Abstract
How neuroinflammation affects signaling pathways leading to human blood–brain barrier (BBB) dysfunction during HIV-1 infection is incompletely understood. We previously demonstrated that signal transducers and activators of transcription-1 (STAT1) signaling is involved in HIV-1 induced BBB damage and is relevant to viral neuropathogenesis. The objective of this study was to delineate the signaling pathways upstream and downstream of STAT1 involved in HIV-1-induced endothelial dysfunction. We show that HIV-1 activation of STAT1 and STAT3 in human brain microvascular endothelial cells (HBMEC) is associated with induction of promoter activity of the interferon-stimulated response element (ISRE)/interferon-γ-activated sequence (GAS). The STAT1 inhibitor fludarabine diminished HIV-1-induced ISRE/GAS promoter activity. CCR5 neutralizing antibodies and the phosphoinositide-3-kinase (PI3K) inhibitor LY-294002 diminished HIV-1-induced phosphorylation of STAT1 and STAT3, significantly diminished HIV-1-induced ISRE/GAS promoter activity, and diminished virus-induced monocyte adhesion and transendothelial migration. HIV-1 infection did not phosphorylate janus kinases but induced activation of the phosphoinositide-dependent kinase-1 (PDK1) and the serine-threonine protein kinase AKT, both downstream effectors of PI3K. CCR5 antibodies also diminished virus-induced phosphorylation of PDK1 and AKT. These results suggest that the chemokine receptor CCR5 is partially involved in HIV-1 binding to HBMEC and show cross-talk between STAT1 and PI3K pathways in HIV-1-induced BBB dysfunction.
Keywords: HIV-1, brain endothelial cell injury, CCR5, PI3K, STAT1
Human brain microvascular endothelial cells (HBMEC) are the major component of blood–brain barrier (BBB) function and integrity and are connected by tight junctions (TJ) that limit paracellular flux and restrict permeability (Hawkins and Davis, 2005; Kanmogne et al., 2005). Under normal physiologic conditions, the BBB functions as an interface between the blood and the brain parenchyma, strictly regulating influx of ions, molecules, and leukocytes into the central nervous system (CNS). However, during progressive HIV-1 infection, the BBB is compromised, and its barrier function breaks down, causing enhanced penetration of cell-free virus and HIV-infected leukocytes into the CNS. This viral infection of the CNS commonly results in behavioral, motor, and cognitive impairments termed HIV-1-associated neurocognitive disorders (HAND; Ghafouri et al., 2006; Antinori et al., 2007). HIV-induced BBB dysfunction has been documented in laboratory animal models, human clinical observations, and autopsy studies (Burger et al., 1997; Dallasta et al., 1999; Kanmogne et al., 2005, 2007). However, the mechanisms of HIV-1-induced BBB dysfunction are poorly understood. Elucidation of the signaling pathways mediating BBB compromise and how they affect ongoing disease may help in the development of new therapies to prevent viral entry into the brain and neuro-AIDS.
In the HIV/AIDS disease process, inflammatory cytokines such as of IL-8, IL-6, IP-10, MCP-1, and Gro-α are produced at high levels and allow the BBB to be more easily breached, enhancing infiltration of infected cells into the CNS (Toborek et al., 2005; Banks et al., 2006; Eugenin et al., 2006). Most of these cytokines signal through the signal transducers and activators of transcription (STAT) pathway, which has been shown to play a prominent role in cytokine-mediated inflammatory responses and has been implicated in the pathogenesis of HIV infection and disease progression (Bovolenta et al., 1999; Decker, 1999). In the classical STAT pathway, STAT proteins are phosphorylated by janus kinases (JAK) in response to cytokines. There is also evidence that phosphorylation of STAT1 and STAT3 can be mediated by phosphoinositide-3-kinase (PI3K) and mitogen-activated protein kinase (MAPK) cascades independent of JAK (Nguyen et al., 2001; Haq et al., 2002). The phosphorylated STATs form dimers and associate with the interferon (IFN)-stimulated gene factor 3γ to form a complex transcription factor that translocates to the nucleus, binds to the IFN-stimulated response element (ISRE)/interferon-γ-activated site (GAS), and subsequently activates the transcription of interferon or cytokine stimulated genes (Schindler and Brutsaert, 1999).
HIV enters target cells using the CD4 receptor and/or coreceptors such as CCR5 and CXCR4 (for a recent review see Moore et al., 2004). Our previous works showed that primary HBMEC do not express CD4 but do express the chemokine receptors CCR5 and CXCR4 (Kanmogne et al., 2000, 2007). We also demonstrated that STAT1 signaling modulates HIV-1-induced inflammatory responses and leukocyte transmigration across the BBB (Chaudhuri et al., 2008b). The current study investigates the effect of HIV-1 on STAT-1 transcriptional activity in endothelial cells. We further determined the cellular receptor used by HIV-1 to bind and activate STAT proteins in HBMEC and delineated the signaling pathways upstream of STAT1 involved in HIV-1-induced endothelial dysfunction.
MATERIALS AND METHODS
Brain Endothelial Cell Culture and Cytotoxicity Assays
Primary HBMEC were isolated from brain tissue obtained during surgical removal of epileptogenic cerebral cortex in adult patients as described previously (Kanmogne et al., 2007) and were provided by Dr. Marlys Witte and Dr. Michael Bernas (University of Arizona, Tuscon, AZ). These brain tissues were collected under an IRB-approved protocol at the University of Arizona. Routine evaluation for von Willebrand factor, Ulex europeus lectin, and CD31 demonstrated that cells were >99% pure. Freshly isolated cells were cultured as we previously described, and cells at passages 2–4 were used in this study. To determine any potential toxic effects of STAT1 and PI3K inhibitors on the brain endothelium, HBMEC were exposed to 1–100 μM fludarabine (Sigma, St. Louis, MO) or LY-294002 (LY; Sigma), and cytotoxicity was assessed over 24 hr with an alamarBlue assay (AbD Serotec, Raleigh, NC) according to the manufacturer’s instructions. To determine any direct effect of HIV-1 on endothelial cell viability and phenotype, HBMEC were exposed to HIV-1 virions [HIV-1ADA at multiplicity of infection (MOI) 0.01] for 30 min to 2 hr, and cell morphology was assessed by microscopy.
Monocyte Isolation and HIV-1 Infection
Human monocytes were obtained from HIV-1-, HIV-2-, and hepatitis B-seronegative donor leukopaks and separated by countercurrent centrifugal elutriation as previously described (Kanmogne et al., 2007). Cells were identified as >98% pure monocytes by Wright staining and CD68 immunostaining (at 1:50 dilution; Dako, Carpentaria, CA). Monocytes were used for adhesion and transendothelial migration assays within 24 hr of elutriation. For infection, monocytes were exposed to HIV-1ADA (MOI 0.01) for 4 hr, pelleted by centrifugation (300g, 10 min), and washed twice with serum-free medium to eliminate viral inoculum. These infected and control noninfected monocytes were used for adhesion and migration experiments. The HIV-1ADA used in this study came from our departmental stock of viral isolates and is also available through the NIH AIDS Research and Reference Reagent Program.
Monocyte Adhesion and Migration Through an In Vitro BBB Model
For adhesion assays, HBMEC were plated on 96-well collagen-coated black plates and cultured to confluence. For experiments testing the involvement of CCR5 and PI3K pathways, HBMEC were exposed to CCR5 neutralizing antibodies (20 μg/ml, R&D Systems, Minneapolis, MN) or LY (50 μM) for 30 min before adhesion. For experiments testing the effects of cytokines, HBMEC were treated with interleukin (IL)-6 (100 ng/ml; Cell Signaling, Danvers, MA) or IL-8 (100 ng/ml, R&D Systems) for 2 hr before adhesion. Cells were rinsed to remove all inhibitors and cytokines and were exposed for 15 min to 2.5 × 105 control noninfected monocytes or HIV-1-infected monocytes labeled with calcein-AM (Invitrogen, Carlsbad, CA) at 5 μM/1 × 106 cells for 45 min. After 15 min of adhesion, HBMEC were washed, and the number of adherent monocytes was quantified by spectrophotometry (absorbance 494 nm; emission 517 nm), with a standard curve derived from a serial dilution of a known number of calcein-labeled cells. Migration experiments were performed as we previously described (Chaudhuri et al., 2008b). For migration experiments testing the involvement of CCR5 and effects of PI3K inhibitors, HBMEC were exposed to CCR5 antibodies and LY for 30 min before migration.
Luciferase Reporter Construct
Oligonucleotides consisting of a combination of ISRE/GAS element (Cramer et al., 2000) were cloned into the reporter plasmid pGL4.26 (Promega, Madison, WI). The synthesized oligonucleotide contained three ISRE/GAS elements ligated to the frame and additional restriction site bases to facilitate the cloning. Individual clones were sequenced at the High-Throughput DNA Sequencing and Genotyping Core Facility, University of Nebraska Medical Center, to confirm the presence of the inserted fragment. The fragment sequence was as follows: top strand 5′-tcgagGGCCGCTTTCGATTTC GCTTTCCCCTAAATGGCTGAGGGCCGCTTTCGATTT CGCTTTCCCCTAAATGGCTGAGGGCCGCTTTCGATT TCGCTTTCCCCTAAATGGCTGAGa-3′; bottom strand 5′-agcttCTCAGCCATTTAGGGGAAAGCGAAATCGAAAG CGGCCCTCAGCCATTTAGGGGAAAGCGAAATCGAAA GCGGCCCTCAGCCATTTAGGGGAAAGCGAAATCGAA AGCGGCCc-3′. Lower case letters on both ends represent additional bases for the restriction enzymes XhoI and HindIII (New England Biolabs, Ipswich, MA).
Transient Transfection and Luciferase Activity Assay
HBMEC were transfected using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s protocol. Briefly, 90–95% confluent HBMEC in 12-well plates were transfected with 1 μg reporter construct DNA using 2 μl lipofectamine 2000 diluted into 100 μl Opti-MEM I reduced serum medium (Invitrogen). After 24 hr, transfected cells were stimulated with cytokines (IFN-γ, 6 hr; IL-6, 2 hr; IL-8, 2 hr; or IP-10, 2 hr) or HIV-1 (MOI 0.01) for 30 min to 2 hr. Stimulation of transfected cells with IFN-γ served as positive control to evaluate activation of the STAT pathway, and the 6-hr stimulation time point was as recommended by the manufacturer of the reporter construct. The other time points (30 min to 2 hr) were selected because our previous studies showed optimal/maximal HIV-1-induced STAT1 phosphorylation between 30 min and 2 hr, and maximal cytokine-induced monocyte adhesion and migration at 2 hr (Chaudhuri et al., 2008a,b; Yang et al., 2009). After transfection and stimulation, cell lysates were prepared, and the fire-fly luciferase activity was measured using the luciferase assay system kit (Promega) according to the manufacturer’s protocol. Each transfection experiment was performed in triplicate, and each experiment was repeated two or three times. For each sample, the luciferase activity was normalized to the sample protein concentration.
Protein Extraction and Western Blot Analysis
CCR5 antibodies and isotype-matched control antibody were from R&D Systems, and all other antibodies were obtained from Cell Signaling. HIV-1 virions were obtained by ultracentrifugation (19,000g, 2 hr) of culture supernatant of HIV-1-infected macrophages; after ultracentrifugation, the pellet (virions) was resuspended in culture media. To determine the MOI, we performed a serial dilution (from 1:1 to 1:10,000) of an aliquot of the virus solution obtained, infected new macrophages with serially diluted virus, and determined their infection efficiency by reverse transcriptase assay. HBMEC were exposed to media containing purified virus at MOI 0.01 for the indicated times, then washed twice with PBS to remove virus. HBMEC were then harvested, and protein extraction, quantification, and Western blot analyses were performed as we previously described (Kanmogne et al., 2005, 2007). For Western blot assays with phosphorylated antibodies, membranes were stripped as we previously described (Chaudhuri et al., 2008b), reblotted with the corresponding total antibody, then stripped again and reblotted with β-actin antibody. Results were expressed as ratio of relative intensity of the target phosphorylated protein to that of the total protein or the internal β-actin standard. For experiments with inhibitors or CCR5 antibodies, HBMEC were exposed for 30 min to HIV-1 with or without CCR5 antibodies (20 μg/ml), isotype-matched control antibodies (20 μg/ml), or LY (50 μM), and protein was extracted and analyzed as described above.
Statistical Analyses
Statistical analyses were performed by one- or two-way ANOVA, followed by Tukey’s multiple-comparisons tests, using GraphPad Prism 4.0. (GraphPad Software, La Jolla, CA). These statistical procedures test differences among several means for significance without increasing type I error rate. Threshold of significance level was 0.05.
RESULTS
CCR5 Antibodies and PI3K Inhibitors Diminished HIV-1-Induced Activation and Expression of STAT1 and STAT3
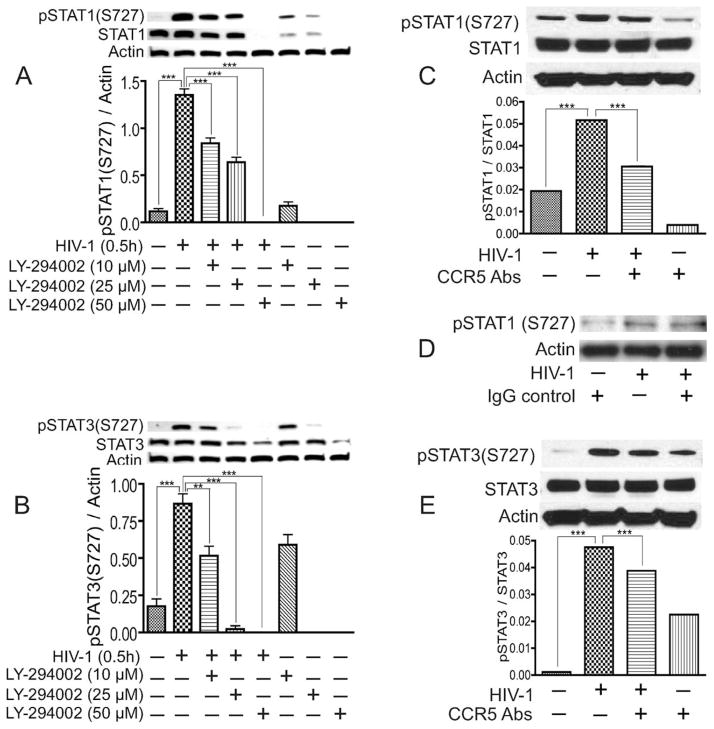
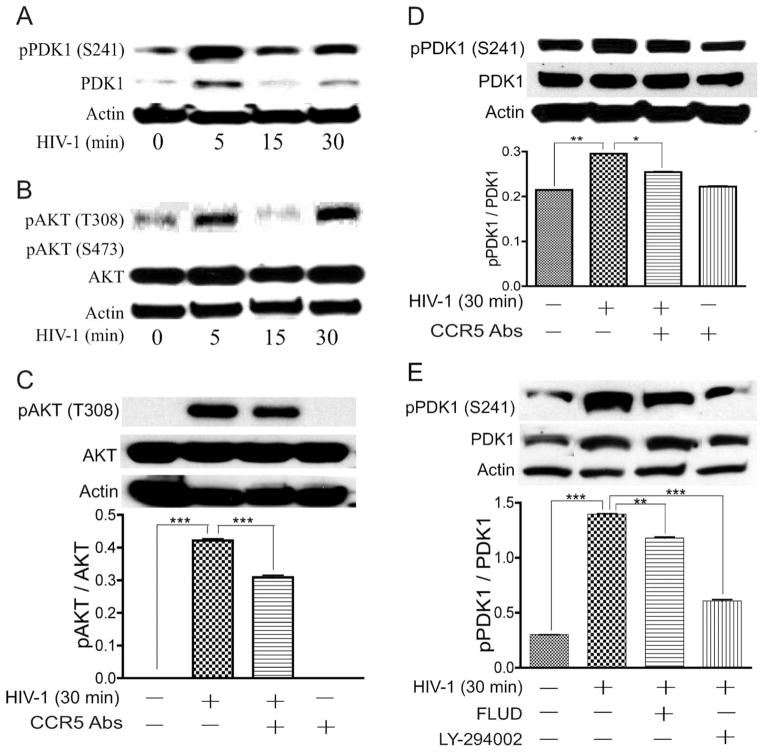
We previously demonstrated that STAT1 signaling modulates HIV-1-induced inflammation in HBMEC and BBB dysfunction (Chaudhuri et al., 2008a,b). We further showed that HIV-1 activates STAT1 and STAT3 in HBMEC, with maximal STAT1 phosphorylation occurring after 30 min of viral exposure, and the STAT1 inhibitor fludarabine prevented viral induced STAT1 phosphorylation, inflammation, and BBB dysfunction (Chaudhuri et al., 2008b). To determine the effectors activated by HIV-1 upstream of STAT1, we analyzed the effect of HIV-1 on the activation and expression of JAK1, JAK2, JAK3, and TYK2. HIV-1 did not induce activation of JAK1, JAK2, JAK3, or TYK2 in HBMEC (data not shown). Evidence suggests that cross-talk exists between the JAK/STAT and PI3K pathways (Nguyen et al., 2001). Thus, we tested the effect of the PI3K inhibitor (LY) on virus-induced phosphorylation of STAT1 and STAT3. Exposure of HBMEC to HIV-1 virions induced the phosphorylation of STAT1 and STAT3 at serine-727 (Fig. 1A,B). No activation of STAT1 and STAT3 at tyrosine residues was detected (data not shown), which is consistent with our previous observations and studies from other laboratories showing that STAT1 serine phosphorylation and signaling can occur independently of its tyrosine phosphorylation (Wen et al., 1995; Zhu et al., 1997; Chaudhuri et al., 2008b). The PI3K inhibitor LY diminished virus-induced activation of STAT1 and STAT3 in a dose-dependent manner; 10 μM and 25 μM LY diminished HIV-1-induced activation and expression of STAT1 by 38% and 53% respectively, and 50 μM LY completely inhibited virus-induced phosphorylation of STAT1 (P < 0.001; Fig. 1A). Similarly, 10 μM and 25 μM LY diminished HIV-1-induced activation of STAT3 by 40% and 97% respectively, and 50 μM LY completely inhibited virus-induced phosphorylation of STAT3 (P < 0.001; Fig. 1B).
Fig. 1.
PI3K inhibitor and CCR5 antibodies diminished HIV-1-induced activation of STAT1 and STAT3. Exposure of HBMEC to infectious viral particles induced the phosphorylation of STAT1 (A,C,D) and STAT3 (B,E) at serine-727. PI3K inhibitors diminished HIV-1 induced activation and expression of STAT1 (A) and STAT3 (B) in a dose-dependent manner, with maximal inhibition at 50 μM. CCR5 antibodies (20 μg/ml) significantly diminished virus-induced phosphorylation of STAT1 (C) and STAT3 (E), whereas isotype-matched antibody control had no effect (D). ★★P < 0.01, ★★★P < 0.001. Representative data from three independent experiments. Primary HBMEC from different human donors were used for these experiments, and the minor differences observed in basal levels of phosphorylated STAT are due to interdonor variability.
We previously showed that HBMEC do not express CD4 but do express CXCR4 and CCR5, two chemokine receptors used by T-lymphocyte-tropic and macrophage-tropic HIV isolates, respectively, to enter target cells (Kanmogne et al., 2000, 2007). Because the HIV-1ADA used in this study is macrophage-tropic, we determined whether CCR5 is involved in virus-induced activation of STAT1 and STAT3 in HBMEC. CCR5 antibodies diminished virus-induced phosphorylation of STAT1 and STAT3 by 37.7% (Fig. 1C) and 31% (Fig. 1E), respectively (P < 0.001), whereas isotype-matched control antibodies showed no effect (Fig. 1D). Exposure of HBMEC to 10 μM LY or CCR5 antibodies also increased STAT3 phosphorylation at S-727 (P < 0.001; Fig. 1B,E); this increase in STAT3 phosphorylation was observed only in cells obtained from some donors; cells from other donors showed no independent effect of LY and CCR5 antibodies on STAT3 phosphorylation. These differential effects, as well as the minor differences in basal levels of activated STAT depictedd in Figure 1 are due to interdonor variability, because primary HBMEC from different human donors were used in these experiments.
HIV-1 Induced ISRE/GAS Promoter Activity in HBMEC, and CCR5 Antibodies and STAT1 and PI3K Inhibitors Diminished Virus-Induced ISRE/GAS Promoter Activity
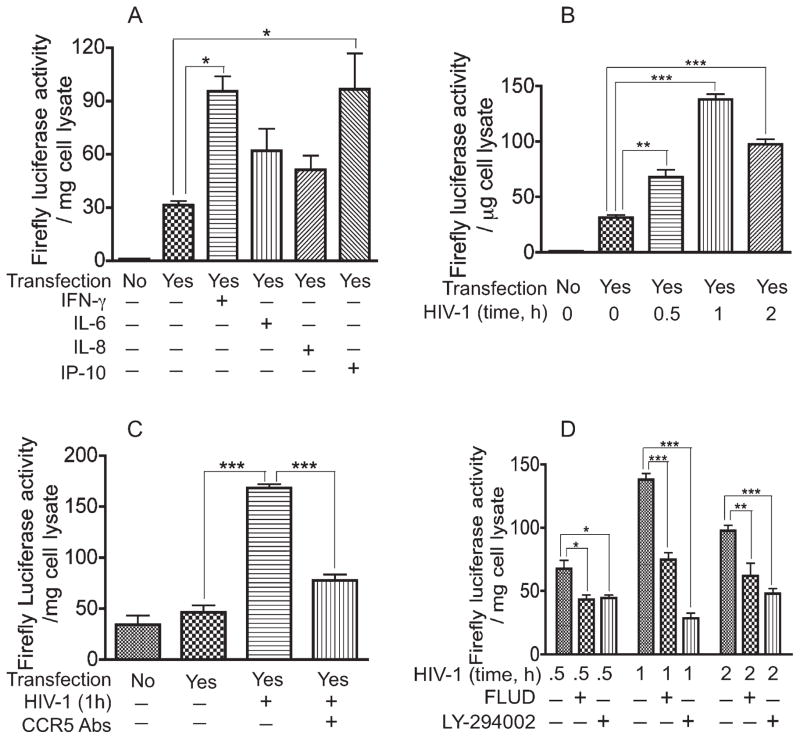
In the STAT signaling pathway, the activated STAT complex translocates to the nucleus and binds to the ISRE/GAS promoter to initiate the transcription of interferon or cytokine stimulated genes (Schindler and Brutsaert, 1999). It has been demonstrated that STAT1 signaling can occur through serine phosphorylation and nuclear translocation of STAT1, independent of tyrosine phosphorylation (Wen et al., 1995; Zhu et al., 1997). To determine whether HIV-1 induces ISRE/GAS promoter activity, we ligated the isolated ISRE/GAS element into a minimal promoter firefly luciferase reporter plasmid and transfected into HBMEC. After cloning and transfection, we tested the transfection efficiency and effectiveness of the construct by measuring the ISRE/GAS promoter activity in transfected HBMEC exposed to IFN-γ, a cytokine known to signal via the JAK/STAT pathway. Compared with unstimulated transfected cells, exposure of transfected HBMEC to IFN-γ increased the ISRE/GAS promoter activity threefold (P < 0.05; Fig. 2A). We previously demonstrated that exposure of HBMEC to HIV-1 induced up-regulation of several proinflammatory cytokines, including IL-6, IL-8, and IP-10 (Chaudhuri et al., 2008a,b). Therefore, we evaluated the effects of these cytokines on the ISRE/GAS promoter activity. IP-10 increased STAT1 transcriptional activity by 3.1-fold compared with transfected cells not exposed to cytokines (P < 0.05; Fig. 2A). IL-6 and IL-8 also increased STAT1 transcriptional activity by 2-fold and 1.6-fold respectively, compared with transfected cells not exposed to cytokines, but the difference was not statistically significant.
Fig. 2.
HIV-1 induced ISRE/GAS promoter activity. CCR5 antibodies and inhibitors of STAT1 and PI3K significantly diminished virus-induced ISRE/GAS promoter activity. The ISRE/GAS element was cloned upstream of a minimal promoter firefly luciferase reporter plasmid, and HBMEC transfection and luciferase assays were performed as described in Materials and Methods. A: Exposure of transfected HBMEC to cytokines (IFN-γ, IL-6, IL-8, or IP-10; 100 ng/ml) for 2–6 hr increases firefly luciferase activity. B: Exposure of transfected HBMEC to HIV-1 for 30 min to 2 hr significantly increase firefly luciferase activity, with maximal luciferase activity observed at 1 hr of viral exposure. CCR5 antibodies (C), STAT1 inhibitor (fludarabine; FLUD), and PI3K inhibitor (LY-294002; D) significantly diminished HIV-1-induced luciferase activity (★P < 0.05, ★★P < 0.01, ★★★P < 0.001). Negative controls consisted of untransfected cells and transfected cells not exposed to cytokines or virus. For all experiments, each experimental condition was performed in triplicate. Representative data from three independent experiments.
Next, we determined the direct effect of HIV-1 on the ISRE/GAS promoter activity. Exposure of HBMEC to viral particles for 30 min, 1 hr, and 2 hr increased STAT1 transcriptional activity by 2.2-fold (P < 0.01), 4-fold (P < 0.001), and 3.1-fold (P < 0.001), respectively, compared with unstimulated transfected cells (Fig. 2B). Using antibodies to the chemokine receptor CCR5, we investigated whether CCR5 is involved in HIV-induced up-regulation of STAT1 transcriptional activity. Exposure of HBMEC to HIV-1 for 1 hr increased the ISRE/GAS promoter activity by 3.6-fold compared with transfected cells not exposed to the virus (Fig. 2C). CCR5 antibodies decreased virus-induced up-regulation of the ISRE/GAS promoter activity by 2.2-fold (P < 0.001; Fig. 2C).
The STAT1 and PI3K inhibitors significantly diminished HIV-1-induced activation of STAT1 and STAT3 (Fig. 1). Therefore, we tested the effects of these inhibitors on HIV-1-induced up-regulation of ISRE/GAS promoter activity. After transfection with reporter plasmid containing the ISRE/GAS element, HBMEC were exposed to HIV-1 for 30 min, 1 hr, and 2 hr with or without the presence of fludarabine or LY. At 30 min, fludarabine and LY had decreased the HIV-1-induced increase in reporter gene activity by 36% (P < 0.05) and 34%, respectively (P < 0.05; Fig. 2D). The maximal HIV-1-induced increase in STAT1 transcriptional activity occurred at 1 hr of viral exposure, and fludarabine and LY decreased the HIV-1-induced increase in STAT1 transcriptional activity by 45.7% and 79.3%, respectively (P < 0.001; Fig. 2D). At 2 hr, fludarabine and LY had decreased the HIV-1-induced increase in reporter gene activity by 36.6% (P < 0.01) and 51% (P < 0.001), respectively (Fig. 2D).
Effect of HIV-1, CCR5 Antibodies, and STAT1 and PI3K Inhibitors on HBMEC Phenotype and Viability
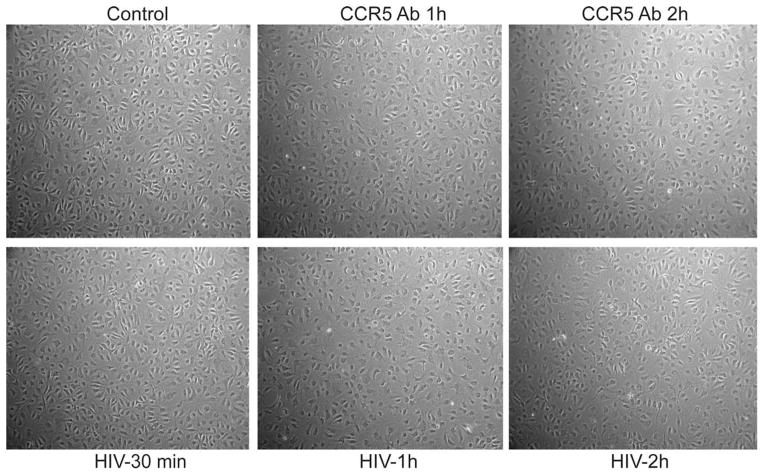
To determine whether HIV-1 and CCR5 antibodies have a direct effect on HBMEC morphology, primary HBMEC were exposed to HIV-1 or CCR5 antibodies for 30 min to 2 hr, and cell morphology was assessed by microscopy. Results showed that exposure of HBMEC to HIV-1 or CCR5 antibodies for up to 2 hr does not change HBMEC morphology (Fig. 3).
Fig. 3.
Effect of HIV-1 and CCR5 antibodies on HBMEC phenotype. Microscopic examination showed that exposure of HBMEC to HIV-1 or CCR5 neutralizing antibodies (CCR5 Ab) for 30 min to 2 hr does not change endothelial cell morphology.
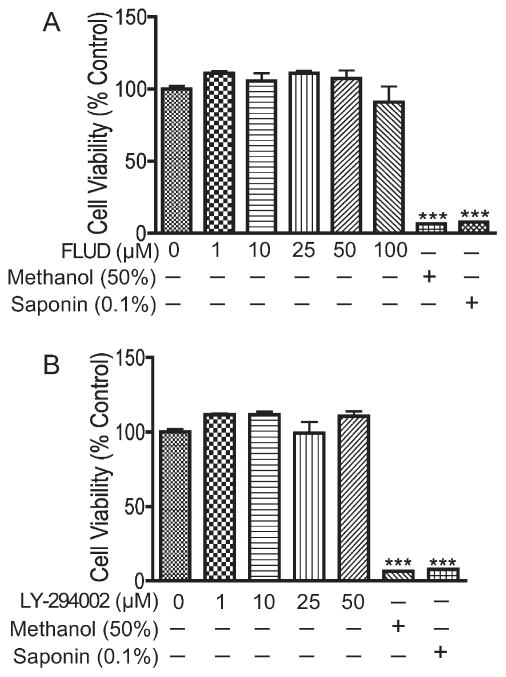
To assess any adverse effect that STAT1 and PI3K inhibitors may have on HBMEC viability and barrier function, we determined the effects of fludarabine and LY on HBMEC viability and BBB tightness. Results of alamarBlue assay showed that exposure of HBMEC to fludarabine (1–100 μM) or LY (1–50 μM) for up 24 hr did not alter endothelial cells viability (Fig. 4). Live recording of transendothelial electrical resistance (TEER) also showed that fludarabine and LY did not alter endothelial barrier tightness (data not shown).
Fig. 4.
STAT1 and PI3K inhibitors did not alter HBMEC viability. AlamarBlue assay show that 24-hr exposure of HBMEC to the STAT1 inhibitor fludarabine (FLUD; 1–100 μM; A) or the PI3K inhibitor LY-294002 (1–50 μM; B) did not induce cytotoxicity. Control represents untreated cells. HBMEC treated with methanol (50%) and saponin (0.1%) were used as positive control (★★★P < 0.001).
CCR5 Antibodies and PI3K Inhibitors Diminished Monocyte Adhesion and Migration Across In Vitro BBB Models
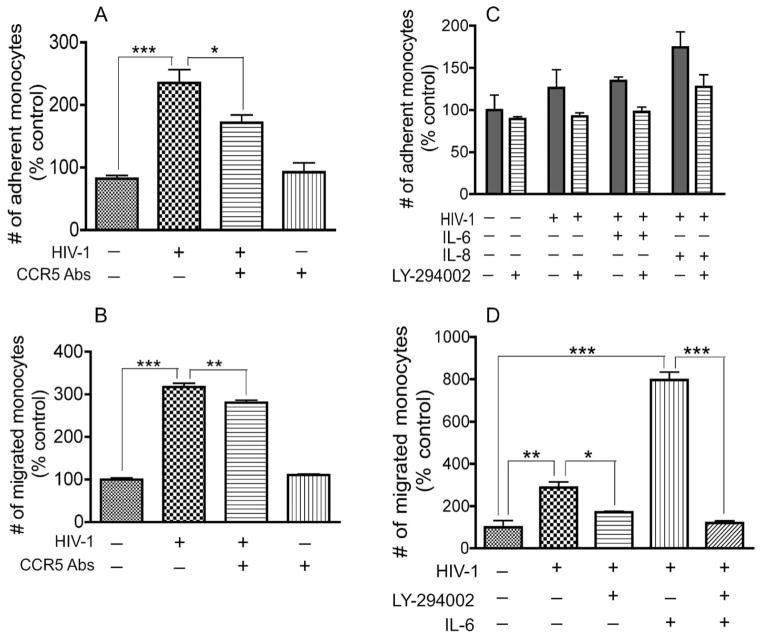
To determine whether the chemokine receptor CCR5 is involved and to investigate further the crosstalk with the PI3K pathway, we analyzed the effects of CCR5 antibodies and LY on HIV-1- and cytokine-induced adhesion and migration of monocytes across an in vitro BBB model. In comparison with noninfected monocytes, increased adhesion to HBMEC was observed with HIV-1 infected monocytes, but the magnitude of adhesion varied with monocyte donor. Primary monocytes from some human donors showed a significant increase in monocyte adhesion following HIV-1 infection (Fig. 5A), whereas monocytes from other donors showed a more modest, nonstatistically significant increase in HIV-1- and cytokine-induced monocyte adhesion (Fig. 5C). CCR5 antibodies significantly diminished virus-induced adhesion and migration of monocytes across the BBB (Fig. 5A,B). The PI3K inhibitor also diminished HIV-1-, IL-6-, and IL-8-induced monocyte adhesion to HBMEC monolayers (Fig. 5C) and significantly decreased HIV-1- and IL-6-induced monocyte migration across in vitro BBB models (Fig. 5D). In comparison with noninfected monocytes, HIV-1 infection and IL-6 increased monocyte transen-dothelial migration by 2.9-fold (P < 0.01) and 6.3-fold (P < 0.001), respectively (Fig. 5D). LY diminished HIV-1-induced monocyte migration by 40.5% (P < 0.05; Fig. 5D) and diminished the migration induced by both HIV-1 and IL-6 by 81% (P < 0.001; Fig. 5D).
Fig. 5.
CCR5 antibodies and PI3K inhibitors diminished HIV-1-and cytokine-induced monocyte adhesion and migration across in vitro BBB models. HIV-1 infection increased monocytes adhesion (A,C) and transendothelial migration (B,D). CCR5 antibodies (20 μg/ml) significantly diminished virus-induced monocyte adhesion (A) and migration across in vitro BBB models (B). The PI3K inhibitor (LY-294002, 50 μM) diminished viral-, IL-6-, and IL-8-induced monocyte adhesion, but the decrease was not statistically significant (C). IL-6 further increased the transendothelial migration of infected monocytes, and the PI3K inhibitor significantly diminished HIV-1-and IL-6-induced monocyte migration (D). Data are expressed as percentage of adherent or migrated monocytes in control untreated cells. For adhesion experiments, n = 6 for each experimental condition; for migration experiments, each experimental condition was performed quadruply. Representative data from three independent experiments. Primary human monocytes were used for each experiment, and the differences observed in the magnitude of monocyte adhesion (A,C) are due to interdonor variability.
CCR5 Antibodies and Inhibitors of STAT1 and PI3K Diminished HIV-1-Induced Phosphorylation of PDK-1 and AKT in HBMEC
Our mechanistic and functional studies suggest that, in HIV-1-induced BBB dysfunction, proteins belonging to the PI3K/AKT pathways are the upstream effectors that phosphorylate STAT1 and STAT3. To identify those effectors, we analyzed the effect of HIV-1 exposure on the activation and expression of phosphoinositide-dependent kinase-1 (PDK1), phosphatase and tensin homologue deleted on chromosome 10 (PTEN), and serine-threonine protein kinase AKT. HIV-1 induced expression and activation of PDK1 in HBMEC. Exposure of HBMEC to HIV-1 increased phosphorylation of PDK1 at serine-241 by 2.6–9.9-fold and also increased the level of total PDK1 (Fig. 6A). CCR5 antibodies and inhibitors of PI3K and STAT1 diminished virus-induced PDK1 phosphorylation (Fig. 6D,E). Exposure of HBMEC to HIV-1 virions did not induce AKT phosphorylation at serine-473 but increased the phosphorylation of AKT at threonine-308 by 4.1–6.14-fold (Fig. 6B); CCR5 antibodies diminished virus-induced AKT phosphorylation (Fig. 6C). HIV-1 also increased the phosphorylation of PTEN at serine-380 in HBMEC, but the basal levels of phosphorylated PTEN in HBMEC not exposed to virus were very high (data not shown).
Fig. 6.
HIV-1 induced phosphorylation of PDK1 and AKT in HBMEC. CCR5 antibodies and inhibitors of STAT1 and PI3K diminished virus-induced PDK1 and AKT phosphorylation. Exposure of HBMEC to HIV-1 increased phosphorylation of PDK1 at serine-241 (A,D,E) and AKT at threonine-308 (B,C). No phosphorylation of AKT at serine-473 was detected (B). CCR5 antibodies, fludarabine (FLUD), and LY-294002 diminished HIV-induced phosphorylation of AKT (C) and PDK1 (D,E). ★P < 0.05, ★★P < 0.01, ★★★P < 0.001. Data are representative of two independent experiments.
DISCUSSION
HIV-induced BBB dysfunction often occurs in the early stages of infection and plays a central role in the pathogenesis of neuro-AIDS; it allows virus and HIV-infected cells to infiltrate the CNS, where they infect brain macrophages and microglia and cause neuronal injury and HIV encephalitis. Therefore, elucidating the mechanisms through which HIV invades the CNS and the signaling pathways responsible for HIV-induced BBB compromise are key to understanding viral neuropathogenesis better and offer opportunities to develop therapies to combat HIV-induced CNS disorders. Data described in this report build upon previous research performed in our laboratory, which showed that STAT1 signaling modulates HIV-1-induced inflammatory responses and BBB dysfunction (Chaudhuri et al., 2008a,b). What remained poorly understood was the cellular receptor and signaling pathway upstream and downstream of STAT1 involved in HIV-1-induced BBB dysfunction. In the current work, we demonstrate a cross-talk between STAT1 and PI3K signaling in HIV-1-induced endothelial dysfunction and also show that this is partially mediated by the G-protein-coupled chemokine (C-C motif) receptor CCR5.
CCR5 is present in immune cells and plays a major role in HIV-1 entry and cellular infection. In fact, mutation in the CCR5 gene is associated with resistance to HIV-1 infection (Samson et al., 1996). CCR5 antagonists showed potent anti-HIV-1 activity in vitro and in vivo and have been approved for clinical use in humans as anti-HIV drugs (for recent review see Bredeek and Harbour, 2007; Dhami et al., 2009). CCR5 also has a major impact on the natural history of HIV infection and neuro-AIDS. CCR5-tropic HIV predominates in many infected individuals, including those with dual/mixed-tropic viruses (Irlbeck et al., 2008), and the majority of these infected patients have CCR5-using viruses in their cerebrospinal fluids (Soulie et al., 2009). Furthermore, chemokine receptors play a functional role in HIV neuropathogenesis; most neurotropic viral isolates are macrophage-tropic and use principally CCR5 as coreceptor to enter and/or infect brain cells (Gabuzda and Wang, 1999).
Previous studies from other laboratories and our laboratory showed that HBMEC express CCR5 (Berger et al., 1999; Kanmogne et al., 2000, 2007). Our current data show that CCR5 plays a role in HIV-1-induced BBB compromise. CCR5 neutralizing antibodies significantly diminished virus-induced phosphorylation of STAT1, STAT3, PDK-1, and AKT and diminished virus-induced up-regulation of STAT1 transcriptional activity as well as virus-induced adhesion and migration of monocytes across in vitro BBB models. In our current study, HIV-1 infection increased monocyte adhesion and migration, but the magnitude of HIV-1- and cytokine-induced monocyte adhesion varied with human monocyte donor. The significant increase in HIV-1- and IL-6-induced monocyte migration, compared with a more modest increase in monocyte adhesion, may also be due to the difference between the two experimental procedures. In our migration assays using fluoroblock inserts, the fluorescence measured include the fluorescence of calcein-labeled monocytes that have already passed through the pores of the inserts and monocytes still attached to the lower (abluminal) side of the insert. In adhesion assays, after the 15-min monocyte adhesion, HBMEC are washed three times to remove nonadherent monocytes before fluorescence measurement to quantify the number of adherent monocytes. It is likely that these stringent washes remove loosely adherent monocytes, thus decreasing the fluorescence intensity in adhesion assays compared with migration assays.
In the present study, CCR5 only partially mediated HIV-1 effects on the brain endothelium; neutralizing CCR5 antibodies did not totally block HIV-induced phosphorylation of STAT1, STAT3, PDK-1, or AKT and only partially reversed HIV-1-induced monocyte adhesion and transendothelial migration. Nevertheless, our data suggest that HIV-1 uses CCR5 to bind/enter HBMEC, and blocking this receptor could diminish HIV-1-induced BBB injury and viral entry into the CNS. Although CCR5 antibodies also induced a slight increase in STAT3 phosphorylation in HBMEC, CCR5 antibodies alone did not alter monocyte adhesion or migration through the BBB and did not alter the phosphorylation of kinases upstream of STAT. Furthermore, our current and previous data show that, of all the eight STAT family members, it is STAT1 that is primarily involved in HIV-1-induced blood–brain barrier dysfunction (Chaudhuri et al., 2008a,b; Yang et al., 2009).
Previous studies showed that serine-727 phosphorylation of STAT1 can lead to formation of STAT dimers, nuclear translocation, and STAT1 signaling, independently of tyrosine phosphorylation (Wen et al., 1995; Zhu et al., 1997). There is also evidence that serine-727 phosphorylation of STAT1 and STAT3 can occur independently of JAK and tyrosine phosphorylation (Decker and Kovarik, 2000; Nguyen et al., 2001). Our current study is in agreement with these pervious observations and demonstrated cross-talk between STAT1 and PI3K pathways in HIV-induced endothelial dysfunction. Pretreatment of HBMEC with fludarabine, a specific STAT1 inhibitor (Frank et al., 1999), or a pharmacological inhibitor of PI3K significantly diminished virus-induced up-regulation of STAT1 luciferase activity; diminished virus-induced activation of STAT1, STAT3, and PDK1; and diminished HIV-1- and cytokine-induced adhesion and migration of monocytes across in vitro BBB models. There is also evidence that PI3K is involved in STAT1 serine phosphorylation and gene activation, cytoskeletal remodeling, and expression and function of tight junction proteins (Nguyen et al., 2001; Gonzalez-Mariscal et al., 2008). Exposure of HIV-1 Tat proteins to brain endothelial cells also reduced tight junction protein expression via PI3K/AKT signaling (Andras et al., 2005).
PI3K enzymes are activated by cell membrane receptors and phosphorylate the 3′-OH position of the inositol ring of phosphatidylinoside, thus converting phosphatidylinositol(4,5)biphosphate [PI(4,5)P2] into phosphatidylinositol(1,4,5)triphosphate [PI(1,4,5)P3]. AKT is a key serine/threonine kinase that mediates PI3K action. The presence of PI(1,4,5)P3 triggers the translocation of AKT from the cytosol to the plasma membrane, where is it phosphorylated on threonine-308 in the kinase activation loop or on serine-473 in the hydrophobic region of the C-terminus (Cantrell, 2001). It is well established that PI3K regulates PKD1 activity and that PKD1 is the upstream kinase that phosphorylates AKT on threonine-308 (Alessi et al., 1997; Cantrell, 2001; Storz and Toker, 2002).
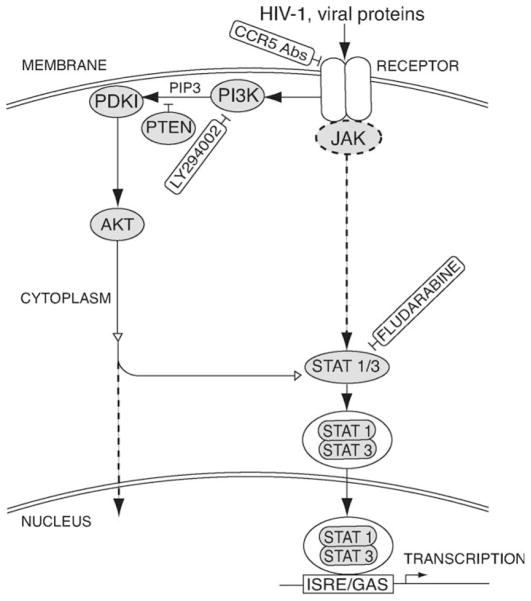
Our present study demonstrates that exposure of HBMEC to HIV-1 induced phosphorylation of PKD1 at serine-241, AKT at threonine-308, and STAT1 and STAT3 at serine-727 and increased IRSE/GAS promoter activity. These results are in agreement with known sequences of events in the PI3K and STAT signaling pathways (Fig. 7) and confirm previous observations that serine-727 phosphorylation of STAT1 and STAT3, independently of tyrosine phosphorylation, can stimulate the promoter activity of IFN- or cytokine-inducible genes (Wen et al., 1995; Zhu et al., 1997; Decker and Kovarik, 2000; Nguyen et al., 2001). There is also evidence that CCR5 is responsible for HIV-1 gp120-induced PI3K activation and cytokine production in human macrophages (Lee et al., 2005), and our current study also demonstrates that CCR5 partially mediates virus-induced PI3K and STAT1 signaling in HBMEC, and CCR5 antibodies partially reversed HIV-induced endothelial cell dysfunction. Our subsequent studies will determine whether blocking the CCR5 receptor in combination with STAT1 and/or PI3K pharmacological inhibitors can better protect the brain endothelium against HIV-induced effects.
Fig. 7.
Hypothetical model for cross-talk of PI3K/AKT with HIV-1-induced STAT1 signaling. The arrows with solid lines indicate activation. Dotted line represents the classical JAK/STAT or PI3K pathway. Open arrows lines represent cross-talk between the PI3K and JAK/STAT pathways. The ⊥ symbol indicates an inhibition effect.
A limitation of this study is the minor differences observed on baseline protein activation between experiments and monocyte adhesion, which were due to inter-donor variability because we use primary HBMEC and freshly isolated monocytes from different human donors. However, using primary human cells and HIV-1 strains isolated from an infected human is also a strength of this study, insofar as results are more indicative of what happens in the brain microvasculature of HIV-infected humans.
In summary, our data show that HIV-1-induced BBB compromise involve cross-talk between STAT1 and PI3K pathways. The results further suggests that CCR5 neutralizing antibodies or other pharmacologic inhibitors of CCR5, STAT1, and PI3K can protect the brain endothelium from injury, providing a therapeutic strategy for preventing viral entry into the brain and the development of neuro-AIDS.
Acknowledgments
Contract grant sponsor: National Institutes of Health; Contract grant number: RO1 MH081780 (to G.D.K.).
The authors thank Ms Regina Eide for graphic support and Dr. Lee Mosley and Ms. Robin Taylor for critical reading of the manuscript.
References
- Alessi DR, James SR, Downes CP, Holmes AB, Gaffney PR, Reese CB, Cohen P. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Balpha. Curr Biol. 1997;7:261–269. doi: 10.1016/s0960-9822(06)00122-9. [DOI] [PubMed] [Google Scholar]
- Andras IE, Pu H, Tian J, Deli MA, Nath A, Hennig B, Toborek M. Signaling mechanisms of HIV-1 Tat-induced alterations of claudin-5 expression in brain endothelial cells. J Cereb Blood Flow Metab. 2005;25:1159–1170. doi: 10.1038/sj.jcbfm.9600115. [DOI] [PubMed] [Google Scholar]
- Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, Clifford DB, Cinque P, Epstein LG, Goodkin K, Gisslen M, Grant I, Heaton RK, Joseph J, Marder K, Marra CM, McArthur JC, Nunn M, Price RW, Pulliam L, Robertson KR, Sacktor N, Valcour V, Wojna VE. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69:1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks WA, Ercal N, Price TO. The blood–brain barrier in neuro-AIDS. Curr HIV Res. 2006;4:259–266. doi: 10.2174/157016206777709447. [DOI] [PubMed] [Google Scholar]
- Berger O, Gan X, Gujuluva C, Burns AR, Sulur G, Stins M, Way D, Witte M, Weinand M, Said J, Kim KS, Taub D, Graves MC, Fiala M. CXC and CC chemokine receptors on coronary and brain endothelia. Mol Med. 1999;5:795–805. [PMC free article] [PubMed] [Google Scholar]
- Bovolenta C, Camorali L, Lorini AL, Ghezzi S, Vicenzi E, Lazzarin A, Poli G. Constitutive activation of STATs upon in vivo human immunodeficiency virus infection. Blood. 1999;94:4202–4209. [PubMed] [Google Scholar]
- Bredeek UF, Harbour MJ. CCR5 antagonists in the treatment of treatment-naive patients infected with CCR5 tropic HIV-1. Eur J Med Res. 2007;12:427–434. [PubMed] [Google Scholar]
- Burger DM, Boucher CA, Meenhorst PL, Kraayeveld CL, Portegies P, Mulder JW, Hoetelmans RM, Beijnen JH. HIV-1 RNA levels in the cerebrospinal fluid may increase owing to damage to the blood–brain barrier. Antivir Ther. 1997;2:113–117. [PubMed] [Google Scholar]
- Cantrell DA. Phosphoinositide 3-kinase signalling pathways. J Cell Sci. 2001;114:1439–1445. doi: 10.1242/jcs.114.8.1439. [DOI] [PubMed] [Google Scholar]
- Chaudhuri A, Duan F, Morsey B, Persidsky Y, Kanmogne GD. HIV-1 activates proinflammatory and interferon-inducible genes in human brain microvascular endothelial cells: putative mechanisms of blood–brain barrier dysfunction. J Cereb Blood Flow Metab. 2008a;28:697–711. doi: 10.1038/sj.jcbfm.9600567. [DOI] [PubMed] [Google Scholar]
- Chaudhuri A, Yang B, Gendelman HE, Persidsky Y, Kanmogne GD. STAT1 signaling modulates HIV-1-induced inflammatory responses and leukocyte transmigration across the blood–brain barrier. Blood. 2008b;111:2062–2072. doi: 10.1182/blood-2007-05-091207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer LA, Nelson SL, Klemsz MJ. Synergistic induction of the Tap-1 gene by IFN-gamma and lipopolysaccharide in macrophages is regulated by STAT1. J Immunol. 2000;165:3190–3197. doi: 10.4049/jimmunol.165.6.3190. [DOI] [PubMed] [Google Scholar]
- Dallasta LM, Pisarov LA, Esplen JE, Werley JV, Moses AV, Nelson JA, Achim CL. Blood–brain barrier tight junction disruption in human immunodeficiency virus-1 encephalitis. Am J Pathol. 1999;155:1915–1927. doi: 10.1016/S0002-9440(10)65511-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker T. Introduction: STATs as essential intracellular mediators of cytokine responses. Cell Mol Life Sci. 1999;55:1505–1508. doi: 10.1007/s000180050390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker T, Kovarik P. Serine phosphorylation of STATs. Oncogene. 2000;19:2628–2637. doi: 10.1038/sj.onc.1203481. [DOI] [PubMed] [Google Scholar]
- Dhami H, Fritz CE, Gankin B, Pak SH, Yi W, Seya MJ, Raffa RB, Nagar S. The chemokine system and CCR5 antagonists: potential in HIV treatment and other novel therapies. J Clin Pharmacol Ther. 2009;34:147–160. doi: 10.1111/j.1365-2710.2008.00978.x. [DOI] [PubMed] [Google Scholar]
- Eugenin EA, Osiecki K, Lopez L, Goldstein H, Calderon TM, Berman JW. CCL2/monocyte chemoattractant protein-1 mediates enhanced transmigration of human immunodeficiency virus (HIV)-infected leukocytes across the blood–brain barrier: a potential mechanism of HIV-CNS invasion and neuroAIDS. J Neurosci. 2006;26:1098–1106. doi: 10.1523/JNEUROSCI.3863-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank DA, Mahajan S, Ritz J. Fludarabine-induced immunosuppression is associated with inhibition of STAT1 signaling. Nat Med. 1999;5:444–447. doi: 10.1038/7445. [DOI] [PubMed] [Google Scholar]
- Gabuzda D, Wang J. Chemokine receptors and virus entry in the central nervous system. J Neurovirol. 1999;5:643–658. doi: 10.3109/13550289909021293. [DOI] [PubMed] [Google Scholar]
- Ghafouri M, Amini S, Khalili K, Sawaya BE. HIV-1 associated dementia: symptoms and causes. Retrovirology. 2006;3:28. doi: 10.1186/1742-4690-3-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Mariscal L, Tapia R, Chamorro D. Cross-talk of tight junction components with signaling pathways. Biochim Biophys Acta. 2008;1778:729–756. doi: 10.1016/j.bbamem.2007.08.018. [DOI] [PubMed] [Google Scholar]
- Haq R, Halupa A, Beattie BK, Mason JM, Zanke BW, Barber DL. Regulation of erythropoietin-induced STAT serine phosphorylation by distinct mitogen-activated protein kinases. J Biol Chem. 2002;277:17359–17366. doi: 10.1074/jbc.M201842200. [DOI] [PubMed] [Google Scholar]
- Hawkins BT, Davis TP. The blood–brain barrier/neurovascular unit in health and disease. Pharmacol Rev. 2005;57:173–185. doi: 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- Irlbeck DM, Amrine-Madsen H, Kitrinos KM, Labranche CC, Demarest JF. Chemokine (C-C motif) receptor 5-using envelopes predominate in dual/mixed-tropic HIV from the plasma of drug-naive individuals. AIDS. 2008;22:1425–1431. doi: 10.1097/QAD.0b013e32830184ba. [DOI] [PubMed] [Google Scholar]
- Kanmogne GD, Grammas P, Kennedy RC. Analysis of human endothelial cells and cortical neurons for susceptibility to HIV-1 infection and co-receptor expression. J Neurovirol. 2000;6:519–528. doi: 10.3109/13550280009091952. [DOI] [PubMed] [Google Scholar]
- Kanmogne GD, Primeaux C, Grammas P. HIV-1 gp120 proteins alter tight junction protein expression and brain endothelial cell permeability: implications for the pathogenesis of HIV-associated dementia. J Neuropathol Exp Neurol. 2005;64:498–505. doi: 10.1093/jnen/64.6.498. [DOI] [PubMed] [Google Scholar]
- Kanmogne GD, Schall K, Leibhart J, Knipe B, Gendelman HE, Persidsky Y. HIV-1 gp120 compromises blood–brain barrier integrity and enhances monocyte migration across blood–brain barrier: implication for viral neuropathogenesis. J Cereb Blood Flow Metab. 2007;27:123–134. doi: 10.1038/sj.jcbfm.9600330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C, Tomkowicz B, Freedman BD, Collman RG. HIV-1 gp120-induced TNF-α production by primary human macrophages is mediated by phosphatidylinositol-3 (PI-3) kinase and mitogen-activated protein (MAP) kinase pathways. J Leukoc Biol. 2005;78:1016–1023. doi: 10.1189/jlb.0105056. [DOI] [PubMed] [Google Scholar]
- Moore JP, Kitchen SG, Pugach P, Zack JA. The CCR5 and CXCR4 coreceptors—central to understanding the transmission and pathogenesis of human immunodeficiency virus type 1 infection. AIDS Res Hum Retroviruses. 2004;20:111–126. doi: 10.1089/088922204322749567. [DOI] [PubMed] [Google Scholar]
- Nguyen H, Ramana CV, Bayes J, Stark GR. Roles of phosphatidylinositol 3-kinase in interferon-gamma-dependent phosphorylation of STAT1 on serine 727 and activation of gene expression. J Biol Chem. 2001;276:33361–33368. doi: 10.1074/jbc.M105070200. [DOI] [PubMed] [Google Scholar]
- Samson M, Libert F, Doranz BJ, Rucker J, Liesnard C, Farber CM, Saragosti S, Lapoumeroulie C, Cognaux J, Forceille C, Muyldermans G, Verhofstede C, Burtonboy G, Georges M, Imai T, Rana S, Yi Y, Smyth RJ, Collman RG, Doms RW, Vassart G, Parmentier M. Resistance to HIV-1 infection in caucasian individuals bearing mutant alleles of the CCR-5 chemokine receptor gene. Nature. 1996;382:722–725. doi: 10.1038/382722a0. [DOI] [PubMed] [Google Scholar]
- Schindler C, Brutsaert S. Interferons as a paradigm for cytokine signal transduction. Cell Mol Life Sci. 1999;55:1509–1522. doi: 10.1007/s000180050391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soulie C, Tubiana R, Simon A, Lambert-Niclot S, Malet I, Canestri A, Brunet C, Murphy R, Katlama C, Calvez V, Marcelin AG. Presence of HIV-1 R5 viruses in cerebrospinal fluid even in patients harboring R5X4/X4 viruses in plasma. J Acquir Immune Defic Syndr. 2009;51:60–64. doi: 10.1097/QAI.0b013e31819fb903. [DOI] [PubMed] [Google Scholar]
- Storz P, Toker A. 3′-Phosphoinositide-dependent kinase-1 (PDK-1) in PI 3-kinase signaling. Front Biosci. 2002;7:d886–902. doi: 10.2741/storz. [DOI] [PubMed] [Google Scholar]
- Toborek M, Lee YW, Flora G, Pu H, Andras IE, Wylegala E, Hennig B, Nath A. Mechanisms of the blood–brain barrier disruption in HIV-1 infection. Cell Mol Neurobiol. 2005;25:181–199. doi: 10.1007/s10571-004-1383-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen Z, Zhong Z, Darnell JE., Jr Maximal activation of transcription by Stat1 and Stat3 requires both tyrosine and serine phosphorylation. Cell. 1995;82:241–250. doi: 10.1016/0092-8674(95)90311-9. [DOI] [PubMed] [Google Scholar]
- Yang B, Akhter S, Chaudhuri A, Kanmogne GD. HIV-1 gp120 induces cytokine expression, leukocyte adhesion, and transmigration across the blood–brain barrier: modulatory effects of STAT1 signaling. Microvasc Res. 2009;77:212–219. doi: 10.1016/j.mvr.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Wen Z, Xu LZ, Darnell JE., Jr Stat1 serine phosphorylation occurs independently of tyrosine phosphorylation and requires an activated Jak2 kinase. Mol Cell Biol. 1997;17:6618–6623. doi: 10.1128/mcb.17.11.6618. [DOI] [PMC free article] [PubMed] [Google Scholar]