Abstract
Background
The American Stroke Association/American Heart Association recommended the criteria for diagnosis of vascular cognitive impairment and memory impairment (MI) is a feature in the classification of vascular mild cognitive impairment (VaMCI). VaMCI patients with MI may differ in terms of infarct location or demographic features, so we evaluated the clinical characteristics associated with MI in patients with VaMCI.
Methods
A prospective multicenter study enrolled 353 acute ischemic stroke patients who underwent evaluation using the Korean Vascular Cognitive Impairment Harmonization Standard Neuropsychological Protocol at three months after onset. The association between MI and demographic features, stroke risk factors, and infarct location was assessed.
Results
VaMCI was diagnosed in 141 patients, and 58 (41.1%) exhibited MI. Proportions of men and of left side infarcts were higher in VaMCI with MI than those without (75.9 vs. 57.8%, P = 0.03, 66.7 vs. 47%, P = 0.02). Multiple logistic analyses revealed that male sex (odds ratio [OR] 3.07, 95% confidence interval [95% CI] 1.12-8.42), left-side infarcts (OR 3.14, 95% CI 1.37-7.20), and basal ganglia/internal capsule infarcts (OR 4.53, 95% CI 1.55-13.22) were associated with MI after adjusting other demographic variables, vascular risk factors, and subtypes of stroke.
Conclusions
MI is associated with sex and infarct location in VaMCI patients.
Background
Vascular mild cognitive impairment (VaMCI) is a subtype of vascular cognitive impairment (VCI) and commonly follows stroke [1,2]. While VaMCI sometimes improves with time, it is associated with an elevated risk of dementia [3,4]. The American Stroke Association/American Heart Association (ASA/AHA) recommended the following criteria for VaMCI: patient exhibits impairment in at least 1 of 4 cognitive domains (memory, executive/activation, visuospatial, language); display normal or mild impairments in daily living activities; imaging results suggest cerebrovascular disease; a temporal relationship exists between the stroke and the cognitive symptoms. Moreover, it is recommended to apply these criteria in the study of VaMCI after stroke [1,5].
Cognitive impairment after stroke is characterized by disturbance of frontal or executive function, however, several previous studies also indicated that clinical stroke causes subsequent poorer performance in multiple cognitive domains including memory [6-8]. Memory impairment (MI) is a prerequisite for a diagnosis of vascular dementia according to DSM-IV criteria and a feature in the classification of VaMCI by ASA/AHA recommendation [1,9]. Verbal memory declines over 3 years after stroke [10] and impairment of verbal memory is associated with progression to dementia or vascular death [4,11]. Memory domain has been considered as a key domain among all cognitive domains, however, little is known about the clinical characteristics of VaMCI with MI.
The prevalence of MI is about 23–55% from a review paper [12] and is 21.4–66.5% from previous studies about VaMCI or VCI without dementia at 3–6 months after stroke [4,6]. Hippocampal atrophy is generally not associated with VCI [13] and the correlation of MI with white matter hyperintensities, infarct location or laterality were reported after stroke, so the mechanisms of MI in VaMCI or VCI may be multifactorial [12]. Interestingly, previous studies showed some difference in age, gender, risk factors, or hippocampal atrophy according to the subtypes of VaMCI or VCI, but these differences were not evaluated in the manner of multivariate analysis [4,14]. Thus, we hypothesize that VaMCI patients with MI may differ in terms of infarct location or demographic features after adjusting the other demographic features, vascular risk factors, and infarct subtypes or locations.
In addition, neuropsychological protocols previously used to assess VCI have varied with respect to examined cognitive domains [2,4,15]. VCI harmonization standards (VCIHS) recommend the evaluation of four specified cognitive domains to conform with ASA/AHA diagnostic guidelines [16]. The Korean VCIHS neuropsychological protocol (K-VCIHS-NP) assesses four cognitive domains and has been validated for use in acute stroke patients [17]. Therefore, the present study uses the K-VCIHS-NP to investigate the associations between MI and the clinical characteristics of VaMCI patients.
Methods
Participants
Six hundred twenty acute ischemic stroke patients from 12 hospitals were enrolled in the study within 7 days of symptom onset from October 2007 to August 2008. Exclusion criteria and the exact number of patients for each stage of the study are shown in Figure 1. A detailed description of the methodology has been published elsewhere [17]. Briefly, we conducted a baseline evaluation within two weeks of stroke onset to determine demographic characteristics, vascular risk factors, stroke subtype [18], and functional status. Among these 620 patients, 353 completed the 60-min K-VCIHS-NP evaluation at three months post-stroke.
Figure 1.
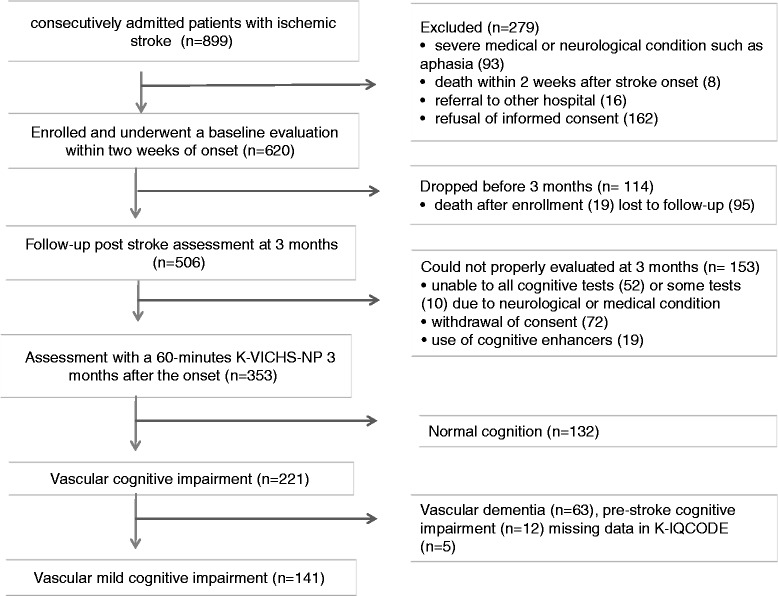
Patient flow chart. K-VCIHS-NP, The Korean VCIHS neuropsychological protocol; K-IQCODE, the Korean version of the Informant Questionnaire of Cognitive Decline in the Elderly.
Cognitive evaluation
The 60-min K-VCIHS-NP includes 7 tests that assess 4 cognitive domains: the Hopkins Verbal Learning Test for memory; Semantic (animal) and phonemic fluency tests, the Digit Symbol Coding, and the Trail Making Test for executive function/activation; the Rey Complex Figure Test-Copy for visuospatial skills; and the Boston Naming Test for language [19].
We also administered the Korean version of the Informant Questionnaire on Cognitive Decline in the Elderly (K-IQCODE) to examine pre-morbid history of cognitive impairment; the Instrumental Activities of Daily Living scale (IADL) to assess instrumental complex daily functioning; and the Korean version of the Mini-Mental Status Examination (K-MMSE) and Clinical Dementia Rating (CDR) as supplementary tests of cognitive function at 3 months post-stroke. All tests and scales were previously validated for the Korean language and standardized in Korean subjects by age and educational levels [20,21].
Diagnosis of VaMCI
The diagnostic procedure included a clinical interview and the administration of the K-VCIHS-NP, K-IQCODE, and IADL. Impaired cognitive function was defined as impairment in at least one cognitive domain on the K-VCIHS-NP, as indicated by a score below the 10th percentile using normative corrections for an individual domain. For executive function/activation domains, which consisted of 4 tests, patients who scored below the 10th percentile on more than 2 tests were identified as having impaired functions. Abnormal functional status was indicated by a score greater than 0.43 on the Korean IADL [20]. Pre-stroke cognitive impairment was considered present if the K-IQCODE score exceeded 3.6 [21]. A vascular cause was presumed when cognitive impairment emerged at 3 months after the stroke in the absence of pre-stroke impairment. VaMCI was defined as impairment in at least 1 of 4 cognitive domains, combined with normal or mild impairments in daily living activities and temporal evidence of vascular causation [1].
Statistical analysis
We compared the demographic features and characteristics of index infarcts of VaMCI patients based on the presence of MI, using a Mann-Whiteny test for continuous variables and chi-square tests for categorical variables. Univariate and multivariate logistic analyses examined the influence of baseline demographic features, infarct locations, and classification of stroke subtypes on memory impairment in VaMCI at 3-month after stroke. We entered age, sex, duration of education, all locations and subtypes of ischemic stroke in the logistic model for detailed description. Analyses were performed with SPSS (Windows version 18.0).
Ethics statement
This study was approved by the Institutional Review Board/Ethics Committee of each participating hospital (Hallym University Sacred Heart Hospital IRB 2007–137 http://hallym.hallym.or.kr/irb/). All subjects provided informed consents. This study followed Good Clinical Practice guidelines and was consistent with the International Conference on Harmonization of ethical principles for medical research involving human subjects.
Results
At 3 months post stroke, 506 patients were follow-up and 353 patients completed K-VCI-HP. Cognitive impairments were present in 221 patients, and VaMCI was diagnosed in 141 patients (mean age: 65.5 ± 11.1 years, 92 men and 49 women; Figure 1). Causes of follow-up loss at 3 months were death (n =19) and move away or transfer to another hospitals (n = 95) and the causes of not completing K-VCIHS-NP at 3 months are deteriorated neurological or medical conditions (n =52), withdrawal of consent (n = 72), missing data of cognitive test (n = 10), and use of cognitive enhancers (n = 19). Among the 141 VaMCI patients, MI was present in 58 patients (41.1%). Executive/activation function was most commonly impaired (96, 68.1%), followed by visuospatial function (60, 42.6%), and language function (38, 27%). MI was more frequent in men than women (47.8% vs. 28.6%, P = 0.03), while no sex differences were observed in the other domains.
Sixty-nine patients (48.9%) exhibited impairments in two or more domains: There were 17 amnestic single domain VaMCI patients, 41 amnestic multiple domain VaMCI patients, 55 non-amnestic single domain VaMCI patients, and 28 non-amnestic multiple domain VaMCI patients. Combined memory and executive/activation impairments were present in 35 patients, but these were not correlated (r = 0.139, P = 0.10). Correlations were observed between language and memory impairments (r = 0.888, P = 0.01) and language and visuospatial impairments (r = 0.221, P = 0.009).
Baseline demographic features, vascular risk factors, location, laterality, multiplicity, classification of index ischemic stroke are shown in Table 1. Proportions of men and of left side infarcts were higher in VaMCI with MI than those without (75.9 vs. 57.8%, P = 0.03, 66.7 vs. 47%, P = 0.02). VaMCI with MI tended to have a higher chance of having basal ganglia/internal capsule (36.2 vs.21.7%, P = 0.06) or cerebellum (13.8 vs.4.8%, P = 0.06) as the location of index stroke than those without. The two groups did not significantly differ in education levels; the vascular risk factors; the other location or the number of infarcts; TOAST classification (Table 1).
Table 1.
Comparison of baseline demographic features, vascular risk factors, and location of cerebral infarcts between vascular mild cognitive impairment patients with and without memory impairment
| Memory impairment | P v alue | ||
|---|---|---|---|
| Present (n = 58) | Absent (n = 83) | ||
| Age | 64.2 ± 9.2 | 66.4 ± 12.2 | 0.09 |
| Sex, men (%) | 44 (75.9) | 48 (57.8) | 0.03 |
| Previous stroke (%) | 8 (13.8) | 8 (9.6) | 0.44 |
| Hypertension (%) | 35 (60.3) | 55 (66.3) | 0.47 |
| Diabetes (%) | 25 (43.1) | 30 (36.1) | 0.40 |
| Hyperlipidemia (%) | 15 (25.9) | 12 (14.5) | 0.09 |
| Duration of education (yr) | 4.9 ± 1.8 | 4.7 ± 1.6 | 0.34 |
| NIH stroke scale score | 3.9 ± 3.46 | 4.5 ± 4.85 | 0.99 |
| Presense of left infarct (%) | 38 (66.7)* | 39 (47) | 0.02 |
| Location of infarcts | |||
| Cortex (%) | 21 (36.2) | 39 (47.0) | 0.20 |
| Corona radiata (%) | 15 (25.9) | 27 (32.5) | 0.39 |
| Basal ganglia/internal capsule (%) | 21 (36.2) | 18 (21.7) | 0.06 |
| Thalamus (%) | 9 (15.5) | 6 (7.2) | 0.12 |
| Brainstem (%) | 7 (12.1) | 7 (8.4) | 0.48 |
| Cerebellum (%) | 8 (13.8) | 4 (4.8) | 0.06 |
| Multiple infarcts (%) | 25 (43.1) | 28 (52.8) | 0.26 |
| TOAST classification | 0.36 | ||
| Large artery disease | 23 (39.7) | 38 (45.8) | |
| Small vessel occlusion | 17 (29.3) | 18 (21.7) | |
| Cardioembolim | 12 (20.7) | 12 (14.5) | |
| Other or undetermined | 6 (10.3) | 15 (18.1) | |
yr, year; NIH, National Institutes of Health; TOAST, Trial of Org 10172 in Acute Stroke Treatment * one is missing in the information about stoke laterality, so a total of 57 patients with MI was analyzed.
VaMCI with MI had a higher score in sum of box of CDR (2.0 ± 1.9 vs.1.3 ± 1.3, P = 0.04) and showed a tendency of lower score in K-MMSE (24.1 ± 5.3 vs. 25.7 ± 3.4, P = 0.14) three months after stroke. There are no difference in K-IQCODE (3.0 ± 0.53 vs. 3.0 ± 0.49) and instrumental ADL (0.19 ± 0.37 vs. 0.19 ± 0.32) between two groups.
Logistic analyses with baseline demographic features, vascular risk factors, and location and classification of stroke revealed that male sex (odds ratio [OR] 3.07, 95% confidence interval [95% CI] 1.12-8.42), left-side infarct location (OR 3.14, 95% CI 1.37-7.20), and basal ganglia or internal capsule infarct location (OR 4.53, 95% CI 1.55-13.22) were associated with MI (Table 2). Additional logistic analyses with all above variables and K-IQCODE showed similar results: Male sex (OR 3.48, 95% CI 1.21-10.02), left-side infarct location (OR 3.15, 95% CI 1.37-7.24), and basal ganglia or internal capsule infarct location (OR 4.86, 95% CI 1.64-14.43) were associated with MI, while no such association was observed for other variables (data not shown).
Table 2.
Univariate and multivariate analysis for predictor of memory impairment
| Univariate | Multivariate | |||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| Age | 0.98 (0.95-1.01) | 0.25 | 0.98 (0.93-1.02) | 0.27 |
| Men | 2.29 (1.09-4.81) | 0.03 | 3.07 (1.12-8.42) | 0.03 |
| Education, year | 1.07 (0.87-1.31) | 0.53 | 0.83 (0.61.-1.12) | 0.22 |
| Previous stroke | 1.50 (0.53-4.26) | 0.45 | 2.04 (0.55-7.57) | 0.29 |
| Hypertension | 0.78 (0.39-1.55) | 0.47 | 1.17 (0.46-2.93) | 0.97 |
| Diabetes | 1.34 (0.67-2.65) | 0.41 | 1.49(0.65-3.39) | 0.34 |
| Hyperlipidemia | 2.01 (0.88-4.82) | 0.09 | 1.93 (0.69-5.45) | 0.21 |
| Left infarct | 2.26 (1.12-4.54) | 0.02 | 3.14 (1.37-7.20) | 0.007 |
| TOAST classification | ||||
| Large artery disease | 0.78 (0.39-1.54) | 0.47 | 0.90 (027–3.03) | 0.86 |
| Small vessel occlusion | 1.45 (0.69-3.23) | 0.30 | 2.12 (0.53-8.49) | 0.29 |
| Cardioembolim | 1.54 (0.64-3.73) | 0.34 | 2.20 (0.47-10.60) | 0.32 |
| Infarct location | ||||
| Cortex | 0.64 (0.32-1.27) | 0.20 | 0.97(0.32-2.91) | 0.95 |
| Corona radiata | 0.72 (0.34-1.53) | 0.40 | 0.71 (0.26-1.92) | 0.50 |
| Basal ganglia/internal capsule | 2.05 (0.97-4.33) | 0.06 | 4.53 (1.55-13.22) | 0.006 |
| Thalamus | 2.36 (0.79-7.03) | 0.12 | 2.70 (0.71-10.22) | 0.14 |
| Brainstem | 1.49 (0.49-4.50) | 0.48 | 1.78 (0.37-8.57) | 0.47 |
| Cerebellum | 3.16 (0.90-11.05) | 0.07 | 4.05 (0.81-20.14) | 0.09 |
OR, odds ratio; 95% CI, 95% confidence interval; TOAST, Trial of Org 10172 in Acute Stroke Treatment.
Discussion
The present study showed that, after adjusting for age, sex, stroke risk factors, education levels, and infarct location, VaMCI patients with MI were more likely to be male and have left side, basal ganglia, or internal capsule infarcts. The follow-up rate with K-VCIHS-NP of patients in prospective acute stroke cohort was 69.8% among 506 patients who were followed-up at 3 months after stroke in this study (56.9% among baseline 620 patients) and the proportion of cognitive assessing was somewhat low compared to the previous studies [15,22]. This discrepancy might be explained by the difference in clinical setting as a multicenter setting and in study design excluding those with cognitive enhancer or in extensiveness of the neuropsychological protocol [17].
Sex differences in VaMCI have been indicated by several studies [4,14,23]. For example, males are more likely to have amnestic MCI [4,14], and females recover more quickly from VaMCI [23]. While age-related cognitive decline, vascular risk factors, and stroke are more common in men, it is unclear whether males are more susceptible to cognitive insult by vascular burden or aging [24-26]. In addition, males were at subtle disadvantage when performing this memory test in a previous paper; thus, this should be considered during the rehabilitation or cognitive testing of stroke patients [27].
Infarct location influenced the domain of cognitive impairment. Basal ganglia and thalamic infarcts have been associated with MI in previous studies [7,28-31]. The basal ganglia is known to play a some role in the regulation of memory and the association between MI and basal ganglia infarcts is a line with the previous studies [29,30,32]. MI was related to left-side infarcts and was correlated with language impairment in this study. While verbal memory tasks rely on intact language function, an association between left-side infarct and MI was also found when memory was assessed using non-verbal memory tests [10,33]. Thalamic infarcts were not related to MI in this study. In a study with acute stroke patients, associations between infrequent infarct location and cognitive impairment may be difficult to detect if comparisons are not made with participants who have not suffered a stroke.
The strength of this study is that it investigated the clinical details of VaMCI with MI using ASA/AHA guidelines for VaMCI diagnosis and VCIHS protocols [1,16]. The exclusion of subjects with pre-stroke vascular dementia or cognitive impairment does not guarantee that the included participants did not have Alzheimer’s disease or another form of dementia that contributed to their MI. MI in VaMCI patients in this study may occur from the current ischemic stroke or underlying degenerative disease, but the VaMCI with MI is worthy to evaulate because of high risk of future dementia and death [12,13].
This study has several limitations. First, we did not measure white matter or infarct volume; or Alzheimer’s biomarkers, such as hippocampal volume and apolipoprotein E subtypes [13,14,34]. The study about MI in VaMCI should be adjusted by these variables to confirm the associations. Second, our results were not validated by the long-term VaMCI outcome. Third, we only used the Hopkins Verbal Learning Test to assess memory. While the Hopkins Verbal Learning Test is the only VCIHS-recommended memory test [35,36], adding visual memory or composite score of several memory tests would be informative as an index of MI in stroke patients [30]. Fourth, the wide confidence interval of the association between MI and stroke location may be related to the relatively small number of patients in each location. The association between MI and this parameter should be interpreted with the caution.
Conclusions
Sex and stroke location differ between VaMCI with MI and those without MI. VaMCI with MI was more frequently men and associated with infarcts on the left side or within the basal ganglia or internal capsule. These differences might be considered in the interpretation of cognitive testing or rehabilitation of VaMCI patients.
Acknowledgments
Korean VCIHS study group members also include Jae-Kwan Cha, Ki-Hyun Cho, Dong-Eog Kim, Hahn-Young Kim, Oeun-Kyu Kim, Yong-Jae Kim, Sun-Uck Kwon, Seung- Hoon Lee, Soo-Ju Lee, Jong-Moo Park, and Joon-Hyun Shin. This study was partially supported by Korea Healthcare technology R&D Project, Ministry of Health & Welfare, Republic of Korea (HI10C2020) and Eisai Co. Ltd. Korea.
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
CSJ, YKH, and LBC participated in conception and design. YKH, OMS, LJH, KIS, and LBC carried out the acquisition of data. CSJ, JS, and LJH participated in analysis and interpretation of data. CSJ, BHJ, and KY have been involved in drafting the manuscript or revising it critically for important intellectual content. All authors read and approved the final manuscript.
Contributor Information
Soo-Jin Cho, Email: dowonc@naver.com.
Kyung-Ho Yu, Email: ykh1030@hallym.or.kr.
Mi Sun Oh, Email: iyyar@hallym.or.kr.
San Jung, Email: neurojs@hallym.ac.kr.
Ju-Hun Lee, Email: leeforte@medimail.co.kr.
Im-Seok Koh, Email: neukoh@naver.com.
Hee-Joon Bae, Email: braindoc@snu.ac.kr.
Yeonwook Kang, Email: ykang913@gmail.com.
Byung-Chul Lee, Email: ssbrain@hallym.ac.kr.
References
- 1.Gorelick PB, Scuteri A, Black SE, Decarli C, Greenberg SM, Iadecola C, Launer LJ, Laurent S, Lopez OL, Nyenhuis D, Petersen RC, Schneider JA, Tzourio C, Arnett DK, Bennett DA, Chui HC, Higashida RT, Lindquist R, Nilsson PM, Roman GC, Sellke FW, Seshadri S, American Heart Association Stroke Council. Council on Epidemiology and Prevention. Council on Cardiovascular Nursing. Council on Cardiovascular Radiology and Intervention. Council on Cardiovascular Surgery and Anesthesia Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American heart association/American stroke association. Stroke. 2011;42:2672–2713. doi: 10.1161/STR.0b013e3182299496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Delgado C, Donoso A, Orellana P, Vasquez C, Diaz V, Behrens MI. Frequency and determinants of poststroke cognitive impairment at three and twelve months in Chile. Dement Geriatr Cogn Disord. 2010;29:397–405. doi: 10.1159/000305097. [DOI] [PubMed] [Google Scholar]
- 3.Williamson JB, Nyenhuis DL, Pedelty L, Byrd S, Jhaveri M, Wang C, deToledo-Morrell L, Sripathirathan K, Gorelick P. Baseline differences between vascular cognitive impairment no dementia reverters and non-reverters. J Neurol Neurosurg Psychiatry. 2008;79:1208–1214. doi: 10.1136/jnnp.2007.137554. [DOI] [PubMed] [Google Scholar]
- 4.Narasimhalu K, Ang S, De Silva DA, Wong MC, Chang HM, Chia KS, Auchus AP, Chen C. Severity of CIND and MCI predict incidence of dementia in an ischemic stroke cohort. Neurology. 2009;73:1866–1872. doi: 10.1212/WNL.0b013e3181c3fcb7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gauthier S, Patterson C, Chertkow H, Gordon M, Herrmann N, Rockwood K, Rosa-Neto P, Soucy JP, participants C 4th Canadian Consensus Conference on the Diagnosis and Treatment of Dementia. Can J Neurol Sci. 2012;39(Suppl 5):1–8. doi: 10.1017/s0317167100015183. [DOI] [PubMed] [Google Scholar]
- 6.Sachdev PS, Brodaty H, Valenzuela MJ, Lorentz L, Looi JC, Wen W, Zagami AS. The neuropsychological profile of vascular cognitive impairment in stroke and TIA patients. Neurology. 2004;62:912–919. doi: 10.1212/01.WNL.0000115108.65264.4B. [DOI] [PubMed] [Google Scholar]
- 7.Moorhouse P, Rockwood K. Vascular cognitive impairment: current concepts and clinical developments. Lancet Neurol. 2008;7:246–255. doi: 10.1016/S1474-4422(08)70040-1. [DOI] [PubMed] [Google Scholar]
- 8.Weinstein G, Preis SR, Beiser AS, Au R, Kelly-Hayes M, Kase CS, Wolf PA, Seshadri S. Cognitive performance after stroke - The Framingham Heart Study. Int J Stroke. 2014;9(Suppl A100):48–54. doi: 10.1111/ijs.12275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 10.Sachdev PS, Lipnicki DM, Crawford JD, Wen W, Brodaty H. Progression of cognitive impairment in stroke/TIA patients over 3 years. J Neurol Neurosurg Psychiatry. 2014;86:1324–1330. doi: 10.1136/jnnp-2013-306776. [DOI] [PubMed] [Google Scholar]
- 11.Lowry J, Austin A, Al-Sayegh H, Yan F, Liu F, Zhang J. Impaired verbal memory is a significant predictor of early cerebral-cardiovascular death, an 18-year follow-up of a national cohort. Int J Geriatr Psychiatry. 2014;29:837–845. doi: 10.1002/gps.4068. [DOI] [PubMed] [Google Scholar]
- 12.Snaphaan L, de Leeuw FE. Poststoke memory function in nondemented patients: a systematic review on frequency and neuroimaging correlates. Stroke. 2007;38:198–203. doi: 10.1161/01.STR.0000251842.34322.8f. [DOI] [PubMed] [Google Scholar]
- 13.Sachdev PS, Chen X, Joscelyne A, Wen W, Altendorf A, Brodaty H. Hippocampal size and dementia in stroke patients: the Sydney stroke study. J Neurol Sci. 2007;260:71–77. doi: 10.1016/j.jns.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 14.Kim BJ, Oh MY, Jang MS, Han MK, Lee J, Lee J, Kang Y, Yu KH, Lee BC, Kim S, Yoon BW, Bae HJ. Medial temporal atrophy and memory dysfunction in poststroke cognitive impairment-no dementia. J Clin Neurol. 2012;8:43–50. doi: 10.3988/jcn.2012.8.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Serrano S, Domingo J, Rodriguez-Garcia E, Castro MD, del Ser T. Frequency of cognitive impairment without dementia in patients with stroke: a two-year follow-up study. Stroke. 2007;38:105–110. doi: 10.1161/01.STR.0000251804.13102.c0. [DOI] [PubMed] [Google Scholar]
- 16.Hachinski V, Iadecola C, Petersen RC, Breteler MM, Nyenhuis DL, Black SE, Powers WJ, DeCarli C, Merino JG, Kalaria RN, Vinters HV, Holtzman DM, Rosenberg GA, Wallin A, Dichgans M, Marler JR, Leblanc GG. National institute of neurological disorders and stroke-Canadian stroke network vascular cognitive impairment harmonization standards. Stroke. 2006;37:2220–2241. doi: 10.1161/01.STR.0000237236.88823.47. [DOI] [PubMed] [Google Scholar]
- 17.Yu KH, Cho SJ, Oh MS, Jung S, Lee JH, Shin JH, Koh IS, Cha JK, Park JM, Bae HJ, Kang Y, Lee BC, Korean-Vascular Cognitive Impairment Harmonization Standards Study Group Cognitive impairment evaluated with Vascular Cognitive Impairment Harmonization Standards in a multicenter prospective stroke cohort in Korea. Stroke. 2013;44:786–788. doi: 10.1161/STROKEAHA.112.668343. [DOI] [PubMed] [Google Scholar]
- 18.Adams HP, Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, Marsh EE., 3rd Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41. doi: 10.1161/01.STR.24.1.35. [DOI] [PubMed] [Google Scholar]
- 19.Kang YW, Na DL. Professional manual; Seoul Neuropsychological Screening Battery. Seoul, Korea: Human brain research and consulting; 2003. [Google Scholar]
- 20.Kang SJ, Choi SH, Lee BH, Kwon JC, Na DL, Han SH. The reliability and validity of the Korean instrumental activities of daily living (K-IDAL) J Korean Neurol Assoc. 2002;20:8–14. [Google Scholar]
- 21.Lee DW, Lee JY, Ryu SG, Cho SJ, Hong CH, Lee JH, Choi YM, Kim BS, Park EJ, Park SH. Validity of the Korean version of informant questionnaire on cognitive decline in the elderly. J Korean Geriatr Soc. 2005;9:196–204. [Google Scholar]
- 22.Sachdev PS, Brodaty H, Valenzuela MJ, Lorentz L, Looi JC, Berman K, Ross A, Wen W, Zagami AS. Clinical determinants of dementia and mild cognitive impairment following ischemic stroke: The Sydney Stroke Study. Dement Geriatr Cogn Disord. 2006;21:275–283. doi: 10.1159/000091434. [DOI] [PubMed] [Google Scholar]
- 23.Rasquin SM, Lodder J, Verhey FR. Predictors of reversible mild cognitive impairment after stroke: a 2-year follow-up study. J Neurol Sci. 2005;229–230:21–25. doi: 10.1016/j.jns.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 24.Lipnicki DM, Sachdev PS, Crawford J, Reppermund S, Kochan NA, Trollor JN, Draper B, Slavin MJ, Kang K, Lux O, Mather KA, Brodaty H. Risk factors for late-life cognitive decline and variation with age and sex in the Sydney Memory and Ageing Study. PLoS One. 2013;8:e65841. doi: 10.1371/journal.pone.0065841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Albert M, Massaro J, DeCarli C, Beiser A, Seshadri S, Wolf PA, Au R. Profiles by sex of brain MRI and cognitive function in the framingham offspring study. Alzheimer Dis Assoc Disord. 2010;24:190–193. doi: 10.1097/WAD.0b013e3181c1ed44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seshadri S, Wolf PA. Lifetime risk of stroke and dementia: current concepts, and estimates from the Framingham Study. Lancet Neurol. 2007;6:1106–1114. doi: 10.1016/S1474-4422(07)70291-0. [DOI] [PubMed] [Google Scholar]
- 27.Norman MA, Moore DJ, Taylor M, Franklin D, Jr, Cysique L, Ake C, Lazarretto D, Vaida F, Heaton RK, HNRC Group Demographically corrected norms for African Americans and Caucasians on the Hopkins Verbal Learning Test-Revised, Brief Visuospatial Memory Test-Revised, Stroop Color and Word Test, and Wisconsin Card Sorting Test 64-Card Version. J Clin Exp Neuropsychol. 2011;33:793–804. doi: 10.1080/13803395.2011.559157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vermeer SE, Prins ND, den Heijer T, Hofman A, Koudstaal PJ, Breteler MM. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med. 2003;348:1215–1222. doi: 10.1056/NEJMoa022066. [DOI] [PubMed] [Google Scholar]
- 29.Giroud M, Lemesle M, Madinier G, Billiar T, Dumas R. Unilateral lenticular infarcts: radiological and clinical syndromes, aetiology, and prognosis. J Neurol Neurosurg Psychiatry. 1997;63:611–615. doi: 10.1136/jnnp.63.5.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Benisty S, Gouw AA, Porcher R, Madureira S, Hernandez K, Poggesi A, van der Flier WM, Van Straaten EC, Verdelho A, Ferro J, Pantoni L, Inzitari D, Barkhof F, Fazekas F, Chabriat H, the LADIS Study group Location of lacunar infarcts correlates with cognition in a sample of non-disabled subjects with age-related white-matter changes: the LADIS study. J Neurol Neurosurg Psychiatry. 2009;80:478–483. doi: 10.1136/jnnp.2008.160440. [DOI] [PubMed] [Google Scholar]
- 31.Park KC, Yoon SS, Chang DI, Chung KC, Ahn TB, Ku BD, Adair JC, Na DL. Amnesic syndrome in a mammillothalamic tract infarction. J Korean Med Sci. 2007;22:1094–1097. doi: 10.3346/jkms.2007.22.6.1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vakil E, Blachstein H, Soroker N. Differential effect of right and left basal ganglionic infarctions on procedural learning. Cogn Behav Neurol. 2004;17:62–73. doi: 10.1097/01.wnn.0000094553.44085.25. [DOI] [PubMed] [Google Scholar]
- 33.Madureira S, Guerreiro M, Ferro JM. Dementia and cognitive impairment three months after stroke. Eur J Neurol. 2001;8:621–627. doi: 10.1046/j.1468-1331.2001.00332.x. [DOI] [PubMed] [Google Scholar]
- 34.Roh JH, Lee JH. Recent updates on subcortical ischemic vascular dementia. J Stroke. 2014;16:18–26. doi: 10.5853/jos.2014.16.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wong A, Xiong YY, Wang D, Lin S, Chu WW, Kwan PW, Nyenhuis D, Black SE, Wong KS, Mok V. The NINDS-Canadian stroke network vascular cognitive impairment neuropsychology protocols in Chinese. J Neurol Neurosurg Psychiatry. 2013;84:499–504. doi: 10.1136/jnnp-2012-304041. [DOI] [PubMed] [Google Scholar]
- 36.Pendlebury ST, Mariz J, Bull L, Mehta Z, Rothwell PM. MoCA, ACE-R, and MMSE versus the National Institute of Neurological Disorders and Stroke-Canadian Stroke Network Vascular Cognitive Impairment Harmonization Standards Neuropsychological Battery after TIA and stroke. Stroke. 2012;43:464–469. doi: 10.1161/STROKEAHA.111.633586. [DOI] [PMC free article] [PubMed] [Google Scholar]


