Abstract
The rewarding property of opioids likely contributes to their abuse potential. Therefore, determining the genetic basis of opioid reward could aid in understanding the neurobiological mechanisms of opioid addiction, provided that it is a heritable trait. Here, we characterized the rewarding property of the widely abused prescription opioid oxycodone (OXY) in the conditioned place preference (CPP) assay using LG/J and SM/J parental inbred mouse strains and 17 parent-offspring families of a LG/J × SM/J F47/F48 advanced intercross line (AIL). Following OXY training (5 mg/kg, i.p.), SM/J mice and AIL mice, but not LG/J mice, showed an increase in preference for the OXY-paired side, suggesting a genetic basis for OXY-CPP. SM/J mice showed greater locomotor activity than LG/J mice in response to both saline and OXY. LG/J, SM/J, and AIL mice all exhibited robust OXY-induced locomotor sensitization. Narrow-sense heritability (h2) estimates of the phenotypes using linear regression and maximum likelihood estimation showed good agreement (r = 0.91). OXY-CPP was clearly not a heritable trait whereas drug free- and OXY-induced locomotor activity and sensitization were significantly and sometimes highly heritable (h2 = 0.30–0.84). Interestingly, the number of transitions between the saline- and OXY-paired sides emerged as a reliably heritable trait following OXY training (h2 = 0.46–0.66) and could represent a genetic component of drug seeking behavior. Thus, although OXY-CPP does not appear to be amenable to genome-wide quantitative trait locus (QTL) mapping, this protocol will be useful for mapping other traits potentially relevant to opioid abuse.
Keywords: addiction, locomotion, opiate, Pavlovian, place conditioning, reward
INTRODUCTION
Prescription opioid drug abuse, in particular the non-prescription misuse of Oxycontin® (a sustained release formula of oxycodone; OXY) is a major socioeconomic problem in the United States. There was a nine-fold increase in the amount of OXY prescribed in the U.S. between 1997 and 2007 (Manchikanti, et al 2010), which drastically increased the potential for its diversion to non-medical use. The surge in OXY prescriptions coincided with a six-fold increase in the number of opioid-related emergency room visits, with OXY making the largest contribution in recent years (Manchikanti, et al 2010). The epidemic in non-prescription opioid abuse highlights the timely and warranted need for determining the underlying factors that contribute to it.
Opioid addiction is a heritable complex trait (Goldman, et al 2005; Ho, et al 2010), i.e., a proportion of the phenotypic variance can be explained by genetic factors. The primary molecular site of action for the motivational and analgesic properties of opioids is the Gi/Go coupled mu opioid receptor (Matthes, et al 1996) and genetic variants of OPRM1 have been associated with opioid addiction (Levran, et al 2012). While many candidate gene association studies of opioid addiction have been reported for other genes (Ho, et al 2010), unbiased, comprehensive genome-wide association studies are lacking.
Addiction can be defined by a repertoire of behaviors, each of which could vary in its presence and degree among individuals and across time. The individual phenotypes are presumably mediated by different (although not necessarily independent) sets of alleles, making the identification of genetic and biological factors more difficult. One way to reduce this complexity is to focus on individual phenotypes that are clearly heritable and associated with opioid abuse. An attractive, intuitive phenotype that may play a role in the early stage of the addiction process is the subjective degree to which a person likes a drug (drug liking), which can predict later patterns of opioid abuse (Haertzen, et al 1983) and is frequently used to evaluate the abuse potential of opioids, including different OXY formulations (Setnik, et al 2011; Webster, et al 2012). There is substantial variation among individuals with regard to the motivational properties of opioids. In a recent human study examining the effects of the mu opioid receptor agonist alfentanil, many of the participants reported an initial positive experience followed by a negative one whereas others reported a predominantly positive experience, a neutral experience, or a predominantly negative one (Angst, et al 2012). Whether this variation can in part be explained by genetic variation is not known; however the authors reported that opioid liking was not a heritable trait.
In both rodents and humans, the motivational properties of opioids can be assessed using a Pavlovian conditioning procedure known as the conditioned place preference (CPP) assay (Childs and de Wit 2009; Childs and de Wit 2011; Tzschentke 2007). Here, distinct cues are paired explicitly with an environment where a drug or saline (SAL) is administered and then subjects are given a free choice between environments (rodents) or asked which environment they prefer (humans). A preference for the drug-paired environment is thought to represent the rewarding properties of the drug and is predicted by self-reported measures of drug liking in humans (Childs and de Wit 2009)
We recently examined methamphetamine (MA)-induced CPP (MA-CPP) and other concomitant behaviors in a LG/J × SM/J F45/F46 advanced intercross line (AIL) of mice. The origin and maintenance of this line was recently summarized (Bryant, et al 2012a). AIL mice have a much greater number of historical recombination events than a typically used F2 cross which can yield higher resolution quantitative trait loci (QTL) for complex traits (Cheng, et al 2010; Darvasi and Soller 1995). AIL mice demonstrated a significant MA-CPP but in contrast to some of the locomotor phenotypes, MA-CPP was not a heritable trait (Bryant, et al 2012a).
In the present study, we wished to estimate the heritability of OXY-CPP and the concomitant phenotypes of the CPP assay. We first examined OXY-CPP in the LG/J and SM/J parental inbred strains of the AIL mice and subsequently phenotyped the parents and offspring of 17 families of AIL mice. The addition of seven extra families compared to our previous study (Bryant, et al 2012a) was included to increase our power to detect heritability. Next, we generated a correlation matrix of the behavioral phenotypes and assessed the relationship of OXY-CPP with these behaviors. We estimated the narrow-sense heritability of OXY-CPP and the concomitant phenotypes to assess the suitability of this population for conducting a genome-wide QTL mapping study. Such efforts have been applied successfully toward other phenotypes associated with opioid addiction in mice, including liquid oral opioid consumption (Berrettini, et al 1994; Doyle, et al 2008; Ferraro, et al 2005) and opioid withdrawal (Kest, et al 2004; Kest, et al 2009).
MATERIALS AND METHODS
Mice
All experiments were performed in accordance with the National Institutes of Health Guidelines for the Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee at the University of Chicago. A 12h/12h light/dark cycle (lights on at 0600 h) was used in the mouse colony room. Behavioral testing was conducted between the hours of 1200 to 1700 h. Mice were same-sex housed in standard shoebox cages with corncob bedding in groups of two to five per cage.
30 LG/J mice (15 females, 15 males) and 30 SM/J mice (15 females, 15 males) were purchased from The Jackson Laboratory (Bar Harbor, ME) at 6 weeks of age and tested at 9–11 weeks of age. The LG × SM F47/F48 advanced intercross line (AIL) was originally obtained from Dr. James Cheverud’s laboratory at Washington University in St. Louis at the F33 generation and has since been bred and maintained in Dr. Abraham Palmer’s laboratory at the University of Chicago by breeding 50–70 families per generation whereby the breeding pairs are systematically chosen in a manner that minimizes relatedness. We phenotyped 209 AIL mice (97 females, 112 males) for OXY-CPP. Thirty-four of these individuals (17 females, 17 males) were F47 mice, each of which was from a different F46 breeder pair. These mice were first phenotyped for OXY-CPP and then paired for breeding. Because OXY-CPP was our primary phenotype of interest, we chose breeder pairs with similar OXY-CPP values after each CPP experiment as indicated by the change in time spent on the OXY-paired side between initial and final preference assessment (r = 0.7 between dam and sire values) and by the time spent on the OXY-paired side during final preference assessment (r = 0.4 between dam and sire values). The purpose of phenotypic selection as opposed to random mating was to avoid any unintended clustering of midparent average values that would have narrowed the phenotypic distribution. A total of 17 families (17 dams, 17 sires) were established and 175 offspring (80 females, 95 males) were generated from these families (N = 5–15 per family). Thus, many of these offspring were siblings.
Conditioned place preference
The CPP procedure was recently described (Bryant, et al 2012a). Briefly, we employed a two-chamber design whereby a plastic black divider containing a mouse entryway separated the two sides of the open field (37.5 cm × 37.5 cm; AccuScan Instruments, Columbus, OH). The two sides were distinguished by visual and tactile cues. The entryway was flipped upside down during training to confine the mice to one side. Mice were always administered OXY (5 mg/kg, i.p.) on the left side of the box which was on average, the slightly less preferred side in AIL mice (48%). The dose of OXY was chosen based on previous studies employing this dose (Der-Avakian, et al 2007; Liu, et al 2009) and on pilot studies indicating that this dose was the most effective at inducing CPP. All test sessions and training sessions lasted 30 min.
On the first day (Day 1; D1), mice were assessed for initial preference for the OXY-paired side whereby mice were administered an injection of SAL (10 ml/kg, i.p.), placed on the SAL-paired side facing the open entryway to the OXY-paired side, and allowed free access to both sides. The time spent on the OXY-paired side (s) and the total distance traveled (cm) were recorded using the automated Versamax conditioned place preference and activity programs (AccuScan). Twenty-four h later, mice received two injections of OXY separated by 48 h (D2, D4) and two injections of SAL separated by 48 h (D3, D5) and were confined to the appropriate side for each training session. Mice were then returned to their home cages and left undisturbed on D6-D7. On D8, final preference for the OXY-paired side was assessed by administering a SAL injection (10 ml/kg, i.p.) to the mice, placing them on the SAL-paired side facing the entryway to the OXY-paired side, and providing free access to both sides. OXY-CPP is presented as the time spent on the OXY-paired side on D1 (D1 CPP) versus D8 (D8 CPP) and as the difference in time spent on the OXY-paired side between D1 and D8 (D8-D1 CPP). The main reason we chose to report both CPP measures was to provide as much information as possible in interpreting the findings with the parental strains and the correlation among the variables in AIL mice. Because our main focus was CPP, we wanted to fully dissect the phenotype and to provide heritability estimates for all components of CPP.
Behavioral analysis
After establishing that sex did not interact with strain for each behavior, we combined sexes for analyses using repeated measures ANOVA (day was the repeated measure), unpaired t-tests to determine the source of strain effects, and paired t-test to determine the source of effects caused by day. The significance level was set to 0.05 and was Bonferroni-corrected in reporting significant post-hoc results. Pearson’s correlation coefficients (r) were reported for the correlation matrix of the behavioral phenotypes with the significance level set to 0.05 (Bonferonni-corrected for the 66 pairwise tests (p < 0.0008).
Heritability estimates
We estimated the narrow-sense heritability (h2), or the proportion of phenotypic variance explained by additive genetic variance, using two methods. First, we used midparent-offspring linear regression of the F47 and F48 generations of AIL mice whereby h2 equaled the slope of the regression line of the offspring averages of the 17 families regressed onto the midparent averages. The standard error of the regression coefficient represented the standard error of the h2 estimate (S.E.). Second, we used a mixed model that accounted for genetic relatedness among individuals by taking advantage of the complete AIL pedigree that we have available (F1-F49) to calculate the kinship coefficients, partition the variance into genetic and non-genetic components, and estimate the heritability using the maximum likelihood estimation of the variance components (Abney, et al 2000). The S.E. for maximum likelihood analysis was calculated using the the jackknife (delete-1) resampling method (Shao and Tu 1995).
RESULTS
OXY-CPP in LG/J and SM/J mice
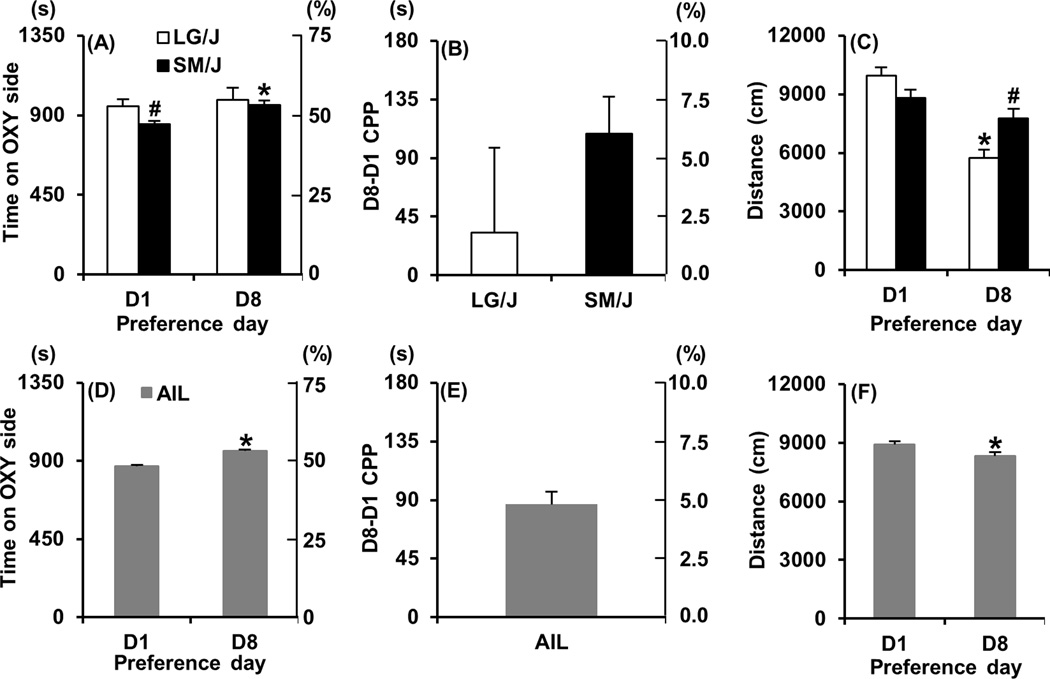
The 8-day OXY-CPP protocol is illustrated in Figure 1 and is essentially a shorter version of our previously published protocol (Bryant, et al 2012a). In examining LG/J and SM/J strain differences in OXY-CPP, repeated measures ANOVA (D1, D8) indicated an effect of day (F1,58 = 3.96; p = 0.05) but no significant effect of strain (F1,58 = 1.76; p = 0.19). SM/J showed an initially lower preference for the OXY-paired side on D1 compared to LG/J (t58 = 2.47; p = 0.017; “#”; Figure 2A) and a significant increase in time spent on the OXY-paired side on D8 (t29 = 3.79; p = 0.0007; “*”; Figure 2A). In contrast, LG/J mice showed no significant change in preference for the OXY-paired side (t29 < 1). In examining OXY-CPP as measured by D8-D1 over the 30 min session (D8-D1 CPP), there was no significant strain difference (t58 = 1.06; p = 0.29; Figure 2B). However, when considering the first 5 min of D8-D1 CPP assessment, SM/J mice clearly showed a significantly greater OXY-CPP than LG/J mice as measured by D8-D1 (t58 = 3.48; p < 0.0001 data not shown).
Figure 1. OXY-CPP protocol.
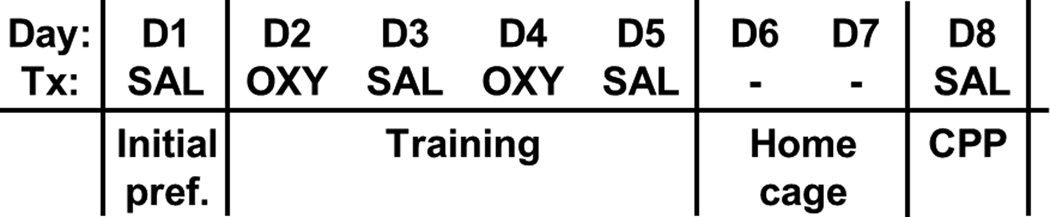
We used an 8-day protocol whereby mice were assessed for initial preference for the OXY-paired side on day 1 (D1, initial preference; Initial pref.), trained over four days with alternating injections of OXY (D2, D4) and SAL (D3, D5) in the respective contexts, and assessed for OXY-CPP on D8. Each day represents a 30 min session. Day = day of the protocol; Tx = treatment for that day; SAL = saline treatment; OXY = oxycodone treatment; - = mice were left undisturbed in their home cage in the vivarium on these days.
Figure 2. OXY-CPP in LG/J, SM/J, and AIL mice.
Panels A, D. Time spent on the OXY-paired side before training (Day 1; D1) and following OXY-CPP training (Day 8; D8) in LG/J (white bars), SM/J (black bars), and AIL mice (gray bars). The y-axes represent both the seconds (s) and % time on the OXY-paired side. Panels B, E: Change in time spent on the OXY-paired side between D1 and D8 (D8-D1 CPP). The y-axes represent both the difference in seconds and the difference in % time on the OXY-paired side. Panels C, F: Total distance traveled on D1 and D8 in LG/J (white bars), SM/J, (black bars) and AIL mice (gray bars). * = significantly different from D1 (Panels A, C, D, F). “#” = significantly different from LG/J (Panels A, C). Data are represented as the mean ± S.E.M.
In examining strain differences in locomotor activity during preference assessment on D1 and D8, repeated measures ANOVA indicated a significant strain × day interaction (F1,58 = 20.54; p < 0.0001). LG/J mice showed a significant decrease in activity from D1 to D8 (t29 = 8.15; p<0.0001; “*”; Figure 2C) which contributed to the strain difference on D8 whereby SM/J mice showed significantly greater locomotor activity than LG/J mice (t58 = 3.04; p = 0.0036; “#”; Figure 2C).
OXY-CPP in AIL mice
Similar to SM/J mice, AIL mice showed a comparable increase in time spent on the OXY-paired side on D8 (t208 = 8.95; p < 0.0001; “*”; Figure 2D, E), indicating OXY-CPP. There was a small but significant decrease in locomotor activity during preference assessment from D1 to D8 (t208 = 3.61; p < 0.0001; “*”; Figure 2F).
Locomotor activity during OXY and SAL training days in LG/J and SM/J mice
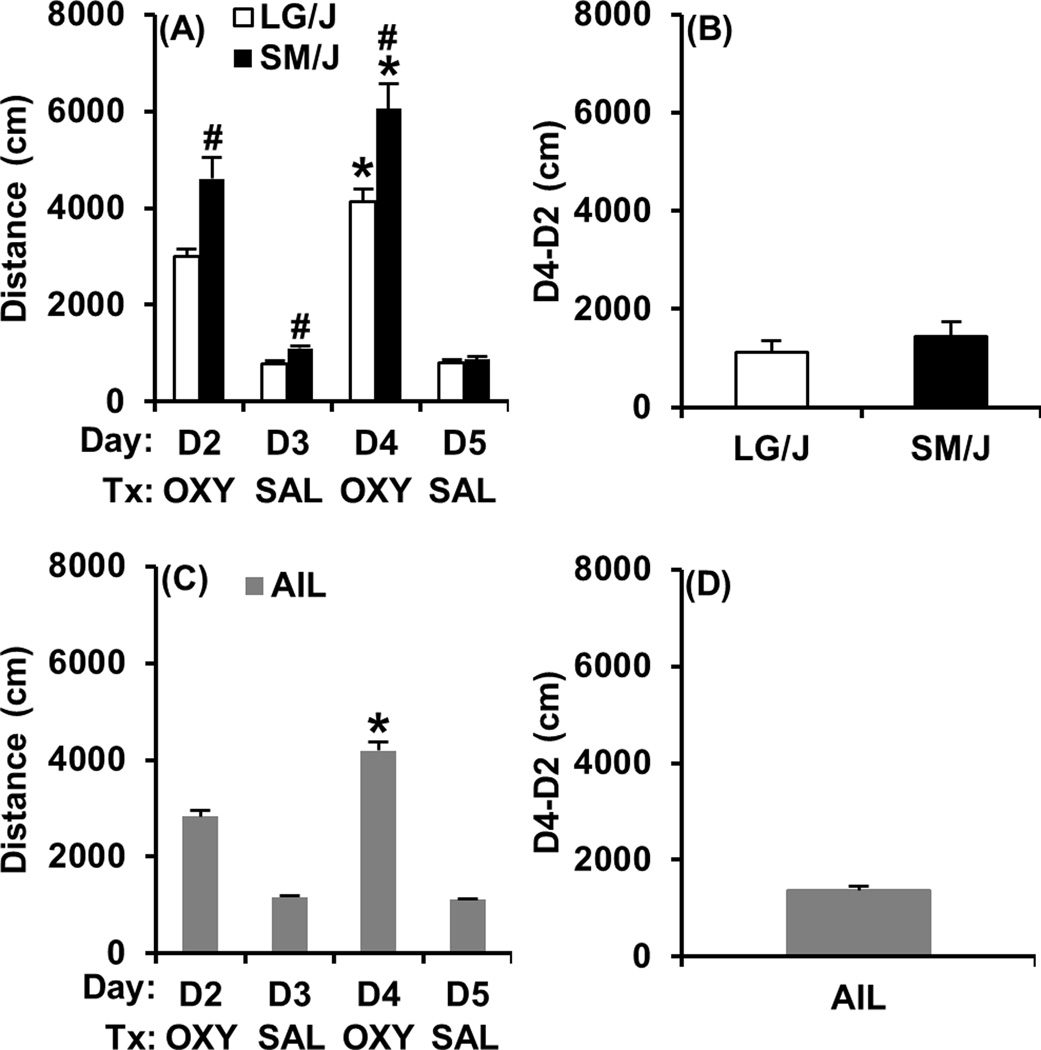
In examining locomotor activity across days in LG/J and SM/J mice, there was a main effect of strain (F1,58 = 13.97; p = 0.0004) day (F3,174 = 185.18; p < 0.0001), and a strain × day interaction (F3,174 = 8.86; p < 0.0001). The strain × day interaction was in part explained by SM/J mice showing significantly greater locomotor activity than LG/J mice on D2, D3, and D4 (t58 = 3.41, 4.18; 3.35; p < 0.0013; “#”; Figure 3A), but not on D5 (t58 < 1). The effect of day was in part explained by both LG/J and SM/J showing a robust locomotor stimulant response to OXY on D2 relative to SAL treatment on D3 (t29 = 13.56, 8.23; p < 0.0001; Figure 3A) and on D4 relative to D5 (t29 = 12.96, 10.71; p < 0.0001; Figure 3A). Furthermore, both LG/J and SM/J mice showed a significantly greater locomotor response to the second OXY injection on D4 relative to D2 (t29 = 5.03, 4.78; p < 0.0001; “*”; Figure 3A), indicating locomotor sensitization. In examining strain differences in the degree of locomotor sensitization as measured via the D4-D2 subtraction measure, there was no significant difference (t58 < 1; Figure 3B).
Figure 3. Locomotor activity during OXY and SAL training days in LG/J, SM/J, and AIL mice.
Panels A, C: Locomotor activity in response to OXY (D2, D4) and SAL (D3, D5) in LG/J, SM/J, and AIL mice. Panels B, D: OXY-induced locomotor sensitization in LG/J, SM/J, and AIL mice as indicated by the difference in locomotor activity between D2 and D4 (D4-D2). “*” = significant increase from D2 to D4 (Panels A, C). “#” = significantly different from LG/J (Panel A). Data are represented as the mean ± S.E.M.
Locomotor activity during OXY and SAL training days in AIL mice
In examining locomotor activity across days, AIL mice showed a robust locomotor stimulant response to OXY on D2 relative to SAL treatment on D3 (t208 = 12.91; p < 0.0001; Figure 3C) and on D4 relative to D5 (t208 =18.82; p < 0.0001; Figure 3C). Furthermore, AIL mice showed a significantly greater locomotor response to the second OXY injection on D4 relative to D2 (t208 = 18.82; p < 0.0001; “*”; Figure 3C), indicating locomotor sensitization. The degree of locomotor sensitization in AIL mice as measured via the D4-D2 subtraction measure is presented in Figure 3D.
Correlation matrix of OXY-CPP and concomitant phenotypes
A correlation matrix of OXY-CPP and the concomitant phenotypes is shown in Table 1. Notably, there was a small but significant positive correlation between D1 CPP and D8 CPP (r = 0.26), indicating that a lower initial preference for the OXY-paired side in part predicted a lower final preference for the OXY-paired side. However, when considering the negative correlation between D1 CPP and D8-D1 CPP (r = −0.51), it was evident that a lower initial preference likely permitted a larger increase in preference. D8 CPP also showed a positive correlation with D8-D1 CPP (r=0.7), indicating that the D8-D1 measure can largely be explained by the final preference for the OXY-paired side on D8 (Table 1).
Table 1.
Correlation matrix of OXY-CPP and concomitant phenotypes in AIL mice.
| Phenotype | D1 CPP |
D8 CPP |
D8- D1 CPP |
D1 ACT |
D8 ACT |
D2 ACT |
D3 ACT |
D4 ACT |
D5 ACT |
D4-D2 ACT |
SD CH D1 |
SD CH D8 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D1 CPP | 1 | |||||||||||
| D8 CPP | 0.26 | 1 | ||||||||||
| D8-D1 CPP | −0.51 | 0.7 | 1 | |||||||||
| D1 ACT | .15 | 0.12 | 0.00 | 1 | ||||||||
| D8 ACT | −0.03 | −0.03 | 0.00 | 0.62 | 1 | |||||||
| D2 ACT | −0.09 | −0.05 | 0.02 | 0.11 | 0.30 | 1 | ||||||
| D3 ACT | 0.08 | 0.08 | 0.01 | 0.47 | 0.51 | 0.22 | 1 | |||||
| D4 ACT | −0.05 | 0.00 | 0.04 | 0.13 | 0.24 | 0.81 | 0.16 | 1 | ||||
| D5 ACT | 0.09 | 0.07 | −0.01 | 0.45 | 0.55 | 0.18 | 0.80 | 0.17 | 1 | |||
| D4-D2 ACT | 0.03 | 0.07 | 0.04 | 0.08 | 0.00 | 0.02 | −0.03 | 0.61 | 0.04 | 1 | ||
| SD CH D1 | 0.13 | 0.04 | −0.06 | 0.57 | 0.40 | 0.11 | 0.36 | 0.13 | 0.33 | 0.08 | 1 | |
| SD CH D8 | −0.05 | −0.07 | −0.02 | 0.37 | 0.67 | 0.29 | 0.39 | 0.23 | 0.44 | −0.01 | 0.53 | 1 |
Bolded values indicate significant correlations (Pearson’s; p < 0.05), corrected for the 66 comparisons (p < 0.0008).
Initial locomotor activity in the freely accessible CPP apparatus on D1 (D1 ACT) predicted only the degree of activity exhibited following SAL treatment (and not OXY treatment) which was expressed either in the confined chamber on D3 and D5 (D3 ACT, D5 ACT; r = 0.47, 0.45) or following final preference assessment on D8 (D8 ACT; r = 0.62). D1 ACT also correlated with the transitions between the SAL- and OXY-paired sides on D1 (r = 0.57) and to a lesser extent, on D8 (r = 0.37; Table 1).
Locomotor activity between OXY training days (D2 ACT, D4 ACT) and between SAL training days (D3 ACT, D5 ACT) were highly correlated within the same treatment (r = 0.81, 0.8 respectively) but not across treatments (r = 0.16–0.22; p > 0.0008; Table 1), suggesting that the genetic basis of OXY-induced locomotor activity is largely distinct from locomotor activity following SAL injections. In further support, D1 ACT did not correlate with D2 ACT or D4 ACT (r = 0.11, 0.13; p > 0.0008; Table 1). However, D8 ACT did correlate with D2 ACT and D4 ACT (r = 0.3, 0.24; Table 1) which might be explained by opioid-induced, Pavlovian conditioned behavior on D8 (Bryant, et al 2009).
We observed a significant degree of OXY-induced locomotor sensitization in AIL mice as evidenced by the increase in activity from D2 to D4 (Figure 3C, D). Therefore, we wanted to determine the relationship of locomotor sensitization as measured by the difference in locomotor activity between D2 and D4 (D4-D2 ACT) with the other phenotypes. Importantly, the only variable that correlated with D4-D2 ACT was D4 ACT (r = 0.61) and not D2 ACT (r = 0.02) or drug-free locomotor activity (r = −0.03–0.08; Table 1), indicating that OXY-induced locomotor sensitization is a phenotype that is distinct from acute OXY sensitivity.
One final variable we were interested in was the number of transitions between the SAL- and OXY-paired sides (side changes; “SD CH”) as this could represent a component of drug seeking behavior (see Discussion). This behavior on D1 (SD CH D1) and D8 (SD CH D8) correlated with D1 ACT and D8 ACT (r=0.37–0.67; p > 0.0008; Table 1), reflecting that transitioning between sides necessitates an increase in activity. The most interesting observation regarding this behavior was that only SD CH D8 correlated with OXY-induced locomotor activity on D2 and D4 (r = 0.29, 0.23; p < 0.0008) and not SD CH D1 (r = 0.11, 0.13; p > 0.0008; Table 1), indicating that this behavior is predicted by the acute and sensitized drug response and could represent a component of conditioned drug seeking behavior.
Heritability of OXY-CPP and conconmitant phenotypes in AIL mice
Table 2 lists the heritability estimates obtained from regression analysis and maximum likelihood estimation. There was good agreement among the variables between the two methods of estimation (r = 0.91). OXY-CPP as measured both via the time spent on the OXY-paired side on D8 (D8 CPP) and via the difference in time spent on the OXY-paired side between training and test (D8-D1 CPP) was not significantly heritable (h2 = −0.11–0.14; Table 2). Because we observed a clear peak in D8-D1 CPP during the first 5 min of assessment in SM/J and AIL mice (data not shown), we also examined heritability for this time point but it was not significant either (data not shown). In contrast, most measures of drug free- and OXY-induced locomotor activity were significantly and in some cases highly heritable (h2 = 0.30–0.83; Table 2). Specifically, locomotor activity during SAL training days (D3, D5) exhibited the highest degree of heritability among the phenotypes (h2 = 0.50–0.83) whereas OXY-induced locomotor activity (D2 ACT, D4 ACT) and activity during preference assessment days (D1, D8) exhibited lower, but still reliable h2 estimates that ranged from 0.30 to 0.59 (Table 2). Interestingly, the number of transitions between the OXY- and SAL-paired sides was not consistently heritable before OXY training (SD CH D1; h2 = 0.18–0.4) but emerged as a highly heritable trait following OXY training on D8 (SD CH D8; h2 = 0.46–66; Table 2).
Table 2.
Heritability of OXY-CPP and concomitant phenotypes in AIL mice.
| Phenotype | h2 regression (S.E.) |
h2 maximum likelihood (S.E.) |
|---|---|---|
| D1 CPP | 0.01 (0.07) | 0 (0) |
| D8 CPP | −0.11 (0.1) | 0 (0) |
| D8-D1 CPP | 0.14 (0.11) | 0 (0) |
| D1 ACT | 0.36 (0.15) | 0.49 (0.20) |
| D8 ACT | 0.35 (0.18) | 0.58 (0.16) |
| D2 ACT | 0.32 (0.14) | 0.58 (0.14) |
| D3 ACT | 0.66 (0.16) | 0.83 (0.10) |
| D4 ACT | 0.30 (0.13) | 0.59 (0.11) |
| D5 ACT | 0.50 (0.11) | 0.72 (0.11) |
| D4-D2 ACT | 0.17 (0.19) | 0.51 (0.13) |
| SD CHG D1 | 0.18 (0.15) | 0.40 (0.16) |
| SD CHG D8 | 0.46 (0.16) | 0.66 (0.11) |
The heritability estimates (h2) and standard errors (S.E.) of OXY-CPP and the concomitant phenotypes derived from regression analysis and maximum likelihood estimation are listed. Bolded values indicate estimates that were significantly different from zero.
DISCUSSION
We established a significant OXY-CPP in SM/J and AIL mice, but not in LG/J mice (Figure 2A, D; “*”). Because D1 preference and CPP were negatively correlated in AIL mice (Table 1), the significant OXY-CPP observed in the parental SM/J strain might be explained by an initially lower preference for the OXY-paired side (Figure 2A; “#”). Similar to our previous findings with MA-CPP (Bryant, et al 2012a) and a previous study involving cocaine-CPP (Philip, et al 2010), OXY-CPP was not a heritable trait as measured via D8 CPP or D8-D1 CPP (Table 2). This observation strongly suggests that genetic variation does not contribute to the conditioned rewarding properties of opioids, at least under these conditions in AIL mice. It is possible that additional training sessions may induce a more robust OXY-CPP that is heritable. However, in our previous study, we did not find any change in MA-CPP or improvement in heritability estimates following an additional week of CPP training in AIL mice (Bryant et al., 2012).
The lack of heritability of opioid reward in mice supports a recent twin study in humans where the subjective liking of the highly selective mu opioid receptor agonist alfentanil was not heritable (h2 = 0–0.05) and contrasted with other traits that were heritable such as opioid disliking (h2 = 0.36) and opioid nausea (h2 = 0.56–0.59) (Angst, et al 2012). However, it is important to consider that although we are using OXY-CPP to infer opioid reward, the underlying mechanisms of CPP extend well beyond hedonic “hot spots” that mediate acute reward processing and could involve additional circuitry that mediates, e.g., incentive salience and approach behavior toward drug-associated stimuli (Berridge 2009) or more general Pavlovian conditioning processes. Thus, OXY reward may prove to be a heritable phenotype under conditions where it can be specifically measured. Last, it is possible that the aversive properties resulting from higher opioid doses could be heritable in mice, just as opioid disliking is in humans and that mapping this phenotype could lead to the identification of alleles that are protective against opioid abuse.
In agreement with our previous findings in AIL mice (Bryant, et al 2012a), locomotor activity in response to a SAL injection (D1 ACT, D8 ACT, D3 ACT, D5 ACT; Figure 2F; Figure 3C) was moderately to highly heritable (h2 = 0.35–0.66; Table 2), demonstrating the reliability of our estimates. This is important because both studies were potentially underpowered to detect significant heritability with only 10–17 parent-offspring observations (Klein, et al 1973; Klein 1974). Here, we utilized 17 observations which decreased the S.E. of the phenotypes listed above from a range of 0.19–0.33 (Bryant, et al 2012a) to a range of 0.08–0.2 (Table 2).
Sensitivity to OXY-induced locomotor activity on D2 and D4 in AIL mice (Figure 3C) was reliably heritable (h2 = 0.30–0.59; Table 2) and supports previous estimates obtained for morphine-induced locomotor stimulation in an inbred strain panel (Belknap, et al 1998) and cocaine-induced locomotor activity in a recombinant inbred strain panel (Philip, et al 2010). We previously identified QTLs on chromosome 11 and 15 that influenced opioid -induced locomotor activity (Bryant, et al 2009; Bryant, et al 2012b), further supporting a genetic contribution to this trait. Additionally, LG/J, SM/J, and AIL mice exhibited a robust locomotor sensitization response to 5 mg/kg OXY, showing a large increase in distance traveled from D2 to D4 (Figure 3). This phenotype was also heritable in AIL mice as estimated via maximum likelihood (h2 = 0.51) but not via linear regression (h2 = 0.17; Table 2). QTLs for locomotor sensitization to cocaine and ethanol have been identified (Cunningham 1995; Philip, et al 2010; Phillips, et al 1995; Phillips, et al 1998; Tolliver, et al 1994; Vendruscolo, et al 2009); thus, we believe that QTLs for oxycodone-induced locomotor sensitization will also be identified. Inbred strain differences in sensitization to heroin-induced locomotor stimulation (Szumlinski, et al 2005) further support the heritability of opioid sensitization and cross-sensitization experiments between ethanol, cocaine, and opioids in mice (Lessov and Phillips 2003) implicate shared genetic mechanisms.
Locomotor sensitization to drugs of abuse following repeated administration is hypothesized to reflect a hypersensitive dopaminergic circuit that increases the incentive motivational properties of drugs (“wanting”) and the incentive salience toward drug-conditioned stimuli (Robinson and Berridge 2008). Because locomotor sensitization to OXY correlated only with OXY-induced locomotor activity on D4 (r = 0.61) and not on D2 (r = 0.02) or any other activity measures following SAL treatment (r = −0.03–0.08; Table 1), this indicates that phenotypic variation in OXY-induced locomotor sensitization is caused by alleles that regulate neuroplasticity as a consequence of OXY exposure and not by alleles that induce pre-existing neurobiological differences to affect, e.g., acute OXY sensitivity. Thus, OXY sensitization could be a specific and genetically tractable behavioral proxy of the neurobiological adaptations accompanying the early addictive process and could be combined with heritable intermediate phenotypes relevant to sensitization (e.g., striatal dopamine release).
An interesting finding of this study was that the number of transitions between the OXY- and SAL-paired sides emerged as a reliably heritable trait following OXY training (SD CH D8; h2 = 0.46–0.66; Table 2) and correlated with OXY-induced locomotor activity on D2 and D4 (r = 0.29, 0.23; Table 1). That the acute and sensitized drug response predicted this behavior unveiled a Pavlovian-conditioned property of this trait because it showed that the intensity of the unconditioned stimulus predicted the degree of conditioned response. A previous study analyzing the location of rats during the expression of morphine-CPP found that the degree of CPP could largely be accounted for by the number of visits to the morphine-paired side, rather than the length of each visit (German and Fields 2007). In our study, there was no correlation between SD CH D8 and OXY-CPP. It should be noted that German and Fields (2007) employed a three-chamber design in rats which provided a neutral chamber and allowed the rats to choose the morphine-paired side relevant to a neutral condition rather than, e.g., choose the morphine-paired side to avoid the non-morphine side (which could be associated with drug withdrawal). Employing a three-chamber design would permit the isolation of approach behavior toward OXY-paired stimuli and this could be a heritable trait associated with OXY-CPP. It would also allow us to distinguish between whether strains or individual mice specifically avoid entering one particular side or if they exhibit a general avoidance of both the OXY- and SAL-paired sides. This could affect the strain differences and individual differences in OXY-CPP as well as the relationship among the concomitant variables.
A perplexing result we observed in this study was that LG/J mice showed a significantly higher initial preference for the drug-paired side compared to the MA study (p = 0.0007) which could contribute to the lack of OXY-CPP in this strain. The source of this strain × experiment interaction is not clear but could be explained by any number of factors. First, in the previous study, mice were tested in the morning through the afternoon hours (0800 h – 01700 h) whereas in the present study, mice were only tested in the afternoon hours. The detection of mouse strain differences in behavioral phenotypes has been shown to be circadian-dependent (Hossain, et al 2004). Second, different experimenters tested the parental strains: In the previous study, C.D.B. phenotyped the LG/J and SM/J strains whereas in the present study, L.A.K. phenotyped these strains. A previous study demonstrated the importance of experimenter on behavioral phenotypes (Chesler et al., 2002) which could potentially interact with genotype. Importantly, despite the discrepancy in the parental strain data, the D1 CPP values for AIL mice were not significantly different between the MA study and the OXY study (t312 < 1), supporting the reproducibility of this phenotype in experiments that employed a much larger sample size (N = 105–209) than used for the parental strains (N = 30–31).
Our findings add to the growing number of studies demonstrating that CPP is not a heritable trait and thus may not be amenable to forward genetic analysis. We have shown that several other phenotypes were heritable, including sensitivity the acute locomotor stimulant response, locomotor sensitization, and transitions between the SAL- and OXY-paired sides which may represent a heritable component of drug seeking behavior. Because of the high resolution afforded by QTL mapping in AIL mice, the use of this protocol could rapidly lead to the identification of alleles important for the initial behavioral and neural plasticity that may be important for the transition to opioid addiction. Last, because heritability estimates can depend on the population that is ascertained (Visscher, et al 2008), employing additional mapping populations with a greater degree of genetic diversity (Svenson, et al 2012) may reflect a greater genetic contribution to CPP than what is currently appreciated.
ACKNOWLEDGEMENTS
We would like to acknowledge Dr. Abraham A. Palmer for providing the infrastructure used to execute this study and Ms. Diane Trahanas for maintaining the colony of AIL mice. This work was supported by K99DA029635.
Footnotes
DISCLOSURE/CONFLICT OF INTEREST
The authors have no financial interests to disclose.
AUTHOR CONTRIBUTION
C.D.B. designed the study, supervised the execution of the experiments, analyzed the data, and wrote the manuscript. M.A.G. and L.A.K. phenotyped the mice and helped write the manuscript. R.C. conducted the heritability analyses for maximum likelihood estimation and helped write the manuscript.
References
- Abney M, McPeek MS, Ober C. Estimation of variance components of quantitative traits in inbred populations. Am J Hum Genet. 2000;66:629–650. doi: 10.1086/302759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angst MS, Lazzeroni LC, Phillips NG, Drover DR, Tingle M, Ray A, Swan GE, Clark JD. Aversive and Reinforcing Opioid Effects: A Pharmacogenomic Twin Study. Anesthesiology. 2012;117:22–37. doi: 10.1097/ALN.0b013e31825a2a4e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belknap JK, Riggan J, Cross S, Young ER, Gallaher EJ, Crabbe JC. Genetic determinants of morphine activity and thermal responses in 15 inbred mouse strains. Pharmacol Biochem Behav. 1998;59:353–360. doi: 10.1016/s0091-3057(97)00421-8. [DOI] [PubMed] [Google Scholar]
- Berrettini WH, Ferraro TN, Alexander RC, Buchberg AM, Vogel WH. Quantitative trait loci mapping of three loci controlling morphine preference using inbred mouse strains. Nature genetics. 1994;7:54–58. doi: 10.1038/ng0594-54. [DOI] [PubMed] [Google Scholar]
- Berridge KC. Wanting and Liking: Observations from the Neuroscience and Psychology Laboratory. Inquiry (Oslo) 2009;52:378. doi: 10.1080/00201740903087359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant CD, Chang HP, Zhang J, Wiltshire T, Tarantino LM, Palmer AA. A major QTL on chromosome 11 influences psychostimulant and opioid sensitivity in mice. Genes Brain Behav. 2009;8:795–805. doi: 10.1111/j.1601-183X.2009.00525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant CD, Kole LA, Guido MA, Cheng R, Palmer AA. Methamphetamine-induced conditioned place preference in LG/J and SM/J mouse strains and an F45/F46 advanced intercross line. Front Genet. 2012a;3:126. doi: 10.3389/fgene.2012.00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant CD, Parker CC, Zhou L, Olker C, Chandrasekaran RY, Wager TT, Bolivar VJ, Loudon AS, Vitaterna MH, Turek FW, Palmer AA. Csnk1e is a genetic regulator of sensitivity to psychostimulants and opioids. Neuropsychopharmacology. 2012b;37:1026–1035. doi: 10.1038/npp.2011.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant CD, Roberts KW, Culbertson CS, Le A, Evans CJ, Fanselow MS. Pavlovian conditioning of multiple opioid-like responses in mice. Drug and alcohol dependence. 2009;103:74–83. doi: 10.1016/j.drugalcdep.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng R, Lim JE, Samocha KE, Sokoloff G, Abney M, Skol AD, Palmer AA. Genome-wide association studies and the problem of relatedness among advanced intercross lines and other highly recombinant populations. Genetics. 2010;185:1033–1044. doi: 10.1534/genetics.110.116863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs E, de Wit H. Amphetamine-induced place preference in humans. Biol Psychiatry. 2009;65:900–904. doi: 10.1016/j.biopsych.2008.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs E, de Wit H. Contextual conditioning enhances the psychostimulant and incentive properties of d-amphetamine in humans. Addict Biol . 2011 doi: 10.1111/j.1369-1600.2011.00416.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham CL. Localization of genes influencing ethanol-induced conditioned place preference and locomotor activity in BXD recombinant inbred mice. Psychopharmacology (Berl) 1995;120:28–41. doi: 10.1007/BF02246142. [DOI] [PubMed] [Google Scholar]
- Darvasi A, Soller M. Advanced intercross lines, an experimental population for fine genetic mapping. Genetics. 1995;141:1199–1207. doi: 10.1093/genetics/141.3.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Der-Avakian A, Bland ST, Rozeske RR, Tamblyn JP, Hutchinson MR, Watkins LR, Maier SF. The effects of a single exposure to uncontrollable stress on the subsequent conditioned place preference responses to oxycodone, cocaine, and ethanol in rats. Psychopharmacology (Berl) 2007;191:909–917. doi: 10.1007/s00213-006-0678-7. [DOI] [PubMed] [Google Scholar]
- Doyle GA, Furlong PJ, Schwebel CL, Smith GG, Lohoff FW, Buono RJ, Berrettini WH, Ferraro TN. Fine mapping of a major QTL influencing morphine preference in C57BL/6 and DBA/2 mice using congenic strains. Neuropsychopharmacology. 2008;33:2801–2809. doi: 10.1038/npp.2008.14. [DOI] [PubMed] [Google Scholar]
- Ferraro TN, Golden GT, Smith GG, Martin JF, Schwebel CL, Doyle GA, Buono RJ, Berrettini WH. Confirmation of a major QTL influencing oral morphine intake in C57 and DBA mice using reciprocal congenic strains. Neuropsychopharmacology. 2005;30:742–746. doi: 10.1038/sj.npp.1300592. [DOI] [PubMed] [Google Scholar]
- German PW, Fields HL. How prior reward experience biases exploratory movements: a probabilistic model. J Neurophysiol. 2007;97:2083–2093. doi: 10.1152/jn.00303.2006. [DOI] [PubMed] [Google Scholar]
- Goldman D, Oroszi G, Ducci F. The genetics of addictions: uncovering the genes. Nat Rev Genet. 2005;6:521–532. doi: 10.1038/nrg1635. [DOI] [PubMed] [Google Scholar]
- Haertzen CA, Kocher TR, Miyasato K. Reinforcements from the first drug experience can predict later drug habits and/or addiction: results with coffee, cigarettes, alcohol, barbiturates, minor and major tranquilizers, stimulants, marijuana, hallucinogens, heroin, opiates and cocaine. Drug and alcohol dependence. 1983;11:147–165. doi: 10.1016/0376-8716(83)90076-5. [DOI] [PubMed] [Google Scholar]
- Ho MK, Goldman D, Heinz A, Kaprio J, Kreek MJ, Li MD, Munafo MR, Tyndale RF. Breaking barriers in the genomics and pharmacogenetics of drug addiction. Clin Pharmacol Ther. 2010;88:779–791. doi: 10.1038/clpt.2010.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain SM, Wong BK, Simpson EM. The dark phase improves genetic discrimination for some high throughput mouse behavioral phenotyping. Genes Brain Behav. 2004;3:167–177. doi: 10.1111/j.1601-183x.2004.00069.x. [DOI] [PubMed] [Google Scholar]
- Kest B, Palmese CA, Juni A, Chesler EJ, Mogil JS. Mapping of a quantitative trait locus for morphine withdrawal severity. Mamm Genome. 2004;15:610–617. doi: 10.1007/s00335-004-2367-3. [DOI] [PubMed] [Google Scholar]
- Kest B, Smith SB, Schorscher-Petcu A, Austin JS, Ritchie J, Klein G, Rossi GC, Fortin A, Mogil JS. Gnao1 (G alphaO protein) is a likely genetic contributor to variation in physical dependence on opioids in mice. Neuroscience. 2009;162:1255–1264. doi: 10.1016/j.neuroscience.2009.05.027. [DOI] [PubMed] [Google Scholar]
- Klein TW. Heritability and genetic correlation: statistical power, population comparisons, and sample size. Behav Genet. 1974;4:171–189. doi: 10.1007/BF01065758. [DOI] [PubMed] [Google Scholar]
- Klein TW, DeFries JC, Finkbeiner CT. Heritability and genetic correlation: standard errors of estimates and sample size. Behav Genet. 1973;3:355–364. doi: 10.1007/BF01070218. [DOI] [PubMed] [Google Scholar]
- Lessov CN, Phillips TJ. Cross-sensitization between the locomotor stimulant effects of ethanol and those of morphine and cocaine in mice. Alcohol Clin Exp Res. 2003;27:616–627. doi: 10.1097/01.ALC.0000062760.17530.74. [DOI] [PubMed] [Google Scholar]
- Levran O, Yuferov V, Kreek MJ. The genetics of the opioid system and specific drug addictions. Hum Genet. 2012;131:823–842. doi: 10.1007/s00439-012-1172-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YL, Yan LD, Zhou PL, Wu CF, Gong ZH. Levo-tetrahydropalmatine attenuates oxycodone-induced conditioned place preference in rats. Eur J Pharmacol. 2009;602:321–327. doi: 10.1016/j.ejphar.2008.11.031. [DOI] [PubMed] [Google Scholar]
- Manchikanti L, Fellows B, Ailinani H, Pampati V. Therapeutic use, abuse, and nonmedical use of opioids: a ten-year perspective. Pain Physician. 2010;13:401–435. [PubMed] [Google Scholar]
- Matthes HW, Maldonado R, Simonin F, Valverde O, Slowe S, Kitchen I, Befort K, Dierich A, Le Meur M, Dolle P, Tzavara E, Hanoune J, Roques BP, Kieffer BL. Loss of morphine-induced analgesia, reward effect and withdrawal symptoms in mice lacking the mu-opioid-receptor gene. Nature. 1996;383:819–823. doi: 10.1038/383819a0. [DOI] [PubMed] [Google Scholar]
- Philip VM, Duvvuru S, Gomero B, Ansah TA, Blaha CD, Cook MN, Hamre KM, Lariviere WR, Matthews DB, Mittleman G, Goldowitz D, Chesler EJ. High-throughput behavioral phenotyping in the expanded panel of BXD recombinant inbred strains. Genes Brain Behav. 2010;9:129–159. doi: 10.1111/j.1601-183X.2009.00540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips TJ, Huson M, Gwiazdon C, Burkhart-Kasch S, Shen EH. Effects of acute and repeated ethanol exposures on the locomotor activity of BXD recombinant inbred mice. Alcohol Clin Exp Res. 1995;19:269–278. doi: 10.1111/j.1530-0277.1995.tb01502.x. [DOI] [PubMed] [Google Scholar]
- Phillips TJ, Huson MG, McKinnon CS. Localization of genes mediating acute and sensitized locomotor responses to cocaine in BXD/Ty recombinant inbred mice. J Neurosci. 1998;18:3023–3034. doi: 10.1523/JNEUROSCI.18-08-03023.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Review. The incentive sensitization theory of addiction: some current issues. Philos Trans R Soc Lond B Biol Sci. 2008;363:3137–3146. doi: 10.1098/rstb.2008.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setnik B, Roland CL, Cleveland JM, Webster L. The abuse potential of Remoxy((R)), an extended-release formulation of oxycodone, compared with immediate- and extended-release oxycodone. Pain Med. 2011;12:618–631. doi: 10.1111/j.1526-4637.2011.01093.x. [DOI] [PubMed] [Google Scholar]
- Shao J, Tu D. The Jackknife and Bootstrap. In: Bickel P, Diggle PJ, Fienberg SE, Gather U, Olkin I, Zeger S, editors. Springer Series in Statistics. 1st ed. . Springer-Verlag, Inc.; 1995. p. 540. [Google Scholar]
- Svenson KL, Gatti DM, Valdar W, Welsh CE, Cheng R, Chesler EJ, Palmer AA, McMillan L, Churchill GA. High-resolution genetic mapping using the Mouse Diversity outbred population. Genetics. 2012;190:437–447. doi: 10.1534/genetics.111.132597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szumlinski KK, Lominac KD, Frys KA, Middaugh LD. Genetic variation in heroin-induced changes in behaviour: effects of B6 strain dose on conditioned reward and locomotor sensitization in 129-B6 hybrid mice. Genes Brain Behav. 2005;4:324–336. doi: 10.1111/j.1601-183X.2004.00111.x. [DOI] [PubMed] [Google Scholar]
- Tolliver BK, Belknap JK, Woods WE, Carney JM. Genetic analysis of sensitization and tolerance to cocaine. J Pharmacol Exp Ther. 1994;270:1230–1238. [PubMed] [Google Scholar]
- Tzschentke TM. Measuring reward with the conditioned place preference (CPP) paradigm: update of the last decade. Addict Biol. 2007;12:227–462. doi: 10.1111/j.1369-1600.2007.00070.x. [DOI] [PubMed] [Google Scholar]
- Vendruscolo LF, Vendruscolo JC, Terenina E, Ramos A, Takahashi RN, Mormede P. Marker-assisted dissection of genetic influences on motor and neuroendocrine sensitization to cocaine in rats. Genes Brain Behav. 2009;8:267–274. doi: 10.1111/j.1601-183X.2008.00465.x. [DOI] [PubMed] [Google Scholar]
- Visscher PM, Hill WG, Wray NR. Heritability in the genomics era--concepts and misconceptions. Nat Rev Genet. 2008;9:255–266. doi: 10.1038/nrg2322. [DOI] [PubMed] [Google Scholar]
- Webster LR, Bath B, Medve RA, Marmon T, Stoddard GJ. Randomized, double-blind, placebo-controlled study of the abuse potential of different formulations of oral oxycodone. Pain Med. 2012;13:790–801. doi: 10.1111/j.1526-4637.2012.01380.x. [DOI] [PubMed] [Google Scholar]