Abstract
Melatonin receptors have been identified in several retinal cell types, including photoreceptors, horizontal cells, amacrine cells, and ganglion cells. Recent reports suggest that melatonin potentiates signaling from rods to inner retinal neurons. However, the organization of the melatonin receptors mediating this action in the outer plexiform layer (OPL) is not clear. To assess melatonin receptor localization in the OPL, double-label confocal immunohistochemistry for Mel1a or Mel1b melatonin receptors was performed in combination with markers for cone photoreceptors (calbindin, XAP-1) and ON bipolar cells (guanine nucleotide binding protein alpha, Goα) on the retina of Xenopus laevis. Both Mel1a and Mel1b receptors were specifically associated with processes contacting the pedicles of cones, but localized to processes from different sets of second-order neurons. Mel1a receptors localized to the large axonal processes of horizontal cells, while Mel1b receptors localized to the dendrites of OFF bipolar cells. Both receptors also localized to third-order amacrine and ganglion cells and their processes in the inner plexiform layer. This study indicates that Mel1a and Mel1b melatonin receptors are expressed specifically in the Xenopus OPL to modulate transmission from cones to horizontal cells and OFF bipolar cells, respectively; they are second-order neurons that predominantly contact ribbon synapses and display OFF responses to light. When combined with results from recent physiological studies, the current results suggest a conserved function for melatonin in enhancing transmission from rods to second-order neurons across species, although the precise mechanisms by which melatonin enhances this transmission are likely to vary in a species-dependent manner.
INDEXING TERMS: photoreceptor, bipolar cell, horizontal cell, dark-adaptation
Melatonin, the major hormone of the pineal gland, is also synthesized by retinal photoreceptors at night and provides a circadian paracrine and/or intracrine signal by binding to specific receptors in the retina (Cahill et al., 1991; Wiechmann and Summers, 2008). The circadian changes that occur in the rate of retinal melatonin synthesis suggest that melatonin may modulate diurnal events that occur in the retina such as photoreceptor outer segment disc shedding and phagocytosis, photomechanical movements, and retinal sensitivity to light (Baba et al., 2009; see Wiechmann and Summers, 2008, for a review).
Three melatonin receptor subtypes (Mel1a, Mel1b, and Mel1c) have been identified in the South African clawed frog Xenopus laevis (Ebisawa et al., 1994; Reppert et al., 1995a,b). The Xenopus Mel1a receptor is the ortholog of the mammalian MT1 receptor, and the Xenopus Mel1b receptor is the ortholog of the mammalian MT2 receptor. The melatonin-related receptor GPR50 appears to be the mammalian ortholog of the Mel1c receptor of fish and amphibians, but does not bind melatonin (Dufourny et al., 2008). All three subtypes are expressed in the Xenopus retina (Wiechmann et al., 1999, 2004; Wiechmann and Smith, 2001; Wiechmann, 2003; Wiechmann and Summers, 2008). Immunohistochemical studies on the melatonin receptor subtypes show that they are differentially expressed among retinal neurons. Horizontal cells in several species express Mel1a receptors (Fujieda et al., 2000; Meyer et al., 2002; Scher et al., 2002; Huang et al., 2005), and Mel1a, Mel1b, and Mel1c receptors are all expressed by subpopulations of amacrine and ganglion cells and are abundant in the inner plexiform layer (Fujieda et al., 2000; Meyer et al., 2002; Scher et al., 2002; Wiechmann, 2003; Wiechmann et al., 2004; Wiechmann and Summers, 2008).
Details of the specific cell types expressing each subtype of melatonin receptor and how these expression patterns relate to the functional organization of retinal circuits are lacking. A recent electrophysiological study in carp retina demonstrated that melatonin specifically potentiates rod signals to ON type bipolar cells, via activation of the melatonin MT2 (Mel1b) receptor (Ping et al., 2008). This finding indicates that melatonin may modulate the function of specific retinal circuits based on the differential distribution of its receptors.
In this study we used immunohistochemical methods to investigate the relationship of melatonin receptor subtypes to the rod-cone and ON-OFF circuits of the outer plexiform layer (OPL) of the X. laevis retina. We describe our discovery of selective Mel1a and Mel1b melatonin receptor expression in cone circuits, with Mel1b receptor expression by OFF-bipolar cells, and Mel1a receptors by horizontal cell processes specifically at cone terminals. These observations indicate that melatonin receptor subtypes do, in fact, show differential, cell-specific patterns of expression that are likely to underlie differential functional modulation of specific retinal pathways.
MATERIALS AND METHODS
Animals and tissue preparation
Adult Xenopus laevis (African clawed frogs) were obtained from Xenopus Express (Dexter, MI) and maintained in aquaria at 20°C on a daily 12:12-hour light–dark schedule. Frogs were deeply anesthetized by immersion in tricaine methanesulfonate (MS-222) and killed by decapitation. In two early experiments, eyes were obtained at 4-hour intervals during a 24-hour period, and in five subsequent experiments eyes were obtained in the early and late dark period or early light period and mid-dark period and processed together. Since no obvious temporal differences in melatonin receptor labeling could be discerned, all subsequent specimens were obtained in the early or mid-light period. Eyes from 43 frogs were used in this study and subjected to analyses with various combinations of antibodies. Anterior segments (cornea, iris, and lens) were dissected away from the posterior segments (sclera, choroid, retinal pigment epithelium [RPE], and neural retina), and these eyecups were immersion-fixed for 2–18 hours at 4°C in 4% paraformaldehyde in 0.1 M phosphate buffer, pH 7.4. Eyecups were rinsed with 0.1 M phosphate-buffered saline (PBS), pH 7.4. For immunocytochemistry of cryostat sections, eyecups were transferred to 30% sucrose in phosphate buffer for 16–20 hours at 4°C, and then mounted in Tissue-Tek O.C.T. mounting matrix (Sakura Finetek, Torrance, CA). Sagittal 10-μm sections were cut on a cryostat microtome and collected on glass slides. For whole-mount immunocytochemistry, neural retinas were peeled away from the RPE and were placed separately into 2.0-ml microcentrifuge tubes containing PBS and processed for immunocytochemistry. Animal care procedures were in accordance with the guidelines of the Public Health Service Policy on Humane Care and Use of Laboratory Animals and approved by the Oklahoma University Health Sciences Center Institutional Animal Care and Use Committee.
Antisera and antibodies
A panel of antibodies directed against Xenopus Mel1a and Mel1b receptors and well known cell-specific markers was used for these studies (Table 1). Labeling patterns observed were consistent with previous reports in the retina of X. laevis and other species (Harris and Messersmith, 1992; Dhingra et al., 2000; Wiechmann and Smith, 2001; Wiechmann et al., 2003, 2004; Zhang and Wu, 2003; Morona et al., 2007; Wiechmann and Summers, 2008).
TABLE 1.
List of Primary Antibodies Used for Immunolabeling
| Antigen | Immunogen | Manufacturer (catalog #) | Host | Dilution/concentration |
|---|---|---|---|---|
| Calbindin | Chicken, full length purified from gut | SWANT; Bellinzona, Switzerland (Catalog # 300) | Mouse monoclonal (clone McAB 300/301) | 1:1,000 |
| Goα | Purified Goα from bovine brain | Millipore, Billerica, MA (Catalog # MAB3073) | Mouse monoclonal (clone 2A) | 1:500 |
| Melatonin receptor 1a | Xenopus laevis, aa 231-243 (HHQTWPYNIHGFI) | Dr. Allan Wiechmann, OUHSC, Oklahoma City, OK | Chicken polyclonal | 2.3 μg/ml |
| Melatonin receptor 1b | Xenopus laevis, aa 221-233 (KPRMKQSDFRNFL) | Dr. Allan Wiechmann, OUHSC, Oklahoma City, OK | Rabbit polyclonal | 2.3 μg/ml |
| XAP-1 | Crude homogenate of Xenopus retina and optic nerve | Developmental Studies Hybridoma Bank, U Iowa, Iowa City, IA (Catalog # XAP-1) | Mouse monoclonal (Clone 3D2) | 1:10-1:20 |
Chicken polyclonal anti-X. laevis Mel1a receptor (Wiechmann, 2003) was raised against a peptide sequence (residues 231–243; HHQTWPYNIHGFI; Reppert et al., 1995b) and affinity-purified as described previously (Wiechmann, 2003). The antibody recognizes a band of ≈42 kDa in western blot analysis of Xenopus neural retina homogenate (unpubl. results). Two additional bands of ≈50 kDa and 76 kDa were also observed, and may represent a glycosylated form of the Mel1a receptor or dimerized receptors, respectively; a pattern that has been previously observed to occur with Xenopus melatonin receptors in the retina and tectum (Wiechmann et al., 2004; Prada et al., 2005). Preincubation with the inoculating peptide blocks all labeling of the western blot, as expected. Preincubation of the antibody with the inoculating peptide also eliminates immunolabeling of tissue sections (Wiechmann, 2003). Immunolabeling observed with this antibody was consistent with previous studies (Wiechmann and Smith, 2001; Wiechmann et al., 2003, 2004).
Rabbit polyclonal anti-X. laevis Mel1b receptor (Wiechmann et al., 2004) was raised against a peptide sequence (residues 221–233; VKSEFKPRMQSDF) with no identity to the corresponding region of the Xenopus Mel1a or Mel1c receptors (Reppert et al., 1995b) and affinity-purified as described previously (Wiechmann et al., 2004). Preincubation of the antibody with the inoculating peptide eliminates labeling and western blotting reveals a protein band of the appropriate size in Xenopus retinal tissue (Wiechmann et al., 2004). Immunolabeling observed with this antibody was consistent with previous studies (Wiechmann and Smith, 2001; Wiechmann et al., 2004) and in situ hybridization studies to identify retinal cells expressing mRNA for the Mel1b receptor (Wiechmann and Smith, 2001).
Mouse monoclonal (clone McAB 300/301; SWANT, Bellinzona, Switzerland) anti-chicken calbindin recognizes a single band of 28 kDa in western blots of brain homogenates from wildtype mice, but no bands in blots from calbindin knockout mice (Celio et al., 1990; Airaksinen et al., 1997). Anti-calbindin antibodies specifically label Xenopus cone photoreceptors (Morona et al., 2007), consistent with labeling patterns observed in this report.
Mouse monoclonal anti-guanine nucleotide binding protein (G protein) alpha (Goα, clone 2A; Millipore, Billerica, MA) was raised against purified Goα from bovine brain and recognizes a single band of 39–42 kDa on western blots of bovine and rat brain membrane homogenates (Li et al., 1995). Knockout of Goα eliminates immunolabeling (Dhingra et al., 2000). This antibody labels retinal ON-bipolar cells (Dhingra et al., 2000; Zhang and Wu, 2003), consistent with labeling patterns observed in this report.
Mouse monoclonal antibody XAP-1 (Clone 3D2; Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA) was raised against a crude homogenate of Xenopus retina and optic nerve (Harris and Messersmith, 1992) and recognizes Grp78, a protein in the interphotoreceptor matrix, as identified by western blotting and mass spectrometry (Nookala et al., 2010). The XAP-1 antibody specifically labels the interphotoreceptor matrix surrounding the outer segments and terminals of cones in the Xenopus retina (Harris and Messersmith, 1992), consistent with labeling patterns observed in this report.
Confocal immunohistochemistry procedures
For single labeling immunohistochemical localization of melatonin receptors in X. laevis retina, cryostat sections or whole retinas were rinsed in PBS, then incubated in incubation buffer (2% bovine serum albumin [Sigma, St Louis, MO], 0.2% Triton X-100, and 0.004% sodium azide in PBS) for 30 minutes at room temperature (RT). Sections were incubated either with chicken anti-Xenopus Mel1a melatonin receptor antibody or rabbit anti-Xenopus Mel1b melatonin receptor antibody at a concentration of 2.3 μg/ml in incubation buffer for 3 days at 4°C. For negative controls, tissue sections were incubated in incubation buffer lacking the primary antibody. Following incubation with the primary antibody, specimens were rinsed in PBS and incubated in 5 μg/ml of goat anti-chicken antibody or goat anti-rabbit anti-body conjugated to Alex-aFluor 488 (Molecular Probes, Eugene, OR) for 1 hour at RT. Sections were rinsed in PBS, then incubated with 0.0005% 4′, 6-diamidino-2-phenylindole (DAPI; Invitrogen, Carlsbad, CA) nuclear stain for 10 seconds at RT, followed by a final rinse in PBS. Retinal whole mounts were incubated in 0.0005% DAPI for 10 minutes at RT, followed by a rinse in PBS. Neural retinas were mounted onto glass slides by making 4–5 slits from peripheral to central retina with scissors and then compressing the tissue under the coverslips after the mounting matrix was applied to achieve a flat-mounted neural retina. Coverslips were mounted onto the slides with Prolong Gold antifade reagent containing DAPI (Molecular Probes).
For double-label immunohistochemistry, a sequential labeling procedure was used. The first primary antibody, either the Mel1a or the Mel1b melatonin receptor antibody, was applied and subsequently labeled with a fluorescent secondary antibody for 1 hour at RT (5 μg/ml goat anti-chicken conjugated to AlexaFluor 568 or 488 for Mel1a antibody, or goat anti-rabbit conjugated to AlexaFluor 568 or 488 for Mel1b antibody; secondary antibodies from Molecular Probes). Following rinses in PBS, specimens were incubated in the complementary anti-melatonin receptor antibody, or one of the following marker antibodies: mouse anti-XAP-1, mouse anti-calbindin, or mouse anti-Goα for 3 days at 4°C. Following incubation with the second primary antibody, specimens were rinsed in PBS and incubated in 5 μg/ml of goat anti-rabbit or goat anti-mouse antibody conjugated to AlexaFluor 488 or 568 (Molecular Probes) for 1 hour at RT. Sections were rinsed in PBS, then incubated with 0.0005% DAPI nuclear stain for 10 seconds at RT, followed by a final rinse in PBS. Whole retinas were incubated in 0.0005% DAPI for 10 minutes at RT, followed by a rinse in PBS. Coverslips were mounted onto the slides with Prolong Gold antifade reagent containing DAPI. Specimens were viewed by confocal microscopy using an Olympus FluoView 1000 laser-scanning confocal microscope (Olympus, Center Valley, PA). The pinhole (confocal aperture diameter) conditions were fixed at 105 μm in all images generated in this study. The objective lens used in this study was an Olympus PlanApo N 60×/1.42 oil lens (8/0.17/FN26.5). In all cases, image scale was calibrated and brightness and contrast were adjusted if necessary to highlight specific labeling. Adjustments in brightness, contrast, and scale were made to images as necessary to optimize for viewing using the Adobe Photoshop CS5 (v. 12.0.4) software program (Adobe Systems, San Jose, CA).
RESULTS
Mel1a and Mel1b receptors are differentially localized in the outer plexiform layer (OPL)
Sections of Xenopus retina double-labeled for Mel1a and Mel1b revealed that both receptors were expressed in the OPL (see Table 2 for abbreviations used in figure legends), but had distinct distributions (Fig. 1). Examination of single optical sections (Fig. 1B–D) confirmed that labeling for Mel1a and Mel1b had distinct distributions in the OPL. Strong labeling for Mel1a localized to a set of large processes with the characteristic morphology of horizontal cell processes (Witkovsky et al., 1988) coursing along the border between the OPL and the inner nuclear layer (INL). In contrast, Mel1b labeling localized to periodic clusters of processes in the distal OPL and in slender processes coursing through the thickness of the OPL. The Mel1b-positive processes arose from a subpopulation of cells in the INL having the characteristic morphology of bipolar cells (also see Fig. 4). Weak labeling for Mel1b also was observed occasionally in photoreceptor cell bodies in the outer nuclear layer (ONL). Together, these findings indicated that Mel1a and Mel1b were differentially distributed in the OPL, with Mel1a localizing to horizontal cell processes and Mel1b localizing to bipolar cell processes.
TABLE 2.
List of Abbreviations in Figure Legends
| AC | amacrine cell |
| BC | bipolar cell |
| Goα | guanine nucleotide binding protein (G protein) alpha |
| HC | horizontal cell |
| INL | inner nuclear layer |
| IPL | inner plexiform layer |
| Mel1a | melatonin receptor 1a |
| Mel1b | melatonin receptor 1b |
| NFL | nerve fiber layer |
| OFF | OFF bipolar cell |
| ON | ON bipolar cell |
| ONL | outer nuclear layer |
| OPL | outer plexiform layer |
| PH | photoreceptor cell |
Figure 1.
Mel1a and Mel1b receptors are differentially distributed in the outer retina. A: Mel1a immunoreactivity (red) in the outer plexiform layer (OPL) is present in large processes (arrows) morphologically similar to horizontal cell axons. Mel1b immunoreactivity (green) in the OPL is present in a distinct set of slender processes that form periodic clusters (arrowheads). Mel1b labeling is also present in the cell bodies of bipolar and amacrine cells (BC and AC, respectively) in the inner nuclear layer (INL). Mel1b labeling is also present at the level of the photoreceptor inner segments (PH). Mel1a and Mel1b receptors show differential distribution in the inner plexiform layer (IPL). Mel1b receptors are diffusely distributed throughout the IPL, but Mel1a receptors are found in discrete puncta (small arrows). The box in (A) indicates the area that is shown at higher magnification in (B–D). The confocal image is comprised of 16 optical slices of ≈400 nm. B–D: Examination of single confocal optical planes confirms that Mel1a and Mel1b localize to distinct processes in the OPL. Single optical slice of ≈400 nm is shown in B–D. B: Mel1b is present in slender processes arising from bipolar cells (BC) that form clusters in the distal OPL (large arrowhead). Mel1b labeling also is present in bipolar cell axons (arrow). C: Mel1a labeling is present in relatively large processes (small arrowheads). D: Overlay of panels B,C. Nuclei are counterstained with DAPI (blue) in all panels. Scale bars = 10 μm in all panels.
Figure 4.
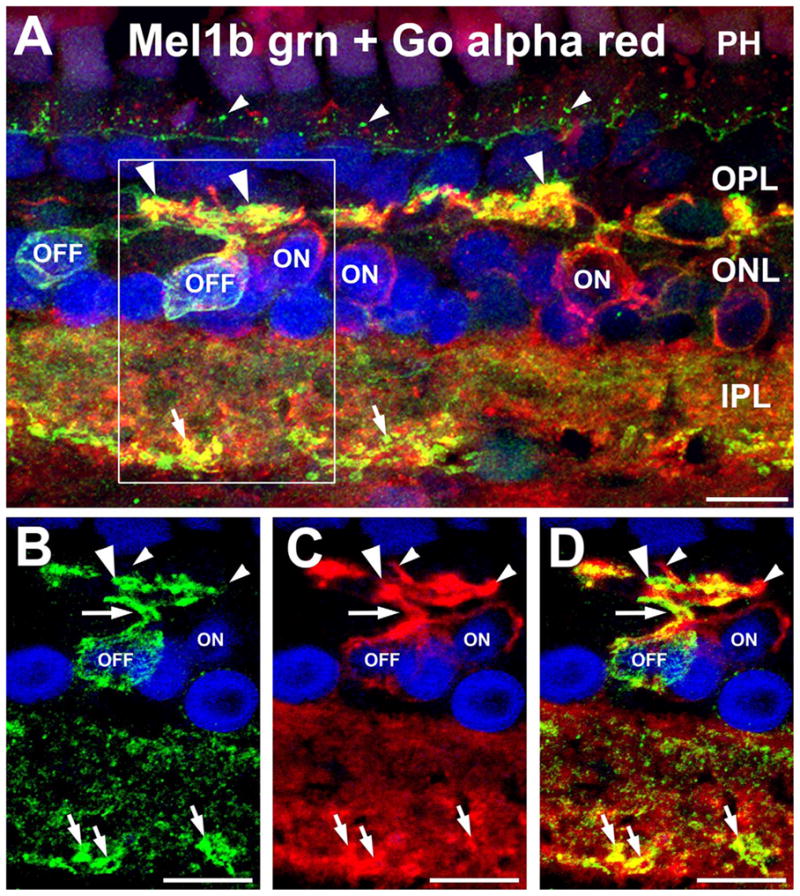
Mel1b receptors are expressed by OFF bipolar cells. A: Double labeling for Mel1b receptors (green) and the ON bipolar cell marker Goα (red) shows that Mel1b receptor-immunoreactivity is absent from the cell bodies of ON bipolar cells (ON), identifying the Mel1b receptor-immunoreactive bipolar cells as OFF bipolar cells (OFF). Confocal image stack comprised of seven optical slices of 400 nm each. Apparent colocalization of Mel1b and Goα immunoreactivity in processes in the outer plexiform layer (OPL) is due to their close proximity and the relative thickness of the image stack, and does not represent genuine colocalization (see panels B–D, below). Immuno-labeling for both Mel1b and Goα is present in the inner plexiform layer (IPL), with strongly Mel1b-positive processes (small arrows) present along the inner margin of the layer. Mel1b immunoreactive puncta (small arrowheads) are also present at the level of the outer limiting membrane (OLM). The box in (A) indicates area shown in panels B–D. B–D: Examination of a single confocal optical plane confirms that Mel1b labeling does not colocalize with labeling for Goα in ON bipolar cells. Images in panels B–D represent a single optical slice of ≈400 nm. B: Mel1b immunoreactivity in the cell body and primary and secondary dendrites (arrow and large arrowhead respectively) of a bipolar cell (OFF). C: ON bipolar cell dendrites labeled for Goα (small arrowheads). D: Overlay of panels B,C showing that ON bipolar cell dendrites are devoid of Mel1b receptor labeling. Nuclei are counterstained with DAPI (blue) in all panels. INL, inner nuclear layer; PH, photoreceptor inner segments. Scale bars = 10 μm in all panels.
Features of Mel1a and Mel1b immunolabeling elsewhere in the retina were consistent with previous reports (Fig. 1; Wiechmann and Smith, 2001; Wiechmann, 2003; Wiechmann et al., 2003, 2004). Mel1a labeling was distributed diffusely throughout the inner plexiform layer (IPL). Abundant labeling for Mel1b also was present in the inner segments of the photoreceptors. Some amacrine cells located in the innermost INL also showed Mel1b labeling. Punctate Mel1b labeling also was present in the IPL.
Mel1b receptors are expressed selectively by cone-driven OFF-bipolar cells
The distribution of Mel1b labeling suggested that a specific subpopulation of bipolar cells expressed the Mel1b receptor. Bipolar cells can be divided into several subtypes, with key functional subclasses determined by the type of photoreceptor that provides their primary input (rod vs. cone bipolar cells) and whether the cell depolarizes or hyperpolarizes in response to light falling in the receptive field (ON vs. OFF bipolar cells, respectively; Wu et al., 2000; see Wu, 2010, for a review).
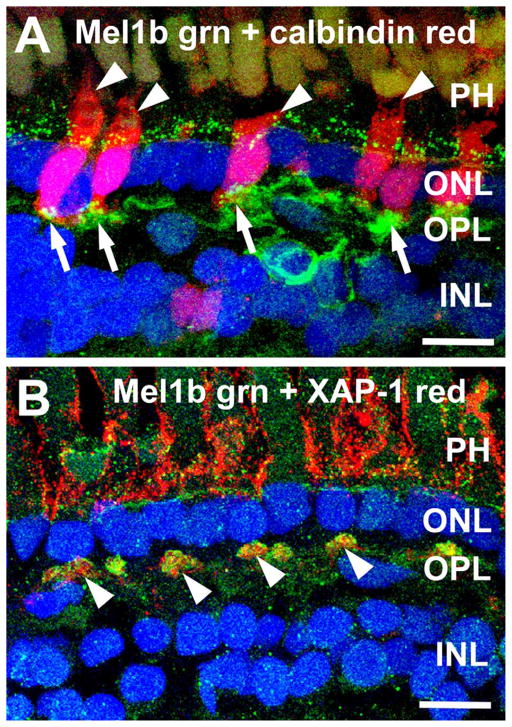
The patchy distribution of Mel1b immunolabeled processes in the OPL suggested that the Mel1b-positive bipolar cell dendrites might selectively contact cone terminals. Assessment of the spatial distribution of Mel1b labeling in the distal OPL of retinal flatmounts confirmed that the Mel1b-immunoreactive processes formed discrete clusters as expected of processes that selectively contact cone terminals (Fig. 2). The Mel1b-immunoreactive clusters also showed a distinctive 2D organization into rings within the OPL, implying selective interactions with a specific set of photoreceptor terminals. Double labeling of retinal sections for Mel1b in combination with calbindin or XAP-1, specific markers for Xenopus cones (Harris and Messersmith, 1992; Morona et al., 2007), showed that the clusters of Mel1b-immunoreactive bipolar cell processes corresponded specifically to the locations of cone terminals where they made contact (Fig. 3).
Figure 2.
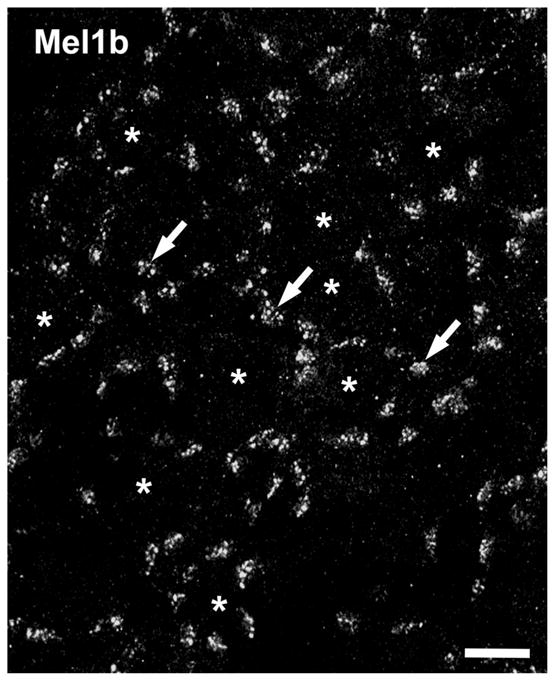
Patchy Mel1b receptor distribution in the OPL resembles the distribution of cone terminals. Mel1b receptor labeling in retinal flatmounts confirms the localization of Mel1b to discrete patches of processes (arrows) in the OPL, reminiscent of cone terminal distribution. The patches of Mel1b receptor labeling tend to form annuli surrounding a center region devoid of Mel1b labeling (*). Confocal image stack comprised of 18 optical slices of ≈400 nm each. Scale bar = 10 μm.
Figure 3.
Mel1b receptor-immunoreactive processes contact cone photoreceptor terminals in the outer plexiform layer (OPL). A: Mel1b-immunoreactive (green) processes (arrows) in the OPL specifically contact cone photoreceptor terminals (arrows) labeled for calbindin (red). Punctate Mel1b immunoreactivity is also present at the level of the outer limiting membrane (OLM) and photo-receptor inner segments (arrowheads, PH), just distal to the outer nuclear layer (ONL). Confocal image stack comprised of 22 optical slices of ≈400 nm each. B: Double labeling for Mel1b receptors (green) and XAP-1 (red), a marker for the extracellular matrix surrounding photoreceptor inner and outer segments (PH) and cone terminals (arrowheads) in the OPL, confirms the interaction of Mel1b-positive processes and cone terminals. Confocal image stack comprised of 11 optical slices of ≈400 nm each. Nuclei are counterstained with DAPI (blue) in both panels. INL, inner nuclear layer. Scale bars = 10 μm in both panels.
To better understand the distribution of Mel1b receptors in bipolar cells, we performed double immunolabeling to identify the ON/OFF characteristics of Mel1b-positive bipolar cells. Double labeling for Mel1b in combination with Goα, a marker specific for all types of ON bipolar cells (Dhingra et al., 2000; Zhang and Wu, 2003), showed that ON bipolar cells did not express Mel1b. Thus, the Mel1b-immunoreactive bipolar cells represented a set of OFF bipolar cells (Fig. 4). Examination of single optical sections confirmed that Mel1b-labeled bipolar cell dendrites in the OPL were distinct from the Goα-positive ON bipolar cell dendrites (Fig. 4B–D). It was also noted that the Mel1b-positive OFF bipolar cell processes often projected further distally into the OPL than the Goα-positive dendrites of ON bipolar cells (Fig. 4A).
Examination of the OPL in retinal flatmounts double-labeled for Mel1b and Goα showed that the Mel1b-positive dendrites of OFF cone bipolar cells had a more restricted distribution than the Goα-positive ON bipolar cell dendrites (Fig. 5). Our results in vertical sections showed that large clusters of Goα-positive ON bipolar cell dendrites were specifically associated with cone terminals, allowing positive identification of cone terminals in retinal flat-mounts on the basis of tightly clustered Goα-positive ON bipolar cell dendrites. As appropriate, Goα-positive ON bipolar cell dendrites were present in large clusters corresponding to cone terminals as well as in the spaces occupied by rod terminals between the larger cone terminals in flatmount preparations. Mel1b-positive OFF cone bipolar cell dendrites were found selectively in the large clusters corresponding to cone terminals, as observed in vertical sections. However, some cone terminals, identified by tightly clustered Goα-positive ON bipolar cell dendrites, did not receive contacts from Mel1b-positive OFF bipolar cell dendrites. This result suggests that the Mel1b-positive OFF bipolar cells did not contact the terminals of all cones. These studies also revealed that the Mel1b- and Goα-positive bipolar cell dendrites were spatially organized within the cluster of processes contacting a cone terminal, with the Mel1b-positive OFF-cone bipolar cell dendrites tending to occupy a more central position within the cluster than the Goα-positive ON bipolar cell dendrites.
Figure 5.
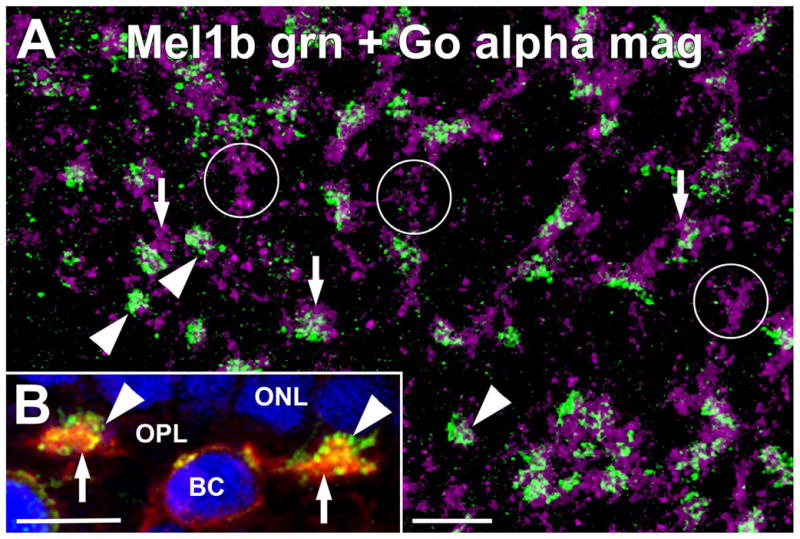
Mel1b receptor-immunoreactive OFF bipolar cell dendrites and ON bipolar cell dendrites are spatially segregated at cone terminals. A: Immunolabeling for Mel1b (green) and Goα (magenta) in a retinal flatmount preparation focused at the level of the outer plexiform layer (OPL). Mel1b-immunoreactive OFF bipolar cell dendrites (arrowheads) and Goα-immunoreactive ON bipolar cell dendrites (arrows) both contact the same cone terminals, but tend to contact cone terminals in discrete locations. Occasionally, cone terminals receiving numerous contacts from Goα-immunoreactive ON bipolar cell dendrites, but little or no contacts from Mel1b-immunoreactive OFF bipolar cell dendrites were observed (circles), suggesting that Mel1b-immunoreactive OFF bipolar cells may not contact all cone types equally. Confocal image stack comprised of nine optical slices of ≈400 nm each. B: Mel1b (green) and Goα (red) immunolabeling in the OPL in vertical frozen sections of retina shows that Mel1b-immunoreactive OFF bipolar cell dendrites (arrowheads) penetrated slightly deeper into the cone terminals than Goα-immunoreactive ON bipolar cell dendrites (arrows). An ON bipolar cell body (BC) labeled for Goα is also visible. Nuclei are counter- stained with DAPI (blue). Confocal image stack comprised of seven optical slices of 400 nm each. Scale bars = 10 μm for both panels.
Mel1a-positive horizontal cell axons contact cone terminals
To better understand the anatomical organization of Mel1a receptors in the Xenopus OPL, we also performed double labeling for Mel1a in conjunction with known cell-specific markers. Double labeling for Mel1a and Goα confirmed that Mel1a labeling in the OPL was not associated with the dendrites of ON bipolar cells, although the two sets of processes often were located near each other (Fig. 6). This result, when combined with the findings that; 1) Mel1b labeling in the OPL is associated with the dendrites of OFF bipolar cells, 2) does not colocalize with Mel1a, 3) and the morphological characteristics of the Mel1a-positive processes, identify the large Mel1a-positive processes as the axons of horizontal cells.
Figure 6.
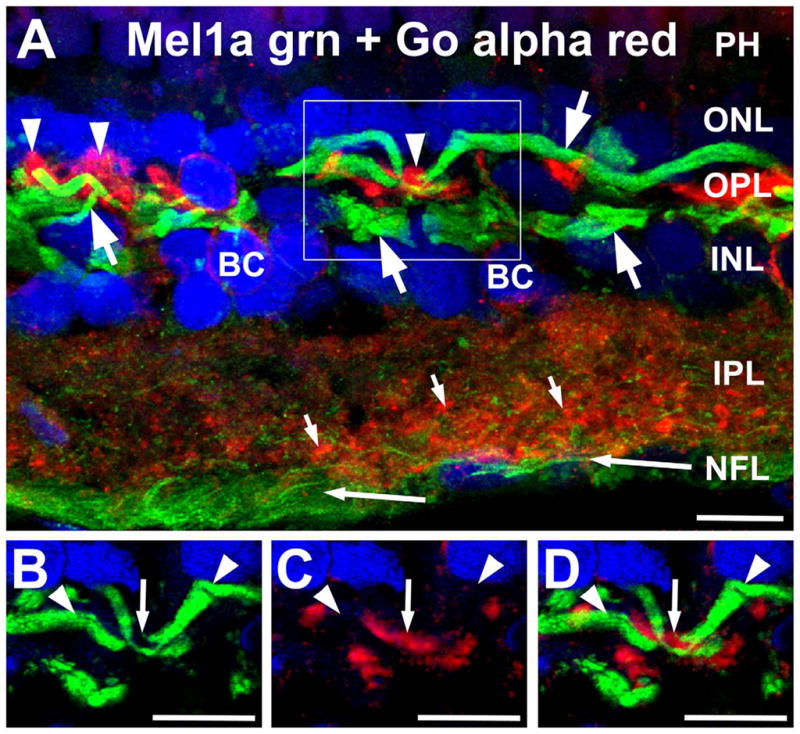
Mel1a receptors are not expressed in ON bipolar cell dendrites. A: Labeling for Mel1a receptor (green) and Goα (red) localize to different processes in the outer plexiform layer (OPL). Mel1a receptors are expressed in distinctive processes morphologically similar to horizontal cell axons (large arrows). These processes are distinct from the Goα-immunoreactive ON bipolar cell dendrites (arrowheads). Apparent colocalization of Mel1b and Goα immunoreactivity in processes in the outer plexiform layer (OPL) is due to their close proximity and the relative thickness of the image stack, and does not represent genuine colocalization (see panels B–D, below). Mel1a immunoreactivity also is present in puncta throughout the inner plexiform layer (IPL; small arrows) and in ganglion cell axons (long arrows) in the nerve fiber layer (NFL). Confocal image stack comprised of 15 optical slices of ≈400 nm each. The box indicates the area shown in panels B–D. B–D: Examination of thin stacks of confocal optical planes confirms that Mel1a labeling is not localized to ON bipolar cell dendrites. Images in panels B–D are comprised of five optical slices of ≈400 nm each. B: Mel1a immunoreactivity in horizontal cell axons (arrow-heads) in the OPL. C: ON bipolar cell dendrites labeled for Goα (small arrow). D: Overlay of panels B,C showing that ON bipolar cell dendrites (Goα-positive) and Mel1a receptor-positive processes are not colocalized. Nuclei are counterstained with DAPI (blue) in all panels. BC, Bipolar cell; PH, photoreceptors; IPL, inner plexiform layer; NFL, nerve fiber layer. Scale bars = 10 μm in all panels.
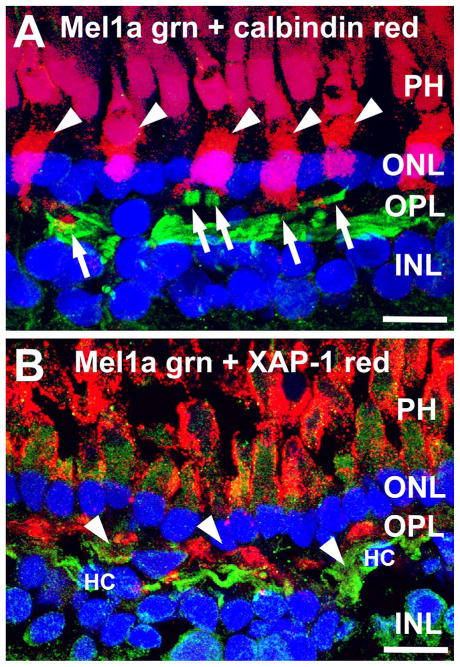
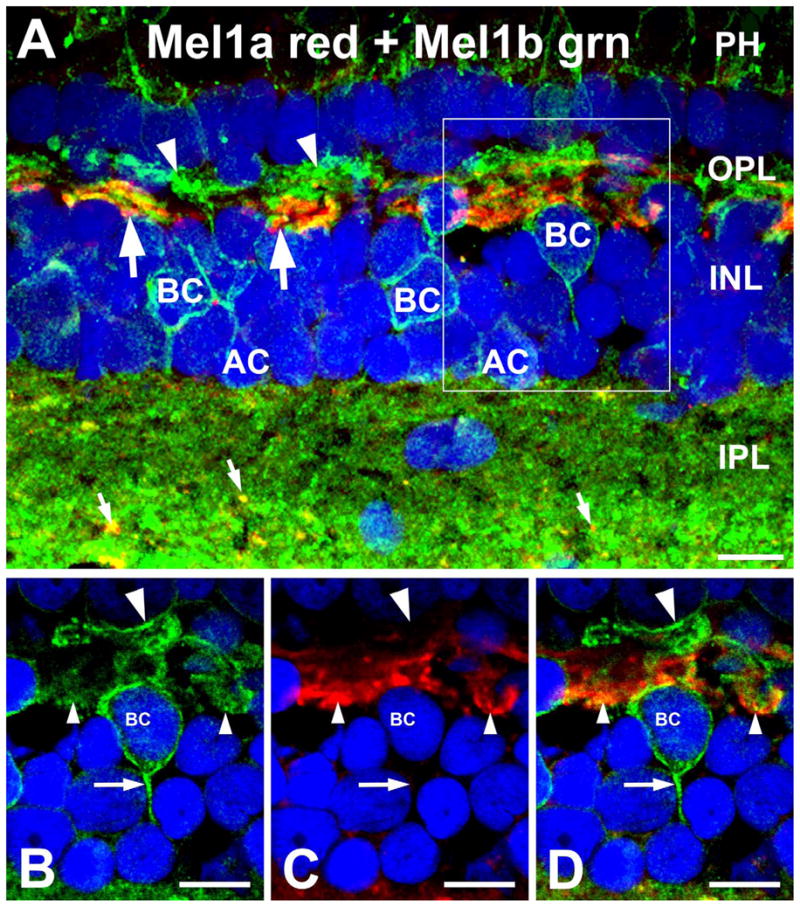
To assess potential photoreceptor inputs to the large Mel1a-positive horizontal cell axons, we performed double labeling for Mel1a in combination with the cone-specific markers calbindin and XAP-1. The Mel1a-positive horizontal cell axons gave rise to distally oriented projections that contacted cone terminals (Fig. 7). Examination of retinal flatmounts double-labeled for Mel1a and Mel1b confirmed that Mel1a-positive horizontal cell processes selectively contacted cone terminals (Fig. 8). Mel1a-positive processes from horizontal cell axons and Mel1b-positive OFF-cone bipolar cell processes clustered together closely at the same cone terminals.
Figure 7.
Mel1a melatonin receptor-immunoreactive processes in the outer plexiform layer (OPL) contact cone photoreceptor terminals. A: Branches of Mel1a-immunoreactive processes (green; arrows) ascend through the OPL to contact the terminals of cal-bindin-immunoreactive cone photoreceptors (red; arrowheads) terminals. Confocal image stack comprised of 11 optical slices of ≈400 nm each. B: Mel1a melatonin receptor-immunoreactive processes (green; arrowheads) in the OPL contact XAP-1-immuno-reactive cone photoreceptor terminals (red). The extracellular matrix surrounding the inner and outer segments of the photoreceptors is also XAP-1-immunoreactive. Confocal image stack comprised of 10 optical slices of ≈400 nm each. Nuclei are counterstained with DAPI (blue) in both panels. PH, photoreceptor inner segments; ONL, outer nuclear layer; INL, inner nuclear layer. Scale bars = 10 μm in both panels.
Figure 8.
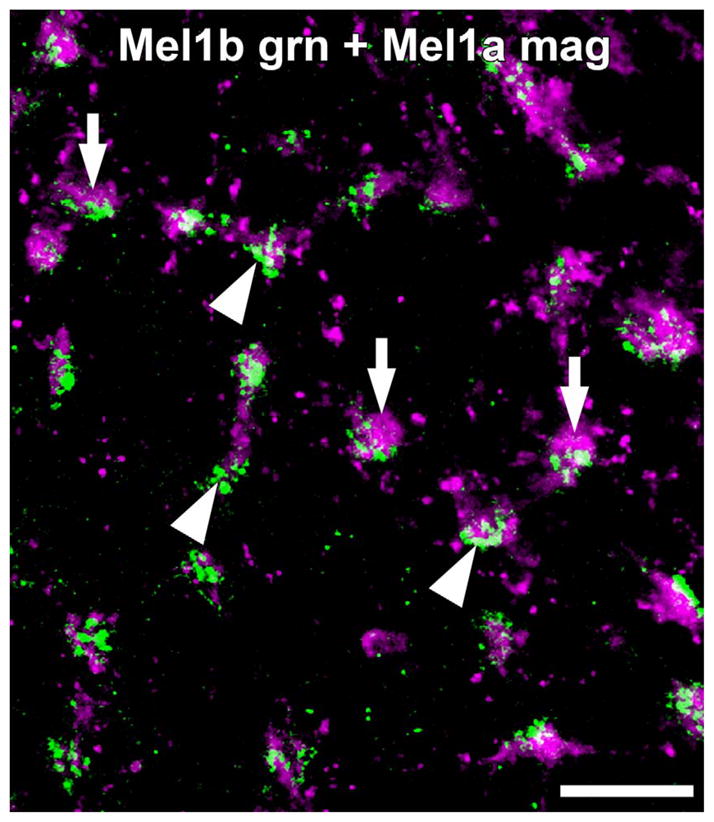
Mel1b receptor-immunoreactive OFF bipolar cell dendrites and Mel1a receptor-immunoreactive horizontal cell axons contact the same cone terminals. Immunolabeling for Mel1b (green) and Mel1a (magenta) in a retinal flatmount focused at the level of the outer plexiform layer (OPL). Mel1b-immunoreactive OFF bipolar cell dendrites (arrowheads) and Mel1a-immunoreactive horizontal cell axons (arrows) selectively contact the same cone terminals. Confocal image stack comprised of seven optical slices of ≈400 nm each. Scale bar = 10 μm.
DISCUSSION
These studies indicate that Mel1a and Mel1b receptors are both present in the OPL of the Xenopus retina and localize to processes of second-order neurons that have OFF responses to light. However, the two receptor types show differential distribution. Mel1a receptors localize to horizontal cell axons, while Mel1b receptors are found on the dendrites of OFF bipolar cells, but not ON bipolar cells. Furthermore, these studies show that Mel1a and Mel1b receptors localize preferentially to processes contacting cone terminals. Together, these studies suggest that melatonin signaling in the outer retina is positioned specifically to play a role in modulating cone-driven signals in OFF circuits in the OPL.
Mel1a and Mel1b receptors localize differentially to horizontal and bipolar cell processes in the OPL
Previous studies have shown that Mel1a and Mel1b receptors are present in the retina, including the OPL (Wiechmann, 2003; Wiechmann et al., 2003, 2004). However, those studies did not identify the specific cells expressing each receptor. The current study establishes that Mel1a and Mel1b receptors in the OPL are differentially distributed on the processes of second-order horizontal and bipolar cells in the Xenopus OPL, respectively. Mel1a receptors localized to a set of processes in the OPL that showed the highly distinctive morphology of horizontal cell axons (Witkovsky et al., 1988), consistent with a report from carp retina suggesting that MT1 (Mel1a) receptors are present on horizontal cells (Huang et al., 2005). Double labeling for Mel1a and cone terminal markers showed that Mel1a receptor-immunoreactive branches from the horizontal cell axons selectively clustered at cone terminals, although the cone terminals themselves did not possess Mel1a receptors. In contrast, Mel1b receptors localized to a set of dendrites in the OPL arising from a subset of cells in the INL with the characteristic features of bipolar cells: dendrites projecting to the OPL, cell body in the INL, and an axonal process projecting to the IPL. Double labeling for Mel1b receptors and cone terminal markers showed that Mel1b receptor-immunoreactive bipolar cell dendrites also clustered at cone terminals; however, the cone terminals themselves showed no Mel1b receptor labeling.
Organization of Mel1a and Mel1b receptors in OPL circuits
Experiments to identify specific types of second-order neurons and their relationship with photoreceptors showed that Mel1a and Mel1b receptors are both associated specifically with second-order neurons that show OFF-type light responses and receive input from cones.
Mel1b receptors in the OPL were associated specifically with dendrites arising from bipolar cells. Double labeling studies showed that Mel1b receptors did not colocalize with labeling for Goα, a definitive marker for all ON bipolar cells (Dhingra et al., 2000; Zhang and Wu, 2003). Thus, Mel1b receptors are expressed specifically by OFF bipolar cells. Mel1a receptors were present in processes with the distinctive morphology of horizontal cell axons, and did not colocalize with either Mel1b receptors or Goα on the dendrites of OFF or ON bipolar cells, respectively. Thus, Mel1a receptors were expressed on the axons of horizontal cells, which show hyperpolarizing OFF responses to light (Witkovsky and Stone, 1983; Witkovsky et al., 1988). This arrangement places Mel1a and Mel1b receptors specifically on the processes of second-order neurons that have OFF-type light responses.
Double labeling studies combining labeling for Mel1a or Mel1b receptors with markers for cone terminals showed that Mel1a and Mel1b receptors were localized to processes that clustered selectively at cone terminals. The cone-selective pattern of contact by the Mel1a receptor-positive branches of horizontal cell axons and Mel1b receptor-positive bipolar cell dendrites was confirmed in flatmount preparations. These studies also demonstrated that the Mel1a- and Mel1b-positive processes both contacted the same cone terminals. The annular pattern of Mel1a- and Mel1b-positive contacts with cone terminals observed in flatmount preparations and the observation that some cone terminals received contacts from Goα-positive ON bipolar cell dendrites, but not Mel1a- or Mel1b-positive processes, suggests that the Mel1a and Mel1b receptors may localize selectively at contacts with specific subtypes of cones. However, the precise relationship between specific types of cones and the Mel1a- and Mel1b-positive processes of OFF bipolar cells and horizontal cells is not known.
OFF bipolar cell dendrites and horizontal cell axons are known to contact both rod and cone terminals in the Xenopus retina (Witkovsky and Stone, 1983; Witkovsky et al., 1988). Therefore, the selective localization of Mel1a and Mel1b receptors to contacts at cone terminals indicates that some mechanism must exist in OFF bipolar cell and horizontal cell processes for specifically trafficking or sequestering Mel1a and Mel1b receptors at contacts with cone terminals, but not rod terminals.
Organization of Mel1a and Mel1b receptors with respect to the functional organization of OFF bipolar, ON bipolar, and horizontal cell processes at cone terminals
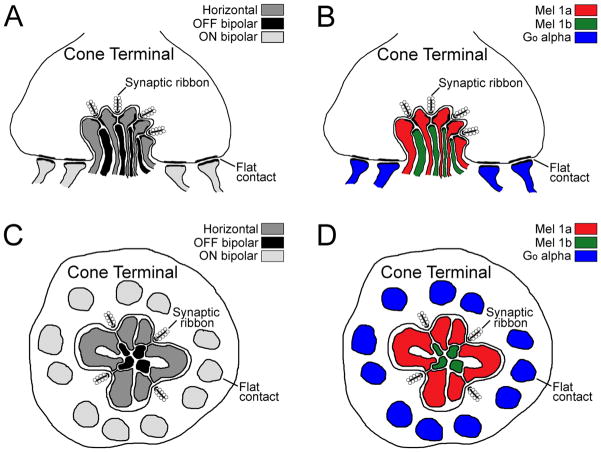
The relationship between the ultrastructural and physiological arrangement of contacts between photoreceptors and second-order neurons in Xenopus and other amphibian species has been investigated in some detail (Lasansky, 1973, 1978; Witkovsky and Powell, 1981; Witkovsky and Stone, 1983; Witkovsky et al., 1988; Wilhelm and Gábriel, 1999; Witkovsky, 2000; Wu et al., 2000; Zhang and Wu, 2009; Wu, 2010). The general pattern that emerges from these studies is that amphibian bipolar and horizontal cells receive mixed inputs from rods and cones, although specific bipolar and horizontal cell types can preferentially contact specific subsets of rods and/or cones. Careful ultrastructural reconstruction of contacts made by physiologically identified salamander horizontal and bipolar cells indicates that the physiology of the cell correlates closely with the type of contact made with photoreceptor terminals (Lasansky, 1978). The processes from horizontal cell dendrites and axons preferentially contact photoreceptor synaptic ribbons as lateral or central processes of the “triad” (typically comprised of a bipolar cell dendrite flanked by two horizontal cell processes). OFF bipolar cell dendrites preferentially contact synaptic ribbons as central or lateral processes of the triad (≈80% of contacts), but also make flat contacts onto the base of the photoreceptor terminal (about 20% of contacts). ON bipolar cells preferentially make flat contacts onto the base of the photoreceptor terminals (about 80% of contacts), but also contact synaptic ribbons as lateral processes in the triad (about 20% of contacts). Contacts made by Xenopus horizontal and bipolar cells have not been analyzed at this level of detail, but appear to follow a similar organization in the postmetamorphic frog (Witkovsky and Powell, 1981; Witkovsky and Stone, 1983; Witkovsky et al., 1988; Wilhelm and Gábriel, 1999; Witkovsky, 2000). Based on this preferred ultrastructural organization and our findings with respect to the localization of Mel1a receptors, Mel1b receptors, and Goα, we have constructed an idealized model of the relationship between melatonin receptors and functional organization of connections among Xenopus cones and bipolar and horizontal cell processes (Fig. 9).
Figure 9.
Schematic model of the relationship between melatonin receptors and functional organization of connections among Xenopus cones and bipolar and horizontal cell processes. A,C: Schematic summary of the predicted organization of horizontal, OFF bipolar, and ON bipolar cell processes at cone terminals in the Xenopus retina shown in the vertical (A) and horizontal (C) orientation. B,D: Schematic summary of the organization of Mel1a and Mel1b receptors at cone terminals in the Xenopus retina shown in the vertical (B) and horizontal (D) orientation.
Although melatonin is known to modulate a number of rhythmic processes in the retina including photoreceptor outer segment disk shedding (Besharse and Dunis, 1983; White and Fisher, 1989), photomechanical movements (Pierce et al., 1985), and dopamine release (Dubocovich, 1983), less is known about the specific functions of melatonin signaling in the circuits of the OPL. The selective localization of Mel1a and Mel1b receptors to contacts with cone terminals implies a functional role for melatonin in directly modulating signaling in cone circuits in the Xenopus OPL. Given that retinal melatonin levels are highest in darkness, when visual function is dominated by rods, Mel1a and Mel1b receptor activation would be expected to lead to enhanced signaling in rod pathways in some manner. Based on the selective localization of Mel1a and Mel1b receptors in the Xenopus OPL specifically to OFF bipolar and horizontal cell processes contacting cone terminals, these receptors may serve to reduce noise from signaling by cone terminals in darkness. This would be particularly important in a species such as Xenopus, in which bipolar and horizontal cells receive mixed rod and cone inputs. Consistent with this idea, a study performed in the carp retina showed that melatonin reduces signals to cone-driven horizontal cells, most likely through activation of MT1 (Mel1a) receptors (Huang et al., 2005). A second study performed in carp retina showed that melatonin enhances rod signals to rod-dominated ON bipolar cells, most likely through activation of MT2 (Mel1b) receptors (Ping et al., 2008). It is unlikely that this mechanism is active in the Xenopus OPL, as we found Mel1b receptors to be selectively expressed by OFF bipolar cell dendrites contacting cones. In this case, we predict that the Mel1b receptors on the Xenopus OFF bipolar cells may act similarly to the Mel1a receptors on carp horizontal cells (and presumably Xenopus horizontal cells) to reduce noise arising from cones during darkness.
Melatonin also appears to function as a key modulator of the balance of signaling through rod and cone pathways in the mammalian retina. In the mouse retina, the MT1 receptor (ortholog to the Xenopus Mel1a receptor) is required for the normal circadian increase in light sensitivity during the dark period (Baba et al., 2009). Melatonin increases the amplitude and sensitivity of the a- and b-waves of the dark-adapted electroretinogram (ERG) in wild-type mice, but not in MT1-deficient mice. The precise mechanism underlying this change in sensitivity is unclear. In situ hybridization shows high levels of MT1 mRNA in photoreceptors and many cells in the INL, but the expression and localization of the receptor itself has not been assessed. The changes in ERG a-wave suggest that at least some of the change in visual sensitivity arises from the photoreceptors themselves, but contributions from other locations in the retina including circuits in the OPL or more proximal sites in the retina where melatonin receptors also are abundant (Scher et al., 2003; Baba et al., 2009; Zhao et al., 2010), is a possibility that should be considered.
Acknowledgments
Grant sponsor: Oklahoma Center for the Advancement of Science and Technology (OCAST); Grant numbers: HR06-125 (to A.F.W.), HR08-149S (to D.M.S.); Grant sponsor: National Center for Research Resource (NRCC); Grant number: P20RR017703; Grant sponsor: National Eye Institute (NEI); Grant number: P30EY012190; Grant sponsor: University of Oklahoma Health Sciences Center.
The XAP-1 monoclonal antibody developed by Harris and Messersmith (1992) was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by the University of Iowa, Department of Biological Sciences, Iowa City, IA. The authors thank Jennifer Graef for technical assistance and Dr. Jody Summers for use of the confocal microscope. Some of the results have previously been published in abstract form: Wiechmann AF, Sherry DM. 2010. Invest Ophthalmol Vis Sci Abstr 52: ARVO E-Abstract 4132.
LITERATURE CITED
- Airaksinen MS, Eilers J, Garaschuk O, Thoenen H, Konnerth A, Meyer M. Ataxia and altered dendritic calcium signaling in mice carrying a targeted null mutation of the calbindin D28k gene. Proc Natl Acad Sci U S A. 1997;94:1488–1493. doi: 10.1073/pnas.94.4.1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba K, Pozdeyev N, Mazzoni F, Contrertas-Alcantara S, Liu C, Kasamatsu M, Martinez-Merlos T, Strettoi E, Iuvone PM, Tosini G. Melatonin modulates visual function and cell viability via the MT1 melatonin receptor. Proc Natl Acad Sci U S A. 2009;106:15043–15048. doi: 10.1073/pnas.0904400106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besharse JC, Dunis DA. Methoxyindoles and photoreceptor metabolism: activation of rod shedding. Science. 1983;219:1341–1342. doi: 10.1126/science.6828862. [DOI] [PubMed] [Google Scholar]
- Cahill GM, Grace MS, Besharse JC. Rhythmic regulation of retinal melatonin: Metabolic pathways, neurochemical mechanisms, and the ocular circadian clock. Cell Mol Neurobiol. 1991;11:529–560. doi: 10.1007/BF00734814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celio MR, Baier W, Schärer L, Gregersen HJ, de Viragh PA, Norman AW. Monoclonal antibodies directed against the calcium binding protein calbindin D-28k. Cell Calcium. 1990;11:599–602. doi: 10.1016/0143-4160(90)90014-l. [DOI] [PubMed] [Google Scholar]
- Dhingra A, Lyubarsky A, Jiang M, Pugh EN, Jr, Birnbaumer L, Sterling P, Vardi N. The light response of ON bipolar neurons requires Goα. J Neurosci. 2000;20:9053–9058. doi: 10.1523/JNEUROSCI.20-24-09053.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubocovich ML. Melatonin is a potent modulator of dopamine release in the retina. Nature. 1983;306:782–784. doi: 10.1038/306782a0. [DOI] [PubMed] [Google Scholar]
- Dufourny L, Levasseur A, Migaud M, Cellebaut I, Pontarotti P, Malpaux B, Monget P. GPR50 is the mammalian ortholog of Mel1c: Evidence of rapid evolution in mammals. BMC Evol Biol. 2008;8:105–118. doi: 10.1186/1471-2148-8-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebisawa T, Karne S, Lerner MR, Reppert SM. Expression cloning of a high-affinity melatonin receptor from Xenopus dermal melanophores. Proc Natl Acad Sci U S A. 1994;91:6133–6137. doi: 10.1073/pnas.91.13.6133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujieda H, Scher J, Hamadanizadeh SA, Wankiewicz E, Pang SF, Brown GM. Dopaminergic and GABAergic amacrine cells are direct targets of melatonin: immunocytochemical study of mt1 melatonin receptor in guinea pig retina. Visual Neurosci. 2000;17:63–70. doi: 10.1017/s0952523800171068. [DOI] [PubMed] [Google Scholar]
- Harris WA, Messersmith SL. Two cellular inductions involved in photoreceptor determination in the Xenopus retina. Neuron. 1992;9:357–372. doi: 10.1016/0896-6273(92)90174-c. [DOI] [PubMed] [Google Scholar]
- Huang H, Lee SC, Yang XL. Modulation by melatonin of glutamatergic synaptic transmission in the carp retina. J Physiol. 2005;569:857–871. doi: 10.1113/jphysiol.2005.098798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasansky A. Organization of the outer synaptic layer in the retina of the larval tiger salamander. Philos Trans R Soc Lond Ser B Biol Sci. 1973;265:471–489. doi: 10.1098/rstb.1973.0033. [DOI] [PubMed] [Google Scholar]
- Lasansky A. Contacts between receptors and electro-physiologically identified neurones in the retina of the larval tiger salamander retina. J Physiol. 1978;285:531–542. doi: 10.1113/jphysiol.1978.sp012587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Mumby S, Greenwood A, Jope R. Pertussis toxin-sensitive G-protein α-subunits: production of monoclonal antibodies and detection of differential increases upon differentiation of PC12 and LA-N-5 cells. J Neurochem. 1995;64:1107–1117. doi: 10.1046/j.1471-4159.1995.64031107.x. [DOI] [PubMed] [Google Scholar]
- Meyer P, Pache M, Loeffler KU, Brydon L, Jockers R, Flammer J, Wirz-Justice A, Savaskan E. Melatonin MT-1 receptor immunoreactivity in the human eye. Br J Ophthalmol. 2002;86:1053–1057. doi: 10.1136/bjo.86.9.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morona R, Moreno N, López JM, González A. Comparative analysis of calbindin D-28K and calretinin in the retina of anuran and urodele amphibians: colocalization with choline acetyltransferase and tyrosine hydroxylase. Brain Res. 2007;1182:34–49. doi: 10.1016/j.brainres.2007.07.102. [DOI] [PubMed] [Google Scholar]
- Nookala S, Gandrakota R, Wohabrebbi A, Wang XF, Howell D, Giorgianni F, Beranova-Giorgianni S, Desiderio DM, Jablonski MM. In search of the identity of the XAP-1 antigen: a protein localized to cone outer segments. Invest Ophthalmol Vis Sci. 2010;51:2736–2743. doi: 10.1167/iovs.09-4286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce ME, Besharse JC. Circadian regulation of retino-motor movements. I. Interaction of melatonin and dopamine in the control of cone length. J Gen Physiol. 1985;86:671–689. doi: 10.1085/jgp.86.5.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ping Y, Huang H, Zhang X-J, Yang X-L. Melatonin potentiates rod signals to ON type bipolar cells in fish retina. J Physiol. 2008;586:2683–2694. doi: 10.1113/jphysiol.2008.152959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prada C, Udin SB, Wiechmann AF, Zhdanova IV. Stimulation of melatonin receptors decreases calcium levels in Xenopus tectal cells by activating GABAc receptors. J Neurophysiol. 94:968–978. doi: 10.1152/jn.01286.2004. [DOI] [PubMed] [Google Scholar]
- Reppert SM, Godson C, Mahle CD, Weaver DR, Slaugenhaupt SA, Gusella JF. Molecular characterization of a second melatonin receptor expressed in human retina and brain: the Mel1b melatonin receptor. Proc Natl Acad Sci U S A. 1995a;92:8734–8738. doi: 10.1073/pnas.92.19.8734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reppert SM, Weaver DR, Cassone VM, Godson C, Kolakowski LF. Melatonin receptors are for the birds: molecular analysis of two receptor subtypes differentially expressed in chick brain. Neuron. 1995b;15:1003–1015. doi: 10.1016/0896-6273(95)90090-x. [DOI] [PubMed] [Google Scholar]
- Scher J, Wankiewicz E, Brown GM, Fujieda H. MT1 melatonin receptor in the human retina: expression and localization. Invest Ophthalmol Vis Sci. 2002;43:889–897. [PubMed] [Google Scholar]
- White MP, Fisher LJ. Effects of exogenous melatonin on circadian disc shedding in the albino rat retina. Vis Res. 1989;29:167–179. doi: 10.1016/0042-6989(89)90122-3. [DOI] [PubMed] [Google Scholar]
- Wiechmann AF. Differential distribution of Mel1a and Mel1c melatonin receptors in Xenopus laevis retina. Exp Eye Res. 2003;76:99–106. doi: 10.1016/s0014-4835(02)00230-0. [DOI] [PubMed] [Google Scholar]
- Wiechmann AF, Smith AR. Melatonin receptor RNA is expressed in photoreceptors and displays a diurnal rhythm in Xenopus retina. Mol Brain Res. 2001;91:104–111. doi: 10.1016/s0169-328x(01)00134-6. [DOI] [PubMed] [Google Scholar]
- Wiechmann AF, Summers JA. Circadian rhythms in the eye: The physiological significance of melatonin receptors in ocular tissues. Prog Ret Eye Res. 2008;27:137–160. doi: 10.1016/j.preteyeres.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Wiechmann AF, Campbell LD, Defoe DM. Melatonin receptor RNA expression in Xenopus retina. Mol Brain Res. 1999;63:297–303. doi: 10.1016/s0169-328x(98)00292-7. [DOI] [PubMed] [Google Scholar]
- Wiechmann AF, Vrieze MJ, Dighe RK, Hu Y. Direct modulation of rod photoreceptor responsiveness through a Mel1c melatonin receptor in transgenic Xenopus laevis retina. Invest Opthalmol Vis Sci. 2003;44:4522–4531. doi: 10.1167/iovs.03-0329. [DOI] [PubMed] [Google Scholar]
- Wiechmann AF, Udin SB, Summers Rada JA. Localization of Mel1b melatonin receptor-like immunoreactivity in ocular tissues of Xenopus laevis. Exp Eye Res. 2004;79:585–594. doi: 10.1016/j.exer.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Wilhelm M, Gábriel R. Functional anatomy of the photoreceptor and second-order cell mosaics in the retina of Xenopus laevis. Cell Tissue Res. 1999;297:35–46. doi: 10.1007/s004410051331. [DOI] [PubMed] [Google Scholar]
- Witkovsky P. Photoreceptor classes and transmission at the photoreceptor synapses in the retina of the clawed frog, Xenopus laevis. Microsc Res Tech. 2000;50:338–346. doi: 10.1002/1097-0029(20000901)50:5<338::AID-JEMT3>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Witkovsky P, Powell CC. Synapse formation and modification by neurons in larval and juvenile Xenopus. Proc R Soc Lond B. 1981;211:373–389. doi: 10.1098/rspb.1981.0012. [DOI] [PubMed] [Google Scholar]
- Witkovsky P, Stone S. Rod and cone inputs to bipolar and horizontal cells of the Xenopus retina. Vision Res. 1983;23:1251–1258. doi: 10.1016/0042-6989(83)90100-1. [DOI] [PubMed] [Google Scholar]
- Witkovsky P, Stone S, MacDonald ED. Morphology and synaptic connections of HRP-filled, axon-bearing horizontal cells in the Xenopus retina. J Comp Neurol. 1988;275:29–38. doi: 10.1002/cne.902750104. [DOI] [PubMed] [Google Scholar]
- Wu SM. Synaptic organization of the vertebrate retina: general principles and species-specific variations: The Friedenwald lecture. Invest Ophthalmol Vis Sci. 2010;51:1263–1274. doi: 10.1167/iovs.09-4396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu SM, Gao F, Maple BR. Functional architecture of synapses in the inner retina: segregation of visual signals by stratification of bipolar cell axon terminals. J Neurosci. 2000;20:4462–4470. doi: 10.1523/JNEUROSCI.20-12-04462.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Wu SM. Goα labels ON bipolar cells in the tiger salamander retina. J Comp Neurol. 2003;461:276–289. doi: 10.1002/cne.10704. [DOI] [PubMed] [Google Scholar]
- Zhang J, Wu SM. Immunocytochemical analysis of photoreceptors in the tiger salamander retina. Vision Res. 2009;49:64–73. doi: 10.1016/j.visres.2008.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W-J, Zhang M, Miao Y, Yang X-L, Wang Z. Melatonin potentiates glycine currents through a PLC/PKC signalling pathway in rat retinal ganglion cells. J Physiol. 2010;588:2605–2619. doi: 10.1113/jphysiol.2010.187641. [DOI] [PMC free article] [PubMed] [Google Scholar]