Abstract
Background
Current total disc replacement (TDR) for lumbar spine requires an anterior approach for implantation but presents inherent limitations, including risks to the abdominal structures, as well as resection of the anterior longitudinal ligament. By approaching the spine laterally, it is possible to preserve the stabilizing ligaments, which are a natural restraint to excessive rotations and translations, and thereby help to minimize facet stresses. This less invasive approach also offers a biomechanical advantage of placement of the device over the ring apophysis bilaterally; importantly, it also offers a greater opportunity for safer revision surgery, if necessary, by avoiding scarring of the anterior vasculature. We present the clinical and radiologic results of a lateral TDR device from a prospective single-center study.
Methods
A new metal-on-metal TDR device designed for implantation through a true lateral, retroperitoneal, transpsoatic approach (extreme lateral interbody fusion) was implanted in 36 patients with discography-confirmed 1- or 2-level degenerative disc disease. Clinical (pain and function) and radiographic (range of motion) outcome assessments were prospectively collected preoperatively, postoperatively, and serially up to a minimum of 36 months’ follow-up.
Results
Between December 2005 and December 2006, 36 surgeries were performed in 16 men and 20 women (mean age, 42.6 years). These included 15 single-level TDR procedures at L3-4 or L4-5, 3 2-level TDR procedures spanning L3-4 and L4-5, and 18 hybrid procedures (anterior lumbar interbody fusion) at L5-S1 and TDR at L4-5 (17) or L3-4 (1). Operative time averaged 130 minutes, with mean blood loss of 60 mL and no intraoperative complications. Postoperative X-rays showed good device placement, with restoration of disc height, foraminal volume, and sagittal balance. All patients were up and walking within 12 hours of surgery, and all but 9 were discharged the next day (7 of those 9 were hybrid TDR–anterior lumbar interbody fusion cases). Postoperatively, 5 of 36 patients (13.8%) had psoas weakness and 3 of 36 (8.3%) had anterior thigh numbness, with both symptoms resolving within 2 weeks. Of the 36 patients, 4 (11%) had postoperative facet joint pain, all in hybrid cases. Visual analog scale pain scores and Oswestry Disability Index scores improved by 74.5% and 69.2%, respectively, from preoperatively to 3-year follow-up. Range of motion at 3 years postoperatively averaged 8.1°. Signals of heterotopic ossification were present in 5 patients (13.9%), and 2 patients (5.5%) were considered to have fusion after 36 months.
Conclusions
The clinical and radiographic results of a laterally placed TDR have shown maintenance of pain relief and functional improvement over a long-term follow-up period. The benefits of the lateral access—minimal morbidity, avoidance of mobilization of the great vessels, preservation of the anterior longitudinal ligament, biomechanically stable orientation, and broader revision options—promote a new option for motion-preservation procedures.
Keywords: Arthroplasty, Total disc replacement, XLIF, Minimally invasive, Lateral approach
Artificial disc replacement surgery has developed as a motion-preservation alternative to fusion procedures for the treatment of pain and instability associated with degenerative disc disease. Currently, all devices have been implanted through an anterior approach, with inherent limitations, including considerable collateral damage to the surrounding tissues and risk of vascular and visceral injuries. Anterior fusion surgeries have shown a complication rate of 38.3%, with complications including sympathetic dysfunction, vascular injury, somatic neural injury, sexual dysfunction, prolonged ileus, wound incompetence, deep vein thrombosis, acute pancreatitis, and bowel injury.1 Studies of anterior total disc replacement (TDR) surgeries corroborate these approach-related complications.2 To reduce or even avoid these potential complications, the lateral approach is required for a less invasive device implantation.
The lateral approach has been indicated for anterior fusion of the thoracolumbar spine. Previous studies have reported the safety and effectiveness of the extreme lateral interbody fusion (XLIF) approach, with few approach-related complications and minimal morbidity with rapid recovery.3–7 The placement of an artificial disc replacement device by the lateral approach allows less invasive access to the degenerated disc, preserving the stabilizing ligaments and providing greater endplate support, with positioning of the device at the vertebral apophyseal ring.8 We present the clinical and radiographic results of a lateral TDR device (XL-TDR; NuVasive, Inc., San Diego, California) after 36 months from a prospective single-center study.
Methods
A prospective nonrandomized study was conducted to evaluate the clinical and radiographic outcomes of a TDR procedure using a lateral approach. All patients provided informed consent to participate. Inclusion/exclusion criteria (partially listed in Table 1) were similar to those previously cited for other lumbar TDR studies.2, 9–12 Because of the inability to access the L5-S1 disc level by the lateral approach, this level was excluded.
Table 1.
A selective (non-comprehensive) list of some of the more relevant inclusion/exclusion criteria for the study
| Inclusion criteria |
| Age 18–60 y |
| Symptomatic lumbar degenerative disease: magnetic resonance imaging–confirmed disc desiccation, loss of disc height, and bridging osteophytes |
| Symptomatic level L1-2, L2-3, L3-4, or L4-5 |
| Preoperative Oswestry Disability Index score ≥ 30 points |
| Unresponsive to conservative treatment for > 6 mo or presence of progressive neurologic symptoms |
| Willing and able to comply with requirements defined in protocol for duration of study |
| Signed and dated informed consent form |
| Exclusion criteria |
| Prior lumbar fusion surgery at operative level |
| Prior lumbar laminectomy at operative level |
| Prior complete lumbar facetectomy at operative level |
| Prior bilateral retroperitoneal surgery |
| Radiographic signs of significant instability at operative level (> 3-mm translation, > 11° angulation different from adjacent level) |
| Bridging osteophytes or absence of motion < 2° |
| Radiographic confirmation of significant facet joint disease or degeneration |
| Pars defect, facet abnormality, or other compromise of posterior elements |
| Spondylolisthesis (greater than grade 1) |
| Osteopenia, osteoporosis, or osteomalacia to a degree that spinal instrumentation would be contraindicated |
| Body mass index > 40 |
| Active local or systemic infection, including AIDS and hepatitis |
Surgical technique
The approach was the standard XLIF technique for fusion,3, 4, 13 with care taken to maintain the anterior longitudinal ligament (ALL) intact. The ALL provides an anterior restraint not only to extension but also to axial rotation. It has been shown that resection of the ALL leads to hypermobility of the segment and potential facet arthrosis at the same level and adjacent levels.10, 14–16
A discectomy was performed, reaching the contralateral margin and releasing the contralateral annulus. The device must be positioned in proper sagittal and coronal alignment, permitting the placement of the prosthesis on both sides of the ring apophysis. Studies of endplate strength have shown that the apophyseal ring is the strongest area and that the center of the endplate, where most anterior implants are currently placed, is the weakest17 and is susceptible to subsidence.18
For proper insertion, sequential sizing was used, and the lateral TDR device (XL-TDR) was inserted. The device consists of a superior endplate and an inferior endplate with a metal-on-metal (cobalt-chromium-molybdenum alloy) ball-and-socket articulation (Fig. 1). The surfaces of the endplates have spikes to increase primary fixation into the vertebral bone and are also coated with a dual-layer titanium plasma spray and hydroxyapatite plasma spray to facilitate bone on-growth for secondary fixation. The device covers more than 50% of the endplate area and spans the ring apophysis on both sides. The device must be in the midline, providing ideal placement of the prosthesis because of the position of its kinematic center of rotation.
Fig. 1.
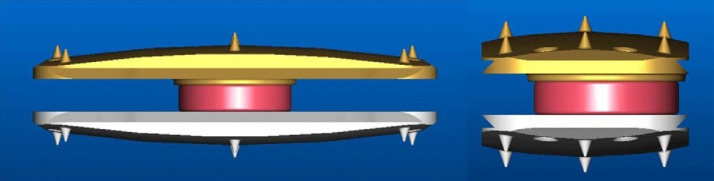
Anteroposterior and lateral views of prosthesis (XL-TDR).
Clinical and radiographic evaluations
Patients were evaluated clinically and radiographically before surgery, at discharge, and at 6 weeks and 3, 6, 12, 24, and 36 months after surgery. At every follow-up visit, patients provided a visual analog scale score for back and leg pain; in addition, function was determined with the Oswestry Disability Index. Physical examinations measured motor and sensory function in the lower limbs. Radiographic evaluation included range-of-motion (ROM) measurements, subsidence, and heterotopic bone formation from flexion-extension x-ray images. Surgical data—operative time, blood loss, complications, and length of hospital stay—were compiled. Descriptive statistics were used to characterize the patient population and results. Paired and unpaired Student's t tests, χ2 tests, and analysis of variance tests were used, where appropriate, to compare results over time or between groups.
Results
Demographics
Thirty-six patients with back pain, with or without leg pain and/or motor or sensory deficits, underwent TDR through an XLIF approach. Patients comprised 16 men and 20 women with a mean age of 42.6 years (range, 22–60 years).
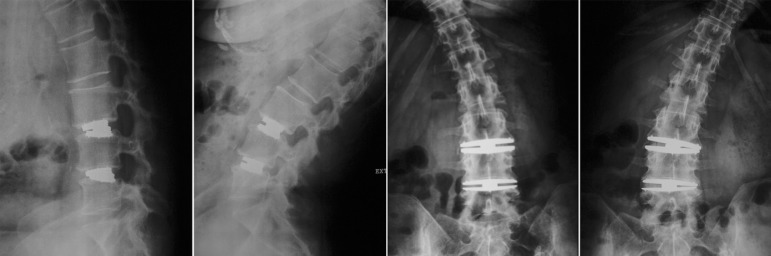
Surgeries included 14 single-level TDR procedures at L4-5 and 1 single-level procedure at L3-4. Three procedures included 2 levels of TDR at L3-4 and L4-5. The other 18 surgeries included an anterior lumbar interbody fusion at L5-S1 and TDR at L4-5 (16) or L3-4 (2). A 2-level example is shown in Fig. 2. Operative time averaged 130 minutes (range, 90–300 minutes) and blood loss averaged 60 mL (range, 30–150 mL), with no intraoperative complications.
Fig. 2.
Case example of 2-level lumbar arthroplasty showing dynamic X-rays at 36-month follow-up.
Patients were encouraged to walk the same day and were discharged after 1.36 days on average. Because of the transpsoatic approach, arthrogenous muscle inhibition19 was clinically observed in 5 patients (13.8%) and 3 patients (8.3%) had anterior thigh numbness postoperatively, with both symptoms resolving within 2 weeks. At the last follow-up point, no neurologic symptoms were observed.
Clinical outcomes
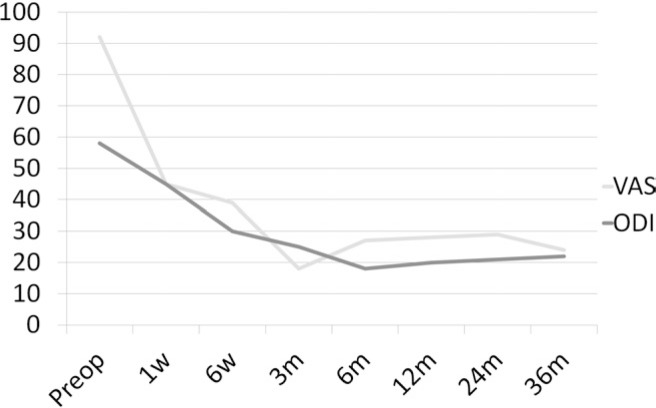
Visual analog scale pain scores statistically improved from a mean of 92.5 preoperatively to 23.4 immediately postoperatively and were maintained at 23.7 at 3 years (P < .0001). Oswestry Disability Index scores statistically improved from a mean of 57.3 preoperatively to 29.8 at 6 weeks, with continued improvement to 17.6 at 3 years (P < .0001) (Fig. 3). There were no statistical differences among patients treated with single-level, 2-level, or hybrid procedures, and they did not differ with regard to age, gender, or level treated (P > .1).
Fig. 3.
Clinical outcomes up to 36 months. Postoperative scores were statistically significantly better (P .05). (ODI, Oswestry Disability Index; VAS, visual analog scale.)
Revision rate
Removal of the TDR device and revision to fusion by the same surgical approach were required in 2 cases (5.6%). The first patient presented with postoperative imaging showing that the caudal endplate of the TDR device was slightly oblique. At the 12-month follow-up visit, the patient opted to have the device removed because of back and leg pain, which was previously reported at her 6-month visit and treated by conservative care during this period. The TDR device was easily removed; the level was then fused and supplemented with bilateral pedicle screws.
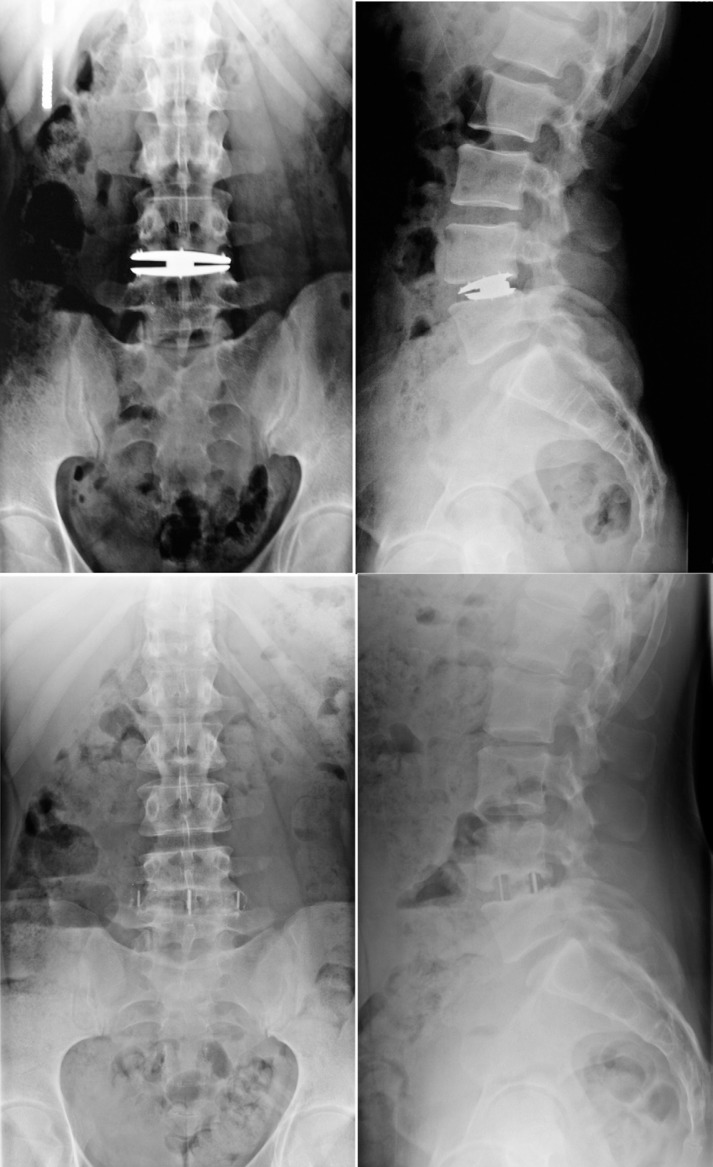
The second patient presented with axial rotation of the superior endplate that initially did not affect pain and function. At the 6-month follow-up visit, the patient reported a significant increase in pain and conservative care was administered. Twenty months after surgery, the patient decided to undergo revision of the prosthesis through the XLIF approach (Fig. 4).
Fig. 4.
Case example of lateral disc revision showing 24-month follow-up images (top) and post-XLIF images (bottom).
Radiographic outcomes
By the lateral approach, it was possible to indirectly decompress the neural structures by disc height restoration and ligamentotaxis, also improving sagittal balance. The total ROM was statistically maintained after 36 months in comparison with preoperative values (P = .6859) and was not a statistically significant factor in clinical success (P = .6730). There was a significant difference among groups, with mean ROM of 12.8° for single-level constructs, 5.3° for 2-level constructs, and 7.3° for hybrid constructs (P = .0181).
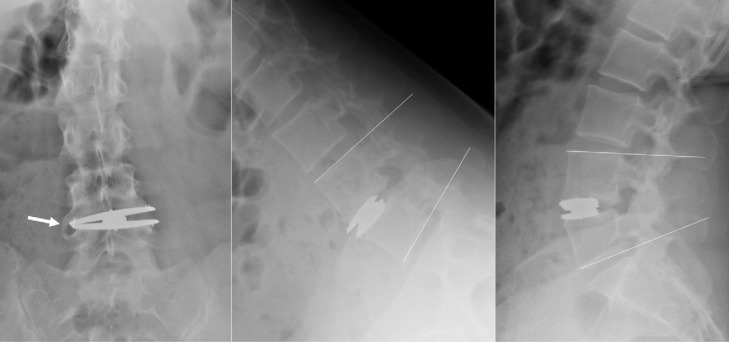
The incidence of contralateral bone formation was seen in 5 patients (13.9%) after 36 months’ follow-up. These formations did not affect clinical results or mean ROM. Two patients were considered to have fusion because no movement was seen on flexion/extension X-rays at 36 months’ follow-up (Fig. 5).
Fig. 5.
Case example of grade IV heterotopic ossification. Contralateral bone formation is shown by the arrow. Fusion at the index level is evidenced by the dynamic X-rays.
Discussion
The lateral TDR presents a minimally invasive alternative to standard anteriorly placed lumbar arthroplasties. Biomechanically, the retention of an intact ALL increases stabilizing effects. Lateral device insertion leads to tensioning of the anterior and posterior longitudinal ligaments and remaining annulus. A previous biomechanical study has shown that the XL-TDR presents less ROM than intact spine, and the motion was found to be more controlled, with a more natural neutral zone.20 However, the XLIF approach has inherent limitations, and because of obstruction by the iliac crest, it is impossible to access the L5-S1 level. Our experience shows that for multilevel pathologies that include L5-S1, a mini–anterior lumbar interbody fusion was conducted for fusion with no statistical differences in clinical outcomes between hybrid and nonhybrid constructions.
The lateral transpsoatic minimally invasive technique avoids complications related to the anterior approach, including sympathetic dysfunction, vascular injury, somatic neural injury, sexual dysfunction, prolonged ileus, wound incompetence, deep venous thrombosis, acute pancreatitis, and bowel injury,1 but it is not risk free. The lumbar plexus within the psoas muscle needs to undergo real-time, stimulus-evoked electromyography through the lateral access. By identifying these nerves during the approach, the surgeon can avoid postoperative neurologic deficits. The results found in our study show no significant differences compared with previous studies with anterior TDRs2, 10, 21, 22 and the lateral approach in relation to neurologic damage.
Primary placement of the device by the lateral approach also allows a safer surgical approach option in case of prosthesis removal or revision,23 as evidenced by the 2 uneventful revisions. It is possible to access the lumbar spine by a contralateral approach with the same technique; in addition, the anterior approach can easily be performed because the primary procedure did not create scars and adhesions in the peritoneum and great vessels.
One of the complications described in the literature regarding TDR is heterotopic ossification.24 The presence of heterotopic bone formation was only seen on the contralateral side of the endplate. It should be pointed out that during the discectomy, the contralateral osteophytes were removed, and this should have accelerated the bone formation seen in this study. Only 2 patients presented with high degrees of heterotopic ossification subsequently associated with loss of movement at the operated level. Low-grade heterotopic ossification was seen in 5 patients and was not associated with loss of ROM. In all 7 cases, there was no relation to worsening in clinical outcomes.
Previous reports have shown that the ALL is an important retainer in extension and axial rotation and that its resection leads to hypermobility and facet arthrosis at the same level and adjacent levels.16, 25, 26 The maintenance of the ALL, preserved by the laterally placed TDR, generates a superior biomechanical construction that prevents anterior displacement and excessive loading of the facet joints, improving ligamentotaxis and sagittal balance, which leads to a more natural neutral zone and a more constrained movement of the lumbar spine.
Clinical and radiologic outcomes at present support the success of the procedure, maintaining motion and relieving pain. Longer-term follow-up is required to determine whether the present clinical success is permanent, as suggested by our results.
Conclusions
The TDR with maintenance of the ALL provides inherent advantages over anterior approaches. While preserving ligaments with minimal morbidity, the XLIF procedure avoids mobilization of the great vessels and gives revision options. The clinical and radiologic results to date suggest a minimally invasive motion-preservation alternative to lumbar disc arthroplasty.
References
- 1.Rajaraman V, Vingan R, Roth P, Heary R, Conklin L, Jacobs G. Visceral and vascular complications resulting from anterior lumbar interbody fusion. J Neurosurg Spine. 1999;1(Suppl):60–4. doi: 10.3171/spi.1999.91.1.0060. [DOI] [PubMed] [Google Scholar]
- 2.Blumenthal S, McAfee PC, Guyer RD, et al. A prospective, randomized, multicenter Food and Drug Administration investigational device exemptions study of lumbar total disc replacement with the CHARITE artificial disc versus lumbar fusion: part I: evaluation of clinical outcomes. Spine (Phila Pa 1976) 2005;30:1565–75. doi: 10.1097/01.brs.0000170587.32676.0e. [DOI] [PubMed] [Google Scholar]
- 3.Ozgur BM, Aryan HE, Pimenta L, Taylor WR. Extreme lateral interbody fusion (XLIF): a novel surgical technique for anterior lumbar interbody fusion. Spine J. 2006;6:435–43. doi: 10.1016/j.spinee.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 4.Rodgers WB, Cox CS, Gerber EJ. Experience and early results with a minimally invasive technique for anterior column support through eXtreme Lateral Interbody Fusion (XLIF) US Musculoskelet Rev. 2007;1:28–32. [Google Scholar]
- 5.Rodgers WB, Cox CS, Gerber EJ. Minimally invasive treatment (XLIF) of adjacent segment disease after prior lumbar fusions. Internet J Minim Invasive Spinal Technol. 2009;3 [Google Scholar]
- 6.Rodgers WB, Cox CS, Gerber EJ. Early complications of extreme lateral interbody fusion in the obese. J Spinal Disord Tech. 2010;23:393–7. doi: 10.1097/BSD.0b013e3181b31729. [DOI] [PubMed] [Google Scholar]
- 7.Rodgers WB, Cox CS, Gerber EJ. Intraoperative and early postoperative complications in extreme lateral interbody fusion (XLIF): an analysis of 600 cases. Spine (Phila Pa 1976) 2011;36:26–32. doi: 10.1097/BRS.0b013e3181e1040a. [DOI] [PubMed] [Google Scholar]
- 8.Pimenta L, Oliveira L, Schaffa T, Coutinho E, Marchi L. Lumbar total disc replacement from an extreme lateral approach: clinical experience with a minimum of 2 years’ follow-up. J Neurosurg Spine. 2011;14:38–45. doi: 10.3171/2010.9.SPINE09865. [DOI] [PubMed] [Google Scholar]
- 9.Bertagnoli R, Yue JJ, Shah RV, et al. The treatment of disabling single-level lumbar discogenic low back pain with total disc arthroplasty utilizing the ProDisc prosthesis: a prospective study with 2-year minimum follow-up. Spine (Phila Pa 1976) 2005;30:2230–6. doi: 10.1097/01.brs.0000182217.87660.40. [DOI] [PubMed] [Google Scholar]
- 10.Le Huec JC, Mathews H, Basso Y, et al. Clinical results of Maverick lumbar total disc replacement: two-year prospective follow-up. Orthop Clin North Am. 2005;36:315–22. doi: 10.1016/j.ocl.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 11.Sasso RC, Foulk DM, Hahn M. Prospective, randomized trial of metal-on-metal artificial lumbar disc replacement: initial results for treatment of discogenic pain. Spine (Phila Pa 1976) 2008;33:123–31. doi: 10.1097/BRS.0b013e31816043af. [DOI] [PubMed] [Google Scholar]
- 12.Zigler J, Delamarter R, Spivak JM, et al. Results of the prospective, randomized, multicenter Food and Drug Administration investigational device exemption study of the ProDisc-L total disc replacement versus circumferential fusion for the treatment of 1-level degenerative disc disease. Spine (Phila Pa 1976) 2007;32:1155–62. doi: 10.1097/BRS.0b013e318054e377. [DOI] [PubMed] [Google Scholar]
- 13.Pimenta L, Schaffa TD. Surgical technique: eXtreme lateral interbody fusion. In: Goodrich JA, Volcan IJ, editors. eXtreme Lateral Interbody Fusion (XLIF) St Louis: Quality Medical Publishing; 2008. pp. 87–104. [Google Scholar]
- 14.Huang RC, Tropiano P, Marnay T, Girardi FP, Lim MR, Cammisa FP. Range of motion and adjacent level degeneration after lumbar total disc replacement. Spine J. 2006;6:242–7. doi: 10.1016/j.spinee.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 15.Lemaire JP, Carrier H, Ali EHS, Skalli W, Lavasate F, Lavaste F. Clinical and radiological outcomes with the Charité artificial disc: a 10-year minimum follow-up. J Spinal Disord Tech. 2005;18:353–9. doi: 10.1097/01.bsd.0000172361.07479.6b. [DOI] [PubMed] [Google Scholar]
- 16.Denozière G, Ku DN. Biomechanical comparison between fusion of two vertebrae and implantation of an artificial intervertebral disc. J Biomech. 2006;39:766–75. doi: 10.1016/j.jbiomech.2004.07.039. [DOI] [PubMed] [Google Scholar]
- 17.Marshman LAG, Friesem T, Rampersaud YR, Le Huec JC, Krishna M. Subsidence and malplacement with the Oblique Maverick Lumbar Disc Arthroplasty: technical note. Spine J. 2008;8:650–5. doi: 10.1016/j.spinee.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 18.Grant JP, Oxland TR, Dvorak MF. Mapping the structural properties of the lumbosacral vertebral endplates. Spine (Phila Pa 1976) 2001;26:889–96. doi: 10.1097/00007632-200104150-00012. [DOI] [PubMed] [Google Scholar]
- 19.Hurley MV, Newham DJ. The influence of arthrogenous muscle inhibition on quadriceps rehabilitation of patients with early, unilateral osteoarthritic knees. Br J Rheumatol. 1993;32:127–31. doi: 10.1093/rheumatology/32.2.127. [DOI] [PubMed] [Google Scholar]
- 20.Oliveira L, Marchi L, Coutinho E, Pimenta L. Biomechanics of disc arthroplasty: what can be done to improve results: present and future perspectives. The Spine Journal. 2010;10:S132. [Google Scholar]
- 21.Zeegers WS, Bohnen LMU, Laaper M, Verhaegen MJ. Artificial disc replacement with the modular type SB Charité III: 2-year results in 50 prospectively studied patients. Eur Spine J. 1999;8:210–17. doi: 10.1007/s005860050160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Geisler FH, Blumenthal SL, Guyer RD, McAfee PC, Regan JJ, Johnson JP, Mullin B. Neurological complications of lumbar artificial disc replacement and comparison of clinical results with those related to lumbar arthrodesis in the literature: results of a multicenter, prospective, randomized investigational device exemption study of Charité intervertebral disc. Invited submission from the Joint Section Meeting on Disorders of the Spine and Peripheral Nerves, March 2004. J Neurosurg Spine. 2004;1:143–54. doi: 10.3171/spi.2004.1.2.0143. [DOI] [PubMed] [Google Scholar]
- 23.Pimenta L, Díaz RC, Guerrero LG. Charité lumbar artificial disc retrieval: use of a lateral minimally invasive technique. Technical note. J Neurosurg Spine. 2006;5:556–61. doi: 10.3171/spi.2006.5.6.556. [DOI] [PubMed] [Google Scholar]
- 24.Park SJ, Kang KJ, Shin SK, Chung SS, Lee CS. Heterotopic ossification following lumbar total disc replacement. Int Orthop. 2011;35:1197–201. doi: 10.1007/s00264-010-1095-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rundell SA, Auerbach JD, Balderston RA, Kurtz SM. Total disc replacement positioning affects facet contact forces and vertebral body strains. Spine (Phila Pa 1976) 2008;33:2510–7. doi: 10.1097/BRS.0b013e318186b258. [DOI] [PubMed] [Google Scholar]
- 26.White A, Panjabi M. Clinical Biomechanics of the Spine. 2nd ed. Philadelphia: JB Lippincott; 2001. [Google Scholar]