Abstract
Cartilage injuries are frequently recognized as a source of significant morbidity and pain in patients with previous knee injuries. The majority of patients who undergo routine knee arthroscopy have evidence of a chondral defect. These injuries represent a continuum of pathology from small, asymptomatic lesions to large, disabling defects affecting a major portion of one or more compartments within the knee joint. In comparison to patients with osteoarthritis, individuals with isolated chondral surface damage are often younger, significantly more active, and usually less willing to accept limitations in activities that require higher impact. At the present time, a variety of surgical procedures exist, each with their unique indications. This heterogeneity of treatment options frequently leads to uncertainty regarding which techniques, if any, are most appropriate for patients. The purpose of this review is to describe the workup and discuss the management techniques for cartilage injuries within the adult knee.
Keywords: articular cartilage, epidemiology, knee, outcome measures
Introduction
Chondral injuries occur commonly within the knee. In a study reviewing over 30,000 arthroscopic procedures, approximately 60% of patients were found to have high-grade cartilaginous defects with lesion depths involving 50% or more of the cartilage surface.1,2 In addition, chondral defects result in higher contact stresses in the healthy adjacent cartilage, which may lead to degenerative knee arthritis.3 While a critical defect size predisposing the knee to early arthritis has yet to be elucidated, there is a growing recognition within the orthopedic community that early intervention may be warranted to prevent future problems and to reduce symptoms of cartilaginous lesions.
The present article will review the various surgical options in detail and discuss their indications. In general, all cartilage resurfacing procedures can be broadly categorized into 2 main groups. The first group, called marrow stimulation techniques, involves utilizing the body’s own pluripotent marrow stem cells to create reparative tissue consisting of fibrocartilage, which predominately consists of type 1 collagen. These procedures include microfracture, abrasion chondroplasty, and subchondral drilling to allow marrow stem cells to cover the area devoid of cartilage. These frequently utilized techniques remain popular due to their simplicity, low cost, potential to relieve symptoms, and their ability to fill the defect, at least partially, with a type of repair tissue. The main drawbacks of fibrocartilage repair tissue are its poor wear characteristics and questionable durability over time.
The second group of procedures aims to restore the injured area with normal or near-normal joint cartilage. These include osteochondral autografts (OATs) and osteochondral allografts, as well as autologous chondrocyte implantation (ACI) procedures. The goal of these techniques is to restore the normal articular contour of the joint and to provide a more resilient, hyaline-like cartilage surface. The proposed advantages of these procedures must be weighted carefully against the disadvantages, which include a technically more demanding operation, increased costs, longer rehabilitation, and potential complications.
Basic Science
Normal adult cartilage has a limited potential to regenerate. Chondrocytes do not replicate in the face of injury like other tissues, such as epidermal skin cells, for example.4 Cartilage is devoid of neural and vascular tissue and relies on diffusion for its nutrition and homeostasis.
Articular cartilage is composed of several distinct zones (Fig. 1). The shallowest layer is called the superficial layer or tangential zone and makes up only 10% of the cartilage thickness. This layer is primarily responsible for resisting shear stress as the chondrocytes are flattened and the collagen fibers are oriented parallel to the joint surface.
Figure 1.

Normal anatomical layers found within articular cartilage.
The next layer beneath the superficial layer is the transitional zone. It is appropriately named for the function it serves as a transition between the shearing forces above and the compressive forces below in the deeper cartilage layers. In the deeper radial zone, collagen fibers are oriented perpendicularly to the joint surface and thus resist the high compressive loads encountered in this layer.
The deepest zone of cartilage is called the calcified cartilage layer, and it contains the tidemark or the boundary between the calcified and uncalcified cartilage. The subchondral plate lies just below the calcified layer, creating a barrier to the marrow cells that exist beneath it in the metaphysis.
The architectural organization of articular cartilage has important implications in the various cartilage repair strategies used in the management of symptomatic chondral defects. For instance, marrow stimulation techniques such as microfracture rely on the creation of small holes that breach the subchondral bone plate, allowing marrow stem cells to migrate into the blood clot that forms within the defect. On the other hand, techniques such as ACI require removal of the damaged cartilage layers without violation of the subchondral plate in order to ensure the viability and maturation of the transplanted chondrocytes.
Evaluation
The key to providing optimal management for patients with chondral injuries is a thorough workup and early detection. Often, these lesions go unrecognized for months to years, leading to delayed diagnosis, worsening symptoms, and the potential for further damage to occur within the knee. Thus, the first step in the evaluation is a thorough history and physical examination. During the patient interview, the provider should assess when the injury (if any) occurred and if symptoms are present. Previous treatment including all prior surgical intervention involving the knee joint should be carefully documented, as some of these treatments can affect the outcome of subsequent cartilage resurfacing procedures.5
Some common symptoms of chondral damage include swelling, activity-related pain, limping, catching, locking, or feelings of instability. Although these symptoms are often nonspecific, they should be assessed to determine the severity of the problem and to search for frequent coexisting pathology such as meniscal tears and ligament injuries.
During the physical examination, a knee effusion may be evident in the presence of a chondral lesion. Point tenderness within the joint line or just above the joint line, immediately over the weightbearing condyle, helps to localize the lesion. Furthermore, a catching or grinding sensation of crepitus can sometimes be felt if the lesion is deep or synovial scar tissue has formed. Lesions involving the patella or trochlea often cause symptoms due to shear stress, and corresponding physical signs of apprehension or pain can be reproduced by patellar grind testing and knee flexion. There may also be point tenderness in the medial or lateral parapatellar gutters. Quadriceps weakness and vastus medialis atrophy may be present in more chronic cases and should alert the physician to a significant ongoing injury. A painful or antalgic gait may or may not be present on examination, but the history will frequently reveal that a limp is present after high-impact activities or prolonged weightbearing.
The next step in the evaluation process is to obtain a complete set of radiographs. This includes a weightbearing anteroposterior (AP) and lateral view as well as an axial view of the patella such as a sunrise or Merchant view. In addition, the Rosenberg view, which is a weightbearing posteroanterior (PA) view of the knee in flexion, is often helpful. This specific view allows better assessment of the lateral and posterior aspects of the joint space, and it is more sensitive for evaluating certain patterns of early knee arthritis or meniscal loss, which may be contraindications to cartilage resurfacing techniques.
Lastly, a full-length weightbearing AP view of the entire extremity from the hip to the ankle taken on a 54-inch cassette should be obtained on any patient who is being considered for cartilage resurfacing. The mechanical weightbearing axis should be drawn, and the line should pass within the center or adjacent to the center of the knee. Weightbearing axes that demonstrate varus or valgus malalignment may be an indication for a high tibial osteotomy or a distal femoral osteotomy, which can be performed prior to or at the same setting as the proposed cartilage resurfacing technique in an effort to reduce the contact stresses on the cartilage defect. Again, it is crucial that the physical examination be performed carefully and all the radiographs scrutinized closely to search for pre-existing instability or malalignment, which can negatively affect the patient’s outcome.
Often, advanced imaging such as computed tomography (CT) arthrogram or magnetic resonance imaging (MRI) is utilized for diagnostic assessment and surgical planning. Computed tomography arthrogram is used in some centers to evaluate the cartilage integrity and to diagnose subtle patellar femoral maltracking, which may require a distal tibial tubercle osteotomy in an effort to unload an area of cartilage repair tissue within the patellofemoral joint. However, the main disadvantage of CT technology is the ionizing radiation exposure, and therefore, the benefits of CT scanning must be weighed against the risks for each patient.
Magnetic resonance imaging is commonly used to confirm the presence of chondral defects and search for associated pathology such as meniscal and cruciate ligament tears. Furthermore, using cartilage-sensitive sequences and higher Tesla magnets found in many of today’s modern MRI machines allows excellent visualization of all cartilage surfaces within the knee (Fig. 2). Magnetic resonance imaging assists the surgeon in assessing whether the problem is an isolated chondral or osteochondral defect or if a more global issue exists, such as chondromalacia and early arthritis. In addition, the presence of bone marrow edema beneath a cartilage defect seen on an MRI study is an indication of ongoing stress and helps to correlate a patient’s symptoms to a specific area within the knee.
Figure 2.
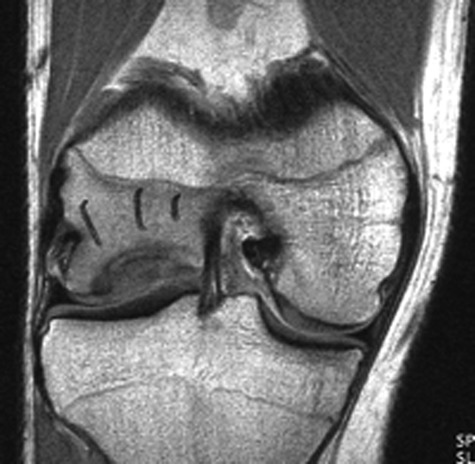
Magnetic resonance image demonstrating a cartilaginous lesion of the lateral femoral condyle.
Lastly, diagnostic arthroscopy remains the “gold standard” for evaluating the joint surfaces and provides the most accurate means of diagnosis. Although this is a surgical procedure with associated risks, it can be readily utilized to diagnose as well as treat cartilaginous injuries of the knee at the same setting or as a staged procedure such as the case with ACI.
Not all patients are candidates for cartilage resurfacing surgery. Many factors need to be taken into account prior to deciding on surgery. These factors include the following:
limb alignment;
pre-existing concomitant injury or arthritis within the knee;
patient expectations and rehabilitation potential;
instability and ligament damage;
previous total or subtotal meniscectomy;
age of the patient;
size, number, and location of defect(s); and
body mass index (BMI).
As just alluded to, some patients may be best treated nonoperatively. For example, those with evidence of osteoarthritis are often better served with another procedure or nonoperative management. In addition, if significant limb malalignment exists leading to stress overload in the compartment with an osteochondral defect, a realigning osteotomy in conjunction with cartilage replacement may be indicated.
Ability to comply with rehabilitation protocols is another factor that must be considered prior to performing surgery. Most cartilage resurfacing techniques require 6 to 12 weeks of protected weightbearing, with some requiring extensive rehabilitation and further restriction beyond 1 year postoperatively (Table 1). This must be thoroughly discussed with the patient ahead of time to reasonably align their expectations and to assess whether surgery is indicated.
Table 1.
Cartilage Repair Rehabilitation Chart
| Rehabilitation | Microfracture33 | Autologous Cultured Chondrocytes33 | Osteochondral Autograft33 | Osteochondral Allograft34 |
|---|---|---|---|---|
| CPM | Begin postoperatively 8-12 hours on day 1 and 6-8 h/d up to 6 weeks | Begin postoperatively 8-12 hours on day 1 and 6-8 h/d up to 6 weeks | Begin postoperatively 8-12 hours on day 1 and 6-8 h/d up to 6 weeks | Not specified |
| Nonweightbearing | Weeks 0-2 | Weeks 1-2 | Weeks 2-4 | Weeks 6-12 |
| Partial weightbearing | Weeks 0-4 | Weeks 2-8 | Weeks 3-7 | Weeks 8-16 |
| Full weightbearing | Weeks 4-8 | Weeks 6-9 | Weeks 8-14 | Weeks 12-16 |
| Low-impact sports | 2-3 months | 6 months | 6-8 months | 6 months |
| Higher impact sports(running, aerobics) | 4-5 months | 8-12 months | 8-10 months | Not specified |
| High-impact pivotal sports | 6-8 months | 12-18 months | 12-18 months | 6 months |
CPM = continuous passive motion.
Source: CARTICEL Web site: http://www.carticel.com/healthcare/about/compare-knee-surgery-options.aspx. © 2010 Genzyme Corp.
Ligament laxity such as a complete anterior cruciate ligament (ACL) tear with instability or significant meniscal damage such as a prior total meniscectomy is a contraindication to isolated cartilage resurfacing. In these cases, consideration may be given to ligament reconstruction or meniscal transplant in certain patients. Good results have been obtained with combined ACL reconstruction and cartilage resurfacing previously; however, the treatment must be individualized to each patient.7
Advancing age, while not an absolute contraindication, has been shown to negatively affect the results of common procedures such as microfracture.8,9 The number, size, and location of the defect(s) do affect the prognosis and should be carefully considered prior to surgery. For example, multiple lesions within the knee, patellar defects, and so-called bipolar “kissing lesions” that abut one another have been shown to have inferior results when compared to isolated femoral condyle defects.10
Lastly, BMI should be considered. Although there are no randomized clinical controlled studies assessing the effects of BMI on cartilage replacement, obesity certainly is a concern among most surgeons. A large lower extremity circumference often increases the level of technical difficulty during cartilage surgery. This can prolong the duration of the procedure, which may lead to a higher incidence of infection. In addition, higher body weight will also increase the knee’s joint reactive forces, which places greater stress on the cartilage repair and may ultimately lead to failure.
Nonoperative Management
Although many patients are candidates for one or more of a variety of cartilage-producing procedures, it is certainly worth mentioning nonoperative management for those who wish to delay or avoid surgery or for those who are deemed poor candidates for cartilage replacement. The mainstay of conservative treatment includes activity modification and maintenance of ideal body weight. High-impact activities such as jogging on hard surfaces and jumping should be discouraged in favor of lower impact exercise such as elliptical machines or swimming.
Oral nonsteroidal anti-inflammatory medications or creams can be used for temporary mild swelling and discomfort, while stronger medications such as narcotics are usually discouraged for long-term use due to their significant side effects. Other interventions such as glucosamine and chondroitin sulfate, intra-articular hyaluronic acids, or cortisone have traditionally been used for osteoarthritis, but these may play a role in the nonoperative management of symptomatic osteochondral lesions as well.
Other therapies worth considering include orthotics and bracing, such as medial unloader braces, which essentially mimic the effects of a realigning osteotomy by reducing the stress on the damaged compartment. Although physical and occupational therapy is often prescribed preoperatively and postoperatively, it has little role in the nonoperative management of cartilage injuries in the knee. One exception perhaps to the use of physical therapy in the nonoperative setting is to increase range of motion and quadriceps strength in those with evidence of significant atrophy.
Surgical Management
Marrow Stimulation Techniques
The 2 most common of these procedures are subchondral drilling and microfracture. Of the two, microfracture has a theoretical advantage as there is no concern for heat generation from drilling that could potentially effect the reparative stem cells. In either of these procedures, it is essential to remove all the devitalized or damaged cartilage, leaving a clean bed just above an intact subchondral plate to which pluripotent stem cells can adhere and produce fibrocartilage. The goal is to produce stable vertical walls surrounding the defect. Large flaps of overhanging or loose cartilage should not be left behind as these will invariably become loose bodies and further promote synovitis within the knee as time progresses.
Once a clean bed is created within the lesion site and a good supporting peripheral rim of cartilage exists, small holes are created through the subchondral plate to allow the extravasation of marrow stem cells. The holes should be made systematically with a few millimeters of equal spacing between each hole. Between the holes, the surgeon must leave an intact subchondral bone plate from which the pluripotent cells can adhere to and produce fibrocartilage repair tissue (Fig. 3).
Figure 3.

This diagram depicts the microfracture procedure. Note the equal spacing created by the microfracture awl while preserving the integrity of the subchondral plate between each hole.
The advantages of these techniques are their simplicity, low costs, and technical ease. Marrow stimulation can be performed for small (<2 cm2) or larger lesions. Therefore, they are often utilized as a first-line procedure for cartilaginous defects. However, the durability of the fibrocartilage repair tissue is a concern over the long run. While long-term randomized clinical trials comparing marrow stimulation to other cartilage resurfacing techniques are lacking, one worry among many cartilage surgeons is the resiliency of fibrocartilage11 obtained from the microfracture techniques. The reparative tissue must be able to withstand large forces in the knee over many decades, especially in athletic individuals participating in high-impact activities.
Another specific issue with the microfracture technique is advancing age. The results in patients over 40 years of age have been shown to be inferior to results in younger patients.6 Plausible explanations for this finding are that either the efficacy of the marrow stem cells to produce repair tissue is decreased or the actual number of cells acquired through marrow stimulation techniques is reduced as we age. In either case, older individuals should be counseled accordingly before deciding on microfracture.
Steadman et al.7 reviewed 72 patients with an average follow-up of 11 years. Inclusion criteria included patients without meniscus or ligament injury, those younger than 45 years of age, and defects of any compartment within the knee. Lysholm scores were 59 preoperatively and 89 postoperatively. Tegner scores also showed improvement with a preoperative score of 3.1 and a postoperative score of 5.8. The preoperative pain score (3.4) improved as well after surgery to 1.9. At 7 years after surgery, 80% still showed improvement in their pain beyond baseline. As previously mentioned, younger patients overall did better than older patients in this study.7
In a randomized clinic control trial, Knutsen et al.8 compared the short-term results of microfracture and ACI in 80 patients with isolated femoral condyle defects measuring between 2 and 10 cm2. Exclusion criteria included patients younger than 18 or older than 45 years of age, ligament instability, malalignment, BMI above 30, or evidence of osteoarthritis. Results were compared using International Cartilage Repair Society (ICRS), Lysholm, Tegner, and Short Form-36 (SF-36) scores, which were recorded preoperatively as well as at 2 years’ follow-up.
In general, no significant outcome differences were found between ACI and microfracture patients at 2 years. Of note, individuals younger than 30 years did better in both groups. Furthermore, there was a tendency towards more hyaline-like cartilage in the ACI group versus the microfracture group in those undergoing second-look biopsies. However, the autologous chondrocyte implant group demonstrated a greater number of repeat arthroscopies for further debridement when compared to the microfracture group.8
Cartilage Resurfacing
Osteochondral autografts and ACI are 2 common procedures used in biological resurfacing of cartilage defects within the knee. Both have the potential to repair cartilage defects with hyaline-like or normal hyaline cartilage tissue. Each surgical technique is technically more demanding than marrow stimulation, and both ACI and OAT require extensive postoperative rehabilitation.
One difference between these 2 procedures is that OAT is performed in a single stage, whereas ACI is currently done as a 2-stage procedure requiring an initial arthroscopic cartilage biopsy prior to reimplantation. In addition, the ACI technique has higher costs associated with the procedure, mainly due to the processing and amplification of the cultured chondrocytes in vitro. On the other hand, the OAT procedures remove healthy cartilage plugs from limited weightbearing areas within the joint and thus potentially lead to donor site morbidity, which is not an issue with ACI. In either case, both surgeries require thorough patient education and proper selection as well as meticulous attention to detail during surgery in order to assure the best possible outcome.
Osteochondral Autograft
This technique is performed in a single stage using an OAT harvester (Fig. 4). After a limited open arthrotomy, the lesion site is prepared and accurately sized with a specialized measuring tamp to determine the exact diameter of the lesion. Plugs of cartilage with underlying bone measuring 15 mm in depth are harvested from limited weightbearing areas within the knee. It is important to keep in mind that all areas within the knee that contain hyaline cartilage are never completely nonweightbearing areas. Thus, the term limited or minimal weightbearing area is more accurate to describe the harvest site.
Figure 4.
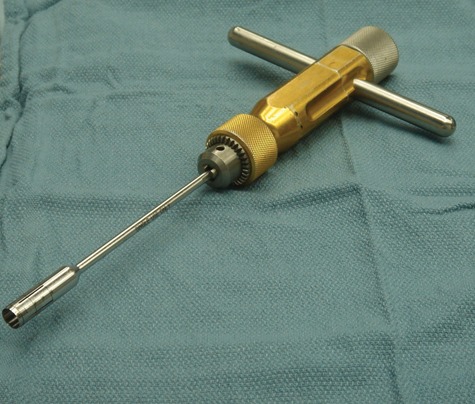
An osteochondral autograft harvester (Arthrex Inc., Naples, FL).
Osteochondral plugs are usually obtained from the medial or lateral trochlear ridge or sometimes from the intercondylar notch (Fig. 5). Since healthy cartilage is harvested from other minimal weightbearing areas within the knee, this technique is generally limited to cartilage defects of 2 × 2 cm or smaller to minimize donor site morbidity.
Figure 5.
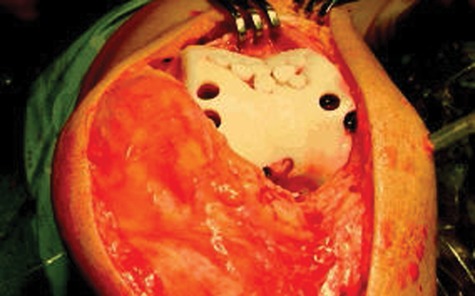
Photograph demonstrating the osteochondral autograft transfer procedure. Note the multiple donor sites along the medial and lateral trochlear ridge.
Once the bed of the lesion site has been prepared and the grafts have been harvested, the autograft plugs are gently impacted with a bone tamp into the osteochondral defect. The key to this technique is to tightly pack the bone plugs into the defect to ensure secure fixation and to reproduce the normal radius of curvature or anatomical contour of the joint. This is the most challenging aspect of the procedure, as it can be difficult to obtain a smooth transition zone between the harvested OAT plugs and the surrounding healthy cartilage. Upon completion of the procedure, the patient is placed on limited weightbearing precautions and will undergo several months of rehabilitation.
Hangody and Fules11 reviewed 831 patients undergoing OAT procedures for focal cartilage lesions (mostly in the knee) between 1 and 4 cm2. The majority of patients had concomitant ACL reconstruction, meniscal repair, or osteotomies performed at the time of the OAT. Multiple outcome ratings were used including Hospital for Special Surgery (HSS), ICRS, Cincinnati, and Lysholm scores. Good to excellent results were found in 92% of femoral, 87% of tibial, and 79% of trochlea/patella lesions. Second-look biopsies in 83 patients demonstrated survival of hyaline cartilage in 69 versus 14 with degeneration of their cartilage. A limitation of this study, however, was that some important data including baseline preoperative scores as well as the average follow-up period were not clearly stated.4
Multiple other studies examining short-term to midterm results of OATs have demonstrated varying success with good to excellent outcomes ranging from 74% to 89%.12-14 Limitations of all these studies include small sample sizes and their retrospective design. Only a few studies have directly compared OAT to ACI.
In one of these direct comparison studies performed by Horas et al.,15 40 patients with isolated, symptomatic femoral condyle lesions ranging between 3.2 and 5.6 cm2 were evaluated. The exclusion criteria included instability, malalignment, age <18 or >45 years, and pre-existing degenerative joint disease. The size of the lesions and the postoperative rehabilitation protocol were similar between the 2 groups. Outcomes were similar in both groups when analyzing the data by Tegner scores. However, the Lysholm score in the OAT group was slightly higher than the ACI group. This result did reach statistical significance, although the clinical significance may be less important.
Furthermore, biopsy specimens revealed hyaline cartilage in the deep layer and fibrocartilage in the central and superficial layers of the ACI group. On the other hand, hyaline cartilage was seen on the surface of the OAT plugs with fibrocartilage found between the plugs. A significant limitation of these results, however, was the limited number of patients who consented to second-look biopsies. Only 6 patients had biopsies in the ACI group versus 5 in the OAT group. In addition, these biopsies were taken randomly between 3 months and 2 years, which could have resulted in potential selection bias.15 Basic science studies have shown that it takes up to 2 years for cartilage cells to undergo maturation during ACI.16 Thus, obtaining cartilage biopsies within the first 3 to 6 months after surgery would likely result in inferior tissue specimens in the ACI group, which was demonstrated in this study.
In another study, Bentley et al.17 selected 100 patients with chronic symptomatic cartilage defects in the knee and randomized them into an ACI group versus an OAT group. Follow-up results were obtained at 1 year postoperatively using modified Cincinnati and ICRS rating systems. The authors showed that both the clinical outcomes and second-look arthroscopic findings were statistically better in the ACI group. However, these findings may be limited by the randomization method and the short-term follow-up used in this study. Ultimately, studies with longer follow-up periods will be needed to better determine which technique, if any, proves to be superior.17
Autologous Chondrocyte Implantation
This procedure is currently performed using a 2-stage technique. The first stage involves a diagnostic arthroscopy to obtain a cartilage biopsy. The specimen is secured with a ring curette or small gouge to obtain a full-thickness cartilage sample. The biopsy sites used in ACI are similar to the OAT procedure, which are along the superior medial or lateral trochlea ridge or within the intercondylar notch. When cartilage is taken from the notch, the gouge is generally placed at the 11-o’clock position for the left knee or the 1-o’clock position for the right knee and advanced down the lateral wall of the intercondylar notch. After harvesting specimens approximately the size of 2 “large tic-tacs” (each 5 × 8 mm), the samples are placed in a sterile transport kit containing gentamycin + Dulbecco’s Modified Eagle’s Medium and sent to the laboratory for processing and cell culture. In approximately 4 to 6 weeks, the cells are often ready for reimplantation.
During the second stage of the technique, an arthrotomy is performed, and the cartilage defect is debrided back to a stable rim of full-thickness surrounding cartilage (Fig. 6). Either a periosteal patch can be harvested (Fig. 7) or one of the newer collagen membrane substitutes can be used to cover the cells. The patch graft is sized to the defect and sutured over this area using 6-0 absorbable suture, placing the knots on the graft tissue rather than on the native cartilage (Fig. 8). The graft covering is tested for a water-tight seal using sterile saline and a small syringe through a small opening in the patch. Next, the saline is aspirated from the graft site, and the cells are injected into the defect. Fibrin glue is used as an adjunct to seal the border between the graft covering and the healthy cartilage to further ensure a water-tight seal.
Figure 6.

A prepared cartilage defect is seen here prior to autologous chondrocyte implantation. The surrounding cartilage rim is healthy, allowing for suture fixation of the patch graft.
Figure 7.

This diagram illustrates harvesting of periosteum as a covering for autologous chondrocyte implantation surgery. Either periosteum or newer mixed collagen membranes can be used as a water-tight barrier for implanted chondrocytes.
Figure 8.

Photograph demonstrating autologous chondrocyte implantation after the patch graft has been secured by suture fixation and fibrin glue.
Technically challenging aspects of this procedure include sizing and creating a patch that conforms anatomically to the articular contour of the joint. In addition, obtaining complete hemostasis prior to injecting the cells underneath the periosteal patch or collagen membrane is paramount. Once the procedure is completed, limited weightbearing and rehabilitation are begun. It can take between 1 to 2 years for cartilage cell maturation to fully occur. Thus, high-impact activities are often restricted accordingly.
Peterson et al.9 reviewed 58 patients with osteochondral defects of the medial femoral condyle averaging 5.7 cm2. Mean time to latest follow-up was approximately 5 years, and outcome measures included Lysholm, Tegner, and Cincinnati scores. They reported good to excellent outcomes in 91% of patients undergoing ACI in this study.9
Minas et al. have published several papers on ACI. Strengths of their studies include larger sample sizes and stratification of results by location of the lesion. Patient satisfaction scores have ranged between 60% to 90% at 2 years depending on the complexity and location of the lesion.18-20
In Minas’ series, patients with patellar lesions, pre-existing osteoarthritis, or bipolar kissing lesions were shown to have lower outcome scores compared to isolated lesions involving the femur.18 Of note, many patients were considered “salvage patients” with large defects who had previously undergone multiple surgeries. In addition, the majority of the patients also underwent concurrent procedures, including osteotomies of the tibia and femur at the time of ACI, making direct comparisons to other studies difficult.
Previously, ACI surgery has been associated with significant complications related to hypertrophy of the periosteal graft. Repeat arthroscopy rates of 20% to 25% are the norm when using autologous periosteum as a cartilage cell covering due to its biological activity.9,18,21,22 However, several authors have recently shown a much lower complication rate when using a type I/type III collagen membrane xenograft from porcine tissue.23-25 A variety of companies now offer these mixed collagen membranes, which at the present time, are still awaiting Food and Drug Administration (FDA) approval in the United States. Repeat arthroscopy rates have been decreased to 5% or less for graft hypertrophy when using the collagen membrane–assisted autologous chondrocyte implantation (C-ACI) technique.23-25
One valid concern held by some skeptics is that the cartilage repair tissue obtained through ACI is not always predictable. Henderson et al.26 performed second-look arthroscopy for persistent mechanical symptoms in 22 knees treated with ACI over a 2-year period. All grafts appeared normal or nearly normal according to the ICRS visual assessment rating scale. Thirteen of 20 biopsies did reveal hyaline or hyaline-like cartilage, while 7 of 20 were fibrous or mixed fibrohyaline cartilage.
Osteochondral Allografts
Similar to OAT procedures, osteochondral allografts also are done in a single stage. The difference is that cadaveric donor tissue is used in the osteochondral allografts, so there is no concern for donor site morbidity. One obvious concern, however, is the possibility of disease transmission. Another issue that is often raised is the durability and viability of the tissue since the cartilage cells are from donor rather than host tissue.
The lesion size and shape of the patient’s condyle can be assessed preoperatively using MRI or CT, and the dimensions may then be used to match a proper fitting allograft. For example, a donor femoral condyle with a similar radius of curvature to the patient’s native condyle is obtained to ensure the best possible fit. After obtaining a proper donor match, the surgery is scheduled ideally within days to preserve chondrocyte viability.
The procedure is performed through an open arthrotomy, and the chondral lesion along with the underlying subchondral bone is reamed away with a counterbore reamer to a depth of 8 to 10 mm. The depth of reaming into subchondral bone is limited in an effort to reduce host-graft immunogenicity and improve the odds of successful allograft incorporation. The prepared lesion is marked and the depth accurately measured at 4 quadrants to aid in matching a core of allograft tissue with similar shape and dimension (Fig. 9). After the lesion site is properly prepared and the allograft is shaped accordingly with the appropriate reamer, it is gently impacted into place with the use of a specialized tamp (Fig. 10).
Figure 9.

Preparation of a large medial femoral condyle defect for a fresh osteochondral allograft.
Figure 10.

Osteochondral allograft (“mega-OAT”) seen after impaction into the femoral condyle.
Gross et al.27 evaluated 60 patients with femoral condyle allografts with an average follow-up of 10 years in one study. In another study, 65 patients with tibial condyle allografts with an average follow-up of 11.8 years were evaluated. Defects were at least 3 cm in diameter, and modified HSS scores as well as telephone surveys were used to assess outcome. Kaplan-Meier survivorship data revealed 95% 5-year survival and 80% to 85% 10-year survival without the need for repeat surgery.27 One critique of these studies was that the treatments rendered were heterogeneous as many patients also had high tibial osteotomies and meniscal allograft transplants along with their allograft procedure.
LaPrade et al.28 evaluated 23 knees that underwent fresh osteochondral allografts at a mean of 20 days after initial refrigeration. The average modified Cincinnati scores increased from 21.9 to 32.5 on the symptom rating scale, and the mean International Knee Documentation Committee scores improved from 52 points to 68.5 points after 3 years of follow-up. Although the sample size of the study was small, the results were found to be statistically significant.28
In a similar retrospective study, Williams et al.29 evaluated 19 symptomatic patients with osteochondral knee defects using Activity of Daily Living and SF-36 scores. Mean graft storage time was 30 days prior to implantation, and the mean follow-up was 4 years after surgery. At final follow-up, the average Activity of Daily Living scores increased from 56 to 70 (P < 0.05), and the SF-36 scores increased from 51 to 66 (P < 0.005). Subsequent MRI evaluation after 2 years postimplantation demonstrated 4 patients with poor graft signal characteristics, while 8 of 18 grafts revealed isointense signals compared to the surrounding cartilage.29
Although osteochondral allografts may not be as readily available as OAT procedures or ACI surgery, they are best suited for large or massive osteochondral defects not amenable to marrow stimulation or OAT techniques. Furthermore, in addition to fresh osteochondral allografts, ACI can also be used for large defects. However, damage to the subchondral plate with significant bone loss as well as massive cartilage lesions can make ACI technically more challenging. Thus, the osteochondral allograft procedure offers another viable option along with ACI for large osteochondral lesions within the knee.
Lastly, rehabilitation protocols vary depending on the type of surgical technique utilized. Each surgeon may also have a different physical therapy regimen based on his or her individual experience and belief. Nevertheless, common recommendations have been summarized into a useful algorithm to help guide physicians concerning the appropriate patient rehabilitation milestones and expected return to activities (Table 1). Specifically, many surgeons recommend early continuous passive range of motion with protected weightbearing for 6 to 12 weeks. This is followed by a supervised muscle strengthening and rehabilitation protocol involving inline activities with increasing contact stresses allowed over time. Finally, pivoting exercises including returning to competitive sports come during the last phase of rehabilitation and may take up to a year or longer depending on the specific therapy protocol.
On the Horizon
Many biological scaffolds are being developed, and some are currently being used today for cartilage defects of the knee.30-32 In Europe, for example, “M-ACI” (matrix-assisted autologous chondrocyte implantation) is being done through minimally invasive incisions and arthroscopy. Cartilage cells obtained through biopsy are amplified and impregnated onto a collagen scaffold for reimplantation within the knee. This reduces the operative time and incision size needed to perform the surgery. Furthermore, these tissues can be secured to the underlying defect with fibrin glue, which may obviate the need for extensive suture fixation of the graft material in the near future.
Recently, reports of particulate cartilage allograft transplantation have also been described, whereby minced human cartilage is placed into a chondral defect and covered using a patch graft technique similar to that used in ACI surgery. Fibrin glue has also been utilized as a scaffold for the particulate cartilage to allow arthroscopic placement and adherence to the underlying chondral defect. At the present time, however, there is no evidence to support or refute this procedure as a principal method for cartilage replacement. Nevertheless, investigators are studying this as a potential treatment for cartilage injuries in the near future.
Lastly, techniques that utilize cartilaginous growth factors and genetic engineering are currently being investigated and may ultimately play a role in cartilage restoration surgery. Although these new techniques are exciting, we will need well-designed studies to assess their long-term benefits.
Summary
The natural history of chondral and osteochondral lesions within the knee has yet to be fully elucidated. However, a correlation between these injuries and an increased risk of degenerative joint disease do exist. Furthermore, the symptoms, morbidity, and loss of livelihood caused by cartilaginous lesions can be significant, especially given that these injuries often occur in younger individuals who are highly active.
Cartilage restorative techniques like OAT and ACI procedures do have some theoretical advantages over simple reparative techniques such as microfracture. The repair tissue from marrow stimulation techniques has been shown to be fibrocartilage, which is less durable than hyaline cartilage. In addition, microfracture or subchondral drilling often yields only a partial fill of the defect, which still leaves an uneven transition zone in the cartilaginous surface. However, the theoretical advantages of cartilage restoration procedures must be weighed against their increased costs and their potential for complications.
General guidelines can be established based on the size of the cartilage lesion within the knee (Table 2). For the smallest symptomatic lesions, microfracture, subchondral drilling, abrasion chondroplasty, and OATs are good options. For large or massive defects, fresh osteochondral allografts and ACI can be utilized. For intermediate-sized lesions, several potential treatments may be indicated, and the selected procedure should be made on a case-by-case basis, taking into account the surgeon’s prior experience and familiarity with the proposed technique.
Table 2.
Cartilage Repair Treatment Algorithm
| Symptomatic Full-Thickness Cartilage Lesion | |||
|---|---|---|---|
| None | Lesion Size: <2 cm2 | Lesion Size: 2-6 cm2 | Lesion Size: >6 cm2 |
| Nonoperative options: | Cartilage repair: | Cartilage repair: | Cartilage repair: |
| Activity modification | Microfracture | Microfracture | Durability issues? |
| Weight reduction | Subchondral drilling | Subchondral drilling | |
| Physical therapy: VMO strengthening | Chondroplasty | Chondroplasty | |
| Bracing | Cartilage restoration: | Cartilage restoration: | Cartilage restoration: |
| NSAIDs | ACI | ACI | |
| Hyaluronic acids | OAT | OAT? (donor site morbidity) | |
| Osteochondral allograft | Osteochondral allograft | ||
VMO = vastus medialis obliquus; NSAIDs = nonsteroidal anti-inflammatory drugs; OAT = osteochondral autograft; ACI = autologous chondrocyte implantation.
Note: The question mark indicates that the treatment with OATs for lesions larger than 2cm squared is of questionable benefit and is not recommended due to donor site morbidity.
Even though cartilage surgery is still in its infancy, good results have been demonstrated over the past 2 decades for many of the current popular methods of cartilage repair and restoration. Most of our current literature is from retrospective studies containing heterogeneous treatments and varying outcome measures. This makes useful comparison between different methods of treatment difficult. We will ultimately need further long-term prospective randomized clinical trials to clarify which procedure, if any, has superior outcomes.
Furthermore, new techniques for cartilage lesions are being developed now, and future studies must keep pace with the constant flux of treatment options in order to provide our patients with the best solutions. Treatments such as biological scaffolds, growth factors, bone morphogenic proteins (BMPs), and gene therapy are rapidly evolving and will likely continue to change the way we treat cartilage lesions in the future.
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the authorship and/or publication of this article.
Acknowledgments and Funding: The authors received no financial support for the research and/or authorship of this article.
References
- 1. Curl WW, Krome J, Gordon ES, Rushing J, Smith BP, Poehling GG. Cartilage injuries: a review of 31,516 knee arthroscopies. Arthroscopy. 1997;13:456-60. [DOI] [PubMed] [Google Scholar]
- 2. Hjelle K, Solheim E, Strand T, Muri R, Brittberg M. Articular cartilage defects in 1,000 knee arthroscopies. Arthroscopy. 2002;18:730-4. [DOI] [PubMed] [Google Scholar]
- 3. Shelbourne K, Jari S, Gray T. Outcome of untreated traumatic articular cartilage defects of the knee: a natural history study. J Bone Joint Surg Am. 2003;85 Suppl 2:8-16. [DOI] [PubMed] [Google Scholar]
- 4. Mankin HJ. The response of articular cartilage to mechanical injury. J Bone Joint Surg Am. 1982;64:460-4. [PubMed] [Google Scholar]
- 5. Minas T, Gomoll AH, Rosenberger R, Royce RO, Bryant T. Increased failure rate of autologous chondrocyte implantation after previous treatment with marrow stimulation techniques. Am J Sports Med. 2009;37:902-8. [DOI] [PubMed] [Google Scholar]
- 6. Amin AA, Bartlett W, Gooding CR, Sood M, Skinner JA, Carrington WJ, et al. The use of autologous chondrocyte implantation following and combined with anterior cruciate ligament reconstruction. Int Orthop. 2006;30:48-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Steadman JR, Briggs KK, Rodrigo JJ, Kocher MS, Gill TJ, Rodkey WG. Outcomes of microfracture for traumatic chondral defects of the knee: average 11-year follow-up. Arthroscopy. 2003;19:477-84. [DOI] [PubMed] [Google Scholar]
- 8. Knutsen G, Engebretsen L, Ludvigsen TC, Drogset JO, Grontvedt T, Solheim E, et al. Autologous chondrocyte implantation compared with microfracture in the knee. J Bone Joint Surg Am. 2004;86:455-64. [DOI] [PubMed] [Google Scholar]
- 9. Peterson L, Minas T, Brittberg M, Nilsson A, Sjogren-Jansson E, Lindahl A. Two to 9-year outcome after autologous chondrocyte transplantation of the knee. Clin Orthop. 2000;374:212-34. [DOI] [PubMed] [Google Scholar]
- 10. Nehrer S, Spector M, Minas T. Histological analysis of failed cartilage repair procedures. Clin Orthop. 1999;365:149-62. [DOI] [PubMed] [Google Scholar]
- 11. Hangody L, Fules P. Autologous osteochondral mosaicplasty for the treatment of full-thickness defects of weight-bearing joints. J Bone Joint Surg Am. 2003;85:25-32. [DOI] [PubMed] [Google Scholar]
- 12. Marcacci M, Zaffagnini S, Visani A. Use of autologous grafts for reconstruction of osteochondral defects of the knee. Orthopedics. 1999;22:595-600. [DOI] [PubMed] [Google Scholar]
- 13. Hangody L, Feczko P, Bartha L, Bodo G, Kish G. Mosaicplasty for the treatment of articular defects of the knee and ankle. Clin Orthop. 2001;391:328-36. [DOI] [PubMed] [Google Scholar]
- 14. Hangody L, Kish G, Karpati Z, Udvarhelyl I, Szigeti I, Bely M. Mosaicplasty for the treatment of articular cartilage defects. Orthopedics. 1998;21:751-60. [DOI] [PubMed] [Google Scholar]
- 15. Horas U, Pelinkovic D, Aigner T, Schnettler R. Autologous chondrocyte implantation and osteochondral cylinder transplantation in cartilage repair of the knee joint. J Bone Joint Surg Am. 2003;85:185-92. [DOI] [PubMed] [Google Scholar]
- 16. Jones D, Peterson L. Autologous chondrocyte implantation. J Bone Joint Surg Am. 2006;88:2502-20. [DOI] [PubMed] [Google Scholar]
- 17. Bentley G, Biant C, Carrington RW, Akmal M, Goldberg A, Williams AM, et al. A prospective randomized comparison of autologous chondrocyte implantation versus mosaicplasty for osteochondral defects in the knee. J Bone Joint Surg Br. 2003;85:223-30. [DOI] [PubMed] [Google Scholar]
- 18. Minas T. Autologous chondrocyte implantation for focal chondral defects of the knee. Clin Orthop. 2001;391:349-61. [DOI] [PubMed] [Google Scholar]
- 19. Minas T, Bryant T. The role of autologous chondrocyte implantation in the patellofemoral joint. Clin Orthop. 2005;436:30-9. [DOI] [PubMed] [Google Scholar]
- 20. Minas T, Gomoll AH, Solhpour S, Rosenberger R, Probst C, Bryant T. Autologous chondrocyte implantation for joint preservation in patients with early osteoarthritis. Clin Orthop. 2009;468:147-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. King PJ, Bryant T, Minas T. Autologous chondrocyte implantation for chondral defects of the knee: indications and technique. J Knee Surg. 2002;15:177-84. [PubMed] [Google Scholar]
- 22. Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331:889-95. [DOI] [PubMed] [Google Scholar]
- 23. Gomoll AH, Probst C, Farr J, Cole BJ, Minas T. Use of a type 1/3 bilayer collagen membrane decreases reoperation rates for symptomatic hypertrophy after autologous chondrocyte implantation. Am J Sports Med. 2009;20:1-4. [DOI] [PubMed] [Google Scholar]
- 24. Bartlett W, Skinner JA, Gooding CR, Carrington RWJ, Flanagan AM, Briggs TWR, Bentley G. Autologous chondrocyte implantation versus matrix-induced autologous chondrocyte implantation for osteochondral defects of the knee: a prospective, randomised study. J Bone Joint Surg Br. 2005;87:640-5. [DOI] [PubMed] [Google Scholar]
- 25. Gooding CR, Bartlett W, Bentley G, Skinner JA, Carrington R, Flanagan A. A prospective randomized study comparing two techniques of autologous chondrocyte implantation for osteochondral defects in the knee: periosteum covered versus type 1/3 collagen covered. Knee. 2006;13:203-10. [DOI] [PubMed] [Google Scholar]
- 26. Henderson, et al. Autologous chondrocyte implantation for treatment of focal chondral defects of the knee–a clinical, arthroscopic, MRI and histologic evaluation at 2 years. Knee. 2005. June;12(3):209-16. [DOI] [PubMed] [Google Scholar]
- 27. Gross AE, Shasha N, Aubin P. Long-term followup of the use of fresh osteochondral allografts for posttraumatic knee defects. Clin Orthop. 2005;435:79-87. [DOI] [PubMed] [Google Scholar]
- 28. LaPrade RF, Botker J, Herzog M, Agel J. Refrigerated osteoarticular allografts to treat articular cartilage defects of the femoral condyles: a prospective outcomes study. J Bone Joint Surg Am. 2009;91:805-11. [DOI] [PubMed] [Google Scholar]
- 29. Williams RJ, Ranawat AS, Potter HG, Carter T, Warren RF. Fresh stored allografts for the treatment of osteochondral defects of the knee. J Bone Joint Surg Am. 2007;89:718-26. [DOI] [PubMed] [Google Scholar]
- 30. Steinwachs M, Kreuz PC. Autologous chondrocyte implantation in chondral defects of the knee with a type 1/3 collagen membrane: a prospective study with 3 year follow-up. Arthoscopy. 2007;23:381-7. [DOI] [PubMed] [Google Scholar]
- 31. Rosenberger RE, Gomoll AH, Bryant T, Minas T. Repair of large chondral defects of the knee with autologous chondrocyte implantation in patients 45 years or older. Am J Sports Med. 2008;36:2336-44. [DOI] [PubMed] [Google Scholar]
- 32. Kreuz PC, Steinwachs M, Erggelet C. Classification of graft hypertrophy after autologous chondrocyte implantation of full thickness cartilage defects in the knee. Osteoarthritis Cartilage. 2007;15:1339-47. [DOI] [PubMed] [Google Scholar]
- 33. Reinold M., et al. Current Concepts in the Rehabilitation Following Articular Cartilage Repair Procedures in the Knee. J Orthop Sports Phys Ther. 2006;36:774-94. [DOI] [PubMed] [Google Scholar]
- 34. Cole BJ, Malek MM. editors. Articular cartilage lesions: a practical guide to assessment and treatment. New York: Springer-Verlag; 2004. [Google Scholar]


