Abstract
Objective:
Many approaches are being taken to generate cartilage replacement materials. The goal of this study was to use a self-aggregating suspension culture model of chondrocytes with mechanical preconditioning.
Design:
Our model differs from others in that it is based on a scaffold-less, self-aggregating culture model that produces a cartilage tissue analog that has been shown to share many similarities with the natural cartilage phenotype. Owing to the known loaded environment under which chondrocytes function in vivo, we hypothesized that applying force to the suspension culture–derived chondrocyte biomass would improve its cartilage-like characteristics and provide a new model for engineering cartilage tissue analogs.
Results:
In this study, we used a specialized hydrostatic pressure bioreactor system to apply mechanical forces during the growth phase to improve biochemical and biophysical properties of the biomaterial formed. We demonstrated that using this high-density suspension culture, a biomaterial more consistent with the hyaline cartilage phenotype was produced without any foreign material added. Unpassaged chondrocytes responded to a physiologically relevant hydrostatic load by significantly increasing gene expression of critical cartilage molecule collagen and aggrecan along with other cartilage relevant genes, CD44, perlecan, decorin, COMP, and iNOS.
Conclusions:
This study describes a self-aggregating bioreactor model without foreign material or scaffold in which chondrocytes form a cartilage tissue analog with many features similar to native cartilage. This study represents a promising scaffold-less, methodological advancement in cartilage tissue engineering with potential translational applications to cartilage repair.
Keywords: bioengineering, cartilage tissue engineering, hydrostatic loading, cartilage repair
Introduction
Osteoarthritis often results from cartilage injuries and overuse. This debilitating joint disease has hallmark qualities of progressive and worsening erosion of joint surfaces. Clearly, osteoarthritis has profound ramifications to both human health and the economy. The functional properties of cartilage are the result of its highly ordered, composite structure of a specialized extracellular matrix (ECM) composed of a number of proteins including proteoglycans and collagen molecules. Any successful repair of cartilage defects or injuries through bioengineering may be dependent on the combination of cellular, molecular, and biophysical characteristics of the material produced.
Bioengineering of cartilage attempts to generate in vitro a biomaterial suitable to repair or replace cartilage tissue that has mechanical and biochemical characteristics similar to native cartilage. The goal of this approach is to generate a biomaterial using a culture model, whereby the cartilage phenotype of cells can be maintained, generating over time a cartilage tissue analog (CTA) with similarities to articular cartilage. Numerous cell, scaffold, and culture technique combinations have yielded a complicated biological and biomechanical design paradigm1-3 with many different outcomes. Frequently used scaffolds or matrices include collagen, fibrin, alginate, agarose, and hyaluronan, and while many have their positive attributes, difficulties arise from the use of these scaffolds including biocompatibility and biodegradability.4-7
In our study, a self-aggregating suspension culture (SASC) model was used to produce a CTA that bears many similarities to natural cartilage. This scaffold-less suspension culture approach has been shown to produce a tissue-engineered structure with a cartilage-like phenotype by our laboratory.8,9 Our model differs from many other approaches in that it does not use a foreign scaffold, perhaps increasing the biocompatibility for cartilage replacement. In our model, chondrocytes are cultured at high density in dishes coated with poly(2-hydroxyethyl methacrylate), which prevents the cells from attaching to the plastic substrate. The role and importance of nonadherence on scaffold-less cultures have been previously studied.10,11 In our model and due to nonadherence, the chondrocytes coalesce and rapidly form a mass that increases over time.12 It has been shown that the cultures continue to produce collagen type II and do not produce collagen type I, which would be indicative of their differentiation to a fibroblastic phenotype.9 In a recent study, we demonstrated that, using this high-density suspension culture, a biomaterial consistent with the hyaline cartilage phenotype in both the expression of collagen type II and aggrecan with biomechanical characteristics was generated and approached that of normal adult cartilage.9 A CTA was generated with properties that were similar to several other studies where scaffolds or starting foreign material was used, whereas our biomaterial does not have this complication.
Hydrostatic pressure has been shown to be beneficial to cartilage in a number of studies.13 Human joints encounter up to 3 to 15 MPa of pressure with normal daily activities.13-15 Cartilage thickness has been shown to coincide with areas exposed to increased hydrostatic pressure in vivo.16-19 Mechanical loading during in vitro culture stimulates biosynthesis and improves the mechanical properties and biochemical composition of tissue-engineered articular cartilage and includes cyclical dynamic loading,20 dynamic shearing,21 and cyclical hydrostatic pressure.13,22 Dynamic loading was shown to increase proteoglycan synthesis and accumulation without increased general protein expression.23,24 Dynamic shear was shown to increase collagen production and stimulate the synthesis of proteoglycans in cartilage explants.25
Hydrostatic pressure is a well-understood method of mechanical stimulation because it provides a uniform stress throughout the material, is similar to the loading pattern in many physiological situations for articular cartilage, and does not impede the transport of nutrients, restrict growth, or damage the surface of the tissue culture, as would occur under compressive loading with a platen.26 A synergistic relationship between hydrostatic pressure and growth factors on the growth of cartilage has also been recently noted.27 While the beneficial effects of loading on cartilage or chondrocytes are clear, no report has taken the approach of combining a physiologically relevant load and a suspension culture model that maintains so closely the cartilage phenotype. Hydrostatic compression of chondrocytes has been shown to enhance matrix synthesis in several different culture systems: cartilage explants,28,29 high-density monolayer,30 agarose gel,31 and collagen sponge.32 Cyclic hydrostatic pressure significantly increases glycosaminoglycan (GAG) content and collagen biosynthesis in chondrocyte pellet cultures.33
In vivo, abnormal joint loading leads to cellular and biochemical changes that can change chondrocyte activity, degrade cartilage, and progress to diseases such as osteoarthritis. Excessive stress and injury to cartilage including that seen in degenerative joint diseases change a number of cell functions including nitric oxide (NO) production.34-37 In this model, we tested if chondrocytes grown under loaded conditions underwent any deleterious stress by examining the production of NO and nitric oxide synthase (iNOS).
The purpose of this study was to establish a viable new approach to generating a cartilage repair biomaterial and, using our SASC model, determine the biochemical and mechanical changes that occur within the CTA that is formed following mechanical loading. Specimens were exposed to hydrostatic pressure and compared with culture-time–matched unloaded control specimens. We hypothesized that using our SASC model and a physiologically relevant loading protocol, an increase in cartilage-specific gene expression can be achieved. Using this SASC model, our study demonstrates that primary chondrocyte CTAs developed without the addition of scaffold or foreign material have some similarities resembling normal cartilage and moreover responded in a positive manner to a physiologically relevant hydrostatic load by generating a cartilage-appearing biomaterial in regard to cell:matrix ratio and by significantly increasing expression of critical cartilage molecules.
Materials and Methods
Cartilage, Chondrocyte Isolation, and Culture Model
Neonatal Yorkshire pigs were obtained within 2 hours of death from a breeding farm associated with the University of Pennsylvania’s New Bolton Center. The Institutional Animal Care and Use Committee (IACUC) reviewed the protocol, exempted it from full review, and approved the procedure. One animal was used for each of the 6 individual studies. Femoral head and condyle articular cartilage were removed under sterile conditions, and the cartilage was held at 4 °C until isolation of chondrocytes was possible (≤18 hours) in Dulbecco’s minimum essential medium (DMEM) (Mediatech, Herndon, VA). The DMEM contained 10% fetal bovine serum (Gibco-BRL, Grand Island, NY), 100 U/mL of penicillin, 100 µg/mL of streptomycin, 2.5 µg/mL of amphotericin B, 2 mM glutamine, and 1% MEM vitamin solution (referred to as “complete medium”) (Invitrogen, Carlsbad, CA). Soft tissue and perichondrium were removed, and the cartilage was isolated free of any growth plate before cutting it into pieces of approximately 3 mm3. The procedure for chondrocyte isolation followed, as previously described.9 Briefly, the articular cartilage was processed through enzymatic digestion with bacterial collagenase initially at 2 mg/mL for 1 hour followed by 0.5 mg/mL overnight in complete medium at 37 °C. The average yield was 4.0 × 107 cells/g of tissue. The isolated chondrocytes were plated at a density of 2 × 107/well/1 mL in a 24-well plate (2.4 cm2/well) treated with polyHEMA (or “low cluster plate”) (Becton, Dickinson and Company, Franklin Lakes, NJ) in a static culture as previously described with n = 3.12 The culture medium used throughout was complete medium with 50 µg/mL of ascorbic acid and was changed every 2 to 3 days. The cells were incubated at 37 °C in 5% CO2; during loading periods, the device was also warmed to 37 °C.
Hydrostatic Loading of the Cartilage Tissue Analog
The chondrocytes self-aggregate and quickly form a mass during the initial culture period of 1 week prior to the initiation of loading; we refer to them as a CTA. Within 1 week, a mass of cells and matrix was formed that could be moved from the well without perturbation. Six CTAs were separated into 2 groups: the unloaded control group and the loaded group. The control specimens remained under the same culture conditions, while the loaded CTAs were placed in a 4-well plate containing complete medium. The cultures were moved to customized vials (1.0-mL cryovials) that previously had the top drilled open and replaced with a flexible membrane. Vial caps were modified replacing the solid surface with a flexible membrane that allowed for the transmission of hydrostatic pressure and protected the CTAs from contamination. The sealed tubes were then placed in a custom-built pressure steel chamber and filled with hydraulic fluid and sealed, and the air was extracted. The chamber was contiguous with a 2.5-kip hydraulic piston, which was mounted onto a materials testing machine (Instron Corp., Canton, MA). The testing machine crosshead compressed the piston and loaded the chamber and the cultures (Fig. 1). Cyclical loading from 0.5 to 5.0 MPa was applied for 3 hours 3 times a week for 3 weeks at a frequency of 0.1 Hz. To maintain the temperature, the chamber was warmed during the procedure to 37 °C by sitting atop a controlled heating block.
Figure 1.
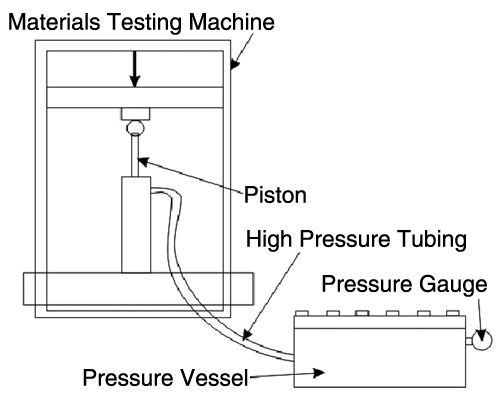
Diagram of the bioreactor used to apply the load. The chamber was contiguous with a 2.5-kip hydraulic piston, which was mounted onto a materials testing machine.
After each loading session, specimens were decompressed, removed from the chamber, and transferred from the vials to the storage wells with fresh complete medium. The loaded CTAs were kept in an incubator between loading sessions. During the 3-week experiment, the medium was replaced for both the loaded and control specimens after each loading session. Spent medium was saved prior to each loading session and stored frozen (–20 °C) with protease inhibitors (1x) (Roche, Basel, Switzerland) for later analysis of collagen and proteoglycan expression changes.
Immunohistochemistry
Six-micrometer sections were prepared, deparaffinized, and blocked with a hydrogen peroxide block and a standard blocking agent from the UltraVision ONE Detection System HRP Polymer & AEC Chromogen kit (LabVision, Fremont, CA). Immunohistochemistry was performed for collagen type II using a commercially available antibody that works with several species (including porcine tissue) by utilizing a conserved epitope (Chondrex, Redmond, WA) followed by an HRP Polymer detection system with AEC Chromogen (LabVision) using the manufacturer’s specifications. Representative images were taken at low (20x) and high (40x) magnification. Thickness measurements were also taken from stained sections using the Image-Pro Plus software (Media Cybernetics, Silver Spring, MD) and a calibrated Nikon microscope (Tokyo, Japan). After collagen type II immunohistochemistry was performed, sections were stained with Alcian blue (1% in 3% acetic acid wt/vol) to identify the anionic GAG characteristic of proteoglycans. Micrographs are shown from a single experiment representative of all experiments. To provide semiquantitative information on the relative intensity of staining for collagen and PG, gray-scale images were used to determine the value for staining above the background. Data are presented in results as percentage change with loading as compared to control unloaded CTA.
Hydroxyproline Assay
Levels of hydroxyproline were determined relative to other amino acids as an estimation of collagen content (in chondrocyte CTAs, the major collagen being collagen type II). A hydroxyproline assay was conducted on both medium and sample tissue. Medium was removed and saved in 25x complete protease inhibitor (Roche). Sample medium (n = 2) was acidified with equal volume 12 N hydrochloric acid (HCl), gassed with nitrogen, and sealed tightly. Hydrolysis took place at 110 °C for 24 hours. The pH was adjusted using Poly-Prep prefilled chromatography columns with H+ cation exchange resin (Bio-Rad Laboratories, Hercules, CA). Amino acids were eluted with 4 M ammonium hydroxide (NH4OH). The samples were dried and resuspended in 20 nM HCl. The entire sample was then run on a high-performance liquid chromatography column (HPLC). Peaks were identified for hydroxyproline and other common amino acids for standardization, and the ratio of hydroxyproline to tyrosine was used to measure relative collagen in each sample.
GAG Assay
GAGs were measured in medium and engineered biomaterial. Frozen samples were thawed and papain digested in a solution consisting of 150 µg/mL type III papain, 20 mM sodium phosphate, 1 mM EDTA, and 2 mM dithiothreitol at pH 6.8. Samples were digested at 60 °C for 24 hours. After centrifugation to remove particulate, samples were adjusted to pH 8 by the addition of 5 M NaOH (4 µL NaOH/mL sample) and heated to 55 °C for 2 hours to dissolve insoluble DNA. GAG and DNA were quantified separately, and the ratio was used to determine a quantity of GAG produced per cell. GAG content was determined in each sample (tissue or medium) using the dimethyl methylene blue binding assay (Blyscan, BioScan, Belfast, Ireland). Fifty microliters of the digest or medium was analyzed following the procedure as described by the manufacturer. Chondroitin 4-sulfate purified from bovine trachea was used to produce a standard curve (0-5 µg) (BioScan). Triplicates were averaged from both the DNA and GAG assay and expressed as GAG/DNA.
DNA Assay
DNA was measured using the Picogreen reagent (Molecular Probes, Eugene, OR). Briefly, 100 µL digest was added in triplicates to a microplate. A standard curve was prepared with 2.0 µg/mL and assayed in triplicates (0.06-2.0 µg/mL). Picogreen reagent was prepared in low light at a 1:200 dilution in 1x TE buffer, added at equal volume (100 µL), and mixed. After 5 minutes, the plate was read at 485/20 excitation and 530/25 emission on a CytoFluor fluorometer (PerSeptive Biosystems, Stafford, TX).
RNA Isolation
Total RNA was isolated using the following procedure. Snap-frozen samples were homogenized using a freezer mill (Spex LLC, Metuchen, NJ). The frozen powder was quickly transferred to a centrifuge tube and solubilized with 1 mL of Trizol Reagent (Invitrogen). Following the manufacturer’s protocol, total RNA was isolated and solubilized in 60 µL DEPC-treated water. The concentration of total RNA was determined by a spectrophotometer. The OD260 was used to determine RNA concentration with OD260:OD280 values between 1.5 and 2.0. To remove DNA contamination and fragments of RNA, the entire volume of RNA from each sample was applied to a silica membrane spin column (Qiagen, Valencia, CA). The samples then were on-column digested with RNase-free DNase-I before being eluted (Qiagen). Using oligo (dT) and random hexamer primer reverse transcriptase, 1 µg total RNA was reverse transcribed into cDNA in 40 µL reactions (iScript, Bio-Rad Laboratories). The RT protocol consisted of 5 minutes at 25 °C, 50 minutes at 42 °C, followed by 5 minutes at 85 °C in an iCycler (Bio-Rad Laboratories).
Quantitative PCR
Levels of mRNA were determined using specific primers for genes of interest (CD44, collagen types II, aggrecan, perlecan, decorin, COMP, iNOS). From cDNA prepared as described above (n = 3), real-time PCR was performed using an ABI 7900 real-time workstation (Carlsbad, California). Each reaction was performed in triplicate and consisted of 35 µL 2x SYBR Green with ROX internal reference dye (iTaq, Bio-Rad Laboratories), 2.1 µL forward and reverse primers, 3.5 µL template, and RNAse-free water up to 70 µL. Using a robotic automatic pipettor, each mixture was distributed into 20 µL triplicates. PCR was performed for cycles using the following parameters: 95 °C for 30 seconds, various optimized annealing temperatures for 30 seconds, and extension at 72 °C for 40 seconds. Primers were designed to span introns using known sequences obtained from Genbank, had products ≤350 base pairs, and were optimized prior to use for efficiency (Table 1). Housekeeping genes (HKG) (GAPDH) were used for relative expression studies to normalize the expression to cDNA loading quantity. A calibrator positive control (CPC) sample was used for ΔΔCT expression for each gene of interest (GOI). The formulas used to determine relative expression were as follows:
Table 1.
Specific primers sequences used and product size used for quantitative PCR
| Primer | Forward Sequence | Reverse Sequence | Product Size |
|---|---|---|---|
| GAPDH | 5’-TCCCTGCTTCTACTGGTGCT-3’ | 5’-TGAGCTTGACAAAGTGGTCG-3’ | 315 |
| Collagen II | 5’-TGTTCTGAGAGGTCTTCCTGGCAA-3’ | 5’-CAGGAGCTCCAGCTTCACCA-3’ | 200 |
| Aggrecan | 5’-AGCCTGAGGAGCCCTTTACATTTG-3’ | 5’-ACACTGCTCGTAGCCTGCTTC-3’ | 361 |
| Decorin | 5’-TGCCCAAAACTCTTCAGGAGC-3’ | 5’-GGTATCAGCAATGCGGATGTAGG-3’ | 188 |
| CD44 | 5’-TACAGCATCTTCCACACCCAC-3’ | 5’-GTTTGCCTCACCTTCTTGACTCC-3’ | 195 |
| Collagen IIA | 5’-TCTGCAGAATGGGCAGAGGTATA-3’ | 5’-TTTGGGTCCTACAATATCCTTGATG-3’ | 253 |
| COMP | 5’-TCATGTGGAAGCAGATGGAG-3’ | 5’-TAGGAACCAGCGGTAGGATG-3’ | 224 |
| iNOS | 5’-GCACTCACCTATTTCCTGGACATC-3’ | 5’-ATGTGGGGCTGTTGGTGA-3’ | 157 |
| Perlecan | 5’-TCGCTCCATCGAGTACAGCC-3’ | 5’-GCAGGCTCTTGGGAACTGGGG-3’ | 324 |
Average CTGOI – Average CTHKG = ΔCTSAMPLE
ΔCTSAMPLE – ΔCTCPC = ΔΔCT
Average and SEM of biological replicate ΔΔCTs taken
2−ΔΔCT = Relative expression.38
Results were confirmed by standard RT-PCR using the iTaq DNA Polymerase kit (Bio-Rad Laboratories). Analysis was performed by densitometry, and relative expression was consistent with the real-time data. Statistical analysis was performed using single-factor analysis of variance (ANOVA). Differences were considered significant at P < 0.05.
Measurement of NO
Samples of medium were saved from all time points in culture and stored at –20 °C in protease inhibitors. The presence of NO in medium would indicate an unfavorable response to load. To measure production of NO by our CTAs in response to loading, we employed the use of the Griess reagent (Sigma, St. Louis, MO). The Griess reagent measures nitrite, the stable end product of NO. NO was measured by established methods that have been previously used.36,37,39 GAG chains present in some media were precipitated out and removed by centrifugation prior to the assay by application of protamine sulfate (10 mg/mL) (Sigma). An equal volume of Griess reagent was added to supernatant, and the OD540 was read after 15 minutes.
Results
Gross Appearance and Immunohistochemistry
The CTAs generated from control and loaded chondrocyte cultures were analyzed for their biomechanical, biochemical, and molecular characteristics. Gross appearance of the CTA formed in unloaded or loaded samples was similar and took a disc-like shape (Fig. 2) that with time appeared more whitish and opaque with characteristics similar to those of samples in previous studies.9 Figure 2 is a representative macroscopic image of CTAs produced from porcine articular chondrocytes. In the experiment shown, chondrocyte CTAs were cut in half prior to the photograph and used for RNA isolation, hence its half-disc appearance. No macroscopic differences were evident in either control (top row) or loaded (bottom row) cultures, and they all were 0.5 to 1.0 cm in diameter. Thickness differed significantly (P < 0.001) after 1 week from unloaded (259.1 ± 22.5 µm) to loaded (184.8 ± 5.6 µm) but not after 3 weeks (197.2 ± 10.6 µm unloaded to 186.6 ± 6.6 µm loaded) (Fig. 2A). Microscopically, it was evident that the chondrocytes maintained their round cell shape and showed varied degrees of organization with time, and over the 3-week culture period, there was an increasing accumulation of ECM. Collagen type II staining was demonstrated in all CTAs assessed. Representative images are presented at 20x and 40x magnification after immunohistochemistry for collagen type II. Figure 3A shows unloaded samples at 1 week (panels I and II) and 3 weeks (panels III and IV). Figure 3B shows loaded samples at 1 week (panels I and II) and 3 weeks (panels III and IV). Collagen type II staining increases noticeably from 1 week to 3 weeks in culture. The staining appears more dense for collagen type II in the loaded samples over controls. In addition, staining is increased in the 3-week specimens over 1-week specimens. The overall staining intensity in all images assessed was between 3% and 5%. It can be noted there is some spatial heterogeneity and regions of even greater collagen at the periphery. Sections were stained with Alcian blue (Fig. 4A-D), and in these representative images, it is clear that staining for GAG is more intense in the loaded CTA. Loading resulted in a 7.6% increase in staining intensity for PG at 1 week and 6.8% increase of staining intensity at 3 weeks. Additionally, loading results in a CTA that appears somewhat more compressed or dense, an observation that is most apparent in the 1-week samples. Images were photographed at the same magnification.
Figure 2.
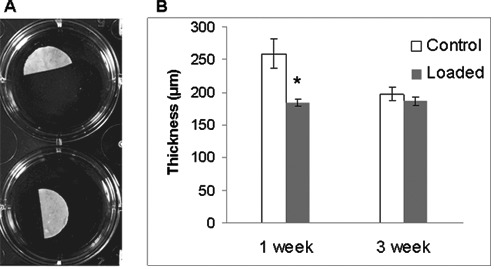
(A) Suspension culture of porcine chondrocytes for 3 weeks (after 1-week preconditioning). Macroscopic pictures of porcine chondrocyte CTAs. This shows examples of typical macroscopic appearance of culture in a 24-well culture plate. Top row, unloaded; bottom row, loaded. CTAs shown were cut in half for RNA isolation prior to photograph. Well diameter is 1.5 cm. (B) Thickness of CTA as measured at 1 week and 3 weeks form control, unloaded CTA, and loaded CTA. Measurements were made microscopically using cross-sections and performed at 4 locations (n = 3). *Significant difference (P < 0.001) after loading at 1 week.
Figure 3.

Paraffin-embedded sections were deparaffinized, and immunohistochemistry was performed for porcine collagen type II. All images are representative and taken at 20x and 40x magnification (e.g., panels I and II or panels III and IV, respectively). (A) Unloaded samples at 1 week (panels I and II) and 3 weeks (panels III and IV). (B) Loaded samples at 1 week (panels I and II) and 3 weeks (panels III and IV).
Figure 4.
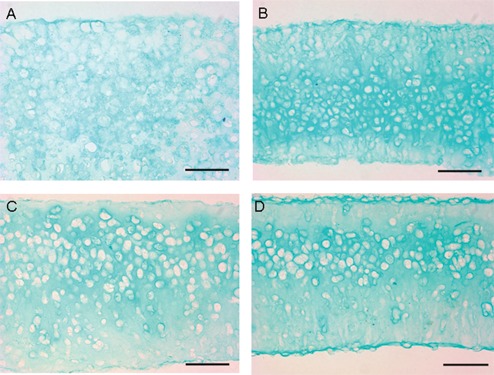
Demonstration of proteoglycan distribution as shown by staining with Alcian blue for GAG chains. Representative CTA at 1 week (A and B) and CTA at 3 weeks (C and D). A and C are unloaded; B and D are loaded. Images were photographed at 40x; bar represents 50 µM.
Extracellular Matrix
The effects of loading chondrocyte CTAs were assessed after various loading durations (1-3 weeks of loading after 1 week in suspension culture). Medium was removed every 2 days for the duration of the study (21 days). Medium and CTAs (at study’s end) were then assessed for hydroxyproline content by hydrolysis followed by HPLC. Hydroxyproline, relative to other amino acids, was used as a measure of total collagen content. Collagen was secreted into the medium more in control samples than loaded samples over a 3-week loading regimen (Fig. 5). Specifically, after 1 week of the loading regimen, unloaded samples released more collagen into the medium over the culture time assessed (1-3 weeks). Measurements of hydroxyproline in loaded CTA showed, on average, a 12% increase in collagen content over control after 1 week of load (data not shown), indicating the loading increased the retention or organization of collagen within the CTA.
Figure 5.

Secreted collagen over time by CTA measured by HPLC HyPro. The effects of loading result in less collagen being released into the medium. In contrast, approximately 12% more is incorporated in the loaded CTA over unloaded (not shown). Loading increased the incorporation of collagen into the ECM.
As with collagen, GAG was released into the medium more in control samples over time (Fig. 6A). After 1 week in the loading regimen, loaded samples demonstrated a decreased GAG level in the medium compared with unloaded controls. When GAG from the CTA was measured relative to DNA content, it was determined that 64% more GAG was incorporated into the matrix per chondrocyte (Fig. 6B). Loaded CTAs more effectively incorporated GAG into the ECM of the growing tissue as a result of loading.
Figure 6.

GAG released into the medium by porcine chondrocyte CTAs (A). Data show that loaded samples appear to incorporate GAG more effectively than control CTAs over time (B). Representative data shown from one experiment (n = 2 at each time point).
Adverse Effects of Hydrostatic Loading
To understand whether chondrocytes were being adversely affected by loading, iNOS gene expression (mRNA) and NO production were studied. iNOS gene expression in chondrocyte CTAs (n = 3) did not differ at any time point, control or loaded (data not shown). The nitrite assay to detect NO release was below the detectable range (0.43-65 µM nitrite) in the medium. These data demonstrate that CTAs benefit from loading without displaying markers of a stressed phenotype, namely, NO production and iNOS upregulation.
Gene Expression
Chondrocyte CTAs were loaded for 1 to 3 weeks, with three 3-hour loading sessions each week. Gene expression in loaded CTAs displayed an overall upregulation of ECM components including collagen type II, aggrecan, COMP, perlecan, and collagen type IIA. Collagen II and aggrecan showed the most increase in expression in response to loading for 3 weeks, and both were significant (P < 0.05 and P < 0.01, respectively) (Fig. 7). CD44 and collagen type II expression increased near significance (P < 0.07) after 2 weeks of loading. Importantly, representative data, although not statistically significant, imply that GAG and collagen type II are incorporated into the CTA ECM at higher levels as a result of loading. If true, this confirms a true increase in relevant cartilage gene expression in this scaffold-less SASC model.
Figure 7.

After 1 week in culture (preconditioning), porcine chondrocyte CTAs were loaded for 1 week, 2 weeks, and 3 weeks. ECM gene expression in porcine chondrocyte CTAs loaded for up to 3 weeks. Experimental conditions were performed in triplicate, and CTAs were collected after each week of loading (i.e., after 1, 2, and 3 weeks of loading 3 times a week). Representative data show the effect on ECM-specific genes in response to hydrostatic load. In general, cartilage-specific genes are upregulated with increased duration of loading regimen. Note that collagen type II and aggrecan, the major macromolecules of cartilage, expression are upregulated at all time points as a result of loading. Relative expression is 2−ΔΔC T. GAPDH serves as the reference gene. Error bars represent SEM. Statistical analysis using ANOVA. *P < 0.05, **P < 0.01, n = 3.
Discussion
The purpose of this study was to determine the effect of applying a physiologically relevant cyclical hydrostatic load to chondrocytes in culture on the molecular and mechanical properties of any CTA formed. Our high-density SASC model results in a CTA with “cartilage-like” characteristics. In this model, devoid of scaffolds or foreign materials, the chondrocytes self-aggregate and form a mass within days that floats at the surface, fundamentally occurring because of a specialized surface coating (polyHEMA), which prevents adherence. We have demonstrated that use of this model can maintain the chondrocytes’ articular cartilage phenotype for extended culture durations9 and that a biomaterial with cartilage-like characteristics is formed that expresses cartilage-specific genes without type I collagen, maintaining the articular cartilage phenotype. Whereas this study focused on using porcine-derived chondrocytes, this SASC approach works equally well with chondrocytes from all tissue sources tested including human, equine, and bovine. Porcine was chosen in part because of the pig being a large animal with future potential for in vivo studies and because there is interest in porcine tissues as a source of xenografts.40,41 In this study, we hypothesized that since chondrocytes grow under a mechanical load in vivo, applying a physiologically relevant mechanical load on chondrocytes during growth would potentially enhance the biomaterial formed and cartilage-like characteristics. Using a specialized SASC model, our results demonstrate an improvement in the CTA’s cartilage-like characteristics with load. Additionally, we ascertained that this loading pressure would not be harmful or induce cell stress in vitro. Considering the importance of how the chondrocyte grows in vivo within an abundant ECM, we preconditioned the chondrocytes for 1 week prior to applying the load. This allowed sufficient time for chondrocytes to aggregate, form a unified mass, and synthesize an ECM. Initially, loading may compress samples as seen at 1 week; however, after 3 weeks, no difference is seen. This could be due to incorporation of a dense matrix resulting from time in culture or loading or both. This study was designed to test the hypothesis that using this model, a natural CTA with cartilage-like qualities could be generated with a loading regimen without the complications of scaffolds and other foreign material, which only confounds the translation of any technology into a clinical application. In this study, we have assessed the molecular and biochemical characteristics of the CTA formed after a 3-week loading regimen.
In a separate study examining the mechanical properties of the CTA, preliminary results show an increase in aggregate modulus and decrease in permeability between the control and loaded specimens (data not shown). Additional studies need to be performed to support these data since the number of specimens tested was not such to obtain statistical significance. More studies are needed to further test various loading regimens such as exposure times and magnitude of hydrostatic pressure and to explore further improvements on the CTA formed, such as its material properties. Taken together with the positive changes in cartilage-relevant molecules, the trend observed in biomechanical tests performed even in a small number is intriguing particularly since this model begins without the benefit of any scaffold.
In contrast to many other model platforms, the properties of the CTA represent matrix formed and organized by the chondrocytes themselves and do not rely on additional exogenously supplied materials to establish or improve load-carrying capabilities. The present study is based on the fact that primary cartilage chondrocytes themselves express the necessary hyaline cartilage constituents, thus over time providing the necessary ECM, that is, scaffold, in which to grow.
In our SASC model, the CTA formed maintains the cartilage-specific phenotype, and constitutive cartilage molecules are upregulated as a result of short periods of physiologically relevant loading. Our biochemical assessment showed that the CTAs incorporate collagen into their ECM more efficiently when in the presence of load. As predicted by previous work,12 CTAs grown in suspension culture show a proliferation up to 3 weeks (comparable with the second week of load) followed by a transition to biosynthesis and an accumulation of ECM. Our previous work, and gene expression data, indicates that the collagen being incorporated is more than likely collagen type II.12 Immunohistochemistry has shown that collagen type II is in fact incorporated well into the matrix. This incorporation appears to increase with time in our culture model and with loading.
The usefulness of this self-aggregating suspension model combined with mechanical loading within a physiological range was demonstrated and presented characteristics that are shared by those of natural cartilage. While equivalence was obviously not achieved, striking similarities were observed using this simple suspension culture model and hydrostatic loading. Other model systems often express similar characteristic molecules; however, many express collagen type I in part because of periods where the cells are permitted to adhere and to change their spherical morphology. Our previous study showed that this model maintained a cartilage-like phenotype and no collagen type I for long periods of time and may, therefore, represent a new scaffold-less approach to generate a cartilage-like biomaterial. Additional improvements such as varying the dose or magnitude of loading or the gene manipulations may improve its cartilage characteristics even further and its suitability for use in cartilage repair.
Footnotes
Acknowledgments and Funding: The authors thank Theresa Michel and Christopher Saeui for exceptional editorial assistance, and Minwook Kim, Aaron Littleton, and Chuanzhao Li for expert technical assistance. The study was sponsored in part by National Institutes of Health (NIH) grant #AR045242, NIH grant #P20RR016458, and NIH grant #P20RR020173 and Nemours.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- 1. Hunziker EB, Driesang IM, Morris EA. Chondrogenesis in cartilage repair is induced by members of the transforming growth factor-beta superfamily. Clin Orthop Rel Res. 2001;391 Suppl:S171-81. [DOI] [PubMed] [Google Scholar]
- 2. Larson CM, Kelley SS, Blackwood AD, Banes AJ, Lee GM. Retention of the native chondrocyte pericellular matrix results in significantly improved matrix production. Matrix Biol. 2002;21:349-59. [DOI] [PubMed] [Google Scholar]
- 3. Lee GM, Poole CA, Kelley SS, Chang J, Caterson B. Isolated chondrons: a viable alternative for studies of chondrocyte metabolism in vitro. Osteoarthritis Cartilage. 1997;5:261-74. [DOI] [PubMed] [Google Scholar]
- 4. Kawabe N, Yoshinao M. The repair of full-thickness articular cartilage defects: immune responses to reparative tissue formed by allogeneic growth plate chondrocyte implants. Clin Orthop Relat Res. 1991;268:279-93. [PubMed] [Google Scholar]
- 5. Haisch A, Wanjura F, Radke C, Leder-Johrens K, Groger A, Endres M, et al. Immunomodulation of tissue-engineered transplants: in vivo bone generation from methylprednisolone-stimulated chondrocytes. Eur Arch Otorhinolaryngol. 2004;261:216-24. [DOI] [PubMed] [Google Scholar]
- 6. Diduch DR, Jordan LC, Mierisch CM, Balian G. Marrow stromal cells embedded in alginate for repair of osteochondral defects. Arthroscopy. 2000;16:571-7. [DOI] [PubMed] [Google Scholar]
- 7. Waldman SD, Grynpas MD, Pilliar RM, Kandel RA. Characterization of cartilagenous tissue formed on calcium polyphosphate substrates in vitro. J Biomed Mater Res. 2002;62:323-30. [DOI] [PubMed] [Google Scholar]
- 8. Reginato AM, Iozzo RV, Jimenez SA. Formation of nodular structures resembling mature articular cartilage in long-term primary cultures of human fetal epiphyseal chondrocytes on a hydrogel substrate. Arthritis Rheum. 1994;37:1338-49. [DOI] [PubMed] [Google Scholar]
- 9. Novotny JE, Turka CM, Jeong C, Wheaton AJ, Li C, Presedo A, et al. Biomechanical and magnetic resonance characteristics of a cartilage-like equivalent generated in a suspension culture. Tissue Eng. 2006;12:2755-64. [DOI] [PubMed] [Google Scholar]
- 10. Hu JC, Athanasiou KA. A self-assembling process in articular cartilage tissue engineering. Tissue Eng. 2006;12:969-79. [DOI] [PubMed] [Google Scholar]
- 11. Ofek G, Revell CM, Hu JC, Allison DD, Grande-Allen KJ, Athanasiou KA. Matrix development in self-assembly of articular cartilage. PLoS One. 2008;3:e2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Estrada LE, Dodge GR, Richardson DW, Farole A, Jimenez SA. Characterization of biomaterial with cartilage-like properties expressing type X collagen generated in vitro using neonatal porcine articular and growth plate chondrocytes. Osteoarthritis Cartilage. 2001;9:169-77. [DOI] [PubMed] [Google Scholar]
- 13. Elder BD, Athanasiou KA. Hydrostatic pressure in articular cartilage tissue engineering: from chondrocytes to tissue regeneration. Tissue Eng Part B Rev. 2009;15:43-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stockwell RA. The interrelationship of cell density and cartilage thickness in mammalian articular cartilage. J Anat. 1971;109:411-21. [PMC free article] [PubMed] [Google Scholar]
- 15. Natoli R, Revell CM, Athanasiou K. Chondroitinase ABC treatment results in increased tensile properties of self-assembled tissue engineered articular cartilage. Tissue Eng Part A. 2009;15:319-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Carter DR, Wong M. The role of mechanical loading histories in the development of diarthrodial joints. J Orthop Res. 1988;6:804-16. [DOI] [PubMed] [Google Scholar]
- 17. Hall AC, Horwitz ER, Wilkins RJ. The cellular physiology of articular cartilage. Exp Physiol. 1996;81:535-45. [DOI] [PubMed] [Google Scholar]
- 18. Wong M, Carter DR. Mechanical stress and morphogenetic endochondral ossification of the sternum. J Bone Joint Surg Am. 1988;70:992-1000. [PubMed] [Google Scholar]
- 19. Wong M, Carter DR. Theoretical stress analysis of organ culture osteogenesis. Bone. 1990;11:127-31. [DOI] [PubMed] [Google Scholar]
- 20. Mauck RL, Soltz MA, Wang CC, Wong DD, Chao PH, Valhmu WB, et al. Functional tissue engineering of articular cartilage through dynamic loading of chondrocyte-seeded agarose gels. J Biomech Eng. 2000;122:252-60. [DOI] [PubMed] [Google Scholar]
- 21. Waldman SD, Spiteri CG, Grynpas MD, Pilliar RM, Kandel RA. Long-term intermittent shear deformation improves the quality of cartilaginous tissue formed in vitro. J Orthop Res. 2003;21:590-6. [DOI] [PubMed] [Google Scholar]
- 22. Sironen R, Elo M, Kaarniranta K, Helminen HJ, Lammi MJ. Transcriptional activation in chondrocytes submitted to hydrostatic pressure. Biorheology. 2000;37:85-93. [PubMed] [Google Scholar]
- 23. Kisiday JD, Jin M, DiMicco MA, Kurz B, Grodzinsky AJ. Effects of dynamic compressive loading on chondrocyte biosynthesis in self-assembling peptide scaffolds. J Biomech. 2004;37:595-604. [DOI] [PubMed] [Google Scholar]
- 24. Bonassar LJ, Grodzinsky AJ, Frank EH, Davila SG, Bhaktav NR, Trippel SB. The effect of dynamic compression on the response of articular cartilage to insulin-like growth factor-I. J Orthop Res. 2001;19:11-7. [DOI] [PubMed] [Google Scholar]
- 25. Jin M, Frank EH, Quinn TM, Hunziker EB, Grodzinsky AJ. Tissue shear deformation stimulates proteoglycan and protein biosynthesis in bovine cartilage explants. Arch Biochem Biophys. 2001;395:41-8. [DOI] [PubMed] [Google Scholar]
- 26. Brown TD. Techniques for mechanical stimulation of cells in vitro: a review. J Biomech. 2000;33:3-14. [DOI] [PubMed] [Google Scholar]
- 27. Elder BD, Athanasiou KA. Synergistic and additive effects of hydrostatic pressure and growth factors on tissue formation. PLoS One. 2008;3:e2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hall AC, Urban JP, Gehl KA. The effects of hydrostatic pressure on matrix synthesis in articular cartilage. J Orthop Res. 1991;9:1-10. [DOI] [PubMed] [Google Scholar]
- 29. Parkkinen JJ, Ikonen J, Lammi MJ, Laakkonen J, Tammi M, Helminen HJ. Effects of cyclic hydrostatic pressure on proteoglycan synthesis in cultured chondrocytes and articular cartilage explants. Arch Biochem Biophys. 1993;300:458-65. [DOI] [PubMed] [Google Scholar]
- 30. Ikenoue T, Trindade MC, Lee MS, Lin EY, Schurman DJ, Goodman SB, et al. Mechanoregulation of human articular chondrocyte aggrecan and type II collagen expression by intermittent hydrostatic pressure in vitro. J Orthop Res. 2003;21:110-6. [DOI] [PubMed] [Google Scholar]
- 31. Toyoda T, Seedhom BB, Kirkham J, Bonass WA. Upregulation of aggrecan and type II collagen mRNA expression in bovine chondrocytes by the application of hydrostatic pressure. Biorheology. 2003;40:79-85. [PubMed] [Google Scholar]
- 32. Mizuno S, Tateishi T, Ushida T, Glowacki J. Hydrostatic fluid pressure enhances matrix synthesis and accumulation by bovine chondrocytes in three-dimensional culture. J Cell Physiol. 2002;193:319-27. [DOI] [PubMed] [Google Scholar]
- 33. Elder SH, Sanders SW, McCulley WR, Marr ML, Shim JW, Hasty KA. Chondrocyte response to cyclic hydrostatic pressure in alginate versus pellet culture. J Orthop Res. 2006;24:740-7. [DOI] [PubMed] [Google Scholar]
- 34. Evans CH, Stefanovic-Racic M, Lancaster J. Nitric oxide and its role in orthopaedic disease. Clin Orthop Relat Res. 1995;312:275-94. [PubMed] [Google Scholar]
- 35. Murrell GA, Doland MM, Jang D, Szabo C, Warren RF, Hannafin JA. Nitric oxide: an important articular free radical. J Bone Joint Surg Am. 1996;78:265-74. [PubMed] [Google Scholar]
- 36. Das P, Schurman DJ, Smith RL. Nitric oxide and G proteins mediate the response of bovine articular chondrocytes to fluid-induced shear. J Orthop Res. 1997;15:87-93. [DOI] [PubMed] [Google Scholar]
- 37. Lee MS, Trindade MC, Ikenoue T, Schurman DJ, Goodman SB, Smith RL. Effects of shear stress on nitric oxide and matrix protein gene expression in human osteoarthritic chondrocytes in vitro. J Orthop Res. 2002;20:556-61. [DOI] [PubMed] [Google Scholar]
- 38. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402-8. [DOI] [PubMed] [Google Scholar]
- 39. Sakurai H, Kohsaka H, Liu MF, Higashiyama H, Hirata Y, Kanno K, et al. Nitric oxide production and inducible nitric oxide synthase expression in inflammatory arthritides. J Clin Invest. 1995;96:2357-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kues WA, Niemann H. The contribution of farm animals to human health. Trends Biotechnol. 2004;22:286-94. [DOI] [PubMed] [Google Scholar]
- 41. Nottle MB, D’Apice AJ, Cowan PJ, Boquest AC, Harrison SJ, Grupen CG. Transgenic perspectives in xenotransplantation, 2001. Xenotransplantation. 2002;9:305-8. [DOI] [PubMed] [Google Scholar]


