Abstract
Purpose:
To present a new method of arthroscopic measurement of the surface and location of condylar lesions.
Methods:
We propose measuring the height of the condylar lesion by using the lesion’s arc (Δ°) obtained from the difference between the angle of flexion at the beginning of the lesion and the angle of flexion at the end of the lesion. The first goal of the study was to determine the intra and inter reliability of the lesion’s arc. Experiment 1: 20 deep lesions were evaluated using the lesional arc by two arthroscopists. Experiment 2: In a second series of 20 lesions, the flexion angles of the knees were recorded using a goniometer. All 10 knees (5 in each series) were then disarticulated and the true lesion arc was checked with a goniometer to assess the validity of the scopic measurements. The second goal was to obtain the height of the lesion from the lesion’s arc. The lesion arc Δ° of the condylar is converted into height (millimeters) on the basis of a table obtained from 5 standard profiles of the lateral X-ray of the knee.
Results:
Experiment 1: The intra observer reliability was good but the inter observer reliability was poor. Experiment 2: The intra and inter observers’ reliability were good. On the anatomic control after disarticulating the knee, the confidence interval was narrower when using the goniometer.
Conclusions:
We propose a simple, reliable method to measure the height of a condylar lesion with the lesion’s arc during arthroscopy.
Keywords: cartilage measurement, cartilage defects, arthroscopy, osteoarthritis
Chondral lesions of the knee are frequent during arthroscopy (63% of cases), and the deep lesions are principally localized on the condylars.1 These lesions are posttraumatic, dystrophic (osteochondritis dissecans), or degenerative (osteoarthritis). The evaluation of depth and size of these chondral lesions is essential when making a therapeutic decision and for the prognosis. The sensitivity of magnetic resonance imaging (MRI) with regard to the depth and dimension of the femorotibial or femoropatellar lesions varies by between 33% and 95% and essentially upon the sequences used, but it is of high specificity.2-6 Advancements in pulse sequence design, specifically the use of fat-suppressed (FS) gradient echo (GRE) images (such as fast low-angle shot [FLASH] and spoiled gradient-recalled echo [SPGR]) have improved the ability to detect lesions.5 Others have reported very good results with fast spin-echo (FSE) sequences.2 McGibbon et al.7 add, “Three-dimensional mapping of cartilage thickness shows great promise for the accurate measurement of focal cartilage defects, though improvement is needed.” Arthroscopy remains the gold standard for direct visualization and classification of the lesions with regard to their severity, and no fewer than 30 classifications in 4 or 5 grades have been described.8 The Outerbridge classification is widely used and considered reliable and reproducible, but all these classifications are unreliable for assessing location and size of the chondral lesions.9
The International Cartilage Research Society (ICRS) proposed an evaluation score of the arthroscopic surface in 1998 (9 zones per condyle, per tibial plateau or for the patella) with the degree of depth in 4 grades.10 ICRS grades 1 (a, b) and 2 are partial-depth injuries concerning less than 50%. ICRS grade 3a includes defects down to more than 50% depth but not to the calcified layer. Grade 3b includes defects down to the calcified layer and grade 3c down to the bone, and grade 3d involves blisters. ICRS grade 4 (a, b) involves the subchondral bone. In 2001, Hunt et al.11 proposed a score of functional zones with an estimate of the percentage of the surface. Ayral et al.12 proposed estimating lesion size as a percentage of cartilage lesion occupied; these measurements are difficult in routine practice and controversial (“statistical artifact”8), and interobserver reliability is poor.12,13 In essence, if the estimation of the severity of the chondropathy seems relatively simple, this is not the case when estimating the size. It is usually done using a graduated probe to obtain the width and the height of the cartilage lesion. If the lesion is quadrangular, the width measurement seems to be accurate with conventional long probes. In contrast, the height, notably on the condylar, is very approximate and often over- or underestimated, as shown with animal studies,14 in cadaveric studies,15 or on plastic models.16 Major sources of variability could be traced to the probe type, the experience of the investigator, and the orientation of the lesion in relation to the probe. Oakley et al.17 proposed using variable angle elongated probes to improve interobserver reliability, but these probes are not available today. The objective of our work is to suggest a new method of arthroscopic measurement of the size and location of focal femoral condylar lesions.
Methods
Principle
We measured the height of the lesion by using the lesion’s arc (Δ°) obtained from the difference between the angle of flexion at the beginning of the lesion and the angle of flexion at the end of the lesion. These records must be taken the same way for each arthroscopy and for each investigator. The lower limb was placed in an arthroscopic leg holder with the thigh horizontal; the standard 4.5-mm arthroscope (30° tip angulation, wide-angle lens) was introduced through a portal inferolateral or inferomedial to the patella. For the reference position, the scope was placed on the homolateral anterior meniscal horn, pointed horizontally toward the damaged condylar, and remained solidly against the tibia during movements. The joint line was horizontal when reading the screen. The knee was completely extended and gently flexed to 120°, the reading being done on the equator of the screen. When the anterior edge of the lesion appeared, the flexion angle of the knee was recorded visually on the lateral side of the limb (A), and then, when the posterior edge of the lesion was on the equator of the screen, the second angular position was recorded (B) (Fig. 1A,B). The lesion’s arc was obtained by the difference (Δ°) between these two positions (B – A). The angles of flexion A and B give information regarding the anterior-posterior location of the condylar lesion.
Figure 1.
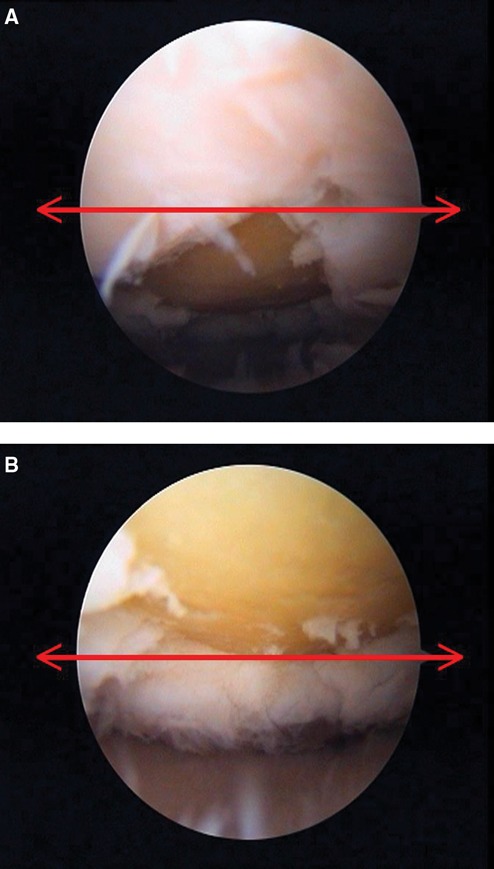
Arthroscopic measurement of the angle of the lesion in the laboratory: on the equator of the screen, the anterior border of the lesion is at 30° (A) and the posterior border is at 70° (B).
Experimental Protocol
We validated this principle in the Anatomical Laboratory at the University of Rennes, France. In the first series, the donor ages were 51, 62, 64, 72, and 77 years, with no degenerative knees on radiograph. Specimens were freshly frozen and thawed at room temperature overnight before preparation and testing. All the specimens studied had a body mass index (BMI) below 35. The thigh was securely fixed in a leg holder with the leg free of flexion/extension movement. Lateral and medial infrapatellar portals were used for visualization, probing, and curetting. No knees had degenerative or local chondral lesions. The knees were filled with water. On each knee, 4 quadrangular artificial lesions, grade 3c on the ICRS scale, were carried out with a curette, under scope view, by an investigator not involved in the work. Two lesions were created on the medial and two on the lateral condyles—thus 4 defects in 1 knee and 20 defects in the first series. Each lesion had been randomly evaluated using the lesional arc checked visually by 2 trained surgeons in arthroscopy (JCL: observer 1, HR: observer 2). Each measurement was randomly repeated after an interval of 1 hour, thus evaluating the intra- and interinvestigator reliability. Then we did a second series of 20 defects in 5 other knees (54, 56, 63, 78, 89 years old), 1 month later, and as before, all the specimens had a BMI below 35. Again each measurement was randomly repeated after an interval of 1 hour. The flexion angles of the knees were measured using a goniometer solidly fixed on the lateral side of the thigh and the leg, the center of the goniometer superimposed on the epicondylar (Fig. 2). All 10 knees (5 in each series) were then disarticulated, and the true lesion arc was checked with a goniometer to assess the validity of the arthroscopic measurements. The center of the goniometer was superimposed on top of the epicondylar, and the lesion arc was read between the condylo-trochlear line (point 0°) and the posterior condyle (point 90°). Each actual lesion’s arc directly measured on the disarticulated knee was compared with the previous results obtained by the 2 arthroscopists.
Figure 2.
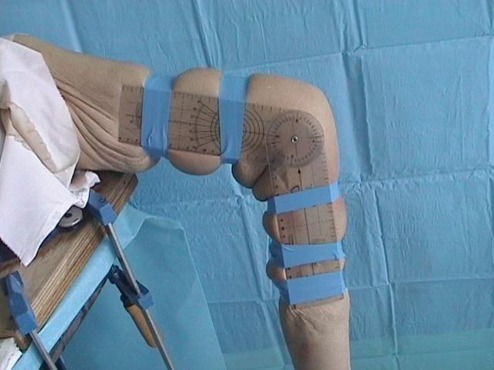
Second series: lateral view of the knee with the goniometer fixed on the lateral side of the knee. The center of the goniometer was superimposed on top of the epicondyle.
The lesion’s arc (Δ°) obtained experimentally has to be converted into height (mm) for a clinical application. For this, we determined, from 30 lateral x-rays in scale 1/1, 5 standard profiles with an increase of 3 mm. For each lateral x-ray, we defined the condylar center according to the methodology of Grood and Suntay18 for the description of the kinematics of bones. Then we measured the length of the arc of flexion from 0° (condylo-trochlear point) to 90° and the length of each arc of 10° by using the Orthogon software (Agfa HealthCare, Greenville, SC) (Fig. 3). Thus, we could obtain an abacus for the calculation of the height of the lesion (mm) for these 5 standard profiles (A to E).
Figure 3.
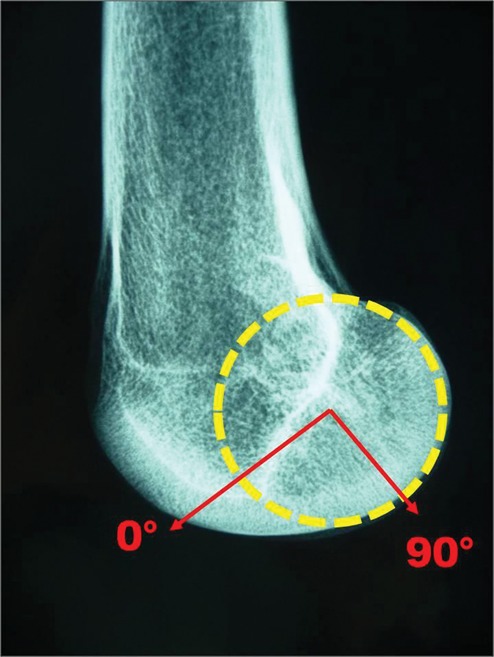
On x-ray, the center of the circle inscribed in the profile of the medial condyle allows an arc to be defined from 0° to 90°, with each arc length of 10° measured with the Orthogon software. Thus, we could obtain an abacus for the calculation of the height of the lesion (mm) for 5 standard profiles (A to E; see also Fig. 8A).
Statistical Analysis
The intraobserver reliability of the measurements of these angles was conducted using the Bland and Altman test limit of agreement (see Figs. 4 and 5).18 The principle of the Bland and Altman test is to compare the difference between a pair of measurements (e.g., between 2 observers or between an observation and a reference) against their mean values. If the measurements are comparable, the differences should be small, be centered around 0, and show no systematic variation with the mean of the measurement pairs. For the analysis of agreement between 2 investigators, we chose the kappa coefficient. Analyses of reproducibility within the observers for the 2 techniques were tested with all angular measurements of each anterior and posterior limit of the lesions (i.e., 2 per lesion). These measurements were transformed into categorical data for kappa agreement by rounding up the value to intervals of 10° ranging from 0° to 90° (0°−10°, 11°−20°, 21°−30°, etc.), which seemed to be an “acceptable error margin” within the experience of the investigators. As each lesion was widened by the instrument once during the experiment, only half of the lesions were available for reading after disarticulation of the knee. All statistics were performed with R software.
Figure 4.
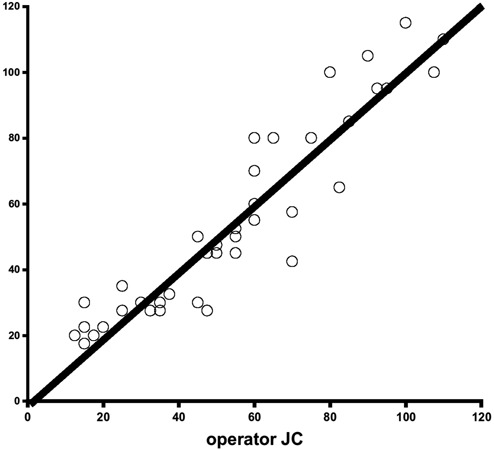
Interobserver reliability for the first series. The kappa coefficient calculated between the measurements of the 2 investigators is .66, indicating poor agreement.
Figure 5.
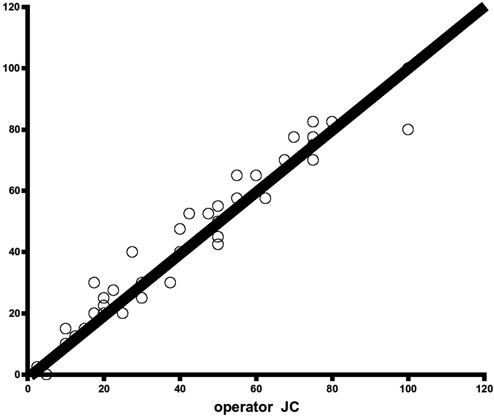
Interobserver reliability for the second series. The kappa coefficient calculated between the measurements of the 2 investigators is .78, indicating good agreement.
Results
For the first series of tests, 20 artificial lesions were evaluated without a goniometer (Table 1): the range for the 95% limit of agreement for the intraobserver analysis showed confidence intervals from −21.82° to 22.32° for observer 1 and −19.36° to 16.36° for observer 2. Means of differences were respectively 0.25° and −1.5°, which shows little bias. In each case, agreement was good.
Table 1.
Study without the Goniometer
| Anterior Limit (°) | Posterior Limit (°) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. Knee | Lesion Location | Observer 1, 1st | Observer 1, 2nd | Observer 2, 1st | Observer 2, 2nd | Control | Observer 1, 1st | Observer 1, 2nd | Observer 2, 1st | Observer 2, 2nd | Control |
| 1 | CM1 | 60 | 70 | 80 | 80 | 75 | 85 | 100 | 100 | ||
| CL1 | 30 | 30 | 30 | 30 | 70 | 50 | 70 | 70 | |||
| CM2 | 60 | 60 | 70 | 90 | 70 | 100 | 100 | 110 | 120 | 100 | |
| CL2 | 30 | 30 | 30 | 30 | 30 | 80 | 85 | 60 | 70 | 70 | |
| 2 | CM1 | 60 | 90 | 80 | 80 | 95 | 120 | 100 | 100 | ||
| CL1 | 30 | 40 | 25 | 30 | 50 | 60 | 45 | 60 | |||
| CM2 | 70 | 50 | 50 | 70 | 90 | 120 | 100 | 100 | 120 | 120 | |
| CL2 | 50 | 45 | 20 | 35 | 30 | 90 | 90 | 100 | 110 | 120 | |
| 3 | CM1 | 20 | 20 | 25 | 20 | 35 | 40 | 35 | 30 | ||
| CL1 | 15 | 15 | 20 | 15 | 25 | 25 | 30 | 25 | |||
| CM2 | 20 | 15 | 20 | 20 | 15 | 45 | 50 | 45 | 45 | 50 | |
| CL2 | 15 | 10 | 20 | 20 | 20 | 55 | 60 | 45 | 45 | 50 | |
| 4 | CM1 | 40 | 30 | 30 | 30 | 60 | 50 | 60 | 40 | ||
| CL1 | 20 | 30 | 40 | 30 | 40 | 50 | 60 | 40 | |||
| CM2 | 35 | 30 | 30 | 25 | 25 | 55 | 55 | 45 | 45 | 40 | |
| CL2 | 15 | 15 | 30 | 30 | 30 | 50 | 60 | 50 | 60 | 60 | |
| 5 | CM1 | 85 | 85 | 85 | 85 | 95 | 95 | 95 | 95 | ||
| CL2 | 50 | 40 | 30 | 30 | 80 | 60 | 45 | 40 | |||
| CM2 | 50 | 50 | 45 | 50 | 60 | 95 | 90 | 95 | 95 | 95 | |
| CL2 | 15 | 15 | 25 | 20 | 30 | 80 | 60 | 55 | 60 | 60 | |
Note: CM1 = first lesion in the medial condyle; CM2 = second lesion in the medial condyle; CL1 = first lesion in the lateral condyle; CL2 = second lesion in the lateral condyle. Observer 1, 1st = first registration by observer 1; observer 1, 2nd = second registration by observer 1; observer 2, 1st = first registration by observer 2; observer 2, 2nd = second registration by observer 2.
For the second series, 20 artificial lesions were evaluated with a goniometer (Table 2). We found a confidence interval from −8.9° to 8.1° and −6.94° to 7.94° for observers 1 and 2, respectively. Differences between the 2 series also gave a good agreement. As the confidence interval was narrower with series with the goniometer for the 2 observers, we can approximately say that intraobserver reproducibility error was smaller with the goniometer.
Table 2.
Study with the Goniometer
| Anterior Limit (°) | Posterior Limit (°) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. Knee | Lesion Location | Observer 1, 1st | Observer 1, 2nd | Observer 2, 1st | Observer 2, 2nd | Control | Observer 1, 1st | Observer 1, 2nd | Observer 2, 1st | Observer 2, 2nd | Control |
| 1 | CM1 | 50 | 55 | 65 | 50 | 60 | 70 | 75 | 80 | ||
| CL1 | 60 | 50 | 50 | 50 | 60 | 75 | 75 | 75 | |||
| CM2 | 40 | 45 | 55 | 50 | 60 | 65 | 70 | 70 | 70 | 80 | |
| CL2 | 50 | 50 | 45 | 40 | 55 | 70 | 80 | 70 | 70 | 80 | |
| 2 | CM1 | 60 | 60 | 65 | 65 | 75 | 75 | 80 | 85 | ||
| CL1 | 20 | 15 | 30 | 30 | 30 | 25 | 40 | 40 | |||
| CM2 | 55 | 45 | 55 | 55 | 40 | 75 | 75 | 75 | 80 | 75 | |
| CL2 | 30 | 30 | 30 | 30 | 35 | 65 | 60 | 55 | 55 | 60 | |
| 3 | CM1 | 15 | 15 | 15 | 15 | 20 | 20 | 20 | 20 | ||
| CL1 | 30 | 30 | 25 | 25 | 40 | 40 | 40 | 40 | |||
| CM2 | 20 | 20 | 15 | 15 | 15 | 40 | 40 | 30 | 30 | 40 | |
| CL2 | 20 | 20 | 25 | 25 | 30 | 50 | 50 | 45 | 45 | 50 | |
| 4 | CM1 | 10 | 10 | 15 | 15 | 20 | 20 | 20 | 20 | ||
| CL1 | 5 | 5 | 0 | 0 | 10 | 10 | 10 | 10 | |||
| CM2 | 10 | 10 | 10 | 10 | 20 | 20 | 20 | 25 | 25 | 30 | |
| CL2 | 5 | 0 | 0 | 0 | 0 | 20 | 20 | 20 | 20 | 20 | |
| 5 | CM1 | 80 | 80 | 80 | 85 | 100 | 100 | 100 | 100 | ||
| CL2 | 25 | 20 | 30 | 25 | 55 | 55 | 70 | 60 | |||
| CM2 | 40 | 40 | 50 | 45 | 45 | 90 | 90 | 80 | 80 | 80 | |
| CL2 | 10 | 10 | 10 | 10 | 10 | 45 | 45 | 50 | 55 | 50 | |
Note: CM1 = first lesion in the medial condyle; CM2 = second lesion in the medial condyle; CL1 = first lesion in the lateral condyle; CL2 = second lesion in the lateral condyle. Observer 1, 1st = first registration by observer 1; observer 1, 2nd = second registration by observer 1; observer 2, 1st = first registration by observer 2; observer 2, 2nd = second registration by observer 2.
The concordance of measurements between observers was tested with the kappa coefficient. All measurements were taken into account in terms of reproducibility. For the series without the goniometer, kappa coefficient was evaluated at 0.66, which could be classified as poor concordance. Calculation for the series with the goniometer showed the kappa coefficient at 0.78, with concordance between observers for this series thus being good.
Comparison to anatomical lesions, available only for half of the measurements, showed for both observers, for the first series, without goniometer, a 95% confidence interval from −27.69° to 21.32° and the mean of these differences around −3.19° (Fig. 6). Agreement was good, and bias was small. With the goniometer, in the second series, the confidence interval of the mean limits of agreement was from −16.69° to 10.81° (Fig. 7). Bias was around 2.94°, which is low. Compared to anatomical lesions, we can hypothesize that reproducibility errors were lower with than without the goniometer.
Figure 6.
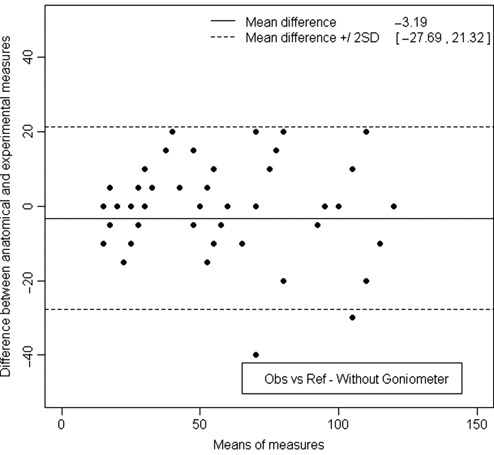
Measurement of the lesion arc, without using the goniometer, after disarticulating the knee. The limits of agreement were 27.69° below and 21.32° above the reference value.
Figure 7.
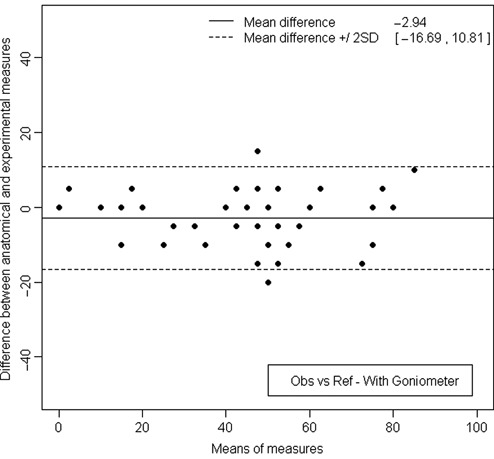
Measurement of the lesion arc, when using the goniometer, after disarticulating the knee. The limits of agreement were 16.69° below and 10.81° above the reference value.
These results show that intra- and interobserver reliability and concordance between observers of reading angular limits of chondral lesions were better with the goniometer.
The use of the abacus is relatively easy from the lesional arc and a strict x-ray profile of the patient’s knee. The height or longitudinal dimension in millimeters of the lesion is obtained by subtraction of angle B and angle A on the abacus. For example, in the case of a lesion extending from 20° to 60° on a condyle sized C, the length of the arc Δ° is 44 – 14.6 = 29.4 mm (see Fig. 8A).
Figure 8.
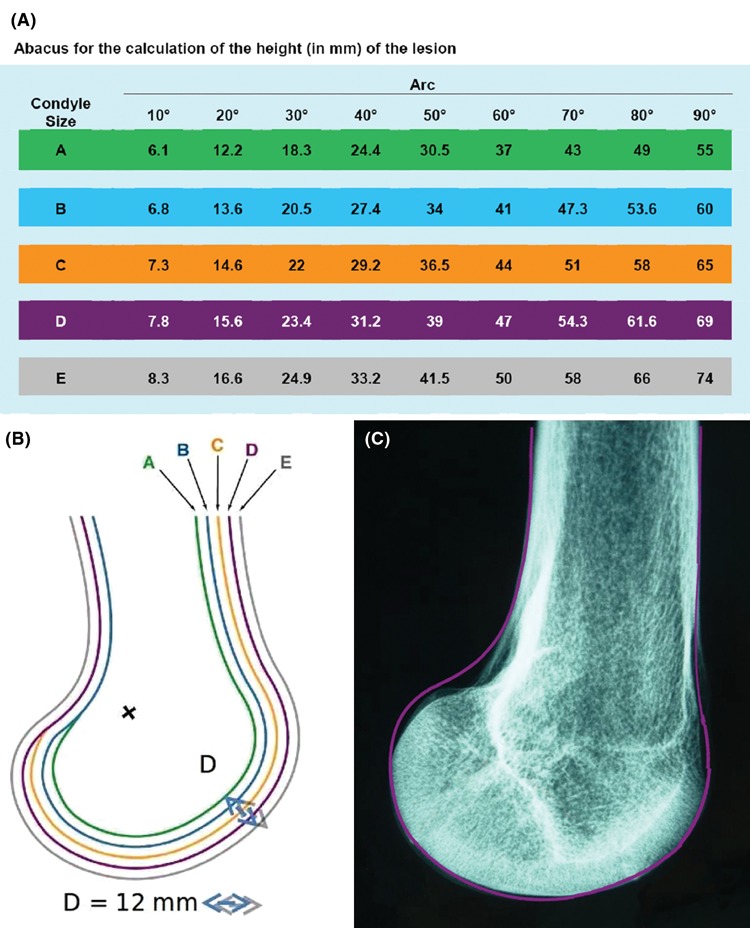
(A) Abacus for calculating lesion height based on condyle size (A to E) and lesion arc. (B) Lateral x-rays of the 5 knees sized from A to E. For a scale 1/1, the D segment has to measure 12 mm long. The patient’s x-ray (scale 1/1) is superimposed on the drawing to determine its size relative to the standard 5 sizes (A to E).
Discussion
The main result of this work is to propose a method for measuring the height of condylar chondral lesions under arthroscopy from a lesion’s arc defined by its anterior and posterior limits.
For Wilson et al.,15 the arthroscopic estimate of the extent of a lesion is considered the “gold standard,” but for Oakley et al.,14 it can contain considerable errors. Several authors have suggested their own techniques but without experimental validation of their accuracy.8,11,19-22 The determination of the surface area in the case of a focal defect is important for the choice of treatment (microfracture, osteochondral graft, chondrocyte implantation). In the case of knee osteoarthritis, it is more important to know the percentage of cartilage damage compared to the total surface of the knee cartilage than in a localized lesion. In this instance, the French Arthroscopic Society composite scoring system proposed by Ayral et al.12 in 1993 has received validation. It is based on the combination of the Beguin and Locker classification for the severity and the percent area estimates for the size; each compartment (medial or lateral femorotibial, femoropatellar) has a score between 0 (normal cartilage) and 100 points (major chondropathy).23 The intraobserver reliability of percent area estimates was evaluated by examination of arthroscopic video recordings and was found to be good, but interobserver reliability was poor, explaining why a single trained observer is used to reread the videos.13 Hunt’s score also uses an evaluation system in percentage terms of each functional lesioned zone, but even the author states that the reliability and accuracy are both insufficient.11 These 2 composite scores require recording percent area estimates for each grade of chondropathy. In a plastic knee model, Oakley et al.16 found that the accuracy and reliability of arthroscopic percent area estimates on the femoral surfaces were generally poor, and there was a clear trend to poorer reliability with larger lesions. It appears to us to be difficult in daily practice, as well as inaccurate for femoral condyles, due to their convexity and their large surface area. The use of variable-angle elongated probes especially designed for measurement of lesion diameter is one possible approach that has not been evaluated to date, to our knowledge.17 We have no direct experience of the commercially available flexible intra-articular rulers (Arthrex, Naples, FL), and although their use is a possibility, they are actually single-use instruments. The extent of lesions is significant in determining the therapeutic option. The location of lesions within cartilage surfaces is an important predictor of clinical outcome. However, there are no data regarding reliability and validity of arthroscopic methods of localization (e.g., comparison of arthroscopy and anatomic dissection).8 Our experimental study led to the development of an abacus based on radiological findings and arthroscopic measurements of the lesion arc. In the operating room, the width or transverse dimension is measured by a long graduated hook (0-20 mm) inserted through the homolateral portal. The depth is estimated with a short angulated probe, of known size, according to the ICRS score in 4 grades, and the height is obtained from the lesion’s arc with the table (Fig. 8A). The lesion surface, similar to a quadrangular dome, is obtained by the following formula: width × radius × Δ° × π/180. This formula does not take into account the spiral in the center of the curvature (evolved Fick curve).24 In practice, we can simply multiply the width by the height to calculate the lesion surface area assimilated to a rectangle after having chosen the exact size of the patient femur by superimposing a printout of Figure 8B on the lateral x-ray (scale 1/1). Figure 8C shows the superimposition of patient x-ray and trace D which best fits this case.
The recording of the lesion’s arc can also be used to locate the lesion on the ICRS map. The location of the condylar lesion between 0° and 90° can be plotted on the 3 areas: anterior, middle, and posterior.
There are two limitations of the work: first, we did not measure the height of the lesion in millimeters after dislocating the knees to validate the abacus, only the lesion arc. This was because we did not have at our disposal at that time a flexible and curved ruler, for example, those manufactured by the Orteq (London, UK). Second, this method cannot be used in a blind procedure. The technique of measuring the lesion’s arc is reproducible, with care taken to respect the conditions required when taking records and using a standard goniometer, solidly fixed on the lateral side of the knee. Care must be taken not to move the stationary arms of the goniometer when taking readings.
By using the x-ray lateral view of the knee with the abacus, we can obtain the height, and a simple formula allows the calculation of the size of the lesion (width × height). The location of the condylar lesion can then be drawn on the ICRS map. Preoperatively, the three-dimensional MR mapping of cartilage surface will permit the accurate measurement of focal defects,21 but arthroscopy remains the standard for assessing small or superficial lesions and the physical properties of cartilage.
Conclusion
We propose a simple, reliable method to measure the height of a condylar lesion with the lesion’s arc during arthroscopy. This method provides the height of the lesion from a simple-to-use abacus.
Footnotes
Acknowledgments and Funding: The authors thank the Anatomy Laboratory, Faculté de Médecine, Rennes, France. The authors received no financial support for the research and/or authorship of this article.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- 1. Curl WW, Krome J, Gordon ES, Rushing J, Smith BP, Poehling G. Cartilage injuries: a review of 31516 arthroscopies. Arthroscopy. 1997;13:456-60. [DOI] [PubMed] [Google Scholar]
- 2. Bredella MA, Tirman PF, Pterfy CG, Zarlingo M, Feller JF, Bost FW, et al. Accuracy of T2-weighted FSE MRI with fat sat saturation in detecting cartilage defect in the knee: comparison with arthroscopy in 130 patients. Am J Roentgology. 1999;172:1073-80. [DOI] [PubMed] [Google Scholar]
- 3. Friemert B, Oberländer Y, Schwarz W, Häberle HJ, Bähren W, Gerngross H, et al. Diagnosis of chondral lesions of the knee joint: can MRI replace arthroscopy? Knee Surg Sports Traumatol Arthrosc. 2004;12:58-64. [DOI] [PubMed] [Google Scholar]
- 4. Potter HG, Linklater JM, Allen AA, Hannafin JA, Haas SB. MRI of articular cartilage in the knee, an evaluation with use of fast spin echo imaging. J Bone Joint Surg. 1998;80A:1276-84. [DOI] [PubMed] [Google Scholar]
- 5. Recht MP, Piraino DW, Paletta GA, Schlis JP, Belhobeck GH. Accuracy of fat-suppressed three-dimensional spoiled gradient-echo FLASH MR imaging in the detection of patellofemoral articular cartilage abnormalities. Radiology. 1996;198:209-11. [DOI] [PubMed] [Google Scholar]
- 6. Drape JL, Pessis E, Auleley G, Chevrot A, Dougados M, Ayral X. Quantitative MR imaging evaluation of chondropathy in osteoarthritic knee. Radiology. 1998;208:49-55. [DOI] [PubMed] [Google Scholar]
- 7. McGibbon CA, Trahan CA. Measurement accuracy of focal cartilage defects from MRI and correlation of MRI graded lesion with histology: a preliminary study. Osteoarthritis Cartilage. 2003;11:483-93. [DOI] [PubMed] [Google Scholar]
- 8. Oakley SP, Lassere MN. A critical appraisal of quantitative arthroscopy as an outcome measure in osteoarthritis of the knee. Semin Arthritis Rheum. 2003;33:83-105. [DOI] [PubMed] [Google Scholar]
- 9. Cameron ML, Briggs KK, Steadman JR. Reproducibility and reliability of the Outerbridge classification for grading chondral lesion of the knee arthroscopically. Am J Sports Med. 2003;31:83-6. [DOI] [PubMed] [Google Scholar]
- 10. Brittberg M, Peterson L. Introduction to an articular cartilage classification. ICRS Newsletter. 1998;1:5-8. www.cartilage.org; ICRS Cartilage Injury Evaluation Package. [Google Scholar]
- 11. Hunt N, Sanchez-Ballester J, Pandit R, Thomas R, Strachan R. Chondral lesion of the knee: a new localization method and correlation with associated pathology. Arthroscopy. 2001;17:481-90. [DOI] [PubMed] [Google Scholar]
- 12. Ayral X, Dougados M, Listrat V, Bonvarlet JP, Simoneau J, Poiraudeau S, et al. A new method for scoring chondropathy. Semin Arthritis Rheum. 1993;22:289-97. [DOI] [PubMed] [Google Scholar]
- 13. Ayral X, Ravaud P. Evaluation de l’arthrose: méthodes d’évaluation objective par l’imagerie médicale et par l’arthroscopie. La Presse médicale. 1998;27:1491-8. [PubMed] [Google Scholar]
- 14. Oakley SP, Portek I, Szomor Z, Appleyard RC, Ghosh P, Kirkham BW, et al. Arthroscopic estimation of the extent of chondropathy. Osteoarthritis Cartilage. 2007;15:506-15. [DOI] [PubMed] [Google Scholar]
- 15. Wilson MA, Nash SD, Wolcott M, Albright JP. Accuracy and reliability of arthroscopic assessments of chondral defects in cadaveric knee specimens. Poster, ICRS Gent, Belgium, May 27-29, 2004. [Google Scholar]
- 16. Oakley SP, Portek I, Szomor Z, Turbull A, Murrell GAC, Kirkham BW, et al. Accuracy and reliability of arthroscopic estimates of cartilage lesion size in a plastic knee simulation model. Arthroscopy. 2003;19:282-9. [DOI] [PubMed] [Google Scholar]
- 17. Oakley S, Portek I, Szomor Z, Turnbull A, Murrell GA, Kirkham BW, et al. Poor accuracy and inter-observer reliability of knee arthroscopy measurements are improved by the use of variable angle elongated probes. Ann Rheum Dis. 2002;61:540-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Grood ES, Suntay WJ. A joint coordinate system for the clinical description of 3D motions: an application to the knee. J Biomech Eng. 1983;105:136-44. [DOI] [PubMed] [Google Scholar]
- 19. Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307-10. [PubMed] [Google Scholar]
- 20. Dzioba RB. The classification and treatment of acute articular cartilage lesions. Arthroscopy. 1988;4:72-80. [DOI] [PubMed] [Google Scholar]
- 21. Lewandrowski KU, Ekkernkamp A, David A, Muhr G, Schollmeier G. Classification of articular cartilage lesions of the knee at arthroscopy. Am J Knee Surg. 1996;9:121-8. [PubMed] [Google Scholar]
- 22. Outerbridge R. The aetiology of chondromalacia patellae. J Bone Joint Surg. 1961;43B:752-6. [DOI] [PubMed] [Google Scholar]
- 23. Beguin J, Heron JF, Sabatier JP, Locker B, Souquieres G. Arthroscopy of the knee, diagnostic value, 1005 cases. Nouv Presse Med. 1982;11:3619-21. [PubMed] [Google Scholar]
- 24. Kapandji IA. Physiologie articulaire, membre inférieur. Paris: Maloine; 1999. [Google Scholar]


