (IGF-I, FGF-2, BMPs, TGF-β),36,127,131,138,143-145,147,150,151,181,184,185 and of transcription factors (SOX family of DNA-binding proteins, i.e., SOX5, SOX6, SOX9).130,136,149,159,167,168 Restoration of cell vitality and activation of proliferation in vitro have been achieved by application of IGF-I and FGF-2,36,90,127,131,168,185 telomerase (hTERT),186 of inhibitors of apoptosis (bcl-2),187 or of HSP70.132 Interestingly, approaches that influence several of these processes have been also successfully attempted, like combining the transfer of inhibitors of catabolism pathways and of activators of anabolic events (IGF-I/IL-1Ra or IGF-I/IL-4),150,151,181 as well as that of activators of anabolic and proliferative processes (FGF-2/SOX9 or FGF-2/IGF-I).168
In Vivo Direct Gene Transfer
The key issue in establishing an efficient therapy against OA is the accessibility of the targets to the treatment when they reside in the joint cavity. The following approaches have been developed to deliver a molecular composition:
systemic delivery, and
intra-articular administration (via injection or arthrotomy).
Systemic approaches are better suited to target diseases that are systemic in nature like rheumatoid arthritis (RA).164,188-190 Local administration of components might be preferable in the case of OA that affects only a limited number of joints without major extra-articular or systemic manifestations. The foreign material may be delivered directly (gene vector preparation) or indirectly (genetically modified cells).
Several lines of evidence have demonstrated that intra-articular injection of most vector types leads to a preferential transduction of the synovium,29,79,81,191 being more suited for strategies aiming at inhibiting inflammatory and catabolic pathways and a common approach employed against experimental RA. Successful attempts towards these goals have been reported by direct application of vectors coding for IL-4,35,192 IL-10,193,194 sTNFR alone162 or combined with IL-10,195 IL-1Ra alone18,20,34,155,196,197 or combined with sTNFR,20 antagonists and inhibitors of TGF-β and of the BMPs,198 HSP70,132 gene expression silencers,199 and kallistatin or thrombospondin-1.200,201
Yet, even if cartilage breakdown can be contained, this will not be sufficient to fully compensate for the loss of matrix elements and cells noted during the disease progression. In this regard, increased synthesis of cartilage matrix components has been documented following injection of vectors carrying genes for anabolic factors (IGF-I).202
Ex Vivo Indirect Gene Transfer
Although more complex, ex vivo gene therapy is considered safer because no free vector particles are introduced in the body. Modified cells can be extensively controlled, tested, and selected while maintained in culture. Administration of cells is also a means to increase the cellularity like needed for severe OA.
Synoviocytes have been predominantly employed to deliver inhibitors of inflammatory and catabolic processes.203-208 Such pathways could be regulated by injecting synoviocytes transduced to overexpress an IL-1Ra alone203-208 or combined with IL-10.208 Also, dermal fibroblasts have been modified for this purpose to overexpress an IL-1Ra, sTNFR, or a combination of both.209
Reduced severity of the induced arthritis was associated with a decrease in cartilage breakdown, but complete resurfacing was not achieved. Successful attempts to promote the formation of new cartilage have been made by administrating dermal fibroblasts modified to express BMP-2.146
Still, preparation of terminally differentiated cells from unaffected sites remains invasive, with a limited supply, and represents an additional burden for the patient. Also, committed cells generally undergo major phenotypic changes upon passaging in culture, especially chondrocytes. Multipotent cells might be more suited for transplantation purposes, possibly leading to the production of a cartilage surface of enhanced quality compared with committed cells that lead to the formation of a poorly differentiated fibrous cartilage. Progenitor cells can be easily isolated from multiple tissues (bone marrow, periosteum, perichondrium, muscle, fat, subdermis, cartilage, bone, synovial membrane, ligaments), even in OA patients, maintaining a multilineage potential with a reliability for differentiation and a capacity for expansion.210,211 Indeed, injection of muscle-derived stem cells modified by combined gene transfer of BMP-4 with sFlt1 (a vascular endothelial growth factor (VEGF) antagonist) allowed for cartilage repair in a rat model of OA.212,213
Osteonecrosis
Osteonecrosis (ON) is primarily a disease of the subchondral bone that secondarily affects the articular cartilage. Initially, a vascular insult is thought to cause an interference of the microcirculation of the subchondral bone, resulting in an edema that leads to an increased intraosseous pressure. This leads to ON of the affected segment of the subchondral bone, which may result in a subchondral insufficiency fracture, destabilizing the overlying articular cartilage and eventually resulting in its collapse and the creation of an osteochondral defect. Treatment options consist of conservative therapy in early stages. Precollapse lesions can be treated with retrograde core decompression, while later-stage lesions presenting with osteochondral defects require osteochondral transplants and/or osteotomies, or ultimately, partial or total knee arthroplasty.214
Possible experimental gene therapy approaches need to be stage dependent, focusing on early stages (when the articular cartilage is not compromised) at the revascularization of the necrotic bone, while at the stage of osteochondral lesion, only gene-enhanced osteochondral transplants might be useful. Katsube et al.214 applied gene transfer of VEGF, to accelerate revascularization of the necrotic bone. Using an adenoviral vector encoding for VEGF, endothelial cells of the rabbit saphenous arteries were transduced. These gene-modified arteries were then placed with its venae comitantes into necrotic iliac crest bone in vivo. Angiogenesis in the necrotic bone was quantified by bone blood flow measurement and assessment of vessel density following microangiography. The extent of neoangiogenesis was significantly greater in the VEGF group than the control group, reflected in an increased capillary density, length of newly formed capillaries, and increased bone blood flow at 1 week postoperatively. While this study was restricted to the bone of the iliac crest, it might serve as a paradigm for the treatment of ON in a subchondral location. Such a therapy may allow the healing of avascular necrosis before fracture and subchondral collapse occur, preventing the articular cartilage from damage. More studies with time points longer than the 1-week evaluation are needed, preferentially performed in animal models of subchondral ON, such as the femoral condyles of the knee joint, its second most common location.
Osteochondritis Dissecans
Osteochondritis dissecans (OCD) usually affects children and young adults and occurs mainly in the knee joint, characteristically in the lateral aspect of the medial femoral condyle. Possible etiological factors beside a genetic predisposition include ischemia and epiphyseal abnormalities with subsequent necrosis. For example, disruption of epiphyseal plate vessels may lead to localized avascular necrosis. Its revascularization usually occurs with the formation of a scar tissue, absorption of necrotic fragments, intertrabecular osteoid deposition, and remodeling with new bone formation. When revascularization is delayed, an OCD lesion can occur. Clinical treatment principles focus on stimulation of revascularization or removal of necrotic subchondral bone together with its restoration (e.g., using autologous bone transplants), beside the surgical fixation of an unstable osteochondral fragment.214
So far, no experimental gene-based treatment has been proposed for the treatment of OCD. In theory, the same principles apply for the revascularization of necrotic subchondral bone, as already outlined for ON with subsequent articular cartilage defects. It may be also possible to enhance the surgical fixation of an osteochondral fragment by applying osteoinductive genes such as the BMPs to the subchondral bone–osteochondral fragment interface to improve integration of the osteochondral fragment. It is unclear whether the integration of a chondral fragment may be achieved, a rare indication currently favored only for surgical refixation of large fragments in juvenile patients.215 Likewise, gene-modified osteochondral transplants may be applied at later stages of deep osteochondral defects.
Meniscal Fibrocartilage
Anatomy, Function, and Pathophysiology
The menisci are semilunar fibrocartilage structures that transmit weightbearing forces and increase stability, facilitate nutrition and provide lubrication for the articular cartilage, and promote knee proprioception.216,217 As the medial meniscus is less mobile during joint motion,218,219 injuries are much more common compared to the lateral meniscus.220 Type I collagen is the predominant collagen of the meniscal tissue.221 It is arranged with a circumferential orientation with interspersed radially oriented fibers.222 The central parts of the menisci are mainly constituted of fibrochondrocytes, whereas fibroblasts are the predominant cell type in the peripheral regions.223 Meniscal blood supply is restricted to the peripheral 10% to 25% of the meniscal tissue.224,225 Nourishment in the central area is provided only by diffusion of the synovial fluid,226 perhaps playing a role in the poor healing capacity of central lesions.225-227 Gene transfer strategies may be applied for the following:
1. meniscal repair, and
2. meniscal reconstruction, using
2.1. meniscal substitutes, and
2.2. meniscal allografts.
Meniscal Repair
Meniscal tears are common228,229 and predispose the affected joint to develop secondary OA.230 Tears of the meniscus in the vascularized peripheral parts can be repaired by sutures, while tears of the central avascular parts are treated by arthroscopic partial meniscectomy.
Gene Transfer Strategies: In Vitro Studies
Gene transfer strategies for the repair of meniscal tears focus on the delivery of therapeutic agents, for example, growth factors, to the site of the meniscal lesion. This can be performed either via direct application of gene vectors or by transplantation of genetically modified cells overexpressing therapeutic genes. Treatment of meniscal fibrochondrocytes with recombinant growth factor proteins such as the platelet-derived growth factor AB (PDGF-AB),231-234 FGF-2,177,235-237 IGF-I,235,238-240 TGF-β1,152,233,239,241,242 BMP-7,233 or TGF-β3235 has been shown to improve the phenotypical and biochemical properties of the cells in vitro. Fibrochondrogenesis of stem cells is enhanced by incubation with growth factors such as TGF-β1243 or TGF-β3 in combination with BMP-4.244 The possible application of gene transfer strategies in meniscal repair has first been investigated by Goto et al.139 The lacZ marker gene was transferred to meniscal cell cultures using retroviral and adenoviral vectors. In a next step, the marker gene was applied to human meniscal fragments and whole lapine menisci using direct adenoviral gene transfer and transplantation of meniscal fibrochondrocytes transduced with a retroviral vector. Transgene expression was detected in meniscal explants following ex vivo gene transfer for at least 20 weeks. Successful transfer of the lacZ marker gene was also achieved by rAAV-mediated transfer into human and lapine fibrochondrocytes in vitro.173 Encouraged by these findings, in 2000, the group of Chris Evans transferred the gene encoding for TGF-β1158 to meniscal cells in vitro, resulting in enhanced synthesis of proteoglycans and collagen. Zhang et al. used a lipid-based gene transfer system to deliver the gene encoding for human IGF-I to meniscal fibrochondrocytes, yielding accelerated proliferation and differentiation of the modified cells.134 Recently, we tested the hypothesis that overexpression of FGF-2 through rAAV vectors leads to detectable metabolic changes in human meniscal fibrochondrocytes and inside defects of human meniscal explants.177 Application of the rAAV-hFGF-2 vector allowed for enhanced cell proliferation and survival in vitro (Figure 3). The idea of applying gene therapy protocols to deliver fibrochondrogenic agents to meniscal tears was supported by a significant reduction of the amplitude of meniscal tears after FGF-2 treatment in this study.177
Gene Therapy: In Vivo Studies
Only few reports have evaluated the feasibility of gene therapy strategies to enhance the repair of meniscal tears in vivo. Experimental studies have shown that repair in the central part of the meniscus can be promoted by various chemotactic and mitogenic stimuli delivered by an autologous fibrin clot245,246 or a free graft of synovium247,248 in vivo. In a sheep model, longitudinal tears of the anterior horn of the medial meniscus were sutured using VEGF-coated sutures. Interestingly, meniscal repair was not enhanced in the VEGF treatment group.249,250 In 1999, methods of direct and indirect gene transfer to meniscal lesions were compared.139 In a lapine model, a suspension of adenoviral vectors carrying the lacZ marker gene was mixed with whole blood, and the clot was inserted into 2-mm-long incisions in the medial meniscus. In the same study, using a canine model, retrovirally transduced allogenic meniscal fibrochondrocytes carrying the lacZ gene were embedded in collagen gels and transferred to partial-thickness circular defects (depth, 3 mm; diameter, 2 mm) in the medial meniscus. Gene expression persisted for at least 3 weeks in the lapine model but for 6 weeks within the transplanted meniscal fibrochondrocytes in the canine model. In another animal study,173 longitudinal incisions were created in the avascular zone of the medial meniscus of rabbits. When rAAV-lacZ constructs were injected intralesionally, X-Gal staining was present by day 20 postoperatively, the longest time point evaluated.
Meniscal Reconstruction
Meniscal Substitutes
Meniscal substitutes have been proposed as a means to overcome problems associated with meniscal allografts and to promote meniscal repair of segmental defects, for example, resulting from a partial meniscectomy.251,252 Meniscal substitutes already in clinical use are based on porous matrices of type I collagen/glycosaminoglycan (Menaflex, ReGen Biologics, Hackensack, NJ)253,254 or polyurethane (Actifit, Orteq, London, UK).255,256
The feasibility of genetic engineering of meniscal fibrochondrocytes has already been described above. However, in the treatment of circumscribed meniscal defects, direct gene vector administration into injured knee joints may be difficult to achieve because a loss of the bradytrophic meniscal tissue may hardly be restored by local cells, even after administration of mitogenic and anabolic genes. Therefore, gene therapy in the treatment of meniscal defects may need to be used in combination with the transplantation of modified cells or tissues.
Tissue engineering involves the combination of cells, engineered extracellular matrices, and biologically active molecules for tissue regeneration.257,258 Over the last 2 decades, numerous tissue engineering strategies have emerged for the replacement of meniscal tissue.259-261 In general, 2 basic approaches for meniscal replacement can be distinguished:
Several concepts for treating circumscribed meniscal defects concentrate on meniscal replacement by acellular matrices,259,266-269 avoiding possible risks associated with transplantation of human allografts (e.g., failure rate, immunoreaction,270 disease transmission271). Different types of meniscal substitutes, such as decellularized allogenic and xenogenic grafts,262,263,272,273 collagen grafts,253,274 permanent synthetic scaffolds,251 and biodegradable scaffolds based on small intestine submucosa,275-278 poly-lactic acid (PLA), or poly-glycolic acid (PGA),279-282 have been used in experimental and clinical studies. However, after transplantation of acellular meniscal constructs into defects, the transplants are populated by synovial fibroblasts, resulting in a scar tissue with poor biomechanical properties.245,283 Therefore, some tissue engineering approaches focus on additional cell-seeding techniques prior to transplantation.251,284 Meniscal cells,282,285 articular chondrocytes,286,287 synovial fibroblasts,288 and MSC289 have been proposed as potential cell sources and have been cultivated in vivo and in vitro on various matrices.267 In addition, different environmental factors such as growth factors have been used to optimize cell proliferation in vitro.290
Gene therapy may aid to further enhance the fibrochondrogenic potential of tissue-engineered transplants. In 2002, Hidaka et al.291 applied a gene transfer protocol to enhance the vascularization and blood supply of cell-seeded bioengineered meniscus transplants. Bovine meniscal cells overexpressing hepatocyte growth factor (HGF) were seeded onto PGA scaffolds and transplanted subcutaneously in athymic nude mice for 8 weeks. Ink injection studies showed that HGF-treated meniscal cells formed a tissue that contained significantly more blood vessels than the controls. In another preliminary ex vivo study, Steinert et al.152 transduced primary meniscus cells and bone marrow–derived MSCs with adenoviral vectors encoding for marker genes or TGF-β1. Modified cells were seeded in type I collagen-glycosaminoglycan (GAG) matrices and transplanted into defects of bovine menisci explants. In vitro, the vectors efficiently transduced meniscal cells and MSCs, and transgene expression remained elevated after incorporation of the cells into matrices. Transfer of TGF-β1 increased the fibrochondrogenic potential of modified cells, and transplantation of the TGF-β1–transduced constructs resulted in satisfactory filling of the lesions ex vivo (Table 3).
Table 3.
Therapeutic Gene Transfer Studies to Meniscal Cells In Vitro or Meniscal Tissue Ex Vivo and In Vivo
| Period of Evaluation | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Gene | Strategy | Vector | Cells | Support | Experimental Model | Min | Max | Major Findings | Ref. | |
| IGF-I | In vitro | Liposome (FuGENE 6) | Meniscal fibrochondrocytes (human) | −/− | −/− | 0 h | 10 d | Transfection efficiency 16% ± 1.2% No cytotoxicity Decrease in PDT from 52.6 to 40.2 h |
134 | |
| IGF-I | In vitro | Liposome (FuGENE 6) | Bone marrow stromal cells (goat) | Calcium alginate gel | Goat | In vitro | In vitro | 292 | ||
| 4 h | 10 d | Transfection efficiency 22.0% ± 2.4% Elevated IGF-I secretion (3.28 v. 1.67 ng/mL by untransfected fibrochondrocytes) |
||||||||
| In vivo | In vivo | In vivo | ||||||||
| 4 wk | 16 wk | Macroscopically and histologically improved repair tissue Elevated GAG content (14.2 v. 13.7 mg/g by untransfected BMSCs) Improved aspect of repair tissue on MRI |
||||||||
| HGF | In vitro | Adenoviral | Meniscal cells (calf) | PGA | Athymic nude mice (subcutaneous pouch) | In vitro | In vitro | 291 | ||
| 48 h | Transduction efficiency N.D. No enhanced proliferation of HGF-transduced cells |
|||||||||
| In vivo | In vivo | In vivo | ||||||||
| 3 d | 8 wk | HGF expression detectable for ≥2 weeks Fibrocartilage with structural limitations (presence and organization of collagen fibrils) Enhanced vascularization of engineered constructs No improved biomechanical properties (compression testing) |
||||||||
| FGF-2 | In vitro | rAAV | Meniscal fibrochondro cytes (human) | −/− (direct vector injection) | Human explants | In vitro | In vitro | 177 | ||
| 0 d | 21 d | Transduction efficiency 53%-59% Efficient FGF-2 transgene expression Enhanced cell proliferation and survival |
||||||||
| Ex vivo | Ex vivo | Ex vivo | ||||||||
| 5 d | 15 d | Enhanced contractile markers (α-SMA) Reduction of meniscal tear amplitude in depth and width (up to 2.4-fold) No stimulation of extracellular matrix components (type I/II collagen, PG) |
||||||||
| TGF-β1 | In vitro | Adenoviral | Primary meniscal cells (calf); bone marrow–derived MSCs (calf) |
Type I collagen-GAG matrix | Bovine explants | In vitro | In vitro | 152 | ||
| 3 d | 3 wk | Transduction efficiency >75% Increased cel lularity and GAG/DNA synthesis Enhanced proteoglycan and type II collagen staining Enhanced meniscal gene expression (COL I, COL II, DCN, BCN) |
||||||||
| Ex vivo | Ex vivo | Ex vivo | ||||||||
| 3 wk | Formation of highly cellular repair tissue; no differences between treatment and control group | |||||||||
| TGF-β1 | In vitro | Retroviral | Meniscal cells (human and canine) | −/− | −/− | 48 h | Transduction efficiency N.D. Increased transgene expression Enhanced collagen and proteoglycan synthesis (up to 15-fold) |
158 | ||
Note: PDT = population doubling time; GAG = glycosaminoglycan; BMSC = bone marrow stromal cell; HGF = hepatocyte growth factor; PGA = polyglycolic acid; α-SMA = alpha-smooth muscle actin; PG = proteoglycan; COL I = type I A2 collagen (537 bp); COL II = type II A1 collagen (580 bp); DCN = decorin (400 bp); BCN = biglycan (165 bp); MSC = mesenchymal stem cells; N.D. = not determined.
FGF = fibroblast growth factor; TGF = transforming growth factor
A recent in vivo work on the use of gene transfer to enhance meniscal repair has been published by Zhang et al.292 Following an indirect gene therapy approach without tissue engineering features, the authors created full-thickness meniscal defects in the avascular area of the anterior horn of the medial meniscus in a goat model. Bone marrow stromal cells were transfected with the gene encoding for human IGF-I using a nonviral transfection system (FuGENE 6) and suspended in calcium alginate prior to injection into the meniscal defects. After 16 weeks, the resulting repair tissue was improved according to MRI and histological and biochemical evaluation and compared with the controls (Table 3).
Meniscal Allografts
Meniscal reconstitution with allografts293-305 is a therapeutic option especially for young and symptomatic patients with a history of lateral meniscectomy in a normally aligned, stable joint without severe degenerative changes of the articular cartilage. A recent review306 suggests that meniscal allograft transplantation improves pain and function in the short and intermediate term.
Application of gene-based strategies has been suggested to improve remodeling of meniscal allografts.307 Martinek et al.308 studied the feasibility of gene transfer in lapine meniscal allografts ex vivo using a retroviral vector encoding the marker gene lacZ. Subsequently, unilateral meniscal replacements were performed with these engineered allografts. Transduced fibrochondrocytes migrated into the depth of the graft, while transgene expression persisted for up to 8 weeks. This investigation suggests potential promise for growth factor delivery in autografts and allografts prior to implantation.
Clinical Gene Therapy Trials
Preclinical data, as those described above, have encouraged the initiation of human clinical trials originally for arthritis. The first studies were based on the ex vivo retroviral gene transfer of a human IL-1Ra sequence in synoviocytes from patients with end-stage RA followed by reinjection of the modified cells in the metacarpophalangeal joint.23,82,309 The aim of these studies was to evaluate the possibility of transferring genes to human joints and expressing them intra-articularly in a safe fashion acceptable to the patients. The use of these protocols has permitted extensive testing of the cells prior to reimplantation, demonstrating successful expression of the transgene locally vis-à-vis control joints, without adverse events related to the treatment but with clinical improvements in some of the patients, encouraging the implementation of phase II studies (pending).1,5,30,310-315 Another protocol has been initiated for intra-articular plasmid316 delivery of the HSV thymidine kinase gene to the synovial lining of RA patients followed by administration of ganciclovir to achieve synovial ablation,1,5,309,311-314 but this protocol has been closed because of a failure to recruit. A new phase I trial for RA involved the direct in vivo intra-articular injection of an rAAV vector carrying the sequence for a fusion protein as sTNFR on an immunoglobulin molecule (tgAAC94 protocol).317 As the study revealed that the treatment was safe and well tolerated in subjects without use of concurrent systemic TNF-α antagonist,1,311,312,314,317 a phase I/II trial was subsequently started318 with the possibility to include patients who were already taking systemic TNF blockers and the administration of a second injection of tgAAC94. As one of the participants who was simultaneously being treated with systemic TNF antagonist and other immunosuppressive medications died after receiving the second injection, the trial was placed on hold by the U.S. Food and Drug Administration (FDA) to investigate, in parallel with the Recombinant DNA Advisory Committee Recombinant DNA Advisory Committee (RAC), the circumstances of the demise of the patient. The death was apparently due to a disseminated infection with Histoplasma capsulatum, a fungus endemic in the region of origin of the volunteer, and to an immunosuppression.312,319-321 Indeed, known serious complications of the particular TNF antagonist are susceptibility to H. capsulatum. The most probable explanation is that the subject was already infected with the fungus when receiving the second injection of tgAAC94. As the committee felt that the gene therapy protocol was very unlikely to have played any significant role in the event based on a large body of data from the independent investigations and since rAAV has been used safely in 47 previous human gene therapy clinical trials, the evaluation has been reopened with some modifications (exclusion of patients with elevated temperature, localized symptoms, fatigue, or with history of opportunistic infection), requiring additional monitoring (repeated blood counts, serum chemistry, vector DNA and transgene product titration, analysis of T-cell responses to AAV), as a possible role of the gene transfer in this course has not been definitely excluded (presence of neutralizing antibodies to the AAV capsid, occasional detection of vector genomes in the blood at the highest vector dose). Regarding OA, a phase I protocol is currently ongoing, based on an ex vivo approach using the retroviral transfer of TGF-β.312
Gene Doping
Although the previously discussed gene-based approaches may have potential value for the treatment of articular cartilage defects and meniscal lesions, some of the therapeutic genes used in these studies have been also implicated for gene doping,322 a term referring to the potential misuse of gene therapy for the purposes of enhancing athletic performance.323-325 Possible genes with such potential include, but are not limited to, growth hormone and IGF-I,326 erythropoietin (Epo),327 VEGF,328 FGF-2, and endorphins.329
IGF-I, the prime target of growth hormone action, is a potential candidate gene. A number of studies have shown that upregulation of IGF-I stimulates muscle growth and improves muscle function.326 Interestingly, this increase in muscle volume is not reflected by detectable increases in circulating IGF-I. While favorable responses have been obtained in animal studies, the transfer of such techniques to humans with the goal of a higher performance still presents many technical challenges.
The hormone Epo is produced by the peritubular capillary endothelial cells in the kidney. Under hypoxic conditions, Epo is produced and secreted, increasing the production of red blood cells. Eero Mäntyranta, a Finnish cross-country skier who won 2 gold medals in the 1964 Olympics, was born with a mutation in the Epo receptor gene that allowed his blood to carry significantly more oxygen than an average person.330 Recombinant Epo has been used already as a performance-enhancing drug. Because of differences in its peptide sequence compared with the endogenous protein, it may be detected in blood. Recently, a viral vector for the release of Epo in response to low oxygen concentrations has been developed under the trade name Repoxygen (Oxford BioMedica, Oxford, UK). The viral vector of undisclosed origin carries the human Epo gene under the control of a hypoxia control element (HRE). At low oxygen concentrations, HRE switches on the expression of the transgene. The vector is designed to be delivered by a simple intramuscular injection, resulting in the synthesis of recombinant Epo by muscle cells, rather than by cells of the liver or kidneys. Initially developed to treat anemia, there have been speculations in the media that it has been already applied for doping purposes.331
Recently, genetically engineered mice have been created with an alteration in energy metabolism based on overexpression of the gene for phosphoenolpyruvate carboxykinases (PEPCK-C). PEPCK-C is an enzyme of the lyase family that plays a role in the metabolic pathway of gluconeogenesis, converting oxaloacetate into phosphoenolpyruvate and carbon dioxide. These transgenic PEPCK-C mice carry a chimeric gene in which a copy of the cDNA for PEPCK-C is placed under control of the skeletal actin gene promoter, directing overexpression of PEPCK-C exclusively to skeletal muscle. PEPCK-C mice were more active, could run longer and faster, and used fatty acids more efficiently and produced far less lactate than control animals.332 Whether these data can be corroborated by studies in large animals remains to be determined.
Taken together, there is an emerging body of results from a number of transgenic and somatic gene transfer studies that suggest the principle of gene transfer may find application to enhance athletic performance. Many of the genes are already cloned in functional vectors, and some of them are being evaluated in clinical trials for the treatment of diseases. However, therapeutic gene transfer to humans is still technically challenging, and no clear evidence has been given that athletes have been using gene technology to enhance their performance. For antidoping authorities, the challenge will be to detect these endogenously produced gene products because of the homology between the transferred cDNA, the homology of the endogenously produced protein, and the limited specificity of indirect detection procedures.333 Further studies in this fíeld are needed since a possible uncontrolled use of these gene vectors imposes potential high risks for both the athlete and the general public.
Outlook
Despite these encouraging data, application of gene transfer approaches in the treatment of articular cartilage and meniscal lesion tears is still in its infancy. Although the use of gene therapy holds great promise, issues that need to be addressed include the duration of transgene expression, further studies in clinically relevant animal models of articular cartilage and meniscal lesions, the benefit of using ex vivo genetically modified cells versus direct gene transfer approaches, and the identification of (an) optimal therapeutic factor(s) for each particular clinical problem. Future studies will also have to shed light on the safety of these approaches regarding the nonlethal nature of these diseases. A successful application of gene therapy for cartilage repair requires the combined effort of orthopedic surgeons continuing to ask clinically relevant questions and of basic scientists further improving the currently available gene transfer systems.
Footnotes
Acknowledgments and Funding: The authors received no financial support for the research and/or authorship of this article.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- 1. Evans CH, Ghivizzani SC, Herndon JH, Robbins PD. Gene therapy for the treatment of musculoskeletal diseases. J Am Acad Orthop Surg. 2005;13(4):230-42. [DOI] [PubMed] [Google Scholar]
- 2. Nakajima A. Application of cellular gene therapy for rheumatoid arthritis. Mod Rheumatol. 2006;16(5):269-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Madry H, Kohn D, Cucchiarini M. [Gene therapy in orthopaedic surgery]. Orthopade. 2006;35(11):1193-202. [DOI] [PubMed] [Google Scholar]
- 4. Ivkovic A, Pascher A, Hudetz D, Jelic M, Haspl M, Windhager R, et al. Current concepts in gene therapy of the musculoskeletal system. Acta Chir Orthop Traumatol Cech. 2006;73(2):115-22. [PubMed] [Google Scholar]
- 5. Robbins PD, Evans CH, Chernajovsky Y. Gene therapy for arthritis. Gene Ther. 2003;10(10):902-11. [DOI] [PubMed] [Google Scholar]
- 6. Abramson SB, Amin A. Blocking the effects of IL-1 in rheumatoid arthritis protects bone and cartilage. Rheumatology (Oxford). 2002;41(9):972-80. [DOI] [PubMed] [Google Scholar]
- 7. Evans CH, Ghivizzani SC, Kang R, Muzzonigro T, Wasko MC, Herndon JH, et al. Gene therapy for rheumatic diseases. Arthritis Rheum. 1999;42(1):1-16. [DOI] [PubMed] [Google Scholar]
- 8. Vervoordeldonk MJ, Tak PP. Gene therapy in rheumatic diseases. Best Pract Res Clin Rheumatol. 2001;15(5):771-88. [DOI] [PubMed] [Google Scholar]
- 9. Burstein H. Gene therapy for rheumatoid arthritis. Curr Opin Mol Ther. 2001;3(4):362-74. [PubMed] [Google Scholar]
- 10. Felgner PL, Gadek TR, Holm M, Roman R, Chan HW, Wenz M, et al. Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Proc Natl Acad Sci U S A. 1987;84(21):7413-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schwendener RA. Liposomes in biology and medicine. Adv Exp Med Biol. 2007;620:117-28. [DOI] [PubMed] [Google Scholar]
- 12. Orth P, Weimer A, Kaul G, Kohn D, Cucchiarini M, Madry H. Analysis of novel nonviral gene transfer systems for gene delivery to cells of the musculoskeletal system. Mol Biotechnol. 2008;38(2):137-44. [DOI] [PubMed] [Google Scholar]
- 13. Hudde T, Rayner SA, Comer RM, Weber M, Isaacs JD, Waldmann H, et al. Activated polyamidoamine dendrimers, a non-viral vector for gene transfer to the corneal endothelium. Gene Ther. 1999;6(5):939-43. [DOI] [PubMed] [Google Scholar]
- 14. Godbey WT, Wu KK, Hirasaki GJ, Mikos AG. Improved packing of poly(ethylenimine)/DNA complexes increases transfection efficiency. Gene Ther. 1999;6(8):1380-8. [DOI] [PubMed] [Google Scholar]
- 15. Chemin I, Moradpour D, Wieland S, Offensperger WB, Walter E, Behr JP, et al. Liver-directed gene transfer: a linear polyethlenimine derivative mediates highly efficient DNA delivery to primary hepatocytes in vitro and in vivo. J Viral Hepat. 1998;5(6):369-75. [DOI] [PubMed] [Google Scholar]
- 16. Ravi Kumar M, Hellermann G, Lockey RF, Mohapatra SS. Nanoparticle-mediated gene delivery: state of the art. Expert Opin Biol Ther. 2004;4(8):1213-24. [DOI] [PubMed] [Google Scholar]
- 17. Graham FL, van der Eb AJ. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973;52(2):456-67. [DOI] [PubMed] [Google Scholar]
- 18. Frisbie DD, Ghivizzani SC, Robbins PD, Evans CH, McIlwraith CW. Treatment of experimental equine osteoarthritis by in vivo delivery of the equine interleukin-1 receptor antagonist gene. Gene Ther. 2002;9(1):12-20. [DOI] [PubMed] [Google Scholar]
- 19. Gelse K, von der Mark K, Aigner T, Park J, Schneider H. Articular cartilage repair by gene therapy using growth factor-producing mesenchymal cells. Arthritis Rheum. 2003;48(2):430-41. [DOI] [PubMed] [Google Scholar]
- 20. Ghivizzani SC, Lechman ER, Kang R, Tio C, Kolls J, Evans CH, et al. Direct adenovirus-mediated gene transfer of interleukin 1 and tumor necrosis factor alpha soluble receptors to rabbit knees with experimental arthritis has local and distal anti-arthritic effects. Proc Natl Acad Sci U S A. 1998;95(8):4613-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hidaka C, Goodrich LR, Chen CT, Warren RF, Crystal RG, Nixon AJ. Acceleration of cartilage repair by genetically modified chondrocytes over expressing bone morphogenetic protein-7. J Orthop Res. 2003;21(4):573-83. [DOI] [PubMed] [Google Scholar]
- 22. Park J, Gelse K, Frank S, von der Mark K, Aigner T, Schneider H. Transgene-activated mesenchymal cells for articular cartilage repair: a comparison of primary bone marrow-, perichondrium/periosteum- and fat-derived cells. J Gene Med. 2006;8(1):112-25. [DOI] [PubMed] [Google Scholar]
- 23. Evans CH, Robbins PD, Ghivizzani SC, Herndon JH, Kang R, Bahnson AB, et al. Clinical trial to assess the safety, feasibility, and efficacy of transferring a potentially anti-arthritic cytokine gene to human joints with rheumatoid arthritis. Hum Gene Ther. 1996;7(10):1261-80. [DOI] [PubMed] [Google Scholar]
- 24. Grande DA, Mason J, Light E, Dines D. Stem cells as platforms for delivery of genes to enhance cartilage repair. J Bone Joint Surg Am. 2003;85-A(Suppl 2):111-6. [DOI] [PubMed] [Google Scholar]
- 25. Mason JM, Breitbart AS, Barcia M, Porti D, Pergolizzi RG, Grande DA. Cartilage and bone regeneration using gene-enhanced tissue engineering. Clin Orthop Relat Res. 2000;(379 Suppl):S171-8. [DOI] [PubMed] [Google Scholar]
- 26. Murphy JM, Fink DJ, Hunziker EB, Barry FP. Stem cell therapy in a caprine model of osteoarthritis. Arthritis Rheum. 2003;48(12):3464-74. [DOI] [PubMed] [Google Scholar]
- 27. Lee KH, Song SU, Hwang TS, Yi Y, Oh IS, Lee JY, et al. Regeneration of hyaline cartilage by cell-mediated gene therapy using transforming growth factor beta 1-producing fibroblasts. Hum Gene Ther. 2001;12(14):1805-13. [DOI] [PubMed] [Google Scholar]
- 28. Gouze E, Pawliuk R, Gouze JN, Pilapil C, Fleet C, Palmer GD, et al. Lentiviral-mediated gene delivery to synovium: potent intra-articular expression with amplification by inflammation. Mol Ther. 2003;7(4):460-6. [DOI] [PubMed] [Google Scholar]
- 29. Gouze E, Pawliuk R, Pilapil C, Gouze JN, Fleet C, Palmer GD, et al. In vivo gene delivery to synovium by lentiviral vectors. Mol Ther. 2002;5(4):397-404. [DOI] [PubMed] [Google Scholar]
- 30. Evans CH, Ghivizzani SC, Robbins PD. The 2003 Nicolas Andry Award: orthopaedic gene therapy. Clin Orthop Relat Res. 2004;(429):316-29. [DOI] [PubMed] [Google Scholar]
- 31. Oligino T, Ghivizzani S, Wolfe D, Lechman E, Krisky D, Mi Z, et al. Intra-articular delivery of a herpes simplex virus IL-1Ra gene vector reduces inflammation in a rabbit model of arthritis. Gene Ther. 1999;6(10):1713-20. [DOI] [PubMed] [Google Scholar]
- 32. Berns KI, Linden RM. The cryptic life style of adeno-associated virus. Bioessays. 1995;17(3):237-45. [DOI] [PubMed] [Google Scholar]
- 33. Flotte TR, Afione SA, Conrad C, McGrath SA, Solow R, Oka H, et al. Stable in vivo expression of the cystic fibrosis transmembrane conductance regulator with an adeno-associated virus vector. Proc Natl Acad Sci U S A. 1993;90(22):10613-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pan RY, Chen SL, Xiao X, Liu DW, Peng HJ, Tsao YP. Therapy and prevention of arthritis by recombinant adeno-associated virus vector with delivery of interleukin-1 receptor antagonist. Arthritis Rheum. 2000;43(2):289-97. [DOI] [PubMed] [Google Scholar]
- 35. Watanabe S, Imagawa T, Boivin GP, Gao G, Wilson JM, Hirsch R. Adeno-associated virus mediates long-term gene transfer and delivery of chondroprotective IL-4 to murine synovium. Mol Ther. 2000;2(2):147-52. [DOI] [PubMed] [Google Scholar]
- 36. Cucchiarini M, Madry H, Ma C, Thurn T, Zurakowski D, Menger MD, et al. Improved tissue repair in articular cartilage defects in vivo by rAAV-mediated overexpression of human fibroblast growth factor 2. Mol Ther. 2005;12(2):229-38. [DOI] [PubMed] [Google Scholar]
- 37. Evans CH, Ghivizzani SC, Smith P, Shuler FD, Mi Z, Robbins PD. Using gene therapy to protect and restore cartilage. Clin Orthop Relat Res. 2000;(379 Suppl):S214-9. [DOI] [PubMed] [Google Scholar]
- 38. Pagnotto MR, Wang Z, Karpie JC, Ferretti M, Xiao X, Chu CR. Adeno-associated viral gene transfer of transforming growth factor-beta1 to human mesenchymal stem cells improves cartilage repair. Gene Ther. 2007;14(10):804-13. [DOI] [PubMed] [Google Scholar]
- 39. Mease PJ, Wei N, Fudman EJ, Kivitz AJ, Schechtman J, Trapp RG, et al. Safety, tolerability, and clinical outcomes after intraarticular injection of a recombinant adeno-associated vector containing a tumor necrosis factor antagonist gene: results of a phase 1/2 study. J Rheumatol. 2010;37(4):692-703. [DOI] [PubMed] [Google Scholar]
- 40. O’Driscoll SW. The healing and regeneration of articular cartilage. J Bone Joint Surg Am. 1998;80(12):1795-812. [PubMed] [Google Scholar]
- 41. Hunziker EB, Michel M, Studer D. Ultrastructure of adult human articular cartilage matrix after cryotechnical processing. Microsc Res Tech. 1997;37(4):271-84. [DOI] [PubMed] [Google Scholar]
- 42. Madry H, van Dijk CN, Mueller-Gerbl M. The basic science of the subchondral bone. Knee Surg Sports Traumatol Arthrosc. 2010;18(4):419-33. [DOI] [PubMed] [Google Scholar]
- 43. Pape D, Filardo G, Kon E, van Dijk CN, Madry H. Disease-specific clinical problems associated with the subchondral bone. Knee Surg Sports Traumatol Arthrosc. 2010;18(4):448-62. [DOI] [PubMed] [Google Scholar]
- 44. Noyes FR, Stabler CL. A system for grading articular cartilage lesions at arthroscopy. Am J Sports Med. 1989;17(4):505-13. [DOI] [PubMed] [Google Scholar]
- 45. Hunziker EB. Articular cartilage repair: basic science and clinical progress. A review of the current status and prospects. Osteoarthritis Cartilage. 2002;10(6):432-63. [DOI] [PubMed] [Google Scholar]
- 46. Furukawa T, Eyre DR, Koide S, Glimcher MJ. Biochemical studies on repair cartilage resurfacing experimental defects in the rabbit knee. J Bone Joint Surg Am. 1980;62(1):79-89. [PubMed] [Google Scholar]
- 47. Shapiro F, Koide S, Glimcher MJ. Cell origin and differentiation in the repair of full-thickness defects of articular cartilage. J Bone Joint Surg Am. 1993;75(4):532-53. [DOI] [PubMed] [Google Scholar]
- 48. Jackson DW, Lalor PA, Aberman HM, Simon TM. Spontaneous repair of full-thickness defects of articular cartilage in a goat model: a preliminary study. J Bone Joint Surg Am. 2001;83-A(1):53-64. [DOI] [PubMed] [Google Scholar]
- 49. Rogachefsky RA, Dean DD, Howell DS, Altman RD. Treatment of canine osteoarthritis with insulin-like growth factor-1 (IGF-1) and sodium pentosan polysulfate. Osteoarthritis Cartilage. 1993;1(2):105-14. [DOI] [PubMed] [Google Scholar]
- 50. Shida J, Jingushi S, Izumi T, Iwaki A, Sugioka Y. Basic fibroblast growth factor stimulates articular cartilage enlargement in young rats in vivo. J Orthop Res. 1996;14(2):265-72. [DOI] [PubMed] [Google Scholar]
- 51. Sellers RS, Peluso D, Morris EA. The effect of recombinant human bone morphogenetic protein-2 (rhBMP-2) on the healing of full-thickness defects of articular cartilage. J Bone Joint Surg Am. 1997;79(10):1452-63. [DOI] [PubMed] [Google Scholar]
- 52. Joyce ME, Roberts AB, Sporn MB, Bolander ME. Transforming growth factor-beta and the initiation of chondrogenesis and osteogenesis in the rat femur. J Cell Biol. 1990;110(6):2195-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hanada K, Solchaga LA, Caplan AI, Hering TM, Goldberg VM, Yoo JU, et al. BMP-2 induction and TGF-beta 1 modulation of rat periosteal cell chondrogenesis. J Cell Biochem. 2001;81(2):284-94. [DOI] [PubMed] [Google Scholar]
- 54. Asahina I, Sampath TK, Hauschka PV. Human osteogenic protein-1 induces chondroblastic, osteoblastic, and/or adipocytic differentiation of clonal murine target cells. Exp Cell Res. 1996;222(1):38-47. [DOI] [PubMed] [Google Scholar]
- 55. Klein-Nulend J, Louwerse RT, Heyligers IC, Wuisman PI, Semeins CM, Goei SW, et al. Osteogenic protein (OP-1, BMP-7) stimulates cartilage differentiation of human and goat perichondrium tissue in vitro. J Biomed Mater Res. 1998;40(4):614-20. [DOI] [PubMed] [Google Scholar]
- 56. Jentzsch KD, Wellmitz G, Heder G, Petzold E, Buntrock P, Oehme P. A bovine brain fraction with fibroblast growth factor activity inducing articular cartilage regeneration in vivo. Acta Biol Med Ger. 1980;39(8-9):967-71. [PubMed] [Google Scholar]
- 57. Hotten GC, Matsumoto T, Kimura M, Bechtold RF, Kron R, Ohara T, et al. Recombinant human growth/differentiation factor 5 stimulates mesenchyme aggregation and chondrogenesis responsible for the skeletal development of limbs. Growth Factors. 1996;13(1-2):65-74. [DOI] [PubMed] [Google Scholar]
- 58. Vortkamp A, Lee K, Lanske B, Segre GV, Kronenberg HM, Tabin CJ. Regulation of rate of cartilage differentiation by Indian hedgehog and PTH-related protein. Science. 1996;273(5275):613-22. [DOI] [PubMed] [Google Scholar]
- 59. Amizuka N, Warshawsky H, Henderson JE, Goltzman D, Karaplis AC. Parathyroid hormone-related peptide-depleted mice show abnormal epiphyseal cartilage development and altered endochondral bone formation. J Cell Biol. 1994;126(6):1611-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Trippel SB, Wroblewski J, Makower AM, Whelan MC, Schoenfeld D, Doctrow SR. Regulation of growth-plate chondrocytes by insulin-like growth-factor I and basic fibroblast growth factor. J Bone Joint Surg Am. 1993;75(2):177-89. [DOI] [PubMed] [Google Scholar]
- 61. Fujimoto E, Ochi M, Kato Y, Mochizuki Y, Sumen Y, Ikuta Y. Beneficial effect of basic fibroblast growth factor on the repair of full-thickness defects in rabbit articular cartilage. Arch Orthop Trauma Surg. 1999;119(3-4):139-45. [DOI] [PubMed] [Google Scholar]
- 62. Trippel SB. Growth factor actions on articular cartilage. J Rheumatol Suppl. 1995;43:129-32. [PubMed] [Google Scholar]
- 63. Nixon AJ, Fortier LA, Williams J, Mohammed H. Enhanced repair of extensive articular defects by insulin-like growth factor-I-laden fibrin composites. J Orthop Res. 1999;17(4):475-87. [DOI] [PubMed] [Google Scholar]
- 64. Trippel SB, Van Wyk JJ, Foster MB, Svoboda ME. Characterization of a specific somatomedin-c receptor on isolated bovine growth plate chondrocytes. Endocrinology. 1983;112(6):2128-36. [DOI] [PubMed] [Google Scholar]
- 65. Erlacher L, Ng CK, Ullrich R, Krieger S, Luyten FP. Presence of cartilage-derived morphogenetic proteins in articular cartilage and enhancement of matrix replacement in vitro. Arthritis Rheum. 1998;41(2):263-73. [DOI] [PubMed] [Google Scholar]
- 66. Katayama R, Wakitani S, Tsumaki N, Morita Y, Matsushita I, Gejo R, et al. Repair of articular cartilage defects in rabbits using CDMP1 gene-transfected autologous mesenchymal cells derived from bone marrow. Rheumatology (Oxford). 2004;43(8):980-5. [DOI] [PubMed] [Google Scholar]
- 67. Bi W, Deng JM, Zhang Z, Behringer RR, de Crombrugghe B. Sox9 is required for cartilage formation. Nat Genet. 1999;22(1):85-9. [DOI] [PubMed] [Google Scholar]
- 68. Inada M, Yasui T, Nomura S, Miyake S, Deguchi K, Himeno M, et al. Maturational disturbance of chondrocytes in Cbfa1-deficient mice. Dev Dyn. 1999;214(4):279-90. [DOI] [PubMed] [Google Scholar]
- 69. Zhao GQ, Eberspaecher H, Seldin MF, de Crombrugghe B. The gene for the homeodomain-containing protein Cart-1 is expressed in cells that have a chondrogenic potential during embryonic development. Mech Dev. 1994;48(3):245-54. [DOI] [PubMed] [Google Scholar]
- 70. Sumarsono SH, Wilson TJ, Tymms MJ, Venter DJ, Corrick CM, Kola R, et al. Down’s syndrome-like skeletal abnormalities in Ets2 transgenic mice. Nature. 1996;379(6565):534-7. [DOI] [PubMed] [Google Scholar]
- 71. Mackie EJ. Tenascin in connective tissue development and pathogenesis. Perspect Dev Neurobiol. 1994;2(1):125-32. [PubMed] [Google Scholar]
- 72. Lanske B, Karaplis AC, Lee K, Luz A, Vortkamp A, Pirro A, et al. PTH/PTHrP receptor in early development and Indian hedgehog-regulated bone growth. Science. 1996;273(5275):663-6. [DOI] [PubMed] [Google Scholar]
- 73. Goldring MB, Fukuo K, Birkhead JR, Dudek E, Sandell LJ. Transcriptional suppression by interleukin-1 and interferon-gamma of type II collagen gene expression in human chondrocytes. J Cell Biochem. 1994;54(1):85-99. [DOI] [PubMed] [Google Scholar]
- 74. Shalom-Barak T, Quach J, Lotz M. Interleukin-17-induced gene expression in articular chondrocytes is associated with activation of mitogen-activated protein kinases and NF-kappaB. J Biol Chem. 1998;273(42):27467-73. [DOI] [PubMed] [Google Scholar]
- 75. Stadler J, Stefanovic-Racic M, Billiar TR, Curran RD, McIntyre LA, Georgescu HI, et al. Articular chondrocytes synthesize nitric oxide in response to cytokines and lipopolysaccharide. J Immunol. 1991;147(11):3915-20. [PubMed] [Google Scholar]
- 76. Pelletier JP, Martel-Pelletier J. [Role of synovial inflammation cytokines and IGF-1 in the physiopathology of osteoarthritis]. Rev Rhum Ed Fr. 1994;61(9 Pt 2):103S-8S. [PubMed] [Google Scholar]
- 77. Blanco FJ, Ochs RL, Schwarz H, Lotz M. Chondrocyte apoptosis induced by nitric oxide. Am J Pathol. 1995;146(1): 75-85. [PMC free article] [PubMed] [Google Scholar]
- 78. Di Cesare PE, Frenkel SR, Carlson CS, Fang C, Liu C. Regional gene therapy for full-thickness articular cartilage lesions using naked DNA with a collagen matrix. J Orthop Res. 2006;24(5):1118-27. [DOI] [PubMed] [Google Scholar]
- 79. Nita I, Ghivizzani SC, Galea-Lauri J, Bandara G, Georgescu HI, Robbins PD, et al. Direct gene delivery to synovium: an evaluation of potential vectors in vitro and in vivo. Arthritis Rheum. 1996;39(5):820-8. [DOI] [PubMed] [Google Scholar]
- 80. Ikeda T, Kubo T, Arai Y, Nakanishi T, Kobayashi K, Takahashi K, et al. Adenovirus mediated gene delivery to the joints of guinea pigs. J Rheumatol. 1998;25(9):1666-73. [PubMed] [Google Scholar]
- 81. Ghivizzani SC, Lechman ER, Tio C, Mule KM, Chada S, McCormack JE, et al. Direct retrovirus-mediated gene transfer to the synovium of the rabbit knee: implications for arthritis gene therapy. Gene Ther. 1997;4(9):977-82. [DOI] [PubMed] [Google Scholar]
- 82. Wehling P, Reinecke J, Baltzer AW, Granrath M, Schulitz KP, Schultz C, et al. Clinical responses to gene therapy in joints of two subjects with rheumatoid arthritis. Hum Gene Ther. 2009;20(2):97-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Pan RY, Xiao X, Chen SL, Li J, Lin LC, Wang HJ, et al. Disease-inducible transgene expression from a recombinant adeno-associated virus vector in a rat arthritis model. J Virol. 1999;73(4):3410-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Watanabe S, Kim KN, Imagawa T, Thornton S, Grom A, Hirsch R. On the mechanism of protection of distal joints after local gene transfer in collagen-induced arthritis. Hum Gene Ther. 2000;11(5):751-8. [DOI] [PubMed] [Google Scholar]
- 85. Tomita T, Hashimoto H, Tomita N, Morishita R, Lee SB, Hayashida K, et al. In vivo direct gene transfer into articular cartilage by intraarticular injection mediated by HVJ (Sendai virus) and liposomes. Arthritis Rheum. 1997;40(5):901-6. [DOI] [PubMed] [Google Scholar]
- 86. Morisset S, Frisbie DD, Robbins PD, Nixon AJ, McIlwraith CW. IL-1ra/IGF-1 gene therapy modulates repair of microfractured chondral defects. Clin Orthop Relat Res. 2007;462:221-8. [DOI] [PubMed] [Google Scholar]
- 87. Madry H, Cucchiarini M, Terwilliger EF, Trippel SB. Recombinant adeno-associated virus vectors efficiently and persistently transduce chondrocytes in normal and osteoarthritic human articular cartilage. Hum Gene Ther. 2003;14(4):393-402. [DOI] [PubMed] [Google Scholar]
- 88. Coutts RD, Healey RM, Ostrander R, Sah RL, Goomer R, Amiel D. Matrices for cartilage repair. Clin Orthop Relat Res. 2001;(391 Suppl):S271-9. [DOI] [PubMed] [Google Scholar]
- 89. Pascher A, Palmer GD, Steinert A, Oligino T, Gouze E, Gouze JN, et al. Gene delivery to cartilage defects using coagulated bone marrow aspirate. Gene Ther. 2004;11(2):133-41. [DOI] [PubMed] [Google Scholar]
- 90. Kaul G, Cucchiarini M, Arntzen D, Zurakowski D, Menger MD, Kohn D, et al. Local stimulation of articular cartilage repair by transplantation of encapsulated chondrocytes overexpressing human fibroblast growth factor 2 (FGF-2) in vivo. J Gene Med. 2006;8(1):100-11. [DOI] [PubMed] [Google Scholar]
- 91. Madry H, Kaul G, Cucchiarini M, Stein U, Zurakowski D, Remberger K, et al. Enhanced repair of articular cartilage defects in vivo by transplanted chondrocytes overexpressing insulin-like growth factor I (IGF-I). Gene Ther. 2005;12(15):1171-9. [DOI] [PubMed] [Google Scholar]
- 92. Fragonas E, Valente M, Pozzi-Mucelli M, Toffanin R, Rizzo R, Silvestri F, et al. Articular cartilage repair in rabbits by using suspensions of allogenic chondrocytes in alginate. Biomaterials. 2000;21(8):795-801. [DOI] [PubMed] [Google Scholar]
- 93. Rahfoth B, Weisser J, Sternkopf F, Aigner T, von der Mark K, Brauer R. Transplantation of allograft chondrocytes embedded in agarose gel into cartilage defects of rabbits. Osteoarthritis Cartilage. 1998;6(1):50-65. [DOI] [PubMed] [Google Scholar]
- 94. Hunter CJ, Levenston ME. Maturation and integration of tissue-engineered cartilages within an in vitro defect repair model. Tissue Eng. 2004;10(5-6):736-46. [DOI] [PubMed] [Google Scholar]
- 95. Kawamura S, Wakitani S, Kimura T, Maeda A, Caplan AI, Shino K, et al. Articular cartilage repair: rabbit experiments with a collagen gel-biomatrix and chondrocytes cultured in it. Acta Orthop Scand. 1998;69(1):56-62. [DOI] [PubMed] [Google Scholar]
- 96. Ikeda T, Kubo T, Nakanishi T, Arai Y, Kobayashi K, Mazda O, et al. Ex vivo gene delivery using an adenovirus vector in treatment for cartilage defects. J Rheumatol. 2000;27(4):990-6. [PubMed] [Google Scholar]
- 97. Perka C, Schultz O, Spitzer RS, Lindenhayn K. The influence of transforming growth factor beta1 on mesenchymal cell repair of full-thickness cartilage defects. J Biomed Mater Res. 2000;52(3):543-52. [DOI] [PubMed] [Google Scholar]
- 98. Driesang IM, Hunziker EB. Delamination rates of tissue flaps used in articular cartilage repair. J Orthop Res. 2000;18(6): 909-11. [DOI] [PubMed] [Google Scholar]
- 99. Yokoo N, Saito T, Uesugi M, Kobayashi N, Xin KQ, Okuda K, et al. Repair of articular cartilage defect by autologous transplantation of basic fibroblast growth factor gene-transduced chondrocytes with adeno-associated virus vector. Arthritis Rheum. 2005;52(1):164-70. [DOI] [PubMed] [Google Scholar]
- 100. Freed LE, Grande DA, Lingbin Z, Emmanual J, Marquis JC, Langer R. Joint resurfacing using allograft chondrocytes and synthetic biodegradable polymer scaffolds. J Biomed Mater Res. 1994;28(8):891-9. [DOI] [PubMed] [Google Scholar]
- 101. Perka C, Sittinger M, Schultz O, Spitzer RS, Schlenzka D, Burmester GR. Tissue engineered cartilage repair using cryopreserved and noncryopreserved chondrocytes. Clin Orthop Relat Res. 2000;(378):245-54. [DOI] [PubMed] [Google Scholar]
- 102. Schaefer D, Martin I, Jundt G, Seidel J, Heberer M, Grodzinsky A, et al. Tissue-engineered composites for the repair of large osteochondral defects. Arthritis Rheum. 2002;46(9):2524-34. [DOI] [PubMed] [Google Scholar]
- 103. Kang R, Marui T, Ghivizzani SC, Nita IM, Georgescu HI, Suh JK, et al. Ex vivo gene transfer to chondrocytes in full-thickness articular cartilage defects: a feasibility study. Osteoarthritis Cartilage. 1997;5(2):139-43. [DOI] [PubMed] [Google Scholar]
- 104. Madry H, Cucchiarini M, Stein U, Remberger K, Menger MD, Kohn D, et al. Sustained transgene expression in cartilage defects in vivo after transplantation of articular chondrocytes modified by lipid-mediated gene transfer in a gel suspension delivery system. J Gene Med. 2003;5(6):502-9. [DOI] [PubMed] [Google Scholar]
- 105. Goomer RS, Deftos LJ, Terkeltaub R, Maris T, Lee MC, Harwood FL, et al. High-efficiency non-viral transfection of primary chondrocytes and perichondrial cells for ex-vivo gene therapy to repair articular cartilage defects. Osteoarthritis Cartilage. 2001;9(3):248-56. [DOI] [PubMed] [Google Scholar]
- 106. Ueblacker P, Wagner B, Kruger A, Vogt S, DeSantis G, Kennerknecht E, et al. Inducible nonviral gene expression in the treatment of osteochondral defects. Osteoarthritis Cartilage. 2004;12(9):711-9. [DOI] [PubMed] [Google Scholar]
- 107. Baragi VM, Renkiewicz RR, Jordan H, Bonadio J, Hartman JW, Roessler BJ. Transplantation of transduced chondrocytes protects articular cartilage from interleukin 1-induced extracellular matrix degradation. J Clin Invest. 1995;96(5):2454-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Mason JM, Grande DA, Barcia M, Grant R, Pergolizzi RG, Breitbart AS. Expression of human bone morphogenic protein 7 in primary rabbit periosteal cells: potential utility in gene therapy for osteochondral repair. Gene Ther. 1998;5(8):1098-104. [DOI] [PubMed] [Google Scholar]
- 109. Adachi N, Sato K, Usas A, Fu FH, Ochi M, Han CW, et al. Muscle derived, cell based ex vivo gene therapy for treatment of full thickness articular cartilage defects. J Rheumatol. 2002;29(9):1920-30. [PubMed] [Google Scholar]
- 110. Hirschmann F, Verhoeyen E, Wirth D, Bauwens S, Hauser H, Rudert M. Vital marking of articular chondrocytes by retroviral infection using green fluorescence protein. Osteoarthritis Cartilage. 2002;10(2):109-18. [DOI] [PubMed] [Google Scholar]
- 111. Mierisch CM, Wilson HA, Turner MA, Milbrandt TA, Berthoux L, Hammarskjold ML, et al. Chondrocyte transplantation into articular cartilage defects with use of calcium alginate: the fate of the cells. J Bone Joint Surg Am. 2003;85-A(9):1757-67. [DOI] [PubMed] [Google Scholar]
- 112. Kobayashi N, Koshino T, Uesugi M, Yokoo N, Xin KQ, Okuda K, et al. Gene marking in adeno-associated virus vector infected periosteum derived cells for cartilage repair. J Rheumatol. 2002;29(10):2176-80. [PubMed] [Google Scholar]
- 113. Baragi VM, Renkiewicz RR, Qiu L, Brammer D, Riley JM, Sigler RE, et al. Transplantation of adenovirally transduced allogeneic chondrocytes into articular cartilage defects in vivo. Osteoarthritis Cartilage. 1997;5(4):275-82. [DOI] [PubMed] [Google Scholar]
- 114. Madry H, Orth P, Kaul G, Zurakowski D, Menger MD, Kohn D, et al. Acceleration of articular cartilage repair by combined gene transfer of human insulin-like growth factor I and fibroblast growth factor-2 in vivo. Arch Orthop Trauma Surg. 2010;130(10):1311-22. [DOI] [PubMed] [Google Scholar]
- 115. Turgeman G, Pittman DD, Muller R, Kurkalli BG, Zhou S, Pelled G, et al. Engineered human mesenchymal stem cells: a novel platform for skeletal cell mediated gene therapy. J Gene Med. 2001;3(3):240-51. [DOI] [PubMed] [Google Scholar]
- 116. Cucchiarini M, Madry H. Gene therapy for cartilage defects. J Gene Med. 2005;7(12):1495-509. [DOI] [PubMed] [Google Scholar]
- 117. Che JH, Zhang ZR, Li GZ, Tan WH, Bai XD, Qu FJ. Application of tissue-engineered cartilage with BMP-7 gene to repair knee joint cartilage injury in rabbits. Knee Surg Sports Traumatol Arthrosc. 2010;18(4):496-503. [DOI] [PubMed] [Google Scholar]
- 118. Gelse K, Muhle C, Franke O, Park J, Jehle M, Durst K, et al. Cell-based resurfacing of large cartilage defects: long-term evaluation of grafts from autologous transgene-activated periosteal cells in a porcine model of osteoarthritis. Arthritis Rheum. 2008;58(2):475-88. [DOI] [PubMed] [Google Scholar]
- 119. Gysin R, Wergedal JE, Sheng MH, Kasukawa Y, Miyakoshi N, Chen ST, et al. Ex vivo gene therapy with stromal cells transduced with a retroviral vector containing the BMP4 gene completely heals critical size calvarial defect in rats. Gene Ther. 2002;9(15):991-9. [DOI] [PubMed] [Google Scholar]
- 120. Vogt S, Wexel G, Tischer T, Schillinger U, Ueblacker P, Wagner B, et al. The influence of the stable expression of BMP2 in fibrin clots on the remodelling and repair of osteochondral defects. Biomaterials. 2009;30(12):2385-92. [DOI] [PubMed] [Google Scholar]
- 121. Goodrich LR, Hidaka C, Robbins PD, Evans CH, Nixon AJ. Genetic modification of chondrocytes with insulin-like growth factor-1 enhances cartilage healing in an equine model. J Bone Joint Surg Br. 2007;89(5):672-85. [DOI] [PubMed] [Google Scholar]
- 122. Kuroda R, Usas A, Kubo S, Corsi K, Peng H, Rose T, et al. Cartilage repair using bone morphogenetic protein 4 and muscle-derived stem cells. Arthritis Rheum. 2006;54(2):433-42. [DOI] [PubMed] [Google Scholar]
- 123. Guo X, Zheng Q, Yang S, Shao Z, Yuan Q, Pan Z, et al. Repair of full-thickness articular cartilage defects by cultured mesenchymal stem cells transfected with the transforming growth factor beta1 gene. Biomed Mater. 2006;1(4):206-15. [DOI] [PubMed] [Google Scholar]
- 124. Evans CH, Liu FJ, Glatt V, Hoyland JA, Kirker-Head C, Walsh A, et al. Use of genetically modified muscle and fat grafts to repair defects in bone and cartilage. Eur Cell Mater. 2009;18:96-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Ivkovic A, Pascher A, Hudetz D, Maticic D, Jelic M, Dickinson S, et al. Articular cartilage repair by genetically modified bone marrow aspirate in sheep. Gene Ther. 2010;17(6):779-89. [DOI] [PubMed] [Google Scholar]
- 126. Gerich TG, Lobenhoffer HP, Fu FH, Robbins PD, Evans CH. [Virally mediated gene transfer in the patellar tendon: an experimental study in rabbits]. Unfallchirurg. 1997;100(5):354-62. [DOI] [PubMed] [Google Scholar]
- 127. Madry H, Zurakowski D, Trippel SB. Overexpression of human insulin-like growth factor-I promotes new tissue formation in an ex vivo model of articular chondrocyte transplantation. Gene Ther. 2001;8(19):1443-9. [DOI] [PubMed] [Google Scholar]
- 128. Goater JJ, O’Keefe RJ, Rosier RN, Puzas JE, Schwarz EM. Efficacy of ex vivo OPG gene therapy in preventing wear debris induced osteolysis. J Orthop Res. 2002;20(2):169-73. [DOI] [PubMed] [Google Scholar]
- 129. Taniyama Y, Tachibana K, Hiraoka K, Aoki M, Yamamoto S, Matsumoto K, et al. Development of safe and efficient novel nonviral gene transfer using ultrasound: enhancement of transfection efficiency of naked plasmid DNA in skeletal muscle. Gene Ther. 2002;9(6):372-80. [DOI] [PubMed] [Google Scholar]
- 130. Tsuchiya H, Kitoh H, Sugiura F, Ishiguro N. Chondrogenesis enhanced by overexpression of sox9 gene in mouse bone marrow-derived mesenchymal stem cells. Biochem Biophys Res Commun. 2003;301(2):338-43. [DOI] [PubMed] [Google Scholar]
- 131. Madry H, Emkey G, Zurakowski D, Trippel SB. Overexpression of human fibroblast growth factor 2 stimulates cell proliferation in an ex vivo model of articular chondrocyte transplantation. J Gene Med. 2004;6(2):238-45. [DOI] [PubMed] [Google Scholar]
- 132. Grossin L, Cournil-Henrionnet C, Pinzano A, Gaborit N, Dumas D, Etienne S, et al. Gene transfer with HSP 70 in rat chondrocytes confers cytoprotection in vitro and during experimental osteoarthritis. FASEB J. 2006;20(1):65-75. [DOI] [PubMed] [Google Scholar]
- 133. Yeh LC, Lee JC. Co-transfection with the osteogenic protein (OP)-1 gene and the insulin-like growth factor (IGF)-I gene enhanced osteoblastic cell differentiation. Biochim Biophys Acta. 2006;1763(1):57-63. [DOI] [PubMed] [Google Scholar]
- 134. Zhang HN, Leng P, Wang YZ, Zhang J. Treating human meniscal fibrochondrocytes with hIGF-1 gene by liposome. Clin Orthop Relat Res. 2009;467(12):3175-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Manning K, Rachakonda PS, Rai MF, Schmidt MF. Co-expression of insulin-like growth factor-1 and interleukin-4 in an in vitro inflammatory model. Cytokine. 2010;50(3):297-305. [DOI] [PubMed] [Google Scholar]
- 136. Li Y, Tew SR, Russell AM, Gonzalez KR, Hardingham TE, Hawkins RE. Transduction of passaged human articular chondrocytes with adenoviral, retroviral, and lentiviral vectors and the effects of enhanced expression of SOX9. Tissue Eng. 2004;10(3-4):575-84. [DOI] [PubMed] [Google Scholar]
- 137. Attur MG, Dave MN, Leung MY, Cipolletta C, Meseck M, Woo SL, et al. Functional genomic analysis of type II IL-1beta decoy receptor: potential for gene therapy in human arthritis and inflammation. J Immunol. 2002;168(4):2001-10. [DOI] [PubMed] [Google Scholar]
- 138. Smith P, Shuler FD, Georgescu HI, Ghivizzani SC, Johnstone B, Niyibizi C, et al. Genetic enhancement of matrix synthesis by articular chondrocytes: comparison of different growth factor genes in the presence and absence of interleukin-1. Arthritis Rheum. 2000;43(5):1156-64. [DOI] [PubMed] [Google Scholar]
- 139. Goto H, Shuler FD, Lamsam C, Moller HD, Niyibizi C, Fu FH, et al. Transfer of lacZ marker gene to the meniscus. J Bone Joint Surg Am. 1999;81(7):918-25. [DOI] [PubMed] [Google Scholar]
- 140. Gerich TG, Kang R, Fu FH, Robbins PD, Evans CH. Gene transfer to the rabbit patellar tendon: potential for genetic enhancement of tendon and ligament healing. Gene Ther. 1996;3(12):1089-93. [PubMed] [Google Scholar]
- 141. Lou J, Kubota H, Hotokezaka S, Ludwig FJ, Manske PR. In vivo gene transfer and overexpression of focal adhesion kinase (pp125 FAK) mediated by recombinant adenovirus-induced tendon adhesion formation and epitenon cell change. J Orthop Res. 1997;15(6):911-8. [DOI] [PubMed] [Google Scholar]
- 142. Mehrara BJ, Saadeh PB, Steinbrech DS, Dudziak M, Spector JA, Greenwald JA, et al. Adenovirus-mediated gene therapy of osteoblasts in vitro and in vivo. J Bone Miner Res. 1999;14(8):1290-301. [DOI] [PubMed] [Google Scholar]
- 143. Nixon AJ, Brower-Toland BD, Bent SJ, Saxer RA, Wilke MJ, Robbins PD, et al. Insulinlike growth factor-I gene therapy applications for cartilage repair. Clin Orthop Relat Res. 2000;(379 Suppl):S201-13. [DOI] [PubMed] [Google Scholar]
- 144. Shuler FD, Georgescu HI, Niyibizi C, Studer RK, Mi Z, Johnstone B, et al. Increased matrix synthesis following adenoviral transfer of a transforming growth factor beta1 gene into articular chondrocytes. J Orthop Res. 2000;18(4):585-92. [DOI] [PubMed] [Google Scholar]
- 145. Brower-Toland BD, Saxer RA, Goodrich LR, Mi Z, Robbins PD, Evans CH, et al. Direct adenovirus-mediated insulin-like growth factor I gene transfer enhances transplant chondrocyte function. Hum Gene Ther. 2001;12(2):117-29. [DOI] [PubMed] [Google Scholar]
- 146. Gelse K, Jiang QJ, Aigner T, Ritter T, Wagner K, Poschl E, et al. Fibroblast-mediated delivery of growth factor complementary DNA into mouse joints induces chondrogenesis but avoids the disadvantages of direct viral gene transfer. Arthritis Rheum. 2001;44(8):1943-53. [DOI] [PubMed] [Google Scholar]
- 147. Saxer RA, Bent SJ, Brower-Toland BD, Mi Z, Robbins PD, Evans CH, et al. Gene mediated insulin-like growth factor-I delivery to the synovium. J Orthop Res. 2001;19(5):759-67. [DOI] [PubMed] [Google Scholar]
- 148. Musgrave DS, Pruchnic R, Bosch P, Ziran BH, Whalen J, Huard J. Human skeletal muscle cells in ex vivo gene therapy to deliver bone morphogenetic protein-2. J Bone Joint Surg Br. 2002;84(1):120-7. [DOI] [PubMed] [Google Scholar]
- 149. Ikeda T, Kamekura S, Mabuchi A, Kou I, Seki S, Takato T, et al. The combination of SOX5, SOX6, and SOX9 (the SOX trio) provides signals sufficient for induction of permanent cartilage. Arthritis Rheum. 2004;50(11):3561-73. [DOI] [PubMed] [Google Scholar]
- 150. Haupt JL, Frisbie DD, McIlwraith CW, Robbins PD, Ghivizzani S, Evans CH, et al. Dual transduction of insulin-like growth factor-I and interleukin-1 receptor antagonist protein controls cartilage degradation in an osteoarthritic culture model. J Orthop Res. 2005;23(1):118-26. [DOI] [PubMed] [Google Scholar]
- 151. Nixon AJ, Haupt JL, Frisbie DD, Morisset SS, McIlwraith CW, Robbins PD, et al. Gene-mediated restoration of cartilage matrix by combination insulin-like growth factor-I/interleukin-1 receptor antagonist therapy. Gene Ther. 2005;12(2):177-86. [DOI] [PubMed] [Google Scholar]
- 152. Steinert AF, Palmer GD, Capito R, Hofstaetter JG, Pilapil C, Ghivizzani SC, et al. Genetically enhanced engineering of meniscus tissue using ex vivo delivery of transforming growth factor-beta 1 complementary deoxyribonucleic acid. Tissue Eng. 2007;13(9):2227-37. [DOI] [PubMed] [Google Scholar]
- 153. Steinert AF, Weber M, Kunz M, Palmer GD, Noth U, Evans CH, et al. In situ IGF-1 gene delivery to cells emerging from the injured anterior cruciate ligament. Biomaterials. 2008;29(7):904-16. [DOI] [PubMed] [Google Scholar]
- 154. Steinert AF, Proffen B, Kunz M, Hendrich C, Ghivizzani SC, Noth U, et al. Hypertrophy is induced during the in vitro chondrogenic differentiation of human mesenchymal stem cells by bone morphogenetic protein-2 and bone morphogenetic protein-4 gene transfer. Arthritis Res Ther. 2009;11(5):R148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Roessler BJ, Hartman JW, Vallance DK, Latta JM, Janich SL, Davidson BL. Inhibition of interleukin-1-induced effects in synoviocytes transduced with the human IL-1 receptor antagonist cDNA using an adenoviral vector. Hum Gene Ther. 1995;6(3):307-16. [DOI] [PubMed] [Google Scholar]
- 156. Baltzer AW, Whalen JD, Muzzonegro T, Georgescu HI, Robbins PD, Evans CH. [In vitro transduction of human osteoblast cell populations with retroviral vectors]. Z Rheumatol. 1999;58(2):88-94. [DOI] [PubMed] [Google Scholar]
- 157. Hildebrand KA, Deie M, Allen CR, Smith DW, Georgescu HI, Evans CH, et al. Early expression of marker genes in the rabbit medial collateral and anterior cruciate ligaments: the use of different viral vectors and the effects of injury. J Orthop Res. 1999;17(1):37-42. [DOI] [PubMed] [Google Scholar]
- 158. Goto H, Shuler FD, Niyibizi C, Fu FH, Robbins PD, Evans CH. Gene therapy for meniscal injury: enhanced synthesis of proteoglycan and collagen by meniscal cells transduced with a TGFbeta(1)gene. Osteoarthritis Cartilage. 2000;8(4):266-71. [DOI] [PubMed] [Google Scholar]
- 159. Tew SR, Li Y, Pothacharoen P, Tweats LM, Hawkins RE, Hardingham TE. Retroviral transduction with SOX9 enhances re-expression of the chondrocyte phenotype in passaged osteoarthritic human articular chondrocytes. Osteoarthritis Cartilage. 2005;13(1):80-9. [DOI] [PubMed] [Google Scholar]
- 160. Jennings K, Miyamae T, Traister R, Marinov A, Katakura S, Sowders D, et al. Proteasome inhibition enhances AAV-mediated transgene expression in human synoviocytes in vitro and in vivo. Mol Ther. 2005;11(4):600-7. [DOI] [PubMed] [Google Scholar]
- 161. Goater J, Muller R, Kollias G, Firestein GS, Sanz I, O’Keefe RJ, et al. Empirical advantages of adeno associated viral vectors in vivo gene therapy for arthritis. J Rheumatol. 2000;27(4):983-9. [PubMed] [Google Scholar]
- 162. Zhang HG, Xie J, Yang P, Wang Y, Xu L, Liu D, et al. Adeno-associated virus production of soluble tumor necrosis factor receptor neutralizes tumor necrosis factor alpha and reduces arthritis. Hum Gene Ther. 2000;11(17):2431-42. [DOI] [PubMed] [Google Scholar]
- 163. Hiraide A, Yokoo N, Xin KQ, Okuda K, Mizukami H, Ozawa K, et al. Repair of articular cartilage defect by intraarticular administration of basic fibroblast growth factor gene, using adeno-associated virus vector. Hum Gene Ther. 2005;16(12):1413-21. [DOI] [PubMed] [Google Scholar]
- 164. Apparailly F, Millet V, Noel D, Jacquet C, Sany J, Jorgensen C. Tetracycline-inducible interleukin-10 gene transfer mediated by an adeno-associated virus: application to experimental arthritis. Hum Gene Ther. 2002;13(10):1179-88. [DOI] [PubMed] [Google Scholar]
- 165. Arai Y, Kubo T, Fushiki S, Mazda O, Nakai H, Iwaki Y, et al. Gene delivery to human chondrocytes by an adeno associated virus vector. J Rheumatol. 2000;27(4):979-82. [PubMed] [Google Scholar]
- 166. Ulrich-Vinther M, Maloney MD, Goater JJ, Soballe K, Goldring MB, O’Keefe RJ, et al. Light-activated gene transduction enhances adeno-associated virus vector-mediated gene expression in human articular chondrocytes. Arthritis Rheum. 2002;46(8):2095-104. [DOI] [PubMed] [Google Scholar]
- 167. Cucchiarini M, Thurn T, Weimer A, Kohn D, Terwilliger EF, Madry H. Restoration of the extracellular matrix in human osteoarthritic articular cartilage by overexpression of the transcription factor SOX9. Arthritis Rheum. 2007;56(1):158-67. [DOI] [PubMed] [Google Scholar]
- 168. Cucchiarini M, Terwilliger EF, Kohn D, Madry H. Remodelling of human osteoarthritic cartilage by FGF-2, alone or combined with Sox9 via rAAV gene transfer. J Cell Mol Med. 2009;13(8B):2476-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Chamberlain JR, Schwarze U, Wang PR, Hirata RK, Hankenson KD, Pace JM, et al. Gene targeting in stem cells from individuals with osteogenesis imperfecta. Science. 2004;303(5661):1198-201. [DOI] [PubMed] [Google Scholar]
- 170. Ito H, Goater JJ, Tiyapatanaputi P, Rubery PT, O’Keefe RJ, Schwarz EM. Light-activated gene transduction of recombinant adeno-associated virus in human mesenchymal stem cells. Gene Ther. 2004;11(1):34-41. [DOI] [PubMed] [Google Scholar]
- 171. Basile P, Dadali T, Jacobson J, Hasslund S, Ulrich-Vinther M, Soballe K, et al. Freeze-dried tendon allografts as tissue-engineering scaffolds for Gdf5 gene delivery. Mol Ther. 2008;16(3):466-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Arsic N, Zacchigna S, Zentilin L, Ramirez-Correa G, Pattarini L, Salvi A, et al. Vascular endothelial growth factor stimulates skeletal muscle regeneration in vivo. Mol Ther. 2004;10(5):844-54. [DOI] [PubMed] [Google Scholar]
- 173. Madry H, Cucchiarini M, Kaul G, Kohn D, Terwilliger EF, Trippel SB. Menisci are efficiently transduced by recombinant adeno-associated virus vectors in vitro and in vivo. Am J Sports Med. 2004;32(8):1860-5. [DOI] [PubMed] [Google Scholar]
- 174. Ito H, Koefoed M, Tiyapatanaputi P, Gromov K, Goater JJ, Carmouche J, et al. Remodeling of cortical bone allografts mediated by adherent rAAV-RANKL and VEGF gene therapy. Nat Med. 2005;11(3):291-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Wang XT, Liu PY, Xin KQ, Tang JB. Tendon healing in vitro: bFGF gene transfer to tenocytes by adeno-associated viral vectors promotes expression of collagen genes. J Hand Surg Am. 2005;30(6):1255-61. [DOI] [PubMed] [Google Scholar]
- 176. Tang JB, Cao Y, Zhu B, Xin KQ, Wang XT, Liu PY. Adeno-associated virus-2-mediated bFGF gene transfer to digital flexor tendons significantly increases healing strength: an in vivo study. J Bone Joint Surg Am. 2008;90(5):1078-89. [DOI] [PubMed] [Google Scholar]
- 177. Cucchiarini M, Schetting S, Terwilliger EF, Kohn D, Madry H. rAAV-mediated overexpression of FGF-2 promotes cell proliferation, survival, and alpha-SMA expression in human meniscal lesions. Gene Ther. 2009;16(11):1363-72. [DOI] [PubMed] [Google Scholar]
- 178. Kafienah W, Al-Fayez F, Hollander AP, Barker MD. Inhibition of cartilage degradation: a combined tissue engineering and gene therapy approach. Arthritis Rheum. 2003;48(3):709-18. [DOI] [PubMed] [Google Scholar]
- 179. Bondeson J, Lauder S, Wainwright S, Amos N, Evans A, Hughes C, et al. Adenoviral gene transfer of the endogenous inhibitor IkappaBalpha into human osteoarthritis synovial fibroblasts demonstrates that several matrix metalloproteinases and aggrecanases are nuclear factor-kappaB-dependent. J Rheumatol. 2007;34(3):523-33. [PubMed] [Google Scholar]
- 180. Kim SH, Kim S, Evans CH, Ghivizzani SC, Oligino T, Robbins PD. Effective treatment of established murine collagen-induced arthritis by systemic administration of dendritic cells genetically modified to express IL-4. J Immunol. 2001;166(5):3499-505. [DOI] [PubMed] [Google Scholar]
- 181. Manning K, Rachakonda PS, Rai MF, Schmidt MF. Co-expression of insulin-like growth factor-1 and interleukin-4 in an in vitro inflammatory model. Cytokine. 2010;50(3): 297-305. [DOI] [PubMed] [Google Scholar]
- 182. Dharmavaram RM, Liu G, Tuan RS, Stokes DG, Jimenez SA. Stable transfection of human fetal chondrocytes with a type II procollagen minigene: expression of the mutant protein and alterations in the structure of the extracellular matrix in vitro. Arthritis Rheum. 1999;42(7):1433-42. [DOI] [PubMed] [Google Scholar]
- 183. Venkatesan N, Barre L, Benani A, Netter P, Magdalou J, Fournel-Gigleux S, et al. Stimulation of proteoglycan synthesis by glucuronosyltransferase-I gene delivery: a strategy to promote cartilage repair. Proc Natl Acad Sci U S A. 2004;101(52):18087-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Lee DK, Choi KB, Oh IS, Song SU, Hwang S, Lim CL, et al. Continuous transforming growth factor beta1 secretion by cell-mediated gene therapy maintains chondrocyte redifferentiation. Tissue Eng. 2005;11(1-2):310-8. [DOI] [PubMed] [Google Scholar]
- 185. Schmal H, Mehlhorn AT, Zwingmann J, Muller CA, Stark GB, Sudkamp NP. Stimulation of chondrocytes in vitro by gene transfer with plasmids coding for epidermal growth factor (hEGF) and basic fibroblast growth factor (bFGF). Cytotherapy. 2005;7(3):292-300. [DOI] [PubMed] [Google Scholar]
- 186. Piera-Velazquez S, Jimenez SA, Stokes D. Increased life span of human osteoarthritic chondrocytes by exogenous expression of telomerase. Arthritis Rheum. 2002;46(3):683-93. [DOI] [PubMed] [Google Scholar]
- 187. Surendran S, Kim SH, Jee BK, Ahn SH, Gopinathan P, Han CW. Anti-apoptotic Bcl-2 gene transfection of human articular chondrocytes protects against nitric oxide-induced apoptosis. J Bone Joint Surg Br. 2006;88(12):1660-5. [DOI] [PubMed] [Google Scholar]
- 188. Chan JM, Villarreal G, Jin WW, Stepan T, Burstein H, Wahl SM. Intraarticular gene transfer of TNFR:Fc suppresses experimental arthritis with reduced systemic distribution of the gene product. Mol Ther. 2002;6(6):727-36. [DOI] [PubMed] [Google Scholar]
- 189. Apparailly F, Verwaerde C, Jacquet C, Auriault C, Sany J, Jorgensen C. Adenovirus-mediated transfer of viral IL-10 gene inhibits murine collagen-induced arthritis. J Immunol. 1998;160(11):5213-20. [PubMed] [Google Scholar]
- 190. Song XY, Gu M, Jin WW, Klinman DM, Wahl SM. Plasmid DNA encoding transforming growth factor-beta1 suppresses chronic disease in a streptococcal cell wall-induced arthritis model. J Clin Invest. 1998;101(12):2615-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Roessler BJ, Allen ED, Wilson JM, Hartman JW, Davidson BL. Adenoviral-mediated gene transfer to rabbit synovium in vivo. J Clin Invest. 1993;92(2):1085-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Kim SH, Evans CH, Kim S, Oligino T, Ghivizzani SC, Robbins PD. Gene therapy for established murine collagen-induced arthritis by local and systemic adenovirus-mediated delivery of interleukin-4. Arthritis Res. 2000;2(4):293-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Lechman ER, Jaffurs D, Ghivizzani SC, Gambotto A, Kovesdi I, Mi Z, et al. Direct adenoviral gene transfer of viral IL-10 to rabbit knees with experimental arthritis ameliorates disease in both injected and contralateral control knees. J Immunol. 1999;163(4):2202-8. [PubMed] [Google Scholar]
- 194. Keravala A, Lechman ER, Nash J, Mi Z, Robbins PD. Human, viral or mutant human IL-10 expressed after local adenovirus-mediated gene transfer are equally effective in ameliorating disease pathology in a rabbit knee model of antigen-induced arthritis. Arthritis Res Ther. 2006;8(4):R91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Kim KN, Watanabe S, Ma Y, Thornton S, Giannini EH, Hirsch R. Viral IL-10 and soluble TNF receptor act synergistically to inhibit collagen-induced arthritis following adenovirus-mediated gene transfer. J Immunol. 2000;164(3):1576-81. [DOI] [PubMed] [Google Scholar]
- 196. Fernandes J, Tardif G, Martel-Pelletier J, Lascau-Coman V, Dupuis M, Moldovan F, et al. In vivo transfer of interleukin-1 receptor antagonist gene in osteoarthritic rabbit knee joints: prevention of osteoarthritis progression. Am J Pathol. 1999;154(4):1159-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Frisbie DD, McIlwraith CW. Evaluation of gene therapy as a treatment for equine traumatic arthritis and osteoarthritis. Clin Orthop Relat Res. 2000;(379 Suppl):S273-87. [DOI] [PubMed] [Google Scholar]
- 198. Scharstuhl A, Vitters EL, van der Kraan PM, van den Berg WB. Reduction of osteophyte formation and synovial thickening by adenoviral overexpression of transforming growth factor beta/bone morphogenetic protein inhibitors during experimental osteoarthritis. Arthritis Rheum. 2003;48(12):3442-51. [DOI] [PubMed] [Google Scholar]
- 199. Chen LX, Lin L, Wang HJ, Wei XL, Fu X, Zhang JY, et al. Suppression of early experimental osteoarthritis by in vivo delivery of the adenoviral vector-mediated NF-kappaBp65-specific siRNA. Osteoarthritis Cartilage. 2008;16(2):174-84. [DOI] [PubMed] [Google Scholar]
- 200. Hsieh JL, Shen PC, Shiau AL, Jou IM, Lee CH, Teo ML, et al. Adenovirus-mediated kallistatin gene transfer ameliorates disease progression in a rat model of osteoarthritis induced by anterior cruciate ligament transection. Hum Gene Ther. 2009;20(2):147-58. [DOI] [PubMed] [Google Scholar]
- 201. Hsieh JL, Shen PC, Shiau AL, Jou IM, Lee CH, Wang CR, et al. Intraarticular gene transfer of thrombospondin-1 suppresses the disease progression of experimental osteoarthritis. J Orthop Res. 2010;28(10):1300-6. [DOI] [PubMed] [Google Scholar]
- 202. Mi Z, Ghivizzani SC, Lechman ER, Jaffurs D, Glorioso JC, Evans CH, et al. Adenovirus-mediated gene transfer of insulin-like growth factor 1 stimulates proteoglycan synthesis in rabbit joints. Arthritis Rheum. 2000;43(11):2563-70. [DOI] [PubMed] [Google Scholar]
- 203. Bandara G, Mueller GM, Galea-Lauri J, Tindal MH, Georgescu HI, Suchanek MK, et al. Intraarticular expression of biologically active interleukin 1-receptor-antagonist protein by ex vivo gene transfer. Proc Natl Acad Sci U S A. 1993;90(22):10764-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Hung GL, Galea-Lauri J, Mueller GM, Georgescu HI, Larkin LA, Suchanek MK, et al. Suppression of intra-articular responses to interleukin-1 by transfer of the interleukin-1 receptor antagonist gene to synovium. Gene Ther. 1994;1(1):64-9. [PubMed] [Google Scholar]
- 205. Makarov SS, Olsen JC, Johnston WN, Anderle SK, Brown RR, Baldwin AS, Jr, et al. Suppression of experimental arthritis by gene transfer of interleukin 1 receptor antagonist cDNA. Proc Natl Acad Sci U S A. 1996;93(1):402-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Otani K, Nita I, Macaulay W, Georgescu HI, Robbins PD, Evans CH. Suppression of antigen-induced arthritis in rabbits by ex vivo gene therapy. J Immunol. 1996;156(9):3558-62. [PubMed] [Google Scholar]
- 207. Pelletier JP, Caron JP, Evans C, Robbins PD, Georgescu HI, Jovanovic D, et al. In vivo suppression of early experimental osteoarthritis by interleukin-1 receptor antagonist using gene therapy. Arthritis Rheum. 1997;40(6):1012-9. [DOI] [PubMed] [Google Scholar]
- 208. Zhang X, Mao Z, Yu C. Suppression of early experimental osteoarthritis by gene transfer of interleukin-1 receptor antagonist and interleukin-10. J Orthop Res. 2004;22(4):742-50. [DOI] [PubMed] [Google Scholar]
- 209. Kim SH, Lechman ER, Kim S, Nash J, Oligino TJ, Robbins PD. Ex vivo gene delivery of IL-1Ra and soluble TNF receptor confers a distal synergistic therapeutic effect in antigen-induced arthritis. Mol Ther. 2002;6(5):591-600. [PubMed] [Google Scholar]
- 210. Yoo JU, Mandell I, Angele P, Johnstone B. Chondrogenitor cells and gene therapy. Clin Orthop Relat Res. 2000;(379 Suppl):S164-70. [DOI] [PubMed] [Google Scholar]
- 211. Barry FP, Murphy JM. Mesenchymal stem cells: clinical applications and biological characterization. Int J Biochem Cell Biol. 2004;36(4):568-84. [DOI] [PubMed] [Google Scholar]
- 212. Matsumoto T, Cooper GM, Gharaibeh B, Meszaros LB, Li G, Usas A, et al. Blocking VEGF as a potential approach to improve cartilage healing after osteoarthritis. J Musculoskelet Neuronal Interact. 2008;8(4):316-7. [PubMed] [Google Scholar]
- 213. Matsumoto T, Cooper GM, Gharaibeh B, Meszaros LB, Li G, Usas A, et al. Cartilage repair in a rat model of osteoarthritis through intraarticular transplantation of muscle-derived stem cells expressing bone morphogenetic protein 4 and soluble Flt-1. Arthritis Rheum. 2009;60(5):1390-405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Katsube K, Bishop AT, Simari RD, Yla-Herttuala S, Friedrich PF. Vascular endothelial growth factor (VEGF) gene transfer enhances surgical revascularization of necrotic bone. J Orthop Res. 2005;23(2):469-74. [DOI] [PubMed] [Google Scholar]
- 215. Nakamura N, Horibe S, Iwahashi T, Kawano K, Shino K, Yoshikawa H. Healing of a chondral fragment of the knee in an adolescent after internal fixation: a case report. J Bone Joint Surg Am. 2004;86-A(12):2741-6. [DOI] [PubMed] [Google Scholar]
- 216. Rath E, Richmond JC. The menisci: basic science and advances in treatment. Br J Sports Med. 2000;34(4):252-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Gray JC. Neural and vascular anatomy of the menisci of the human knee. J Orthop Sports Phys Ther. 1999;29(1):23-30. [DOI] [PubMed] [Google Scholar]
- 218. Messner K, Gao J. The menisci of the knee joint: anatomical and functional characteristics, and a rationale for clinical treatment. J Anat. 1998;193(Pt 2):161-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Chivers MD, Howitt SD. Anatomy and physical examination of the knee menisci: a narrative review of the orthopedic literature. J Can Chiropr Assoc. 2009;53(4):319-33. [PMC free article] [PubMed] [Google Scholar]
- 220. Lengsfeld M, Rudig L, von Issendorff WD, Koebke J. [Significance of shape differences between medial and lateral knee joint menisci for functional change of position]. Unfallchirurgie. 1991;17(6):309-15. [DOI] [PubMed] [Google Scholar]
- 221. Bullough PG, Munuera L, Murphy J, Weinstein AM. The strength of the menisci of the knee as it relates to their fine structure. J Bone Joint Surg Br. 1970;52(3):564-7. [PubMed] [Google Scholar]
- 222. McDevitt CA, Webber RJ. The ultrastructure and biochemistry of meniscal cartilage. Clin Orthop Relat Res. 1990;(252):8-18. [PubMed] [Google Scholar]
- 223. Verdonk PC, Forsyth RG, Wang J, Almqvist KF, Verdonk R, Veys EM, et al. Characterisation of human knee meniscus cell phenotype. Osteoarthritis Cartilage. 2005;13(7):548-60. [DOI] [PubMed] [Google Scholar]
- 224. Arnoczky SP, Warren RF. Microvasculature of the human meniscus. Am J Sports Med. 1982;10(2):90-5. [DOI] [PubMed] [Google Scholar]
- 225. Cooper DE, Arnoczky SP, Warren RF. Meniscal repair. Clin Sports Med. 1991;10(3):529-48. [PubMed] [Google Scholar]
- 226. Arnoczky SP, Warren RF. The microvasculature of the meniscus and its response to injury: an experimental study in the dog. Am J Sports Med. 1983;11(3):131-41. [DOI] [PubMed] [Google Scholar]
- 227. Englund M, Guermazi A, Roemer FW, Aliabadi P, Yang M, Lewis CE, et al. Meniscal tear in knees without surgery and the development of radiographic osteoarthritis among middle-aged and elderly persons: the Multicenter Osteoarthritis Study. Arthritis Rheum. 2009;60(3):831-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Englund M, Lohmander LS. Patellofemoral osteoarthritis coexistent with tibiofemoral osteoarthritis in a meniscectomy population. Ann Rheum Dis. 2005;64(12):1721-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Beaufils P, Hulet C, Dhenain M, Nizard R, Nourissat G, Pujol N. Clinical practice guidelines for the management of meniscal lesions and isolated lesions of the anterior cruciate ligament of the knee in adults. Rev Chir Orthop Traumatol. 2009;95(6):437-42. [DOI] [PubMed] [Google Scholar]
- 230. Ding C, Martel-Pelletier J, Pelletier JP, Abram F, Raynauld JP, Cicuttini F, et al. Meniscal tear as an osteoarthritis risk factor in a largely non-osteoarthritic cohort: a cross-sectional study. J Rheumatol. 2007;34(4):776-84. [PubMed] [Google Scholar]
- 231. Tumia NS, Johnstone AJ. Platelet derived growth factor-AB enhances knee meniscal cell activity in vitro. Knee. 2009;16(1):73-6. [DOI] [PubMed] [Google Scholar]
- 232. Bhargava MM, Hidaka C, Hannafin JA, Doty S, Warren RF. Effects of hepatocyte growth factor and platelet-derived growth factor on the repair of meniscal defects in vitro. In Vitro Cell Dev Biol Anim. 2005;41(8-9):305-10. [DOI] [PubMed] [Google Scholar]
- 233. Lietman SA, Hobbs W, Inoue N, Reddi AH. Effects of selected growth factors on porcine meniscus in chemically defined medium. Orthopedics. 2003;26(8):799-803. [DOI] [PubMed] [Google Scholar]
- 234. Spindler KP, Mayes CE, Miller RR, Imro AK, Davidson JM. Regional mitogenic response of the meniscus to platelet-derived growth factor (PDGF-AB). J Orthop Res. 1995;13(2):201-7. [DOI] [PubMed] [Google Scholar]
- 235. Fox DB, Warnock JJ, Stoker AM, Luther JK, Cockrell M. Effects of growth factors on equine synovial fibroblasts seeded on synthetic scaffolds for avascular meniscal tissue engineering. Res Vet Sci. 2010;88(2):326-32. [DOI] [PubMed] [Google Scholar]
- 236. Tumia NS, Johnstone AJ. Promoting the proliferative and synthetic activity of knee meniscal fibrochondrocytes using basic fibroblast growth factor in vitro. Am J Sports Med. 2004;32(4):915-20. [DOI] [PubMed] [Google Scholar]
- 237. Webber RJ, Harris MG, Hough AJ., Jr Cell culture of rabbit meniscal fibrochondrocytes: proliferative and synthetic response to growth factors and ascorbate. J Orthop Res. 1985;3(1):36-42. [DOI] [PubMed] [Google Scholar]
- 238. Imler SM, Doshi AN, Levenston ME. Combined effects of growth factors and static mechanical compression on meniscus explant biosynthesis. Osteoarthritis Cartilage. 2004;12(9):736-44. [DOI] [PubMed] [Google Scholar]
- 239. Pangborn CA, Athanasiou KA. Growth factors and fibrochondrocytes in scaffolds. J Orthop Res. 2005;23(5):1184-90. [DOI] [PubMed] [Google Scholar]
- 240. Tumia NS, Johnstone AJ. Regional regenerative potential of meniscal cartilage exposed to recombinant insulin-like growth factor-I in vitro. J Bone Joint Surg Br. 2004;86(7):1077-81. [DOI] [PubMed] [Google Scholar]
- 241. Pangborn CA, Athanasiou KA. Effects of growth factors on meniscal fibrochondrocytes. Tissue Eng. 2005;11(7-8):1141-8. [DOI] [PubMed] [Google Scholar]
- 242. Collier S, Ghosh P. Effects of transforming growth factor beta on proteoglycan synthesis by cell and explant cultures derived from the knee joint meniscus. Osteoarthritis Cartilage. 1995;3(2):127-38. [DOI] [PubMed] [Google Scholar]
- 243. Koay EJ, Athanasiou KA. Development of serum-free, chemically defined conditions for human embryonic stem cell-derived fibrochondrogenesis. Tissue Eng Part A. 2009;15(8):2249-57. [DOI] [PubMed] [Google Scholar]
- 244. Hoben GM, Willard VP, Athanasiou KA. Fibrochondrogenesis of hESCs: growth factor combinations and cocultures. Stem Cells Dev. 2009;18(2):283-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Arnoczky SP, Warren RF, Spivak JM. Meniscal repair using an exogenous fibrin clot: an experimental study in dogs. J Bone Joint Surg Am. 1988;70(8):1209-17. [PubMed] [Google Scholar]
- 246. Port J, Jackson DW, Lee TQ, Simon TM. Meniscal repair supplemented with exogenous fibrin clot and autogenous cultured marrow cells in the goat model. Am J Sports Med. 1996;24(4):547-55. [DOI] [PubMed] [Google Scholar]
- 247. Shirakura K, Niijima M, Kobuna Y, Kizuki S. Free synovium promotes meniscal healing: synovium, muscle and synthetic mesh compared in dogs. Acta Orthop Scand. 1997;68(1): 51-4. [DOI] [PubMed] [Google Scholar]
- 248. Jitsuiki J, Ochi M, Ikuta Y. Meniscal repair enhanced by an interpositional free synovial autograft: an experimental study in rabbits. Arthroscopy. 1994;10(6):659-66. [DOI] [PubMed] [Google Scholar]
- 249. Petersen W, Pufe T, Starke C, Fuchs T, Kopf S, Neumann W, et al. The effect of locally applied vascular endothelial growth factor on meniscus healing: gross and histological findings. Arch Orthop Trauma Surg. 2007;127(4):235-40. [DOI] [PubMed] [Google Scholar]
- 250. Petersen W, Pufe T, Starke C, Fuchs T, Kopf S, Raschke M, et al. Locally applied angiogenic factors: a new therapeutic tool for meniscal repair. Ann Anat. 2005;187(5-6):509-19. [DOI] [PubMed] [Google Scholar]
- 251. van Tienen TG, Hannink G, Buma P. Meniscus replacement using synthetic materials. Clin Sports Med. 2009;28(1):143-56. [DOI] [PubMed] [Google Scholar]
- 252. Martinek V, Imhoff A. Das künstliche meniskusimplantat. Arthroskopie. 2008;21:266-70. [Google Scholar]
- 253. Stone KR, Steadman JR, Rodkey WG, Li ST. Regeneration of meniscal cartilage with use of a collagen scaffold: analysis of preliminary data. J Bone Joint Surg Am. 1997;79(12):1770-7. [DOI] [PubMed] [Google Scholar]
- 254. Rodkey WG, DeHaven KE, Montgomery WH, 3rd, Baker CL, Jr, Beck CL, Jr, Hormel SE, et al. Comparison of the collagen meniscus implant with partial meniscectomy: a prospective randomized trial. J Bone Joint Surg Am. 2008;90(7):1413-26. [DOI] [PubMed] [Google Scholar]
- 255. de Groot JH, de Vrijer R, Pennings AJ, Klompmaker J, Veth RP, Jansen HW. Use of porous polyurethanes for meniscal reconstruction and meniscal prostheses. Biomaterials. 1996;17(2):163-73. [DOI] [PubMed] [Google Scholar]
- 256. Welsing RT, van Tienen TG, Ramrattan N, Heijkants R, Schouten AJ, Veth RP, et al. Effect on tissue differentiation and articular cartilage degradation of a polymer meniscus implant: a 2-year follow-up study in dogs. Am J Sports Med. 2008;36(10):1978-89. [DOI] [PubMed] [Google Scholar]
- 257. Langer R, Vacanti JP. Tissue engineering. Science. 1993; 260(5110):920-6. [DOI] [PubMed] [Google Scholar]
- 258. Nerem RM. Cellular engineering. Ann Biomed Eng. 1991; 19(5):529-45. [DOI] [PubMed] [Google Scholar]
- 259. Buma P, Ramrattan NN, van Tienen TG, Veth RP. Tissue engineering of the meniscus. Biomaterials. 2004;25(9):1523-32. [DOI] [PubMed] [Google Scholar]
- 260. Sweigart MA, Athanasiou KA. Toward tissue engineering of the knee meniscus. Tissue Eng. 2001;7(2):111-29. [DOI] [PubMed] [Google Scholar]
- 261. Setton LA, Guilak F, Hsu EW, Vail TP. Biomechanical factors in tissue engineered meniscal repair. Clin Orthop Relat Res. 1999;(367 Suppl):S254-72. [DOI] [PubMed] [Google Scholar]
- 262. Sandmann GH, Eichhorn S, Vogt S, Adamczyk C, Aryee S, Hoberg M, et al. Generation and characterization of a human acellular meniscus scaffold for tissue engineering. J Biomed Mater Res A. 2009;91(2):567-74. [DOI] [PubMed] [Google Scholar]
- 263. Stabile KJ, Odom D, Smith TL, Northam C, Whitlock PW, Smith BP, et al. An acellular, allograft-derived meniscus scaffold in an ovine model. Arthroscopy. 2010;26(7):936-48. [DOI] [PubMed] [Google Scholar]
- 264. Ibarra C, Koski JA, Warren RF. Tissue engineering meniscus: cells and matrix. Orthop Clin North Am. 2000;31(3):411-8. [DOI] [PubMed] [Google Scholar]
- 265. Muller-Rath R, Mumme T, Miltner O, Andereya S, Schneider U. [Meniscus replacement: current aspects in the field of tissue engineering]. Z Orthop Ihre Grenzgeb. 2004;142(5):540-5. [DOI] [PubMed] [Google Scholar]
- 266. Adams SB, Jr, Randolph MA, Gill TJ. Tissue engineering for meniscus repair. J Knee Surg. 2005;18(1):25-30. [DOI] [PubMed] [Google Scholar]
- 267. Arnoczky SP. Building a meniscus: biologic considerations. Clin Orthop Relat Res. 1999;(367 Suppl):S244-53. [PubMed] [Google Scholar]
- 268. Baker BM, Gee AO, Sheth NP, Huffman GR, Sennett BJ, Schaer TP, et al. Meniscus tissue engineering on the nanoscale: from basic principles to clinical application. J Knee Surg. 2009;22(1):45-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269. Steadman JR, Rodkey WG. Tissue-engineered collagen meniscus implants: 5- to 6-year feasibility study results. Arthroscopy. 2005;21(5):515-25. [DOI] [PubMed] [Google Scholar]
- 270. Rodeo SA, Seneviratne A, Suzuki K, Felker K, Wickiewicz TL, Warren RF. Histological analysis of human meniscal allografts: a preliminary report. J Bone Joint Surg Am. 2000;82-A(8):1071-82. [DOI] [PubMed] [Google Scholar]
- 271. Sohn DH, Toth AP. Meniscus transplantation: current concepts. J Knee Surg. 2008;21(2):163-72. [DOI] [PubMed] [Google Scholar]
- 272. Elder BD, Eleswarapu SV, Athanasiou KA. Extraction techniques for the decellularization of tissue engineered articular cartilage constructs. Biomaterials. 2009;30(22):3749-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273. Stapleton TW, Ingram J, Katta J, Knight R, Korossis S, Fisher J, et al. Development and characterization of an acellular porcine medial meniscus for use in tissue engineering. Tissue Eng Part A. 2008;14(4):505-18. [DOI] [PubMed] [Google Scholar]
- 274. Stone KR, Rodkey WG, Webber R, McKinney L, Steadman JR. Meniscal regeneration with copolymeric collagenscaffolds: in vitro and in vivo studies evaluated clinically, histologically, and biochemically. Am J Sports Med. 1992;20(2): 104-11. [DOI] [PubMed] [Google Scholar]
- 275. Gastel JA, Muirhead WR, Lifrak JT, Fadale PD, Hulstyn MJ, Labrador DP. Meniscal tissue regeneration using a collagenous biomaterial derived from porcine small intestine submucosa. Arthroscopy. 2001;17(2):151-9. [DOI] [PubMed] [Google Scholar]
- 276. Welch JA, Montgomery RD, Lenz SD, Plouhar P, Shelton WR. Evaluation of small-intestinal submucosa implants for repair of meniscal defects in dogs. Am J Vet Res. 2002;63(3):427-31. [DOI] [PubMed] [Google Scholar]
- 277. Cook JL, Fox DB, Malaviya P, Tomlinson JL, Kuroki K, Cook CR, et al. Long-term outcome for large meniscal defects treated with small intestinal submucosa in a dog model. Am J Sports Med. 2006;34(1):32-42. [DOI] [PubMed] [Google Scholar]
- 278. Cook JL, Tomlinson JL, Kreeger JM, Cook CR. Induction of meniscal regeneration in dogs using a novel biomaterial. Am J Sports Med. 1999;27(5):658-65. [DOI] [PubMed] [Google Scholar]
- 279. Klompmaker J, Jansen HW, Veth RP, de Groot JH, Nijenhuis AJ, Pennings AJ. Porous polymer implant for repair of meniscal lesions: a preliminary study in dogs. Biomaterials. 1991;12(9):810-6. [DOI] [PubMed] [Google Scholar]
- 280. Tienen TG, Heijkants RG, de Groot JH, Pennings AJ, Schouten AJ, Veth RP, et al. Replacement of the knee meniscus by a porous polymer implant: a study in dogs. Am J Sports Med. 2006;34(1):64-71. [DOI] [PubMed] [Google Scholar]
- 281. Kobayashi M, Toguchida J, Oka M. Development of an artificial meniscus using polyvinyl alcohol-hydrogel for early return to, and continuance of, athletic life in sportspersons with severe meniscus injury. II: animal experiments. Knee. 2003;10(1):53. [DOI] [PubMed] [Google Scholar]
- 282. Kang SW, Son SM, Lee JS, Lee ES, Lee KY, Park SG, et al. Regeneration of whole meniscus using meniscal cells and polymer scaffolds in a rabbit total meniscectomy model. J Biomed Mater Res A. 2006;78(3):659-71. [DOI] [PubMed] [Google Scholar]
- 283. Arnoczky SP, DiCarlo EF, O’Brien SJ, Warren RF. Cellular repopulation of deep-frozen meniscal autografts: an experimental study in the dog. Arthroscopy. 1992;8(4):428-36. [DOI] [PubMed] [Google Scholar]
- 284. Marsano A, Vunjak-Novakovic G, Martin I. Towards tissue engineering of meniscus substitutes: selection of cell source and culture environment. Conf Proc IEEE Eng Med Biol Soc. 2006;1:3656-8. [DOI] [PubMed] [Google Scholar]
- 285. Chiari C, Koller U, Kapeller B, Dorotka R, Bindreiter U, Nehrer S. Different behavior of meniscal cells in collagen II/I,III and Hyaff-11 scaffolds in vitro. Tissue Eng Part A. 2008;14(8):1295-304. [DOI] [PubMed] [Google Scholar]
- 286. Gunja NJ, Athanasiou KA. Effects of co-cultures of meniscus cells and articular chondrocytes on PLLA scaffolds. Biotechnol Bioeng. 2009;103(4):808-16. [DOI] [PubMed] [Google Scholar]
- 287. Kon E, Chiari C, Marcacci M, Delcogliano M, Salter DM, Martin I, et al. Tissue engineering for total meniscal substitution: animal study in sheep model. Tissue Eng Part A. 2008;14(6):1067-80. [DOI] [PubMed] [Google Scholar]
- 288. Tan Y, Zhang Y, Pei M. Meniscus reconstruction through coculturing meniscus cells with synovium-derived stem cells on small intestine submucosa: a pilot study to engineer meniscus tissue constructs. Tissue Eng Part A. 2010;16(1):67-79. [DOI] [PubMed] [Google Scholar]
- 289. Yamasaki T, Deie M, Shinomiya R, Yasunaga Y, Yanada S, Ochi M. Transplantation of meniscus regenerated by tissue engineering with a scaffold derived from a rat meniscus and mesenchymal stromal cells derived from rat bone marrow. Artif Organs. 2008;32(7):519-24. [DOI] [PubMed] [Google Scholar]
- 290. Johns DE, Athanasiou KA. Growth factor effects on costal chondrocytes for tissue engineering fibrocartilage. Cell Tissue Res. 2008;333(3):439-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291. Hidaka C, Ibarra C, Hannafin JA, Torzilli PA, Quitoriano M, Jen SS, et al. Formation of vascularized meniscal tissue by combining gene therapy with tissue engineering. Tissue Eng. 2002;8(1):93-105. [DOI] [PubMed] [Google Scholar]
- 292. Zhang H, Leng P, Zhang J. Enhanced meniscal repair by overexpression of hIGF-1 in a full-thickness model. Clin Orthop Relat Res. 2009;467(12):3165-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293. Arnoczky SP, Warren RF, McDevitt CA. Meniscal replacement using a cryopreserved allograft: an experimental study in the dog. Clin Orthop Relat Res. 1990;(252):121-8. [PubMed] [Google Scholar]
- 294. Cole BJ, Carter TR, Rodeo SA. Allograft meniscal transplantation: background, techniques, and results. Instr Course Lect. 2003;52:383-96. [PubMed] [Google Scholar]
- 295. Dienst M, Greis PE, Ellis BJ, Bachus KN, Burks RT. Effect of lateral meniscal allograft sizing on contact mechanics of the lateral tibial plateau: an experimental study in human cadaveric knee joints. Am J Sports Med. 2007;35(1):34-42. [DOI] [PubMed] [Google Scholar]
- 296. Garrett JC, Steensen RN. Meniscal transplantation in the human knee: a preliminary report. Arthroscopy. 1991;7(1):57-62. [DOI] [PubMed] [Google Scholar]
- 297. Greis PE, Holmstrom MC, Bardana DD, Burks RT. Meniscal injury. II: management. J Am Acad Orthop Surg. 2002;10(3):177-87. [DOI] [PubMed] [Google Scholar]
- 298. Kohn D, Verdonk R, Aagaard H, Seil R, Dienst M. Meniscal substitutes: animal experience. Scand J Med Sci Sports. 1999;9(3):141-5. [DOI] [PubMed] [Google Scholar]
- 299. Messner K. Meniscal regeneration or meniscal transplantation? Scand J Med Sci Sports. 1999;9(3):162-7. [DOI] [PubMed] [Google Scholar]
- 300. Packer JD, Rodeo SA. Meniscal allograft transplantation. Clin Sports Med. 2009;28(2):259-83, viii. [DOI] [PubMed] [Google Scholar]
- 301. Rodeo SA. Meniscal allografts: where do we stand? Am J Sports Med. 2001;29(2):246-61. [DOI] [PubMed] [Google Scholar]
- 302. Siegel MG, Roberts CS. Meniscal allografts. Clin Sports Med. 1993;12(1):59-80. [PubMed] [Google Scholar]
- 303. Verdonk PC, Verstraete KL, Almqvist KF, De Cuyper K, Veys EM, Verbruggen G, et al. Meniscal allograft transplantation: long-term clinical results with radiological and magnetic resonance imaging correlations. Knee Surg Sports Traumatol Arthrosc. 2006;14(8):694-706. [DOI] [PubMed] [Google Scholar]
- 304. Verdonk R. Meniscal transplantation. Acta Orthop Belg. 2002;68(2):118-27. [PubMed] [Google Scholar]
- 305. Verdonk R, Almqvist KF, Huysse W, Verdonk PC. Meniscal allografts: indications and outcomes. Sports Med Arthrosc. 2007;15(3):121-5. [DOI] [PubMed] [Google Scholar]
- 306. Crook TB, Ardolino A, Williams LA, Barlow IW. Meniscal allograft transplantation: a review of the current literature. Ann R Coll Surg Engl. 2009;91(5):361-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307. Huard J, Li Y, Peng H, Fu FH. Gene therapy and tissue engineering for sports medicine. J Gene Med. 2003;5(2):93-108. [DOI] [PubMed] [Google Scholar]
- 308. Martinek V, Usas A, Pelinkovic D, Robbins P, Fu FH, Huard J. Genetic engineering of meniscal allografts. Tissue Eng. 2002;8(1):107-17. [DOI] [PubMed] [Google Scholar]
- 309. Evans CH, Ghivizzani SC, Herndon JH, Wasko MC, Reinecke J, Wehling P, et al. Clinical trials in the gene therapy of arthritis. Clin Orthop Relat Res. 2000;(379 Suppl):S300-7. [DOI] [PubMed] [Google Scholar]
- 310. Evans CH, Gouze JN, Gouze E, Robbins PD, Ghivizzani SC. Osteoarthritis gene therapy. Gene Ther. 2004;11(4):379-89. [DOI] [PubMed] [Google Scholar]
- 311. Evans CH, Ghivizzani SC, Robbins PD. Gene therapy for arthritis: what next? Arthritis Rheum. 2006;54(6):1714-29. [DOI] [PubMed] [Google Scholar]
- 312. Evans CH, Ghivizzani SC, Robbins PD. Arthritis gene therapy’s first death. Arthritis Res Ther. 2008;10(3):110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313. Evans CH, Ghivizzani SC, Oligino TJ, Robbins PD. Gene therapy for autoimmune disorders. J Clin Immunol. 2000;20(5):334-46. [DOI] [PubMed] [Google Scholar]
- 314. Evans CH. Gene therapy: what have we accomplished and where do we go from here? J Rheumatol Suppl. 2005;72:17-20. [PubMed] [Google Scholar]
- 315. Evans CH, Robbins PD, Ghivizzani SC, Wasko MC, Tomaino MM, Kang R, et al. Gene transfer to human joints: progress toward a gene therapy of arthritis. Proc Natl Acad Sci U S A. 2005;102(24):8698-703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316. Sant SM, Suarez TM, Moalli MR, Wu BY, Blaivas M, Laing TJ, et al. Molecular lysis of synovial lining cells by in vivo herpes simplex virus-thymidine kinase gene transfer. Hum Gene Ther. 1998;9(18):2735-43. [DOI] [PubMed] [Google Scholar]
- 317. Mease PJ, Hobbs K, Chalmers A, El-Gabalawy H, Bookman A, Keystone E, et al. Local delivery of a recombinant adenoassociated vector containing a tumour necrosis factor alpha antagonist gene in inflammatory arthritis: a phase 1 dose-escalation safety and tolerability study. Ann Rheum Dis. 2009;68(8):1247-54. [DOI] [PubMed] [Google Scholar]
- 318. Gladman DD, Mease PJ, Ritchlin CT, Choy EH, Sharp JT, Ory PA, et al. Adalimumab for long-term treatment of psoriatic arthritis: forty-eight week data from the adalimumab effectiveness in psoriatic arthritis trial. Arthritis Rheum. 2007;56(2):476-88. [DOI] [PubMed] [Google Scholar]
- 319. Kaiser J. Clinical research: death prompts a review of gene therapy vector. Science. 2007;317(5838):580. [DOI] [PubMed] [Google Scholar]
- 320. Williams DA. RAC reviews serious adverse event associated with AAV therapy trial. Mol Ther. 2007;15(12):2053-4. [DOI] [PubMed] [Google Scholar]
- 321. Williams DA. NIH Recombinant DNA Advisory Committee continues to ponder adverse event associated with AAV gene therapy trial. Mol Ther. 2008;16(3):427-8. [DOI] [PubMed] [Google Scholar]
- 322. Sweeney HL. Gene doping. Sci Am. 2004;291(1):62-9. [DOI] [PubMed] [Google Scholar]
- 323. Adam D. Gene therapy may be up to speed for cheats at 2008 Olympics. Nature. 2001;414(6864):569-70. [DOI] [PubMed] [Google Scholar]
- 324. Friedmann T, Koss JO. Gene transfer and athletics: an impending problem. Mol Ther. 2001;3(6):819-20. [DOI] [PubMed] [Google Scholar]
- 325. Friedmann T, Rabin O, Frankel MS. Ethics: gene doping and sport. Science. 2010;327(5966):647-8. [DOI] [PubMed] [Google Scholar]
- 326. Barton-Davis ER, Shoturma DI, Musaro A, Rosenthal N, Sweeney HL. Viral mediated expression of insulin-like growth factor I blocks the aging-related loss of skeletal muscle function. Proc Natl Acad Sci U S A. 1998;95(26): 15603-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327. Zhou S, Murphy JE, Escobedo JA, Dwarki VJ. Adeno-associated virus-mediated delivery of erythropoietin leads to sustained elevation of hematocrit in nonhuman primates. Gene Ther. 1998;5(5):665-70. [DOI] [PubMed] [Google Scholar]
- 328. Baumgartner I, Pieczek A, Manor O, Blair R, Kearney M, Walsh K, et al. Constitutive expression of phVEGF165 after intramuscular gene transfer promotes collateral vessel development in patients with critical limb ischemia. Circulation. 1998;97(12):1114-23. [DOI] [PubMed] [Google Scholar]
- 329. Baoutina A, Alexander IE, Rasko JE, Emslie KR. Potential use of gene transfer in athletic performance enhancement. Mol Ther. 2007;15(10):1751-66. [DOI] [PubMed] [Google Scholar]
- 330. de la Chapelle A, Traskelin AL, Juvonen E. Truncated erythropoietin receptor causes dominantly inherited benign human erythrocytosis. Proc Natl Acad Sci U S A. 1993;90(10):4495-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331. Slot O. Apocalypse now: fears of gene doping are realised. The Times. 2006. February 2. [Google Scholar]
- 332. Hanson RW, Hakimi P. Born to run: the story of the PEPCK-Cmus mouse. Biochimie. 2008;90(6):838-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333. Baoutina A, Alexander IE, Rasko JE, Emslie KR. Developing strategies for detection of gene doping. J Gene Med. 2008;10(1):3-20. [DOI] [PubMed] [Google Scholar]