Abstract
Neurotrophin-3 (NT-3) regulates oligodendrocyte (OLG) differentiation by mechanisms that remain poorly understood. Exposure of OLGs to NT-3 induces a significant increase in the levels of myelin basic protein (MBP). However, we found that this stimulation occurs in the absence of measurable effects on MBP gene promoter activation or mRNA expression, suggesting that NT-3 up-regulates MBP protein expression by a posttranscriptional mechanism. Furthermore, NT-3 also causes an increase in the levels of myelin associated glycoprotein (MAG) and myelin oligodendrocyte glycoprotein (MOG), raising the possibility of a more general effect on myelin protein synthesis. Surprisingly, 35S-methionine incorporation into total OLG proteins demonstrated a 50% increase in labeling following only a brief, 15 minute treatment with NT-3. Such a remarkably fast response is unlikely due to transcriptional activation, reinforcing the possibility that NT-3 may play a crucial role in regulating protein expression by a posttranscriptional mechanism. In support of this idea, we found that NT-3 stimulates the phosphorylation of essential regulators of the initiation machinery, eukaryotic initiation factor 4E (eIF4E) and its inhibitory binding partner 4E binding protein 1 (4EBP1), two crucial players in controlling cap-dependent protein synthesis. This stimulation involves the activation of pathways mediated by ERK1/2 and PI3K/mTOR, implicating these two kinase systems as modulators of protein synthesis in developing OLGs. Altogether, these observations show for the first time that NT-3 has the capacity of targeting the translational machinery and suggest a potential stimulatory effect of this neurotrophin on myelination by direct action on protein translation in the OLGs.
Keywords: neurotrophin-3, oligodendrocytes, myelination, translational regulation
INTRODUCTION
Myelin, the insulating sheath that wraps around the axons facilitating the rapid saltatory conduction of impulses, is made in the central nervous system (CNS) by the oligodendrocytes (OLGs), cells which also play a crucial role in establishing axoglial interactions responsible for clustering of sodium channels at the nodes of Ranvier (Dupree et al. 1999; Rasband and Trimmer 2001). The importance of the OLGs and myelin is underscored by the clinical disability resulting from demyelinating diseases such as multiple sclerosis (MS). Because a single OLG forms multiple myelin internodes, loss of even few cells leads to demyelination of several axons. Thus, the ideal MS treatment should address the loss of both myelin and functional myelinating OLGs. During embryogenesis, OLGs arise from a pool of progenitors within the ventricular and subventricular zone (SVZ) (LeVine and Goldman 1988). These highly proliferative cells migrate throughout the CNS and undergo a series of well established maturational steps prior to becoming fully differentiated myelin-producing OLGs (Armstrong 1998). Importantly, subsets of undifferentiated cells persist within the mature CNS as adult OLG progenitors that can be recruited to demyelinated areas in experimental demyelination and in MS (Chang et al. 2000; Keirstead and Blakemore 1999). However, myelin regeneration is often incomplete, a problem aggravated by the fact that remyelination protects from axonal degeneration (Irvine and Blakemore 2008), a major cause of disability and a hallmark of progressive MS (Bjartmar et al. 2003). Failure to remyelinate has been attributed in part to aging (Irvine and Blakemore 2006) and decreased efficiency in recruitment of progenitors and their differentiation into OLGs (Sim et al. 2002). However, the mechanisms that control these processes remain poorly understood. OLG development is influenced by a variety of molecules, including platelet derived growth factor, basic fibroblast growth factor, insulin-like growth factor-1 and neurotrophins (Barres et al. 1993; Dubois-Dalcq and Murray 2000; McKinnon et al. 1990; McKinnon et al. 1993; Rogister et al. 1999).
Neurotrophin-3 (NT-3) in particular, has positive effects at multiple stages of OLG development. NT-3 induces survival and proliferation of OLG progenitors both in vitro and in vivo (Barres and Raff 1994; Barres et al. 1994). Developing OLGs express the NT-3 receptor TrkC and knockout mice lacking TrkC or NT-3 have fewer numbers of OLG progenitors as well as attenuated expression of OLG markers (Cohen et al. 1996; Kahn et al. 1999; Kumar et al. 1998). NT-3 has been shown to diminish the susceptibility of cultured OLGs to glutamate induced excitotoxicity (Kavanaugh et al. 2000), and studies with animal models indicate that NT-3 may play an important role regulating OLG number and remyelination following CNS injury and demyelination (Jean et al. 2003; McTigue et al. 1998). Importantly, NT-3 was also shown to influence OLG differentiation (Heinrich et al. 1999) and cultured OLG precursor cells transfected with the NT-3 gene showed a dramatic increase in myelin production (Rubio et al. 2004). Nevertheless, the molecular mechanisms responsible for NT-3 action on OLG maturation and myelin formation have yet to be elucidated.
We now found that NT-3 induces in OLGs a simultaneous stimulation in the expression of several myelin proteins, including the four major myelin basic protein (MBP) isoforms, myelin associated glycoprotein (MAG) and myelin OLG glycoprotein (MOG). Moreover, the present results suggest that NT-3 could play a major role in upregulating myelin protein synthesis by targeting the initiation factor 4E (eIF4E) and its inhibitory protein 4E binding protein 1(4EBP1), two key players of the translation initiation machinery. Phosphorylation of these factors in response to NT-3 involves the activation of phosphatidylinositol-3-kinase (PI3K)/mammalian target of rapamycin (mTOR) and extracellular signal-regulated kinase (ERK)-dependent signaling cascades. These results show for the first time that NT-3 has a direct effect on components of the initiation complex and uncover potential pathways that may be crucial in stimulating protein synthesis in OLGs both during normal development and remyelination.
MATERIALS AND METHODS
Materials
Percoll and culture medium components were from Sigma-Aldrich (St Louis, MO). Reduced-growth factor Matrigel was from Becton Dickinson (Franklin Lakes, NJ). NT-3 was from Peprotech (Rocky Hill, NJ). LY294002, PD98059 and rapamycin were from Calbiochem (San Diego, CA). Anti-protein kinase B (Akt), anti-phosphorylated Akt (P-Akt), anti-phosphorylated eukaryotic initiation factor 4E (P-eIF4E), anti-phosphorylated 4E binding protein1 (P-4EBP1), anti-eIF4E, and anti-4EBP1 antibodies were from Cell Signaling Technology (Danvers, MA). Anti-MBP and anti-MAG antibodies were from Chemicon (Temecula, CA). Anti-MOG antibody (8-18C5) was a kind gift from Dr. Jeff Dupree. Anti-β-actin was from Sigma-Aldrich. Anti-phosphorylated ERK, anti-ERK and all secondary antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Electrophoresis reagents were from Bio-Rad Laboratories (Hercules, CA).
Isolation and culture of OLGs
OLGs were isolated from 7-day-old Sprague–Dawley (Harlan Laboratories) rat brains using a Percoll gradient and differential adhesion (Sato-Bigbee et al. 1999). OLGs were plated in plates coated with Matrigel and maintained overnight in chemically defined medium (CDM) [Dulbecco’s modified Eagle’s medium (DMEM)/F-12 medium (1:1; Invitrogen, Grand Island, NY) with 1 mg/ml fatty acid-free bovine serum albumin, 50 μg/ml transferrin, 5 μg/ml insulin, 30 nM sodium selenite, 0.11 mg/ml sodium pyruvate, 10 nM biotin, 20 nM progesterone, 15 nM triiodothyronine, and 100 μM putrescine]. These cultures are comprised of immature OLGs many of which can be already labeled with the O4 antibody (Sato-Bigbee et al. 1999). Astroglial contamination, assessed by glial fibrillary acid protein staining, was less than 5%. Animal use was conducted in accordance with guidelines from the National Institutes of Health and Virginia Commonwealth University Animal Care and Use Committee.
Treatment with NT-3
One day after isolation, cells were incubated for various times in CDM with or without different concentrations of NT-3. Clultures were then processed for western blotting, promoter reporter assays or real-time RT-PCR as described below. In experiments evaluating the role of different kinases, cells were pre-incubated for 10 min with the following specific kinase inhibitors: PD98059 (mitogen extracellular signal regulated kinase kinase (MEK) inhibitor, 10 μM), LY294002 (PI3K inhibitor, 30 μM) or rapamycin (mTOR inhibitor, 25 nM). Cultures were then incubated for 15 min in the presence of either 50 ng/ml NT-3 or the kinase inhibitor or a combination of both. Control media contained the same volume of vehicle in which inhibitors were dissolved (DMSO). Inhibitor concentrations were in agreement with those previously used to inhibit these kinases in OLGs (Baron et al. 2000; Coelho et al. 2007; Cui et al. 2006; Flores et al. 2000; Sato-Bigbee et al. 1999).
MBP promoter reporter assay
The -1323-luc construct containing MBP gene sequences from −1,323 to +30 upstream of the luciferase coding region in pGL3Basic was a generous gift of Dr. Robin Miskimins (Miskimins et al. 2002). For transfection, 60% confluent cultures in 24 well plates were incubated for 3 hrs. with 1 μg/well luciferase-MBP construct and 1.5 μl GeneJammer reagent (Stratagene, La Jolla, CA), following the manufacturer’s recommendations. After overnight incubation in fresh CDM, the cells were incubated for various times in CDM with or without 50 ng/ml NT-3. Cell extracts were then prepared and assayed for luciferase activity (Luciferase Assay System, Promega, Madison, WI). β-Actin levels were determined by western blotting to ensure equal cell numbers for each condition.
Real-time reverse transcriptase polymerase chain reaction (RT-PCR)
Total RNA was isolated from OLG cultures using the RNeasy Micro Kit (Qiagen, Valencia, CA). For real-time qRT-PCR, oligo(dT)-primed cDNAs were synthesized using the Sensiscript RT kit (Qiagen). PCR was performed on a Chromo 4 Four-Color Real-Time System (BioRad) using the iQ SYBR Green Supermix. The following primer pairs were used at 60°C annealing temperatures: MBP (exon 2 containing isoforms) forward (5′-ACTTGGCCACAGCAAGTACCATGGACC-3′), reverse (5′-TTG TAC ATG TGG CAC AGC CCG GAC-3′) and MBP (all isoforms) forward (5′-GTG ACA CCT CGT ACA CCC CCT CCA T-3′), reverse (5′-GCT AAA TCT GCT GAG GGA CAG GCC T-3′). For normalization, amplification of cyclophilin was performed: forward (5′-GGA GAC GA ACCT GTA GGA CG -3′), reverse (5′-GAT GCT CTT TCC TCC TGT GC-3′), 60°C. PCR conditions were as follows: 95°C for 15 min followed by 34 cycles at 94°C for 15 sec, annealing temperature for 20 s, and 72°C for 20 s. The ΔΔCT method was used (Livak and Schmittgen 2001) for relative comparison of MBP mRNA levels in the presence or absence of NT-3.
Western blotting
Cultures containing equivalent numbers of cells per well were lysed in 60 mM Tris-HCl buffer (pH 6.8) with 10% glycerol, 2% sodium dodecyl sulfate (SDS), and 5% 2-mercaptoethanol. Samples for analysis of MAG were prepared under non-reducing conditions in absence of 2-mercaptoethanol. Fifteen μl samples were subjected to SDS-polyacrylamide gel electrophoresis in 12% acrylamide, proteins were electrotransferred to nitrocellulose, and the membranes were then subjected to immunoblot analysis (Saini et al. 2005). Non-specific antibody binding was blocked by incubation for 1 hr. in 10 mM Na2HPO4, 2.7 mM KCl and 137 mM NaCl, pH 7.4, phosphate buffered saline (PBS) containing 3% nonfat dry milk and 0.05% Tween-20 (blocking solution). Blots were then incubated overnight with one of the following primary antibodies: anti-MBP (dil. 1:100), anti-MAG (dil. 1:1,000), anti-MOG (dil. 1:25), anti-phospho-Akt (dil. 1:1,000), anti-Akt (dil. 1:1,000), anti-phospho-ERK (dil. 1:1,000), anti-ERK (dil. 1:2,000), anti-phospho-eIF4E (dil. 1:1,000), anti-phospho-4EBP1 (dil. 1:1,000), anti-eIF3E (dil:1,000), or anti-4EBP1 (1:1000). After rinsing with PBS, blots were incubated for 30 min in blocking solution, and for 3 hrs with the appropriate horseradish peroxidase (HRP)-conjugated secondary antibody (dil.1:1,000) in blocking buffer. After extensive washing, the immunoreactive bands were detected by chemiluminescence with Super Signal West Dura reagent (Pierce, Rockford, IL). β-Actin levels detected with anti-β-actin antibody (dil. 1:2,000) were used as controls to normalize results for protein loading. The relative amount of immunoreactive protein in each band was determined by scanning densitometric analysis of the X-ray films using the NIH Image J program.
In vitro measurement of protein synthesis using [35S]Methionine
OLGs were plated in 24-well plates coated with 25 μl/well Matrigel and after overnight incubation, the CDM was replaced by F12 medium. After 3 hrs, the cells were incubated for 15 min in medium containing 10μCi 35S- Methionine (1,000 Ci/mmol sp. activity) with or with 50 ng/ml NT-3. Cultures were then rinsed three times with ice cold PBS and lysed by incubation for 30 min in 250 μl 0.5M NaOH at 37° C. Lysates were then collected in microcentrifuge tubes and proteins were precipitated by addition of 250 μl 20% trichloroacetic acid (TCA) followed by incubation on ice for 1–2 hrs. After centrifugation, the pellet was washed twice with 5% TCA and solubilized by addition of 100 μl 70% formic acid and incubation for 1 hr at 37° C. Aliquots were used to determine the radioactivity by liquid scintillation counting.
Statistical Analysis
Statistical analysis was performed by one-way analysis of variance (ANOVA), ad hoc Tukey–Kramer test and Student’s t-test (GraphPad Prism). Unless otherwise indicated, results are the mean from 3 independent experiments carried out in triplicate and standard errors were calculated considering n:3. Differences were considered statistically significant when p-values were < 0.05.
RESULTS
Treatment of OLGs with NT-3 upregulates MBP expression without altering MBP gene promoter activation or MBP mRNA levels
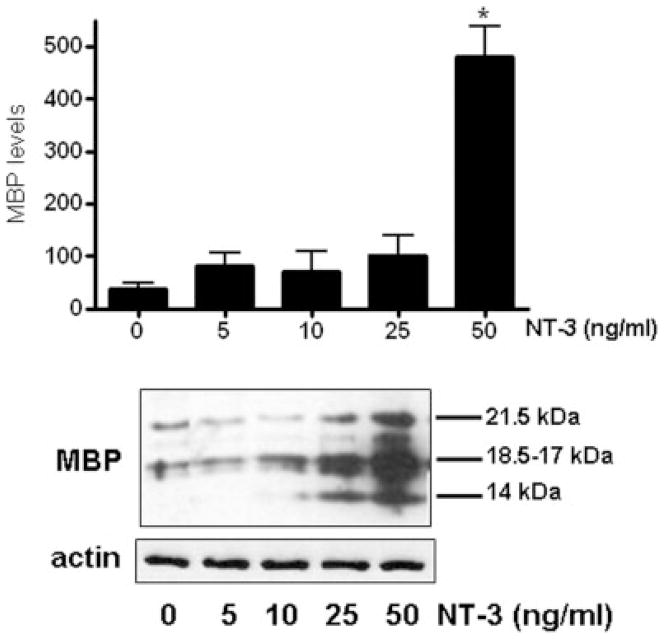
In agreement with previous reports (Du et al. 2003; Rubio et al. 2004), our results showed that NT-3 is a potent inducer of MBP expression. In these experiments, developing OLGs were cultured for 3 days in the presence or absence of different concentrations of NT-3. As shown in Figure 1, treatment of the cells with this neurotrophin causes a dose-dependent increase in MBP expression, attaining levels that were about 10-fold higher than those observed in controls.
Figure 1. Treatment of OLGs with NT-3 results in increased MBP expression.
OLGs were incubated for 3 days in CDM with or without 5, 10, 25, 50 ng/ml NT-3. MBP levels were determined by western blotting using β-actin levels as loading controls. Figures correspond to representative experiments. Results in the bar graph are expressed as percentage of controls (0 ng/ml NT-3) and represent the mean ± SEM from 3 independent experiments performed in triplicate. * p<0.005
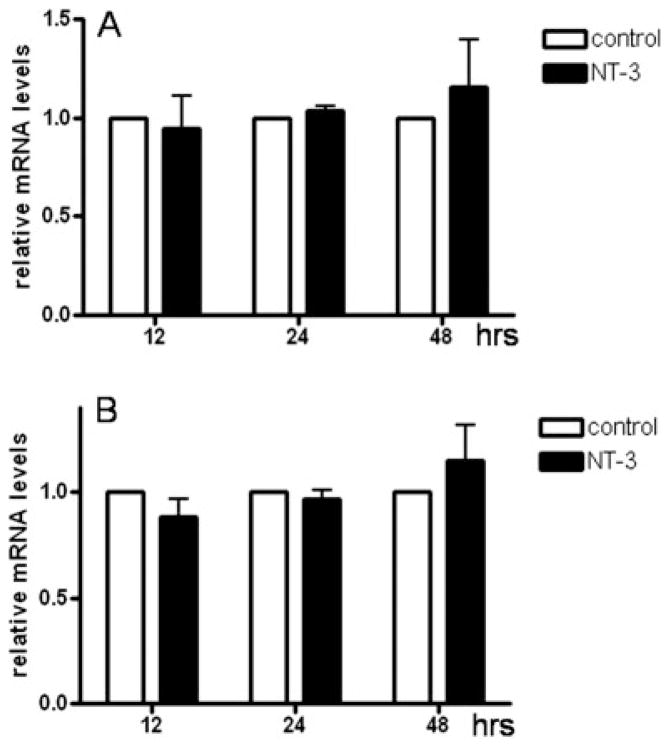
To understand the mechanisms underlying this stimulatory action of NT-3, we next investigated whether this neurotrophin could have an effect on MBP gene activity. For this, cells were transfected with a reporter construct containing the luciferase gene under the control of the MBP gene promoter, and were then incubated for different times in media with or without NT-3. Since the cells were exposed to a medium that induces OLG differentiation (Sato-Bigbee et al. 1999), an expected time-dependent increase in MBP gene promoter activity was observed, even in controls cultured in absence of NT-3 (Figure 2). Surprisingly, the results indicated that in spite of the dramatic elevation in MBP protein levels observed in cultures treated with NT-3, this neurotrophin did not cause any significant increase in MBP gene promoter activity at any time point studied.
Figure 2. NT-3 does not induce significant changes in MBP gene promoter activity.
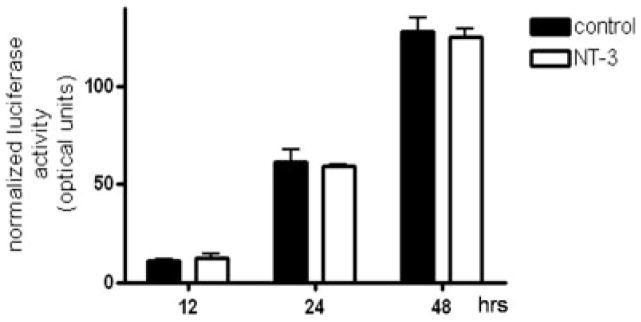
OLGs were transfected with a reporter construct containing the luciferase gene under control of the MBP gene promoter, and incubated for 12, 24 and 48 hrs in CDM in the absence (control) or presence of NT-3 (50 ng/ml). The luciferase activity in the samples is expressed as optical units. The results are the mean ± SEM from 3 independent experiments performed in triplicate.
Furthermore, comparison with controls demonstrated that OLGs treated with NT-3 did not exhibit any major differences in MBP mRNA levels (Figure 3). The different MBP isoforms are generated by alternative splicing of a single gene (de Ferra et al. 1985) and both MBP gene activity and splicing are developmentally regulated (Campagnoni 1988). Because MBP species expressed during early OLG maturation are predominantly represented by the exon-2 containing isoforms, real-time RT-PCR analysis in these studies used two different sets of primers to distinguish between all MBPs and exon-2 containing MBP mRNA transcripts. As shown in Figure 3, no major differences in MBP mRNA levels between controls and treated cells could be detected at any time of NT-3 exposure and regardless of isoform-specific exon expression. As with the above luciferase reporter experiments, additional treatment times with NT-3 failed to reveal any significant effects (data not shown).
Figure 3. Treatment of OLG cultures with NT-3 does not have a significant effect on MBP mRNA levels.
OLGs cultures were incubated for 12, 24 and 48 hrs in CDM with or without NT-3 (50 ng/ml). MBP mRNA levels were determined by real-time RT-PCR using appropriate primers. Cyclophilin mRNA levels were used for normalization. Bar graphs represent steady-state levels of MBP mRNA relative to controls (not treated with NT-3) which have been set to 1. (A) Total MBP mRNA levels, (B) Exon-2 containing MBP mRNA levels. Results are the mean ± SD from two independent experiments done in duplicates.
The stimulatory effect of NT-3 is not restricted to MBP expression
The lack of promoter activation or significant changes in mRNA levels, suggested that NT-3 up-regulates MBP expression by a posttranscriptional mechanism. Such possibility also raised the question of whether NT-3 could have a more general effect, also stimulating the expression of other oligodendroglial proteins. To test this possibility, we next investigated the action of NT-3 on the expression of two additional myelin proteins, MAG and MOG.
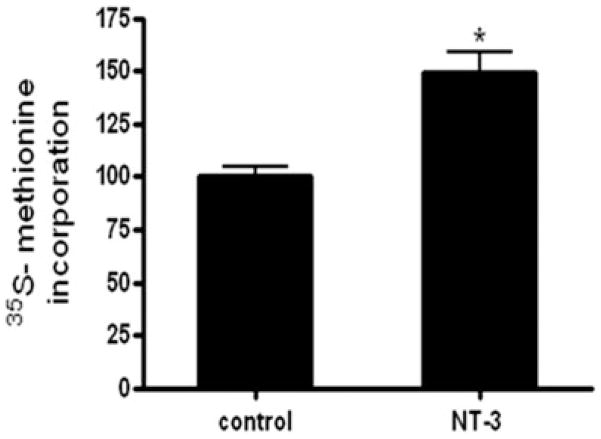
Similar to the results observed for MBP, exposure to NT-3 resulted in a significant increase in the levels of both MAG and MOG (Figure 4). Furthermore, a 35S-methionine incorporation assay demonstrated that, even within only 15 min of NT-3 treatment, there is already a 50% increase in 35S labeling of the total OLG protein (Figure 5). Such a remarkably fast response and measurable increase in protein synthesis is unlikely due to transcriptional activation or reduced turnover. Although this necessarily short-term protocol is not sensitive enough to label individual OLG proteins, these results and our previous observations reinforced the possibility that NT-3 may play a crucial role in regulating protein expression by a posttranscriptional mechanism.
Figure 4. Treatment of OLGs with NT-3 results in increased expression of MAG and MOG.
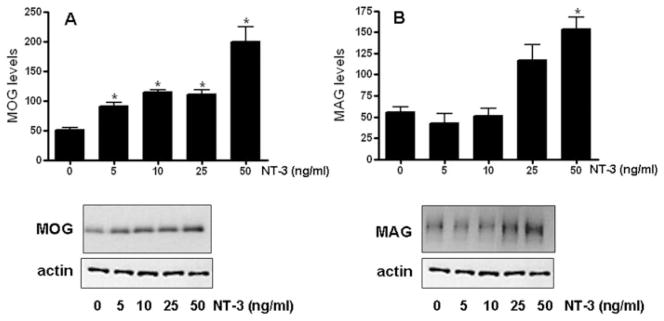
OLGs were incubated for 3 days in CDM with or without 5, 10, 25, 50 ng/ml NT-3. MAG and MOG levels were determined by western blotting. Figures correspond to representative experiments. Results are expressed as percentage of controls (0 ng/ml NT-3) and represent the mean ± SEM from two independent experiments performed in triplicate. * p< 0.05, **p<0.005
Figure 5. NT-3 increases 35S- methionine incorporation in OLG proteins.
OLGs were incubated for 15 min in F12 medium containing 10 μCi/well 35S- methionine, in the presence or absence of 50 ng/ml NT-3. 35S methionine incorporation into total protein was estimated by scintillation counting as described under “Materials and Methods”. Results are the mean ± SEM from 3 independent experiments done in triplicate. *p<0.005
NT-3 induces the phosphorylation of factors that control the initiation of mRNA translation
The results described above lead us to investigate whether NT-3 could stimulate some of the steps involved in mRNA translation, in particular the initiation phase, a key regulatory step in eukaryotic protein synthesis (Sonenberg and Gingras 1998). Among the factors that regulate initiation, eukaryotic initiation factor 4E (eIF4E) and its inhibitory binding partner 4E binding protein 1 (4EBP1) are known to be essential players in mediating cap-dependent protein synthesis (Svitkin et al. 2005). Phosphorylation of 4EBP1 releases eIF4E into the cytoplasm, step which is followed by eIF4E phosphorylation and binding to the mRNA cap. This cascade of events triggers the initiation machinery, allowing the beginning of translation (Raught and Gingras 1999).
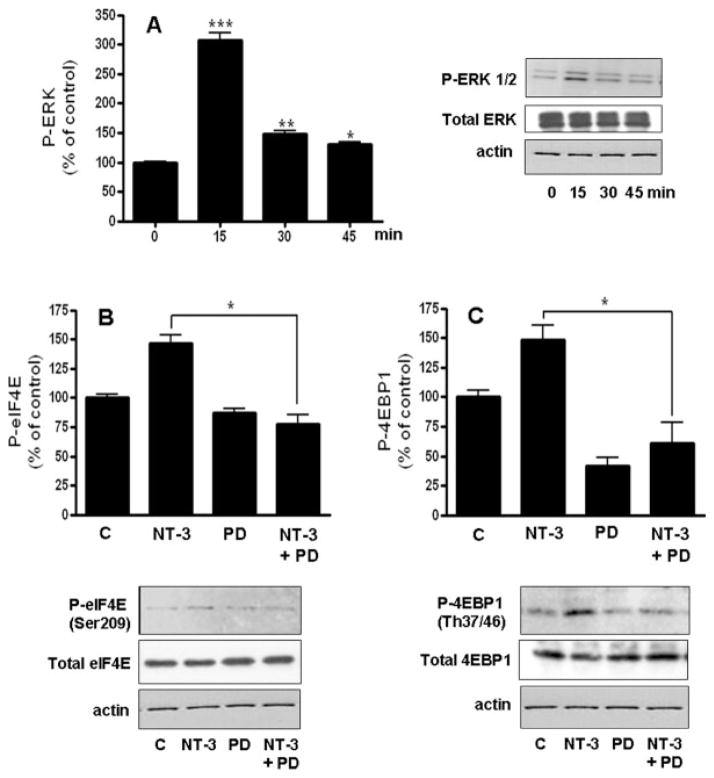
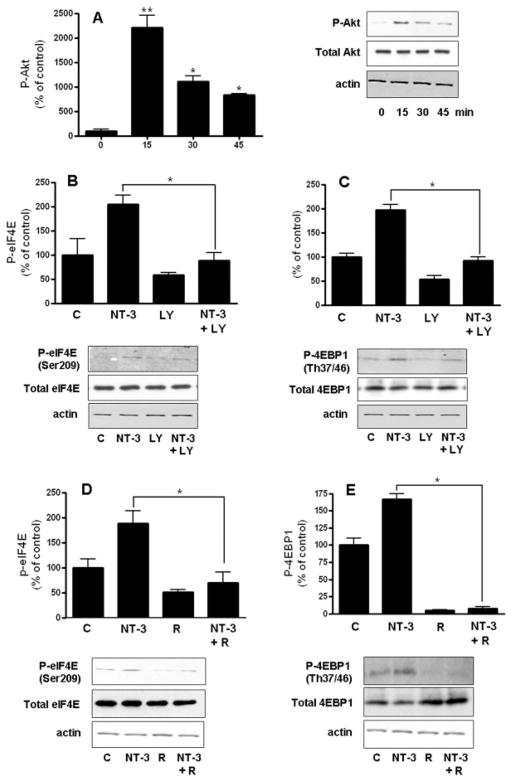
In support of a role for NT-3 in stimulating protein synthesis initiation, we found that OLGs incubated with this neurotrophin exhibited a sustained increased in eIF4E phosphorylation (Figure 6A). Furthermore, treatment with NT-3 was also accompanied by a robust increase in phosphorylation of 4EBP1 (Figure 6B). On the other hand, exposure to NT-3 did not cause any significant changes in the total levels of these two regulatory factors.
Figure 6. Treatment of OLGs with NT-3 induces phosphorylation of eIF4E and 4EBP1.
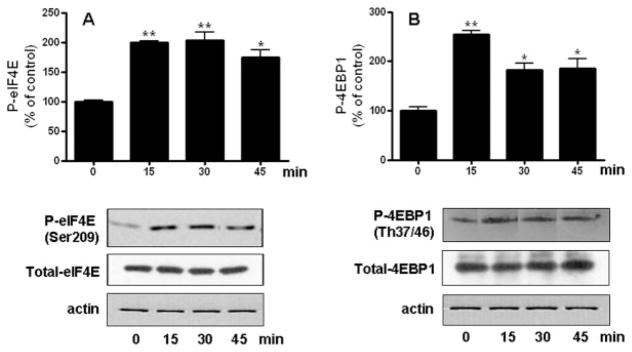
Cells were incubated in DMEM/F12 with or without 50 ng/ml NT-3. P-eIF4E and eIF4E (A), or P-4EBP1 and 4EBP1 (B) levels were determined by western blotting. Figures correspond to representative experiments. Results are expressed as percentage of controls (0 time) and represent the mean ± SEM from 3 independent experiments performed in triplicate. *p<0.05, **p<0.005
NT-3 stimulates eIF4E and 4EBP1 phosphorylation by ERK and PI3K/mTOR mediated pathways
In agreement with previous results from this and other laboratories (Cohen et al. 1996; Johnson et al. 2000; Kumar et al. 1998; Ness et al. 2002), treatment of OLGs with NT-3 induces rapid activation of ERK1/2 and Akt (Figures 7A and 8A). However, the role of these two kinases as regulators of protein synthesis in OLGs has never been investigated before. Because both ERK1/2 and Akt were implicated in the control of the initiation phase of protein synthesis in a variety of cells, we next examined their involvement as mediators in the induction of eIF4E and 4EBP1 phosphorylation by NT-3. Studies in cells transformed by ras- or src- oncogenes demonstrated a role of the MAPK/ERK pathway in eIF4E phosphorylation (Frederickson et al. 1991; Rinker-Schaeffer et al. 1992). In agreement with this function, the capacity of NT-3 to induce eIF4E phosphorylation in the OLGs was significantly reduced by PD98059, an inhibitor that blocks MEK, the kinase that activates ERK1/2 (Fig 7B). Unexpectedly, inhibition of MEK also blocked NT-3 ability to stimulate 4EBP1 phosphorylation (Fig 7C), suggesting that in OLGs, signaling through an ERK mediated pathway also plays a key role in regulating 4EBP1.
Figure 7. The stimulation of eIF4E and 4EBP1 phosphorylation by NT-3 involves an ERK-dependent pathway.
Cells were incubated for 15 min with or without 50 ng/ml NT-3 in the presence or absence of 10 μM PD98059 (MEKi). P-ERK and ERK (A), P-eIF4E and eIF4E (B), and P-4EBP1and 4EBP1 (C) levels were determined by western blotting. Results are expressed as percentage of controls (0 time for A, DMEM/F12 alone for B and C) and represent the mean ± SEM from 3 independent experiments performed in triplicate. *p<0.05, **p<0.005, ***p<0.0001
Figure 8. The NT-3-dependent induction of eIF4E and 4EBP1 phosphorylation is inhibited by LY294002 and rapamycin.
Cells were incubated for 15 min with or without 50 ng/ml NT-3 in the presence or absence of 30 μM LY294002 (PI3K inhibitor) (B and C) or 25 nM rapamycin (mTOR inhibitor) (D and E). P-Akt and Akt (A), P-eIF4E and eIF4E (B and D), and P-4EBP1 and 4EBP1 (C and D) levels were determined by western blotting. Results are expressed as percentage of controls (0 time for A, DMEM/F12 alone for B, C, D and E) and represent the mean ± SEM from 3 independent experiments performed in triplicate. *p<0.05, **p<0.005
Several lines of evidence also showed that the intracellular signaling cascade leading to 4EBP1 phosphorylation involves components of the PI3K pathway and its downstream effector Akt (Gingras et al. 1998). In addition, 4EBP1 phosphorylation was also shown to be dependent upon the FKBP12-rapamycin associated protein/mammalian target of rapamycin (FRAP/mTOR) kinase (Brunn et al. 1997a; Brunn et al. 1997b; Burnett et al. 1998; Hara et al. 1997). Therefore, we used LY294002, a specific inhibitor of the PI3K/Akt pathway as well as the mTOR inhibitor rapamycin to investigate the role of these two kinase systems in the induction of phosphorylation of 4EBP1 by NT-3. The results indicated that both LY294002 (Figure 8C) and rapamycin (Figure 8E) prevented the stimulation of 4EBP1 phosphorylation by NT-3. Interestingly, rapamycin also dramatically decreased the control values to negligible levels of detection, suggesting that an mTOR mediated pathway also plays a crucial role in maintaining basal levels of protein synthesis in the OLGs.
To our knowledge, the involvement of the Akt and mTOR pathways in controlling eIF4E activation remains unknown. However, our results demonstrated a significant reduction in the NT-3-mediated activation of eIF4E in the presence of LY294002 (Fig 8B) and rapamycin (Fig 8D), indicating that Akt and mTOR pathways do play a role in regulating eIF4E activation in response to NT-3 stimulation.
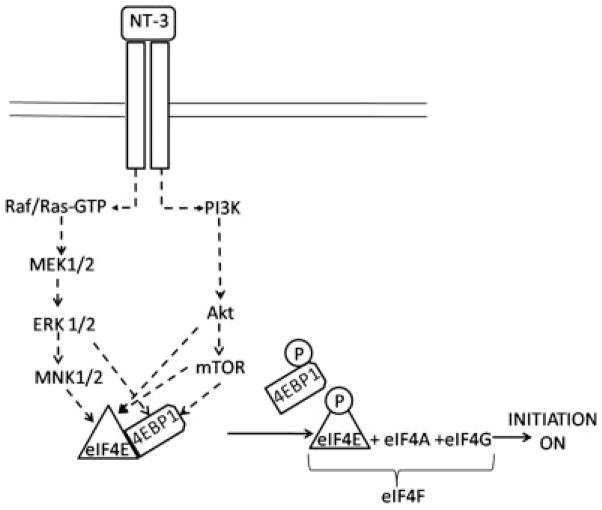
Altogether, these results indicate the involvement of both ERK and PI3K/mTOR mediated cascades in regulating the translation initiation machinery in OLGs, with both of these pathways participating in the mechanisms leading to phosphorylation of eIF4E and 4EBP1 in response to NT-3 stimulation (Figure 9). Furthermore, these observations suggest that NT-3 could play a crucial role in upregulating protein expression in myelinating OLGs by modulating the activity of factors critical to the control of protein synthesis initiation.
Figure 9. Proposed mechanism of NT-3 action as a stimulator of translation initiation in OLGs.
Binding of NT-3 to the TrkC receptor activates a classical ERK pathway involving Ras-GTP/Raf, MEK1/2, ERK 1/2 and MNK 1/2 as well as the PI3K/Akt/mTOR cascade leading to the phosphorylation of eIF4E and 4EBP1. Phosphorylation of 4EBP1 releases eIF4E allowing the binding of this factor to eIF4A and eIF4G to form the eIF4F initiation complex and resulting in the initiation of protein translation.
DISCUSSION
NT-3 plays multiple roles in OLG development, ranging from stimulation of survival and proliferation (Barres et al. 1994; Barres et al. 1993; Bertollini et al. 1997; Johnson et al. 2000; Kumar et al. 1998; Robinson and Miller 1996; Wilson et al. 2003) to the enhancement of cell differentiation (Coelho et al. 2007; Du et al. 2003; Heinrich et al. 1999; Yan and Wood 2000). However, while several studies investigated the signaling pathways mediating effects of NT-3 on survival and proliferation (Cohen et al. 1996; Heinrich et al. 1999; Johnson et al. 2000; Ness et al. 2002; Saini et al. 2005; Saini et al. 2004), little is known about the mechanisms underlying the actions of this neurotrophin on OLG maturation and myelination.
In our attempt to investigate the signals involved in increasing protein expression during OLG differentiation in response to NT-3, we have now uncovered a novel effect of this neurotrophin as a regulator of the eukaryotic initiation factor eIF4E and its inhibitory partner 4EBP1. These two proteins are key players in the initiation phase, the first and rate controlling step of protein translation (Hershey 1991). The initiation phase is a multi-step process that begins with recruitment of the heterotrimeric complex eIF4F to the mRNA 5′-end cap, finally causing unwinding of the mRNA and recruitment of the 40S ribosomal subunit. Binding of the eIF4F complex to the mRNA is mediated by its cap-binding subunit eIF4E (Gingras et al. 1999), a factor that is normally maintained at limiting levels by controlled gene transcription (Lynch et al. 2005; Schmidt 2004) and proteosome-dependent degradation (Murata and Shimotohno 2006).
The present observation of a stimulatory effect of NT-3 on eIF4E phosphorylation at serine 209 is particularly important because phosphorylation at this site was shown to increase both eIF4E affinity for capped mRNA and its binding to eIF4G, an associated scaffolding protein required in the assembly of the initiation machinery (Bu et al. 1993; Minich et al. 1994). Furthermore, we showed that NT-3 could also stimulate eIF4E activity and concomitant translational initiation through the observed increase in 4EBP1 phosphorylation. 4EBP1 plays a crucial role as a negative regulator of translation because it dimerizes with eIF4E, competitively blocking its binding to eIF4G (reviewed by Gingras et al. 1999). However, this inhibitory effect on the assembly of the translational machinery is known to be reversed by 4EBP1 phosphorylation, a process that results in disruption of eIF4E-4EBP1 complex formation.
In agreement with different studies investigating other cell systems and stimuli (Brunn et al. 1997a; Brunn et al. 1997b; Burnett et al. 1998; Hara et al. 1997), we found that NT-3 upregulates 4EBP1 phosphorylation in the OLGs by a PI3K/mTOR dependent pathway. Interestingly, our results showed that the NT-3 dependent phosphorylation of 4EBP1 is also dependent on MEK, suggesting that at least in OLGs, an ERK mediated pathway also controls 4EBP1 activity. Likewise, we found that both ERK and PI3K/mTOR dependent pathways are also involved in the NT-3 dependent phosphorylation of eIF4E. Studies have shown that an ERK-mediated pathway can induce eIF4E phosphorylation by activation of MAP kinase interacting kinases 1 and 2 (reviewed by Gingras et al. 1999). It is possible to hypothesize that the observed participation of a PI3K/mTOR pathway on eIF4E phosphorylation reflects the sequestration of this factor by 4EBP1. This is because a decrease in 4EBP1 phosphorylation due to inhibition of the PI3K/mTOR pathway would result in increased 4EBP1-eIF4E complex formation.
The present results in OLGs add to a growing list of studies supporting the role of translation initiation as a crucial regulatory mechanism in CNS development and function. Another neurotrophin, BDNF, was shown to upregulate local protein synthesis in primary cultured neurons by inducing phosphorylation of eIF4E and 4EBP1 (Takei et al. 2001). Moreover, translational control by both ERK and mTOR signaling has been implicated in the induction of long-lasting synaptic plasticity and memory (Banko et al. 2006; Banko and Klann 2008; Gelinas et al. 2007; Kelleher et al. 2004; Tang et al. 2002). It is important to point out that although several lines of evidence indicate that translational control mechanisms play a crucial role in cell development (de Moor and Richter 2001), little is known about the importance of these regulatory processes in OLG biology and myelination. Interestingly, recent studies from Lin et al. (Lin et al. 2008) showed that another component of the initiation machinery, eIF2α, a complex that interacts with the methionyl initiator tRNA, was involved in the regulation of the integrated stress response in OLGs, a process that is responsible for the protective effects of interferon-γ (IFN-γ) in experimental autoimmune encephalomyelitis, an animal model of MS (Lin et al. 2007). These authors demonstrated that IFN-γ could promote OLG survival in immune-mediated demyelination by activation of the pancreatic endoplasmic reticulum kinase (PERK) and subsequent phosphorylation of the α subunit of the eIF2 complex. Moreover, the importance of translational initiation in OLGs is underscored by the observation that alterations in eIF2α kinases in these cells have been linked to schizophrenia (Carter 2007), and defects of initiation eIF2B complex in OLGs may be responsible for childhood ataxia with CNS hypomyelination or vanishing white matter leukoencephalopathy, a fatal brain disorder (Richardson et al. 2004).
The present results show for the first time that ERK and PI3K/mTOR mediated pathways target the translation initiation machinery in developing OLGs. The PI3K/Akt pathway has been previously implicated as a mediator of survival signals in OLGs (Cui and Almazan 2007; Ebner et al. 2000; Flores et al. 2000; Ness et al. 2002). Interestingly, a recent report showed that transgenic mice overexpressing constitutively active Akt in OLGs exhibit enhanced myelination in the absence of changes in proliferation or survival of the cells (Flores et al. 2008). Based on our results, it may be possible to speculate that this enhancement of myelination may in part reflect upregulation of protein translation downstream of Akt.
Myelination by OLGs involves a dramatic upregulation of protein synthesis and while several studies have focused on understanding the transcriptional control of this process, there is a lack of information regarding the role of translational mechanisms. The present findings support the idea that activation of the translational machinery by NT-3 could play a crucial role stimulating protein synthesis during OLG maturation and myelination. These observations offer the possibility of new targets to stimulate efficient myelin protein synthesis, and therefore remyelination, in demyelinating diseases such as MS.
Acknowledgments
This work was supported by grants RG3432A2 from the National Multiple Sclerosis Society (C. S-B) and NIH 5RO1NS045883 (B.F.). The authors would like to thank Karen Gorse for technical assistance with the analysis of MBP mRNA levels and Fatemah S. Afshari for helpful discussions.
References
- Armstrong RC. Isolation and characterization of immature oligodendrocyte lineage cells. Methods. 1998;16(3):282–92. doi: 10.1006/meth.1998.0685. [DOI] [PubMed] [Google Scholar]
- Banko JL, Hou L, Poulin F, Sonenberg N, Klann E. Regulation of eukaryotic initiation factor 4E by converging signaling pathways during metabotropic glutamate receptor-dependent long-term depression. J Neurosci. 2006;26(8):2167–73. doi: 10.1523/JNEUROSCI.5196-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banko JL, Klann E. Cap-dependent translation initiation and memory. Prog Brain Res. 2008;169:59–80. doi: 10.1016/S0079-6123(07)00004-0. [DOI] [PubMed] [Google Scholar]
- Baron W, Metz B, Bansal R, Hoekstra D, de Vries H. PDGF and FGF-2 signaling in oligodendrocyte progenitor cells: regulation of proliferation and differentiation by multiple intracellular signaling pathways. Mol Cell Neurosci. 2000;15(3):314–29. doi: 10.1006/mcne.1999.0827. [DOI] [PubMed] [Google Scholar]
- Barres BA, Raff MC. Control of oligodendrocyte number in the developing rat optic nerve. Neuron. 1994;12(5):935–42. doi: 10.1016/0896-6273(94)90305-0. [DOI] [PubMed] [Google Scholar]
- Barres BA, Raff MC, Gaese F, Bartke I, Dechant G, Barde YA. A crucial role for neurotrophin-3 in oligodendrocyte development. Nature. 1994;367(6461):371–5. doi: 10.1038/367371a0. [DOI] [PubMed] [Google Scholar]
- Barres BA, Schmid R, Sendnter M, Raff MC. Multiple extracellular signals are required for long-term oligodendrocyte survival. Development. 1993;118(1):283–95. doi: 10.1242/dev.118.1.283. [DOI] [PubMed] [Google Scholar]
- Bertollini L, Ciotti MT, Cherubini E, Cattaneo A. Neurotrophin-3 promotes the survival of oligodendrocyte precursors in embryonic hippocampal cultures under chemically defined conditions. Brain Res. 1997;746(1–2):19–24. doi: 10.1016/s0006-8993(96)01199-7. [DOI] [PubMed] [Google Scholar]
- Bjartmar C, Wujek JR, Trapp BD. Axonal loss in the pathology of MS: consequences for understanding the progressive phase of the disease. J Neurol Sci. 2003;206(2):165–71. doi: 10.1016/s0022-510x(02)00069-2. [DOI] [PubMed] [Google Scholar]
- Brunn GJ, Fadden P, Haystead TA, Lawrence JC., Jr The mammalian target of rapamycin phosphorylates sites having a (Ser/Thr)-Pro motif and is activated by antibodies to a region near its COOH terminus. J Biol Chem. 1997a;272(51):32547–50. doi: 10.1074/jbc.272.51.32547. [DOI] [PubMed] [Google Scholar]
- Brunn GJ, Hudson CC, Sekulic A, Williams JM, Hosoi H, Houghton PJ, Lawrence JC, Jr, Abraham RT. Phosphorylation of the translational repressor PHAS-I by the mammalian target of rapamycin. Science. 1997b;277(5322):99–101. doi: 10.1126/science.277.5322.99. [DOI] [PubMed] [Google Scholar]
- Bu X, Haas DW, Hagedorn CH. Novel phosphorylation sites of eukaryotic initiation factor-4F and evidence that phosphorylation stabilizes interactions of the p25 and p220 subunits. J Biol Chem. 1993;268(7):4975–8. [PubMed] [Google Scholar]
- Burnett PE, Barrow RK, Cohen NA, Snyder SH, Sabatini DM. RAFT1 phosphorylation of the translational regulators p70 S6 kinase and 4E-BP1. Proc Natl Acad Sci U S A. 1998;95(4):1432–7. doi: 10.1073/pnas.95.4.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campagnoni AT. Molecular biology of myelin proteins from the central nervous system. J Neurochem. 1988;51(1):1–14. doi: 10.1111/j.1471-4159.1988.tb04827.x. [DOI] [PubMed] [Google Scholar]
- Carter CJ. eIF2B and oligodendrocyte survival: where nature and nurture meet in bipolar disorder and schizophrenia? Schizophr Bull. 2007;33(6):1343–53. doi: 10.1093/schbul/sbm007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang A, Nishiyama A, Peterson J, Prineas J, Trapp BD. NG2-positive oligodendrocyte progenitor cells in adult human brain and multiple sclerosis lesions. J Neurosci. 2000;20(17):6404–12. doi: 10.1523/JNEUROSCI.20-17-06404.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelho RP, Payne SG, Bittman R, Spiegel S, Sato-Bigbee C. The immunomodulator FTY720 has a direct cytoprotective effect in oligodendrocyte progenitors. J Pharmacol Exp Ther. 2007;323(2):626–35. doi: 10.1124/jpet.107.123927. [DOI] [PubMed] [Google Scholar]
- Cohen RI, Marmur R, Norton WT, Mehler MF, Kessler JA. Nerve growth factor and neurotrophin-3 differentially regulate the proliferation and survival of developing rat brain oligodendrocytes. J Neurosci. 1996;16(20):6433–42. doi: 10.1523/JNEUROSCI.16-20-06433.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui QL, Almazan G. IGF-I-induced oligodendrocyte progenitor proliferation requires PI3K/Akt, MEK/ERK, and Src-like tyrosine kinases. J Neurochem. 2007;100(6):1480–93. doi: 10.1111/j.1471-4159.2006.04329.x. [DOI] [PubMed] [Google Scholar]
- Cui QL, Fogle E, Almazan G. Muscarinic acetylcholine receptors mediate oligodendrocyte progenitor survival through Src-like tyrosine kinases and PI3K/Akt pathways. Neurochem Int. 2006;48(5):383–93. doi: 10.1016/j.neuint.2005.11.014. [DOI] [PubMed] [Google Scholar]
- de Ferra F, Engh H, Hudson L, Kamholz J, Puckett C, Molineaux S, Lazzarini RA. Alternative splicing accounts for the four forms of myelin basic protein. Cell. 1985;43(3 Pt 2):721–7. doi: 10.1016/0092-8674(85)90245-4. [DOI] [PubMed] [Google Scholar]
- de Moor CH, Richter JD. Translational control in vertebrate development. Int Rev Cytol. 2001;203:567–608. doi: 10.1016/s0074-7696(01)03017-0. [DOI] [PubMed] [Google Scholar]
- Du Y, Fischer TZ, Lee LN, Lercher LD, Dreyfus CF. Regionally specific effects of BDNF on oligodendrocytes. Dev Neurosci. 2003;25(2–4):116–26. doi: 10.1159/000072261. [DOI] [PubMed] [Google Scholar]
- Dubois-Dalcq M, Murray K. Why are growth factors important in oligodendrocyte physiology? Pathol Biol (Paris) 2000;48(1):80–6. [PubMed] [Google Scholar]
- Dupree JL, Girault JA, Popko B. Axo-glial interactions regulate the localization of axonal paranodal proteins. J Cell Biol. 1999;147(6):1145–52. doi: 10.1083/jcb.147.6.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebner S, Dunbar M, McKinnon RD. Distinct roles for PI3K in proliferation and survival of oligodendrocyte progenitor cells. J Neurosci Res. 2000;62(3):336–45. doi: 10.1002/1097-4547(20001101)62:3<336::AID-JNR3>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Flores AI, Mallon BS, Matsui T, Ogawa W, Rosenzweig A, Okamoto T, Macklin WB. Akt-mediated survival of oligodendrocytes induced by neuregulins. J Neurosci. 2000;20(20):7622–30. doi: 10.1523/JNEUROSCI.20-20-07622.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores AI, Narayanan SP, Morse EN, Shick HE, Yin X, Kidd G, Avila RL, Kirschner DA, Macklin WB. Constitutively active Akt induces enhanced myelination in the CNS. J Neurosci. 2008;28(28):7174–83. doi: 10.1523/JNEUROSCI.0150-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederickson RM, Montine KS, Sonenberg N. Phosphorylation of eukaryotic translation initiation factor 4E is increased in Src-transformed cell lines. Mol Cell Biol. 1991;11(5):2896–900. doi: 10.1128/mcb.11.5.2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelinas JN, Banko JL, Hou L, Sonenberg N, Weeber EJ, Klann E, Nguyen PV. ERK and mTOR signaling couple beta-adrenergic receptors to translation initiation machinery to gate induction of protein synthesis-dependent long-term potentiation. J Biol Chem. 2007;282(37):27527–35. doi: 10.1074/jbc.M701077200. [DOI] [PubMed] [Google Scholar]
- Gingras AC, Kennedy SG, O’Leary MA, Sonenberg N, Hay N. 4E-BP1, a repressor of mRNA translation, is phosphorylated and inactivated by the Akt(PKB) signaling pathway. Genes Dev. 1998;12(4):502–13. doi: 10.1101/gad.12.4.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingras AC, Raught B, Sonenberg N. eIF4 initiation factors: effectors of mRNA recruitment to ribosomes and regulators of translation. Annu Rev Biochem. 1999;68:913–63. doi: 10.1146/annurev.biochem.68.1.913. [DOI] [PubMed] [Google Scholar]
- Hara K, Yonezawa K, Kozlowski MT, Sugimoto T, Andrabi K, Weng QP, Kasuga M, Nishimoto I, Avruch J. Regulation of eIF-4E BP1 phosphorylation by mTOR. J Biol Chem. 1997;272(42):26457–63. doi: 10.1074/jbc.272.42.26457. [DOI] [PubMed] [Google Scholar]
- Heinrich M, Gorath M, Richter-Landsberg C. Neurotrophin-3 (NT-3) modulates early differentiation of oligodendrocytes in rat brain cortical cultures. Glia. 1999;28(3):244–55. [PubMed] [Google Scholar]
- Hershey JW. Translational control in mammalian cells. Annu Rev Biochem. 1991;60:717–55. doi: 10.1146/annurev.bi.60.070191.003441. [DOI] [PubMed] [Google Scholar]
- Irvine KA, Blakemore WF. Age increases axon loss associated with primary demyelination in cuprizone-induced demyelination in C57BL/6 mice. J Neuroimmunol. 2006;175(1–2):69–76. doi: 10.1016/j.jneuroim.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Irvine KA, Blakemore WF. Remyelination protects axons from demyelination-associated axon degeneration. Brain. 2008;131(Pt 6):1464–77. doi: 10.1093/brain/awn080. [DOI] [PubMed] [Google Scholar]
- Jean I, Lavialle C, Barthelaix-Pouplard A, Fressinaud C. Neurotrophin-3 specifically increases mature oligodendrocyte population and enhances remyelination after chemical demyelination of adult rat CNS. Brain Res. 2003;972(1–2):110–8. doi: 10.1016/s0006-8993(03)02510-1. [DOI] [PubMed] [Google Scholar]
- Johnson JR, Chu AK, Sato-Bigbee C. Possible role of CREB in the stimulation of oligodendrocyte precursor cell proliferation by neurotrophin-3. J Neurochem. 2000;74(4):1409–17. doi: 10.1046/j.1471-4159.2000.0741409.x. [DOI] [PubMed] [Google Scholar]
- Kahn MA, Kumar S, Liebl D, Chang R, Parada LF, De Vellis J. Mice lacking NT-3, and its receptor TrkC, exhibit profound deficiencies in CNS glial cells. Glia. 1999;26(2):153–65. [PubMed] [Google Scholar]
- Kavanaugh B, Beesley J, Itoh T, Itoh A, Grinspan J, Pleasure D. Neurotrophin-3 (NT-3) diminishes susceptibility of the oligodendroglial lineage to AMPA glutamate receptor-mediated excitotoxicity. J Neurosci Res. 2000;60(6):725–32. doi: 10.1002/1097-4547(20000615)60:6<725::AID-JNR4>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Keirstead HS, Blakemore WF. The role of oligodendrocytes and oligodendrocyte progenitors in CNS remyelination. Adv Exp Med Biol. 1999;468:183–97. doi: 10.1007/978-1-4615-4685-6_15. [DOI] [PubMed] [Google Scholar]
- Kelleher RJ, 3rd, Govindarajan A, Jung HY, Kang H, Tonegawa S. Translational control by MAPK signaling in long-term synaptic plasticity and memory. Cell. 2004;116(3):467–79. doi: 10.1016/s0092-8674(04)00115-1. [DOI] [PubMed] [Google Scholar]
- Kumar S, Kahn MA, Dinh L, de Vellis J. NT-3-mediated TrkC receptor activation promotes proliferation and cell survival of rodent progenitor oligodendrocyte cells in vitro and in vivo. J Neurosci Res. 1998;54(6):754–65. doi: 10.1002/(SICI)1097-4547(19981215)54:6<754::AID-JNR3>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- LeVine SM, Goldman JE. Spatial and temporal patterns of oligodendrocyte differentiation in rat cerebrum and cerebellum. J Comp Neurol. 1988;277(3):441–55. doi: 10.1002/cne.902770309. [DOI] [PubMed] [Google Scholar]
- Lin W, Bailey SL, Ho H, Harding HP, Ron D, Miller SD, Popko B. The integrated stress response prevents demyelination by protecting oligodendrocytes against immune-mediated damage. J Clin Invest. 2007;117(2):448–56. doi: 10.1172/JCI29571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W, Kunkler PE, Harding HP, Ron D, Kraig RP, Popko B. Enhanced integrated stress response promotes myelinating oligodendrocyte survival in response to interferon-gamma. Am J Pathol. 2008;173(5):1508–17. doi: 10.2353/ajpath.2008.080449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25(4):402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lynch M, Chen L, Ravitz MJ, Mehtani S, Korenblat K, Pazin MJ, Schmidt EV. hnRNP K binds a core polypyrimidine element in the eukaryotic translation initiation factor 4E (eIF4E) promoter, and its regulation of eIF4E contributes to neoplastic transformation. Mol Cell Biol. 2005;25(15):6436–53. doi: 10.1128/MCB.25.15.6436-6453.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinnon RD, Matsui T, Dubois-Dalcq M, Aaronson SA. FGF modulates the PDGF-driven pathway of oligodendrocyte development. Neuron. 1990;5(5):603–14. doi: 10.1016/0896-6273(90)90215-2. [DOI] [PubMed] [Google Scholar]
- McKinnon RD, Smith C, Behar T, Smith T, Dubois-Dalcq M. Distinct effects of bFGF and PDGF on oligodendrocyte progenitor cells. Glia. 1993;7(3):245–54. doi: 10.1002/glia.440070308. [DOI] [PubMed] [Google Scholar]
- McTigue DM, Horner PJ, Stokes BT, Gage FH. Neurotrophin-3 and brain-derived neurotrophic factor induce oligodendrocyte proliferation and myelination of regenerating axons in the contused adult rat spinal cord. J Neurosci. 1998;18(14):5354–65. doi: 10.1523/JNEUROSCI.18-14-05354.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minich WB, Balasta ML, Goss DJ, Rhoads RE. Chromatographic resolution of in vivo phosphorylated and nonphosphorylated eukaryotic translation initiation factor eIF-4E: increased cap affinity of the phosphorylated form. Proc Natl Acad Sci U S A. 1994;91(16):7668–72. doi: 10.1073/pnas.91.16.7668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miskimins R, Srinivasan R, Marin-Husstege M, Miskimins WK, Casaccia-Bonnefil P. p27(Kip1) enhances myelin basic protein gene promoter activity. J Neurosci Res. 2002;67(1):100–5. doi: 10.1002/jnr.10080. [DOI] [PubMed] [Google Scholar]
- Murata T, Shimotohno K. Ubiquitination and proteasome-dependent degradation of human eukaryotic translation initiation factor 4E. J Biol Chem. 2006;281(30):20788–800. doi: 10.1074/jbc.M600563200. [DOI] [PubMed] [Google Scholar]
- Ness JK, Mitchell NE, Wood TL. IGF-I and NT-3 signaling pathways in developing oligodendrocytes: differential regulation and activation of receptors and the downstream effector Akt. Dev Neurosci. 2002;24(5):437–45. doi: 10.1159/000069050. [DOI] [PubMed] [Google Scholar]
- Rasband MN, Trimmer JS. Developmental clustering of ion channels at and near the node of Ranvier. Dev Biol. 2001;236(1):5–16. doi: 10.1006/dbio.2001.0326. [DOI] [PubMed] [Google Scholar]
- Raught B, Gingras AC. eIF4E activity is regulated at multiple levels. Int J Biochem Cell Biol. 1999;31(1):43–57. doi: 10.1016/s1357-2725(98)00131-9. [DOI] [PubMed] [Google Scholar]
- Richardson JP, Mohammad SS, Pavitt GD. Mutations causing childhood ataxia with central nervous system hypomyelination reduce eukaryotic initiation factor 2B complex formation and activity. Mol Cell Biol. 2004;24(6):2352–63. doi: 10.1128/MCB.24.6.2352-2363.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinker-Schaeffer CW, Austin V, Zimmer S, Rhoads RE. Ras transformation of cloned rat embryo fibroblasts results in increased rates of protein synthesis and phosphorylation of eukaryotic initiation factor 4E. J Biol Chem. 1992;267(15):10659–64. [PubMed] [Google Scholar]
- Robinson S, Miller R. Environmental enhancement of growth factor-mediated oligodendrocyte precursor proliferation. Mol Cell Neurosci. 1996;8(1):38–52. doi: 10.1006/mcne.1996.0042. [DOI] [PubMed] [Google Scholar]
- Rogister B, Ben-Hur T, Dubois-Dalcq M. From neural stem cells to myelinating oligodendrocytes. Mol Cell Neurosci. 1999;14(4–5):287–300. doi: 10.1006/mcne.1999.0790. [DOI] [PubMed] [Google Scholar]
- Rubio N, Rodriguez R, Arevalo MA. In vitro myelination by oligodendrocyte precursor cells transfected with the neurotrophin-3 gene. Glia. 2004;47(1):78–87. doi: 10.1002/glia.20035. [DOI] [PubMed] [Google Scholar]
- Saini HS, Coelho RP, Goparaju SK, Jolly PS, Maceyka M, Spiegel S, Sato-Bigbee C. Novel role of sphingosine kinase 1 as a mediator of neurotrophin-3 action in oligodendrocyte progenitors. J Neurochem. 2005;95(5):1298–310. doi: 10.1111/j.1471-4159.2005.03451.x. [DOI] [PubMed] [Google Scholar]
- Saini HS, Gorse KM, Boxer LM, Sato-Bigbee C. Neurotrophin-3 and a CREB-mediated signaling pathway regulate Bcl-2 expression in oligodendrocyte progenitor cells. J Neurochem. 2004;89(4):951–61. doi: 10.1111/j.1471-4159.2004.02365.x. [DOI] [PubMed] [Google Scholar]
- Sato-Bigbee C, Pal S, Chu AK. Different neuroligands and signal transduction pathways stimulate CREB phosphorylation at specific developmental stages along oligodendrocyte differentiation. J Neurochem. 1999;72(1):139–47. doi: 10.1046/j.1471-4159.1999.0720139.x. [DOI] [PubMed] [Google Scholar]
- Schmidt EV. The role of c-myc in regulation of translation initiation. Oncogene. 2004;23(18):3217–21. doi: 10.1038/sj.onc.1207548. [DOI] [PubMed] [Google Scholar]
- Sim FJ, Zhao C, Penderis J, Franklin RJ. The age-related decrease in CNS remyelination efficiency is attributable to an impairment of both oligodendrocyte progenitor recruitment and differentiation. J Neurosci. 2002;22(7):2451–9. doi: 10.1523/JNEUROSCI.22-07-02451.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonenberg N, Gingras AC. The mRNA 5′ cap-binding protein eIF4E and control of cell growth. Curr Opin Cell Biol. 1998;10(2):268–75. doi: 10.1016/s0955-0674(98)80150-6. [DOI] [PubMed] [Google Scholar]
- Svitkin YV, Herdy B, Costa-Mattioli M, Gingras AC, Raught B, Sonenberg N. Eukaryotic translation initiation factor 4E availability controls the switch between cap-dependent and internal ribosomal entry site-mediated translation. Mol Cell Biol. 2005;25(23):10556–65. doi: 10.1128/MCB.25.23.10556-10565.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takei N, Kawamura M, Hara K, Yonezawa K, Nawa H. Brain-derived neurotrophic factor enhances neuronal translation by activating multiple initiation processes: comparison with the effects of insulin. J Biol Chem. 2001;276(46):42818–25. doi: 10.1074/jbc.M103237200. [DOI] [PubMed] [Google Scholar]
- Tang SJ, Reis G, Kang H, Gingras AC, Sonenberg N, Schuman EM. A rapamycin-sensitive signaling pathway contributes to long-term synaptic plasticity in the hippocampus. Proc Natl Acad Sci U S A. 2002;99(1):467–72. doi: 10.1073/pnas.012605299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson HC, Onischke C, Raine CS. Human oligodendrocyte precursor cells in vitro: phenotypic analysis and differential response to growth factors. Glia. 2003;44(2):153–65. doi: 10.1002/glia.10280. [DOI] [PubMed] [Google Scholar]
- Yan H, Wood PM. NT-3 weakly stimulates proliferation of adult rat O1(−)O4(+) oligodendrocyte-lineage cells and increases oligodendrocyte myelination in vitro. J Neurosci Res. 2000;62(3):329–35. doi: 10.1002/1097-4547(20001101)62:3<329::AID-JNR2>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]