Abstract
Pitch plays a fundamental role in audition, from speech and music perception to auditory scene analysis. Congenital amusia is a neurogenetic disorder that appears to affect primarily pitch and melody perception. Pitch is normally conveyed by the spectro-temporal fine structure of low harmonics, but some pitch information is available in the temporal envelope produced by the interactions of higher harmonics. Using 10 amusic subjects and 10 matched controls, we tested the hypothesis that amusics suffer exclusively from impaired processing of spectro-temporal fine structure. We also tested whether the inability of amusics to process acoustic temporal fine structure extends beyond pitch by measuring sensitivity to interaural time differences, which also rely on temporal fine structure. Further tests were carried out on basic intensity and spectral resolution. As expected, pitch perception based on spectro-temporal fine structure was impaired in amusics; however, no significant deficits were observed in amusics' ability to perceive the pitch conveyed via temporal-envelope cues. Sensitivity to interaural time differences was also not significantly different between the amusic and control groups, ruling out deficits in the peripheral coding of temporal fine structure. Finally, no significant differences in intensity or spectral resolution were found between the amusic and control groups. The results demonstrate a pitch-specific deficit in fine spectro-temporal information processing in amusia that seems unrelated to temporal or spectral coding in the auditory periphery. These results are consistent with the view that there are distinct mechanisms dedicated to processing resolved and unresolved harmonics in the general population, the former being altered in congenital amusia while the latter is spared.
Keywords: Pitch, auditory perception, spectro-temporal fine structure, ITD, ILD, auditory filter bandwith
1. Introduction
Pitch is the perceptual correlate of the periodicity (or repetition rate) of acoustic waveforms. It is a salient characteristic of sounds that plays a fundamental role in music and speech perception, as well as in auditory stream segregation, the mechanism by which we are able to hear out single auditory events within mixtures of sounds. Vocal and instrumental sounds that produce pitch are mostly harmonic complex tones that can be decomposed into a series of sinusoids (or partials) with discrete frequencies at integer multiples of a fundamental frequency (F0). It has long been known that lower-order harmonics (e.g., harmonics 1-5) within a complex can be heard out as separate tones under certain circumstances (e.g., von Helmholtz, 1877; Plomp, 1964; Bernstein and Oxenham 2003). These lower-order “resolved” harmonics generally provide a strong, or salient, pitch when presented together, and have been shown to dominate the overall pitch of natural harmonic complexes (Plomp, 1967; Moore et al., 1985; Dai, 2000). Listeners can perceive differences in F0 between two successive harmonic complex comprising resolved harmonics that are an order of magnitude smaller than the semitone, the smallest scale step used in Western music (e.g., Micheyl et al., 2006). In contrast, higher-order harmonics (higher than about the 10th) are not readily heard out individually (Bernstein and Oxenham 2003), and they produce a generally weak pitch sensation, with the minimum detectable change in F0 being much poorer than for resolved harmonics, and often larger than a semitone (Houtsma and Smurzynski, 1990; Bernstein and Oxenham 2003).
The difference in pitch salience and accuracy produced by resolved and unresolved harmonics has been ascribed to how they are coded in the peripheral auditory system. The cochlea provides a frequency decomposition, resulting in a frequency-to-place mapping of the content of the incoming sounds. Cochlear encoding is thus often likened to a bank of bandpass filters, with bandwidths that are roughly proportional to the center frequency of the filter (Glasberg and Moore, 1990; Shera et al., 2002). The lower harmonics have been termed “resolved” because they are spaced sufficiently far apart in frequency to fall into distinct auditory filters. Resolved harmonics are thus thought to be represented by the spectro-temporal fine-structure cues associated with the cochlear response to the individual harmonics. In contrast, the pitch of unresolved complexes is thought to be extracted from the repetition rate of the temporal envelope produced by the interactions of multiple harmonics in a given auditory filter. Overall, pitch extraction is a complex process which details are not yet fully understood despite many years of research (see Moore and Gockel, 2011; Oxenham, 2012 for recent reviews).
Although pitch plays an important role in speech perception in both tone (Gandour, 1983) and non-tone (Nooteboom, 1997; Binns and Culling, 2007; Miller et al., 2010) languages, it is in music that the demands on pitch processing are highest. In contrast to prosody, where variations as large as an octave can be observed within an utterance (Fitzsimons et al., 2001), differences in pitch as small as one semitone (or a change of 6% in frequency) are both common (Vos and Troost, 1989) and meaningful in music. For instance changes of a semitone define the distinction between the major and minor mode in Western tonal music. Therefore, deficits in pitch processing can lead to a wide variety of deficits in music-related tasks. Here we study pitch perception in congenital amusia, a life-long disorder of music perception that, in its most common form, is thought to reflect impaired pitch processing (sometimes also called tone-deafness).
Impairments related to amusia have been reported for pitch discrimination (Ayotte et al., 2002; Peretz et al., 2003; Foxton et al., 2004; Hyde and Peretz, 2004; Tillmann et al., 2009; Liu et al., 2010), memory for pitch (Gosselin et al., 2009; Tillmann et al., 2009; Williamson et al., 2010; but see Jiang et al., 2013), melody recognition (Ayotte et al., 2002), singing in tune (Dalla Bella et al., 2009), and dissonance perception (Ayotte et al., 2002; Cousineau et al., 2012). The condition was originally described as a strictly musical deficit, but this specificity has been challenged by recent evidence for deficits in prosody perception in both non-tonal (Patel et al., 1998; Patel et al., 2008; Thompson et al., 2012) and tonal (Jiang et al., 2010; Jiang et al., 2012a) languages, as well as categorical perception of language tones in Mandarin speakers (Jiang et al., 2010; 2012b). The diagnostic tool most widely used for congenital amusia is the Montreal Battery of Evaluation of amusia (MBEA, Peretz et al., 2003), which consists of six tests that assess musical pitch and rhythm discrimination, meter perception and memory. The prevalence of the condition has been estimated to be around 4% of the general population (Kalmus and Fry, 1980; though see Henry and McAuley, 2010).
Here, we use psychoacoustic methods to investigate the origin of the pitch deficit observed in this population. Our main hypothesis, tested in the first experiment, is that the deficits in pitch perception observed in congenital amusia arise from abnormal processing of spectro-temporal fine-structure cues associated with the individual resolved harmonics. The hypothesis is motivated by the observation that the classic deficits found in amusia (pitch discrimination thresholds larger than a semitone and poor melody recognition) can also be observed in normal listeners when the access to these cues is reduced by removing resolved harmonics (Moore and Rosen, 1979; Shackleton and Carlyon, 1994; Kaernbach and Bering, 2001). Accordingly, amusics are expected to perform poorly with single pure tones and low-order resolved harmonics, but normally with high-order unresolved harmonics. Additionally, if amusics suffer from impaired access to the fine spectro-temporal information associated with pure tones and resolved harmonics, then increasing stimulus uncertainty (via pitch transposition) and reducing tone duration will be more deleterious to thresholds for resolved complexes in amusics than in controls. This prediction is based on the hypothesis that amusics use a mechanism to process resolved harmonics that is similar to that used by controls with unresolved complexes and the observation that both these manipulations increase thresholds for unresolved complexes in the normal population (White and Plack, 1998; Cousineau et al., 2009).
Our second experiment tested the hypothesis that the deficits in fine spectro-temporal coding found in amusia reflect a deficit in fine peripheral spectral processing. The processing of low-order resolved harmonics has been shown to relate to frequency selectivity, with poorer frequency selectivity leading to fewer resolved harmonics and poorer pitch discrimination (Bernstein and Oxenham, 2006). If pitch discrimination deficits observed in amusia are related to a peripheral deficit in spectral resolution, then amusics should also show deficits in basic measures of spectral resolution.
Our third experiment tested the hypothesis that deficits in fine spectro-temporal coding found in amusia reflect poor temporal fine-structure coding in the auditory periphery. Spatial hearing in humans relies on the coding of very fine timing differences between the ears in the microsecond range. It has been hypothesized that the same specialized neural mechanisms that permit this exquisite sensitivity to timing may also subserve the processing of periodicity and pitch (Licklider, 1951; Meddis and Hewitt, 1991a; b). If amusia is associated with a deficit in processing temporal fine structure, this deficit may also affect spatial hearing.
The three hypotheses were tested in a series of experiments using the same group of amusic subjects and matched control subjects. In the first experiment, we measured pitch discrimination using either low-order (resolved) or high-order (unresolved) harmonics. In the second experiment, we estimated spectral resolution, or frequency selectivity, using a masking paradigm, known as the “notched-noise method” (Patterson, 1976; Glasberg and Moore, 1990; Oxenham and Shera, 2003). In the third experiment, we measured sensitivity to interaural time differences (ITDs) and interaural level differences (ILDs).
2. Material and methods
2.1 Subjects
Ten amusics and ten matched controls participated in this study. The two groups did not differ significantly in age, years of education, years of musical training, or audiometric thresholds (see Tables 1 and 2). Each amusic participant scored at least 2 SD below the mean of the general population when tested with the MBEA (Peretz et al., 2003; see Henry and McAuley, 2013 for further qualifications).
Table 1.
Control and amusic group characteristics. The audiogram data are mean values of thresholds at .5, 1, 2, 3, 4 and 8 kHz in the two ears. Values are group means ± standard deviation.
| Group | |||
|---|---|---|---|
| Amusics (N=10) | Controls (N=10) | p-value of t-test | |
| Demographic characteristics | |||
| Age (years) | 60.5 ± 5.6 | 59.5 ± 3.8 | 0.65 (n.s.) |
| Gender | 6 female | 7 female | - |
| Musical education (years) | 0.7 ± 0.5 | 1.2 ± 1.5 | 0.30 (n.s.) |
| Audiogram (dB hearing loss) | 17.0 ± 7.3 | 19.3 ± 9.4 | 0.55 (n.s.) |
Table 2.
Individual scores of the congenital amusics and the controls for each of the individual tests of the Montreal Battery of Evaluation of Amusia (MBEA), as well as the global score (mean of the 6 subtests). Each test is scored on 30 points. The cut off on the global score (mean of the six subtests) is the criterion used for diagnosis of congenital amusia (Ayotte et al., 2002).
| Scale | Contour | Interval | Rhythm | Metric | Memory | Global (cut off = 23.4) | |
|---|---|---|---|---|---|---|---|
| A1 | 17 | 20 | 12 | 21 | 14 | 19 | 17.2 |
| A2 | 22 | 20 | 22 | 26 | 28 | 22 | 23.3 |
| A3 | 20 | 26 | 22 | 22 | 22 | 21 | 22.2 |
| A4 | 16 | 16 | 16 | 19 | 22 | 20 | 18.2 |
| A5 | 14 | 16 | 14 | 26 | 19 | 22 | 18.5 |
| A6 | 17 | 20 | 22 | 29 | 21 | 22 | 21.8 |
| A7 | 15 | 15 | 15 | 15 | 17 | 15 | 15.3 |
| A8 | 16 | 17 | 20 | 24 | 17 | 20 | 19 |
| A9 | 22 | 16 | 19 | 20 | 27 | 21 | 20.8 |
| A10 | 22 | 19 | 18 | 28 | 16 | 23 | 21 |
| Amusics mean (std) | 18.1 (3.1) | 18.5 (3.3) | 18 (3.6) | 23 (4.4) | 20.3 (4.6) | 20.5 (2.3) | 19.8 (2.50) |
| C1 | 30 | 29 | 29 | 24 | 30 | 26 | 28 |
| C2 | 28 | 30 | 27 | 26 | 22 | 29 | 27 |
| C3 | 30 | 27 | 28 | 28 | 28 | 24 | 27,5 |
| C4 | 29 | 28 | 30 | 29 | 19 | 29 | 27.3 |
| C5 | 29 | 25 | 29 | 29 | 23 | 23 | 26.3 |
| C6 | 26 | 26 | 27 | 25 | 25 | 24 | 25.5 |
| C7 | 28 | 29 | 25 | 27 | 17 | 27 | 25.5 |
| C8 | 29 | 25 | 27 | 27 | 25 | 23 | 26 |
| C9 | 30 | 29 | 29 | 27 | 26 | 27 | 28 |
| C10 | 28 | 28 | 27 | 26 | 29 | 29 | 27.8 |
| Controls mean (std) | 28.7 (1.3) | 27.6 (1.8) | 27.8 (1.5) | 26.8 (1.6) | 24.4 (4.2) | 26.1 (2.5) | 26.9 (1.0) |
2.2 Stimuli and Procedure
Sound delivery and presentation
All stimuli were delivered via an external soundcard (RME Fireface 800) with a 44.1-kHz sampling rate and 16-bit resolution. Sounds were delivered using closed headphones (Beyerdynamic DT 770 Pro). Participants were tested individually in a double-walled sound-attenuating booth. Responses were collected using a keyboard, and feedback was provided following each trial in all experiments. The overall testing time was approximately three hours per participant.
2.2.1 Experiment 1 – F0 difference limens
Fundamental frequency difference limens (F0DLs) were measured using a dual-pair task with a 2-down, 1-up adaptive method to track the 70.7% correct point on the psychometric function. In this task, subjects are presented with two pairs of sounds, one that contains two identical sounds and one that contains sounds that differ with respect to the attribute tested. The different pair is presented first or second with equal a priori probability and subjects are asked to judge which pair contained the difference. In the pair containing the difference, the difference in F0 was varied adaptively. Initially the F0 difference was 110% and the difference was varied by a factor of 2. After the second reversal in the adaptive procedure, the factor was reduced to 1.2. The run was terminated after 8 reversals, and the mean of the 4 last was taken as the estimate of the threshold for the run.
The sounds were click trains, band-pass filtered between 1500 and 3500 Hz. This frequency region was selected to be high enough so that sounds with low, but still musically relevant (> 50 Hz), F0s would contain only unresolved harmonics when filtered, but low enough to incorporate the range in which the normal auditory system is still very sensitive to small frequency differences (Moore, 1973; Micheyl et al., 2010).
In the normal population, sensitivity to F0 differences is high so long as some harmonics below about the 10th are present (Houtsma and Smurzynski, 1990; Shackleton and Carlyon, 1994; Bernstein and Oxenham 2003). Our two F0s of 62.5 Hz and 250 Hz were selected to ensure that the lower F0 had no harmonics in that range and the higher F0 had several harmonics in that range. Thus, we expected F0DLs in the control group to be low (good) in the 250-Hz F0 condition, which contained resolved harmonics (PR condition), and F0DLs to be high (poor) in the 62.5-Hz condition, which contained only unresolved harmonics (PU condition). A background pink noise was added to all conditions to mask possible distortion products (Pressnitzer and Patterson, 2001). The overall level of the noise was set at 6 dB below the overall level of the tones. The overall level of the tones plus noise was set close to 65 dB SPL, which was judged to be a comfortable listening level. The tone plus noise compounds were gated on and off with 25-ms raised-cosine ramps.
To examine the effect of transposition, thresholds were measured with and without transposition between the two pairs of each trial with the dual pair task. When transposition was present, on each trial, the reference in one of the pairs was moved up (higher in frequency) by 20% of the reference value. Finally, to evaluate the role of tone duration on pitch discrimination abilities, thresholds were measured for tone durations of 150 and 350 ms. Thresholds were thus measured in four different subconditions (transposed and not transposed, along with short and long durations; see Figure 1A) for each of the two experimental conditions (PR and PU). The sounds in the pairs were always presented contiguously, and the two pairs in each trial were separated by a 500-ms silent delay. Subjects completed two or three experimental runs in each condition, depending on the time available. The mean number of experimental runs did not differ significantly between the two groups (amusics mean = 2.64, controls mean = 2.75; t-test p= 0.63) and the mean of all experimental runs was kept as the final estimate of threshold for each condition and subject.
Figure 1.
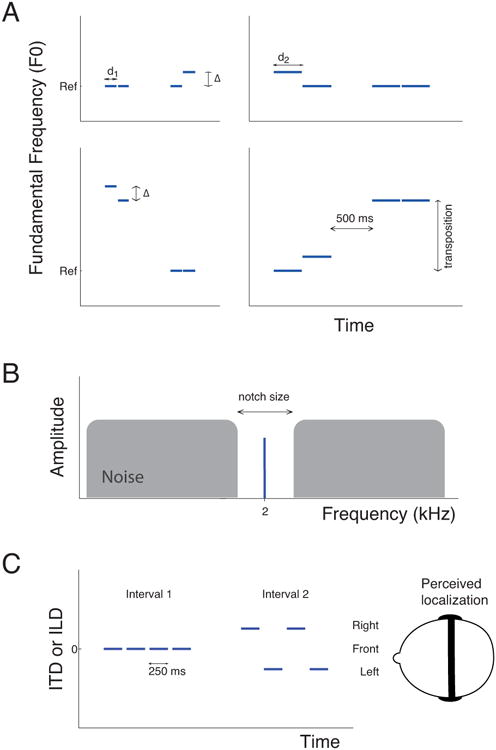
Schematic representation of the stimuli. A) Experiment 1. Representation of a single trial of the dual-pair task in which subjects are presented with two pairs of sounds, one of which contains a F0 difference (Δ) to be reported. This task was used in four different subcondition including short (left panels, d1 = 150 ms) and long (right panels, d2 = 350 ms) tone durations and absence (top panels) or presence (bottom panels) of transposition between the two pairs. The reference frequency (Ref) was 250 Hz for resolved pitch and 62.5 Hz for unresolved pitch B) The notched-noise method allows the shape of the auditory filter to be derived from the measurement of thresholds for a pure tone embedded in a broadband noise with various widths of spectral notches in the noise around the frequency of the tone. On each trial, subjects are presented with two intervals, one containing only the noise and one containing the tone embedded in the noise, as illustrated here. C) Experiment 2. Each interval of the 2I-2AFC procedure included four segments of noise ramped on and off. In the signal interval (interval 2 in this example), localization cues (ITD or ILD) were added to the noise segments in alternate polarity, resulting in a repetitive movement from one side of the head to the other. Subjects had to report which of the two intervals contained a moving sound.
2.2.2 Experiment 2 - Auditory filter bandwidth
Masked thresholds for a 2-kHz pure tone were measured in the presence of a spectrally notched Gaussian noise (Patterson, 1976) using a 2-interval, 2-alternative forced-choice method. On each trial, subjects were presented with two consecutive noises, separated by a 500-ms gap. One of the noises (with equal a priori probability) contained the 2-kHz tonal signal (See Figure 1B). Each noise was 700 ms long and was gated on and off with 10-ms raised-cosine ramps. The signal was 500 ms long, was gated on and off with 30-ms raised-cosine ramps, and was presented in the temporal center of the noise. Detection thresholds for the tone in noise were measured with three different widths of a spectral notch, symmetrically centered around the signal frequency of 2 kHz, with total widths of 0, 0.4fs, and 0.8fs, where fs is the tone frequency. The signal level was fixed at 45 dB SPL and the level of the noise masker was adaptively varied using a 3-up 1-down procedure that tracks the 79.4% point on the psychometric function. All stimuli were presented diotically (same to both ears).
2.2.3 Experiment 3 - Localization cues
Difference limens were measured for ITDs and ILDs using a 2-interval 2-alternative forced-choice task and with a 3-down, 1-up adaptive procedure. On each trial subjects were presented with two consecutive bursts of lowpass-filtered noise (cutoff frequency of 1 kHz). Each noise burst comprised four segments of 250 ms each that were ramped on and off with 50-ms raised-cosine ramps, with no silent interval between the four segments. In the “reference” interval, there were no interaural differences in any of the four segments; in the “signal” interval, an ITD or ILD was introduced, which was reversed between alternating noises, producing a sensation of moving back and forth between left and right (see Figure 1C). Subjects had to report which interval contained the moving sound. Subjects completed two runs in each condition and the mean was kept as the final estimate of the threshold.
3. Results
3.1 Experiment 1: F0 discrimination with resolved and unresolved harmonics
Figure 2 shows the F0-discrimination thresholds obtained by amusic and control participants for stimuli comprising resolved harmonics (PR; left) or solely unresolved harmonics (PU; right). An initial mixed-model analysis of variance (ANOVA) was performed on the log-transformed thresholds obtained in Experiment 1 with group (amusics vs. controls) as a between-subjects factor and condition (PR vs. PU), transposition (present vs. absent) and duration (150 ms vs. 350 ms) as within-subjects factors. The ANOVA revealed significant main effects of group [F(1,18) = 17.4, p = 0.0005, effect size generalized-η2 =0.28], condition [F(1,18) = 386.9, p < 0.0001, η2 = 0.74], as well as a significant interaction between the two factors [F(1,12) = 14.6, p = 0.001, η2 = 0.095]. All values for this ANOVA are reported in Table 3. For clarity, results from the PR and PU conditions are discussed separately below.
Figure 2.
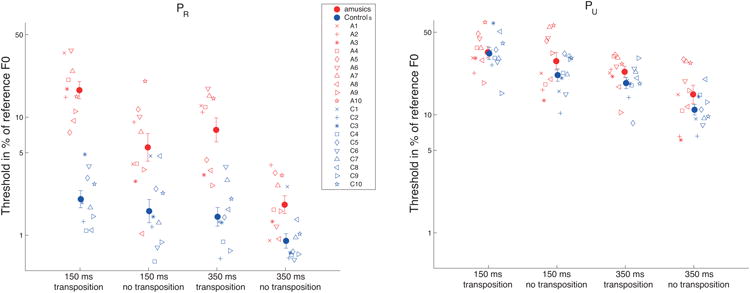
Results of Experiment 1. A) Mean and individual difference limens for the resolved pitch (PR) stimuli. In both panels, the closed symbols represent mean results for the amusic (red) and the control (blue) groups. The open symbols represent individual data and are consistent throughout all the figures of the paper. Results of the four different subconditions are plotted: from left to right 1) short tone duration (150 ms) and presence of transposition; 2) short tone duration and absence of transposition; 3) long tone duration (350 ms) and presence of transposition; 4) long tone duration and absence of transposition. Here and in subsequent figures, error bars represent +/- standard error about the mean. B) Same for unresolved pitch stimuli (PU).
Table 3.
Results of the initial mixed-model analysis of variance (ANOVA) performed on the log-transformed thresholds obtained in Experiment 1 with Group (amusics vs. controls) as a between-subjects factor and condition (PR vs. PU), transposition (present vs. absent) and duration (150 ms vs. 350 ms) as within-subjects factors.
| Effect | DFn | DFD | F | p | p<.05 | generalized- η2 |
|---|---|---|---|---|---|---|
| Group | 1 | 18 | 17.374 | 5.775220e-04 | * | 2.812435e-01 |
| Cond | 1 | 18 | 386.92 | 1.283056e-13 | * | 7.363539e-01 |
| Transp | 1 | 18 | 35.724 | 1.181329e-05 | * | 2.162334e-01 |
| Dur | 1 | 18 | 90.653 | 1.889937e-08 | * | 2.942587e-01 |
| Group×Cond | 1 | 18 | 14.607 | 1.248786e-03 | * | 9.538394e-02 |
| Group×Transp | 1 | 18 | 2.2954 | 1.471155e-01 | 1.741830e-02 | |
| Group×Dur | 1 | 18 | 2.9096 | 1.052489e-01 | 1.320568e-02 | |
| Cond×Transp | 1 | 18 | 0.2670 | 6.116109e-01 | 1.475726e-03 | |
| Cond×Dur | 1 | 18 | 14.367 | 1.339631e-03 | * | 2.857875e-02 |
| Transp×Dur | 1 | 18 | 0.2490 | 6.237656e-01 | 7.011815e-04 | |
| Group:Cond×Transp | 1 | 18 | 13.195 | 1.903958e-03 | * | 6.805869e-02 |
| Group×Cond×Dur | 1 | 18 | 8.7800 | 8.326395e-03 | * | 1.766103e-02 |
| Group×Transp×Dur | 1 | 18 | 0.4408 | 5.151049e-01 | 1.240480e-03 | |
| Cond×Transp×Dur | 1 | 18 | 0.0043 | 9.482110e-01 | 1.342776e-05 | |
| Group×Cond×Transp ×Dur | 1 | 18 | 1.5406 | 2.304529e-01 | 4.746081e-03 |
The left panel of Figure 2 displays the mean thresholds obtained for the PR stimuli expressed as a percentage of the reference F0. A mixed-model ANOVA of only data from the PR condition, with duration and transposition as within-subject factors and group as a between-subjects factor revealed that all three main effects were significant [group: F(1,18) = 51.8, p < 0.0001, η2 = 0.57; duration: F(1,18) = 84.9, p < 0.0001, η2 = 0.25; transposition: F(1,18) = 23.9, p = 0.0001, η2 = 0.32]. The main effect of group confirms the poorer overall performance of amusics compared with controls. More interestingly, among all possible interactions, the only two that were significant were group × duration [F(1,18) = 23.6, p = 0.0001, η2 =0.08] and group × transposition [F(1,18) = 12.2, p = 0.002, η2 = 0.19], showing that decreasing duration and imposing transposition between trials both had stronger deleterious effects on the performance of amusics than of controls. Values for non-significant interactions in the ANOVA were as follows: transposition × duration [F(1,18) = 0.13, p = 0.72, η2 = 0.0008]; group × transposition × duration [F(1,18) = 0.29, p = 0.59, η2 = 0.002]. Independent sample t-tests revealed that amusics were significantly poorer than controls in all four conditions (150 ms with transposition: p<0.00001; 150 ms without transposition: p=0.004; 350 ms with transposition: p=0.0001; 350 ms without transposition: p=0.008).
The right panel of Figure 2 displays the mean thresholds obtained using the PU stimuli, expressed as a percentage of the reference F0, in the four different configurations tested. As demonstrated in the initial ANOVA, and consistent with previous studies (e.g. Shackleton and Carlyon, 1994), complexes with only unresolved harmonics produced significantly larger (worse) thresholds than did the complexes with resolved harmonics. Considering just the data from the PU condition, a mixed-model ANOVA with duration and transposition as within-subject factors and group as a between-subjects factor revealed that only the main effects of duration and transposition were significant [duration: F(1,18) = 62.7, p < 0.0001, η2 = 0.33; transposition: F(1,18) = 17.9, p = 0.0004, η2 = 0.15]. Neither the main effect of group nor its interaction with the other factors was significant [group: F(1,18) = 2.15, p = 0.16, η2 = 0.06; group × duration: F(1,18) = 0.03, p = 0.86, η2 = 0.0002; group × transposition: F(1,18) = 1.42, p = 0.24, η2 = 0.013; group × transposition × duration F(1,18) = 1.38, p = 0.25, η2 = 0.008]. Thus, in contrast to the findings with the resolved harmonic complexes, the amusic group showed no significant deficit in F0 discrimination, relative to the control group, when judging complexes with only unresolved harmonics.
3.2 Experiment 2: Spectral resolution
Masked thresholds in the three spectral notch conditions (see methods) were used to fit a simple symmetric rounded-exponential filter shape to the data from each subject. The filter is described by the equation:
Where W(f) is the filter weighting function as a function of frequency, f, p is the single free parameter determining the steepness of the filter slope, and fc is the center frequency of the filter. The resulting filter shape is not expected to provide an accurate estimate of the underlying filter shape, but provides a one-parameter fit that should at least vary monotonically with the underlying filter bandwidth. A least-square-errors minimization routine, using the dB difference between the predicted and actual masked thresholds, was used to determine the best-fitting value of p for each subject (Oxenham and Simonson, 2006). The resulting filter shapes and the equivalent rectangular bandwidths corresponding to the best-fitting p values for each subject are shown in the top panels of Figure 3. An ANOVA performed on the estimated ERBs revealed no significant difference between ERBs of amusics and controls [F(1,18)= 0.31, p = 0.58, η2 = 0.017], suggesting no significant difference in basic auditory frequency selectivity.
Figure 3.
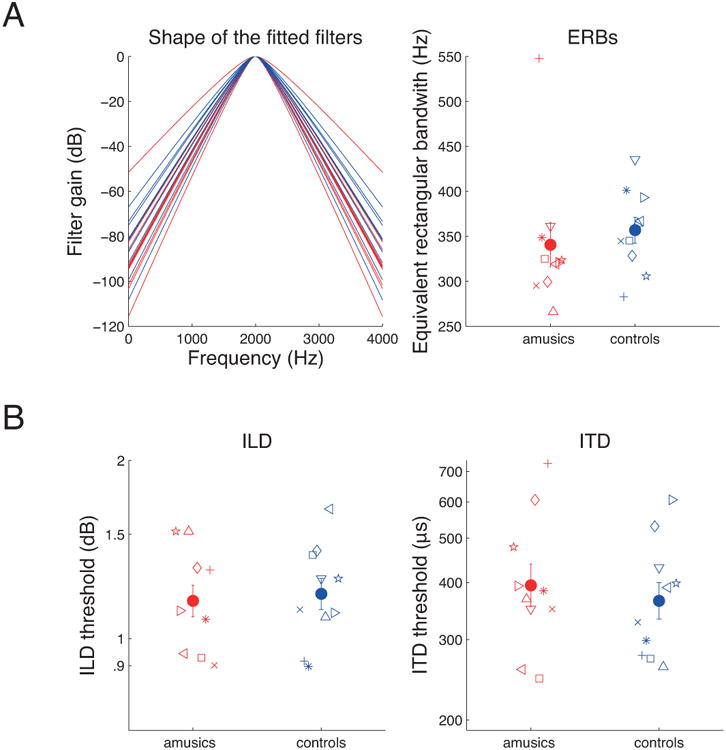
Results of Experiments 2 and 3. A) Masked thresholds in the three spectral notch conditions (see methods) were used to fit a simple symmetric rounded-exponential filter shape to the data from each subject. Individual filter shapes are depicted in the left panel, and mean (closed symbols) and individual (open symbols) equivalent rectangular bandwidth are depicted in the right panel in red for amusics and in blue for controls. B) Mean (closed symbols) and individual (open symbols) difference limens for Interaural Level Difference (ILD, left panel) and Interaural Time Difference (ITD, right panel).
3.3 Experiment 3: Interaural time and level differences
The thresholds for the detection of ITD and ILD are shown in Figure 3 (bottom panels). Separate ANOVAs performed on ILD and ITD data, with group as a between-subjects factor revealed no significant differences, either for ILD [F(1,18)=0.10, p = 0.75, η2 = 0.005] or for ITD [F(1,18)=0.31, p = 0.58, η2 = 0.017] thresholds. Thus, congenital amusia seems to have no significant effect on auditory intensity coding (based on the ILD results) or on the coding of temporal fine structure (based on the ITD results).
3.4 Evaluation of null results
Some of the most important aspects of the results presented here depend on the failure to reject the null hypothesis that performance of amusic and normal subjects is not different in some conditions. Because of this outcome, it is particularly important to consider the statistical power of the tests associated with the null results and whether a larger sample might lead to a different outcome. The primary null results involved the lack of difference between the amusic and control groups in i) F0DLs for unresolved harmonics (Experiment 1), ii) estimated auditory filter bandwidths (Experiment 2), and iii) interaural intensity and temporal acuity as measured with ILDs and ITDs, respectively (Experiment 3). In order to further investigate these results, we performed minimum-effect tests (Murphy and Myors, 1999). The idea behind these methods is that instead of testing the hypothesis that a factor has no effect whatsoever, the hypothesis tested is that the effect of the factor is so small that it can be labeled “negligibly small”. As for the regular uses of the F statistic, the test is done by comparing the F value obtained in a study to F values tabled in terms of degrees of freedom. Test of minimum-effect are however based on the non-central F distribution, that is also determined by the degrees of freedom, but additionally by a non-centrality parameter that allows to specifically test that a factor accounts for no more than a fixed percentage of the variance (see Murphy and Myors, 1999 for more details). Performed on the present data, these tests revealed that in the three conditions listed above, the non-significant main effect of group accounted for 1% or less of the variance, with a .01 α value.
There is an inherent difficulty in drawing strong conclusions based on negative results. Nevertheless, given the outcome of the minimum-effects tests, in addition to the small effect sizes (which yielded η2 values between 0.005 and 0.06), it seems unlikely that a significant effect would have been obtained even with a substantially larger sample size. Thus, it seems that any remaining effects of amusia in the dimensions of unresolved pitch discrimination, and basic spectral, intensity, and temporal coding are unlikely to be of behavioral relevance.
4. Discussion
4.1 Summary of results
This study tested basic psychoacoustic aspects of spectral and temporal processing in people with congenital amusia. Pitch perception was tested by measuring F0 difference limens (F0DLs) with harmonic complexes containing either resolved harmonics or only unresolved harmonics. Sensitivity to temporal fine structure was probed independently of pitch by measuring ITD thresholds, intensity resolution was probed by measuring ILD thresholds, and spectral resolution was probed by estimating the width of auditory filters using the notched-noise masking method. It was found that amusics show impaired thresholds compared to controls only when pitch perception involves extracting the spectro-temporal fine structure of resolved harmonics; no significant difference was found between amusics and normal controls when the complexes contained only unresolved harmonics. Similarly, no significant difference was found between the amusic and control groups for any of the non-pitch measures of basic auditory function.
4.2 Peripheral auditory coding is intact in amusia
The normal performance observed in people with congenital amusia in all non-pitch tasks suggests that basic temporal, spectral, and intensity coding is intact in the amusic auditory system. A deficit at an early stage of auditory processing would be expected to have a general effect on all tasks that rely on any particular form of processing. The lack of a deficit for tasks not related to pitch perception suggests that the deficits observed in congenital amusia are not peripheral in nature.
4.3 Pitch deficits in congenital amusia are stimulus specific
Consistent with the existing literature on amusia, we found elevated thresholds in amusics compared to matched controls for pitch discrimination when using complex tones containing low-numbered, resolved harmonics. In contrast, for tones consisting solely of higher, unresolved harmonics, pitch discrimination by amusics was normal. This outcome is consistent with our initial hypothesis that impaired pitch perception observed in congenital amusia is due to abnormal processing of the fine spectro-temporal cues provided by resolved harmonics, whereas the mechanisms involved in extracting periodicity from the temporal envelope of sounds is spared in congenital amusia.
In the conditions involving resolved harmonics that revealed a deficit related to amusia, the size of the deficit was affected by both tone duration and the presence of transposition. These effects of parameter choices may help explain some of the variability found in the existing literature. Indeed, where some studies have shown no overlap between F0 discrimination thresholds of amusics and normal controls for stimuli comprising resolved harmonics (Ayotte et al., 2002; Peretz et al., 2003; Hyde and Peretz, 2004), others have found some amusic individuals with F0 discrimination thresholds in the range of normal performance for the same type of stimuli (Foxton et al., 2004; Tillmann et al., 2009; Liu et al., 2010). Our study suggests that introducing random variation (roving or transposition) in pitch between intervals of a discrimination task helps to separate the amusic from the normal population.
4.4 Implications for the neuro-functional origin of the pitch perception deficit in amusia
As hypothesized, our results show that the deficits in pitch perception associated with congenital amusia are related specifically to abnormal processing of the spectro-temporal fine-structure cues associated with low-order resolved harmonics. These cues are the basis for efficient pitch extraction in the general population and, taken in isolation, the results imply that the deficit may not be specific to music, but rather that it is observed in music because of the need for fine-grained pitch processing. Indeed, although initial studies suggested that the musical pitch deficits observed in amusics did not extend to the perception of pitch contours in speech (Ayotte et al., 2002), later studies that equated the degree of pitch variation in both the musical and speech tasks found similar performance deficits in both (Patel et al., 2008; Jiang et al., 2010; Jiang et al., 2012a; Thompson et al., 2012). The relative effect of amusia on speech and music is still a matter of debate; for example, pitch discrimination is found to be poorer for tone than for syllable material (Tillmann et al., 2011). Nevertheless, it is now widely accepted that the deficits associated with amusia cannot be considered exclusive to music.
The results of Experiments 2 and 3 strongly suggest normal peripheral coding of both temporal and spectral cues in amusics. The results also indicate intact processing of localization cues in the auditory brainstem – the first stage of binaural interactions, but they do not speak to pitch extraction at this level: ILDs and ITDs are believed to be extracted in the lateral and medial superior olivary complexes, respectively (Grothe et al., 2010), whereas representations of periodicity can be found in the cochlear nucleus (Winter, 2005). It thus cannot be excluded that the pitch extraction deficit found in amusia can be observed as early as the brainstem. Such differences in the frequency following response (FFR, a sustained brainstem response elicited by pitched sounds), have been reported for other cognitive deficits such as dyslexia (Hornickel and Kraus, 2013).
The most investigated hypothesis on the origin of the pitch perception deficit in amusia is currently that of a disconnection syndrome. On gross inspection, amusic brains do not exhibit neurological abnormality. However, subtle neural anomalies have been detected when a series of amusic brains are examined with fine-grained automated analyses of magnetic resonance images. Compared to controls, amusic brains show anomalous proportions of grey and white matter in the right inferior frontal gyrus (IFG; BA 47) and right auditory cortex (BA 22), suggesting the presence of cortical malformations that may have compromised the normal development of the right fronto-temporal pathways (Hyde et al., 2006; Hyde et al., 2007; Albouy et al., 2013). These reports on congenital amusia are in line with neural disconnection reports made for other cognitive disorders such as prosopagnosia (Avidan and Behrmann, 2009) or dyslexia (Vandermosten et al., 2012). It has remained unclear, however, whether these higher-level abnormalities reflected deficits that were already established at a lower level of processing. The present work excludes a peripheral origin of the deficit. A future goal is to investigate the integrity of subcortical relays on the pitch processing pathway. Towards this goal, the current study provides evidence that increasing stimulus variability (by introducing transposition or roving of the reference F0) or processing load (by decreasing the duration of the stimuli and thus increasing the presentation rate) can accentuate the differences in performance between amusic and control subjects in a pitch perception task.
4.5 Implications for the theories of pitch perception in the general population
Some researchers have proposed the existence of two distinct mechanisms for the perception of complex tones (Houtsma and Smurzynski, 1990; Shackleton and Carlyon, 1994), one for resolved harmonics and the other for unresolved harmonics. This idea is motivated by the fact that, while resolved harmonics provide fine spectro-temporal information from which the pitch of the complex can be extracted, unresolved harmonics can only be derived from the temporal envelope. The results obtained here with congenital amusics, whose perception of complex tones composed solely of unresolved harmonics is spared while they show increased thresholds for resolved tones, is consistent with the existence of two distinct mechanisms. The greater impact of short durations and transposition on resolved pitch thresholds in amusics is also consistent with the idea that amusics extract pitch from resolved complexes using a mechanism similar to that used for unresolved complex tones. It does not however completely rule out other possibilities (Gockel et al., 2004; Micheyl and Oxenham, 2004).
An alternative interpretation is that amusia is a deficit that impairs only very fine pitch discrimination (Hyde and Peretz, 2004), regardless of the stimulus configuration. When pitch discrimination is already poor, due to the availability only of the temporal envelope cues associated with unresolved harmonics, it may be that the coding “noise” associated with unresolved harmonics swamps the “noise” associated with amusia. Thus, the lack of a deficit in the amusic subjects with unresolved harmonics may just reflect the higher thresholds, rather than anything specific about the nature of unresolved harmonics. In other words, if performance is already reduced by other factors, it is not further worsened by amusia. This alternative could be tested by measuring pitch discrimination in other conditions that worsen performance, such as very short tone durations or very high frequencies (Moore, 1973; Moore and Ernst, 2012).
Highlights.
We used psychoacoustical methods to investigate pitch perception in congenital amusia
The pitch perception deficit in congenital amusia is restricted to resolved harmonics
Spectral and temporal peripheral coding is not significantly impaired in congenital amusia
The results are consistent with two mechanisms theories of pitch perception
Acknowledgments
This work was supported by a postdoctoral grant from the Fyssen Foundation, by NIH grant R01 DC005216 (AJO), grants from the Natural Sciences and Engineering Research Council of Canada, the Canadian Institutes of Health Research, and the Canada Research Chairs (IP). Collaboration was facilitated by an exchange funded by the Erasmus Mundus Auditory Cognitive Neuroscience Network.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albouy P, Mattout J, Bouet R, Maby E, Sanchez G, Aguera PE, Daligault S, Delpuech C, Bertrand O, Caclin A. Impaired pitch perception and memory in congenital amusia: the deficit starts in the auditory cortex. Brain. 2013;136:1639–1661. doi: 10.1093/brain/awt082. [DOI] [PubMed] [Google Scholar]
- Avidan G, Behrmann M. Functional MRI reveals compromised neural integrity of the face processing network in congenital prosopagnosia. Current Biology. 2009;19:1146–1150. doi: 10.1016/j.cub.2009.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayotte J, Peretz I, Hyde K. Congenital amusia: a group study of adults afflicted with a music-specific disorder. Brain. 2002;125:238–251. doi: 10.1093/brain/awf028. [DOI] [PubMed] [Google Scholar]
- Bernstein JGW, Oxenham AJ. Pitch discrimination of diotic and dichotic tone complexes: harmonic resolvability or harmonic number? The Journal of the Acoustical Society of America. 2003;113:3323–3334. doi: 10.1121/1.1572146. [DOI] [PubMed] [Google Scholar]
- Bernstein JGW, Oxenham AJ. The relationship between frequency selectivity and pitch discrimination: Sensorineural hearing loss. The Journal of the Acoustical Society of America. 2006;120:3929–3945. doi: 10.1121/1.2372452. [DOI] [PubMed] [Google Scholar]
- Binns C, Culling JF. The role of fundamental frequency contours in the perception of speech against interfering speech. The Journal of the Acoustical Society of America. 2007;122:1765–1776. doi: 10.1121/1.2751394. [DOI] [PubMed] [Google Scholar]
- Cousineau M, Demany L, Pressnitzer D. What makes a melody: The perceptual singularity of pitch sequences. The Journal of the Acoustical Society of America. 2009;126:3179–3187. doi: 10.1121/1.3257206. [DOI] [PubMed] [Google Scholar]
- Cousineau M, McDermott JH, Peretz I. The basis of musical consonance as revealed by congenital amusia. Proceedings of the National Academy of Sciences. 2012;109:19858–19863. doi: 10.1073/pnas.1207989109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai H. On the relative influence of individual harmonics on pitch judgment. The Journal of the Acoustical Society of America. 2000;107:953–959. doi: 10.1121/1.428276. [DOI] [PubMed] [Google Scholar]
- Dalla Bella S, Giguère JF, Peretz I. Singing in congenital amusia. The Journal of the Acoustical Society of America. 2009;126:414–424. doi: 10.1121/1.3132504. [DOI] [PubMed] [Google Scholar]
- Fitzsimons M, Sheahan N, Staunton H. Gender and the integration of acoustic dimensions of prosody: implications for clinical studies. Brain and language. 2001;78:94–108. doi: 10.1006/brln.2000.2448. [DOI] [PubMed] [Google Scholar]
- Foxton JM, Dean JL, Gee R, Peretz I, Griffiths TD. Characterization of deficits in pitch perception underlying ‘tone deafness’. Brain. 2004;127:801–810. doi: 10.1093/brain/awh105. [DOI] [PubMed] [Google Scholar]
- Gandour J. Tone perception in far eastern languages. Journal of Phonetics. 1983;11:149–175. [Google Scholar]
- Glasberg BR, Moore BC. Derivation of auditory filter shapes from notched-noise data. Hearing research. 1990;47:103–138. doi: 10.1016/0378-5955(90)90170-t. [DOI] [PubMed] [Google Scholar]
- Gockel H, Carlyon RP, Plack CJ. Across-frequency interference effects in fundamental frequency discrimination: questioning evidence for two pitch mechanisms. The Journal of the Acoustical Society of America. 2004;116:1092–1104. doi: 10.1121/1.1766021. [DOI] [PubMed] [Google Scholar]
- Gosselin N, Jolicœur P, Peretz I. Impaired memory for pitch in congenital amusia. Annals of the New York Academy of Sciences. 2009;1169:270–272. doi: 10.1111/j.1749-6632.2009.04762.x. [DOI] [PubMed] [Google Scholar]
- Grothe B, Pecka M, McAlpine D. Mechanisms of sound localization in mammals. Physiological Reviews. 2010;90:983–1012. doi: 10.1152/physrev.00026.2009. [DOI] [PubMed] [Google Scholar]
- Henry MJ, McAuley JD. On the prevalence of congenital amusia. Music Perception: An Interdisciplinary Journal. 2010;25:413–418. [Google Scholar]
- Henry MJ, McAuley JD. Failure to apply signal detection theory to the Montreal Battery of Evaluation of Amusia may misdiagnose amusia. Music Perception: An Interdisciplinary Journal. 2013;30:480–496. [Google Scholar]
- Hornickel J, Kraus N. Unstable representation of sound: a biological marker of dyslexia. The Journal of Neuroscience. 2013;33:3500–3504. doi: 10.1523/JNEUROSCI.4205-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houtsma AJM, Smurzynski J. Pitch Identification and Discrimination for Complex Tones with Many Harmonics. The Journal of the Acoustical Society of America. 1990;87:304–310. [Google Scholar]
- Hyde KL, Lerch JP, Zatorre RJ, Griffiths TD, Evans AC, Peretz I. Cortical thickness in congenital amusia: when less is better than more. The Journal of Neuroscience. 2007;27:13028–13032. doi: 10.1523/JNEUROSCI.3039-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde KL, Peretz I. Brains that are out of tune but in time. Psychological Science. 2004;15:356–360. doi: 10.1111/j.0956-7976.2004.00683.x. [DOI] [PubMed] [Google Scholar]
- Hyde KL, Zatorre RJ, Griffiths TD, Lerch JP, Peretz I. Morphometry of the amusic brain: a two-site study. Brain. 2006;129:2562–2570. doi: 10.1093/brain/awl204. [DOI] [PubMed] [Google Scholar]
- Jiang C, Hamm JP, Lim VK, Kirk IJ, Chen X, Yang Y. Amusia results in abnormal brain activity following inappropriate intonation during speech comprehension. PloS one. 2012a;7:e41411. doi: 10.1371/journal.pone.0041411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang C, Hamm JP, Lim VK, Kirk IJ, Yang Y. Processing melodic contour and speech intonation in congenital amusics with Mandarin Chinese. Neuropsychologia. 2010;48:2630–2639. doi: 10.1016/j.neuropsychologia.2010.05.009. [DOI] [PubMed] [Google Scholar]
- Jiang C, Hamm JP, Lim VK, Kirk IJ, Yang Y. Impaired categorical perception of lexical tones in Mandarin-speaking congenital amusics. Memory & cognition. 2012b;40:1109–1121. doi: 10.3758/s13421-012-0208-2. [DOI] [PubMed] [Google Scholar]
- Jiang C, Lim VK, Wang H, Hamm JP. Difficulties with Pitch Discrimination Influences Pitch Memory Performance: Evidence from Congenital Amusia. PloS one. 2013;8:e79216. doi: 10.1371/journal.pone.0079216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaernbach C, Bering C. Exploring the temporal mechanism involved in the pitch of unresolved harmonics. The Journal of the Acoustical Society of America. 2001;110:1039–1048. doi: 10.1121/1.1381535. [DOI] [PubMed] [Google Scholar]
- Kalmus H, Fry DB. On tune deafness (dysmelodia): frequency, development, genetics and musical background. Annals of human genetics. 1980;43:369–382. doi: 10.1111/j.1469-1809.1980.tb01571.x. [DOI] [PubMed] [Google Scholar]
- Licklider JC. A duplex theory of pitch perception. Experientia. 1951;7:128–134. doi: 10.1007/BF02156143. [DOI] [PubMed] [Google Scholar]
- Liu F, Patel AD, Fourcin A, Stewart L. Intonation processing in congenital amusia: discrimination, identification and imitation. Brain. 2010;133:1682–1693. doi: 10.1093/brain/awq089. [DOI] [PubMed] [Google Scholar]
- Meddis R, Hewitt MJ. Virtual pitch and phase sensitivity of a computer model of the auditory periphery. I: Pitch identification. The Journal of the Acoustical Society of America. 1991a;89:2866–2882. [Google Scholar]
- Meddis R, Hewitt MJ. Virtual pitch and phase sensitivity of a computer model of the auditory periphery. II: Phase sensitivity. The Journal of the Acoustical Society of America. 1991b;89:2883–2894. [Google Scholar]
- Micheyl C, Delhommeau K, Perrot X, Oxenham AJ. Influence of musical and psychoacoustical training on pitch discrimination. Hearing research. 2006;219:36–47. doi: 10.1016/j.heares.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Micheyl C, Keebler MV, Oxenham AJ. Pitch perception for mixtures of spectrally overlapping harmonic complex tones. The Journal of the Acoustical Society of America. 2010;128:257–269. doi: 10.1121/1.3372751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micheyl C, Oxenham AJ. Sequential F0 comparisons between resolved and unresolved harmonics: No evidence for translation noise between two pitch mechanisms. The Journal of the Acoustical Society of America. 2004;116:3038–3050. doi: 10.1121/1.1806825. [DOI] [PubMed] [Google Scholar]
- Miller SE, Schlauch RS, Watson PJ. The effects of fundamental frequency contour manipulations on speech intelligibility in background noise. The Journal of the Acoustical Society of America. 2010;128:435–443. doi: 10.1121/1.3397384. [DOI] [PubMed] [Google Scholar]
- Moore BCJ. Frequency difference limens for short-duration tones. The Journal of the Acoustical Society of America. 1973;54:610–619. doi: 10.1121/1.1913640. [DOI] [PubMed] [Google Scholar]
- Moore BCJ, Ernst SMA. Frequency difference limens at high frequencies: Evidence for a transition from a temporal to a place code. The Journal of the Acoustical Society of America. 2012;132:1542–1547. doi: 10.1121/1.4739444. [DOI] [PubMed] [Google Scholar]
- Moore BCJ, Glasberg BR, Peters RW. Relative dominance of individual partials in determining the pitch of complex tones. The Journal of the Acoustical Society of America. 1985;77:1853–1860. [Google Scholar]
- Moore BCJ, Gockel HE. Resolvability of components in complex tones and implications for theories of pitch perception. Hearing research. 2011;276:88–97. doi: 10.1016/j.heares.2011.01.003. [DOI] [PubMed] [Google Scholar]
- Moore BCJ, Rosen SM. Tune recognition with reduced pitch and interval information. The Quarterly journal of experimental psychology. 1979;31:229–240. doi: 10.1080/14640747908400722. [DOI] [PubMed] [Google Scholar]
- Murphy KR, Myors B. Testing the hypothesis that treatments have negligible effects: Minimum-effect tests in the general linear model. Journal of Applied Psychology. 1999;84:234–248. [Google Scholar]
- Nooteboom S. The Prosody of Speech: Melody and Rhythm. In: Hardcastel HJ, Laver H, editors. The Handbook of Phonetic Sciences. Wiley: Blackwell; 1997. pp. 640–673. [Google Scholar]
- Oxenham AJ. Pitch perception. The Journal of Neuroscience. 2012;32:13335–13338. doi: 10.1523/JNEUROSCI.3815-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxenham AJ, Shera CA. Estimates of human cochlear tuning at low levels using forward and simultaneous masking. Journal of the Association for Research in Otolaryngology. 2003;4:541–554. doi: 10.1007/s10162-002-3058-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxenham AJ, Simonson AM. Level dependence of auditory filters in nonsimultaneous masking as a function of frequency. The Journal of the Acoustical Society of America. 2006;119:444–453. doi: 10.1121/1.2141359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel AD, Peretz I, Tramo M, Labreque R. Processing prosodic and musical patterns: a neuropsychological investigation. Brain and language. 1998;61:123–144. doi: 10.1006/brln.1997.1862. [DOI] [PubMed] [Google Scholar]
- Patel AD, Wong M, Foxton JM, Lochy A, Peretz I. Speech intonation perception deficits in musical tone deafness (congenital amusia) Music Perception. 2008;25:357–368. [Google Scholar]
- Patterson RD. Auditory filter shapes derived with noise stimuli. The Journal of the Acoustical Society of America. 1976;59:640–654. doi: 10.1121/1.380914. [DOI] [PubMed] [Google Scholar]
- Peretz I, Champod AS, Hyde K. Varieties of musical disorders. The Montreal Battery of Evaluation of Amusia. Annals of the New York Academy of Sciences. 2003;999:58–75. doi: 10.1196/annals.1284.006. [DOI] [PubMed] [Google Scholar]
- Plomp R. The ear as a frequency analyzer. The Journal of the Acoustical Society of America. 1964;36:1628–1636. doi: 10.1121/1.1910894. [DOI] [PubMed] [Google Scholar]
- Plomp R. Pitch of complex tones. The Journal of the Acoustical Society of America. 1967;41:1526. doi: 10.1121/1.1910515. [DOI] [PubMed] [Google Scholar]
- Pressnitzer D, Patterson R. Distortion products and the perceived pitch of harmonic complex tones. Physiological and psychophysical bases of auditory function. 2001:97–104. [Google Scholar]
- Shackleton TM, Carlyon RP. The role of resolved and unresolved harmonics in pitch perception and frequency modulation discrimination. The Journal of the Acoustical Society of America. 1994;95:3529–3540. doi: 10.1121/1.409970. [DOI] [PubMed] [Google Scholar]
- Shera CA, Guinan JJ, Oxenham AJ. Revised estimates of human cochlear tuning from otoacoustic and behavioral measurements. Proceedings of the National Academy of Sciences. 2002;99:3318–3323. doi: 10.1073/pnas.032675099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson WF, Marin MM, Stewart L. Reduced sensitivity to emotional prosody in congenital amusia rekindles the musical protolanguage hypothesis. Proceedings of the National Academy of Sciences. 2012;109:19027–19032. doi: 10.1073/pnas.1210344109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillmann B, Rusconi E, Traube C, Butterworth B, Umiltà C, Peretz I. ine-grained pitch processing of music and speech in congenital amusia. Journal of the Acoustical Society of America. 2011;130:4089–4096. doi: 10.1121/1.3658447. [DOI] [PubMed] [Google Scholar]
- Tillmann B, Schulze K, Foxton JM. Congenital amusia: A short-term memory deficit for non-verbal, but not verbal sounds. Brain and cognition. 2009;71:259–264. doi: 10.1016/j.bandc.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Vandermosten M, Boets B, Poelmans H, Sunaert S, Wouters J, Ghesquière P. A tractography study in dyslexia: neuroanatomic correlates of orthographic, phonological and speech processing. Brain. 2012;135:935–948. doi: 10.1093/brain/awr363. [DOI] [PubMed] [Google Scholar]
- von Helmholtz H. On the sensations of tone (English translation AJ Ellis, 1885, 1954) New York: Dover; 1877. [Google Scholar]
- Vos PG, Troost JM. Ascending and descending melodic intervals: Statistical findings and their perceptual relevance. Music Perception. 1989;6:383–396. [Google Scholar]
- White LJ, Plack CJ. Temporal processing of the pitch of complex tones. The Journal of the Acoustical Society of America. 1998;103:2051–2063. doi: 10.1121/1.421352. [DOI] [PubMed] [Google Scholar]
- Williamson VJ, McDonald C, Deutsch D, Griffiths TD, Stewart L. Faster decline of pitch memory over time in congenital amusia. Advances in Cognitive Psychology. 2010;6:15–22. doi: 10.2478/v10053-008-0073-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter IM. The neurophysiology of pitch. In: Plack CJ, Oxenham AJ, Fay RR, Popper AN, editors. Pitch: Neural Coding and Perception. Springer; New York: 2005. pp. 99–146. [Google Scholar]


