Abstract
Background
The treatment of painful osteoporotic vertebral compression fractures with transpedicular cement augmentation has grown significantly over the last 20 years. There is still uncertainty about long-term and midterm effects of polymethyl methacrylate in trabecular bone. Preservation of the trabecular structures, as well as interdigitation of the cement with the surrounding bone, therefore has been gaining increasing attention. Interdigitation of cement is likely relevant for biological healing and the biomechanical augmentation process. In this study a cutting and grinding technique was used to evaluate the interdigitation for 4 augmentation techniques.
Methods
By use of a standardized protocol, wedge fractures were created in vertebrae taken from a fresh-frozen spine. Thereafter the vertebrae were assigned to 1 of 4 similar groups with regard to the vertebral size and force required to produce the fracture. The 4 groups were randomized to the following augmentation techniques: balloon kyphoplasty, radiofrequency (RF) kyphoplasty, shield kyphoplasty, and vertebral stenting. Histologic analysis was designed to examine the bone structure and interdigitation after the augmentation.
Results
For the void-creating procedures, the distance between bone and cement was 341.4 ± 173.7 µm and 413.6 ± 167.6 µm for vertebral stenting and balloon kyphoplasty, respectively. Specifically, the trabecular bone was condensed around the cement, forming a shield of condensed bone. The procedures without a balloon resulted in shorter distances of 151.2 ± 111.4 µm and 228.1 ± 183.6 µm for RF and shield kyphoplasty, respectively. The difference among the groups was highly significant (P < .0001). The percentage of interdigitation was higher for the procedures that did not use a balloon: 16.7% ± 9.7% for balloon kyphoplasty, 20.5% ± 12.9% for vertebral stenting, 66.45% ± 12.35% for RF kyphoplasty, and 48.61% ± 20.56% for shield kyphoplasty. The difference among the groups was highly significant (P < .00001).
Conclusions
Cavity-creating procedures reduce the cement interdigitation significantly and may accordingly reduce the effectiveness of the augmentation procedures.
Keywords: Vertebral compression fractures, Vertebroplasty, Kyphoplasty, Interdigitation, Bone-cement interface
Vertebroplasty and kyphoplasty, the percutaneous injection of various bone cements into affected vertebral bodies, have been used in painful osteoporotic vertebral fractures. The technique of percutaneous injection was first described by Galibert et al.1 Since the introduction of the balloon kyphoplasty2 in which a void in the vertebral body is created using an inflatable balloon before cement augmentation, treatment numbers of both procedures have been significantly rising.
According to the criteria of evidence-based medicine, there is only 1 level Ib evidence study3 that shows the benefits of balloon kyphoplasty compared with conservative treatment especially in the early course after osteoporotic fractures. However, the study results are limited to clinical benefits, such as pain, and not to kyphotic correction. The level Ib studies comparing vertebroplasty and conservative treatment show inconsistent results and have been discussed in detail elsewhere.4, 5 In addition, in several systematic reviews it was possible to observe the efficacy with regard to significant pain reduction in 87% of the patients undergoing vertebroplasty and in 92% of the patients undergoing kyphoplasty.2 Overall, the complication rate of both procedures is considered low.6–8
There are different types of cements that are used for kyphoplasty and vertebroplasty. Polymethyl methacrylate (PMMA) and calcium phosphate cements are the main groups. The cements are premixed with all kinds of radiopaque agents (eg, barium, tungsten, and iodine) or “osteoinductive” materials (eg, hydroxyl apatite). The calcium phosphate cements are not as mechanically stable as PMMA,9 and there is a lack of long-term observations, both of which are considered disadvantages. For years, PMMA has been successfully used in the fields of orthopedics and traumatology.
Besides cement composition, viscosity has been claimed to be important.10, 11 In the early days of vertebroplasty, low-viscosity cements were used, leading to increased leakage rates. A higher viscosity of the cement has been recognized to reduce cement leakage. The main rationale is that the cement sticks together and is not flushed into draining vessels. The use of high-viscosity cement makes higher pressures for injection necessary. Almost all the pressure is used to neutralize the resistance in the working cannulae.12
There is still uncertainty about midterm and long-term effects of PMMA in the trabecular bone and about how the distribution of cements could influence the outcome of the patients. The pain reduction and stabilization of the broken vertebra, which are the primary goals of the treatment, appear independent of the volume applied to the vertebral body.13 Ideally, the cement should provide a high primary stability. The interdigitation between the cement and trabecular bone might have an influence on the stability. A subsidence of the treated vertebra was observed after both balloon kyphoplasty and percutaneous vertebroplasty.
To allow better for biological healing, unfractured trabecular bone should remain intact as much as possible. To heal the fractured bone, it is important to ensure the supply of nutrients to the tissue. This supply to the trabecular parts of the bone is strongly affected by the destruction of the anatomic structures. The lack of nutrients could lead to prolonged fracture healing or even osteonecrosis.
Accordingly, the purpose of this study was to examine the cement interdigitation into the trabecular bone in a fractured vertebral model and to evaluate 4 existing augmentation techniques with respect to the cement interdigitation and distributions.
Methods
Specimens
To analyze the characteristics of different distribution patterns, it was necessary to create similar conditions for all devices. For this reason, all devices were investigated in vertebral bodies of the same fresh-frozen human cadaveric spine. One intact fresh human cadaveric spine (T3-L5) was used. The donor was a man aged 91 years. The specimen was stored at −20°C. Before surgery, a computed tomography scan of the spine was performed to identify pathologies, especially pre-existent vertebral fractures or deformities. Osteoporosis was defined according to the World Health Organization criteria: bone mineral density of more than 2.5 SDs below the mean of a young healthy reference population of the same gender (T score). Bone density measurement showed a T score of −5.31, representing substantial osteoporosis. The spine was dissected into single vertebral bodies, and the surrounding soft tissue, laminae, and spinal processes were removed. Altogether, 13 undamaged vertebral bodies were prepared (third thoracic to third lumbar vertebral body). Vertebral heights were measured at the anterior and posterior walls of the vertebral bodies. The caudal and cranial endplates were embedded in a cold-curing resin for surface testing and impressions (Technovit 3040; Heraeus Kulzer, Wehrheim, Germany).
Fracture generation and experimental groups
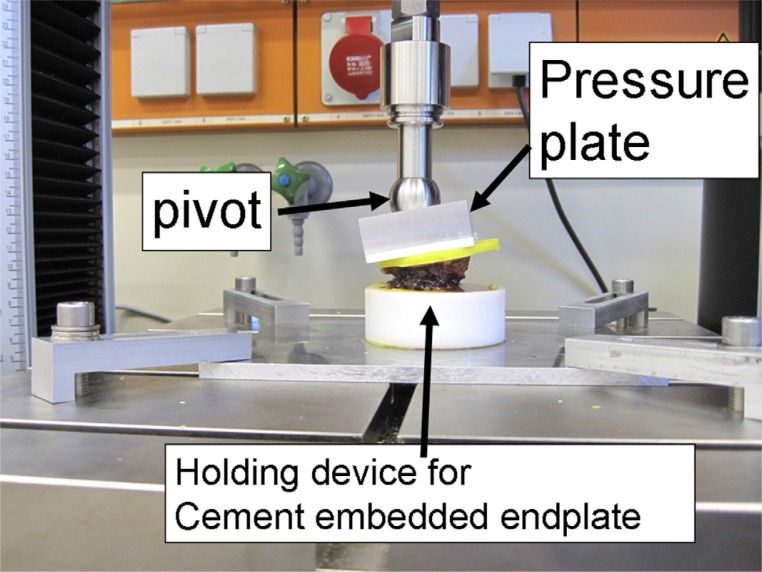
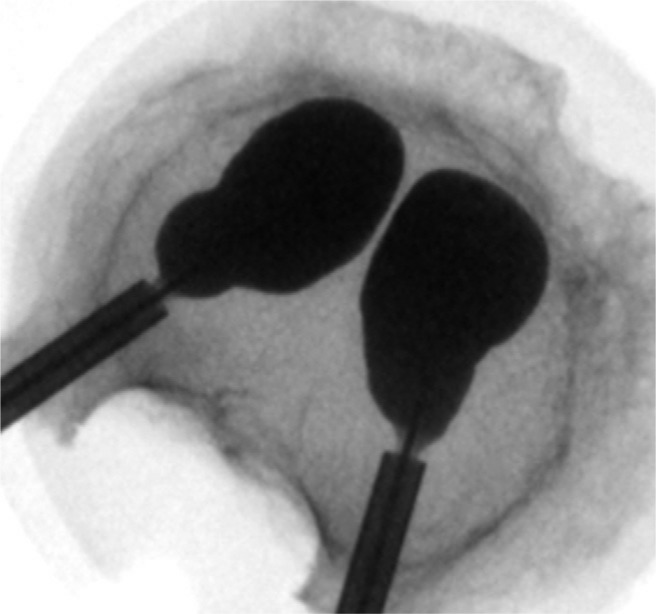
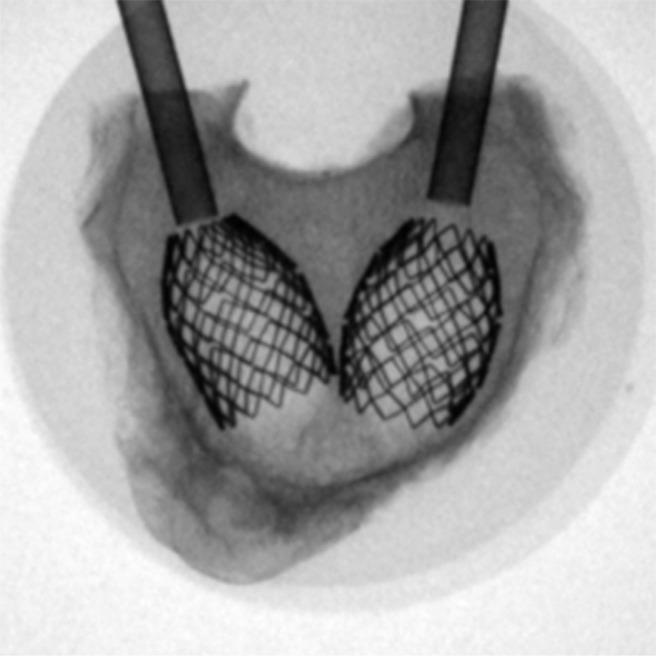
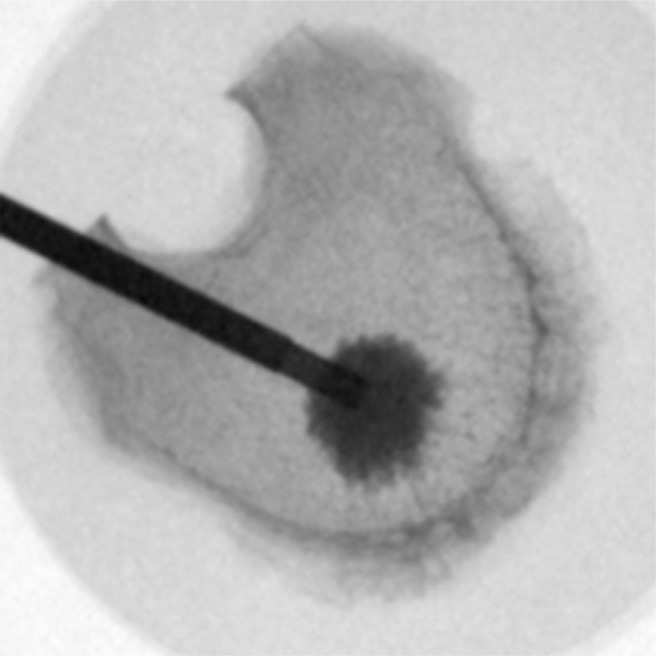
Vertebral wedge compression fractures were created by a material testing machine (Universal Testing Machine 5566; Instron, Norwood, Massachusetts). The load was transferred by a pivot-mounted pressure plate on the superior vertebral endplate. To create wedge compression of the anterior wall of the vertebral body, the main vector of the axial force was centered on the transition from the middle to the anterior third of the vertebral body (Fig. 1). The axial load was continuously increased (1-mm/min load application velocity) until the height of the anterior edge of the vertebral body was reduced to 50% of the initial measured values. The load was maintained for 15 minutes. After creation of the wedge fractures, the vertebral bodies were assigned into 1 of 4 similar groups concerning size and force needed to produce the fracture. The 4 groups were randomized to different cementing techniques. The augmentation techniques used were balloon kyphoplasty (Kyphon; Medtronic, Memphis, Tennessee) (Fig. 2), vertebral stenting (VBS; DePuy Synthes, West Chester, Pennsylvania) (Fig. 3), radiofrequency (RF) kyphoplasty (StabiliT; DFINE, San Jose, California) (Fig. 4), and shield kyphoplasty (Soteira, Natick, Massachusetts) (Fig. 5).
Fig. 1.
Fracture generation. The endplates are embedded in Technovit and placed on a holding device. The length of the sagittal midline is divided into thirds. The center of the pivot is aligned at the transition between the middle and anterior thirds to create vertebral wedge compression fractures (Orthopaedic Trauma Association type A 1.2).
Fig. 2.
Top view of vertebral body with cavity-creating balloons in place (Kyphon).
Fig. 3.
Top view of vertebral stents in place. The stents are expanded using a balloon catheter that is removed afterward. The next step would be filling the void with cement (VBS).
Fig. 4.
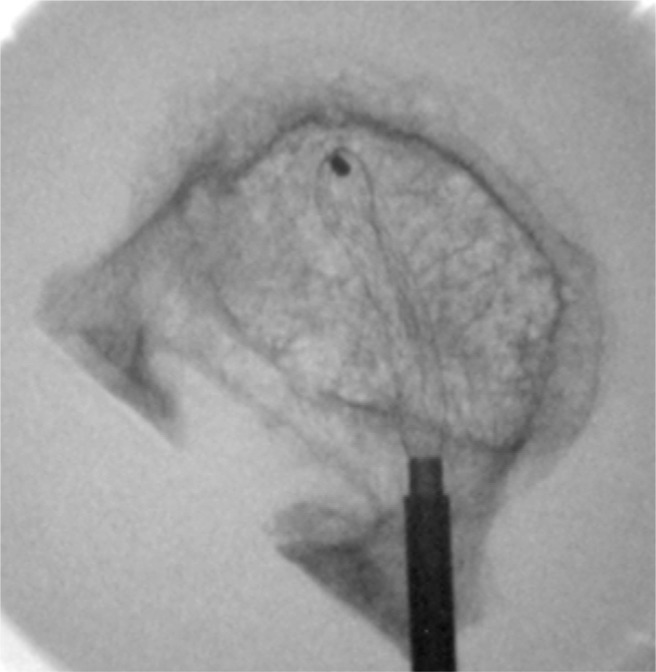
Top view of RF kyphoplasty. A unipedicular approach is used. High-viscosity cement (StabiliT) is applied after a small cavity has been created.
Fig. 5.
Top view of shield kyphoplasty stent in place. After cavity creation, this covered stent is placed and subsequently filled with cement.
Techniques
Balloon kyphoplasty
In balloon kyphoplasty 2 guidewires are placed through both pedicles by use of Jamshidi needles. After insertion of 2 working cannulae, 2 inflatable bone tamps are advanced into the collapsed vertebral body. They are inflated, creating 2 cavities, squeezing the surrounding trabeculae to the periphery. The bone tamps are then deflated and withdrawn. The cavity is filled with KyphX High Viscosity Radiopaque Bone Cement (Medtronic).
Vertebral body stenting
The VBS system consists of a balloon-expandable metal stent that is mounted on a balloon catheter. Access and application are the same as in balloon kyphoplasty. After withdrawal of the balloon catheters, the stents stay in place. The cavities are filled with Vertecem Radiopaque Vertebral Fixation Cement after use of a viscosimeter to ensure ideal viscosity for injection of the cement.
RF kyphoplasty
The access for the RF kyphoplasty system to the vertebral body is through 1 pedicle. After insertion of a guidewire through a Jamshidi needle, a working cannula is placed. After reaming and void creation in the midline and anterior third of the vertebral body, an injection cannula is placed. To achieve ultrahigh viscosity of the cement, it is heated by RF and is injected by a hydraulic device.
Shield kyphoplasty
The access for the shield kyphoplasty system to the vertebral body is again through 1 pedicle. After insertion of a guidewire through a Jamshidi needle, a working cannula is placed. Through this working cannula, a curved drill is inserted in the anterior third of the vertebral body. On the way back out, a cavity is reamed by a curette. The void created is filled by a covered self-expanding stent that only has openings to the anterior surfaces. After curing of the cement, the stent is filled. Once the stent is completely filled, additional cement can only extrude through the anterior perforations, directing the cement away from the dorsal parts of the vertebral body.
Histologic and data analysis
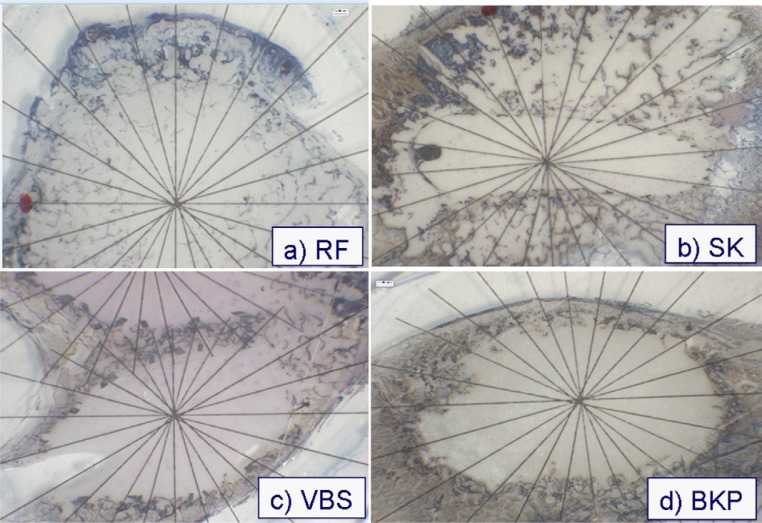
All procedures were performed by the same 2 surgeons using an image intensifier. The procedures were monitored by fluoroscopic control in 3 planes (anterior-posterior, lateral, and craniocaudal) by turning the vertebral bodies under the image intensifier. To attain a relevant result, clinical judgment was used to proceed or stop the cement injection. After the procedures, all vertebral bodies were fixed in 10% formalin and cut in 2-mm slices parallel to the caudal endplates with a diamond band saw (Exakt Medical Instruments, Inc., Oklahoma City, Oklahoma). Dehydration and degasification were performed in a precooled desiccator. The probes were embedded in Technovit 7200 (Heraeus Kulzer) by use of blue light polymerization. The polymerized tissue blocks were removed from the embedding mold and trimmed to the needed size with a diamond band saw. The blocked surface of the embedded tissue probes was ground with a micro-grinding machine according to the cutting and grinding technique described by Donath and Breuner.14 After the probes were ground to an appropriate thickness, staining was performed (Figs. 6–8). A protocol for histologic analysis was performed. From the center of the cement, an actinomorphic grid was placed on the specimens (24 radii, every 15°) (Fig. 9).
Fig. 6.
Top view of different cement augmentation techniques. The center of the actinomorphic grid is placed in the center of the cement. (A) RF kyphoplasty (RF). (B) Shield kyphoplasty (SK). (C) Vertebral stenting (VBS). (D) Balloon kyphoplasty (BKP).
Fig. 8.
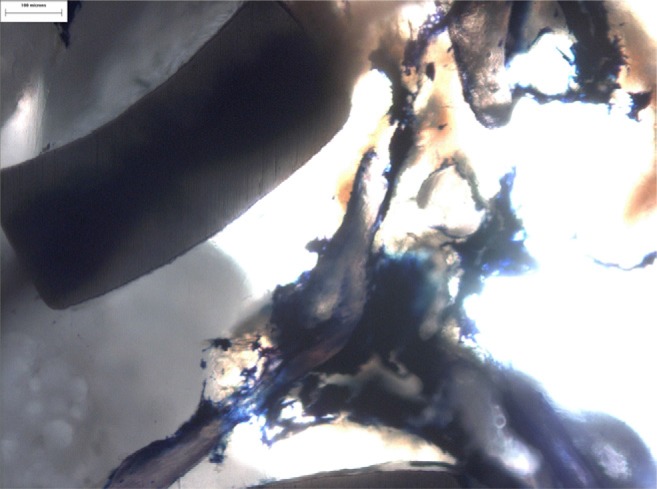
Detailed top view after Giemsa staining (Vertebral Body Stenting [VBS]). The stent, cement, and compressed cancellous bony structures are clearly visible.
Fig. 9.
Top view of specimen after cement augmentation (StabiliT RF kyphoplasty). From the center of the cement, an actinomorphic grid was placed on the specimens (24 radii, every 15°).
Fig. 7.
Detailed top view after unspecific staining of cancellous bone (StabiliT RF kyphoplasty). The preserved trabecular structures should be noted.
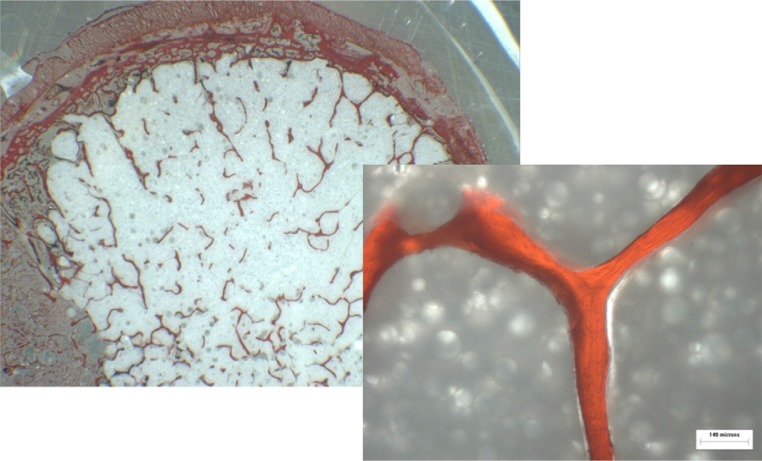
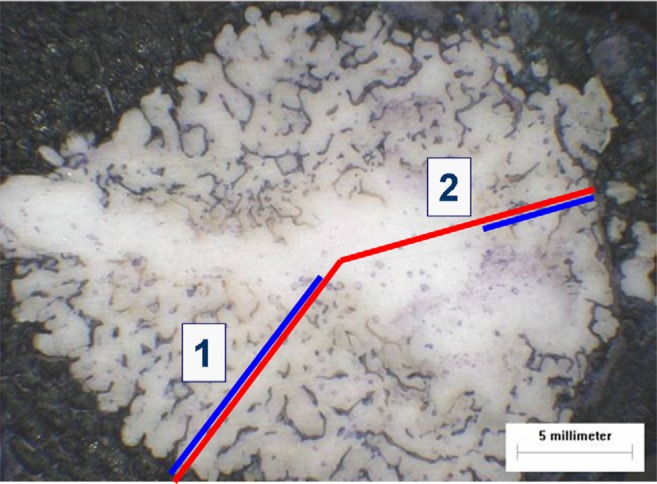
To objectively measure the interdigitation, the relation between the length of the radius (center to border of cement) and preserved cancellous bone along the radius was measured for all specimens (Fig. 10). In addition, the distance between the cement and trabecular bone (bone-cement interface) at the exterior radius was ascertained with a microscope and calculated by use of Image-Pro Plus (Media Cybernetics, Bethesda, Maryland).
Fig. 10.
To objectify interdigitation, the relation between the length of the radius (center to cement-bone interface [red line]) and preserved trabecular bone along the radius (blue line) was measured for all specimens (1 = 90% and 2 = 45%).
To evaluate the density and structure of the trabecular bone surrounding the cement, bone density and integrity of the trabecular bone were measured at +1 mm and +3 mm exterior as well as at −1 mm and −3 mm inside the bone-cement interface along the radii described in detail earlier (Fig. 5). The density was expressed as the percentage of bony structure in 1 visual field of the microscope at 63-fold magnification.
To objectify and compare the integrity of the bony structures, a numeric score was established. The score ranges from 1 to 5 (1, intact bony structures without any destruction; 2, <50% destruction; 3, >50% destruction; 4, complete destruction of bony structures; and 5, no bony structures visible) (Table 1).
Table 1.
Numeric scale to objectify integrity of trabecular bone
| Value | Morphologic characterization of integrity of bony structures |
|---|---|
| 1 | Intact bony structures |
| 2 | <50% destruction |
| 3 | >50% destruction |
| 4 | Complete destruction of bony structures |
| 5 | No bony structures visible |
All data were expressed as means and standard deviations. For all group analyses, we used the D'Agostino and Pearson omnibus normality test, followed by the Kruskal-Wallis test.
Results
In all vertebral bodies, vertebral wedge fractures could be established. According to the Orthopaedic Trauma Association classification, they were all type A 1.2 fractures.15 The mean force needed was 2,164.25 ± 514.35 N. All surgical procedures could be performed without technical problems. All the equipment worked without technical difficulties. It was possible to adapt the cutting and grinding technique described by Donath and Breuner14 to vertebral bodies that have been treated by cement augmentation. It was possible to cut the specimens with cement and implants in situ. No decalcification or removal of the implants became necessary before staining of the probes.
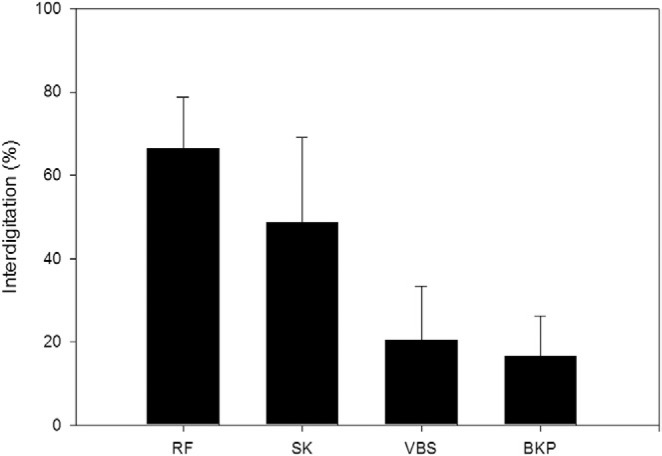
The percentage of interdigitation was higher for the procedures that did not create a void before augmentation: 66.5% ± 12.4% for RF kyphoplasty and 48.6% ± 20.6% for shield kyphoplasty. In comparison, the percentages for those that create a void were 16.7% ± 9.7% for balloon kyphoplasty and 20.6% ± 12.9% for vertebral stenting. The difference among the groups was highly significant (P < .0001) (Fig. 11).
Fig. 11.
Cement interdigitation of different procedures (mean values and standard deviations). (BKP, balloon kyphoplasty; RF, RF kyphoplasty; SK, shield kyphoplasty; VBS, vertebral stenting.)
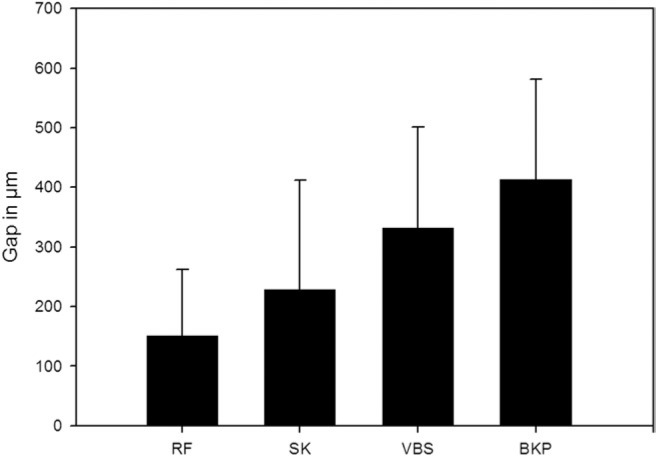
In the procedures that use a balloon to create a void in the vertebral body, the mean distance between bone and cement was 341.4 ± 173.7 µm for vertebral stenting and 413.6 ± 167.6 µm for balloon kyphoplasty. The procedures without a balloon resulted in distances of 151.2 ± 111.4 µm for RF kyphoplasty and 228.1 ± 183.6 µm for shield kyphoplasty. The difference among the groups was highly significant (P < .0001) (Fig. 12).
Fig. 12.
Bone-cement interface: gap between outer surface of cement and surrounding cancellous bone in micrometers (mean values and standard deviations). (BKP, balloon kyphoplasty; RF, RF kyphoplasty; SK, shield kyphoplasty; VBS, vertebral stenting.)
Bone density
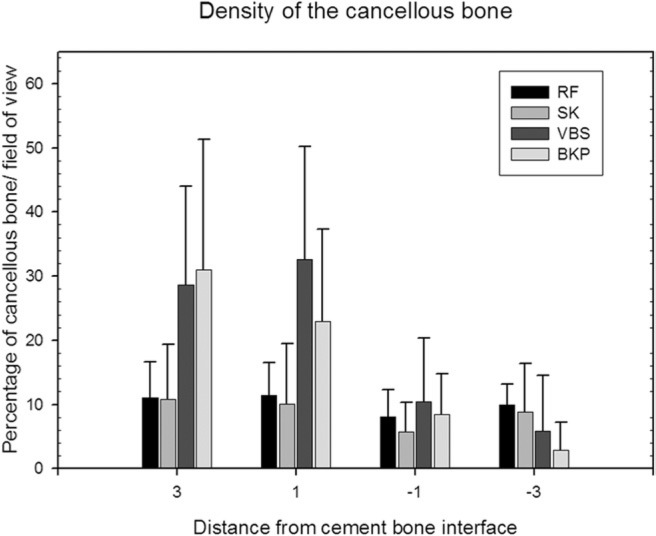
The bone densities of the trabecular bone at 1 and 3 mm exterior and −1 and −3 mm interior to the bone-cement interface are listed in detail in Table 2 and Fig. 9. The values represent the percentage of trabecular bone in 1 visual field of the microscope at 63-fold magnification.
Table 2.
Density of trabecular bone at defined distances from cement-bone interface
| 3 mm from cement-bone interface | 1 mm from cement-bone interface | −1 mm from cement-bone interface | −3 mm from cement-bone interface | |
|---|---|---|---|---|
| RF-Kyphoplasty (%) | 11.0 (5.6) | 11.4 (5.2) | 8.1 (4.2) | 9.9 (3.2) |
| Shield-Kyphoplasty (%) | 10.8 (8.6) | 10.1 (9.4) | 5.7 (4.6) | 8.8 (7.6) |
| VBS (%) | 28.6 (15.4) | 32.6 (17.5) | 10.5 (9.8) | 5.9 (8.6) |
| Balloon-Kyphoplasty (%) | 31.0 (20.4) | 23.0 (14.4) | 8.5 (6.3) | 2.9 (4.4) |
| Kruskal-Wallis | P < .0001 | P < .0001 | P < .0006 | P < .0001 |
Abbreviation: VBS, vertebral stenting.
NOTE. Data are presented as mean (standard deviation).
The density of the bone at +3 mm outside of the bonecement interface is significantly higher with the void-creating procedures: 28.6% ± 15.4% for vertebral stenting and 31.0% ± 20.4% for balloon kyphoplasty compared with 11.0% ± 5.6% for RF kyphoplasty and 10.8% ± 8.6% for shield kyphoplasty (P < .0001).
The densities inside the bone-cement interface at −3 mm are significantly lower for the void-creating procedures: 5.9% ± 8.6% for vertebral stenting and 2.9% ± 4.4% for balloon kyphoplasty compared with 9.9% ± 3.2% for RF kyphoplasty and 8.8% ± 7.6% for shield kyphoplasty (P < .0001) (Fig. 13). It is noteworthy that no trabecular structures are preserved in the void that is created by the balloons. The densities inside and outside the bone-cement interface differ to a greater extent for the procedures that create a void.
Fig. 13.
Density of cancellous bone at defined distances from cement/bone transition (mean values and standard deviations). Table 2 shows detailed values. (BKP, balloon kyphoplasty; RF, RF kyphoplasty; SK, shield kyphoplasty; VBS, vertebral stenting.)
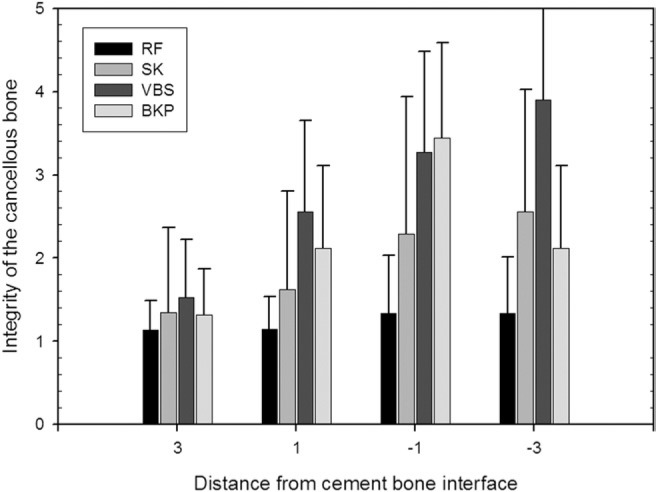
Integrity of bony structures
The integrity of the bony structures was scored according to the scale listed in Table 1 at +1 and +3 mm exterior and −1 and −3 mm interior to the bone-cement interface. The integrity at −3 mm was significantly higher for the procedures that did not create a void: 1.33 ± 0.68 for RF kyphoplasty and 2.56 ± 1.48 for shield kyphoplasty compared with 3.90 ± 1.13 for vertebral stenting and 4.32 ± 0.98 for balloon kyphoplasty (P < .0001). The integrity at −1 mm was also significantly higher for the procedures that did not create a void: 1.33 ± 0.70 for RF kyphoplasty and 2.29 ± 1.65 for shield kyphoplasty compared with 3.27 ± 1.22 for vertebral stenting and 3.44 ± 1.15 for balloon kyphoplasty (P < .0001).
Outside the bone-cement interface, the differences in the mean values were smaller but significant. The integrity at +1 mm was also significantly higher for the procedures that did not create a void: 1.14 ± 0.40 for RF kyphoplasty and 1.62 ± 1.19 for shield kyphoplasty compared with 2.56 ± 1.09 for vertebral stenting and 2.16 ± 1.00 for balloon kyphoplasty (P < .0001). At 3 mm outside the bone-cement interface, the difference was still significant, at 1.14 ± 0.35 for RF kyphoplasty and 1.35 ± 1.02 for shield kyphoplasty versus 1.53 ± 0.69 for vertebral stenting and 1.32 ± 0.55 for balloon kyphoplasty (P < .0005). The results are described in detail in Table 3 and Fig. 14.
Table 3.
Integrity of trabecular bone at defined distances from cement-bone interface
| 3 mm from cement-bone interface | 1 mm from cement-bone interface | −1 mm from cement-bone interface | −3 mm from cement-bone interface | |
|---|---|---|---|---|
| RF-Kyphoplasty | 1.14 (0.35) | 1.14 (0.40) | 1.33 (0.70) | 1.33 (0.68) |
| Shield-Kyphoplasty | 1.35 (1.02) | 1.62 (1.19) | 2.29 (1.65) | 2.56 (1.48) |
| VBS | 1.53 (0.69) | 2.56 (1.09) | 3.27 (1.22) | 3.90 (1.13) |
| Balloon-Kyphoplasty | 1.32 (0.55) | 2.16 (1.00) | 3.44 (1.15) | 4.32 (0.98) |
| Kruskal-Wallis | P < .0005 | P < .0001 | P < .0001 | P < .0001 |
Abbreviation: VBS, vertebral stenting.
NOTE. Data are presented as mean (standard deviation). Integrity was scored according to the scale in Table 1.
Fig. 14.
Integrity of cancellous bone at defined distances from cement/bone transition (mean values and standard deviations). Table 3 shows detailed values. (BKP, balloon kyphoplasty; RF, RF kyphoplasty; SK, shield kyphoplasty; VBS, vertebral stenting.)
The values of the RF kyphoplasty showed the smallest differences among the 4 subgroups, but the difference was significant (P < .0003). The histologic morphologic analysis showed that the anatomic structures were respected and almost no damage to the trabecular bone occurred. The destruction of the trabecular bone increased from shield kyphoplasty to balloon kyphoplasty and vertebral stenting (Fig. 10).
Discussion
This article has both a methodologic aim and a scientific aim. Methodologically, the objective was to develop and transfer the cutting and grinding technique described by Donath and Breuner14 to cement-augmented vertebrae. Scientifically, the focus was on examining the interdigitation and distribution patterns of 4 techniques commonly used in vertebral augmentation procedures.
To our knowledge, this is the first comparative study that examines cement interdigitation on a histologic basis. The cutting and grinding technique described by Donath and Breuner14 was used to evaluate the cement interdigitation and distribution patterns. An important advantage of this method is that no decalcification or removal of the implants was necessary before histologic staining. By this method, a detailed analysis of the interaction of cement, implants, and trabecular bone is possible. An additional advantage of the cutting and grinding technique pertains to the possibility of histologic and immunohistochemical staining. In future studies, the cement and the interdigitation and healing processes can be evaluated in living tissues (eg, animal studies). The first aim of this study was to establish this method, and we found that it was possible to evaluate all vertebral bodies without any technical pitfalls.
A number of complications related to the use of PMMA in bone augmentation procedures have been reported in the literature. Progressive collapses are experienced especially in cases where PMMA conglomerated without contiguous bone interdigitation. 16 To avoid this complication, we advise clinicians to sufficiently inject cement that must infiltrate the contiguous bone to create strong support and anchorage.
The gaps in void-assisted interventions potentially lead to immediate mechanical instability. This mechanical instability could immediately lead to persistent pain. The long-term effects could be nonunion of the fracture and progressive collapse of the treated vertebrae. Therefore it becomes even more important in these procedures to sufficiently fill the voids with cement. Furthermore, it must be taken into account that PMMA is shrinking over time because of water loss, which enhances the instability effect.
An additional negative impact of bone destructive techniques that use a balloon (balloon kyphoplasty and VBS) represents the stress-shielding effect, which has been described in the literature.17 Good interdigitation and distribution of the cement within the vertebrae like in vertebroplasty procedures seem not to have this effect.18 Combining this previous knowledge with our results, which (like the effect of stress shielding) point toward persisting instability, bone resorption, and cement dislocation, whether one should use balloon kyphoplasty–like procedures seems debatable.
The long-discussed and cited positive effects of balloon kyphoplasty, such as being a safer procedure, are being challenged by new cements and cementing techniques, leaving the potential of height restoration as the only indication for balloon kyphoplasty and similar procedures. However, because this effect has not been proven clinically to be related to the outcome, the negative side effects discussed earlier overshadow the theoretic advantage of height restoration.
One point of criticism for our study is that we did not use an eggshell technique in balloon kyphoplasty, which may have a positive effect on the interdigitation. This potential beneficial effect is not possible with vertebral body stenting.
Interdigitation and filling patterns, as well as cement leakage, are strongly dependent on the viscosity of the cement. It has been shown in various studies that the use of high-viscosity cement results in lower leakage rates.10, 19, 20 In addition, high-viscosity cement seems to stabilize cement flow.10
The clinical significance of our study cannot be ascertained easily. Balloon kyphoplasty was designed to reduce the risk of cement leakage during vertebroplasty. By creating a void, cement extravasations were significantly reduced compared with percutaneous vertebroplasty.6 To create a void with the balloon, the trabecular structures in the vertebral body have to be displaced and damaged. By inflating the balloon, unfractured spongious bone is damaged for procedural security reasons.
New cementing techniques focus on the preservation of the trabecular bone, assuming that intact trabecula can help in biological healing of the fractured bone around the cement. The importance of good interdigitation between the cement and the trabecular bone has been shown in various studies dealing with cemented total joint replacement.21–23 It was shown that a reduced bone-cement interface leads to a higher rate of implant loosening. The biological and physiological reasons remain unclear. It seems that the knowledge gathered in total joint replacement can be transferred to cementing of vertebral fractures. Additional studies, especially animal studies, could show whether the observed differences between the techniques have an influence on the trabecular healing of the bone. These questions could not be answered by our in vitro study.
Conclusion
To summarize, we designed a study using cadaveric vertebra to examine the cement interdigitation between the cement mass and trabecular bone. The protocols for creating wedge fractures, as well as the cutting-and-grinding technique and microscopic evaluation, led to reproducible results and effects.
Void-assisted interventions led to compaction of the bone around the cement mass, as well as a gap of around 300 µm. The interdigitation of cement and trabecular bone seems better for the procedures that do not create a void before augmentation.
The clinical implications include potential primary instability of the cement implant, and this may relate to the clinical outcome and the biological healing process. Our results may point toward a safer and more efficient stabilization with vertebroplasty techniques over the bone-destroying techniques with balloons.
References
- 1.Galibert P, Deramond H, Rosat P, Le Gars D. Preliminary note on the treatment of vertebral angioma by percutaneous acrylic vertebroplasty [in French] Neurochirurgie. 1987;33:166–8. [PubMed] [Google Scholar]
- 2.Wong W, Reiley MA, Garfin S. Vertebroplasty/kyphoplasty. J Womens Imaging. 2000;2:117–24. [Google Scholar]
- 3.Wardlaw D, Cummings SR, Van Meirhaeghe J, et al. Efficacy and safety of balloon kyphoplasty compared with non-surgical care for vertebral compression fracture (FREE): A randomised controlled trial. Lancet. 2009;373:1016–24. doi: 10.1016/S0140-6736(09)60010-6. [DOI] [PubMed] [Google Scholar]
- 4.Buchbinder R, Osborne RH, Ebeling PR, et al. A randomized trial of vertebroplasty for painful osteoporotic vertebral fractures. N Engl J Med. 2009;361:557–68. doi: 10.1056/NEJMoa0900429. [DOI] [PubMed] [Google Scholar]
- 5.Kallmes DF, Comstock BA, Heagerty PJ, et al. A randomized trial of vertebroplasty for osteoporotic spinal fractures. N Engl J Med. 2009;361:569–79. doi: 10.1056/NEJMoa0900563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hulme PA, Krebs J, Ferguson SJ, Berlemann U. Vertebroplasty and kyphoplasty. A systematic review of 69 clinical studies. Spine (Phila Pa 1976) 2006;31:1983–2001. doi: 10.1097/01.brs.0000229254.89952.6b. [DOI] [PubMed] [Google Scholar]
- 7.Taylor RS, Fritzell P, Taylor RJ. Balloon kyphoplasty in the management of vertebral compression fractures: An updated systematic review and meta-analysis. Eur Spine J. 2007;16:1085–100. doi: 10.1007/s00586-007-0308-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taylor RS, Taylor RJ, Fritzell P. Balloon kyphoplasty and vertebroplasty for vertebral compression fractures. A comparative systematic review of efficacy and safety. Spine (Phila Pa 1976) 2006;31:2747–55. doi: 10.1097/01.brs.0000244639.71656.7d. [DOI] [PubMed] [Google Scholar]
- 9.Wilke HJ, Mehnert U, Claes LE, Bierschneider MM, Jaksche H, Boszczyk BM. Biomechanical evaluation of vertebroplasty and kyphoplasty with polymethyl methacrylate or calcium phosphate cement under cyclic loading. Spine (Phila Pa 1976) 2006;31:2934–41. doi: 10.1097/01.brs.0000248423.28511.44. [DOI] [PubMed] [Google Scholar]
- 10.Baroud G, Crookshank M, Bohner M. High-viscosity cement significantly enhances uniformity of cement filling in vertebroplasty. An experimental model and study on cement leakage. Spine (Phila Pa 1976) 2006;31:2562–8. doi: 10.1097/01.brs.0000240695.58651.62. [DOI] [PubMed] [Google Scholar]
- 11.Baroud G, Yahia FB. A finite element rheological model for polymethylmethacrylate flow: Analysis of the cement delivery in vertebroplasty. Proc Inst Mech Eng H. 2004;218:331–8. doi: 10.1243/0954411041932827. [DOI] [PubMed] [Google Scholar]
- 12.Baroud G, Bohner M, Heini P, Steffen T. Injection biomechanics of bone cements used in vertebroplasty. Biomed Mater Eng. 2004;14:487–504. [PubMed] [Google Scholar]
- 13.Kaufmann TJ, Trout AT, Kallmes DF. The effects of cement volume on clinical outcomes of percutaneous vertebroplasty. AJNR Am J Neuroradiol. 2006;27:1933–7. [PMC free article] [PubMed] [Google Scholar]
- 14.Donath K, Breuner G. A method for the study of undecalcified bones and teeth with attached soft tissues. The Säge-Schliff (sawing and grinding) technique. J Oral Pathol. 1982;11:318–26. doi: 10.1111/j.1600-0714.1982.tb00172.x. [DOI] [PubMed] [Google Scholar]
- 15.Marsh JL, Slongo TF, Agel J, et al. Fracture and dislocation classification compendium—2007. Orthopaedic Trauma Association classification, database and outcomes committee. J Orthop Trauma. 2007;21:S1–133. doi: 10.1097/00005131-200711101-00001. [DOI] [PubMed] [Google Scholar]
- 16.Shin DA, Kim KN, Shin HC, Kim SH, Yoon DH. Progressive collapse of PMMA-augmented vertebra: A report of three cases. Zentralbl Neurochir. 2008;69:43–6. doi: 10.1055/s-2007-992137. [DOI] [PubMed] [Google Scholar]
- 17.Dabirrahmani D, Becker S, Hogg M, Appleyard R, Baroud G, Gillies M. Mechanical variables affecting balloon kyphoplasty outcome—A finite element study. Comput Methods Biomech Biomed Engin. 2012;15:211–20. doi: 10.1080/10255842.2010.522183. [DOI] [PubMed] [Google Scholar]
- 18.Rohlmann A, Boustani HN, Bergmann G, Zander T. A probabilistic finite element analysis of the stresses in the augmented vertebral body after vertebroplasty. Eur Spine J. 2010;19:1585–95. doi: 10.1007/s00586-010-1386-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Georgy BA. Clinical experience with high-viscosity cements for percutaneous vertebral body augmentation: Occurrence, degree, and location of cement leakage compared with kyphoplasty. AJNR Am J Neuroradiol. 2010;31:504–8. doi: 10.3174/ajnr.A1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nieuwenhuijse MJ, Muijs SP, van Erkel AR, Dijkstra SP. A clinical comparative study on low versus medium viscosity polymethylmetacrylate bone cement in percutaneous vertebroplasty. Viscosity associated with cement leakage. Spine (Phila Pa 1976) 2010;35:E1037–44. doi: 10.1097/BRS.0b013e3181ddd262. [DOI] [PubMed] [Google Scholar]
- 21.Breer S, Krause M, Busse B, et al. Analysis of retrieved hip resurfacing arthroplasties reveals the interrelationship between interface hyperosteoidosis and demineralization of viable bone trabeculae. J Orthop Res. 2012;30:1155–61. doi: 10.1002/jor.22035. [DOI] [PubMed] [Google Scholar]
- 22.Krause M, Breer S, Hahn M, et al. Cementation and interface analysis of early failure cases after hip-resurfacing arthroplasty. Int Orthop. 2012;36:1333–40. doi: 10.1007/s00264-011-1464-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zustin J, Hahn M, Morlock MM, Rüther W, Amling M, Sauter G. Femoral component loosening after hip resurfacing arthroplasty. Skeletal Radiol. 2010;39:747–56. doi: 10.1007/s00256-009-0862-z. [DOI] [PubMed] [Google Scholar]