Abstract
AIM: To investigate the potential for early gestation placenta-derived mesenchymal stromal cells (PMSCs) for fetal tissue engineering.
METHODS: PMSCs were isolated from early gestation chorionic villus tissue by explant culture. Chorionic villus sampling (CVS)-size tissue samples (mean = 35.93 mg) were used to test the feasibility of obtaining large cell numbers from CVS within a clinically relevant timeframe. We characterized PMSCs isolated from 6 donor placentas by flow cytometry immunophenotyping, multipotency assays, and through immunofluorescent staining. Protein secretion from PMSCs was examined using two cytokine array assays capable of probing for over 70 factors in total. Delivery vehicle compatibility of PMSCs was determined using three common scaffold systems: fibrin glue, collagen hydrogel, and biodegradable nanofibrous scaffolds made from a combination of polylactic acid (PLA) and poly(lactic-co-glycolic acid) (PLGA). Viral transduction of PMSCs was performed using a Luciferase-GFP-containing lentiviral vector and efficiency of transduction was tested by fluorescent microscopy and flow cytometry analysis.
RESULTS: We determined that an average of 2.09 × 106 (SD ± 8.59 × 105) PMSCs could be obtained from CVS-size tissue samples within 30 d (mean = 27 d, SD ± 2.28), indicating that therapeutic numbers of cells can be rapidly expanded from very limited masses of tissue. Immunophenotyping by flow cytometry demonstrated that PMSCs were positive for MSC markers CD105, CD90, CD73, CD44, and CD29, and were negative for hematopoietic and endothelial markers CD45, CD34, and CD31. PMSCs displayed trilineage differentiation capability, and were found to express developmental transcription factors Sox10 and Sox17 as well as neural-related structural proteins NFM, Nestin, and S100β. Cytokine arrays revealed a robust and extensive profile of PMSC-secreted cytokines and growth factors, and detected 34 factors with spot density values exceeding 103. Detected factors had widely diverse functions that include modulation of angiogenesis and immune response, cell chemotaxis, cell proliferation, blood vessel maturation and homeostasis, modulation of insulin-like growth factor activity, neuroprotection, extracellular matrix degradation and even blood coagulation. Importantly, PMSCs were also determined to be compatible with both biological and synthetic material-based delivery vehicles such as collagen and fibrin hydrogels, and biodegradable nanofiber scaffolds made from a combination of PLA and PLGA. Finally, we demonstrated that PMSCs can be efficiently transduced (> 95%) with a Luciferase-GFP-containing lentiviral vector for future in vivo cell tracking after transplantation.
CONCLUSION: Our findings indicate that PMSCs represent a unique source of cells that can be effectively utilized for in utero cell therapy and tissue engineering.
Keywords: Placenta, Mesenchymal stromal cells, Chorionic villus, Fetal surgery, Tissue engineering
Core tip: In this study we characterize mesenchymal stromal cells derived from early gestation human placenta chorionic villi (PMSCs) for the purpose of fetal tissue engineering. We examine cell expansion in early passages from chorionic villus sampling-size tissue samples, as well as PMSC surface marker expression, multipotency, intracellular protein expression, protein secretion, and compatibility with delivery vehicles and tracking methods often used for in vivo experimentation. We show that early gestation PMSCs are excellent candidates for future tissue engineering studies, particularly as it applies to in utero therapy for congenital anomalies.
INTRODUCTION
Over the past three decades, fetal surgery has emerged as an effective treatment option for congenital diseases, including spina bifida, congenital diaphragmatic hernia, sacrococcygeal teratoma and cardiac malformations[1,2]. In utero intervention allows clinicians to treat disease quickly after its onset and prevent debilitating symptoms before they ever occur[3,4]. Mesenchymal stromal cell (MSC) therapy can augment existing in utero surgical techniques, and treatment with MSCs has been shown to promote wound healing[5,6], protect damaged tissues[7-11], and modulate the immune system[12-15]. Cells obtained via methods such as chorionic villus sampling (CVS) allow for the potential development of cell therapies that are autologous (derived from the patient’s cells) to the fetus[16-18]. Autologous therapies are potentially preferable to allogeneic (not patient specific) therapies, as using the patient’s own cells in a therapy should not elicit an immune response[19]. However, where autologous therapies are not required, allogeneic early gestation chorionic villus tissue can be an outstanding cell source for cell therapy as well.
The chorionic villi of early gestation placenta present an ideal source of autologous and allogeneic stromal cells. Placenta-derived mesenchymal stromal cells (PMSCs) are analogous to the MSCs routinely obtained from bone marrow in both surface marker expression and multipotency[20,21]. Investigators interested in utilizing PMSCs or other fetal-derived mesenchymal stromal cells have demonstrated their potential for engineering bone[22-24], cartilage[25-27], and muscle[28-30], as well as pancreatic[31], neural[7,17] and cardiac tissues[18,32-34]. The gestational age of the source tissue may affect the therapeutic capabilities of the cells. One study comparing cells obtained from term chorionic villus tissue vs those from first trimester tissue noted that the cells from earlier gestation tissue had more stem-like properties, faster growth kinetics, and improved wound-healing capability in vivo[35]. These data indicate that cells obtained from earlier gestation chorionic villus tissue may be preferential to term cells for tissue engineering purposes.
In this study we isolate PMSCs from chorionic villus tissue of early gestation placenta (GA 12-18 wk). We detail the initial growth phase of cells isolated from CVS-sized tissue samples using an explant culture method. PMSCs were characterized by flow cytometry immunophenotyping, multipotency assays and immunofluorescent staining for stem cell related transcription factors and intracellular markers, as well as by examining secreted proteins by membrane array. We demonstrate that these cells are compatible with multiple delivery matrices critical to their local distribution upon surgical transplantation. Lastly, PMSCs are shown to be efficiently transduced with a Luciferase-GFP-containing lentiviral vector which allows for cell tracking data to be obtained from in vivo transplantation experiments. These data provide further support for the use of early gestation PMSCs for in vivo tissue engineering purposes, and sets the stage for further investigation of the therapeutic profile of transplantable cell-seeded matrices.
MATERIALS AND METHODS
Isolation and culture of PMSCs from human early gestation placenta
Donated placental tissues were collected at the UC Davis Medical Center with approval from the Institutional Review Board. PMSCs were isolated from placentas of varying ages early in gestation (GA 12-18 wk) using an explant culture method. Chorionic villus tissue was dissected into small pieces (roughly 5 mm) and washed in sterile 1X phosphate-buffered saline (PBS) containing 100 UI/mL of penicillin, 100 μg/mL of streptomycin. Tissue was then dissected into even smaller pieces and evenly spread across tissue culture-treated dishes. Cells were allowed to migrate from tissue onto the dish before being harvested for subculture. Cells were cryopreserved in a freeze media containing fetal bovine serum (FBS) and 10% dimethyl sulfoxide beginning at passages 3-5 and thawed for later characterization. Our standard culture media for all experiments was DMEM high glucose with 5% FBS, 100 UI/mL of penicillin, 100 μg/mL of streptomycin, 20 ng/mL recombinant human bFGF (Peprotech) and 20 ng/ml recombinant human EGF (Peprotech).
Early passage growth from CVS-size samples
Cell growth curves were generated from PMSCs obtained from CVS-size tissue samples in order to test the feasibility of obtaining significant cell numbers from a limited tissue mass. Early gestation chorionic villus tissue was carefully dissected and washed in sterile 1X PBS containing 100 UI/mL of penicillin and 100 μg/mL of streptomycin. Dissected tissue was then further cut into smaller pieces (about 20-50 mg) analogous to the amount of tissue obtained in routine chorionic villus sampling (CVS). The CVS-size samples were weighed before being further dissected and spread evenly across an individual well of a 6-well cell culture plate previously coated with CELLStart xeno-free substrate for 1 h at 37 °C. When cells reached 70%-80% confluence, individual wells were passaged and villus tissue removed. Cells were counted at each passage up to the third passage.
Flow cytometry immunophenotyping of PMSCs
Cells were detached for flow cytometry using Accutase (Invitrogen) and viable cell counts obtained using Trypan Blue staining. Cells were then fractioned into tubes containing approximately 1 × 106 cells per sample before staining with the following antibodies: FITC-CD44 (560977), PECy5-CD90 (555597), PE-CD73 (561014), Alexa Fluor 647-CD105 (561439), PE-CD29 (561795), PE-CD34 (560941), Alexa Fluor 647-CD31 (561654), FITC-CD45 (560976) or appropriate isotype controls (all from B.D. Biosciences). Prior to antibody staining, viability staining was completed using LIVE/DEAD® Fixable Aqua Dead Cell Stain Kit (Molecular Probes) to detect dead cells. After viability and antibody staining, cells were fixed in 10% formalin for 30 min prior to analysis. Cell samples were analyzed on a BD Fortessa LSR Cell Analyzer and further data analysis and gating was performed using FlowJo software (Treestar, Inc).
Multipotency assays
Trilineage differentiation capability of PMSCs was assessed using the assays described below.
For osteogenic differentiation, PMSCs were grown in media consisting of DMEM containing 10% FBS, 10 mmol/L β-glycerol phosphate (Sigma Aldrich), 0.1 µmol/L dexamethasone (Sigma Aldrich) and 200 μmol/L ascorbic acid (Sigma Aldrich) for a minimum of two weeks. Upon differentiation cells were fixed in formalin and osteogenesis confirmed using Alizarin Red staining (Sigma-Aldrich).
For chondrogenic differentiation, PMSCs were grown as cell pellets in suspension in media consisting of DMEM containing 10% FBS, 10 ng/mL transforming growth factor beta-3 (Peprotech), and 200 μmol/L ascorbic acid (Sigma Aldrich) for a minimum of 3 wk. Chondrogenic pellets were then fixed in formalin before subsequently being embedded in Optimal Cutting Temperature compound (Fisher Scientific) and frozen prior to sectioning. Pellet cross sections were then stained with Alcian Blue (Sigma-Aldrich) and Nuclear Fast Red (Sigma Aldrich) to confirm presence of glycosaminoglycans.
For adipogenic differentiation, PMSCs were grown in media consisting of DMEM containing 10% FBS, 1 μmol/L dexamethasone (Sigma Aldrich), 10 μg/mL insulin (Sigma Aldrich), 5 μmol/L isobutylxanthine (AdipoGen), and 200 μmol/L indomethacin (MP Biomedicals) for a minimum of 3 wk Upon differentiation cells were fixed in formalin and stained with Oil Red O (Sigma-Aldrich) to identify lipid formation followed by hematoxylin to stain cell nuclei.
Immunofluorescent staining of PMSCs
Cells were fixed in formalin and permeabilized using 0.5% Triton X-100 in PBS prior to immunofluorescent staining. Non-specific binding of primary antibodies was blocked with 1% bovine serum albumin (BSA) in 1X PBS for one hour at room temperature. Primary antibody staining consisted of an overnight incubation with each of following primary antibodies at 4 °C in a solution of 1% BSA in PBS: Sox10 (R and D Systems, MAB2864), Sox17 (R and D Systems, MAB1924), Nestin (Millipore, MAB5326), S100β (Sigma-Aldrich, S2532), or NFM (Santa Cruz Biotech, SC-16143). Following primary antibody incubation, cells were then incubated with AlexaFluor 546-conjugated secondary antibodies (Molecular Probes, A10040) for 1 h at room temperature. Lastly, cell nuclei were counterstained using DAPI (Biotium, 40011). Stained samples were imaged using a Carl Zeiss Axio Observer D1 inverted microscope and post-processed with ImageJ software.
Cytokine array analysis of PMSC secreted proteins
Secreted factors were analyzed from the culture supernatant of PMSCs from a single donor (14 wk GA) using two cytokine array kits (Human Cytokine Array Panel A and Human Angiogenesis Array, both from RD Systems). PMSCs were seeded at a density of 7.5 × 105 per 100 mm culture dish. Culture supernatant was collected at 96 h, centrifuged to remove particulates and then the assays performed according to the manufacturer’s instructions. Stained membrane blots were imaged on a Bio-Rad ChemiDoc MP, and images were analyzed using ImageJ software with the Dot Blot Analysis plugin. Integrated density values obtained from membrane images were then plotted using Microsoft Excel.
Delivery vehicle compatibility testing
A single PMSC line (15 wk GA) was tested for compatibility in three delivery vehicles: collagen and fibrin hydrogels, as well as aligned nanofiber scaffold made from synthetic polymers. Fibrin gels were formed using EVICEL® Fibrin Sealant kit. PMSCs were resuspended in DMEM and mixed with 70 mg/mL fibrinogen solution and 1000 U/mL thrombin. The final composition of fibrin hydrogel was 5 mg/mL fibrinogen, 5 U/mL thrombin and 8.3 × 104 cells/mL. Fibrin gel with embedded cells were seeded in 96-well plates at a volume of 100 μL per well and incubated at 37 °C for 1 h to ensure complete gelation. After gelation was complete, 100 μL of culture media was added to each well to cover the hydrogel layer. Collagen hydrogel was prepared by mixing stock 5 mg/mL bovine collagen I solution (Advanced BioMatrix) in DMEM containing PMSCs, yielding a final concentration of 2 mg/mL collagen I and 1.5 × 105 cells/mL. The collagen/cell solution was then seeded in 24-well plates at a volume of 500 μL per well and allowed to gel at 37 °C for 1 h before the addition of 500 μL culture media to cover the hydrogel. Aligned nanofiber scaffold has a two-layer structure and was prepared using electrospinning technology. The scaffold consists of polylactic acid (PLA) and poly(lactic-co-glycolic acid) (PLGA) polymers each of them making up 15% weight/volume of the final product. A rectangular piece of nanofiber scaffold (150 mm2 surface area) was attached to the bottom of a 35 mm culture dish using double sided bonding tape 5 × 105 cells in 400 μL of DMEM were carefully pipetted on the membrane and incubated at 37 °C for 1 h to allow cells to adhere. After 1 h, 1 mL of culture medium was added to the 35 mm dish to cover the scaffold with cells. Cell viability in each system was assessed using LIVE/DEAD® Viability/Cytotoxicity Kit (Molecular Probes) after 24-72 h. Stained constructs were imaged using a Carl Zeiss Axio Observer D1 inverted microscope, and images further processed with ImageJ software.
Lentiviral vector transduction
To allow for long term labeling for future in vivo transplantation applications, PMSCs from a single donor (17 wk GA) were transduced using a Luciferase-GFP-containing lentiviral vector. The viral vector used was a pCCLc-MNDU3-LUC-PGK-EGFP-WPRE construct obtained from the UC Davis/CIRM Institute for Regenerative Cures, Sacramento, CA. Cells were seeded overnight at a density of 7.5 × 105 cells per 100 mm dish, and the next morning were treated with transduction media containing 8 µg/mL protamine sulfate and 10 µL/mL viral vector for 6 hours. After incubation with the viral vector, cells were washed twice and normal culture media reintroduced. Cells were imaged with fluorescent microscopy to detect successful GFP expression using a Carl Zeiss Axio Observer D1 inverted microscope. At 5 d post-transduction the GFP expression was quantified by flow cytometry using a BD Fortessa LSR Cell Analyzer and collected data further analyzed using FlowJo software (Treestar, Inc).
RESULTS
Early passage growth from CVS-size Samples
Cell growth from CVS-size tissue samples demonstrated the feasibility of obtaining cells from biopsied early gestation chorionic villus tissue within a timeframe meaningful for autologous in utero cellular therapy, as shown in Figure 1. Expansion of PMSCs from CVS-size tissue samples (mean = 35.93 mg) was found to yield over 2 × 106 cells on average by the third passage (n = 6 samples). The first passage of cells to a monolayer occurred at an average of 18.8 d with a minimum and maximum time of 14 and 23 d until passage one, respectively. The cultures reached passage three by day 27 on average, and the longest culture time until passage three was observed to be 29 d.
Figure 1.
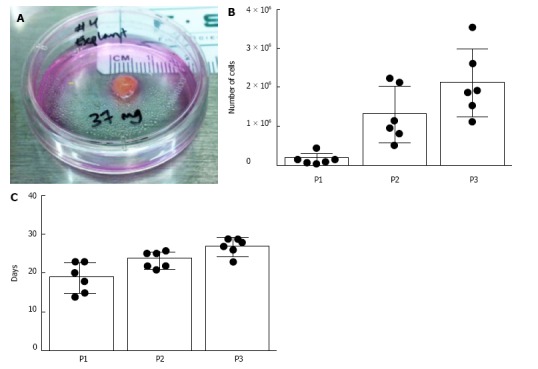
Early passage growth of placenta-derived mesenchymal stromal cells from chorionic villus sampling-size tissues. Placenta-derived mesenchymal stromal cells (PMSCs) were isolated and expanded from chorionic villus sampling (CVS)-size tissue samples (n = 6). A: Photograph of a representative CVS-size mass of human early gestation chorionic villus tissue. The average tissue mass used in this experiment was 35.9 mg; B: Cell expansion data from the first three passages show that an average of 2.09 × 106 cells were obtained by the third passage; C: Average days in vitro until passages 1, 2, and 3 were 18.83, 23.5, and 27 d, respectively.
PMSC display typical MSC surface marker profile and multipotency
Flow cytometry immunophenotyping displayed a profile for PMSCs similar to that typically assigned to mesenchymal stem cells from various sources. As shown in Figure 2A and 2B, cells were observed positive for well-established MSC surface markers CD105 (97.13%), CD90 (96.98%), CD73 (99.28%), CD44 (98.93%), and CD29 (99.58%). Additionally, they were mostly negative for hematopoietic and endothelial-related surface markers CD31 (0.62%), CD34 (1.96%), and CD45 (0.53%) (n = 6). Trilineage differentiation potential was observed for all cell lines assayed and results displayed in Figure 2C. PMSCs exposed to induction media for 2 wk were capable of differentiating into osteocytes and adipocytes, confirmed by Alizarin Red staining to observe calcium deposition and Oil Red staining for lipid accumulation, respectively. In addition, PMSCs were capable of forming chondrocytes when cultured as pellets in induction-specific media for 3 wk, as confirmed by Alcian Blue staining. These results indicate that these cells are multipotent, which is another widely accepted hallmark of traditionally characterized MSCs.
Figure 2.
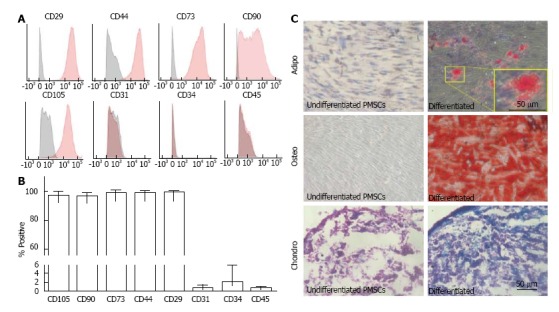
Placenta-derived mesenchymal stromal cells express typical mesenchymal stromal cells surface markers and are multipotent. A, B: Placenta-derived mesenchymal stromal cells (PMSCs) were found to be mostly positive for well-established MSC markers CD29, CD44, CD73, CD90, and CD105, and mostly negative for hematopoietic and endothelial cell markers CD31, CD34, and CD45 (n = 6); C: Cells were multipotent and were capable of differentiating into adipocytes, osteocytes and chondrocytes in vitro. Images in the left column show negative control samples (normal media only) while those on the right display cultures grown in induction media. Yellow boxes provide a magnified view of the selected area. Scale bar = 50 μm.
Immunofluorescence staining of PMSC reveals stem cell phenotype
PMSCs were probed for developmentally significant transcription factors and stem cell-related intracellular proteins in order to describe their phenotype in a developmental context. All cell lines examined displayed positivity for transcription factors Sox10 and Sox17, as well as for intracellular stem cell-related proteins Nestin, S100β, and neurofilament medium (NFM) (Figure 3).
Figure 3.
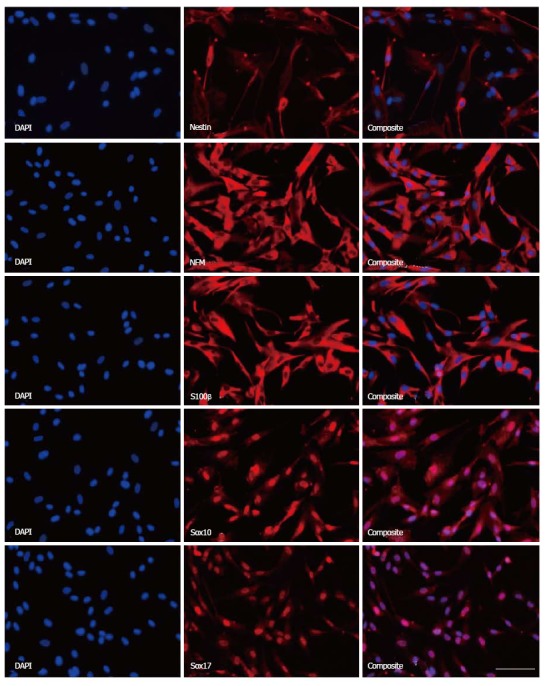
Immunofluorescence staining of placenta-derived mesenchymal stromal cells reveals stem cell phenotype. Placenta-derived mesenchymal stromal cells (PMSCs) were probed using immunofluorescence and found to express intracellular structural proteins Nestin, NFM, and S100β that are often associated with neural-lineage phenotypes. Additionally, PMSCs were positive for developmental transcription factors Sox10 and Sox17. Scale bar = 100 μm. NFM: Neurofilament medium.
Robust cytokine and angiogenic protein secretion by PMSCs
Supernatant from one PMSC line was collected and secreted cytokines were detected using two cytokine array kits together capable of probing for over 70 cytokines. A total of 34 factors with spot density values above 103 were detected in the culture supernatant in both assays combined (Figure 4). Density values for each array showed variable range, with negative control spots having values of around 500. Density maximums were near 57000 in both kits (IL-8 for the angiogenesis array and MCP-1 for the Panel A array). With the angiogenesis array we found 13 of the 30 detected factors (43.3%) to have densities above 10000, compared to 6 of 7 detected factors (85.7%) detected in the Panel A array. The functions of these factors are quite expansive and are detailed in Table 1. Among the most highly detected factors for both kits were cytokines and angiogenic factors such as MCP-1, IL-8, IL-6, GROα, MIF, HGF, Angiopoietin-1, FGF-7, coagulation factor III and VEGF. Inhibitors of angiogenesis such as TIMP-1, Thrombospondin-1, and Pentraxin-3 were notably present as well, indicating the cells can modulate angiogenesis. IGF-binding proteins 2 and 3 were also highly detected, as was extracellular matrix degradation protease μPA and its inhibitor Serpin E1. The presence of an additional 18 factors was observed at lower levels (integrated density < 10000). While most function in some way to modulate angiogenesis, many also have specific function beyond this process. Some of the more unique functions include vessel homeostasis and maturation (Angiopoietin 1 and 2), ribonuclease activity (Angiogenin), and initiating coagulation (Coagulation Factor III). Additional capabilities such as chemotaxis, stimulation of cell growth, and immunomodulation are shared among many of the detected factors.
Figure 4.
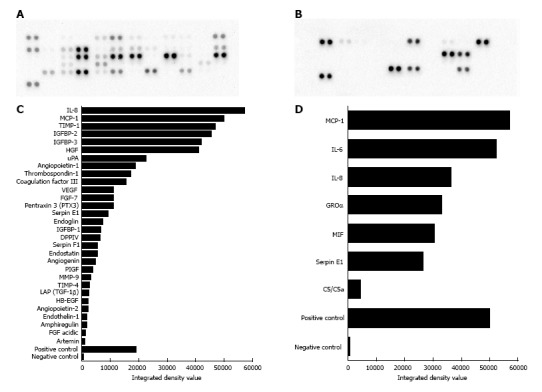
Robust paracrine secretion by placenta-derived mesenchymal stromal cells. A, C: Results from the human angiogenesis array kit detected 30 secreted factors with integrated density values above 103. A: Original membrane image from the Angiogenesis array, and C: corresponding graph showing measured integrated density values of detected spots. Functions of proteins identified here include modulation of angiogenesis, chemotaxis, stimulation of cell proliferation, blood vessel maturation, blood coagulation, and extracellular matrix remodeling. B, D: Results from the panel A array kit detected 7 secreted factors with integrated density values above 103. B: Original membrane image from the Panel A array; D: Corresponding graph showing measured integrated density values of detected spots. Many of these proteins have distinct functions related to the cell chemotaxis and immune response. TGF: Transforming growth factor; IL:Interleukin; VEGF: Vascular endothelial growth factor; GROα: Growth regulated oncogene alpha; MCP-1: Monocyte chemotactic protein 1; uPA: Urokinase-type plasminogen activator.
Table 1.
Functions of placenta-derived mesenchymal stromal cells secreted factors
| Factor | Array | Function | Ref. |
| Amphiregulin | Angiogenesis | Related to EGF, autocrine mitogen, cancer cell inhibition | [51,52] |
| Angiogenin | Angiogenesis | Angiogenesis, ribonuclease, mitogen | [53] |
| Angiopoietin-1 | Angiogenesis | Angiogenesis, TIE2 agonist, vessel homeostasis/maturation | [54] |
| Angiopoietin-2 | Angiogenesis | Angiogenesis, TIE2 antagonist, vessel homeostasis/maturation | [54] |
| Artemin | Angiogenesis | Neurotrophin, endothelial cell migration and proliferation | [55,56] |
| Tissue Factor | Angiogenesis | Angiogenesis, activation of coagulation cascade | [57,58] |
| DPPIV | Angiogenesis | Angiogenesis via ECM degradation and EC invasion | [59] |
| Endoglin | Angiogenesis | Angiogenesis, modulation of effects of TGF-β1 | [60] |
| Endostatin | Angiogenesis | Angiogenesis inhibition by inhibition of EC proliferation | [61] |
| Endothelin-1 | Angiogenesis | Angiogenesis by stimulating EC proliferation and invasion | [62] |
| FGF acidic | Angiogenesis | Angiogenesis, EC mitogen | [63-65] |
| FGF-7 | Angiogenesis | Angiogenesis, neuroprotection, wound repair | [66,67] |
| HB-EGF | Angiogenesis | Chemotaxis, cell growth, angiogenesis | [68] |
| HGF | Angiogenesis | Angiogenesis through EC motility and growth, neuroprotection | [65,69] |
| IGFBP-1 | Angiogenesis | Modulation of IGF activity, cell chemotaxis and adhesion | [70,71] |
| IGFBP-2 | Angiogenesis | Modulation of IGF activity, mitogen, osteogenesis | [70-72] |
| IGFBP-3 | Angiogenesis | Modulation of IGF activity, inhibition of cell proliferation | [70,71] |
| LAP (TGF-1β) | Angiogenesis | Angiogenesis, abundant and varied cell functions | [73,74] |
| MMP-9 | Angiogenesis | Angiogenesis, ECM proteolysis aiding EC migration | [75] |
| Pentraxin 3 (PTX3) | Angiogenesis | Angiogenesis inhibition by binding FGF2, innate immunity | [76,77] |
| PIGF | Angiogenesis | Angiogenesis, related to VEGF family | [78,79] |
| Serpin F1 | Angiogenesis | Angiogenesis inhibition, cell survival, immunomodulation | [80] |
| Thrombospondin-1 | Angiogenesis | Angiogenesis inhibition | [81,82] |
| TIMP-1 | Angiogenesis | Angiogenesis inhibition, neuroprotection | [83-85] |
| TIMP-4 | Angiogenesis | Modulation of angiogenesis, EC migration | [86] |
| uPA | Angiogenesis | ECM proteolysis by converting plasminogen to plasmin | [87] |
| VEGF | Angiogenesis | Angiogenesis, EC migration and proliferation | [88,89] |
| IL-8 | Both | Angiogenesis, leukocyte chemotaxis | [90-92] |
| MCP-1 | Both | Monocyte chemotaxis, macrophage infiltration, angiogenesis | [93,94] |
| Serpin E1 | Both | mPA inhibitor, wound healing, atherosclerosis | [95,96] |
| C5/C5a | Panel A | Anaphylatoxin, chemotaxis, immunomodulation | [97] |
| GROα | Panel A | Chemotaxis, cell growth, thrombin-induced angiogenesis | [98] |
| IL-6 | Panel A | Both pro- and anti-inflammation, myokine, cell metabolism | [99,100] |
| MIF | Panel A | Adaptive/innate immune response, inflammation modulation | [101] |
TGF: Transforming growth factor; IL:Interleukin; VEGF: Vascular endothelial growth factor; GROα: Growth regulated oncogene alpha; MCP-1: Monocyte chemotactic protein 1; uPA: Urokinase-type plasminogen activator.
PMSCs are compatible with delivery vehicle matrices and transducible for in vivo tracking
Cell loading experiments demonstrated that PMSCs are viable on both biological and synthetic delivery vehicle systems. Viable non-transduced cells were observed with green fluorescence in all vehicles tested as well as the two-dimensional control culture (Figure 5A). PMSCs seeded on the scaffolds had normal morphology indicating that cells were well adhered. Little to no red fluorescent staining was observed in each condition, indicating that cells seeded on each matrix remained viable with few, if any, dead cells. Additionally, PMSCs were readily transduced with the lentiviral vector (97.3% of total cell population) as confirmed by microscopy images for GFP-expression as well as by flow cytometry analysis of transduced and non-transduced cells (Figure 5B).
Figure 5.
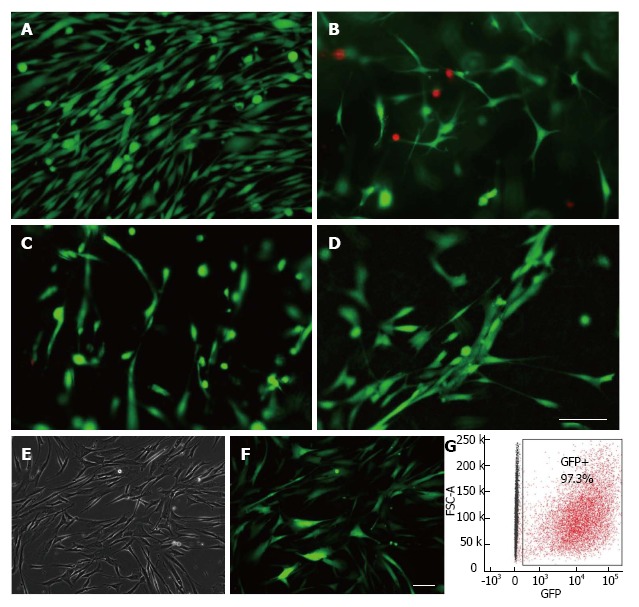
Placenta-derived mesenchymal stromal cells are compatible with biological and synthetic delivery vehicle matrices. A-D: Cell viability was assessed using Molecular Probes Live/Dead fluorescent assay kit for PMSCs cultured in various delivery vehicles. Results show that PMSCs were viable when culture in A: two-dimensional culture (control); B: 2 mg/mL collagen hydrogel; C: 5 mg/mL fibrin glue, D: an aligned PLLA/PLGA nanofiber scaffold. E-G: PMSCs can be efficiently transduced with Luciferase-GFP-containing lentiviral vector; E: phase contrast; F: green fluorescence images from the same field of view of transduced PMSCs; G: Flow cytometry analysis of transduced PMSCs indicate that they were transduced with an efficiency of 97.3%. Scale bars = 100 μm. PMSCs: Placenta-derived mesenchymal stromal cell.
DISCUSSION
Early gestation chorionic villus tissue is a unique cell source, yielding robust mesenchymal stromal cells well-suited for autologous and allogeneic in utero cell therapy and tissue engineering. PMSCs isolated from CVS-size samples were consistently able to generate populations on the order of 106 cells in less than four weeks, indicating that simple explant culture provides feasible means of obtaining cells for an autologous fetal cell therapy or tissue engineering. For example, in utero repair of spina bifida typically occurs before 26 wk gestational age[36], and our data show that CVS tissues obtained early in the second-trimester can produce sufficient cell numbers well before they are needed for the repair. Additionally, other structural and congenital defects such as cardiac malformations and congenital diaphragmatic hernia (CDH) may also be potential targets for autologous, tissue engineered constructs populated with PMSCs[18,32,37].
The in vitro characteristics of early second-trimester PMSCs presented in this study are analogous to those of MSCs isolated from various source tissues[38] in terms of surface marker expression and multipotency. The data also corroborate previous studies aimed at characterizing chorionic villus stromal cells from first and third trimester placentas[20,39,40], showing that PMSCs described here contain similarities to cells typically obtained from placentas younger and older in gestation.
Immunofluorescent staining revealed that PMSCs expressed developmentally significant transcription factors Sox10 and Sox17. Sox10 is critical for neurogenesis and maintenance of multipotency[41-43], while Sox17 functions in the development of definitive endoderm and vasculogenesis[44,45]. The uniformity of expression of these transcription factors in PMSC cultures may reflect the developmental origins of these cells and could serve as predictors of some related functional properties. All examined PMSC lines also expressed structural proteins Nestin, NFM, and S100β. These proteins are often associated with a neural phenotype, but their appearance in cultures of non-fetal MSCs has been previously reported elsewhere[46,47]. Taken in total, these results provide a characteristic phenotypic profile of these cells similar to that of traditionally studied bone marrow MSCs. Still, more work is necessary to better understand how the expression of proteins described above may impact the cell function after in vivo transplantation.
It is widely understood that MSCs derive many of their therapeutic properties from paracrine signaling[48,49]; thus we employed cytokine array assays in order to examine PMSC cytokine secretion. This approach allowed us to identify an abundance of secreted factors with a wide array of functions. Many of the factors function directly or indirectly in the process of angiogenesis, either to stimulate or inhibit its onset. This seems to indicate that PMSCs are able to modulate the angiogenic process, possibly in response to other environmental cues. Indeed, the enormous variety of function in the factors detected seems to indicate that these cells are capable of exerting an extensive paracrine effect on their environment. In the context of the field of tissue engineering where it is necessary to “restore, maintain, or improve tissue function”[50], PMSCs delivered locally to damaged tissue may be capable of enhancing the endogenous wound response through their paracrine function.
Cell fate and a suitable delivery vehicle must be determined in the course of developing a new cell therapy. Of crucial importance in this study is the examination of cell compatibility with several delivery systems capable of carrying and supporting PMSCs for transplantation. We have found that PMSCs are exceptionally well-suited for seeding in several systems capable of delivering cells such as collagen hydrogel and fibrin glue, as well as nanofiber scaffolds made of synthetic polymers. These materials have distinct physical properties in terms of rigidity and biodegradability, making them ideal for tailored approaches to tissue engineering with cell-seeded matrices. These data demonstrate cell attachment and survivability on each of these matrices, which is critical to their effective use for in utero tissue engineering. Support for further investigation into the use of PMSCs for tissue engineering purposes is given by the efficiency at which they can be transduced with viral vectors. We show here that PMSCs are readily transduced (97.3% of total cell population) with a luciferase-GFP-containing lentiviral vector that can aid in tracking the cells after in vivo transplantation. PMSCs transduced with this viral vector can be tracked in two ways: by detection of luciferase expression in live animals injected with the luciferin substrate, and by GFP expression in histological sections after the transplanted animal is sacrificed. Future investigations into the therapeutic function of PMSCs in vivo are enhanced by this technique in that it may allow researchers to gain insight into the ultimate fate of transplanted cells.
In this report, the characterization of early gestation PMSC populations establishes some basic properties of the cells in their surface marker expression and multipotency. Early gestation PMSCs appear to be an excellent candidate cell source for an autologous in utero cellular therapy, especially given their compatibility with several delivery vehicles. However, much work still needs to be completed to determine their potential therapeutic applications and to optimize relevant parameters for transplantation, such as delivery vehicle and cell dosage. In addition, more detailed characterization of cytokine secretion across various cell lines and gestational ages is needed in order to determine the optimal time frame for tissue collection and ex vivo cell expansion. It will also be necessary to characterize changes in cell secretion patterns for cells seeded on delivery vehicles in comparison to normal two-dimensional culture. Still, there is abundant potential for PMSCs to be utilized as a therapeutic cell source for transplantation, especially in the form of an autologous in utero cell therapy.
ACKNOWLEDGMENTS
The authors would like to acknowledge C. Pivetti, B. Keller, M. Miguelino, for their contributions in manuscript review, editing and figure preparation.
COMMENTS
Background
In utero surgery has emerged in recent years as an effective option for early repair of many congenital anamolies and malformations. Additionally, many diseases for which in utero surgery is available may be amenable to augmentation with tissue engineered repair strategies using mesenchymal stromal cells (MSCs). MSCs have been shown to promote wound healing, protect damaged tissues, and modulate the immune system via paracrine secretion. The placenta has recently been described as an ethically unobjectionable and minimally invasive source of fetal MSCs.
Research frontiers
As the field of in utero surgery continues to expand, it is likely that future advances in regenerative medicine such as tissue engineering, stem cell and gene therapy will in turn be incorporated into clinical practice. The placenta represents a unique cell source for both autologous and allogeneic fetal stem cell therapies. Due to the developmental nature of many congenital malformations, fetal-derived cells may confer greater benefit to the fetus than traditional adult multipotent cells such as bone marrow or adipose-derived MSCs.
Innovations and breakthroughs
Early gestation placenta-derived mesenchymal stromal cells (PMSCs) were shown to be expandable to clinicaly relevant populations from chorionic villus sampling (CVS)-sized samples on a timeline applicable to autologous in utero therapy for disorders treated mid-gestation. The sheer variety of factors secreted by PMSCs reported here underscores their ability to affect a broad number of physiologic and cellular processes. Additionally, their compatibility with numerous delivery vehicle systems demonstrate that PMSCs can be surgically applied locally to damaged or injured tissues.
Applications
The results obtained in this study suggest that PMSCs have the potential to be highly therapeutic when used to augment current fetal surgical procedures.
Terminology
The chorionic villi are a functionally and anatomically distinct portion of the placenta. They consist of small, tree-like projections that facilitate nutrient transportation between the maternal and fetal blood supplies. CVS is a technique by which a small portion of chorionic villus tissue can be obtained in a minimally invasive procedure as early as 8 wk of gestation.
Peer reviews
This report from Lankford et al demonstrates the extraction of stromal cells from chorionic-villi suitable for manipulation, expansion, and reimplanation with the ultimate goal of tissue engineering and repair of multiple congenital disease, namely those of the heart and spinal cord. These findings are particularly relevant to the translational field of medicine, as heart conditions and spinal bifida have recently been described as promising targets of in utero repair; thus the cells described here would be an excellent source for repair of these congenital anomalies. The authors have detailed an exciting area of research with solid findings that will be of significant interest to multiple investigators and clinicians in the field.
Footnotes
P- Reviewer: Chen CP, Richardson C, Sharma GT S- Editor: Qi Y L- Editor: A E- Editor: Lu YJ
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: September 6, 2014
First decision: October 14, 2014
Article in press: December 16, 2014
References
- 1.Deprest JA, Flake AW, Gratacos E, Ville Y, Hecher K, Nicolaides K, Johnson MP, Luks FI, Adzick NS, Harrison MR. The making of fetal surgery. Prenat Diagn. 2010;30:653–667. doi: 10.1002/pd.2571. [DOI] [PubMed] [Google Scholar]
- 2.Harrison MR. The University of California at San Francisco Fetal Treatment Center: a personal perspective. Fetal Diagn Ther. 2004;19:513–524. doi: 10.1159/000080165. [DOI] [PubMed] [Google Scholar]
- 3.Adzick NS, Thom EA, Spong CY, Brock JW, 3rd , Burrows PK, Johnson MP, Howell LJ, Farrell JA, Dabrowiak ME, et al. A randomized trial of prenatal versus postnatal repair of myelomeningocele. N Engl J Med. 2011;364:993–1004. doi: 10.1056/NEJMoa1014379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biard JM, Johnson MP, Carr MC, Wilson RD, Hedrick HL, Pavlock C, Adzick NS. Long-term outcomes in children treated by prenatal vesicoamniotic shunting for lower urinary tract obstruction. Obstet Gynecol. 2005;106:503–508. doi: 10.1097/01.AOG.0000171117.38929.eb. [DOI] [PubMed] [Google Scholar]
- 5.Maxson S, Lopez EA, Yoo D, Danilkovitch-Miagkova A, Leroux MA. Concise review: Role of mesenchymal stem cells in wound repair. Stem Cells Transl Med. 2012;1:142–149. doi: 10.5966/sctm.2011-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nuschke A. Activity of mesenchymal stem cells in therapies for chronic skin wound healing. Organogenesis. 2014;10:29–37. doi: 10.4161/org.27405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calzarossa C, Bossolasco P, Besana A, Manca MP, De Grada L, De Coppi P, Giardino D, Silani V, Cova L. Neurorescue effects and stem properties of chorionic villi and amniotic progenitor cells. Neuroscience. 2013;234:158–172. doi: 10.1016/j.neuroscience.2012.12.038. [DOI] [PubMed] [Google Scholar]
- 8.Green EC. Diarrhea and the social marketing of oral rehydration salts in Bangladesh. Soc Sci Med. 1986;23:357–366. doi: 10.1016/0277-9536(86)90078-x. [DOI] [PubMed] [Google Scholar]
- 9.Pittenger MF, Martin BJ. Mesenchymal stem cells and their potential as cardiac therapeutics. Circ Res. 2004;95:9–20. doi: 10.1161/01.RES.0000135902.99383.6f. [DOI] [PubMed] [Google Scholar]
- 10.Farge D, Turner MW, Roy DR, Jothy S. Dyazide-induced reversible acute renal failure associated with intracellular crystal deposition. Am J Kidney Dis. 1986;8:445–449. doi: 10.1016/s0272-6386(86)80173-1. [DOI] [PubMed] [Google Scholar]
- 11.Fleiss B, Guillot PV, Titomanlio L, Baud O, Hagberg H, Gressens P. Stem cell therapy for neonatal brain injury. Clin Perinatol. 2014;41:133–148. doi: 10.1016/j.clp.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 12.Lee JM, Jung J, Lee HJ, Jeong SJ, Cho KJ, Hwang SG, Kim GJ. Comparison of immunomodulatory effects of placenta mesenchymal stem cells with bone marrow and adipose mesenchymal stem cells. Int Immunopharmacol. 2012;13:219–224. doi: 10.1016/j.intimp.2012.03.024. [DOI] [PubMed] [Google Scholar]
- 13.Ringden O, Keating A. Mesenchymal stromal cells as treatment for chronic GVHD. Bone Marrow Transplant. 2011;46:163–164. doi: 10.1038/bmt.2010.275. [DOI] [PubMed] [Google Scholar]
- 14.Manochantr S, U-pratya Y, Kheolamai P, Rojphisan S, Chayosumrit M, Tantrawatpan C, Supokawej A, Issaragrisil S. Immunosuppressive properties of mesenchymal stromal cells derived from amnion, placenta, Wharton’s jelly and umbilical cord. Intern Med J. 2013;43:430–439. doi: 10.1111/imj.12044. [DOI] [PubMed] [Google Scholar]
- 15.Jang MJ, Kim HS, Lee HG, Kim GJ, Jeon HG, Shin HS, Chang SK, Hur GH, Chong SY, Oh D, et al. Placenta-derived mesenchymal stem cells have an immunomodulatory effect that can control acute graft-versus-host disease in mice. Acta Haematol. 2013;129:197–206. doi: 10.1159/000345267. [DOI] [PubMed] [Google Scholar]
- 16.Gucciardo L, Lories R, Ochsenbein-Kölble N, Done’ E, Zwijsen A, Deprest J. Fetal mesenchymal stem cells: isolation, properties and potential use in perinatology and regenerative medicine. BJOG. 2009;116:166–172. doi: 10.1111/j.1471-0528.2008.02005.x. [DOI] [PubMed] [Google Scholar]
- 17.Portmann-Lanz CB, Schoeberlein A, Huber A, Sager R, Malek A, Holzgreve W, Surbek DV. Placental mesenchymal stem cells as potential autologous graft for pre- and perinatal neuroregeneration. Am J Obstet Gynecol. 2006;194:664–673. doi: 10.1016/j.ajog.2006.01.101. [DOI] [PubMed] [Google Scholar]
- 18.Schmidt D, Mol A, Breymann C, Achermann J, Odermatt B, Gössi M, Neuenschwander S, Prêtre R, Genoni M, Zund G, et al. Living autologous heart valves engineered from human prenatally harvested progenitors. Circulation. 2006;114:I125–I131. doi: 10.1161/CIRCULATIONAHA.105.001040. [DOI] [PubMed] [Google Scholar]
- 19.Isakova IA, Lanclos C, Bruhn J, Kuroda MJ, Baker KC, Krishnappa V, Phinney DG. Allo-reactivity of mesenchymal stem cells in rhesus macaques is dose and haplotype dependent and limits durable cell engraftment in vivo. PloS one. 2014;9:e87238. doi: 10.1371/journal.pone.0087238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poloni A, Rosini V, Mondini E, Maurizi G, Mancini S, Discepoli G, Biasio S, Battaglini G, Berardinelli E, Serrani F, et al. Characterization and expansion of mesenchymal progenitor cells from first-trimester chorionic villi of human placenta. Cytotherapy. 2008;10:690–697. doi: 10.1080/14653240802419310. [DOI] [PubMed] [Google Scholar]
- 21.Poloni A, Maurizi G, Serrani F, Mancini S, Discepoli G, Tranquilli AL, Bencivenga R, Leoni P. Human AB serum for generation of mesenchymal stem cells from human chorionic villi: comparison with other source and other media including platelet lysate. Cell Prolif. 2012;45:66–75. doi: 10.1111/j.1365-2184.2011.00799.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raynaud CM, Maleki M, Lis R, Ahmed B, Al-Azwani I, Malek J, Safadi FF, Rafii A. Comprehensive characterization of mesenchymal stem cells from human placenta and fetal membrane and their response to osteoactivin stimulation. Stem Cells Int. 2012:2012. doi: 10.1155/2012/658356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mohr S, Portmann-Lanz CB, Schoeberlein A, Sager R, Surbek DV. Generation of an osteogenic graft from human placenta and placenta-derived mesenchymal stem cells. Reprod Sci. 2010;17:1006–1015. doi: 10.1177/1933719110377471. [DOI] [PubMed] [Google Scholar]
- 24.Jin J, Wang J, Huang J, Huang F, Fu J, Yang X, Miao Z. Transplantation of human placenta-derived mesenchymal stem cells in a silk fibroin/hydroxyapatite scaffold improves bone repair in rabbits. J Biosci Bioeng. 2014;118:593–598. doi: 10.1016/j.jbiosc.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 25.Hsu SH, Huang TB, Cheng SJ, Weng SY, Tsai CL, Tseng CS, Chen DC, Liu TY, Fu KY, Yen BL. Chondrogenesis from human placenta-derived mesenchymal stem cells in three-dimensional scaffolds for cartilage tissue engineering. Tissue Eng Part A. 2011;17:1549–1560. doi: 10.1089/ten.TEA.2010.0419. [DOI] [PubMed] [Google Scholar]
- 26.Li F, Chen YZ, Miao ZN, Zheng SY, Jin J. Human placenta-derived mesenchymal stem cells with silk fibroin biomaterial in the repair of articular cartilage defects. Cell Reprogram. 2012;14:334–341. doi: 10.1089/cell.2012.0002. [DOI] [PubMed] [Google Scholar]
- 27.Zhang X, Mitsuru A, Igura K, Takahashi K, Ichinose S, Yamaguchi S, Takahashi TA. Mesenchymal progenitor cells derived from chorionic villi of human placenta for cartilage tissue engineering. Biochem Biophys Res Commun. 2006;340:944–952. doi: 10.1016/j.bbrc.2005.12.091. [DOI] [PubMed] [Google Scholar]
- 28.Arakawa R, Aoki R, Arakawa M, Saito K. Human first-trimester chorionic villi have a myogenic potential. Cell Tissue Res. 2012;348:189–197. doi: 10.1007/s00441-012-1340-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Longo UG, Loppini M, Berton A, La Verde L, Khan WS, Denaro V. Stem cells from umbilical cord and placenta for musculoskeletal tissue engineering. Curr Stem Cell Res Ther. 2012;7:272–281. doi: 10.2174/157488812800793054. [DOI] [PubMed] [Google Scholar]
- 30.Park TS, Gavina M, Chen CW, Sun B, Teng PN, Huard J, Deasy BM, Zimmerlin L, Peault B. Placental perivascular cells for human muscle regeneration. Stem Cells Dev. 2011;20:451–463. doi: 10.1089/scd.2010.0354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kadam S, Govindasamy V, Bhonde R. Generation of functional islets from human umbilical cord and placenta derived mesenchymal stem cells. Methods Mol Biol. 2012;879:291–313. doi: 10.1007/978-1-61779-815-3_17. [DOI] [PubMed] [Google Scholar]
- 32.Weber B, Zeisberger SM, Hoerstrup SP. Prenatally harvested cells for cardiovascular tissue engineering: fabrication of autologous implants prior to birth. Placenta. 2011;32 Suppl 4:S316–S319. doi: 10.1016/j.placenta.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 33.Lisi A, Briganti E, Ledda M, Losi P, Grimaldi S, Marchese R, Soldani G. A combined synthetic-fibrin scaffold supports growth and cardiomyogenic commitment of human placental derived stem cells. PloS one. 2012;7:e34284. doi: 10.1371/journal.pone.0034284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Makhoul G, Chiu RC, Cecere R. Placental mesenchymal stem cells: a unique source for cellular cardiomyoplasty. Ann Thorac Surg. 2013;95:1827–1833. doi: 10.1016/j.athoracsur.2012.11.053. [DOI] [PubMed] [Google Scholar]
- 35.Jones GN, Moschidou D, Puga-Iglesias TI, Kuleszewicz K, Vanleene M, Shefelbine SJ, Bou-Gharios G, Fisk NM, David AL, De Coppi P, et al. Ontological differences in first compared to third trimester human fetal placental chorionic stem cells. PloS one. 2012;7:e43395. doi: 10.1371/journal.pone.0043395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saadai P, Runyon T, Farmer DL. Fetal neurosurgery: Current state of the art. Future Neurol. 2011;6:165–171. doi: 10.2217/fnl.11.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fuchs JR, Kaviani A, Oh JT, LaVan D, Udagawa T, Jennings RW, Wilson JM, Fauza DO. Diaphragmatic reconstruction with autologous tendon engineered from mesenchymal amniocytes. J Pediatr Surg. 2004;39:834–838; discussion 834-838. doi: 10.1016/j.jpedsurg.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 38.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 39.Abumaree MH, Al Jumah MA, Kalionis B, Jawdat D, Al Khaldi A, AlTalabani AA, Knawy BA. Phenotypic and functional characterization of mesenchymal stem cells from chorionic villi of human term placenta. Stem Cell Rev. 2013;9:16–31. doi: 10.1007/s12015-012-9385-4. [DOI] [PubMed] [Google Scholar]
- 40.Igura K, Zhang X, Takahashi K, Mitsuru A, Yamaguchi S, Takashi TA. Isolation and characterization of mesenchymal progenitor cells from chorionic villi of human placenta. Cytotherapy. 2004;6:543–553. doi: 10.1080/14653240410005366-1. [DOI] [PubMed] [Google Scholar]
- 41.Britsch S, Goerich DE, Rietchmacher D, Peirano RI, Rossner M, Nave KA, Birchmeier C, Wegner M. The transcription factor sox10 is a key regulator of peripheral glial development. Genes Dev. 2001;15:66–78. doi: 10.1101/gad.186601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kelsh RN. Sorting out Sox10 functions in neural crest development. Bioessays. 2006;28:788–798. doi: 10.1002/bies.20445. [DOI] [PubMed] [Google Scholar]
- 43.Kim J, Lo L, Dormand E, Anderson DJ. SOX10 maintains multipotency and inhibits neuronal differentiation of neural crest stem cells. Neuron. 2003;38:17–31. doi: 10.1016/s0896-6273(03)00163-6. [DOI] [PubMed] [Google Scholar]
- 44.Lee SH, Lee S, Yang H, Song S, Kim K, Saunders TL, Yoon JK, Koh GY, Kim I. Notch pathway targets proangiogenic regulator Sox17 to restrict angiogenesis. Circ Res. 2014;115:215–226. doi: 10.1161/CIRCRESAHA.115.303142. [DOI] [PubMed] [Google Scholar]
- 45.Tam PP, Kanai-Azuma M, Kanai Y. Early endoderm development in vertebrates: lineage differentiation and morphogenetic function. Curr Opin Genet Dev. 2003;13:393–400. doi: 10.1016/s0959-437x(03)00085-6. [DOI] [PubMed] [Google Scholar]
- 46.Deng J, Petersen BE, Steindler DA, Jorgensen ML, Laywell ED. Mesenchymal stem cells spontaneously express neural proteins in culture and are neurogenic after transplantation. Stem Cells. 2006;24:1054–1064. doi: 10.1634/stemcells.2005-0370. [DOI] [PubMed] [Google Scholar]
- 47.Tondreau T, Lagneaux L, Dejeneffe M, Massy M, Mortier C, Delforge A, Bron D. Bone marrow-derived mesenchymal stem cells already express specific neural proteins before any differentiation. Differentiation. 2004;72:319–326. doi: 10.1111/j.1432-0436.2004.07207003.x. [DOI] [PubMed] [Google Scholar]
- 48.Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circulation Res. 2008;103:1204–1219. doi: 10.1161/CIRCRESAHA.108.176826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Murphy MB, Moncivais K, Caplan AI. Mesenchymal stem cells: Environmentally responsive therapeutics for regenerative medicine. Exp Mol Med. 2013;45:e54. doi: 10.1038/emm.2013.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Langer R, Vacanti JP. Tissue engineering. Science. 1993;260:920–926. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 51.Shoyab M, Plowman GD, McDonald VL, Bradley JG, Todaro GJ. Structure and function of human amphiregulin: a member of the epidermal growth factor family. Science. 1989;243:1074–1076. doi: 10.1126/science.2466334. [DOI] [PubMed] [Google Scholar]
- 52.Plowman GD, Green JM, McDonald VL, Neubauer MG, Disteche CM, Todaro GJ, Shoyab M. The amphiregulin gene encodes a novel epidermal growth factor-related protein with tumor-inhibitory activity. Mol Cell Biol. 1990;10:1969–1981. doi: 10.1128/mcb.10.5.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shestenko OP, Nikonov SD, Mertvetsov NP. [Angiogenin and its role in angiogenesis] Mol Biol (Mosk) 2001;35:349–371. [PubMed] [Google Scholar]
- 54.Augustin HG, Koh GY, Thurston G, Alitalo K. Control of vascular morphogenesis and homeostasis through the angiopoietin-Tie system. Nat Rev Mol Cell Biol. 2009;10:165–177. doi: 10.1038/nrm2639. [DOI] [PubMed] [Google Scholar]
- 55.Baloh RH, Tansey MG, Lampe PA, Fahrner TJ, Enomoto H, Simburger KS, Leitner ML, Araki T, Johnson EM, Milbrandt J. Artemin, a novel member of the GDNF ligand family, supports peripheral and central neurons and signals through the GFRalpha3-RET receptor complex. Neuron. 1998;21:1291–1302. doi: 10.1016/s0896-6273(00)80649-2. [DOI] [PubMed] [Google Scholar]
- 56.Banerjee A, Wu ZS, Qian PX, Kang J, Liu DX, Zhu T, Lobie PE. Artemin promotes de novo angiogenesis in er negative mammary carcinoma through activation of twist1-vegf-a signalling. PLoS one. 2012;7:e50098. doi: 10.1371/journal.pone.0050098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Butenas S, Orfeo T, Mann KG. Tissue factor activity and function in blood coagulation. Thromb Res. 2008;122 Suppl 1:S42–S46. doi: 10.1016/S0049-3848(08)70018-5. [DOI] [PubMed] [Google Scholar]
- 58.Ruf W, Yokota N, Schaffner F. Tissue factor in cancer progression and angiogenesis. Thrombosis Res. 2010;125 Suppl 2:S36–S38. doi: 10.1016/S0049-3848(10)70010-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ghersi G, Zhao Q, Salamone M, Yeh Y, Zucker S, Chen WT. The protease complex consisting of dipeptidyl peptidase iv and seprase plays a role in the migration and invasion of human endothelial cells in collagenous matrices. Cancer Res. 2006;66:4652–4661. doi: 10.1158/0008-5472.CAN-05-1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nassiri F, Cusimano MD, Scheithauer BW, Rotondo F, Fazio A, Yousef GM, Syro LV, Kovacs K, Lloyd RV. Endoglin (CD105): a review of its role in angiogenesis and tumor diagnosis, progression and therapy. Anticancer Res. 2011;31:2283–2290. [PubMed] [Google Scholar]
- 61.O’Reilly MS, Boehm T, Shing Y, Fukai N, Vasios G, Lane WS, Flynn E, Birkhead JR, Olsen BR, Folkman J. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell. 1997;88:277–285. doi: 10.1016/s0092-8674(00)81848-6. [DOI] [PubMed] [Google Scholar]
- 62.Salani D, Taraboletti G, Rosano L, Di Castro V, Borsotti P, Giavazzi R, Bagnato A. Endothelin-1 induces an angiogenic phenotype in cultured endothelial cells and stimulates neovascularization in vivo. Am J Pathol. 2000;157:1703–1711. doi: 10.1016/S0002-9440(10)64807-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rosengart TK, Budenbender KT, Duenas M, Mack CA, Zhang QX, Isom OW. Therapeutic angiogenesis: a comparative study of the angiogenic potential of acidic fibroblast growth factor and heparin. J Vasc Surg. 1997;26:302–312. doi: 10.1016/s0741-5214(97)70193-9. [DOI] [PubMed] [Google Scholar]
- 64.Klagsbrun M. The fibroblast growth factor family: structural and biological properties. Prog Growth Factor Res. 1989;1:207–235. doi: 10.1016/0955-2235(89)90012-4. [DOI] [PubMed] [Google Scholar]
- 65.Hossain MA, Russell JC, Gomez R, Laterra J. Neuroprotection by scatter factor/hepatocyte growth factor and FGF-1 in cerebellar granule neurons is phosphatidylinositol 3-kinase/akt-dependent and MAPK/CREB-independent. J Neurochem. 2002;81:365–378. doi: 10.1046/j.1471-4159.2002.00837.x. [DOI] [PubMed] [Google Scholar]
- 66.Gillis P, Savla U, Volpert OV, Jimenez B, Waters CM, Panos RJ, Bouck NP. Keratinocyte growth factor induces angiogenesis and protects endothelial barrier function. J Cell Sci. 1999;112(Pt 12):2049–2057. doi: 10.1242/jcs.112.12.2049. [DOI] [PubMed] [Google Scholar]
- 67.Beer HD, Gassmann MG, Munz B, Steiling H, Engelhardt F, Bleuel K, Werner S. Expression and function of keratinocyte growth factor and activin in skin morphogenesis and cutaneous wound repair. J Investig Dermatol Symp Proc. 2000;5:34–39. doi: 10.1046/j.1087-0024.2000.00009.x. [DOI] [PubMed] [Google Scholar]
- 68.Ongusaha PP, Kwak JC, Zwible AJ, Macip S, Higashiyama S, Taniguchi N, Fang L, Lee SW. HB-EGF is a potent inducer of tumor growth and angiogenesis. Cancer Res. 2004;64:5283–5290. doi: 10.1158/0008-5472.CAN-04-0925. [DOI] [PubMed] [Google Scholar]
- 69.Bussolino F, Di Renzo MF, Ziche M, Bocchietto E, Olivero M, Naldini L, Gaudino G, Tamagnone L, Coffer A, Comoglio PM. Hepatocyte growth factor is a potent angiogenic factor which stimulates endothelial cell motility and growth. J Cell biol. 1992;119:629–641. doi: 10.1083/jcb.119.3.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rajaram S, Baylink DJ, Mohan S. Insulin-like growth factor-binding proteins in serum and other biological fluids: regulation and functions. Endocr Rev. 1997;18:801–831. doi: 10.1210/edrv.18.6.0321. [DOI] [PubMed] [Google Scholar]
- 71.Firth SM, Baxter RC. Cellular actions of the insulin-like growth factor binding proteins. Endocr Rev. 2002;23:824–854. doi: 10.1210/er.2001-0033. [DOI] [PubMed] [Google Scholar]
- 72.Hamidouche Z, Fromigue O, Ringe J, Haupl T, Marie PJ. Crosstalks between integrin alpha 5 and igf2/igfbp2 signalling trigger human bone marrow-derived mesenchymal stromal osteogenic differentiation. BMC Cell Biol. 2010;11:44. doi: 10.1186/1471-2121-11-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Barnard JA, Lyons RM, Moses HL. The cell biology of transforming growth factor beta. Biochim Biophys Acta. 1990;1032:79–87. doi: 10.1016/0304-419x(90)90013-q. [DOI] [PubMed] [Google Scholar]
- 74.Ferrari G, Cook BD, Terushkin V, Pintucci G, Mignatti P. Transforming growth factor-beta 1 (tgf-beta1) induces angiogenesis through vascular endothelial growth factor (vegf)-mediated apoptosis. J Cell Physiol. 2009;219:449–458. doi: 10.1002/jcp.21706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.van Hinsbergh VW, Koolwijk P. Endothelial sprouting and angiogenesis: matrix metalloproteinases in the lead. Cardiovasc Res. 2008;78:203–212. doi: 10.1093/cvr/cvm102. [DOI] [PubMed] [Google Scholar]
- 76.Rusnati M, Camozzi M, Moroni E, Bottazzi B, Peri G, Indraccolo S, Amadori A, Mantovani A, Presta M. Selective recognition of fibroblast growth factor-2 by the long pentraxin ptx3 inhibits angiogenesis. Blood. 2004;104:92–99. doi: 10.1182/blood-2003-10-3433. [DOI] [PubMed] [Google Scholar]
- 77.Bottazzi B, Bastone A, Doni A, Garlanda C, Valentino S, Deban L, Maina V, Cotena A, Moalli F, Vago L, et al. The long pentraxin PTX3 as a link among innate immunity, inflammation, and female fertility. J Leukoc Biol. 2006;79:909–912. doi: 10.1189/jlb.1005557. [DOI] [PubMed] [Google Scholar]
- 78.Park JE, Chen HH, Winer J, Houck KA, Ferrara N. Placenta growth factor. Potentiation of vascular endothelial growth factor bioactivity, in vitro and in vivo, and high affinity binding to Flt-1 but not to Flk-1/KDR. J Biol Chem. 1994;269:25646–25654. [PubMed] [Google Scholar]
- 79.Luttun A, Tjwa M, Carmeliet P. Placental growth factor (plgf) and its receptor flt-1 (vegfr-1): Novel therapeutic targets for angiogenic disorders. Ann N Y Acad Sci. 2002;979:80–93. doi: 10.1111/j.1749-6632.2002.tb04870.x. [DOI] [PubMed] [Google Scholar]
- 80.Filleur S, Nelius T, de Riese W, Kennedy RC. Characterization of pedf: A multi-functional serpin family protein. J Cell Biochem. 2009;106:769–775. doi: 10.1002/jcb.22072. [DOI] [PubMed] [Google Scholar]
- 81.Klenotic PA, Page RC, Li W, Amick J, Misra S, Silverstein RL. Molecular basis of antiangiogenic thrombospondin-1 type 1 repeat domain interactions with cd36. Arterioscler Thromb Vasc Biol. 2013;33:1655–1662. doi: 10.1161/ATVBAHA.113.301523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lawler J. Thrombospondin-1 as an endogenous inhibitor of angiogenesis and tumor growth. J Cell Mol Med. 2002;6:1–12. doi: 10.1111/j.1582-4934.2002.tb00307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ikenaka Y, Yoshiji H, Kuriyama S, Yoshii J, Noguchi R, Tsujinoue H, Yanase K, Namisaki T, Imazu H, Masaki T, et al. Tissue inhibitor of metalloproteinases-1 (timp-1) inhibits tumor growth and angiogenesis in the timp-1 transgenic mouse model. J Int Cancer. 2003;105:340–346. doi: 10.1002/ijc.11094. [DOI] [PubMed] [Google Scholar]
- 84.Reed MJ, Koike T, Sadoun E, Sage EH, Puolakkainen P. Inhibition of TIMP1 enhances angiogenesis in vivo and cell migration in vitro. Microvasc Res. 2003;65:9–17. doi: 10.1016/s0026-2862(02)00026-2. [DOI] [PubMed] [Google Scholar]
- 85.Tejima E, Guo S, Murata Y, Arai K, Lok J, van Leyen K, Rosell A, Wang X, Lo EH. Neuroprotective effects of overexpressing tissue inhibitor of metalloproteinase timp-1. J Neurotrauma. 2009;26:1935–1941. doi: 10.1089/neu.2009.0959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fernández CA, Moses MA. Modulation of angiogenesis by tissue inhibitor of metalloproteinase-4. Biochem Biophys Res Commun. 2006;345:523–529. doi: 10.1016/j.bbrc.2006.04.083. [DOI] [PubMed] [Google Scholar]
- 87.Vincenza Carriero M, Franco P, Vocca I, Alfano D, Longanesi-Cattani I, Bifulco K, Mancini A, Caputi M, Stoppelli MP. Structure, function and antagonists of urokinase-type plasminogen activator. Front Biosci (Landmark Ed) 2009;14:3782–3794. doi: 10.2741/3488. [DOI] [PubMed] [Google Scholar]
- 88.Hoeben A, Landuyt B, Highley MS, Wildiers H, Van Oosterom AT, De Bruijn EA. Vascular endothelial growth factor and angiogenesis. Pharmacol Rev. 2004;56:549–580. doi: 10.1124/pr.56.4.3. [DOI] [PubMed] [Google Scholar]
- 89.Lamalice L, Le Boeuf F, Huot J. Endothelial cell migration during angiogenesis. Circ Res. 2007;100:782–794. doi: 10.1161/01.RES.0000259593.07661.1e. [DOI] [PubMed] [Google Scholar]
- 90.Li A, Dubey S, Varney ML, Dave BJ, Singh RK. IL-8 directly enhanced endothelial cell survival, proliferation, and matrix metalloproteinases production and regulated angiogenesis. J Immunol. 2003;170:3369–3376. doi: 10.4049/jimmunol.170.6.3369. [DOI] [PubMed] [Google Scholar]
- 91.Baggiolini M, Clark-Lewis I. Interleukin-8, a chemotactic and inflammatory cytokine. FEBS Lett. 1992;307:97–101. doi: 10.1016/0014-5793(92)80909-z. [DOI] [PubMed] [Google Scholar]
- 92.Mukaida N, Harada A, Yasumoto K, Matsushima K. Properties of pro-inflammatory cell type-specific leukocyte chemotactic cytokines, interleukin 8 (IL-8) and monocyte chemotactic and activating factor (MCAF) Microbiol Immunol. 1992;36:773–789. doi: 10.1111/j.1348-0421.1992.tb02080.x. [DOI] [PubMed] [Google Scholar]
- 93.Mulé SJ, Casella GA. Quantitation and confirmation of the diazolo- and triazolobenzodiazepines in human urine by gas chromatography/mass spectrometry. J Anal Toxicol. 1989;13:179–184. doi: 10.1093/jat/13.3.179. [DOI] [PubMed] [Google Scholar]
- 94.Ohta M, Kitadai Y, Tanaka S, Yoshihara M, Yasui W, Mukaida N, Haruma K, Chayama K. Monocyte chemoattractant protein-1 expression correlates with macrophage infiltration and tumor vascularity in human gastric carcinomas. Int J Oncol. 2003;22:773–778. [PubMed] [Google Scholar]
- 95.Durand MK, Bødker JS, Christensen A, Dupont DM, Hansen M, Jensen JK, Kjelgaard S, Mathiasen L, Pedersen KE, Skeldal S, et al. Plasminogen activator inhibitor-I and tumour growth, invasion, and metastasis. Thromb Haemost. 2004;91:438–449. doi: 10.1160/TH03-12-0784. [DOI] [PubMed] [Google Scholar]
- 96.Lijnen HR. Pleiotropic functions of plasminogen activator inhibitor-1. J Thromb Haemost. 2005;3:35–45. doi: 10.1111/j.1538-7836.2004.00827.x. [DOI] [PubMed] [Google Scholar]
- 97.Perez HD. Biologically active complement (C5)-derived peptides and their relevance to disease. Crit Rev Oncol Hematol. 1984;1:199–225. doi: 10.1016/s1040-8428(84)80012-8. [DOI] [PubMed] [Google Scholar]
- 98.Bechara C, Chai H, Lin PH, Yao Q, Chen C. Growth related oncogene-alpha (GRO-alpha): roles in atherosclerosis, angiogenesis and other inflammatory conditions. Med Sci Monit. 2007;13:RA87–RA90. [PubMed] [Google Scholar]
- 99.Scheller J, Chalaris A, Schmidt-Arras D, Rose-John S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta. 2011;1813:878–888. doi: 10.1016/j.bbamcr.2011.01.034. [DOI] [PubMed] [Google Scholar]
- 100.Pedersen BK, Febbraio MA. Muscle as an endocrine organ: focus on muscle-derived interleukin-6. Physiol Rev. 2008;88:1379–1406. doi: 10.1152/physrev.90100.2007. [DOI] [PubMed] [Google Scholar]
- 101.Baugh JA, Donnelly SC. Macrophage migration inhibitory factor: a neuroendocrine modulator of chronic inflammation. J Endocrinol. 2003;179:15–23. doi: 10.1677/joe.0.1790015. [DOI] [PubMed] [Google Scholar]


