Abstract
Aims
To test the efficacy and safety of varenicline as an aid to smoking cessation in methadone maintained smokers.
Design
Multicenter, randomized, double-blind, placebo-controlled trial with random assignment to 12 weeks of varenicline 1 mg twice daily (n=57) or matched placebo (n=55), with in-person and telephone counseling.
Setting
Urban methadone programmes in the Bronx, New York City, New York, USA.
Participants: Methadone maintenance patients, smoking ≥5 cigarettes/day, interested in quitting, stable in methadone treatment, without current axis I psychiatric disorders, suicidal ideation, or recent suicide attempts.
Measurements
Seven-day point prevalence abstinence verified by expired carbon monoxide (CO) < 8 p.p.m at week 12 (primary outcome); CO-verified abstinence, cigarettes/day, incident axis I psychiatric illness, suicidal ideation or serious adverse events (SAEs) at weeks 2, 4, 8, 12 or 24 (secondary outcomes).
Findings
Baseline demographic, smoking and clinical factors were similar between groups. Retention at 24 weeks was 90%. Subjects receiving varenicline were more likely than those receiving placebo to achieve abstinence (10.5% v 0%, p = .03; effect size 10.5%, 95% CI 4.4 – 19.3%) and to reduce smoking (median 5 v 2 cigarettes/day, p<.001) at 12 weeks. These effects were not maintained after drug treatment ceased. Incident psychiatric illness (OR = 0.84, 95% CI 0.16, 4.4) and suicidality (OR = 0.88, 95% CI 0.2, 3.9) were not different between groups. There were no psychiatric or cardiac SAEs.
Conclusions
Varenicline can aid short-term smoking abstinence in methadone maintained smokers.
INTRODUCTION
Opioid dependent persons have a high tobacco use prevalence, and suffer high rates of tobacco-related disease and mortality (1–6). Despite this, little is known about how to achieve smoking cessation among opioid-dependent patients receiving methadone maintenance. To date, three randomized trials have evaluated behavioral interventions in conjunction with nicotine replacement therapy in methadone maintenance patients; all demonstrated significant tobacco cessation at treatment end which was not sustained over follow-up (7–9). Two controlled trials have evaluated varenicline among methadone patients (10, 11). In one, a significant reduction in cigarettes smoked was observed, but the number of varenicline-treated patients was small (10). The other evaluated extended varenicline treatment, with low varenicline adherence and varenicline-associated abstinence at six months (11).
Reasons for the scant provision of smoking cessation alternatives in substance abuse treatment programs are multifaceted (12–16), and include limited treatment capacity, unfounded fears that smoking cessation treatment might jeopardize recovery, and particularly regarding varenicline, concerns about psychiatric risks for patients with substantial psychiatric co-morbidity. Despite an FDA boxed warning about varenicline-associated psychiatric risks, observational data among methadone patients (17) and growing research on varenicline among persons with psychiatric co-morbidity (18–23) suggest that varenicline is associated with minimal adverse psychiatric events.
Our objective in this randomized, placebo-controlled, double-blind trial was to test the efficacy and safety of varenicline for smoking cessation among opioid dependent methadone maintenance patients.
METHODS
Design Overview
Between 8/2009 and 9/2011, we conducted a phase IV, multicenter, parallel-design randomized controlled trial. Methadone maintained smokers were randomized to receive 12 weeks of varenicline 1 mg twice daily or matched placebo, in combination with in-person and telephone counseling. Research assessments occurred at baseline, and at weeks 2, 4, 8, 12 and 24. Subjects were reimbursed $15 per research visit. The protocol was approved by the Einstein Committee on Clinical Investigations. We obtained a Certificate of Confidentiality from the National Institutes on Drug Abuse to protect against disclosure of research information.
Setting and Participants
We recruited smokers enrolled in methadone maintenance treatment at three urban outpatient substance abuse treatment programs in the Bronx, NY. Eligible persons were: 18 years or older; smoking five or more cigarettes per day; interested in quitting smoking with a plan to quit in ≤ six months (Ladder of Change score 6–8); had not taken varenicline in the past 30 days; enrolled in methadone treatment for at least three months without change in dose for the prior two weeks; English speaking; able to provide informed consent; and if female, not pregnant, breastfeeding, or trying to conceive. Unstable liver, cardiac, pulmonary, renal or infectious diseases were exclusionary. Psychiatric exclusion criteria included current major depressive or manic episode, current psychotic disorder, past year suicide attempt or psychiatric hospitalization, or current suicidal ideation with plan or intent. Though we had originally planned to exclude individuals with any history of a suicide attempt or a Brief Symptom Inventory score indicating a “case” of psychiatric illness (24), we (S.N., K.R., J.A.) expanded our eligibility criteria after enrolling the first ten subjects to enhance generalizability.
Subjects were recruited by research assistants in methadone clinic waiting areas, as well as by word of mouth, posted fliers, and counseling and medical staff referral. RAs conducted a brief screening interview, then abstracted laboratory and treatment data from charts to further assess eligibility. Eligibility was confirmed only after the study physician (S.N.) completed a clinical and structured psychiatric interview using the Mini-International Neuropsychiatric Interview 6.0.0 and Columbia Suicide Severity Scale, and conducted a physical examination and urine pregnancy test (if applicable). Interviews were completed in private offices in the methadone clinics. All subjects signed written informed consent.
We based our effect size estimation on the observation that the efficacy of smoking cessation medications in methadone patients has been half that observed in general (7, 8, 25). We thus anticipated an abstinence rate at 12 weeks in the varenicline arm of 22%. We estimated a 2% abstinence rate in the placebo arm given findings that opioid dependent smokers not receiving cessation treatment quit at negligible rates (9, 26). Allowing a type 1 error of 0.05, we estimated that 50 subjects in each arm would give us 80% power to detect this difference, and we therefore recruited 112 subjects to account for loss to follow-up. Interim analyses were not performed, and did not guide recruitment decisions.
Randomization and Interventions
Subjects were randomized to receive 12 weeks of varenicline or placebo in a 1:1 ratio. Treatment group allocation was computer-generated, and stratified by the three clinic sites in blocks of six within each stratum. All subjects, research assistants, counselors, and physicians were blinded to treatment assignment. To ensure blinding, a central data manager concealed the allocation sequence using a password-protected file, assigned subjects to treatment groups, and faxed pre-printed medication orders to the study pharmacist. The pharmacist prepared the research medication by compounding varenicline tablets or placebo lactose powder to create identical-appearing capsules. The pharmacist marked medication bottles with subjects’ study ID numbers, and delivered medications for individual study subjects to clinical sites, where they were distributed to each subject by the research assistant. Varenicline subjects received standard varenicline dosing: 0.5 mg/d (days one to three), 0.5 mg twice daily (days four to seven), then 1 mg twice daily (through week 12); placebo subjects received matched capsules at an identical frequency.
All subjects set a quit date one week following treatment initiation, and were offered structured, brief (≤ 10-minute), individual, in-person counseling by a physician or masters-level tobacco treatment specialist at the baseline, two, four, eight and 12 week visits. Counseling was standardized and based on Public Health Service guidelines. Counselors completed a within-session checklist to document adherence to the counseling procedural manual. Counseling sessions were audiotaped, and a random sample of recordings were reviewed to provide feedback and monitor fidelity. All subjects were also offered referral to the New York State Quitline. This is a free, proactive quitline, which contacts smokers and provides telephone counseling.
Outcomes and Follow-up
Varenicline efficacy
Our primary outcome was carbon monoxide (CO)-validated 7-day point prevalence abstinence at 12 weeks. Our original primary outcome was defined as including both self-reported smoking abstinence and salivary cotinine ≤ 15 ng/ml (27). Because of the high rate of missing data (6 of 51 cases of self-reported abstinence not verified by cotinine, due to research assistant error, participant refusal, and test strip undersaturation), and because the semi-quantitative assay we used (Nymox NicAlert™) did not use a ≤ 15 ng/ml threshold (10 of 51 cases fell in the 10–30 ng/ml range), all authors decided to verify abstinence with carbon monoxide (CO) < 8 p.p.m. (Micro Smokerlyzer®, Bedfont Scientific) prior to unblinding and data analysis. Self-reported tobacco abstinence was measured at all visits by asking, “Have you smoked at least part of a cigarette in the past seven days, even a puff?” We chose the CO threshold of < 8 p.p.m. for comparability with other smoking cessation trials among methadone maintained smokers (7, 8). We prespecified abstinence at 12 weeks rather than long-term abstinence as our primary outcome to evaluate proximal intervention effects in a group with modest cessation success in prior studies (28, 29). Our secondary outcome measures included: 1) CO-verified 7-day point prevalence abstinence at each follow-up visit; 2) continuous abstinence between weeks 4 and 12; and 3) number of cigarettes smoked per day. In addition, we assessed interest in quitting by: 1) quit attempts lasting ≥ 24 hours; 2) confidence in quitting smoking (1–10 scale); and 3) importance of quitting smoking (1–10 scale).
Psychiatric illness and symptoms
We assessed incident psychiatric symptoms at weeks two, four, eight, 12 and 24 with: 1) the Mini-International Neuropsychiatric Interview (30), a short, structured diagnostic interview for DSM-IV Axis I disorders; 2) the Columbia Suicide Severity Rating Scale, a structured interview which assesses suicidal ideation, plans, intent, and behavior (31); and 3) the Brief Symptom Inventory [BSI, (32)] a self-report psychiatric symptom inventory. We evaluated the BSI global measure of emotional distress (the Global Severity Index), and used a T score ≥ 63 to indicate a “case” of psychiatric illness (24). Medications were discontinued at weeks two, four, or eight if participants met criteria for current major depressive or manic episode, psychotic disorder, or suicidal ideation with plan or intent.
Adverse medication effects
We evaluated adverse events at weeks two, four, eight, and 12 using both a structured questionnaire that assessed the presence of specific symptoms reported among varenicline subjects in published clinical trials, and an open-ended review of symptoms that emerged or increased in intensity following the start of study medication. Serious adverse events were defined as those which resulted in death, were life-threatening, or required inpatient hospitalization.
Statistical Analysis
For abstinence analyses, we used an intent-to-treat approach that counted subjects with missing data as smokers. We first compared the proportion of subjects in each treatment arm with biochemically validated 7-day point prevalence abstinence at the end of the intervention (week 12) using Fisher exact tests. We then assessed the association of biochemically verified abstinence at each follow-up time-point with several potential confounders, including clinical site, gender, and subjects’ baseline cocaine and hazardous alcohol use. Given that there were no significant confounders, we did not conduct multivariate analyses. In post-hoc analyses, we used GEE with exchangeable correlation to assess the interaction of abstinence and time in each group. All analyses were performed using SAS, version 9.3 (SAS Institute, Cary, NC).
Given the potential bias in counting subjects with missing data as smokers (33), we also conducted sensitivity analyses of missing abstinence data. We used SAS PROC MI to construct fully conditional specification multiple imputation models; this modeling approach is valid for monotone or arbitrary missing patterns, and can take into account uncertainties regarding the missing at random mechanism of missing data. We generated ten imputed data sets, using the following predictors: age, sex, cigarettes/day, BSI Global Severity Index score, and methadone dose. We then applied a Fisher exact test to each imputed proportion of abstinent subjects at each time point. Finally, we averaged the resulting proportions and p-values at each time point.
To evaluate differences between groups in our secondary outcomes, we used Fisher Exact or chi-square tests for binary outcomes, and Wilcoxon rank sum tests for continuous outcomes. We then used GEE models with unstructured correlation to estimate the odds of having incident psychiatric disease or suicidal ideation. We report adverse medication effects occurring in over five percent of subjects, or which had statistically significant differences between groups. Serious adverse events are reported descriptively.
RESULTS
Baseline characteristics
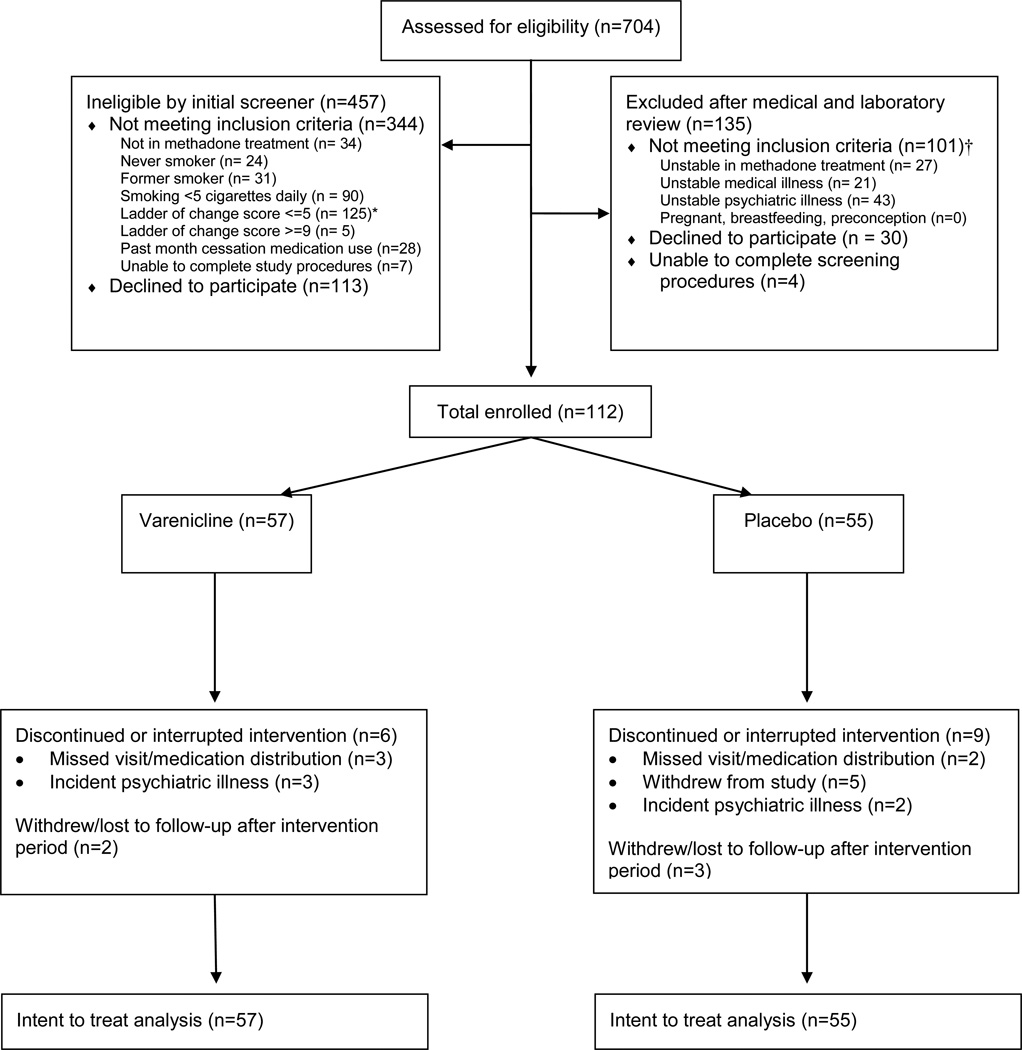
We screened 704 methadone patients, of whom 445 were ineligible, and 147 were unwilling to participate or did not complete screening procedures (Figure 1). Eligible and ineligible subjects were similar with respect to clinical site, age, interest in quitting, and past 30 day use of smoking cessation medication, however eligible subjects smoked more cigarettes per day than ineligible subjects (median 15 vs. 10, p < .0001). A total of 112 subjects were randomized, 57 to the varenicline arm and 55 to the placebo arm. Subjects completed a mean of 1.96 and 1.85 counseling sessions in the varenicline and placebo arms respectively (p = 0.7), but sessions were not normally distributed. This resulted in a marked but non-significant difference between arms, with a median of two sessions (IQR 1, 3) in the varenicline arm and one (IQR = 0, 3) in the placebo arm (p = 0.7). Ninety percent of subjects completed 24-week follow-up, and subjects in both groups completed a median of six (of six) scheduled research visits.
Figure 1.
Flow chart of participant screening, enrollment and follow-up
* Not interested in quitting smoking, or no intent to quit in next 6 months
† Participants may have been excluded for more than one reason
Subjects’ mean age was 48 years, 47% were male, 54% were Latino, and 27% were Black. They smoked a median of 15 cigarettes per day, and had low levels of nicotine dependence (median FTND score = 4). Psychiatric comorbidity was common: 21% had a lifetime history of one or more major depressive episodes. Baseline demographic, smoking and clinical factors were similar between groups (Table 1).
Table 1.
Baseline characteristics of the study population
| Varenicline (n=57) |
Placebo (n=55) |
|
|---|---|---|
| Sociodemographic characteristics | ||
| Age, mean (sd) | 48 (9) | 48 (8) |
| Male sex, n (%) | 26 (46) | 27 (49) |
| Race/ethnicity, n (%) | ||
| Hispanic | 32 (56) | 28 (51) |
| Black | 14 (25) | 17 (31) |
| Non-Hispanic White | 5 (9) | 5 (9) |
| Other | 6 (10) | 5 (9) |
| ≤ High school education, n (%) | 41 (72) | 46 (84) |
| Married or living with partner, n (%)† | 23 (40) | 31 (57) |
| Employed, n (%) | 15 (26) | 17 (31) |
| Lifetime history of incarceration, n (%) | 42 (74) | 31 (56) |
| Unstable housing, n (%)‡ | 21 (37) | 14 (25) |
| Tobacco Use Characteristics | ||
| Cigarettes/day, median (IQR) | 15 (10,20) | 15 (10,20) |
| Carbon monoxide, median (IQR) (n=107) | 10 (6,15) | 9 (7,17) |
| Carbon monoxide < 8 p.p.m., n (%) | 22 (39) | 18 (33) |
| Fagerstrom Test of Nicotine Dependence score, median (IQR) | 4 (2,6) | 4 (3,5) |
| Ladder of change score, median (IQR) | 7 (6,8) | 7 (6,8) |
| Quit importance, median (IQR) | 10 (8,10) | 10 (9,10) |
| Quit confidence, median (IQR) | 8 (5,9) | 8 (5,10) |
| Any past quit attempts, n (%) | 41 (72) | 41 (75) |
| Median duration longest prior quit attempt, weeks (IQR) (n=82) | 9 (1,30) | 4 (1,26) |
| Any other household smoker, n (%) | 34 (60) | 29 (53) |
| Psychiatric comorbidity | ||
| Lifetime major depressive episode, n (%)§ | 12 (21) | 11 (20) |
| Lifetime psychotic disorder, n (%)§ | 9 (16) | 9 (16) |
| Lifetime suicide attempt, n (%) ‖ | 9 (16) | 7 (13) |
| Severe global psychiatric symptoms, n (%)¶ | 9 (16) | 12 (22) |
| Currently receiving psychiatric treatment, n (%) | 24 (42) | 26 (47) |
| Medical comorbidity | ||
| Hypertension, n (%) | 19 (33) | 22 (40) |
| Diabetes, n (%) | 13 (23) | 11 (20) |
| COPD/Asthma, n (%) | 19 (33) | 12 (22) |
| HIV/AIDS | 11 (19) | 10 (18) |
| Substance use characteristics | ||
| Median duration methadone maintenance, years (IQR) | 4 (2,10) | 7 (3,13) |
| Median methadone dose, mg (IQR) | 110 (70,150) | 100 (65,160) |
| Self-reported use of illicit drugs in 30 days prior to baseline, n (%)** | ||
| Heroin | 8 (14) | 5 (9) |
| Other opiates† | 11 (20) | 13 (24) |
| Cocaine (including crack)† | 17 (30) | 8 (15) |
| Marijuana† | 14 (25) | 13 (24) |
| Hazardous alcohol use, n (%)†† | 4 (7) | 9 (16) |
| Clinical sites | ||
| Site 1 | 9 (16) | 7 (13) |
| Site 2 | 32 (56) | 33 (60) |
| Site 3 | 16 (28) | 15 (27) |
n=111, data missing for one participant
Living in a shelter, temporary housing, hotel/motel, or on the street
Assessed using the Mini-International Neuropsychiatric Interview 6.0.0
Assessed using the Columbia Suicide Severity Scale, a structured interview which assesses suicidal ideation, plans, intent, and behavior
Assessed using the Brief Symptom Inventory Global Severity Index, with scores dichotomized at a T score ≥ 63
Assessed using the Addiction Severity Index-Lite. No participants reported amphetamine use
Assessed using the Alcohol Use Disorder Identification Test, with hazardous alcohol use defined as a score >= 4 for women or >=8 for men
Tobacco use
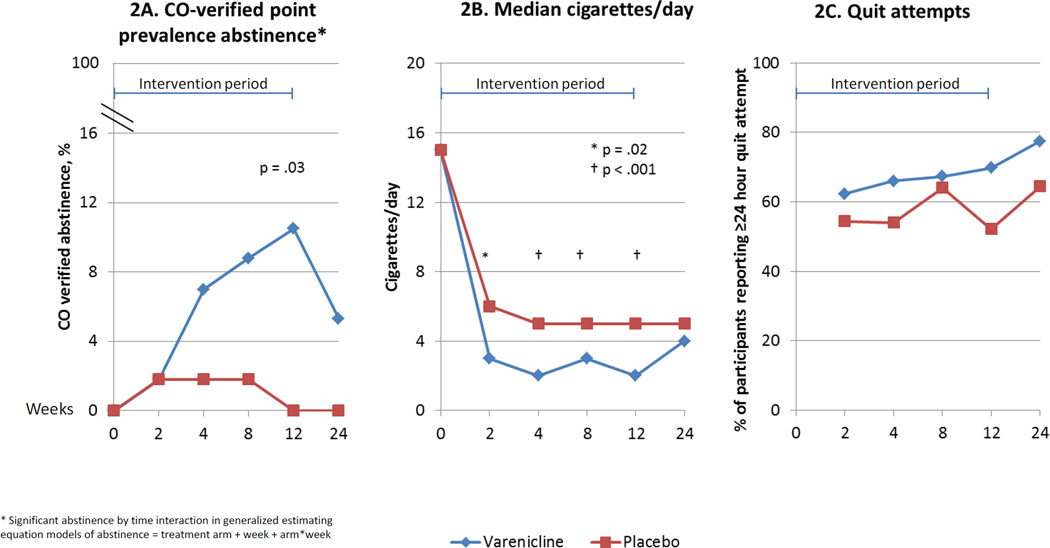
Rates of biochemically verified cessation were 0% in the placebo arm, and significantly higher in the varenicline arm at the end of 12 weeks of treatment (10.5%, 95% CI 4% – 19%; p=.03; Table 2, Figure 2a). Only two subjects, both in the varenicline arm, had continuous abstinence between weeks 4 and 12. Abstinence in the varenicline arm increased significantly over the intervention period. Estimates of cessation at 12 weeks remained significantly higher among varenicline-treated subjects when multiple imputation models were used for missing data (11.2 v 0%, p=.02). Neither clinical site nor gender was associated with abstinence. Varenicline-treated subjects also reported smoking significantly fewer cigarettes per day throughout the intervention period (Figure 2b). The differences in abstinence and smoking reduction between groups were not maintained after varenicline treatment ceased.
Table 2.
Tobacco abstinence outcomes
| Varenicline | Placebo | p value | Percentage Point Difference |
95% CI | |
|---|---|---|---|---|---|
| Seven-day point prevalence abstinence measure | n=57 | n=55 | |||
| Primary outcome: CO-verified abstinence at 12 weeks, missing = smoking, % (n)* | 10.5 (6) | 0 (0) | 0.03 | 10.5 | 4.4 – 19.3 |
| CO-verified abstinence at 12 weeks, multiple imputation for missing data, % (n)† | 11.2 (6) | 0 (0) | 0.02 | 11.2 | 4.4 – 13.6 |
| Secondary outcome: CO-verified abstinence at 24 weeks, missing = smoking, % (n)‡ | 5.3 (3) | 0 (0) | 0.24 | 5.3 | 0.9 – 11.8 |
| CO-verified abstinence at 24 weeks, multiple imputation for missing data, % (n)† | 6.8 (3.9) | 0 (0) | 0.14 | 6.7 | 0.9 – 15.4 |
Primary outcome: 7 day point prevalence abstinence at 12 w eeks, biochemically verified w ith CO<8 p.p.m., w ith missing considered smoking
Sensitivity analyses of CO-verified 7 day point prevalence abstinence outcomes using multiple imputation models with fully conditional specification for missing data
Secondary outcome: 7 day point prevalence abstinence at 24 weeks, biochemically verified with CO<8 p.p.m., with missing considered smoking
Figure 2.
Tobacco use outcomes
* Significant abstinence by time interaction in generalized estimating equation models of abstinence = treatment arm + week + arm*week
At baseline, subjects in both groups reported high levels of quit importance and confidence (median scores 10 and 8, respectively). Median quit importance and confidence scores did not change over the study period, and did not differ between groups. At each follow-up time point, the majority of subjects reported ≥24 hour quit attempts; differences between groups were not significant (Figure 2c).
Psychiatric illness and symptoms
Incident major depressive or manic episodes or psychotic disorders were infrequent, and did not differ between treatment arms (odds ratio (OR) = 1.0, 95% CI 0.4, 2.3). During the intervention period, a small number of subjects reported non-specific wishes to be dead, only one in each treatment arm had thoughts of killing themselves, and none had suicidal ideation with plan or intent. There were no differences in odds of suicidal ideation between groups (OR = 0.88, 95% CI 0.2, 3.9).
Adverse events
More varenicline- than placebo-treated subjects experienced treatment-emergent nausea (51 v 28%, p = .01) or constipation (40 v 18%, p = .01). Overall, there were high rates of gastrointestinal symptoms (including change in taste, vomiting and gas); neurologic symptoms (including change in concentration and headache); and sleep disturbance, but these were similar between groups (Table 3).
Table 3.
Participants experiencing treatment emergent adverse effects over intervention period
| Varenicline (n=57) |
Placebo (n=55) |
p value* | |
|---|---|---|---|
| Psychiatric outcomes, n (%) | |||
| Incident major depressive episode† | 2 (4) | 1 (2) | 1 |
| Incident manic episode† | 0 | 0 | NA |
| Incident psychotic disorder† | 1 (2) | 3 (6) | 0.34 |
| Suicidal ideation‡ | 3 (5) | 4 (8) | 0.71 |
| Severe global psychiatric symptoms§ | 12 (21) | 17 (33) | 0.15 |
| Medical symptoms, n (%)‖ | |||
| Change in taste | 18 (32) | 14 (25) | 0.64 |
| Dry mouth | 27 (47) | 23 (45) | 0.81 |
| Change in appetite | 29 (51) | 18 (35) | 0.1 |
| Nausea | 29 (51) | 14 (27) | 0.01 |
| Vomiting | 11 (19) | 8 (16) | 0.62 |
| Gas | 19 (33) | 15 (29) | 0.66 |
| Constipation | 23 (40) | 9 (18) | 0.01 |
| Change in concentration | 6 (11) | 6 (12) | 0.84 |
| Headache | 11 (19) | 18 (35) | 0.06 |
| Fatigue | 15 (26) | 13 (24) | 0.92 |
| Insomnia | 15 (26) | 13 (24) | 0.92 |
| Dizziness | 9 (16) | 8 (15) | 0.99 |
| Irritability | 10 (18) | 8 (15) | 0.8 |
| Vivid/more frequent dreams | 18 (32) | 22 (43) | 0.21 |
p value for difference between varenicline and placebo groups
Assessed using the Mini-International Neuropsychiatric Interview 6.0.0
Wishes to be dead, or thoughts of killing self, assessed using the Columbia Suicide Severity Scale. No cases of suicidal ideation with plan or intent.
Assessed using the Brief Symptom Inventory Global Severity Index, with scores dichotomized at a T score ≥ 63
Structured questionnaire, assessing specific symptoms reported in varenicline clinical trials, and open-ended assessment
During the intervention period, serious adverse events among varenicline-treated subjects included one hypoglycemic episode in a subject with a preceding increase in long-acting insulin dose; one hospitalization for alcohol and cocaine rehabilitation; and one total knee replacement following mechanical fall. SAEs among placebo subjects during the intervention period included one hospitalization for chest pain (with negative cardiac and pulmonary tests); and one hospitalization for alcohol detoxification. During post-intervention follow-up, varenicline subjects were hospitalized for alcohol rehabilitation, acute cholecystitis, and asthma exacerbation (two subjects); one placebo group participant was hospitalized for a total hip replacement.
DISCUSSION
In this randomized, double-blind, placebo-controlled trial of varenicline’s efficacy in methadone maintained smokers, 12 weeks of varenicline treatment was associated with a significantly higher point-prevalence tobacco abstinence rate compared to placebo. Varenicline-treated subjects also reported smoking significantly fewer cigarettes per day throughout the intervention period. These effects were not maintained after medication treatment ceased. Varenicline treatment was not associated with adverse psychiatric effects, but treatment-emergent nausea and constipation were more common.
There are two published randomized trials which have evaluated varenicline for smoking cessation among opioid dependent smokers. In one, 31 cocaine-using smokers in methadone treatment were randomized to varenicline or placebo (10); of the 13 varenicline-group subjects, two self-reported tobacco abstinence. In the other, 315 methadone maintained smokers were randomized to varenicline, nicotine patch and gum, or placebo. There was no significant varenicline effect, and CO-verified abstinence was 3.7% in the varenicline group at 6 months (11). However this was an extended (6 month) intervention, medication adherence rates were low, and whether varenicline had more proximal therapeutic effects (e.g., inducing cessation) was not described. To our knowledge, ours is the first randomized trial to demonstrate a significant difference in tobacco abstinence between varenicline- and placebo-treated methadone-maintained smokers.
The 10.5% cessation rate we observed among varenicline-treated subjects is approximately one quarter that in varenicline phase 3 clinical trials. However, this modest cessation rate is comparable to that achieved in trials using other smoking cessation medications among opioid dependent smokers. Specifically, a trial of smokers treated with nicotine patches and randomized to receive individualized motivational counseling or brief cessation counseling achieved seven day abstinence rates of 4.7 – 5.3% at six months (7). A second trial, which employed a nicotine patch platform and 2 × 2 design to test behavioral treatments found that 14% of those who received nicotine patches alone were abstinent at 12 weeks (8). In another randomized trial of nicotine patch therapy and group counseling compared to substance abuse treatment as usual, active group participants achieved cessation rates of 10–11% during treatment and 5–6% at 13- and 26-week follow-up (9). Finally, a single-arm study of the combination of bupropion, nicotine gum, and motivational counseling found that 14% of methadone-maintained smokers were abstinent at six months (25). Observational studies and standard care arms of controlled trials provide another important comparison point—without smoking cessation treatment, cessation rates among opioid dependent smokers are negligible (9, 26).
The very low cessation rates among our placebo group, despite in-person and telephone counseling, suggest that medication is an important treatment component. Because varenicline treatment with brief smoking cessation counseling can easily be implemented by medical providers in addiction treatment settings, even a relatively modest treatment effect could have a profound impact because of the high prevalence of smoking in these settings.
Novel treatment interventions, including varenicline “pretreatment” two to four weeks prior to target quit date, have been shown to enhance varenicline efficacy (34, 35). All subjects in our trial were instructed to quit one week after initiating medication, and a majority in both arms made initial quit attempts. More varenicline group subjects had biochemically verified cessation at four weeks, and their abstinence rates in later study visits continued to climb relative to placebo arm subjects. Whether further extending varenicline “pretreatment” duration increases cessation among methadone maintained smokers warrants additional investigation. Half the participants who quit relapsed at 24-week follow-up, consistent with other varenicline trials (36, 37). Extending varenicline treatment to six months among varenicline-treated smokers who have quit is associated with greater time to relapse and lower relapse rates (38), and long-term varenicline treatment may have a role in preventing relapse among opioid dependent smokers. Methadone maintenance programs in particular, because of patients’ frequent visits, close contact with counselors and health care providers, and high retention rates, may be ideal settings for extended smoking cessation interventions.
The majority of subjects in both groups reported ≥ 24 hour quit attempts, and also decreased the number of cigarettes smoked per day. These data, however, may be distorted by social desirability bias, as has been demonstrated previously among methadone maintained smokers attempting cessation (7, 11). Alternately, these findings may reflect motivation to quit and changes in participants' smoking behavior. Because smoking reduction does not appear to predict future cessation (39–41) and has only modest health benefits (42), more work is needed to determine how to leverage the health behavior change seen among those who reduce their smoking into sustained smoking cessation.
Importantly, we found that the rates of incident psychiatric illness and suicidal ideation were evenly distributed between the two groups. These data add to the growing literature supporting the safety of varenicline among patients with mental illness (18–23). A more limited evidence base supports the safety of varenicline among substance abuse treatment patients. In a cohort of 70 opioid dependent patients prescribed varenicline, few had documented adverse psychiatric events (17). In one randomized trial of varenicline among methadone-maintained smokers, there were no adverse psychiatric events observed with 12 weeks of varenicline (10). In another, 2 of 137 varenicline-group subjects stopped varenicline due to neurobehavioral effects, but rates of depression were lower among varenicline- than nicotine replacement therapy group subjects (11). Another randomized, placebo-controlled trial of varenicline among 37 cocaine-dependent smokers found a significant decline in depression intensity scores over the course of the trial in both treatment groups (43). Our finding that varenicline treatment is not associated with adverse psychiatric events removes an important barrier to its use among methadone maintenance patients.
Though our randomized trial used objective measures of cessation, and validated, structured psychiatric measures, it has limitations. Self-reported smoking reduction could not be biochemically validated. We did not analyze changes in drug use over time. Our sample size limits our power to detect rare psychiatric events. While we excluded persons with active psychiatric illness, it is possible that a broader population may safely use varenicline. Finally, our findings may not generalize to individuals with higher levels of nicotine dependence, less motivation to quit, or those who are not in substance abuse treatment. We recruited a large proportion of racial and ethnic minority smokers, and our findings should generalize to racially/ethnically diverse, urban drug treatment settings.
In summary, among methadone maintained smokers, varenicline was associated with increased smoking cessation at 12 weeks compared to placebo, which was not maintained at 24 weeks. We observed no association between varenicline and adverse psychiatric effects. Given the disproportionate burden of tobacco use and the limited efficacy of other smoking cessation treatments among substance abuse treatment patients, varenicline should be considered for substance abuse treatment patients who smoke.
Acknowledgements
The authors thank Steven Bernstein, Amanda Carter, Sarah Church, Michael Ciofoletti, Xia Ha, Joe Hecht, Moonseong Heo, Marla Keller, David Lounsbury, Xuan Li, Alain Litwin, Juan Martinez, Samantha Miller, Tanya Nahvi, Tessa Rabinowitz, Ellie Schoenbaum, Lauren Sher, Ingrid Symes, Jeremy Ann Turton, Marie Trombetta, Bryan Wu, Port Morris Pharmacy, Division of Substance Abuse staff, and the Division of General Internal Medicine Substance Abuse Affinity Group for assistance with trial administration, data management, and manuscript preparation and review.
Declarations of interest: This work was supported by the National Center for Research Resources grant UL1 RR025750 to SN, and the National Institute on Drug Abuse grants K23 DA025736 to SN, and R25 DA023021 to SN and JHA. The authors have no connection with the tobacco, alcohol, pharmaceutical or gaming industries or any body substantially funded by one of these organizations. The Albert Einstein College of Medicine Office of Biotechnology and Business Development receives funding from Pfizer, which manufactures varenicline; neither that office nor Pfizer had any role in the study design, analyses or decision to submit the manuscript for publication. None of the authors have direct or indirect funding from Pfizer.
REFERENCES
- 1.Nahvi S, Richter K, Li X, Modali L, Arnsten J. Cigarette smoking and interest in quitting in methadone maintenance patients. Addictive Behaviors. 2006;31:2127–2134. doi: 10.1016/j.addbeh.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 2.Richter KP, Gibson CA, Ahluwalia JS, Schmelzle KH. Tobacco use and quit attempts among methadone maintenance clients. American Journal of Public Health. 2001;91:296–299. doi: 10.2105/ajph.91.2.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hurt RD, Offord KP, Croghan IT, Gomez-Dahl L, Kottke TE, Morse RM, et al. Mortality following inpatient addictions treatment. Role of tobacco use in a community-based cohort. JAMA. 1996;275:1097–1103. doi: 10.1001/jama.275.14.1097. [DOI] [PubMed] [Google Scholar]
- 4.Hser YI, McCarthy WJ, Anglin MD. Tobacco Use as a Distal Predictor of Mortality among Long-Term Narcotics Addicts. Preventive Medicine. 1994;23:61–69. doi: 10.1006/pmed.1994.1009. [DOI] [PubMed] [Google Scholar]
- 5.Smyth B, Hoffman V, Fan J, Hser Y-I. Years of potential life lost among heroin addicts 33 years after treatment. Preventive Medicine. 2007;44:369–374. doi: 10.1016/j.ypmed.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. Current Cigarette Smoking Among Adults -- United States, 2011. Morbidity and mortality weekly report. 2012;61:889–894. [PubMed] [Google Scholar]
- 7.Stein MD, Weinstock MC, Herman DS, Anderson BJ, Anthony JL, Niaura R. A smoking cessation intervention for the methadone-maintained. Addiction. 2006;101:599–607. doi: 10.1111/j.1360-0443.2006.01406.x. [DOI] [PubMed] [Google Scholar]
- 8.Shoptaw S, Rotheram-Fuller E, Yang XW, Frosch D, Nahom D, Jarvik ME, et al. Smoking cessation in methadone maintenance. Addiction. 2002;97:1317–1328. doi: 10.1046/j.1360-0443.2002.00221.x. [DOI] [PubMed] [Google Scholar]
- 9.Reid MS, Fallon B, Sonne S, Flammino F, Nunes EV, Jiang H, et al. Smoking cessation treatment in community-based substance abuse rehabilitation programs. Journal of Substance Abuse Treatment. 2008;35:68–77. doi: 10.1016/j.jsat.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 10.Poling J, Rounsaville B, Gonsai K, Severino K, Sofuoglu M. The Safety and Efficacy of Varenicline in Cocaine Using Smokers Maintained on Methadone: A Pilot Study. The American Journal on Addictions. 2010;19:401–408. doi: 10.1111/j.1521-0391.2010.00066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stein MD, Caviness CM, Kurth ME, Audet D, Olson J, Anderson BJ. Varenicline for smoking cessation among methadone-maintained smokers: A randomized clinical trial. Drug and Alcohol Dependence. 2013;133:486–493. doi: 10.1016/j.drugalcdep.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Friedmann PD, Jiang L, Richter KP. Cigarette smoking cessation services in outpatient substance abuse treatment programs in the United States. Journal of Substance Abuse Treatment. 2008;34:165–172. doi: 10.1016/j.jsat.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hunt JJ, Cupertino AP, Garrett S, Friedmann PD, Richter KP. How is tobacco treatment provided during drug treatment? Journal of Substance Abuse Treatment. 2012;42:4–15. doi: 10.1016/j.jsat.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Knudsen HK, Studts JL, Boyd S, Roman PM. Structural and Cultural Barriers to the Adoption of Smoking Cessation Services in Addiction Treatment Organizations. Journal of Addictive Diseases. 2010;29:294–305. doi: 10.1080/10550887.2010.489446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fuller BE, Guydish J, Tsoh J, Reid MS, Resnick M, Zammarelli L, et al. Attitudes toward the integration of smoking cessation treatment into drug abuse clinics. Journal of Substance Abuse Treatment. 2007;32:53–60. doi: 10.1016/j.jsat.2006.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Richter KP, Choi WS, McCool RM, Harris KJ, Ahluwalia JS. Smoking Cessation Services in U.S. Methadone Maintenance Facilities. Psychiatric Services. 2004;55:1258–1264. doi: 10.1176/appi.ps.55.11.1258. [DOI] [PubMed] [Google Scholar]
- 17.Nahvi S, Wu B, Richter KP, Bernstein SL, Arnsten JH. Low incidence of adverse events following varenicline initiation among opioid dependent smokers with comorbid psychiatric illness. Drug and Alcohol Dependence. 2013;132:47–52. doi: 10.1016/j.drugalcdep.2012.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stapleton JA, Watson L, Spirling LI, Smith R, Milbrandt A, Ratcliffe M, et al. Varenicline in the routine treatment of tobacco dependence: a pre-post comparison with nicotine replacement therapy and an evaluation in those with mental illness. Addiction. 2008;103:146–154. doi: 10.1111/j.1360-0443.2007.02083.x. [DOI] [PubMed] [Google Scholar]
- 19.McClure J, Swan G, Jack L, Catz S, Zbikowski S, McAfee T, et al. Mood, Side-effects and Smoking Outcomes Among Persons With and Without Probable Lifetime Depression Taking Varenicline. Journal of General Internal Medicine. 2009;24:563–569. doi: 10.1007/s11606-009-0926-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shim JC, Jung DU, Jung SS, Seo YS, Cho DM, Lee JH, et al. Adjunctive varenicline treatment with antipsychotic medications for cognitive impairments in people with schizophrenia: a randomized double-blind placebo-controlled trial. Neuropsychopharmacology. 2012;37:660–668. doi: 10.1038/npp.2011.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Williams JM. A Randomized, Double-Blind, Placebo-Controlled Study Evaluating the Safety and Efficacy of Varenicline for Smoking Cessation in Patients With Schizophrenia or Schizoaffective Disorder. The journal of clinical psychiatry. 2012;73:654–660. doi: 10.4088/JCP.11m07522. [DOI] [PubMed] [Google Scholar]
- 22.Anthenelli RM, Morris C, Ramey TS, Dubrava SJ, Tsilkos K, Russ C, et al. Effects of Varenicline on Smoking Cessation in Adults With Stably Treated Current or Past Major DepressionA Randomized Trial. Annals of Internal Medicine. 2013;159:390–400. doi: 10.7326/0003-4819-159-6-201309170-00005. [DOI] [PubMed] [Google Scholar]
- 23.Evins AE, Cather C, Pratt SA, Pachas GN, Hoeppner SS, Goff DC. Maintenance treatment with varenicline for smoking cessation in patients with schizophrenia and bipolar disorder: A randomized clinical trial. JAMA. 2014;311:145–154. doi: 10.1001/jama.2013.285113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Derogatis LR, Spencer PM. The Brief Symptom Inventory (BSI): Administration, scoring, and procedural manual. Baltimore: John Wiley; 2006. [Google Scholar]
- 25.Richter K, McCool RM, Catley D, Hall M, Ahluwalia JS. Dual Pharmacotherapy and Motivational Interviewing for Tobacco Dependence Among Drug Treatment Patients. Journal of Addictive Diseases. 2005;24:79–90. doi: 10.1300/j069v24n04_06. [DOI] [PubMed] [Google Scholar]
- 26.Haas AL, Sorensen JL, Hall SM, Lin C, Delucchi K, Sporer K, et al. Cigarette smoking in opioid-using patients presenting for hospital-based medical services. American Journal on Addictions. 2008;17:65–69. doi: 10.1080/10550490701756112. [DOI] [PubMed] [Google Scholar]
- 27.Benowitz NL, Jacob P, Ahijevych K, Jarvis MJ, Hall S, LeHouezec J, et al. Biochemical verification of tobacco use and cessation. Nicotine & Tobacco Research. 2002;4:149–159. doi: 10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- 28.Baker T, Mermelstein R, Collins L, Piper M, Jorenby D, Smith S, et al. New Methods for Tobacco Dependence Treatment Research. Annals of Behavioral Medicine. 2011;41:192–207. doi: 10.1007/s12160-010-9252-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prochaska JJ, Delucchi K, Hall SM. A meta-analysis of smoking cessation interventions with individuals in substance abuse treatment or recovery. Journal of consulting and clinical psychology. 2004;72:1144–1156. doi: 10.1037/0022-006X.72.6.1144. [DOI] [PubMed] [Google Scholar]
- 30.Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. Journal of Clinical Psychiatry. 1998;59(Suppl 20):22–33. quiz 34–57. [PubMed] [Google Scholar]
- 31.Posner K. The Columbia–Suicide Severity Rating Scale: Initial Validity and Internal Consistency Findings From Three Multisite Studies With Adolescents and Adults. The American journal of psychiatry. 2011;168:1266–1277. doi: 10.1176/appi.ajp.2011.10111704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Derogatis LR, Melisaratos N. The Brief Symptom Inventory: an introductory report. Psychological Medicine. 1983;13:595–605. [PubMed] [Google Scholar]
- 33.Nelson DB, Partin MR, Fu SS, Joseph AM, An LC. Why assigning ongoing tobacco use is not necessarily a conservative approach to handling missing tobacco cessation outcomes. Nicotine & Tobacco Research. 2009;11:77–83. doi: 10.1093/ntr/ntn013. [DOI] [PubMed] [Google Scholar]
- 34.Hawk LW, Ashare RL, Lohnes SF, Schlienz NJ, Rhodes JD, Tiffany ST, et al. The Effects of Extended Pre-Quit Varenicline Treatment on Smoking Behavior and Short-Term Abstinence: A Randomized Clinical Trial. Clin Pharmacol Ther. 2012;91:172–180. doi: 10.1038/clpt.2011.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hajek P, McRobbie HJ, Myers KE, Stapleton J, Dhanji A. Use of varenicline for 4 weeks before quitting smoking: Decrease in ad lib smoking and increase in smoking cessation rates. Archives of Internal Medicine. 2011;171:770–777. doi: 10.1001/archinternmed.2011.138. [DOI] [PubMed] [Google Scholar]
- 36.Jorenby D, Hays JT, Rigotti NA, Azoulay S, Watsky EJ, Williams KE, et al. Efficacy of Varenicline, an alpha4beta2 Nicotinic Acetylcholine Receptor Partial Agonist, vs Placebo or Sustained-Release Bupropion for Smoking Cessation: A Randomized Controlled Trial. JAMA. 2006;296:56–63. doi: 10.1001/jama.296.1.56. [DOI] [PubMed] [Google Scholar]
- 37.Gonzales D, Rennard SI, Nides M, Oncken C, Azoulay S, Billing CB, et al. Varenicline, an alpha4beta2 Nicotinic Acetylcholine Receptor Partial Agonist, vs Sustained-Release Bupropion and Placebo for Smoking Cessation: A Randomized Controlled Trial. JAMA. 2006;296:47–55. doi: 10.1001/jama.296.1.47. [DOI] [PubMed] [Google Scholar]
- 38.Tonstad S, Tonnesen P, Hajek P, Williams KE, Billing CB, Reeves KR, et al. Effect of Maintenance Therapy With Varenicline on Smoking Cessation: A Randomized Controlled Trial. JAMA. 2006;296:64–71. doi: 10.1001/jama.296.1.64. [DOI] [PubMed] [Google Scholar]
- 39.Hughes JR, Lindgren PG, Connett JE, Nides MA. Smoking reduction in the Lung Health Study. Nicotine & Tobacco Research. 2004;6:275–280. doi: 10.1080/14622200410001676297. [DOI] [PubMed] [Google Scholar]
- 40.Hughes JR, Cummings KM, Hyland A. Ability of smokers to reduce their smoking and its association with future smoking cessation. Addiction. 1999;94:109–114. doi: 10.1046/j.1360-0443.1999.9411097.x. [DOI] [PubMed] [Google Scholar]
- 41.Cropsey KL, Jackson DO, Hale GJ, Carpenter MJ, Stitzer ML. Impact of self-initiated pre-quit smoking reduction on cessation rates: Results of a clinical trial of smoking cessation among female prisoners. Addictive Behaviors. 2011;36:73–78. doi: 10.1016/j.addbeh.2010.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pisinger C, Godtfredsen NS. Is There a Health Benefit of Reduced Tobacco Consumption? A Systematic Review. Nicotine & Tobacco Research. 2007;9:631–646. doi: 10.1080/14622200701365327. [DOI] [PubMed] [Google Scholar]
- 43.Plebani JG. Results of an initial clinical trial of varenicline for the treatment of cocaine dependence. Drug and Alcohol Dependence. 2012;121:163–166. doi: 10.1016/j.drugalcdep.2011.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]