Abstract
Objective:
The purpose of our study was to determine the effectiveness of cartilage repair utilizing 1-step surgery with bone marrow aspirate concentrate (BMAC) and a collagen I/III matrix (Chondro-Gide, Geistlich, Wolhusen, Switzerland).
Materials and Methods:
We prospectively followed up for 2 years 15 patients (mean age, 48 years) who were operated for grade IV cartilage lesions of the knee. Six of the patients had multiple chondral lesions; the average size of the lesions was 9.2 cm2. All patients underwent a mini-arthrotomy and concomitant transplantation with BMAC covered with the collagen matrix. Coexisting pathologies were treated before or during the same surgery. X-rays and MRI were collected preoperatively and at 1 and 2 years’ follow-up. Visual analog scale (VAS), International Knee Documentation Committee (IKDC), Knee injury and Osteoarthritis Outcome Score (KOOS), Lysholm, Marx, SF-36 (physical/mental), and Tegner scores were collected preoperatively and at 6, 12, and 24 months’ follow-up. Four patients gave their consent for second-look arthroscopy and 3 of them for a concomitant biopsy.
Results:
Patients showed significant improvement in all scores at final follow-up (P < 0.005). Patients presenting single lesions and patients with small lesions showed higher improvement. MRI showed coverage of the lesion with hyaline-like tissue in all patients in accordance with clinical results. Hyaline-like histological findings were also reported for all the specimens analyzed. No adverse reactions or postoperative complications were noted.
Conclusion:
This study showed that 1-step surgery with BMAC and collagen I/III matrix could be a viable technique in the treatment of grade IV knee chondral lesions.
Keywords: knee, cartilage, BMAC, chondral lesion, Chondro-Gide, Plateltex Act
Introduction
The limited intrinsic healing potential of articular cartilage is attributed to the presence of few and specialized cells with low mitotic activity, to the lack of vessels and of undifferentiated cells that can promote tissue repair. Therefore, once injury occurs, surgical intervention is necessary to achieve repair of the articular surface to obtain good functional outcome and to avoid subsequent cartilage degeneration, which could lead to the development of osteoarthritis (OA).1,2
The incidence of chondral defects is frequent with sporting injuries, especially in patients over 40 years of age, usually resulting in persistent pain. Furthermore, community-based studies have shown that 10% of the population over the age of 55 years has troublesome knee pain, and of those, 25% are severely disabled.3 The social impact of bone and cartilage pathologies entails high costs in terms of therapeutic treatments and loss of income.
Many surgical techniques have been utilized to improve cartilage lesion healing and demonstrated variable results. Autologous chondrocyte implantation (ACI), which was first introduced by Peterson,4 is considered an effective procedure for cartilage defects of the knee restoring hyaline-like cartilage tissue, which is mechanically and functionally stable at long-term follow-up.5-7 However, the need of 2 surgical procedures, the sacrifice of periosteal tissue, the uncertain distribution of chondrocyte solution,5,7-10 and complications such as periosteal patch hypertrophy and arthrofibrosis6,10-13 prompted the scientific community to develop new techniques, namely second-generation ACI. The use of a 3-dimensional scaffold for autologous chondrocyte culture was developed with the aim to improve both the biological performance of chondrogenic autologous cells as well as render the surgical technique easier, and surgeons have been enabled to perform this procedure arthroscopically.12,14-18 However, this technique is still a 2-step procedure including an arthroscopic biopsy, in vitro cell cultivation, and subsequent implantation, either using an arthroscopic technique or mini-arthrotomy.16,17,19,20 Apart from donor site morbidity, the risks of 2 surgical procedures, and the limited quantity of cartilage that could be harvested, the total cost of surgeries, scaffold, and in vitro culture still represents the major limitation of this technique.
Therefore, research has been moving towards the possibility to perform a 1-step surgical procedure. In this regard, the use of bone marrow aspirate concentrate (BMAC) cells, which contain pluripotent mesenchymal stem cells (MSCs) and growth factors (GFs), can represent a possible alternative to regenerate cartilage tissue. In particular, it allows to avoid the first surgery for cartilage biopsy and the subsequent chondrocyte cell cultivation, with a significant reduction of the cost of the total procedure.21-30 The aim of this study was to validate a 1-step procedure for the treatment of large chondral defects of the knee based on BMAC covered with a commercially available collagen I/III matrix. The rationale of this procedure was to paste the BMAC into the cartilage defect and protect the in-growth of the neotissue with a user-friendly scaffold impermeable to cells; furthermore, our technique maximizes cell-to-cell contact and provides a strong chondrogenic environment utilizing a collagen I/III matrix promoting chondrogenic differentiation of MSC and cartilage regeneration. Our hypothesis was that this technique could provide satisfactory clinical results, avoiding biopsy and cell cultivation and reducing the cost of cartilage transplantation procedure.
Materials and Methods
From April 2007, we prospectively followed up 15 symptomatic patients, presenting chronic large full-thickness cartilage lesions, treated at our institution with BMAC pasted—after activation—into the lesions and covered with a collagen type I/III matrix (Chondro-Gide, Geistlich, Wolhusen, Switzerland). Inclusion criteria were patients with knee cartilage injury of International Cartilage Repair Society (ICRS) grade 4; minimum follow-up of 2 years; age between 30 and 60 years; body mass index (BMI) <30; and knee stable or stabilized, normal alignment, or corrected at the time of cartilage repair. Exclusion criteria included tricompartmental arthritis; osteonecrosis; untreated malalignment (varus/valgus >5°); knee instability (no compliance to concomitant stabilization); patients who have had multiple intra-articular injections with steroids in the 3 months preceding the study; hip disorders that led to abnormal gait; general systemic illnesses such as rheumatic diseases, Bechterew syndrome, chondrocalcinosis, gout, and neurovascular diseases; and noncompliance to our rehabilitation protocol.12,14,19
All patients (10 males and 5 females) reached a minimum follow-up of 2 years (range, 24-38 months) and were active in sports but were not professional. The mean age was 48 years, ranging from 32 to 58 years. The BMI of the patients was 24.5 (standard deviation [SD], 2.53). Cartilage lesions were diagnosed by MRI and arthroscopy as grade 4 of ICRS classification. Six patients had multiple chondral lesions; the location of the lesions was 7 patella, 6 trochlea, 4 medial tibial plateau, 6 medial, and 1 lateral femoral condyle. The average cartilage lesion size per patient was 9.2 cm2 (SD, 6.3), ranging from 1.5 to 22 cm2. Twelve of our patients had coexisting pathologies such as tibiofemoral axial alignment, patellofemoral alignment, and ligamentous insufficiency, which were treated before or during the same surgery.31 Detailed demographic data, size and location of lesions, and surgical management of coexisting pathologies are reported in Table 1. All patients followed the same rehabilitation protocol for 8 months, which is similar to rehabilitation after second-generation ACI, based on current knowledge of the graft healing biology and on functional criteria and therapy goal progression (Table 2).
Table 1.
Demographic Data, Lesion Size, Colony-Forming Unit (CFU/mL), and Associated Procedures
| Patient/Side | Age/Sex | BMI | Sport / Activity | Location and Size of Lesions (mm × mm) | Size (cm2) | CFU MSC/mL | Associated Procedures |
|---|---|---|---|---|---|---|---|
| 1 / Right | 45 / M | 24 | Motocross | MFC 50 × 20 | 10 | 4,700 | ACLR |
| 2 / Right | 39 / F | 25 | Gymnastics | Patella 40 × 20 | 8 | 2,600 | Patellar realignment (Fulkerson) |
| 3 / Left | 47 / M | 24 | Tennis | Trochlea 25 × 20 | 5 | 4,600 | Opening wedge osteotomy |
| 4 / Right | 49 / M | 23 | Running | Trochlea 20 × 12 | 2.4 | 4,550 | None |
| 5 / Right | 48 / M | 24 | Tennis | Patella 45 × 15 | 6.75 | 4,600 | Opening wedge osteotomy |
| 6 / Left | 48 / F | 22 | Trekking, cycling | MTP 20 × 10 | 3 | 4,650 | None |
| 7 / Left | 58 / M | 30 | Swimming, cycling | MFC 20 × 30, MTP 13 × 10 | 7.3 | 3,650 | Opening wedge osteotomy |
| 8 / Right | 32 / M | 22 | Soccer | Patella 40 × 20 | 8 | 5,700 | ACLR |
| 9 / Right | 33 / F | 20 | Alpine skiing, trekking | Trochlea 30 × 25, patella 25 × 25, MFC 25 × 20 | 18.75 | 5,700 | Patellar realignment (Fulkerson) |
| 10 / Left | 50 / F | 25 | Gymnastics | Patella 12 × 8, patella 20 × 15 | 3.95 | 2,640 | Lateral release |
| 11 / Left | 41 / M | 28 | Hockey | Trochlea 40 × 30, MFC 18 × 23 | 16.15 | 3,100 | None |
| 12 / Left | 58 / M | 27 | Skiing, hunting | MTP 20 × 30, MFC 40 × 30, trochlea 20 × 20 | 22 | 2,435 | Opening wedge osteotomy |
| 13 / Left | 55 / M | 26 | Trekking, cycling | MTP 20 × 10, MFC 40 × 30, trochlea 15 × 10 | 15.5 | 2,808 | Opening wedge osteotomy |
| 14 / Left | 45 / M | 23 | Skiing | Patella 40 × 25 | 10 | 4,900 | ACLR (allograft) |
| 15 / Right | 53 / F | 25 | Skiing | LFC 11 × 11 | 1.5 | 2,000 | ACLR |
Note: MSC = mesenchymal stem cell; MFC = medial femoral condyle; MTP = medial tibial plateau; LFC = lateral femoral condyle; ACLR = anterior cruciate ligament reconstruction.
Table 2.
Rehabilitation Phases, Objectives, and Criteria to Progress between Phases
| Phase | Objectives | Criteria to Progress |
|---|---|---|
| Phase 1: Protection of the implant | Protect the transplant from excessive loads and shearing forces Decrease pain and effusion Gain full extension and gradual recovery of knee flexionRetard muscle atrophy |
Full active knee extension Knee flexion >120° No or minimum pain and swelling No pain during weightbearing Adequate muscle recruitment (quadriceps) |
| Phase 2: Transition and recovery of gait | Return to normal gait patter Progressive recovery in daily functional activities Increase the strength of the quadriceps and flexors Recovery of full range of motion |
Normal gait Recovery of nearly full range of motion (full extension, flexion >135°) Adequate muscle tone and neuromuscular control No pain or swelling |
| Phase 3: Maturation and recovery of running | Return to a correct running pathway Further increase in strength of quadriceps and flexors muscles Further increase in functional activities level |
Running without pain/swelling at 8 km/h for 10 minutes Adequate recovery of coordination and neuromuscular control Recovery of strength >80% contralateral limb Single-leg hop test >80% contralateral limb |
| Phase 4: Turnover and sport-specific recovery | Sustain high loads and impact activities Recover sport-specific skills Prepare athlete for a return to team and competition with good recovery of the aerobic endurance Maintain a good quality of life, avoiding excess of body fat and preventing risk of reinjury |
Running without pain/effusion at 10 km/h for 15 minutes Recovery of strength > 90% contralateral limb Single-leg hop test >90% contralateral limb Recovery of sport-specific skills |
X-rays and MRI were collected preoperatively and at 1- and 2-year follow-up; the standard radiographic evaluation included a standing anteroposterior long-leg radiograph, including also the hips and ankles, standing anteroposterior/lateral views of knees, skyline patellofemoral, and standing 45° bent-knee views. Visual analog scale (VAS) for pain, International Knee Documentation Committee (IKDC), Knee injury and Osteoarthritis Outcome Score (KOOS), Short-Form Health Survey (SF-36 Physical/Mental), Lysholm, Tegner, and Marx scores were collected preoperatively and at 6-, 12-, and 24-month follow-up. We also studied the difference in improvement between patients with single or multiple lesions as well as between subgroups of our patients according to the size of the lesion: 1) small-medium (1.5-5 cm2), 2) medium-large (5-10 cm2), and 3) large-multiple (>10 cm2). Four patients gave their consent for second-look arthroscopy but only 3 for a concomitant biopsy.
MRI Protocol
MRI assessment was carried out by a 1.5-T system (Quad Knee/8-CH SENSE Knee, Philips, Amsterdam, the Netherlands), and the recommended T1-weighted, T2-weighted, and intermediate-weighted contrast mapping protocol for MRI of the knee by the Hospital for Special Surgery was considered. Series I were performed using T2*-weighted 2-dimensional gradient recalled echo (FFE) sequences in an axial plane, with a TR of 33 milliseconds, TE of 13 milliseconds, flip angle (FA) of 30°, field of view (FOV) of 24 × 24 cm, thickness of 5 mm, and matrix of 256 × 128 (frequency × phase). Series II were carried out using proton density (PD)–weighted 2-dimensional fast/turbo spin echo (TSE) sequences in a coronal plane with TR of 4,000 to 4,500 milliseconds, TE of 34 milliseconds, FOV of 11 to 13 × 11 to 13 cm, thickness of 3.0 mm, intersection gap of 0.0 mm, and matrix of 512 × 288; receiver bandwidth was 125 Hz/pixel (water-fat shift 0.58 pixel at 1.5 T). Series III were performed using PD-weighted 2-dimensional TSE sequences with frequency-selective fat-signal suppression in a sagittal plane with TR of 3,500 to 4,000 milliseconds, TE of 40 milliseconds, FOV of 16 × 16 cm, thickness of 3.5 to 4.0 mm, intersection gap of 0.0 mm, and matrix of 256 × 224. T1-weighted 2-dimensional TSE sequences were performed in a sagittal plane with TR of 620 to 640 milliseconds, TE of 10 to 12 milliseconds, FOV of 16 × 16 cm, thickness of 4.0 mm, intersection gap of 0.4 mm, and matrix of 256 × 192. In MRI, we evaluated the filling of the defects, the restoration of the cartilage layer, the remodeling of the subchondral bone, and presence of hypertrophy of the neotissue.
Surgical Technique
All the procedures were performed under spinal anesthesia and routine sterile preparation and draping; 60 mL of bone marrow aspirate were harvested from the ipsilateral iliac crest using a dedicated aspiration kit and centrifuged using a commercially available system (BMAC Harvest Smart PreP2 System, Harvest Technologies, Plymouth, MA). In order to concentrate the baseline value of the bone marrow cells 4 to 6 times, we followed the method recommended by the manufacturer. Using a batroxobin enzyme (Plateltex Act, Plateltex S.R.O., Bratislava, Slovakia), the bone marrow concentrate was activated in order to produce a sticky clot material (Fig. 1A), which was implanted into the prepared cartilage defect.
Figure 1.
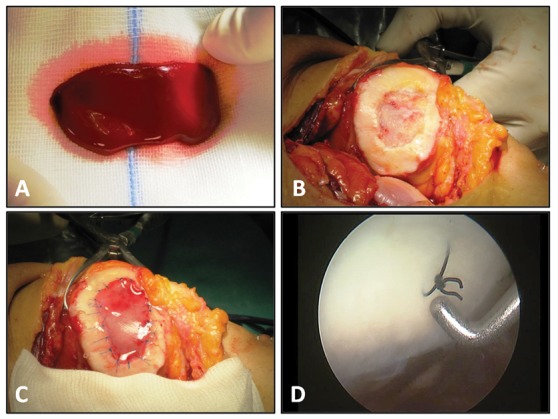
(A) Bone marrow aspirate concentrate (BMAC) clot after activation, (B) grade IV lesion of the patella, (C) covering the lesion with a collagen type I/III matrix after pasting the clot into the lesion, and (D) second-look arthroscopy at 2-year follow-up.
After arthroscopic evaluation, the knee was approached with a mini-arthrotomy, and the chondral defect was prepared and debrided with the use of curettes (Fig. 1B). Specific attention was paid to remove the calcified layer if present, while avoiding penetration of the subchondral bone and reducing the bleeding, as much as possible, from the bottom of the lesion. Damaged cartilage was removed until a contained, shouldered defect remained, which is necessary in order to facilitate suturing the scaffold. The defect was templated and the collagen membrane fashioned according to the defect size. Finally, the prepared clot was pasted into the lesion. In order to protect MSC, the defect was covered with a collagen-based membrane scaffold (Fig. 1C).
The membrane was anchored to the surrounding cartilage using PDS 6-0 and sealed with fibrin glue (Tissucol, Baxter, Rome, Italy); the knee was then ranged through flexion and extension in order to check the stability of the implanted membrane. Coexisting knee pathologies such as tibiofemoral axial alignment, patellofemoral alignment, and ligamentous insufficiency were treated during the same surgery in 12 patients.
Second-Look Arthroscopy
Second-look arthroscopy and biopsy were done in 4 knees after an average of 13.5 months of follow-up, but only 3 patients gave their consent for a biopsy (Fig. 1D). The first knee had a second look after the patient started complaining of mid–joint-line pain after 6 months. Knees 2 and 3 had a second-look arthroscopy in concomitance with hardware removal for a previous medial opening wedge osteotomy at 12 and 24 months. The fourth knee had a second-look arthroscopy in concomitance with an arthroscopy to the opposite knee for a partial meniscectomy at 12 months.
Histochemistry
Biopsies for histological analysis were fixed in 10% buffered formalin and washed and decalcified with a 4% HCl, 5% formic acid decalcificant solution until required. The samples were then dehydrated through a graded series of alcohol and embedded in paraffin. Sections, 4 µm thick, were obtained from the specimens and stored at room temperature. The slides were stained with 0.001% Fast Green and 0.1% Safranin-O (Sigma, St. Louis, MO) to evaluate the cellular morphology, visualize the proteoglycan content of the extracellular matrix, and highlight the presence of hyaline-like tissue. An independent, experienced histologist examined 4 distinct regions within the specimens: a global area, a superficial zone, an intermediate zone, and a deep zone, and calcified layer/bone transition. The knees were assessed using the ICRS visual scoring system.
Immunohistochemistry: Type I and II Collagens
For immunohistochemical analyses, the following primary antibodies were used: mouse monoclonal anti-human type I collagen (Chemicon International, Temecula, CA) and antihuman collagen type II mouse monoclonal antibody (Chemicon International). Paraffin sections were deparaffinized and rehydrated. For epitope unmasking, the samples were treated with 0.1% hyaluronidase (Sigma) in phosphate buffered saline (PBS) at 37 °C for 5 minutes. After washing, the slides for the detection of type I and II collagens were incubated at room temperature for 30 minutes in 1x PBS containing 5% of normal goat serum (NGS) (Dako, Carpinteria, CA) to prevent nonspecific bindings. The slides were incubated with the anti-human type I and II collagen primary antibodies diluted 1:20 in 0.04 M Trizma base saline (TBS), pH 7.6, containing 1% bovine serum albumin (BSA) and 0.1% Triton X-100 for 1 hour at room temperature. The slides were washed 3 times with 0.04 M TBS, pH 7.6, and incubated with goat anti-mouse and antirabbit immunoglobulins labeled with dextran molecules/alkaline phosphatase (Envision, Dako) at room temperature for 30 minutes. After 3 washes with 0.04 M TBS, pH 7.6, the reactions were developed using the new fuchsin kit (Kit New Fuchsin Substrate System, Dako) in the presence of 5 mM levamisole (Sigma) to block endogenous alkaline phosphatase. Negative staining controls were performed either by omitting the primary antibody or using a control isotype-matched antibody. Slides were counterstained with hematoxylin and mounted in glycerol gel. All the samples were visualized with a Zeiss axioscope microscope (Carl Zeiss, Oberkochen, Germany).
Statistical Analysis
For statistical analysis, the SPSS software was used (SPSS 17.0, SPSS, Chicago, IL). Nonparametric analysis was performed with Wilcoxon signed-rank tests in order to analyze the clinical outcome between preoperative and postoperative at 6, 12, and 24 months’ evaluation. Continuous data are described as average mean ± standard error of the mean (SEM). Z score and P values are provided for all the parameters evaluated. Reported P values are 1-tailed with an α level of 0.05 indicating significance.
We also studied the difference in improvement between patients with single or multiple lesions as well as between subgroups of our patients according to the size of the lesion; however, statistical analysis was not performed because of the small size of our subgroups. Therefore, we calculated the percentage of the maximum possible improvement for each score as follows: (score at final follow-up – preoperative score) / (best score – preoperative score) × 100. In particular, for the Tegner score, the preinjury value was considered as the best score, while for the VAS score, the best score was zero.
Results
Patients showed significant improvement in all scores at 6, 12, and 24 months’ follow-up (P < 0.05) (Figs. 2 and 3). No adverse reactions or postoperative complication were noted. Results are summarized in Table 3.
Figure 2.
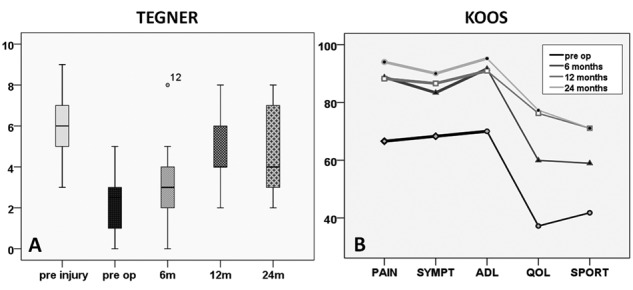
(A) Boxplots showing the significant improvement in Tegner score from preoperative evaluation to 6, 12, and 24 months (P < 0.005); however, the patients did not reach the preinjury value. (B) Diagram showing the significant improvement in Knee injury and Osteoarthritis Outcome Score (KOOS) subgroups from preoperative to 6, 12, and 24 months (P < 0.005).
Figure 3.
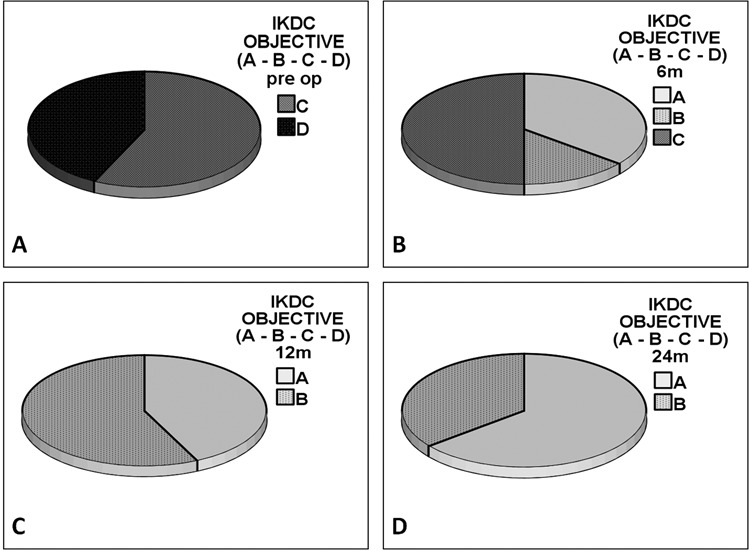
International Knee Documentation Committee (IKDC) objective score showed significant improvement in A and B subgroups from preoperative to 6, 12, and 24 months (P < 0.005).
Table 3.
Summary of Clinical Outcome
| Preoperative | 6-Month Follow-up | 12-Month Follow-up | 24-Month Follow-up | |||||
|---|---|---|---|---|---|---|---|---|
| Variable | Mean ± SEM | Mean ± SEM | P Value / Z | Mean ± SEM | P Value / Z | Mean ± SEM | P Value / Z | |
| VAS | 5.1 ± 0.04 | 0.8 ± 0.3 | 0.001 / −3.307a | 1.0 ± 0.3 | 0.001 / −3.315a | 0.7 ± 0.3 | 0.001 / −3.195a | |
| KOOS pain | 66.2 ± 5.6 | 88.7 ± 2.7 | 0.004 / −2.858a | 87.7 ± 3.8 | 0.016 / −2.415a | 94.0 ± 2.7 | 0.001 / −3.183a | |
| KOOS sym. | 68.2 ± 4.6 | 83.2 ± 2.5 | 0.004 / −2.906a | 86.5 ± 3.7 | 0.016 / −2.419a | 90.0 ± 2.7 | 0.003 / −3.018a | |
| KOOS ADL | 70.0 ± 6.1 | 91.6 ± 2.1 | 0.010 / −2.587a | 91.1 ± 3.9 | 0.023 / −2.272a | 95.1 ± 2.0 | 0.003 / −3.015a | |
| KOOS sport | 41.6 ± 7.9 | 59.0 ± 6.2 | 0.001 / −3.234a | 71.0 ± 5.2 | 0.023 / −2.272a | 71.3 ± 6.7 | 0.023 / −2.278a | |
| KOOS QOL | 37.2 ± 5.4 | 59.6 ± 6.0 | 0.013 / −2.482a | 76.1 ± 4.9 | 0.003 / −2.990a | 77.5 ± 4.4 | 0.002 / −3.030a | |
| IKDC subj. | 43.6 ± 5.6 | 66.9 ± 3.1 | 0.004 / −2.920a | 78.8 ± 3.3 | 0.002 / −3.045a | 80.7 ± 3.7 | 0.002 / −3.107a | |
| IKDC obj. | 8C / 7D | 5A / 8B / 2C | 0.002 / −3.145a | 6A / 9B | 0.001 / −3.442a | 10A / 5B | 0.001 / −3.402a | |
| SF-36 phys. | 39.0 ± 1.3 | 52.6 ± 1.1 | 0.001 / −3.233a | 56.0 ± 0.4 | 0.001 / −3.296a | 55.5 ± 0.9 | 0.001 / −3.296a | |
| SF-36 mental | 46.9 ± 1.7 | 54.1 ± 1.6 | 0.074 / −1.789a | 55.8 ± 1.0 | 0.004 / −2.856a | 54.0 ± 1.3 | 0.011 / −2.542a | |
| Tegner | 2.07 ± 0.3 | 3.2 ± 0.4 | 0.050 / −1.912a | 4.7 ± 0.4 | 0.001 / −3.203a | 4.9 ± 0.5 | 0.003 / −2.956a | |
| Marx | 4.2 ± 1.0 | 5.4 ± 1.1 | 0.406 / −0.831a | 7.8 ± 0.9 | 0.018 / −2.360a | 10.3 ± 1.0 | 0.004 / −2.906a | |
| Lysholm | 60.4 ± 5.5 | 88.3 ± 2.5 | 0.002 / −3.111a | 93.0 ± 2.5 | 0.001 / −3.297a | 92.9 ± 2.4 | 0.001 / −3.297a | |
Note: The variables are described as mean ± SEM (standard error of the mean). All the scores showed significant improvement from preoperative evaluation to 6-, 12-, and 24-month follow-up. VAS = visual analog scale; KOOS = Knee injury and Osteoarthritis Outcome Score; ADL = activities of daily loving; QOL = quality of life; IKDC = International Knee Documentation Committee.
Reported P values are 1-tailed with an α level of 0.05 indicating significance.
At the final follow-up, patients with single lesions showed higher improvement than patients with multiple lesions in all scores (Fig. 4 and Table 4), except for KOOS pain and symptom subgroups. However, the average KOOS values for these subgroups were comparable at final follow-up. Patients with smaller lesion sizes showed higher improvement at final follow-up (Fig. 5).
Figure 4.
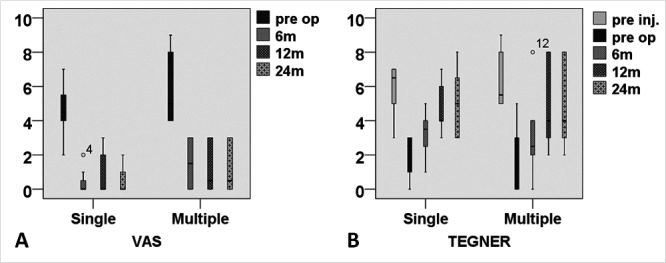
Visual analog scale (VAS) and Tegner score showing higher improvement in single versus multiple lesion patients from preoperative evaluation to final follow-up.
Table 4.
Comparison of the Outcome between Multiple and Single Lesion Patients
| Multiple Lesions | Single Lesions | |||
|---|---|---|---|---|
| Mean | SEM | Mean | SEM | |
| VAS | 79.8% | ± 8.9 | 84.4% | ± 9.5 |
| KOOS pain | 78.8% | ± 8.6 | 74.2% | ± 8.7 |
| KOOS symptoms | 74.9% | ± 7.9 | 67.4% | ± 8.6 |
| KOOS ADL | 75.6% | ± 10.2 | 86.7% | ± 7.6 |
| KOOS sport | 56.3% | ± 9.3 | 62.9% | ± 7.6 |
| KOOS QOL | 69.3% | ± 8.2 | 73.9% | ± 6.8 |
| Tegner | 79.3% | ± 17.8 | 87.0% | ± 16 |
| Marx | 48.9% | ± 6.1 | 66.8% | ± 8.2 |
| IKDC (subj.) | 64.9% | ± 8.3 | 71.6% | ± 7.5 |
| Lysholm | 75.1% | ± 8.7 | 85.1% | ± 7.6 |
Note: SEM = standard error of the mean; VAS = visual analog scale; KOOS = Knee injury and Osteoarthritis Outcome Score; ADL = activities of daily loving; QOL = quality of life; IKDC = International Knee Documentation Committee.
Figure 5.
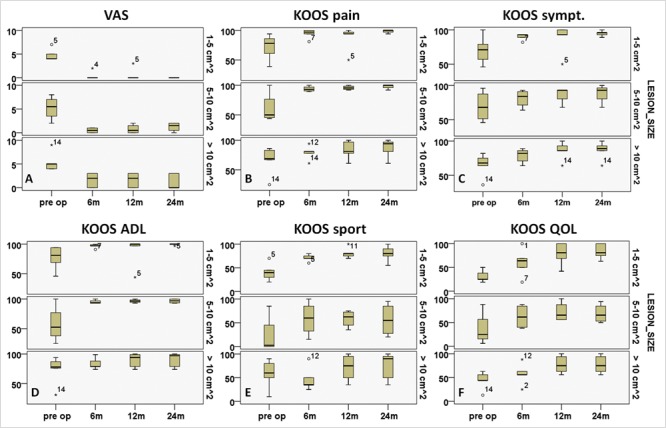
Visual analog scale (VAS) and Knee injury and Osteoarthritis Outcome Score (KOOS) scores showing higher improvement in patients with smaller lesions from preoperative to 6, 12, and 24 months.
After harvesting the bone marrow, we sent a sample to an independent laboratory in order to quantify the colony-forming unit (CFU/mL) of MSC per patient, which is a measurement of the viability of the bone marrow. The average CFU/mL of MSC per patient was 3,904 CFU/mL (SD, 1,232), ranging from 2,000 to 5,700 CFU/mL per patient (Table 1). However, we were unable to standardize patients according to the provided volume of the clot and the size of the lesion (CFU/cm2) because it was not possible to quantify the exact volume of the BMAC clot used to fill each lesion.
MRI showed complete filling of the defect in 12 of 15 patients (80%) and incomplete (<50% of the adjacent cartilage) in 3 of 15 patients (20%), while no signs of hypertrophy were identified. Integration with adjacent cartilage was complete in 14 of 15 patients (93.3%) with restoration of the cartilage layer and subchondral bone (Fig. 6). We also did not identify edema, cysts, or sclerosis of subchondral bone either.
Figure 6.
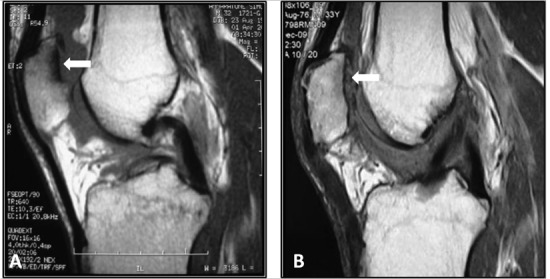
MRI in a 33-year-old amateur soccer player: (A) preoperative T1 sequence in sagittal plane showing a grade IV patellar lesion, (B) T1 sequence in sagittal plane at 2 years’ follow-up showing good coverage of the lesion, preoperative T2 sequence in axial plane showing a grade IV patellar lesion, and T2 sequence in axial plane showing good coverage of the lesion; the associated anterior cruciate ligament reconstruction is evident.
Second-look arthroscopies in 4 knees revealed smooth, newly formed tissue with continuous intact to the healthy surrounding cartilage in all 3 patients; no hypertrophy was identified. The stability of the implant appeared similar to the adjacent tissue as checked with a probe. Macroscopic evaluation showed normal to nearly normal as classified by the ICRS visual scoring system.
Good histological findings were reported for the 3 specimens analyzed, which presented many hyaline-like features. Results of the ICRS histological evaluation score are reported in Table 5. Histochemical and immunohistochemical evaluations of the 3 biopsies are described in Figures 7, 8, and 9.
Table 5.
International Cartilage Repair Society (ICRS) Visual Histological Assessment Scale
| Knee/Patient | I. Surface | II. Matrix | III. Cell Distribution | IV. Cell Population | V. Subchondral Bone | VI. Cartilage Mineralization |
|---|---|---|---|---|---|---|
| 1 / no. 6 | 1 (smooth) | 1 (fibrocartilage) | 0 (individual cells/disorganized) | 2 (viable) | 1 (active remodeling) | 1 (normal) |
| 2 / no. 3 | 1 (smooth) | 3 (hyaline/fibrocartilage) | 2 (mixed: columnar/clusters) | 2 (viable) | 2 (active remodeling) | 1 (normal) |
| 3 / no. 5 | 1 (smooth) | 4 (hyaline) | 3 (columnar) | 2 (viable) | 2 (active remodeling) | 1 (normal) |
Figure 7.
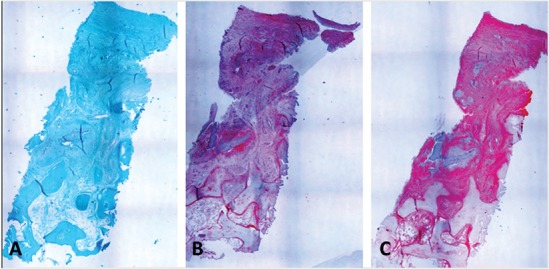
Biopsy of patient no. 6 obtained after 6 months (original magnification, 40x). (A) Safranin-O staining shows a structure, which is not well organized yet and with many fibrous features. The superficial layer is regular. Proteoglycans and cellular components are not represented. (B) Collagen type I immunostaining shows the positivity of the extracellular matrix in line with the presence of fibrous tissue. (C) The presence of type I collagen does not necessarily imply a negative outcome since positive intracellular staining for type II collagen in this case indicates ongoing remodeling.
Figure 8.
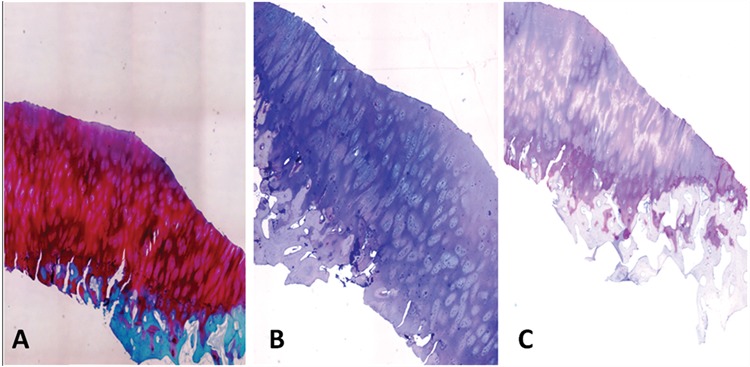
Biopsy of patient no. 3 obtained after 12 months (original magnification, 40x). (A) Safranin-O staining shows a hyaline-like repair tissue. The superficial layer is regular. The subchondral bone shows some signs of ongoing remodeling. Extracellular matrix shows high levels of proteoglycans. Columnar cellular organization of the repair tissue is observed. (B) Immunohistochemical analysis results of collagen type I are completely negative. (C) Collagen type II immunostaining is positive at the extracellular level.
Figure 9.
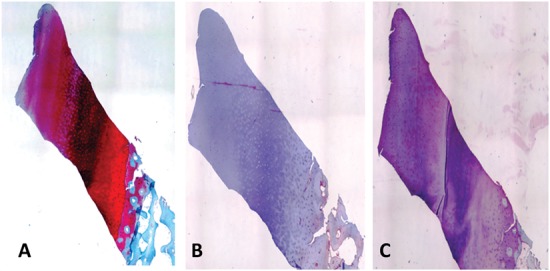
Biopsy of patient no. 5 after 24 months (original magnification, 40x). (A) Safranin-O staining reveals a well-organized cartilage tissue with the typical features of normal articular cartilage. The superficial layer is regular. The tidemark is well evident. The proteoglycan component is well represented, and the cells show regular distribution along the extracellular matrix. The subchondral bone tissue is in a remodeling process. (B) Immunohistochemical analysis results of collagen type I are almost negative with only a few positive cells at the superficial layer. (C) Type II collagen is slightly positive in the extracellular matrix and at the cellular level.
Discussion
The purpose of our study was to determine the effectiveness of cartilage repair utilizing 1-step surgery with BMAC, which represents a cell source of MSCs and GFs, covered after activation with a commercially available collagen type I/III matrix. Our group of patients showed significant improvement in all the scores (<0.005); furthermore, these good outcomes were correlated with MRI, arthroscopy, and available biopsy findings. Although only 15 patients were included in this nonrandomized prospective study, there are no corresponding studies in the literature analyzing a similar 1-step surgery procedure and providing clinical outcome and MRI evaluation. Despite the high number of experimental studies performed, only one study reported the use of BMAC in a single-step surgical procedure in the talus.32 To our knowledge, this is the first report of this 1-step approach for the treatment of large full-thickness cartilage lesions of the knee. Another unique feature of this study is the large average size of lesions (9.2 cm2). Considering that microfractures are usually used to treat lesions smaller than 3 cm2,33 and that the average size of lesions treated with ACI is also smaller (5.3 cm2 in the last report by Peterson et al. on 224 patients),34 our data pave the way to the treatment of large articular cartilage lesions. Another interesting result is that 80% of patients required concomitant procedures, which implies that coexisting pathologies such as tibiofemoral axial alignment, patellofemoral alignment, and ligamentous insufficiency are common in patients with cartilage lesions; in these patients, a concomitant procedure is recommended in order to protect the newly formed tissue.31
This study presents some limitations: the study design neither included a control group treated with an established procedure such as microfracture nor an untreated group for ethical reasons; furthermore, we did not find a control group with a comparable lesion size that could be treated with microfracture. Furthermore, there are possible confounding factors like tibiofemoral axial alignment, patellofemoral alignment, and ligamentous insufficiency, which may affect the outcome of treatment. The present study is a prospective nonrandomized one with a 2-year follow-up period, and only a limited number of patients gave written consent for second-look arthroscopy and biopsy.
Regarding the potential of MSCs for regenerative medicine, recent studies21-23,35 demonstrated that MSCs secrete bioactive molecules that stimulate angiogenesis and mitosis of tissue-specific and intrinsic progenitors and reduce T cell surveillance and inflammation, and some authors have also recognized that the presence of other nucleated cells is able to restore the damaged tissue.36-40 This recently revealed capacity of MSCs to secrete bioactive factors that are both immunomodulatory and regenerative paves the way to strategies that mimic natural tissue repair.41 According to this paradigm, cell selection and cultivation in the laboratory may not be necessary with a significant reduction to the cost of the total procedure, allowing the development of 1-step surgical procedures.
Ochi et al. observed in a rat model that the injection of cultured MSCs combined with microfracture could accelerate the regeneration of cartilage and concluded that this approach could represent an effective and less invasive strategy for the regeneration of articular surfaces.42 In another animal study on rats and rabbits,43,44 the same authors developed a cell delivery system based on MSCs bound to magnetic beads and on the use of an electromagnetic field, demonstrating the feasibility of the MSCs injected into the joint accumulating in the chondral defect, thus improving neocartilage synthesis and reducing the risk of ectopic cartilage formation. Enhanced chondrogenesis and improved cartilage healing have been demonstrated also in equine models after arthroscopic implantation with MSC.30 However, a rapid loss of implanted cells and deterioration in cartilage quality were observed. The authors concluded that the development of a system for intraoperative stem cell isolation, purification for immediate grafting, and cell stabilization into the defect could have significant advantages in time-saving and immediate application of a cell-based approach for cartilage repair. Grigolo et al. transplanted in a rabbit model of an OA knee a hyaluronan-based scaffold seeded with in vitro expanded bone marrow–derived MSCs. They performed histological, histomorphometric, and immunohistological evaluations showing better quality of the regenerated tissue between the implants with scaffolds carrying MSCs compared to the scaffold alone or controls in particular at 6 months.45
Another crucial issue in the clinical application of MSCs for cartilage repair is their phenotype stability.46 In fact, MSC-derived chondrogenic cells still possess a degree of plasticity and the tendency to proceed along the endochondral ossification route that can lead to calcification of the implant.46,47 In this regard, our strategy, based on the use of Chondro-Gide (Geistlich) may provide both the suitable environment to maintain stable cell phenotype and cell stabilization into the defect.48
Histological examination of the biopsies evaluated showed the regeneration of new tissues with many hyaline-like cartilage features such as the presence of a noticeable proteoglycan component around the chondrons and also collagen type II content. The biopsies showed a good organization of proteoglycans and collagens in the extracellular matrix, an intact superficial zone, and a not well-defined tidemark, suggesting that maturation of the neotissue is still undergoing. Specimens also showed a mild positivity for type I collagen, suggesting the presence of some fibrous features. Histological features of the 6-month biopsy demonstrated low cartilaginous quality of the tissue, suggesting that the repair tissue was still undergoing remodeling. Overall, even if biopsy specimens were obtained only from 3 knees, the observed level of maturity, at the latest time point, seems higher than that obtained by other authors with cell suspension autologous chondrocyte transplantation techniques at a similar time point.49,50
Regarding previous experiences with a similar 1-step procedure, Giannini et al. recently showed successful results of bone marrow–derived cell transplantation in talar osteochondral lesions by a 1-step procedure based on concentrated bone marrow–derived cells and collagen powder or hyaluronic acid membrane as scaffolds.32 Despite the differences between this study and ours, these results are in accordance with the data obtained by our present work and suggest a potential for this approach in the treatment of articular cartilage lesions.
This approach presents several positive features: its 1-step nature, the use of a collagen I/III–based matrix (Chondro-Gide, Geistlich), which favors cell concentration in the defect area and also allows early mobilization of the operated knee, and its lower cost if compared to standard 2-step ACI procedures. The good clinical outcome showed that the use of BMAC in full-thickness articular cartilage lesion repair can be a promising option for the treatment of knee cartilage defects; however, an increased sample size and longer term prospective randomized studies are needed to confirm these preliminary results.
Footnotes
Acknowledgments and Funding: The investigation was performed at Orthopaedic Arthroscopy Surgery International, Milan, Italy. The authors received no financial support for the research and/or authorship of this article.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- 1. Hunter W. On the structure and diseases of articulating cartilage. Philos Trans Rsoc Lond B Biol Sci. 1743;9:277. [Google Scholar]
- 2. Mankin HJ. The response of articular cartilage to mechanical injury. J Bone Joint Surg Am. 1982;64(3):460-6. [PubMed] [Google Scholar]
- 3. Nuki G. Osteoarthritis. In: Luqmani R, Robb J, Porter D, et al., editors. Textbook of orthopaedics, trauma and rheumatology. Chicago: Mosby; 2008. p. 193. [Google Scholar]
- 4. Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331(14):889-95. [DOI] [PubMed] [Google Scholar]
- 5. Behrens P, Bitter T, Kurz B, Russlies M. Matrix-associated autologous chondrocyte transplantation/implantation (MACT/MACI): 5-year follow-up. Knee. 2006;13(3):194-202. [DOI] [PubMed] [Google Scholar]
- 6. Peterson L, Brittberg M, Kiviranta I, Akerlund EL, Lindahl A. Autologous chondrocyte transplantation: biomechanics and long-term durability. Am J Sports Med. 2002;30(1):2-12. [DOI] [PubMed] [Google Scholar]
- 7. Peterson L, Vasiliadis HS, Brittberg M, Lindahl A. Autologous chondrocyte implantation: a long-term follow-up. Am J Sports Med. 2010;38(6):1117-24. [DOI] [PubMed] [Google Scholar]
- 8. Henderson IJ, Tuy B, Connell D, Oakes B, Hettwer WH. Prospective clinical study of autologous chondrocyte implantation and correlation with MRI at three and 12 months. J Bone Joint Surg Br. 2003;85(7):1060-6. [DOI] [PubMed] [Google Scholar]
- 9. Henderson I, Francisco R, Oakes B, Cameron J. Autologous chondrocyte implantation for treatment of focal chondral defects of the knee: a clinical, arthroscopic, MRI and histologic evaluation at 2 years. Knee. 2005;12(3):209-16. [DOI] [PubMed] [Google Scholar]
- 10. Minas T, Peterson L. Advanced techniques in autologous chondrocyte transplantation. Clin Sports Med. 1999;18(1):13-44. [DOI] [PubMed] [Google Scholar]
- 11. Bekkers JE, Inklaar M, Saris DB. Treatment selection in articular cartilage lesions of the knee: a systematic review. Am J Sports Med. 2009;37 Suppl 1:148S-55S. [DOI] [PubMed] [Google Scholar]
- 12. Kon E, Gobbi A, Filardo G, Delcogliano M, Zaffagnini S, Marcacci M. Arthroscopic second-generation autologous chondrocyte implantation compared with microfracture for chondral lesions of the knee: prospective nonrandomized study at 5 years. Am J Sports Med. 2009;37(1):33-41. [DOI] [PubMed] [Google Scholar]
- 13. Mandelbaum B, Browne JE, Fu F, Micheli LJ, Moseley JB, Jr, Erggelet C, Anderson AF. Treatment outcomes of autologous chondrocyte implantation for full-thickness articular cartilage defects of the trochlea. Am J Sports Med. 2007;35(6):915-21. [DOI] [PubMed] [Google Scholar]
- 14. Gobbi A, Bathan L. Minimally invasive second-generation autologous chondrocyte implantation. In: Cole BJ, Gomoll A, editors. Biologic joint reconstruction. Thorofare, NJ: SLACK Incorporated; 2009. p. 155-61. [Google Scholar]
- 15. Marcacci M, Kon E, Zaffagnini S, Filardo G, Delcogliano M, Neri MP, et al. Arthroscopic second generation autologous chondrocyte implantation. Knee Surg Sports Traumatol Arthrosc. 2007;15(5):610-9. [DOI] [PubMed] [Google Scholar]
- 16. Marcacci M, Zaffagnini S, Kon E, Visani A, Iacono F, Loreti I. Arthroscopic autologous chondrocyte transplantation: technical note. Knee Surg Sports Traumatol Arthrosc. 2002;10(3):154-9. [DOI] [PubMed] [Google Scholar]
- 17. Nehrer S, Domayer S, Dorotka R, Schatz K, Bindreiter U, Kotz R. Three-year clinical outcome after chondrocyte transplantation using a hyaluronan matrix for cartilage repair. Eur J Radiol. 2006;57(1):3-8. [DOI] [PubMed] [Google Scholar]
- 18. Sgaglione NA, Miniaci A, Gillogly SD, Carter TR. Update on advanced surgical techniques in the treatment of traumatic focal articular cartilage lesions in the knee. Arthroscopy. 2002;18(2 Suppl 1):9-32. [DOI] [PubMed] [Google Scholar]
- 19. Gobbi A, Kon E, Berruto M, Filardo G, Delcogliano M, Boldrini L, et al. Patellofemoral full-thickness chondral defects treated with second-generation autologous chondrocyte implantation: results at 5 years’ follow-up. Am J Sports Med. 2009;37(6):1083-92. [DOI] [PubMed] [Google Scholar]
- 20. Marcacci M, Berruto M, Brocchetta D, Delcogliano A, Ghinelli D, Gobbi A, et al. Articular cartilage engineering with Hyalograft C: 3-year clinical results. Clin Orthop Relat Res. 2005;435:96-105. [DOI] [PubMed] [Google Scholar]
- 21. Fortier LA, Balkman CE, Sandell LJ, Ratcliffe A, Nixon AJ. Insulin-like growth factor-I gene expression patterns during spontaneous repair of acute articular cartilage injury. J Orthop Res. 2001;19(4):720-8. [DOI] [PubMed] [Google Scholar]
- 22. Fortier LA, Mohammed HO, Lust G, Nixon AJ. Insulin-like growth factor-I enhances cell-based repair of articular cartilage. J Bone Joint Surg Br. 2002;84(2):276-88. [DOI] [PubMed] [Google Scholar]
- 23. Fortier LA, Potter H, Rickey E, Schnabel L, Foo L, Chong L, et al. Concentrated bone marrow aspirate improves full-thickness cartilage repair. Proceedings of the 55th ORS Annual Meeting; 2009 Feb 22-25; Las Vegas, NV. [DOI] [PubMed] [Google Scholar]
- 24. Gobbi A. L’impiego delle cellule mesenchimali autologhe e del gel piastrinico per il trattamento delle lesioni cartilaginee. Archivio di Ortopedia e Reumatologia. 2009;120(3-4):29-31. [Google Scholar]
- 25. Johnstone B, Hering TM, Caplan AI, Goldberg VM, Yoo JU. In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp Cell Res. 1998;238(1):265-72. [DOI] [PubMed] [Google Scholar]
- 26. Mackay AM, Beck SC, Murphy JM, Barry FP, Chichester CO, Pittenger MF. Chondrogenic differentiation of cultured human mesenchymal stem cells from marrow. Tissue Eng. 1998;4(4):415-28. [DOI] [PubMed] [Google Scholar]
- 27. Muraglia A, Cancedda R, Quarto R. Clonal mesenchymal progenitors from human bone marrow differentiate in vitro according to a hierarchical model. J Cell Sci. 2000;113:1161-6. [DOI] [PubMed] [Google Scholar]
- 28. Sudo K, Kanno M, Miharada K, Ogawa S, Hiroyama T, Saijo K, Nakamura Y. Mesenchymal progenitors able to differentiate into osteogenic, chondrogenic, and/or adipogenic cells in vitro are present in most primary fibroblast-like cell populations. Stem Cells. 2007;25(7):1610-7. [DOI] [PubMed] [Google Scholar]
- 29. Wakitani S, Imoto K, Yamamoto T, Saito M, Murata N, Yoneda M. Human autologous culture expanded bone marrow mesenchymal cell transplantation for repair of cartilage defects in osteoarthritic knees. Osteoarthritis Cartilage. 2002;10(3):199-206. [DOI] [PubMed] [Google Scholar]
- 30. Wilke MM, Nydam DV, Nixon AJ. Enhanced early chondrogenesis in articular defects following arthroscopic mesenchymal stem cell implantation in an equine model. J Orthop Res. 2007;25(7):913-25. [DOI] [PubMed] [Google Scholar]
- 31. Minas T. Autologous chondrocyte implantation in the osteoarthritic knee. In: Cole BJ, Malek M, editors. Articular cartilage lesions. New York: Springer-Verlag Inc.; 2004. p. 105-18. [Google Scholar]
- 32. Giannini S, Buda R, Vannini F, Cavallo M, Grigolo B. One-step bone marrow-derived cell transplantation in talar osteochondral lesions. Clin Orthop Relat Res. 2009;467(12):3307-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Salzmann GM, Niemeyer P, Steinwachs M, Kreuz PC, Südkamp NP, Mayr HO. Cartilage repair approach and treatment characteristics across the knee joint: a European survey. Arch Orthop Trauma Surg. Epub 2010. January 16. [DOI] [PubMed] [Google Scholar]
- 34. Peterson L, Vasiliadis HS, Brittberg M, Lindahl A. Autologous chondrocyte implantation: a long-term follow-up. Am J Sports Med. 2010;38(6):1117-24. [DOI] [PubMed] [Google Scholar]
- 35. Robey PG, Bianco P. The use of adult stem cells in rebuilding the human face. J Am Dent Assoc. 2006;137(7):961-72. [DOI] [PubMed] [Google Scholar]
- 36. Caplan AI. Why are MSCs therapeutic? New data: new insight. J Pathol. 2009;217:318-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Caplan AI. Mesenchymal stem cells: cell-based reconstructive therapy in orthopedics. Tissue Eng. 2005;11:1198-211. [DOI] [PubMed] [Google Scholar]
- 38. Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98:1076-84. [DOI] [PubMed] [Google Scholar]
- 39. Jones E, McGonagle D. Human bone marrow mesenchymal stem cells in vivo. Rheumatology (Oxford). 2008;47(2):26-31. [DOI] [PubMed] [Google Scholar]
- 40. Wang L, Li Y, Chen X, Chen J, Gautam SC, Xu Y, Chopp M. MCP-1, MIP-1, IL-8 and ischemic cerebral tissue enhance human bone marrow stromal cell migration in interface culture. Hematology. 2002;7(2):113-7. [DOI] [PubMed] [Google Scholar]
- 41. Caplan AI. Mesenchymal stem cells: the past, the present, the future. Cartilage. 2010;1(1):6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ochi M, Adachi N, Nobuto H, Yanada S, Ito Y, Agung M. Articular cartilage repair using tissue engineering technique: novel approach with minimally invasive procedure. Artif Organs. 2004;28(1):28-32. [DOI] [PubMed] [Google Scholar]
- 43. Kobayashi T, Ochi M, Yanada S, Ishikawa M, Adachi N, Deie M, et al. A novel cell delivery system using magnetically labeled mesenchymal stem cells and an external magnetic device for clinical cartilage repair. Arthroscopy. 2008;24(1):69-76. [DOI] [PubMed] [Google Scholar]
- 44. Yanada S, Ochi M, Adachi N, Nobuto H, Agung M, Kawamata S. Effects of CD44 antibody—or RGDS peptide—immobilized magnetic beads on cell proliferation and chondrogenesis of mesenchymal stem cells. J Biomed Mater Res A. 2006;77 (4):773-84. [DOI] [PubMed] [Google Scholar]
- 45. Grigolo B, Lisignoli G, Desando G, Cavallo C, Marconi E, Tschon M, et al. Osteoarthritis treated with mesenchymal stem cells on hyaluronan-based scaffold in rabbit. Tissue Eng Part C Methods. 2009;15(4):647-58. [DOI] [PubMed] [Google Scholar]
- 46. Pelttari K, Steck E, Richter W. The use of mesenchymal stem cells for chondrogenesis. Injury. 2008;39 Suppl 1:S58-65. [DOI] [PubMed] [Google Scholar]
- 47. Scotti C, Tonnarelli B, Papadimitropoulos A, Scherberich A, Schaeren S, Schauerte A, et al. Recapitulation of endochondral bone formation using human adult mesenchymal stem cells as a paradigm for developmental engineering. Proc Natl Acad Sci U S A. 2010;107(16):7251-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Scotti C, Wirz D, Wolf F, Schaefer DJ, Bürgin V, Daniels AU, et al. Engineering human cell-based, functionally integrated osteochondral grafts by biological bonding of engineered cartilage tissues to bony scaffolds. Biomaterials. 2010;31(8):2252-9. [DOI] [PubMed] [Google Scholar]
- 49. Horas U, Pelinkovic D, Herr G, Aigner T, Schnettler R. Autologous chondrocyte implantation and osteochondral cylinder transplantation in cartilage repair of the knee joint: a prospective, comparative trial. J Bone Joint Surg Am. 2003;85-A(2): 185-92. [DOI] [PubMed] [Google Scholar]
- 50. Knutsen G, Engebretsen L, Ludvigsen TC, Drogset JO, Grøntvedt T, Solheim E, et al. Autologous chondrocyte implantation compared with microfracture in the knee: a randomized trial. J Bone Joint Surg Am. 2004;86-A(3):455-64. [DOI] [PubMed] [Google Scholar]


