Abstract
The discovery that adipose tissue represents an interesting source of multipotent stem cells has led to many studies exploring the clinical potential of these cells in cell-based therapies. Recent advances in understanding the secretory capacity of adipose tissue and the role of adipokines in the development of obesity and associated disorders have added a new dimension to the study of adipose tissue biology in normal and diseased states. Subcutaneous adipose tissue forms the interface between the clinical application of regenerative medicine and the establishment of the pathological condition of obesity. These two facets of adipose tissue should be understood as potentially related phenomena. Because of the functional characteristics of adipose stem cells, these cells represent a fundamental tool for understanding how these two facets are interconnected and could be important for therapeutic applications. In fact, adipose tissue stem cells have multiple functions in obesity related to adipogenic, angiogenic and secretory capacities. In addition, we have also previously described a predominance of larger blood vessels and an adipogenic memory in the subcutaneous adipose tissue after massive weight loss subsequent to bariatric surgery (ex-obese patients). Understanding the reversibility of the behavior of adipose stem cells in obeses and in weight loss is relevant to both physiological studies and the potential use of these cells in regenerative medicine.
Keywords: Adipose stem cells, Subcutaneousadipose tissue, Obesity, Weight loss, Regenerative medicine
Core tip: In this mini-review, we summarize recent aspects regarding obese subcutaneous adipose tissue with a focus in adipogenic and secretory capacities of adipose stem cells. In particular, we discuss how the occurrence of obesity and weight loss could alter the properties of stem cells and the consequences of using adipose-derived stem cells in regenerative medicine. Our previous results reveal that, after massive weight loss subsequent to bariatric surgery, stem cells retain an adipogenic memory besides a predominance of larger blood vessels in subcutaneous adipose tissue. Inflammatory subcutaneous adipose tissue microenvironment found in obese patients and even the massive weight loss could alter adipose tissue-derived stem cells phenotype to a non-healthy state.
INTRODUCTION
The discovery that adipose tissue represents an interesting source of multipotent stem cells has led to many studies exploring their clinical potential in cell-based therapies. Recent advances in understanding the secretory capacity of adipose tissue and the role of adipokines in the development of obesity and associated disorders have added a new dimension to adipose tissue biology under normal and diseased states. In the present mini-review, we summarize different aspects of adipose tissue stem cells and the role of these cells in obese subcutaneous adipose tissue. In addition, we discuss how the occurrence of obesity and weight loss after bariatric surgery could alter the properties adipose-derived stem cells and the consequences of using these cells in regenerative medicine.
OBESE SUBCUTANEOUS ADIPOSE TISSUE
White adipose tissue is highly complex and is composed of various cell types that interact dynamically with each other through the secretion of adipokines, including hormones, cytokines and chemokines. This tissue is now recognized as being equivalent to an endocrine organ[1].White adipose tissue is mainly divided into two fat depots: internal or visceral and subcutaneous. In humans, a major contribution of visceral adipose tissue to the development of insulin resistance and to the cardio-metabolic complications in obesity was proposed[2]. The differences observed between visceral and subcutaneous adipose tissues are supported by studies showing that visceral and subcutaneous fat depots exhibit differential gene expression profiles[3]. A recent study has suggested a common embryonic origin from mesothelium and mesothelium-derived cells for up to six internal fat depots, but not for the subcutaneous fat depot[4].
Visceral and subcutaneous adipose tissues can significantly alter their mass according to the amount of triglycerides stored in adipocytes, however is more evident in subcutaneous fat tissues. Some authors assume that the visceral fat depot is associated with metabolic syndromes, while subcutaneous fat has a metabolic buffer function, which means that an increase in the subcutaneous fat depot in the presence of a positive caloric balance is protective[3,5]. However, during the development of obesity, the enormous increase in the subcutaneous fat mass results in a dysfunctional tissue. Tissue homeostasis is disrupted due to adipocyte hypertrophy, decreased adipogenesis and angiogenesis[6].
The cellular content of subcutaneous adipose tissue includes a major population of specialized cells, the adipocytes, and a stromal vascular fraction (SVF) composed of preadipocytes, pericytes or multipotent stem cells, vascular wall and endothelial cells, macrophages[7], lymphocytes[8], eosinophils[9], neutrophils[10], mast cells[11] and hematopoietic progenitor cells[12]. It was initially proposed that adipocyte hypertrophy is the major mechanism by which subcutaneous adipose tissue expands. However, adipocyte hyperplasia also contributes to tissue expansion through the activation of multipotent stem cells, leading to the generation of new cells to sustain the demand for fat storage[13,14]. Therefore, the capacity to dynamically expand or shrink throughout adult life is mediated by adipocytes, preadipocytes and multipotent cells with a regenerative capacity.
Lipid accumulation is not the only trigger for the development of obesity. One of the most important discoveries regarding the development of obesity was the description of the establishment of an inflammatory state characterized by an increase in the levels of local and systemic inflammatory cytokines and macrophage infiltration into subcutaneous adipose tissue[15-18]. Besides, endoplasmic reticulum dysfunction is being given as evidence of adipocyte stress[19], playing an upregulation role in inflammatory scenario of adipose tissue. The accumulated macrophages contribute to the production of inflammatory mediators, thus amplifying the inflammatory state of the adipose tissue and thereby representing an important factor in promoting insulin resistance[17,18]. The macrophage population within inflamed obese adipose tissue can switch phenotypes between inflammatory and non-inflammatory states[20]. Adipose tissues also secrete osteopontin, which also plays a role in the migration and differentiation of cells of the immune system[21-25].
It has been suggested that preadipocytes of mouse adipose tissue could be responsible for most of the secretion of MCP-1[22,24,25], which has been classically described regarding the role of this cytokine in monocyte mobilization from the bone marrow and the migration of monocytes through the peripheral circulation. Therefore, it was proposed that the inflammatory process that is established during the development of obesity could be initiated by preadipocytes that were affected by adipose tissue growth. In this case, both adipocytes and the preadipocytes would be responsible for the migration and, possibly, the activation of macrophages in adipose tissues.
The cellular events that lead to the establishment of this scenario have not been fully elucidated, and the mechanisms by which obesity is associated with other metabolic disorders are not well understood. Because multipotent stem cells are directly involved in subcutaneous adipose tissue homeostasis, it is reasonable to postulate a pleiotropic role for stem cells in the vicious cycle of inflammation. In fact, since 2000, the number of scientific articles in the adipose stem cell field within the context of obesity has been increasing (Figure 1). However, there is only a few analyzing subcutaneous adipose tissue samples after massive weight loss[26-30]; two of them with focus on multipotent stem cells[26,30].
Figure 1.
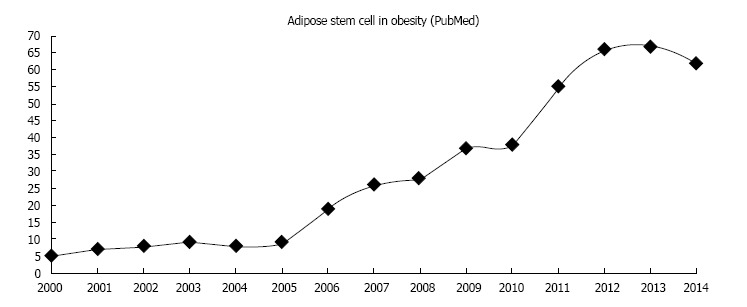
Annual number of publications referenced in the PubMed database between 2000 and 2014 with the sentence “adipose stem cell in obesity”.
HEALTHY AND NON-HEALTHY ADIPOSE STEM CELLS
The term mesenchymal stem cells (MSC) was first used for a multipotent cell population that dwells in adult bone marrow stroma. Based on the concept of bone marrow MSC, this population of perivascular cells has been described in a variety of other adult tissues[31-36], including adipose tissue[37]. MSC contribute to tissue homeostasis, repair and regeneration[38,39]. Data collected during the last 10 years suggest that MSC represent a specific adult tissue stem cell population, the plasticity of which is essential. It is not clear whether all the MSC populations, derived from different tissues throughout the body, represent a substantially similar cell category. Among the different sources of MSC already described, the adipose stem cells have attracted the attention of many scientists and physicians due to the frequently large amounts of subcutaneous adipose tissue that can be easily harvested using liposuction. In addition to being an important tool for tissue regeneration, due to their secretory capacity, adipose stem cells also represent possible regulators in the development of obesity. While many studies have reported on the participation of inflammatory and non-inflammatory macrophages, little is known about the events in obesity that involve resident stem cells.
The in vitro-amplified stem cell population derived from adipose tissue is now termed adipose tissue-derived stem cells (ASC)[40]. ASC share common features with MSC derived from other tissues, which are primarily based on the paracrine activity of these cells. ASC are similar to MSC derived from bone marrow, including the capacity to sustain hematopoiesis[12] and immunomodulatory activities, but most importantly, ASC display specific features, among which are higher angiogenic and adipogenic potentials[41]. Regarding the surface marker profile, ASC are distinguished by CD34 expression[42].
In fact, results from in vitro assays and animal models have supported a beneficial role for ASC in tissue regeneration and immunomodulation via paracrine activity[43]. However, ASC are responsive to inflammatory stimulus. Hoogduijn et al[44] showed in C57BL/6 mice that intravenously infused ASC home to the lung where they first induce an inflammatory response that in turn leads to immunomodulatory effects. Furthermore, after lipopolysaccharide stimulation ASC increases their inflammatory cytokines secretion[45] with clones exhibiting varying degrees of paracrine activity[46]. Stressful conditions on tissue homeostasis can lead to extreme cell behavior changes[47], and specifically in subcutaneous adipose tissue it has been reported that some diseases can alter ASC function. For example, autologous ASC therapy treatment in a mouse model of multiple sclerosis showed no positive results on the disease progression[48]. The angiogenic potential[49,50] and wound healing[51] capacity of ASC is altered by Diabetes.
There is a positive correlation between high body-mass index (BMI) and some types of malignant tumors[52]. MSC may favor tumor development through the release of soluble factors and by direct cell contact, promoting proliferation, survival, and drug resistance. It is postulated that the inflammatory tumor microenvironment could change the phenotype of infiltrating MSC to a pro-inflammatory state[53]. We could speculate that the tumor-derived MSC is in fact a non-healthy state, since they promote tumor progression.
In addition to producing new adipocytes and to participation in the growth of the vascular tree[41], ASC could contribute in other ways to the development of obesity. First, these cells could generate macrophages by direct differentiation or indirectly, by supporting their generation from resident hematopoietic progenitor cells[12]. Murine ASC have a phagocytic activity, both in vitro[54] and in vivo[55]. Human ASC also have this capacity[56]. Recently, a macrophage population that expresses CD34 and is plastic-adherent and multipotent, similar to ASC, was identified in human white adipose tissue[7]. Therefore, the idea that a significant proportion of the inflammatory macrophages present in the adipose tissue of obese patients may be generate in situ cannot be ignored[57]. Second, ASC could also participate in obesity via cytokine secretion function. The endocrine function of adipose tissue depends upon the activity of the adipocytes and the cells from the SVF. Adipocytes are the main source of leptin and adiponectin, while inflammatory cytokines such as IL-6 and TNF-α, are mostly secreted by the stromal-vascular cells[13,17,58], among which are ASC and preadipocytes. Consequently, ASC can be key players in obese subcutaneous adipose tissue as secretory cells.
Oñate et al[59] showed a low capacity for adipogenic and angiogenic differentiation and an upregulation on inflammatory genes[60] in the ASC from obese patients. Once ASC and preadipocytes are exposed to inflammatory adipose tissue microenvironment, they may react in several possible ways including cytokines secretion, dedifferentiation and migration, aiming to reduce tissue damage[47]. In this microenvironment, inflammatory cytokines secretion by non-healthy ASC could represent a failure to evade stress. Indeed, the cellular and molecular events in obesity may alter ASC producing a non-healthy and even inflammatory phenotype that in turn impairs the normal cellular physiology of the subcutaneous adipose tissue (Figure 2). Once a disruption in adipose tissue homeostasis begins, the metabolic buffer function of subcutaneous adipose tissue[3,5] can be lost, thereby worsening the systemic chronic inflammatory condition of obese patients.
Figure 2.
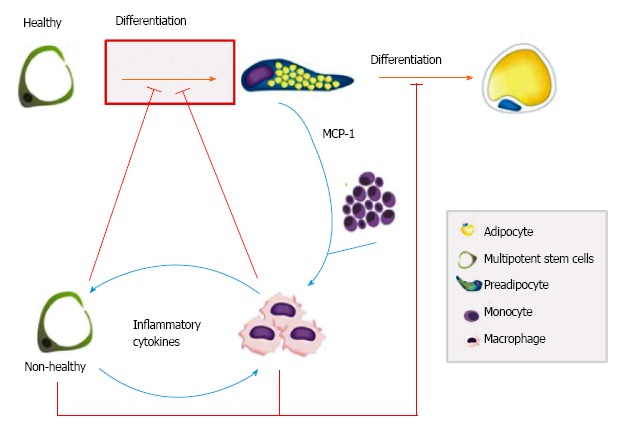
Multipotent stem cells as key players in tissue homeostasis disruption in obesity. The enormous increase in subcutaneous fat mass leads to an increase in secreted molecules such as monocyte chemotactic protein 1 (MCP-1), which causes monocyte infiltration and subsequent macrophage differentiation (blue lines). Inflammatory cytokines may change multipotent stem cells to a non-healthy phenotype (blue lines) that in turn impairs (red lines) the normal adipogenesis of subcutaneous adipose tissue.
This opens a new and fascinating pathway for modulating obesity through the possibility of manipulating the functional capacity of ASC[61]. Massive weight loss after bariatric surgery reduces the inflammation as measured by C-reactive protein and IL-6 plasma levels[62] and even the stress in endoplasmic reticulum of adipocytes[63], however if ASC isolated from subcutaneous adipose tissue could return their healthy state is still unknown.
ADIPOSE STEM CELLS AFTER MASSIVE WEIGHT LOSS: IMPLICATIONS FOR REGENERATIVE MEDICINE
ASC are now considered to be regenerative cells that promote tissue repair and regeneration in vivo, which may occur via different mechanisms, including indirectly by the production of soluble factors or directly by the differentiation of the ASC themselves. The first clinical trial that used expanded ASC involved the treatment of fistulas in Crohn’s auto-immune disease[64-66]. More than a decade’s worth of animal model studies and clinical trials using ASC[67] suggest that cell therapy approaches lead to the reestablishment of function and the remodeling of tissue after injury, mostly via a paracrine mechanism. Paracrine factors include a set of growth factors that once released by the transplanted stem cells may abrogate local apoptosis and stimulate the proliferation and activation of resident cell progenitors[45,46].
Obesity is an inflammatory disease, and relevant changes in the cytokine release profile are expected when comparing obese and non-obese patients and even after massive weight loss (ex-obese patients). In fact, it is known that epigenetic modifications could play a role in chronic diseases[68,69]. The alterations in cellular behavior caused by the inflammatory microenvironment in obese subcutaneous adipose tissue could be partially maintained even after the decrease of inflammation because of the accumulation of epigenetic modifications. Therefore, it is critical to investigate the biology of ASC to understand their potential use in particular therapeutic approaches. If ASC are isolated from ex-obese patients, the regenerative potential of these cells may be modified, and distinct from that observed in the cell populations present in and derived from non-obese patients. Although massive weight loss has been associated with reduced inflammatory markers in plasma[62], bariatric procedures could produce metabolic abnormalities, including the increase of bone resorption[29]. It has been reported that after massive weight loss, subcutaneous adipose tissue returns to a non-inflamatory state with a significant decrease in inflammatory cytokines[28,58] and immune cells infiltration[27,29]. These cytokines are produced mainly by SVF and their decrease was observed during weight stabilization period[58].
Mitterberger et al[30] have showed no alterations in ASC after massive weight loss; however we analyzed few days of adipogenic induction in contrast to previous studies[26,59,60]. We have described a predominance of larger blood vessels in the subcutaneous adipose tissues derived from ex-obese patients (BMI 25-29.9 kg/m2), who had lost weight after treatment with bariatric surgery, compared to that from control patients (BMI 25-29.9 kg/m2), who had never developed morbid obesity. In the same study, ASC isolated from ex-obese patients attained a mature adipocyte phenotype faster than those obtained from non-obese patients[26], suggesting an enrichment of the preadipocyte population.
We currently propose to find a potential biomarker to predict the adipogenic commitment of ASC and, therefore, a biomarker to predict the potential regaining of subcutaneous adipose tissue mass. Recently, Joe et al[70] have described two major subpopulations, based on CD34 surface marker expression, that dwell in murine skeletal muscle tissue. Using cell sorting techniques, the authors have shown that LinnegSca-1posCD34pos cells contain adipogenic progenitors, while LinnegSca-1negCD34neg cells contain osteogenic and chondrogenic activity. In a mouse model of acute lung injury, freshly isolated human umbilical cord blood CD34-positive cells are protective against the inflammation induced by a lipopolysaccharide challenge[71].
The role of the CD34 surface molecule in ASC physiology has been recently discussed[72]. In subcutaneous adipose tissue, the population of preadipocytes can be identified by flow cytometry using the following combination of cell surface markers: CD45neg, CD146neg, CD31neg, CD34pos[42]. Our preliminary results have shown an accumulation of CD34-positive cells in subcutaneous adipose tissue of ex-obese patients. Furthermore, a novel adipose tissue macrophage subpopulation (CD45pos/CD14pos/CD34pos/CD206pos) had characteristics similar to ASC, including adherence, localization, morphology, and mesenchymal multipotency. This subpopulation may have migrated from the bone marrow and, once in the adipose tissue, may play an important role in tissue maintenance[7] and also in the development of obesity.
The mechanisms by which the ASC from ex-obese patients are committed to the adipogenic lineage are unknown, but some results point to the existence of circulating preadipocytes that can be recruited and participate in the enlargement of the adipose tissue mass[73]. Another possible mechanism for the preadipocyte accumulation in ex-obese adipose tissue relies on the fact that adipocytes can dedifferentiate in vitro[41,74]. Considering that patients treated with bariatric surgery massively decrease food intake and increase energy expenditure through physical exercise, hypertrophied adipocytes tend to mobilize lipids through lipolysis and could dedifferentiate into preadipocytes (Figure 3). The enrichment of preadipocytes in the adipose tissues of ex-obese patients and the fact that the blood vessels in these tissues do not return to a normal diameter[26]may play roles in accelerated weight regain.
Figure 3.
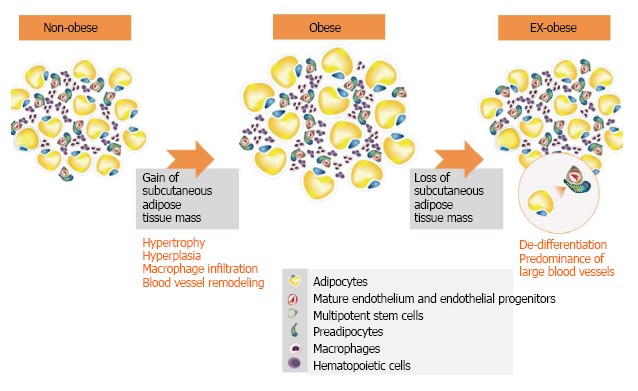
The enrichment of preadipocytes and the density of blood vessels in the adipose tissues of ex-obese patients may play roles in accelerated weight regain. Even after massive weight loss, ex-obese adipose tissue does not return to a normal cellular state. This adipose tissue is characterized by a higher percentage of CD34 cells and a greater number of small and large blood vessels[26].
Due to the high demand for breast augmentation, replacing devices with autologous fat and ASC is a fair option[75]. However, this approach cannot be followed if previous breast cancer has occurred. Moreover, if the ASC from ex-obese patients have an adipogenic memory of obesity development, we could also postulate an inflammatory memory or even altered inflammatory properties. It is thus reasonable to consider that the ASC from ex-obese patients may worsen the evolution of a tumor.
Due to ASC plasticity, studies of the function and contribution of ASC during the development of obesity are fundamental to developing new therapeutic approaches to prevent or treat obesity and its complications. Evidence of the alteration of ASC function during the development of obesity is beginning to be evaluated[59,60], but information is still scarce[61], specially related to patients who lost weight after bariatric surgery. In fact, inflammatory subcutaneous adipose tissue microenvironment could change ASC phenotype to a non-healthy state, possibly maintained by epigenetic alterations even after massive weight loss. We believe that the use of cell sorting approaches is imperative to validate the use of ASC from obese and ex-obese patients for autologous cellular therapy.
CONCLUSION
In conclusion, subcutaneous adipose tissue forms the interface between the clinical application of regenerative medicine and the establishment of the pathological condition of obesity. These two facets of adipose tissue should be understood as potentially related phenomena. Due to their functional characteristics, ASC represent a fundamental tool for understanding how these two facets are interconnected and could be important for therapeutic applications. Efforts to increase the understanding of the biology of ASC isolated from adipose tissues with normal physiology and in disease states may thus lead to the appropriate use of these cells in regenerative medicine and will provide novel therapeutic insights regarding obesity and weight regain.
ACKNOWLEDGMENTS
The authors thank the Instituto Nacional de Metrologia, Qualidade e Tecnologia (Inmetro) and Excellion – Biomedical Services, for laboratory facilities and Professor Cesar Claudio-da-Silva, MD, PhD and Marcelo Aniceto, MD, MSc for providing the human samples.
Footnotes
P- Reviewer: Bernhardt GA, Shen J, Swierczynski JT S- Editor: Tian YL L- Editor: A E- Editor: Lu YJ
Supported by Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ); Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq); Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES)
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: July 26, 2014
First decision: August 28, 2014
Article in press: December 16, 2014
References
- 1.Gustafson B. Adipose tissue, inflammation and atherosclerosis. J Atheroscler Thromb. 2010;17:332–341. doi: 10.5551/jat.3939. [DOI] [PubMed] [Google Scholar]
- 2.Mathieu P, Lemieux I, Després JP. Obesity, inflammation, and cardiovascular risk. Clin Pharmacol Ther. 2010;87:407–416. doi: 10.1038/clpt.2009.311. [DOI] [PubMed] [Google Scholar]
- 3.Gerhard GS, Styer AM, Strodel WE, Roesch SL, Yavorek A, Carey DJ, Wood GC, Petrick AT, Gabrielsen J, Ibele A, et al. Gene expression profiling in subcutaneous, visceral and epigastric adipose tissues of patients with extreme obesity. Int J Obes (Lond) 2014;38:371–378. doi: 10.1038/ijo.2013.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chau YY, Bandiera R, Serrels A, Martínez-Estrada OM, Qing W, Lee M, Slight J, Thornburn A, Berry R, McHaffie S, et al. Visceral and subcutaneous fat have different origins and evidence supports a mesothelial source. Nat Cell Biol. 2014;16:367–375. doi: 10.1038/ncb2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garg A. Regional adiposity and insulin resistance. J Clin Endocrinol Metab. 2004;89:4206–4210. doi: 10.1210/jc.2004-0631. [DOI] [PubMed] [Google Scholar]
- 6.Patel P, Abate N. Body fat distribution and insulin resistance. Nutrients. 2013;5:2019–2027. doi: 10.3390/nu5062019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eto H, Ishimine H, Kinoshita K, Watanabe-Susaki K, Kato H, Doi K, Kuno S, Kurisaki A, Yoshimura K. Characterization of human adipose tissue-resident hematopoietic cell populations reveals a novel macrophage subpopulation with CD34 expression and mesenchymal multipotency. Stem Cells Dev. 2013;22:985–997. doi: 10.1089/scd.2012.0442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lolmède K, Duffaut C, Zakaroff-Girard A, Bouloumié A. Immune cells in adipose tissue: key players in metabolic disorders. Diabetes Metab. 2011;37:283–290. doi: 10.1016/j.diabet.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 9.Schipper HS, Prakken B, Kalkhoven E, Boes M. Adipose tissue-resident immune cells: key players in immunometabolism. Trends Endocrinol Metab. 2012;23:407–415. doi: 10.1016/j.tem.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 10.Elgazar-Carmon V, Rudich A, Hadad N, Levy R. Neutrophils transiently infiltrate intra-abdominal fat early in the course of high-fat feeding. J Lipid Res. 2008;49:1894–1903. doi: 10.1194/jlr.M800132-JLR200. [DOI] [PubMed] [Google Scholar]
- 11.Anderson EK, Gutierrez DA, Hasty AH. Adipose tissue recruitment of leukocytes. Curr Opin Lipidol. 2010;21:172–177. doi: 10.1097/MOL.0b013e3283393867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Toni F, Poglio S, Youcef AB, Cousin B, Pflumio F, Bourin P, Casteilla L, Laharrague P. Human adipose-derived stromal cells efficiently support hematopoiesis in vitro and in vivo: a key step for therapeutic studies. Stem Cells Dev. 2011;20:2127–2138. doi: 10.1089/scd.2011.0044. [DOI] [PubMed] [Google Scholar]
- 13.de Ferranti S, Mozaffarian D. The perfect storm: obesity, adipocyte dysfunction, and metabolic consequences. Clin Chem. 2008;54:945–955. doi: 10.1373/clinchem.2007.100156. [DOI] [PubMed] [Google Scholar]
- 14.Tang QQ, Lane MD. Adipogenesis: from stem cell to adipocyte. Annu Rev Biochem. 2012;81:715–736. doi: 10.1146/annurev-biochem-052110-115718. [DOI] [PubMed] [Google Scholar]
- 15.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 16.Lasselin J, Magne E, Beau C, Ledaguenel P, Dexpert S, Aubert A, Layé S, Capuron L. Adipose inflammation in obesity: relationship with circulating levels of inflammatory markers and association with surgery-induced weight loss. J Clin Endocrinol Metab. 2014;99:E53–E61. doi: 10.1210/jc.2013-2673. [DOI] [PubMed] [Google Scholar]
- 17.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gregor MF, Hotamisligil GS. Thematic review series: Adipocyte Biology. Adipocyte stress: the endoplasmic reticulum and metabolic disease. J Lipid Res. 2007;48:1905–1914. doi: 10.1194/jlr.R700007-JLR200. [DOI] [PubMed] [Google Scholar]
- 20.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fain JN, Madan AK, Hiler ML, Cheema P, Bahouth SW. Comparison of the release of adipokines by adipose tissue, adipose tissue matrix, and adipocytes from visceral and subcutaneous abdominal adipose tissues of obese humans. Endocrinology. 2004;145:2273–2282. doi: 10.1210/en.2003-1336. [DOI] [PubMed] [Google Scholar]
- 22.Nomiyama T, Perez-Tilve D, Ogawa D, Gizard F, Zhao Y, Heywood EB, Jones KL, Kawamori R, Cassis LA, Tschöp MH, et al. Osteopontin mediates obesity-induced adipose tissue macrophage infiltration and insulin resistance in mice. J Clin Invest. 2007;117:2877–2888. doi: 10.1172/JCI31986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zeyda M, Gollinger K, Todoric J, Kiefer FW, Keck M, Aszmann O, Prager G, Zlabinger GJ, Petzelbauer P, Stulnig TM. Osteopontin is an activator of human adipose tissue macrophages and directly affects adipocyte function. Endocrinology. 2011;152:2219–2227. doi: 10.1210/en.2010-1328. [DOI] [PubMed] [Google Scholar]
- 24.Cancello R, Henegar C, Viguerie N, Taleb S, Poitou C, Rouault C, Coupaye M, Pelloux V, Hugol D, Bouillot JL, et al. Reduction of macrophage infiltration and chemoattractant gene expression changes in white adipose tissue of morbidly obese subjects after surgery-induced weight loss. Diabetes. 2005;54:2277–2286. doi: 10.2337/diabetes.54.8.2277. [DOI] [PubMed] [Google Scholar]
- 25.Zhou HR, Kim EK, Kim H, Claycombe KJ. Obesity-associated mouse adipose stem cell secretion of monocyte chemotactic protein-1. Am J Physiol Endocrinol Metab. 2007;293:E1153–E1158. doi: 10.1152/ajpendo.00186.2007. [DOI] [PubMed] [Google Scholar]
- 26.Baptista LS, da Silva KR, da Pedrosa CS, Claudio-da-Silva C, Carneiro JR, Aniceto M, de Mello-Coelho V, Takiya CM, Rossi MI, Borojevic R. Adipose tissue of control and ex-obese patients exhibit differences in blood vessel content and resident mesenchymal stem cell population. Obes Surg. 2009;19:1304–1312. doi: 10.1007/s11695-009-9899-2. [DOI] [PubMed] [Google Scholar]
- 27.Viardot A, Lord RV, Samaras K. The effects of weight loss and gastric banding on the innate and adaptive immune system in type 2 diabetes and prediabetes. J Clin Endocrinol Metab. 2010;95:2845–2850. doi: 10.1210/jc.2009-2371. [DOI] [PubMed] [Google Scholar]
- 28.Moschen AR, Molnar C, Geiger S, Graziadei I, Ebenbichler CF, Weiss H, Kaser S, Kaser A, Tilg H. Anti-inflammatory effects of excessive weight loss: potent suppression of adipose interleukin 6 and tumour necrosis factor alpha expression. Gut. 2010;59:1259–1264. doi: 10.1136/gut.2010.214577. [DOI] [PubMed] [Google Scholar]
- 29.Folli F, Sabowitz BN, Schwesinger W, Fanti P, Guardado-Mendoza R, Muscogiuri G. Bariatric surgery and bone disease: from clinical perspective to molecular insights. Int J Obes (Lond) 2012;36:1373–1379. doi: 10.1038/ijo.2012.115. [DOI] [PubMed] [Google Scholar]
- 30.Mitterberger MC, Mattesich M, Zwerschke W. Bariatric surgery and diet-induced long-term caloric restriction protect subcutaneous adipose-derived stromal/progenitor cells and prolong their life span in formerly obese humans. Exp Gerontol. 2014;56:106–113. doi: 10.1016/j.exger.2014.03.030. [DOI] [PubMed] [Google Scholar]
- 31.De Bari C, Dell’Accio F, Tylzanowski P, Luyten FP. Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis Rheum. 2001;44:1928–1942. doi: 10.1002/1529-0131(200108)44:8<1928::AID-ART331>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 32.Dellavalle A, Sampaolesi M, Tonlorenzi R, Tagliafico E, Sacchetti B, Perani L, Innocenzi A, Galvez BG, Messina G, Morosetti R, et al. Pericytes of human skeletal muscle are myogenic precursors distinct from satellite cells. Nat Cell Biol. 2007;9:255–267. doi: 10.1038/ncb1542. [DOI] [PubMed] [Google Scholar]
- 33.Shi S, Gronthos S. Perivascular niche of postnatal mesenchymal stem cells in human bone marrow and dental pulp. J Bone Miner Res. 2003;18:696–704. doi: 10.1359/jbmr.2003.18.4.696. [DOI] [PubMed] [Google Scholar]
- 34.Akintoye SO, Lam T, Shi S, Brahim J, Collins MT, Robey PG. Skeletal site-specific characterization of orofacial and iliac crest human bone marrow stromal cells in same individuals. Bone. 2006;38:758–768. doi: 10.1016/j.bone.2005.10.027. [DOI] [PubMed] [Google Scholar]
- 35.Kögler G, Sensken S, Airey JA, Trapp T, Müschen M, Feldhahn N, Liedtke S, Sorg RV, Fischer J, Rosenbaum C, et al. A new human somatic stem cell from placental cord blood with intrinsic pluripotent differentiation potential. J Exp Med. 2004;200:123–135. doi: 10.1084/jem.20040440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Longo UG, Loppini M, Berton A, La Verde L, Khan WS, Denaro V. Stem cells from umbilical cord and placenta for musculoskeletal tissue engineering. Curr Stem Cell Res Ther. 2012;7:272–281. doi: 10.2174/157488812800793054. [DOI] [PubMed] [Google Scholar]
- 37.Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 38.Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 39.Dimarino AM, Caplan AI, Bonfield TL. Mesenchymal stem cells in tissue repair. Front Immunol. 2013;4:201. doi: 10.3389/fimmu.2013.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bourin P, Bunnell BA, Casteilla L, Dominici M, Katz AJ, March KL, Redl H, Rubin JP, Yoshimura K, Gimble JM. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: a joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT) Cytotherapy. 2013;15:641–648. doi: 10.1016/j.jcyt.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Planat-Benard V, Silvestre JS, Cousin B, André M, Nibbelink M, Tamarat R, Clergue M, Manneville C, Saillan-Barreau C, Duriez M, et al. Plasticity of human adipose lineage cells toward endothelial cells: physiological and therapeutic perspectives. Circulation. 2004;109:656–663. doi: 10.1161/01.CIR.0000114522.38265.61. [DOI] [PubMed] [Google Scholar]
- 42.Zimmerlin L, Donnenberg VS, Pfeifer ME, Meyer EM, Péault B, Rubin JP, Donnenberg AD. Stromal vascular progenitors in adult human adipose tissue. Cytometry A. 2010;77:22–30. doi: 10.1002/cyto.a.20813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McIntosh KR, Frazier T, Rowan BG, Gimble JM. Evolution and future prospects of adipose-derived immunomodulatory cell therapeutics. Expert Rev Clin Immunol. 2013;9:175–184. doi: 10.1586/eci.12.96. [DOI] [PubMed] [Google Scholar]
- 44.Hoogduijn MJ, Roemeling-van Rhijn M, Engela AU, Korevaar SS, Mensah FK, Franquesa M, de Bruin RW, Betjes MG, Weimar W, Baan CC. Mesenchymal stem cells induce an inflammatory response after intravenous infusion. Stem Cells Dev. 2013;22:2825–2835. doi: 10.1089/scd.2013.0193. [DOI] [PubMed] [Google Scholar]
- 45.Kilroy GE, Foster SJ, Wu X, Ruiz J, Sherwood S, Heifetz A, Ludlow JW, Stricker DM, Potiny S, Green P, et al. Cytokine profile of human adipose-derived stem cells: expression of angiogenic, hematopoietic, and pro-inflammatory factors. J Cell Physiol. 2007;212:702–709. doi: 10.1002/jcp.21068. [DOI] [PubMed] [Google Scholar]
- 46.Sempere JM, Martinez-Peinado P, Arribas MI, Reig JA, De La Sen ML, Zubcoff JJ, Fraga MF, Fernández AF, Santana A, Roche E. Single cell-derived clones from human adipose stem cells present different immunomodulatory properties. Clin Exp Immunol. 2014;176:255–265. doi: 10.1111/cei.12270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shoshani O, Zipori D. Stress as a fundamental theme in cell plasticity. Biochim Biophys Acta. 2014:Jul 16; Epub ahead of print. doi: 10.1016/j.bbagrm.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 48.Zhang X, Bowles AC, Semon JA, Scruggs BA, Zhang S, Strong AL, Gimble JM, Bunnell BA. Transplantation of autologous adipose stem cells lacks therapeutic efficacy in the experimental autoimmune encephalomyelitis model. PLoS One. 2014;9:e85007. doi: 10.1371/journal.pone.0085007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rennert RC, Sorkin M, Januszyk M, Duscher D, Kosaraju R, Chung MT, Lennon J, Radiya-Dixit A, Raghvendra S, Maan ZN, et al. Diabetes impairs the angiogenic potential of adipose-derived stem cells by selectively depleting cellular subpopulations. Stem Cell Res Ther. 2014;5:79. doi: 10.1186/scrt468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Policha A, Zhang P, Chang L, Lamb K, Tulenko T, DiMuzio P. Endothelial differentiation of diabetic adipose-derived stem cells. J Surg Res. 2014;192:656–663. doi: 10.1016/j.jss.2014.06.041. [DOI] [PubMed] [Google Scholar]
- 51.Cianfarani F, Toietta G, Di Rocco G, Cesareo E, Zambruno G, Odorisio T. Diabetes impairs adipose tissue-derived stem cell function and efficiency in promoting wound healing. Wound Repair Regen. 2013;21:545–553. doi: 10.1111/wrr.12051. [DOI] [PubMed] [Google Scholar]
- 52.Bhaskaran K, Douglas I, Forbes H, dos-Santos-Silva I, Leon DA, Smeeth L. Body-mass index and risk of 22 specific cancers: a population-based cohort study of 5·24 million UK adults. Lancet. 2014;384:755–765. doi: 10.1016/S0140-6736(14)60892-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sun Z, Wang S, Zhao RC. The roles of mesenchymal stem cells in tumor inflammatory microenvironment. J Hematol Oncol. 2014;7:14. doi: 10.1186/1756-8722-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cousin B, Munoz O, Andre M, Fontanilles AM, Dani C, Cousin JL, Laharrague P, Casteilla L, Pénicaud L. A role for preadipocytes as macrophage-like cells. FASEB J. 1999;13:305–312. doi: 10.1096/fasebj.13.2.305. [DOI] [PubMed] [Google Scholar]
- 55.Charrière G, Cousin B, Arnaud E, André M, Bacou F, Penicaud L, Casteilla L. Preadipocyte conversion to macrophage. Evidence of plasticity. J Biol Chem. 2003;278:9850–9855. doi: 10.1074/jbc.M210811200. [DOI] [PubMed] [Google Scholar]
- 56.Saillan-Barreau C, Cousin B, André M, Villena P, Casteilla L, Pénicaud L. Human adipose cells as candidates in defense and tissue remodeling phenomena. Biochem Biophys Res Commun. 2003;309:502–505. doi: 10.1016/j.bbrc.2003.08.034. [DOI] [PubMed] [Google Scholar]
- 57.Cousin B, André M, Casteilla L, Pénicaud L. Altered macrophage-like functions of preadipocytes in inflammation and genetic obesity. J Cell Physiol. 2001;186:380–386. doi: 10.1002/1097-4652(2001)9999:9999<000::AID-JCP1038>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 58.Siklova-Vitkova M, Klimcakova E, Polak J, Kovacova Z, Tencerova M, Rossmeislova L, Bajzova M, Langin D, Stich V. Adipose tissue secretion and expression of adipocyte-produced and stromavascular fraction-produced adipokines vary during multiple phases of weight-reducing dietary intervention in obese women. J Clin Endocrinol Metab. 2012;97:E1176–E1181. doi: 10.1210/jc.2011-2380. [DOI] [PubMed] [Google Scholar]
- 59.Oñate B, Vilahur G, Ferrer-Lorente R, Ybarra J, Díez-Caballero A, Ballesta-López C, Moscatiello F, Herrero J, Badimon L. The subcutaneous adipose tissue reservoir of functionally active stem cells is reduced in obese patients. FASEB J. 2012;26:4327–4336. doi: 10.1096/fj.12-207217. [DOI] [PubMed] [Google Scholar]
- 60.Oñate B, Vilahur G, Camino-López S, Díez-Caballero A, Ballesta-López C, Ybarra J, Moscatiello F, Herrero J, Badimon L. Stem cells isolated from adipose tissue of obese patients show changes in their transcriptomic profile that indicate loss in stemcellness and increased commitment to an adipocyte-like phenotype. BMC Genomics. 2013;14:625. doi: 10.1186/1471-2164-14-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cignarelli A, Perrini S, Ficarella R, Peschechera A, Nigro P, Giorgino F. Human adipose tissue stem cells: relevance in the pathophysiology of obesity and metabolic diseases and therapeutic applications. Expert Rev Mol Med. 2012;14:e19. doi: 10.1017/erm.2012.13. [DOI] [PubMed] [Google Scholar]
- 62.Rao SR. Inflammatory markers and bariatric surgery: a meta-analysis. Inflamm Res. 2012;61:789–807. doi: 10.1007/s00011-012-0473-3. [DOI] [PubMed] [Google Scholar]
- 63.Gregor MF, Yang L, Fabbrini E, Mohammed BS, Eagon JC, Hotamisligil GS, Klein S. Endoplasmic reticulum stress is reduced in tissues of obese subjects after weight loss. Diabetes. 2009;58:693–700. doi: 10.2337/db08-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.García-Olmo D, García-Arranz M, Herreros D, Pascual I, Peiro C, Rodríguez-Montes JA. A phase I clinical trial of the treatment of Crohn’s fistula by adipose mesenchymal stem cell transplantation. Dis Colon Rectum. 2005;48:1416–1423. doi: 10.1007/s10350-005-0052-6. [DOI] [PubMed] [Google Scholar]
- 65.Herreros MD, Garcia-Arranz M, Guadalajara H, De-La-Quintana P, Garcia-Olmo D. Autologous expanded adipose-derived stem cells for the treatment of complex cryptoglandular perianal fistulas: a phase III randomized clinical trial (FATT 1: fistula Advanced Therapy Trial 1) and long-term evaluation. Dis Colon Rectum. 2012;55:762–772. doi: 10.1097/DCR.0b013e318255364a. [DOI] [PubMed] [Google Scholar]
- 66.de la Portilla F, Alba F, García-Olmo D, Herrerías JM, González FX, Galindo A. Expanded allogeneic adipose-derived stem cells (eASCs) for the treatment of complex perianal fistula in Crohn’s disease: results from a multicenter phase I/IIa clinical trial. Int J Colorectal Dis. 2013;28:313–323. doi: 10.1007/s00384-012-1581-9. [DOI] [PubMed] [Google Scholar]
- 67.Casteilla L, Planat-Benard V, Laharrague P, Cousin B. Adipose-derived stromal cells: Their identity and uses in clinical trials, an update. World J Stem Cells. 2011;3:25–33. doi: 10.4252/wjsc.v3.i4.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Symonds ME, Budge H, Frazier-Wood AC. Epigenetics and obesity: a relationship waiting to be explained. Hum Hered. 2013;75:90–97. doi: 10.1159/000352009. [DOI] [PubMed] [Google Scholar]
- 69.Wegner M, Neddermann D, Piorunska-Stolzmann M, Jagodzinski PP. Role of epigenetic mechanisms in the development of chronic complications of diabetes. Diabetes Res Clin Pract. 2014;105:164–175. doi: 10.1016/j.diabres.2014.03.019. [DOI] [PubMed] [Google Scholar]
- 70.Joe AW, Yi L, Natarajan A, Le Grand F, So L, Wang J, Rudnicki MA, Rossi FM. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat Cell Biol. 2010;12:153–163. doi: 10.1038/ncb2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Huang X, Sun K, Zhao YD, Vogel SM, Song Y, Mahmud N, Zhao YY. Human CD34+ progenitor cells freshly isolated from umbilical cord blood attenuate inflammatory lung injury following LPS challenge. PLoS One. 2014;9:e88814. doi: 10.1371/journal.pone.0088814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Scherberich A, Di Maggio ND, McNagny KM. A familiar stranger: CD34 expression and putative functions in SVF cells of adipose tissue. World J Stem Cells. 2013;5:1–8. doi: 10.4252/wjsc.v5.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hong KM, Burdick MD, Phillips RJ, Heber D, Strieter RM. Characterization of human fibrocytes as circulating adipocyte progenitors and the formation of human adipose tissue in SCID mice. FASEB J. 2005;19:2029–2031. doi: 10.1096/fj.05-4295fje. [DOI] [PubMed] [Google Scholar]
- 74.Matsumoto T, Kano K, Kondo D, Fukuda N, Iribe Y, Tanaka N, Matsubara Y, Sakuma T, Satomi A, Otaki M, et al. Mature adipocyte-derived dedifferentiated fat cells exhibit multilineage potential. J Cell Physiol. 2008;215:210–222. doi: 10.1002/jcp.21304. [DOI] [PubMed] [Google Scholar]
- 75.Yoshimura K, Asano Y, Aoi N, Kurita M, Oshima Y, Sato K, Inoue K, Suga H, Eto H, Kato H, et al. Progenitor-enriched adipose tissue transplantation as rescue for breast implant complications. Breast J. 2010;16:169–175. doi: 10.1111/j.1524-4741.2009.00873.x. [DOI] [PubMed] [Google Scholar]


