Abstract
BACKGROUND & AIMS
Patients with stroke experience swallowing problems (dysphagia); increased risk of aspiration pneumonia, malnutrition, and dehydration; and have increased mortality. We investigated the behavioral and neurophysiological effects of a new neurostimulation technique (paired associative stimulation [PAS]), applied to the pharyngeal motor cortex, on swallowing function in healthy individuals and patients with dysphagia from stroke.
METHODS
We examined the optimal parameters of PAS to promote plasticity by combining peripheral pharyngeal (electrical) with cortical stimulation. A virtual lesion was used as an experimental model of stroke, created with 1-Hz repetitive transcranial magnetic stimulation over the pharyngeal cortex in 12 healthy individuals. We tested whether hemispheric targeting of PAS altered swallowing performance before applying the technique to 6 patients with severe, chronic dysphagia from stroke (mean of 38.8 ± 24.4 weeks poststroke).
RESULTS
Ten minutes of PAS to the unlesioned pharyngeal cortex reversed (bilaterally) the cortical suppression induced by virtual lesion (lesioned: F1,9 = 21.347, P = .001; contralesional: F1,9 = 9.648, P = .013; repeated-measures analysis of variance) compared with sham PAS. It promoted changes in behavior responses measured with a swallowing reaction time task (F1,7 = 21.02, P = .003; repeated-measures analysis of variance). In patients with chronic dysphagia, real PAS induced short-term bilateral changes in the brain; the unaffected pharyngeal cortex had increased excitability (P = .001; 95% confidence interval, 0.21–0.05; post hoc paired t test) with reduced penetration-aspiration scores and changes in swallowing biomechanics determined by videofluoroscopy.
CONCLUSIONS
The beneficial neurophysiological and behavioral properties of PAS, when applied to unlesioned brain, provide the foundation for further investigation into the use of neurostimulation as a rehabilitative approach for patients with dysphagia from stroke.
Keywords: Swallowing Disorders, Clinical, Therapy, Recovery
The importance of dysphagia as a major complication following neurologic disorders such as stroke is well recognized.1 Moreover, new avenues for rehabilitation based on high-quality evidence-based practice are attracting increased interest. While spontaneous recovery of swallowing takes place during the acute stroke phase,2 it can be a protracted process.3,4 Different compensation strategies believed to promote reorganization in both lesional and perilesional areas after stroke have been proposed.5 In preclinical swallowing studies, cerebral stimulation of the caudal pericentral cortex in monkeys can evoke a full swallow.6 Studies in animals have also shown that cortical stimulation of both hemispheres can evoke swallowing,7 while stimulating vagal and/or glossopharyngeal nerve fibers may induce, initiate, or modulate reflexive swallowing.8 Moreover, several studies with transcranial magnetic stimulation (TMS) showed that the pharyngeal motor cortex (MI) has the ability to reorganize following brain lesions and after experimental stimulation. In the swallowing system, it has been proposed that effective recovery of swallowing function after unilateral stroke is associated with increases in cortical excitability and cortical area map size of the unaffected hemisphere.9 As a consequence, numerous research protocols have attempted to promote these cortical changes in the swallowing network via peripheral sensory stimulation10 and particularly with electrical stimulation.11 These exploratory neurostimulation studies on swallowing performance in health and dysphagia following stroke have introduced novel approaches to dysphagia rehabilitation.12
One promising neurostimulation technique is paired associative stimulation (PAS). This technique induces heterosynaptic plasticity in the motor and somatosensory cortical areas by combining peripheral stimulation to the targeted muscle with cortical stimulation over the representational area of that muscle in the MI. By combining these 2 modalities, peripheral and central, and by separating them with a specific time interval, MI excitation can be strongly induced. Recently, we investigated the brain effects of PAS applied unilaterally to the pharyngeal motor system and explored the involvement of specific neurotransmitters with magnetic resonance spectroscopy.13 However, much less is known about the effects of PAS on swallowing performance and whether the application of PAS to the damaged or undamaged cortex would be effective in treating dysphagia after stroke.
This study aimed to examine PAS as a therapy for dysphagic stroke by (1) investigating the relevant dose parameters for inducing plasticity in the pharyngeal MI, (2) assessing its effects on swallowing performance in healthy and experimentally lesioned pharyngeal cortex, and (3) providing proof-of-principle data to support its use in treating stroke patients with chronic dysphagia.
Our hypothesis was that PAS would have beneficial effects on brain excitability and swallowing behavior when applied to the stronger pharyngeal motor projection in health, whereas when lesioned, PAS targeting the unlesioned hemisphere would be preferential both following virtual and actual brain lesions (stroke).
Methods
No major illnesses were reported by the healthy participants, while stroke-affected patients had their stable medical status confirmed by their general practitioners before participation. Written informed consent was obtained from all participants before the experiments. The exclusion criteria included history of epilepsy; cardiac pacemaker; previous brain or ear, nose, and throat surgery; previous swallowing problems (before stroke); significant medical disorders (any history of dementia or cognitive impairment) or severe communication/aphasic problems; pregnancy; metal in the head or eyes; or use of medication that acts on the central nervous system. The research protocols were approved by Salford and Trafford Research Ethics Committee, and all experiments were undertaken in the clinical laboratories of the Gastrointestinal Sciences at Salford Royal NHS, England, in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Experimental Procedures
TMS
Focal TMS was performed using a flat figure-of-8-shaped magnetic coil (outer diameter, 70 mm) connected with a Magstim BiStim2 magnetic stimulator (Magstim Co, Whitland, Wales, UK), which produced maximal output of 2.2 T. The anterior-posterior direction with the plane of the coil parallel to the scalp surface and the handle/axis of the coil at 45° to midsagittal line was chosen according to previous studies.14
Pharyngeal and thenar electromyographic measurements
Pharyngeal electromyographic measurements after single TMS pulses, termed pharyngeal motor evoked potentials (PMEPs), were recorded through a 3.2-mm-diameter intraluminal catheter (Gaeltec Ltd; Dunvegan, Isle of Skye, Scotland) with a built-in pair of bipolar platinum ring electrodes, which was inserted either nasally (15–17 cm to pair electromyographic electrodes from the nasal flare) or orally (13–15 cm) depending on subject preference. This allowed the recording of PMEPs at the mid-pharyngeal level and likely middle pharyngeal constrictors. As a control (unilaterally innervated) system, thenar motor evoked potentials (TMEPs) from the abductor pollicis brevis muscle were also recorded from MI (see Supplementary Materials and Methods).
PAS
PAS was delivered by pairing a pharyngeal electrical stimulus (0.2-millisecond pulse) with a single TMS pulse on the pharyngeal MI at the intensity of motor threshold (MT) plus 20% of stimulator output. The 2 pulses were delivered in a controlled manner through Signal software (v2.13; Cambridge Electronic Design, Cambridge, England), with an interstimulus interval of 100 milliseconds, based on previous investigations.13 The intraluminal catheter used for PMEPs was connected to a constant current generator (model DS7; Digitimer Welwyn-Garden City, Herts, UK) to deliver pharyngeal electrical stimulation (PES) (see Supplementary Materials and Methods). For the sham intervention, the coil was held tangentially to the skull at a 90° angle to the sagittal plane, and no PES was delivered through the pharyngeal catheter in situ.
Swallowing reaction times
Participants were requested to swallow a 3-mm intraluminal catheter with a built-in pressure transducer (Gaeltec Ltd) positioned in the pharyngeal area. Similarly to previous studies,15 participants’ swallowing performance was assessed by swallowing boluses of 5 mL water, cued by an electrical pulse to the thenar, while using visual feedback desktop software for the reaction time paradigm (see Supplementary Materials and Methods).
Focal cortical suppression
To induce focal cortical suppression or a “virtual lesion,” trains of stimuli were delivered through the figure-of-8 coil connected to a Magstim super rapid stimulator (Magstim Co) with maximal 1.8 T output. The focal cortical suppression was created with 1 Hz repetitive TMS (rTMS) at 120% of pharyngeal MT for 10 minutes with 600 single pulses, as previously described.15
Videofluoroscopy
The examination was conducted with 6 swallows of 5-mL boluses and one of a 50-mL bolus of liquid barium (60% wt/vol, EZ-HD; E-Z-EM Ltd, London, UK), and the images were acquired (Fluorospot H SIRESKOP SX Unit; Siemens, Germany) in real time using continuous fluoroscopy at 30 frames/s (Videomed DI-TV system) and recorded by digital video at 25 frames/s (Sony DHR-1000; Sony Ltd) for later offline analysis. Subjects’ oropharyngeal regions were examined in lateral view without magnification as previously described.16
Experimental Protocols
Protocol 1: Investigating the optimal duration of PAS for neurophysiological changes
Twelve healthy participants (10 female; age, 38.1 ± 3.3 years [mean ± SEM]) were asked to attend the laboratory 4 times. At each attendance, volunteers sat comfortably in a reclining chair with the catheter in situ. The cranial vertex was identified17 and marked on the scalp. The cortical sites characterized as the sites evoking the largest pharyngeal responses in each hemisphere were identified with mapping procedures using single TMS pulses delivered over multiple points over the MI (see Supplementary Materials and Methods).
During the recording of 10 motor evoked potentials (MEPs) at MT plus 20% TMS pulse intensity for each hemispheric site (stronger, weaker pharyngeal MI and thenar representation) at baseline and at each of the post-intervention follow-up time points, the participants were advised to withhold from any swallowing, coughing, talking, or moving their hands or arms.
To assess the effects of PAS duration, the participants were studied on different occasions at least 1 week apart and received the following: 10 minutes of PAS (PAS10min), 20 minutes of PAS (PAS20min), 30 minutes of PAS (PAS30min), or sham (PASSham).
After the previously described interventions, the baseline PMEP and TMEP recordings were repeated immediately and then at 30, 60, and 90 minutes after all interventions on every visit. The 4 different interventions were randomized for all subjects’ visits using block randomization (StatsDirect v2.7; StatsDirect Ltd). The lead researcher performed the recordings and the analysis but was blinded to the interventions, delivered by another researcher. All individuals’ data were kept unidentifiable.
Protocol 2: Investigating the behavioral effects of PAS to the strong and weak pharyngeal projection
Seven healthy participants from protocol 1 (5 female; age, 36.7 ± 2.9 years [mean ± SEM]) attended the laboratory on 4 additional occasions. The intensity of PAS used was based on results from protocol 1. The procedures for the recording PMEPs and TMEPs, randomization, and blinding were the same as in protocol 1. Baseline swallowing reaction times (SRTs) were then measured as described in Experimental Procedures.
The participants were randomized to 4 different states investigating the effects of PAS on the stronger pharyngeal projection with neurophysiological measurements (PAS10ST + TMS), the stronger pharyngeal projection with SRT (PAS10ST + SRT), the weaker pharyngeal representation with neurophysiological measurements (PAS10W + TMS), and the weaker pharyngeal representation with SRT (PAS10W + SRT).
The neurophysiological measurements were repeated with single TMS pulses bilaterally immediately and 30 and 60 minutes post-intervention, similarly to protocol 1, whereas the SRT measurements were assessed immediately and 5, 10, 15, 30, and 60 minutes postintervention.
Protocol 3: Effects of PAS after a unilateral virtual lesion in the pharyngeal MI
Ten healthy participants (9 female, 5 naive participants; age, 38.6 ± 2.3 years [mean ± SEM]) were recruited and randomized by an independent researcher across 6 different study arms investigating the effects of active or sham PAS delivered to either the lesioned or the unlesioned hemisphere after a virtual lesion using 1 Hz rTMS of the stronger pharyngeal projection. After defining the hotspots for PMEPs, TMEPs, and sensory pharyngeal thresholds, neurophysiological and behavioral baseline recordings were obtained as in previous protocols. Then a virtual lesion was created to the stronger pharyngeal projection, and this was followed by PAS to the lesioned hemisphere (neurophysiological measurements [PAS10ST + TMS] and SRT measurements [PAS10ST + SRT]), PAS to the contralesional hemisphere (neurophysiological measurements [PAS10W + TMS] and SRT measurements [PAS10W + SRT]), and sham PAS to the lesioned hemisphere (neurophysiological measurements [PAS10ST + TMS] and SRT measurements [PAS10ST + SRT]).
Similar to the previous protocols, for all study arms (performed on separate days, at least 4 days apart), both cortical excitability measurements with single TMS pulses and SRT measurements were recorded at baseline and then immediately and 5, 10, 15, 30, and 60 minutes after PAS applications.
Protocol 4: PAS in patients with chronic dysphagic stroke: a proof-of-principle study
Six patients (5 male; age, 74.8 ± 2.2 years [mean ± SEM]; number of weeks post-stroke, 38.8 ± 24.4 [mean ± SEM]) with a clinical diagnosis of stroke (cerebral infarcts) and confirmed dysphagia persistent more than 6 weeks post-stroke were recruited from hospitals and clinics across northwestern England. Patients were asked to attend the laboratory for 2 visits. Videofluoroscopy was performed to verify dysphagia and to acquire the baseline measurements. Subjects were asked to hold the 5-mL bolus in their mouth until the command to swallow was given. The procedure was stopped if individuals aspirated greater than 50% of the bolus on 3 consecutive swallows. Although the localization of the lesion was taken into consideration (for those who had neuroimaging examinations in the acute stage), additional TMS mapping was performed following videofluoroscopy. After defining the hotspots for PMEPs and sensory pharyngeal thresholds, baseline PMEPs were recorded at MT plus 20% intensity of the stimulator output using 10 single TMS pulses at each hemispheric site for both pharyngeal projections on each study day. Afterwards, an independent researcher delivered the real or sham PAS, while the researcher collecting and analyzing the data remained blinded. The neurophysiological measurements were repeated immediately and 30 minutes post-intervention, whereas the follow-up videofluoroscopy was performed immediately after the 30-minute PMEP recordings on both the real and sham treatment days.
Data Analysis
Neurophysiological measurements
The peak-to-peak amplitude of MEPs evoked by TMS was used as a measure of cortical excitability. The individual MEPs were reviewed with Signal Software (CED, Cambridge, UK), and an average trace was created at each time point. Then, the latencies and amplitudes of averaged MEPs were determined. Baseline MEP data for all interventions were compared using nonparametric tests (Wilcoxon signed rank test). Data were normalized to baseline and are shown as percentage change from baseline to minimize the interindividual variability. Interindividual factors such as age and sex were therefore equalized. Because the data set for the stroke-affected patients did not show normal distribution, logarithmic transformation of the raw data was used to stabilize the variance of the sample.18
Based on previous studies,13 changes in excitability over time between the different interventional groups were compared with responses after sham for each hemispheric hotspot for each time point except baseline using generalized linear model repeatedmeasures analysis of variance (RmANOVA) (SPSS 14.0, IBM Press).
Behavioral measurements
The mean (raw) values of normal and fast swallowing latencies times were analyzed for each time point before and after interventions for each subject. For challenged swallows, the percentage change of the correctly timed swallows was calculated. Correctly timed swallows were termed the successful swallows within a 150-millisecond target time-window, indicated visually on a desktop. The baseline behavioral data were compared with Wilcoxon’s test. The results of latencies times and challenged swallows were then analyzed separately for each intervention using RmANOVA. Nonsphericity was corrected using Greenhouse-Geisser estimation (adjustment of the numbers of degrees of freedom) when necessary.
For both neurophysiological and behavioral measurements, when a significant interaction was present, separate analyses of variance with time as a within-subject factor were performed to characterize time-dependent changes in performance. Post hoc paired-sample t tests were then performed to explore the strength of main effects and the patterns of interaction between experimental factors. A P value of ≤.05 was used to indicate statistical significance.
Protocol 4
Analysis of the videos took place offline by a speech and language therapist blinded to the randomization, and each swallow was analyzed frame-by-frame for bolus transport timings (see Supplementary Materials and Methods). The safety of all swallows was assessed and scored using a previously developed and validated 8-point Penetration-Aspiration Scale, which describes the severity of airway compromise.19 Because healthy volunteers with normal swallowing are known to score 1 to 2 on the scale,20 the subject was considered to have abnormal laryngeal protection if the swallow was scored >3 on one or more swallows.
All data are presented as group mean ± SEM unless stated otherwise.
Results
Protocol 1: Optimal Duration of PAS
PMEPs were recorded in all subjects without difficulty. Larger pharyngeal responses were found from the right hemisphere in 4 participants, whereas the remaining 8 subjects had larger responses from the left hemisphere. The optimal site for stimulation was between 2.4 and 4.3 cm anterior to the vertex and 3.0 and 4.2 cm lateral to the midline. The mean value of pharyngeal MT for the stronger pharyngeal projection for swallowing hemisphere was 66.6% ± 2.3% of TMS output. PES was delivered at 19.6 ± 2.1 mA.
TMS response amplitudes
Figure 1 shows the group mean percentage change of the PMEP amplitude from baseline following the different durations of PAS for and after sham stimulation (PMEP traces from one participant before and after all PAS durations appear in Supplementary Materials and Methods). Baseline cortical excitability across the different study days remained stable for both the pharyngeal and thenar projections. Moreover, TMEP response amplitudes and latencies from each hotspot across the different PAS durations were unaffected (see Supplementary Materials and Methods).
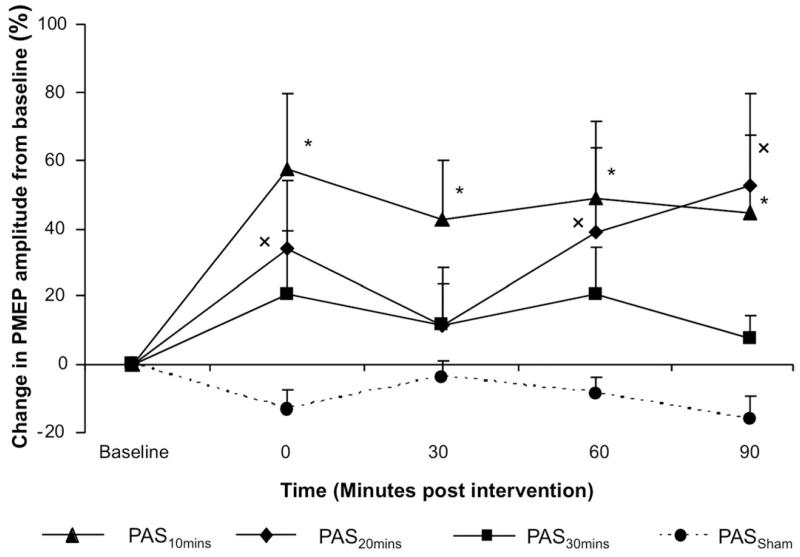
Figure 1.
Group mean percentage change in PMEP amplitude after PAS for 3 different durations and sham (protocol 1). PMEPs are plotted as mean data across all TMS intensities ± SEM. Changes in amplitude are seen after PAS for 10 (▲), 20 ( ◆), and 30 (■) minutes and sham stimulation (●). However, only the increases in electromyographic amplitude after PAS10min and PAS20min were significantly greater than following PAS30min (*,× P < .05).
Changes in PMEP-strong pharyngeal projection
A 2-way RmANOVA on the percentage change with the factors of intervention (PAS10min, PAS20min, PAS30min, PASSham) and time revealed significant time × intervention interaction (F1,11 = 10.41; P = .008) and a significant effect of intervention for PAS10min against PASSham (F1,11 = 11.22; P = .006). A significant effect of intervention for PAS20min compared with PASSham (F1,11 = 4.92; P = .048) was also observed, together with a significant time × intervention interaction (F1,11 = 5.57; P = .038). There was only a trend to significant effect of intervention for excitability changes after PAS30min (F1,11 = 4.19; P = .065) (Figure 1).
Compared with PASSham, both PAS10min and PAS20min increased cortical excitability (maximum of 58% and 53%, respectively). The PMEP amplitudes increased significantly immediately (P = .024; 95% confidence interval, −106.04 to −9.14) and at 30 minutes (P = .03; 95% confidence interval, −80.27 to −4.97) after PAS10min compared with baseline.
Changes in PMEP-weak pharyngeal projection
Contrary to the stronger projection, the nonstimulated, weaker pharyngeal projection showed no change in cortical excitability compared with PASSham (2-way RmANOVA; see Supplementary Materials and Methods).
Protocol 2: Behavioral Effects of PAS to the Strong and Weak Pharyngeal Projections
All participants tolerated the procedures well. Following the optimal duration data from protocol 1, PAS10min was used as the experimental intervention thereafter.
Changes in PMEP-strong pharyngeal projection
A 2-way RmANOVA on the percentage change from baseline values with the factors of intervention (PAS10ST, PAS10W) and time revealed a significant effect of intervention for PAS10ST against PAS10W (F1,6 = 8.329; P = .028) but no significant time × intervention interaction. This difference reflected an increase in excitability in the stronger projection to ipsilateral PAS (PAS10ST) with a maximum increase of 59% ± 28.3%. Following PAS10W, excitability of stronger pharyngeal projection actually decreased by −18% ± 15.5% 30 minutes after (see Supplementary Materials and Methods).
Changes in PMEP-weak pharyngeal projection
The cortical excitability of the weaker pharyngeal projection did not change significantly after the application of the 2 interventions (2-way RmANOVA). Similarly, TMEPs remained unchanged.
Changes in “normal” and “fast” and challenged swallows after PAS10ST and PAS10W
Three separate 2-way RmANOVAs were performed for each of the 3 behavioral tasks (normal, fast, and challenged swallows) with the factors of time and intervention (PAS10ST, PAS10W). There was no effect of intervention on normal or fast swallowing latencies times (F1,6 = .162; P = .701 and F1,6 = 0; P = .994, respectively). However, there was a significant effect of intervention on the challenged swallowing task (F1,6 = 7.615; P = .033) between PAS10ST and PAS10W but no time × intervention interaction. Percentage change of the correct challenged swallows reached a maximum of 55.3% ± 24.4% after PAS10ST, whereas the maximum value after PAS10W was −2.3% ± 15.3% (see Supplementary Materials and Methods).
Protocol 3: Effects of PAS After Unilateral Virtual Lesion
Complete data sets (neurophysiological and behavioral measurements) were analyzed in 8 subjects due to subject dropout from follow-up. The baseline excitability in both hemispheres before the virtual lesion was similar across all 6 arms.
As expected, the excitability of the lesioned hemisphere after sham PAS decreased by −35.5% ± 8.5% at 5 minutes (P = .028, z =−2.197, Wilcoxon), whereas the excitability of the weaker pharyngeal projection (contralesional hemisphere) decreased by −25.5% ± 7.1% at 5 minutes (P = .017, z =−2.395, Wilcoxon), in keeping with the published data for the effects of the virtual lesion on cortical excitability.15
Changes in PMEP-strong pharyngeal projection
Two-way RmANOVA with the factors of intervention (PAS10ST, PAS10W, PASSham) and time performed for the stronger pharyngeal projection (lesioned hemisphere) showed that for PAS10ST there was a clear effect of intervention (F1,9 = 17.561; P = .002) and a significant time × intervention interaction (F1,9 = 9.875; P = .012). Hence, PAS10ST over the lesioned hemisphere significantly increased cortical excitability ipsilaterally (maximum value of 16.8% ± 1.1% at 15 minutes postintervention (Figure 2) (see Supplementary Materials and Methods).
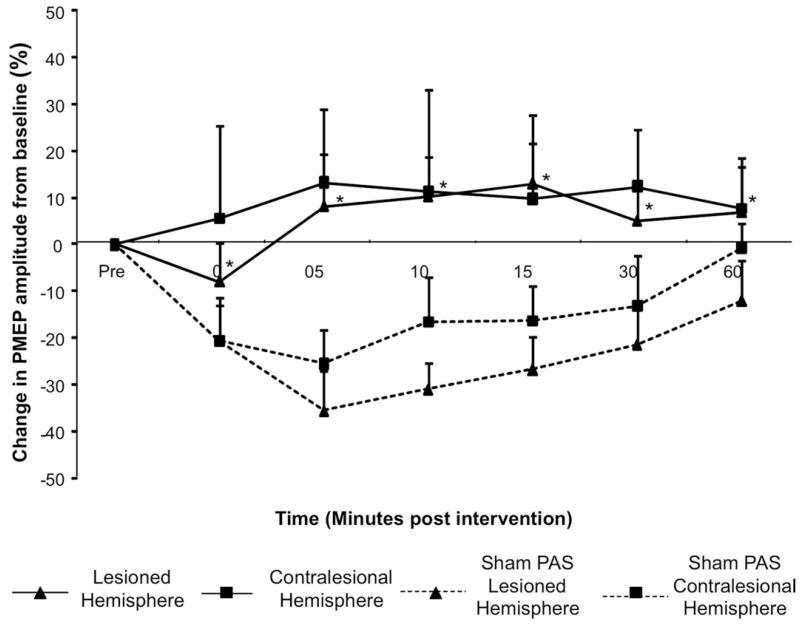
Figure 2.
Group mean percentage of change in PMEP amplitude after PAS10min over the lesioned hemisphere and after PASSham (protocol 4). A significant increase in amplitude is observed ipsilaterally after PAS10min applied to the virtually focal suppressed stronger pharyngeal representation (*P < .005) (▲). However, there was no significant increase in cortical excitability of the contralesional hemisphere (■). Hemispheric responses (lesioned and contralesional) were compared with the responses of each hemisphere after PASSham (dashed lines).
Changes in PMEP-weak pharyngeal projection
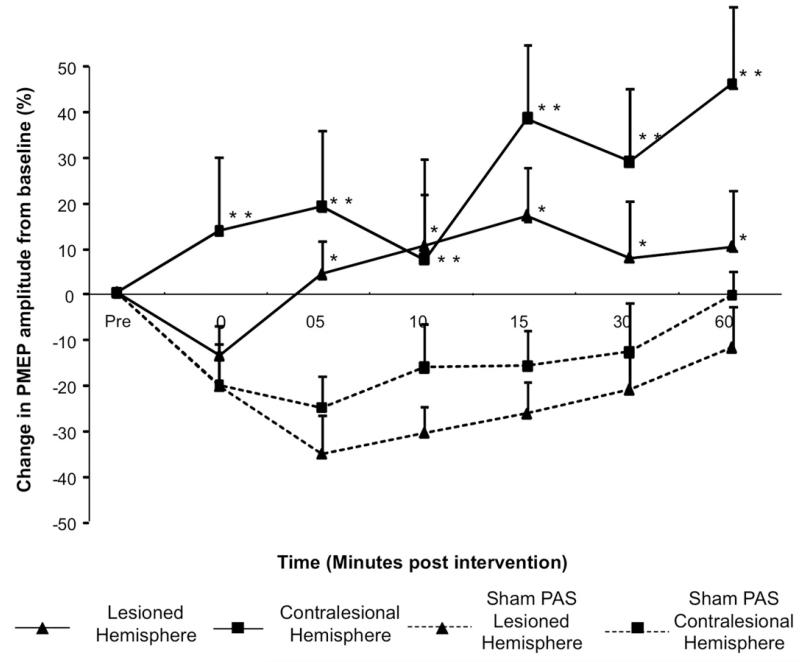
By comparison, following contralesional PAS, the excitability of the lesioned hemisphere increased significantly (maximum 16.8% ± 10.5% at 15 minutes; F1,9 = 21.347; P = .001). There was also an increase in PMEPs in the contralesional site (maximum, 45.9% ± 17%) at 60 minutes and 38.3% ± 16.2% at 15 minutes (F1,9 = 9.648; P = .013) (Figure 3). Hence, PAS10ST over the unlesioned hemisphere increased excitability bilaterally in both hemispheres.
Figure 3.
Group mean percentage of change in PMEP amplitude after PAS10min over the contralesional hemisphere and after PASSham (protocol 4). A significant increase in amplitude after PAS10min to the contralesional hemisphere is observed bilaterally (*,**P < .05) (▲, lesioned hemisphere; ■, contralesional hemisphere). Hemispheric responses were compared with the responses of each hemisphere after PASSham (dashed lines).
Changes in normal, fast, and challenged swallows
Separate 2-way RmANOVA for normal and fast swallows with the factors intervention (PAS10ST, PAS10W, PASSham) and time showed no statistical significant effect or interaction across all follow-up time points as compared with the responses after PASSham. However, 2-way RmANOVA showed a significant effect of intervention in the percentage change of the correct challenged swallows after PAS over the contralesional hemisphere (weak hemispheric projection) (F1,7 = 21.020; P = .003) and a significant time × intervention interaction (F1,7 = 14.615; P = .007). No significant change in successful challenged swallowing tasks after PAS10min to the lesioned hemisphere (F1,7 = 3.298; P = .112) was observed (see Supplementary Materials and Methods).
Protocol 4: PAS in Patients With Chronic Dysphagic Stroke: A Proof-of-Principle Study
Study demographics for the 6 patients with stroke are listed in Table 1. The mean stroke severity (±SD) was 7.3 ± 1.3 on the National Institutes of Health Stroke Scale and total videofluoroscopy screening time was kept less than 80 seconds in all cases, therefore giving a radiation dose ≤0.3 mSv, with no adverse events recorded. However, one patient did aspirate at least half the bolus on 3 consecutive boluses and examination was terminated. Therefore, the remaining noncompleted swallows in that patient were scored at the maximum value on the scale to reflect such severe impairment. All data were analyzed on an intention-to-treat basis.
Table 1.
Demographics of the Stroke Population Studied (Protocol 5)
| Clinical research form | Age (y) | Sex | Diet | Weeks poststroke | Previous stroke | National Institute of Health Stroke Scale score | Side of symptoms |
|---|---|---|---|---|---|---|---|
| A | 83 | Male | Modified | 9 | 0 | 6 | Left |
| B | 67 | Male | PEG | 13 | 0 | 6 | Left |
| C | 73 | Male | PEG | 10 | 0 | 4 | Left |
| D | 77 | Male | PEG/modified | 28 | 1 | 6 | Right |
| E | 72 | Male | PEG/modified | 160 | 1 | 7 | Left |
| F | 77 | Female | PEG/modified | 11 | 0 | 12 | Left |
| Mean ± SD | 74 ± 2.2 | 38.5 ± 24.4 | 7.25 ± 1.3 |
PEG, percutaneous endoscopic gastrostomy.
Change in penetration aspiration scores and bolus transport timings
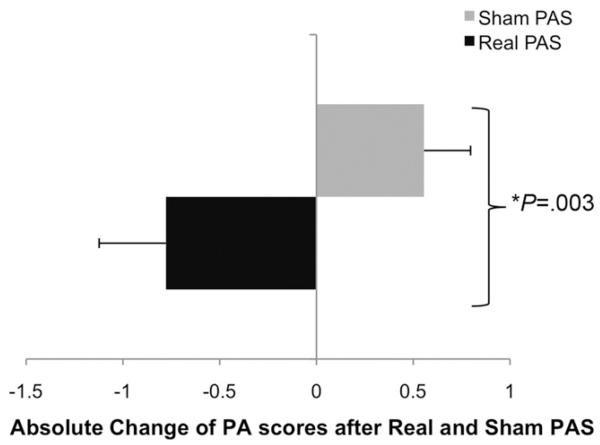
Penetration aspiration scores of discrete swallows before and after real (vs sham) PAS were improved (reduced) (P = .003, z =−2.928, Wilcoxon) and are plotted in Figure 4. The effect size was r =−0.34, indicating a medium effect.21
Figure 4.
Group absolute change of penetration aspiration scores after real and sham PAS of the 6 patients with dysphagic stroke. There was a significant reduction in penetration aspiration scores after real stimulation (P = .003, Wilcoxon).
Some variability was noted in the bolus transport timings before and after real and sham PAS. Nonparametric Wilcoxon test showed shortened pharyngeal response time after real PAS compared with sham PAS (0.2 ± 0.23 vs 0.03 ± 0.18 seconds, P = .020, z =−2.303, respectively). Pharyngeal transit time was also decreased after real PAS compared with sham PAS (0.53 ± 0.28 vs 0.05 ± 0.1 seconds, P = .014, z =−2.433, respectively) (Table 2).
Table 2.
Group Mean Changes in Bolus Transport Timings for 5-mL Boli After Real and Sham PAS
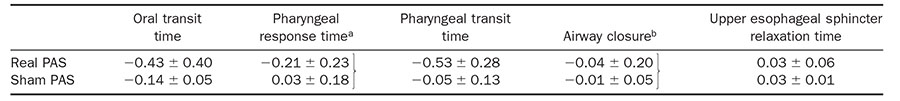
NOTE. All values are expressed in seconds. Significant changes were noted for pharyngeal response time (aP = .02) and pharyngeal transit time (bP = .014) after the single application of PAS protocol to the unaffected hemisphere in the group of patients with severe dysphagia.
Changes in PMEPs: PAS applied to the unaffected pharyngeal projection
PMEPs were recorded in all patients with no adverse effects. Cortical hotspots for stimulation on the right hemisphere were 4.2 ± 1.1 cm anteriorly and 2.9 ± 1.7 cm (mean ± SD) laterally. The mean distance from the vertex for the left hemispheric hot spots was 4.1 ± 1.3 cm anteriorly and 3.1 ± 1.6 cm (mean ± SD) laterally. The motor threshold at the unaffected site was 66.6% ± 10.3% of the stimulator output and at the affected site was 70.5% ± 8.9% (mean ± SD). For all the patients across both days, the intensity for PES was 26.7 ± 1.7 mA (mean ± SD) (see Supplementary Materials and Methods).
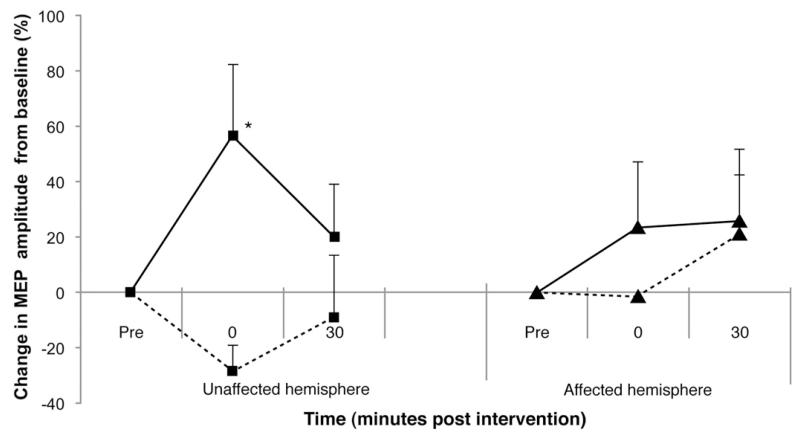
Figure 5 shows the change in cortical excitability in patients with dysphagia from stroke immediately and 30 minutes after real and sham PAS. The excitability of the unaffected hemisphere increased by 56.5% ± 25.7%, whereas for the affected by 25.8% ± 8.25%.
Figure 5.
Group mean percentage of change in PMEP amplitude after real and sham PAS of the 6 patients with dysphagic stroke. Both the unaffected and affected hemisphere excitability was increased after real PAS (solid line) to the unaffected hemisphere compared with sham PAS (dashed line) (*P = .001).
RmANOVA showed significant interaction between hemisphere × time points (F1,59 = 11.767; P = .001) and a significant effect of hemisphere (P = .006; F1,59 = 11.767; r = 0.34) and intervention (F1,59 = 12.54; P = .001; r = 0.41).
A significant difference between real and sham PAS was revealed for the unaffected hemisphere at 30 minutes (t59 =−3.515; P = .001; 95% confidence interval, 0.21-0.05) and a significant difference in excitability of the affected hemisphere 30 minutes after real PAS compared with baseline (t59 =−5.18; P < .001; 95% confidence interval, 0.14-0.06).
There were no significant differences in the response latencies of PMEPs for both sides after real and sham PAS.
Discussion
PAS is a novel neurostimulation technique that promotes plasticity by combining peripheral stimulation in the targeted muscle and cortical stimulation (TMS) over the targeted muscle cortical representational area. Based on the results from previous studies,13 our work has further investigated the parameters for using PAS before its application in dysphagic populations. The cortical stimulation applied for PAS was delivered to the pharyngeal cortical representation, with the intention to induce changes to the pharyngeal corticobulbar projection, because this has been observed to be a highly relevant pathway for swallowing and dysphagia.15,22 Although in animal studies the swallowing motor cortex has been stimulated electrophysiologically with intracortical micro-stimulation,6 there has not been sufficient evidence in the literature for the identification of a topographically distinct swallowing motor cortical map in humans.
Changes in Cortical Excitability Depend on the Duration of the Application
The results from protocol 1 indicate that the duration of the application of PAS is an important parameter for the induction of excitatory plastic changes in healthy participants. We observed that changes in cortical excitability were significantly increased after only 10 minutes of PAS to the stronger pharyngeal projection. The results of this study corroborate the earlier findings by Singh et al,13 providing additional evidence that PAS can produce facilitatory effects in pharyngeal MI. Contrary to expectations, our current study found fewer substantial changes in cortical excitability after the application of PAS30min, which was used in the previously reported study.13 This variability is not surprising, because some variability has been observed in the previous study of PAS30min,13 as well as in the previous study examining the pharyngeal cortical evoked potentials latencies by Gow et al.23 Moreover, these response differences are in accordance with observations in the literature on hand musculature,24 which showed that the intraindividual reproducibility for facilitatory PAS effects may lack stability.
Our finding that PAS10min can induce larger effects in the pharyngeal MI than the longer PAS durations has precedent. Similar observations were found in studies on healthy participants examining the optimal parameters for the frequency, intensity, and duration for PES.25 The results observed in both our PAS duration study and the study for the optimal parameters for PES favoring a shorter period of stimuli may actually be a result of a “saturation” effect on the cortical capacity for synaptic efficacy and long-term potentiation26 and may be indicative of the importance of the peripheral component (pharyngeal stimulation) of PAS as an input to influence the sensory pathways to cortical representation of swallowing musculature.
PAS Can Induce Changes in Complex Swallowing Tasks
In animal studies, swallowing can be evoked by stimulation of both hemispheres,7 suggesting a possible equi-hemispheric contribution to cortical swallowing control. In our results, we observed that the application of PAS10min to the stronger pharyngeal projection resulted in a greater number of correctly timed swallows compared with PAS10min to the weaker projection. Indeed, PAS10min to the weaker pharyngeal projection induced virtually no MEP changes in both the ipsilateral and contralateral hemispheres. This finding adds to the increasing literature showing the functional significance of the stronger hemisphere for swallowing in healthy subjects.17,22,27 The bilateral nature of cortical control of swallowing in humans does not support the existence of strict competitive inhibitory processes between the hemispheres. The results from the current study support the notion that in the healthy (unlesioned) state the hemisphere with the stronger projection to pharyngeal musculature is more functionally important in regulating swallowing; when enhanced by neurostimulation, this translates into subtle improvements in swallowing performance, at least in swallow accuracy when complex tasks are performed. This result provides supportive evidence for the therapeutic translation of PAS to potential treatments that may alter swallowing performance.
Effects of PAS on a Virtual Lesion Model of Brain Suppression
PAS applied contralaterally to a virtual lesion induced with 1 Hz rTMS to the stronger pharyngeal projection, increased excitability bilaterally, and produced changes in behavioral responses to a swallowing reaction task. By contrast, PAS applied to the ipsilateral (lesioned) hemisphere resulted in only modest unilateral increases in excitation and no effects on swallowing performance. This is in contrast to the application of PAS in the uninhibited state (without lesioning), where its application to the stronger projection was able to promote swallowing changes. Taken together, these observations lend further support to the concept that lateralization of swallowing function is relevant to the regulation of behavioral response. Enhancement of the hemispheric system with the stimulation of the stronger pharyngeal projection is able to facilitate swallowing behavior; when this system is damaged or lesioned, greater beneficial effects can be achieved by targeting the previously weaker pathways, which, by virtue of brain injury, become more susceptible to plasticity-inducing inputs in driving recovery and behavioral compensation.
PAS Applied to Patients With Chronic Dysphagic Stroke
The effectiveness of the application of facilitatory PAS to the contralesional cortex in brain injury was further explored in the pilot study of patients with severe dysphagic stroke (mean time poststroke, 38.8 ± 24.4 weeks), 5 of which were tube fed. Although the number of patients with stroke was small, it is of interest that the application of PAS on the contralesional pharyngeal MI significantly increased the cortical excitability of the un-affected hemisphere, which was accompanied by a decrease in penetration aspiration scores and changes in bolus transport timings, with corresponding decreases in the pharyngeal response times and transit times of bolus flow. There was also a small but significant increase in the affected hemisphere when compared with the hemispheric baseline pharyngeal representation excitability, although its relevance is unclear. The level of this effect is unclear because the effects of the intervention on subcortical structures cannot be ruled out (because the real stimulation might have increased output from MI to remote cortical and/or subcortical areas). Interestingly, the use of neurostimulation in patients with chronic dysphagic stroke has been reported before; in one recent study,28 1 Hz rTMS (inhibitory stimulation paradigm) was applied, in a specific regimen, over the intact hemisphere in stroke-affected patients with very mild chronic dysphagia who were a mean of 56 weeks post-stroke. Even though the study was not controlled and the patients were not severely dysphagic, immediate behavioral effects were observed. Thus, the fact that in the current protocol a single application of PAS showed immediate behavioral and neurophysiological effects in patients with chronic dysphagic stroke is of interest and provides additional information to support the role of carefully designed interventions even after long-term swallowing disability.5
Supplementary Material
Acknowledgments
The authors thank all study participants as well as Lisa Renault, Jackie Johnson, and Daniela Burgess (senior radiographers), who helped conduct the videofluoroscopy examinations.
Funding
Supported by the Wellcome Trust (WT081741MA). E.M. was recipient of the Greek State Foundation Scholarship. The study was sponsored by the University of Manchester, which did not have a role in the study design or in the collection, analysis, or interpretation of data.
Abbreviations used in this paper
- MEP
motor evoked potential
- MI
motor cortex
- MT
motor threshold
- PAS
paired associative stimulation
- PES
pharyngeal electrical stimulation
- PMEP
pharyngeal motor evoked potential
- RmANOVA
repeated-measures analysis of variance
- rTMS
repetitive transcranial magnetic stimulation
- SRT
swallowing reaction time
- TMEP
thenar motor evoked potential
- TMS
transcranial magnetic stimulation
Footnotes
Note: To access the supplementary material accompanying this article, visit the online version of Gastroenterology at www.gastrojournal.org, and at doi: 10.1053/j.gastro.2011.09.040.
Conflicts of interest
The authors disclose no conflicts.
References
- 1.Kumar S, Selim MH, Caplan LR. Medical complications after stroke. Lancet Neurol. 2010;9:105–118. doi: 10.1016/S1474-4422(09)70266-2. [DOI] [PubMed] [Google Scholar]
- 2.Mann G, Hankey GJ, Cameron D. Swallowing function after stroke: prognosis and prognostic factors at 6 months. Stroke. 1999;30:744–748. doi: 10.1161/01.str.30.4.744. [DOI] [PubMed] [Google Scholar]
- 3.Terre R, Mearin F. Resolution of tracheal aspiration after the acute phase of stroke-related oropharyngeal dysphagia. Am J Gastroenterol. 2009;104:923–932. doi: 10.1038/ajg.2008.160. [DOI] [PubMed] [Google Scholar]
- 4.Martino R, Foley N, Bhogal S, et al. Dysphagia after stroke: incidence, diagnosis, and pulmonary complications. Stroke. 2005;36:2756–2763. doi: 10.1161/01.STR.0000190056.76543.eb. [DOI] [PubMed] [Google Scholar]
- 5.Murphy TH, Corbett D. Plasticity during stroke recovery: from synapse to behaviour. Nat Rev Neurosci. 2009;10:861–872. doi: 10.1038/nrn2735. [DOI] [PubMed] [Google Scholar]
- 6.Martin RE, Kemppainen P, Masuda Y, et al. Features of cortically evoked swallowing in the awake primate (Macaca fascicularis) J Neurophysiol. 1999;82:1529–1541. doi: 10.1152/jn.1999.82.3.1529. [DOI] [PubMed] [Google Scholar]
- 7.Sumi T. Some properties of cortically-evoked swallowing and chewing in rabbits. Brain Res. 1969;15:107–120. doi: 10.1016/0006-8993(69)90313-8. [DOI] [PubMed] [Google Scholar]
- 8.Jean A. Brain stem control of swallowing: neuronal network and cellular mechanisms. Physiol Rev. 2001;81:929–969. doi: 10.1152/physrev.2001.81.2.929. [DOI] [PubMed] [Google Scholar]
- 9.Hamdy S, Aziz Q, Rothwell JC, et al. Recovery of swallowing after dysphagic stroke relates to functional reorganization in the intact motor cortex. Gastroenterology. 1998;115:1104–1112. doi: 10.1016/s0016-5085(98)70081-2. [DOI] [PubMed] [Google Scholar]
- 10.Steele CM, Miller AJ. Sensory input pathways and mechanisms in swallowing: a review. Dysphagia. 2010;25:323–333. doi: 10.1007/s00455-010-9301-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jayasekeran V, Singh S, Tyrrell P, et al. Adjunctive functional pharyngeal electrical stimulation reverses swallowing disability after brain lesions. Gastroenterology. 2010;138:1737–1746. doi: 10.1053/j.gastro.2010.01.052. [DOI] [PubMed] [Google Scholar]
- 12.Michou E, Hamdy S. Cortical input in control of swallowing. Curr Opin Otolaryngol Head Neck Surg. 2009;17:166–171. doi: 10.1097/MOO.0b013e32832b255e. [DOI] [PubMed] [Google Scholar]
- 13.Singh S, Mistry S, Jefferson S, et al. A magnetic resonance spectroscopy study of brain glutamate in a model of plasticity in human pharyngeal motor cortex. Gastroenterology. 2009;136:417–424. doi: 10.1053/j.gastro.2008.10.087. [DOI] [PubMed] [Google Scholar]
- 14.Hamdy S, Aziz Q, Rothwell JC, et al. The cortical topography of human swallowing musculature in health and disease. Nat Med. 1996;2:1217–1224. doi: 10.1038/nm1196-1217. [DOI] [PubMed] [Google Scholar]
- 15.Mistry S, Verin E, Singh S, et al. Unilateral suppression of pharyngeal motor cortex to repetitive transcranial magnetic stimulation reveals functional asymmetry in the hemispheric projections to human swallowing. J Physiol. 2007;585:525–538. doi: 10.1113/jphysiol.2007.144592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Logemann JA. Manual for the videofluorographic study of swallowing. 2nd ed Pro-Ed; Austin, TX: 1993. [Google Scholar]
- 17.Jasper HH. The 10-20 electrode system of the International Federation. Electroencephalogr Clin Neurophysiol. 1958:371–375. [PubMed] [Google Scholar]
- 18.Bland MJ, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–10. [PubMed] [Google Scholar]
- 19.Rosenbek JC, Robbins JA, Roecker EB, et al. A penetration-aspiration scale. Dysphagia. 1996;11:93–98. doi: 10.1007/BF00417897. [DOI] [PubMed] [Google Scholar]
- 20.Robbins J, Coyle J, Rosenbek J, et al. Differentiation of normal and abnormal airway protection during swallowing using the penetration-aspiration scale. Dysphagia. 1999;14:228–232. doi: 10.1007/PL00009610. [DOI] [PubMed] [Google Scholar]
- 21.Cohen J. Statistical power analysis for the behavioral sciences. Erlbaum; Hillsdale, NJ: 1988. [Google Scholar]
- 22.Jefferson S, Mistry S, Michou E, et al. Reversal of a virtual lesion in human pharyngeal motor cortex by high frequency contralesional brain stimulation. Gastroenterology. 2009;137:841–849.e1. doi: 10.1053/j.gastro.2009.04.056. [DOI] [PubMed] [Google Scholar]
- 23.Gow D, Hobson AR, Furlong P, et al. Characterising the central mechanisms of sensory modulation in human swallowing motor cortex. Clin Neurophysiol. 2004;115:2382–2390. doi: 10.1016/j.clinph.2004.05.017. [DOI] [PubMed] [Google Scholar]
- 24.Fratello F, Veniero D, Curcio G, et al. Modulation of corticospinal excitability by paired associative stimulation: reproducibility of effects and intraindividual reliability. Clin Neurophysiol. 2006;117:2667–2674. doi: 10.1016/j.clinph.2006.07.315. [DOI] [PubMed] [Google Scholar]
- 25.Fraser C, Power M, Hamdy S, et al. Driving plasticity in human adult motor cortex is associated with improved motor function after brain injury. Neuron. 2002;34:831–840. doi: 10.1016/s0896-6273(02)00705-5. [DOI] [PubMed] [Google Scholar]
- 26.Rioult-Pedotti M-S, Friedman D, Donoghue JP. Learning-induced LTP in neocortex. Science. 2000;290:533–536. doi: 10.1126/science.290.5491.533. [DOI] [PubMed] [Google Scholar]
- 27.Hamdy S, Aziz Q, Rothwell JC, et al. Sensorimotor modulation of human cortical swallowing pathways. J Physiol. 1998;506:857–866. doi: 10.1111/j.1469-7793.1998.857bv.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Verin E, Leroi A. Poststroke dysphagia rehabilitation by repetitive transcranial magnetic stimulation: a noncontrolled pilot study. Dysphagia. 2009;24:204–210. doi: 10.1007/s00455-008-9195-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.