Abstract
Sexual selection theory predicts that males in polygynous species of mammals will invest more reproductive effort in mate competition than parental investment. A corollary to this prediction is that males will mount a stress response when their access to mates is threatened. Indeed, numerous studies have shown that males exhibit elevated stress hormones, or glucocorticoids (GCs), when their access to females, or a proxy to this access like dominance rank, is challenged. In contrast, the relationship between stress hormones and paternal effort is less obvious. We report results from a study of wild male chacma baboons indicating that males experienced elevated GC levels during periods of social instability following the immigration of a dominant male. These effects were strongest in males whose mating opportunities were at greatest risk: high-ranking males and males engaged in sexual consortships. Males involved in friendships with lactating females, a form of paternal investment, also experienced high GC levels during these periods of instability. There was a tendency for males with lactating female friends to reduce their time spent in consortships during unstable periods, when the risk of infanticide was high. Thus, even in a highly polygynous mammal, males may have to balance paternal effort with mating effort. Males who invest entirely in mating effort risk losing the infants they have sired to infanticide. Males who invest in paternal care may enhance their offspring's survival, but at the cost of elevated GC levels, the risk of injury, and the loss of mating opportunities.
Introduction
Sexual selection theory predicts that males in polygynous species of mammals will invest more reproductive effort in mate competition than in parental care (Trivers 1972; Queller 1997). A corollary to this prediction is that males will mount a stress response when their access to mates is threatened. Indeed, numerous studies have shown that males exhibit elevated stress hormones, or glucocorticoids (GCs), when their access to females, or a proxy to this access like dominance rank, is challenged (Goymann and Wingfield 2004). For example, in multi-male species of primates, where there is usually a positive correlation between male rank and mating success (reviewed by e.g. Beehner et al. 2009), high-ranking males often have higher glucocorticoid levels (commonly measured by GC metabolites excreted in feces: fGCs) than low-ranking males, particularly when the dominance hierarchy is unstable (baboons, Papio hamadryas spp.: Sapolsky 1992; Bergman et al. 2005; mandrills, Mandrillus sphinx: Setchell et al. 2010; Japanese macaques, Macaca fuscata: Barrett et al. 2002; rhesus macaques, M. mulatta: Higham et al. 2013; chimpanzees, Pan troglodytes: Muller and Wrangham 2004; bonobos, Pan paniscus: Surbeck et al. 2012; but see Ostner et al. 2008 for Assamese macaques, M. assamensis). Among baboons, high-ranking males are particularly likely to exhibit high fGC levels following upheavals at the top of the male dominance hierarchy upon the arrival of a new immigrant (Sapolsky 1992; Bergman et al. 2005; Setchell et al. 2010). In one study of yellow baboons (P.h. cynocephalus), the alpha male exhibited high fGC levels even when the dominance hierarchy was stable, perhaps reflecting the stress of maintaining a rank that is subject to frequent challenges (Gesquiere et al. 2011). There is also a tendency for males to exhibit elevated fGC levels when involved in sexual consortships with oestrous females, a period when males aggressively guard their females against challenges by other males (baboons: Bergman et al. 2005; long-tailed macaques, M. fascicularis: Girard-Buttoz et al. 2014a). Thus, unpredictability and threats to mating opportunities appear to cause physiological stress in males.
In contrast, the relationship between stress hormones and paternal effort, even in species in which males routinely provide some parental care, is less obvious (Wynne-Edwards and Timonin 2007). Some studies have documented a correlation between increases in fGC levels and paternal effort (e.g. meerkats, Suricata suricatta: Carlson et al. 2006; prairie voles, Microtus ochrogaster: Bales et al. 2006), whereas in monogamous primates the pattern is less clear (e.g. marmosets, Callithrix kuhlii: Nunes et al. 2001; Cavanaugh and French 2013). Among male Barbary macaques (M. sylvanus), time spent with infants is associated with elevated fGC levels, but males’ interactions with infants appear to function primarily to facilitate social bonds among males, not protect infants (Henkel et al. 2010).
Although males in polygynous species usually invest less care in offspring than males in socially monogamous or cooperatively breeding species, male parental care is not entirely absent. Such paternal investment can be indirect – for example, when a male defends the territorial integrity of his social group – or direct, such as when a male defends offspring against predation or an infanticidal conspecific (for review, see e.g. Clutton-Brock 1991). Whatever its form, however, males who invest in offspring incur potential costs in time, effort, and the risk of injury.
Baboons exemplify the potential costs of both mating and paternal effort in a polygynous species. In these populations, males compete with one another through aggressive threat displays and escalated fights (Kitchen et al. 2003, 2005), and access to females is skewed toward dominant males (Bulger 1993; Weingrill et al. 2000; Alberts et al. 2006). However, males also show paternal care in the form of ‘friendships’ with lactating females, which function to protect infants against harassment and the threat of infanticide. Males aggressively defend infants during infanticidal attacks and sometimes incur injuries when so doing (Palombit et al. 1997, 2000; Kitchen et al. 2005). Most male friends are long-term residents who are probable or actual fathers of the infants whom they are protecting (Seyfarth 1978; Smuts 1985; Palmobit et al. 1997; Palombit et al. 2000; Weingrill 2000; Buchan et al. 2003; Clarke et al. 2009; Nguyen et al. 2009; Huchard et al. 2010; Moscovice et al. 2010; Onyango et al. 2013). Friendships appear to function primarily to reduce the threat of infanticide and harassment to females. There is currently no evidence that friendships function as a male strategy to enhance future mating opportunities (Palombit et al. 2000; Weingrill et al. 2000; Nguyen et al. 2009; Moscovice et al. 2010). Similarly, the extent to which friendships constrain males’ mating opportunities is not known.
Infanticide occurs at particularly high rates among chacma baboons (P. h. ursinus) (Cheney et al. 2004), and friendships are correspondingly strong. Both observations and playback experiments have shown that male chacma baboons respond strongly to the distress calls of their female friends and their offspring (Palombit et al. 1997; Moscovice et al. 2009). Friendships are potentially costly to males, because they increase the risk of injury during infanticidal attacks (Kitchen et al. 2005). Even in the absence of overt aggression, however, the threat of infanticide following the immigration of a dominant male may exert costs in the form of elevated stress. Lactating and pregnant females exhibit elevated fGC levels when a potentially infanticidal immigrant male enters their group, even though they themselves are at no physical risk (Beehner et al. 2005; Engh et al. 2006; Wittig et al. 2008). However, females with male friends experience significantly lower fGC levels than females without friends, perhaps reflecting the reduced threat to their infants (Beehner et al. 2005; Engh et al. 2006). By contrast, the only previous study to investigate the relation between friendship status and fGC levels in males (Bergman et al. 2005) found a non-significant trend for friends to show higher fGC levels than non-friends after the arrival of an immigrant male, suggesting a cost to paternal care. In the absence of additional data, therefore, the degree to which males in polygynous mammals incur costs when investing in offspring remains uncertain.
Here we attempt to extend previous findings using a larger data set, focusing on challenges to both mating opportunities and paternal care in male chacma baboons. Based on previous work, we predicted that instability in the male dominance hierarchy due to the arrival of a dominant immigrant male would be the primary catalyst influencing male fGC levels, because this event represents a threat both to mating effort, in the form of a loss of dominance rank, and parental effort, in the form of infanticide. Specifically, we predicted that, during unstable periods, males involved in consortships and those maintaining friendships would experience significantly higher fGC levels than uninvolved males. We also investigate whether males experience a trade-off between mating effort and parental effort, particularly during periods of instability. Finally, we attempt to replicate previous findings indicating that high-ranking males exhibit elevated fGC levels when the dominance hierarchy becomes unstable.
Methods
Data were gathered over a 32-month period (April 2003 – November 2005) as part of a long-term study of a group of wild chacma baboons living in the Moremi Game Reserve in the Okavango Delta of Botswana. The group had been observed since 1978 and all animals were fully habituated to human observers on foot. Data on maternal relatedness were available for all natal individuals. Paternity data (obtained from fecal samples; Moscovice et al. 2010) were available for some individuals, but this information was skewed toward infants that survived to weaning, since fecal samples were almost impossible to obtain from unweaned infants. As in other species of Old World monkeys, female baboons remain in their natal groups throughout their lives, while males usually immigrate to neighboring groups after approximately 9 years of age (Kitchen et al. 2003; Cheney et al. 2004). Male dominance is determined primarily by age and fighting ability (Kitchen et al. 2003; Fischer et al. 2004).
During the period of this study, the average number of adult females in the group was 26.7 (range 25-29). The number of adult males fluctuated from 4 to 12, depending on maturation, immigration, emigration, and death. Subjects for this analysis were 20 males > 8 years old. This sample included four natal males, all of whom rose to one of the top two positions in the male dominance hierarchy before emigrating (one male never emigrated). Although eight of the 20 males appeared in the previous study by Bergman et al. (2005), only one remained in the group throughout the current study. All others disappeared within the first 12 months of the current study (four within the first four months). Seven of the 20 males were observed to form friendships. All were long-term residents who had mated with their female friend during her conceptive cycle (Moscovice et al. 2010). Sixteen males were observed in consortships.
The group was followed almost daily and all adults were sampled regularly using focal animal sampling (Altmann 1974). Data on dominance ranks, consortships, and friendships were noted daily according to a common protocol (Palombit et al. 1997; Beehner et al. 2005; Bergman et al. 2005; Engh et al. 2006; see below).
Hormone Collection and Analysis
We collected 654 fecal samples from 20 males (440 samples during ‘stable’ periods and 214 during ‘unstable’ periods; see below). Because males were in the group for different lengths of time, the total number of samples from each male varied (mean N = 34, mode N = 15, range N = 6-93). However, all males were sampled at the same rate (approximately twice per month in 2003 and once per week in 2004 and 2005). The majority of samples (68%) were obtained from males not included in Bergman et al.'s (2005) previous study.
All samples were collected between 6:00am and 12:30pm. Hormones were extracted from feces in the field using methods described by Beehner and Whitten (2004; see also Beehner et al. 2005) and analyzed in the laboratory of Dr. Patricia Whitten (Emory University). All samples were assayed for fGC metabolites using a commercially available radioimmunoassay (RIA) kit. In brief, steroid hormones were extracted from about 0.5 g of the fecal sample and immediately homogenized in 10 ml of methanol/acetone solution (4:1). After 4–10 h, the samples were separated from the fecal matrix using a 0.2-mm polytetrafluoroethylene (PTFE) filter followed by solid-phase extraction. Samples were stored on solid-phase extraction cartridges (Sep-Pak Plus C18 cartridges, Waters Associates, Milford, MA) at a subzero temperature until they were shipped to Emory University. After about two weeks, we recorded the dry weight of the residual fecal matter.
In the lab, steroids were eluted from cartridges (3 ml 100% MeOH) and stored at –80 °C until the time of RIA. Samples were assayed to determine the concentration of fecal fGC (fGC) using a modification of the ImmuChemä Corticosterone 125I RIA kit (MP Biomedicals LLC, Orangeburg, NY, USA). This method has been validated for use with baboons (Beehner and Whitten, 2004) and has been used in several preceding studies of the same population (Beehner et al. 2005; Bergman et al. 2005; see Crockford et al. 2008 for more information about the analysis of the samples included here). The mean inter-assay coefficients of variation for the 245 samples collected in 2003 and the first six months of 2004 were 12.16% (high control, N =9) and 8.82% (low control, N = 9). The mean intra-assay coefficient of variation for a subset of 35 samples was 5.97%. The mean inter-assay coefficients of variation for the 409 samples collected in the latter half of 2004 and 2005 were 14.7% (high control, N =14) and 12.1% (low control, N =14). The mean intra-assay coefficient for a subset of 40 samples was 3.4%.
Methods of obtaining hormones from feces must factor in a delay between hormone secretion (i.e. circulating concentrations) and hormone excretion (i.e. fecal concentrations). In baboons, this lag time for fGCs ranges from 24 to 72 h, with peak excretion occurring at 26 h (Wasser et al. 2000). Based on this estimate, we assumed a 24-h delay between social and demographic events and fecal samples reflecting hormonal changes resulting from these events. All hormone data were log-transformed to meet the assumptions of normality. We removed outliers, defined as samples that deviated by 1.5 standard deviations from a given male's mean fGC concentration during the period (stable or unstable) from which the sample was collected. Outliers represented less than 1% of the total dataset.
Study Design
We used generalized linear mixed models (GLMM) to assess the correlation between fGC concentrations (the dependent variable)(N = 654) and five predictor variables defined below. Because we sampled the same individuals repeatedly, we used a repeated measures model with individual identity as a random factor and a heterogenous co-variance structure. Statistical analyses were conducted with R statistical software (version 2.15.0, R Foundation for Statistical Computing, R Development Core Team 2012). GLMMs were calculated using the function ‘lmer’ of the R package ‘lme4’ (R package version 0.999375e31). Non-parametric statistics were used in post hoc tests.
Predictor Variables
Stable/unstable periods
As in Bergman et al. (2005), stability was coded as a categorical variable that indicated whether the male dominance hierarchy was stable or unstable at the time that a fecal sample was gathered. Because rank changes that occurred among low-ranking individuals did not appear disruptive to the rest of the group (Bergman et al. 2005), we defined instability only in terms of the first and second male rank positions. Periods were determined to be unstable until one week after the top two ranks in the male dominance hierarchy became established. During this study, 21 months were classified as stable and 11 months as unstable.
Immigration/non-immigration periods
This was a categorical variable that indicated the recent immigration by a male who assumed one of the two top dominance rank positions. Immigration periods (N = 12 months) were defined from the date of the male's entry to two months later. All other were termed ‘non-immigration’ periods (N = 20 months).
Dominance rank
Rank was a continuous variable, where the highest-ranking male was ranked one. We determined male dominance ranks through daily observations of approach–retreat interactions (supplants), using both focal animal and ad libitum sampling (Altmann 1974). In this population, rank changes among males were unambiguous and could be determined by a single approach-retreat interaction (Kitchen et al. 2003).
Friendship status
Males were defined as having a friend if they maintained a ‘friendship’ relation (characterized by frequent grooming, approaching, close proximity, infant handling, infant defense; defined in detail in Palombit et al. 1997) with a lactating female who had an unweaned infant. All males who were observed in a friendship had consorted with their female friend during her conceptive cycle and in many cases had sired her infant (Moscovice et al. 2010).
Consort status
Consort status was a categorical variable that indicated whether a male was ‘in consort’ or ‘not in consort’ at the time of the fecal sample. Following Bergman et al. (2005), we considered a male as ‘in consort’ with a female when: (1) the female had visible signs of swelling of the perineal skin; and (2) the male both mated with and guarded the female, followed her and threatened other males that approached her). We classified a fecal sample as an ‘in consort’ sample if the male had been observed with an oestrous female one or two days before the sample collection.
Results
Instability in the male dominance hierarchy
During this study, there were three time periods when a male, or several males within a few weeks of each other, immigrated into the group and competed with resident males and other recent immigrants for the alpha rank position. As in a previous study of the same group (Bergman et al. 2005), fGC levels of all males were significantly higher during these unstable periods than during stable periods (ß = 67.766, se = 8.49, t =7.98, P =0.001). fGC levels were also significantly higher during immigration, as opposed to non-immigration, periods (ß = 37.428, se = 8.31, t = 4.50, P = 0.0001). Because all periods of rank instability were associated with male immigration and vice versa, the two measures were highly correlated (ß = 3.866, se = 0.303, z = 12.749, P < 0.0001). We therefore dropped the ‘immigration’ category from all further analyses, using only stable/unstable as a predictor in our models.
Periods of instability were marked by high rates of infanticide and attempted infanticide. During the 32-month period of this study, we recorded 11 cases of successful infanticide and four additional attacks resulting in injuries to infants. All attacks were perpetrated by males who were not in the group when the infant was conceived (and thus were extremely unlikely to be the sire). The majority (73%) occurred in the first three months following the immigration of a male who assumed the alpha rank position (X2 = 10.08, df = 1, P < 0.01). Thus, periods of instability following the immigration of a dominant male were characterized by critical threats to infants.
Perhaps because males generally experienced significantly higher fGC levels during unstable periods, we could not detect an increase in males’ fGC levels in the days following specific infanticidal attacks themselves. Of the 13 males present during one or more attacks, seven showed an average decrease in fGC levels from the week before to the week after the attack and six showed an increase. Both of the two confirmed fathers of infants in this sample showed an increase in fGC levels.
In contrast to Bergman et al. (2005), we did not find that unstable periods were marked by higher rates of aggression among males. Indeed, rates of aggression derived from focal animal sampling suggested that unstable months were marked by lower mean rates of male-male aggression than stable months (two-tailed Mann-Whitney U test, n1 =19, n2 = 8, U = 35, P < 0.05; months with fewer than 5 focal samples per male were excluded). Similarly, ad libitum records suggested little difference in the frequency of escalated fights during unstable, as opposed to stable, periods. There was also little difference between stable and unstable periods in the frequency with which males received wounds (stable: mean of 2.14 wounds among all males per month; unstable: mean of 2.36).
Interactions between stability/instability, consort status, friendship, and dominance rank
We first performed a GLMM using all predictors and all possible interactions (Table 1). We used a likelihood ratio test to compare the full GLMM against a null model that included only the random effects. The full model fit the data better than the null model (X2 = 79.06, df = 15, P < 0.001). The full model revealed significant interactions between stability/instability and both a male's friendship status and his consort status. There was also a significant interaction between a male's dominance rank and his consort status and a near-significant interaction between his dominance rank and friendship status.
Table 1.
Results of a general linear mixed model with males' fGC concentrations as the dependent variable and stable/unstable period, friendship status, consort status, and dominance rank, plus all possible interactions, as predictor variables
| Estimate (ß) | Std. Error | t value | P | |
|---|---|---|---|---|
| (Intercept) | 2.05309 | 0.04299 | 47.76 | 0.001 |
| stable | 0.06371 | 0.04932 | 1.29 | 0.197 |
| consort | 0.11806 | 0.07896 | 1.50 | 0.134 |
| friend | 0.09163 | 0.04788 | 1.91 | 0.064 |
| dom. rank | 0.10872 | 0.06533 | 1.66 | 0.097 |
| Interactions | ||||
| stable:consort | 0.29018 | 0.14035 | 2.07 | 0.040* |
| stable:friend | 0.18679 | 0.07446 | 2.51 | 0.014* |
| consort:friend | −0.11287 | 0.15319 | −0.74 | 0.461 |
| stable:dom rank | 0.01153 | 0.08230 | 0.14 | 0.835 |
| consort:dom rank | −0.21379 | 0.10604 | −2.02 | 0.049* |
| friend:dom rank | −0.16295 | 0.08334 | −1.96 | 0.053 |
| stable:consort:friend | 0.04237 | 0.28672 | 0.15 | 0.874 |
| stable:consort:dom rank | −0.24944 | 0.19023 | −1.31 | 0.193 |
| stable:friend:dom rank | −0.20302 | 0.14071 | −1.44 | 0.143 |
| consort:friend:dom rank | 0.16505 | 0.22345 | 0.74 | 0.473 |
| stable:consort:friend:dom rank | −0.49521 | 0.47342 | −1.05 | 0.291 |
Significant or near significant results are highlighted.
P < 0.05; See text for definitions
Because the full model contained a number of interactions that were not significant, we next removed these non-significant interactions and ran the model again (Table 2). Results were similar to those obtained in the original full model, revealing significant interactions between stability/instability and friendship status and between dominance rank and both friendship and consort status. Like the full model, the reduced model fit the data better than the null model (X2 = 66.39, df = 8, P < 0.001).
Table 2.
Results of a reduced generalized linear mixed model including only the significant interactions from Table 1 as predictor variables
| Estimate (ß) | Std. error | t value | P | |
|---|---|---|---|---|
| (Intercept) | 2.053 | 0.038 | 53.37 | 0.0001* |
| Stable | 0.079 | 0.026 | 3.01 | 0.003* |
| Consort | 0.147 | 0.059 | 2.50 | 0.016* |
| Friend | 0.106 | 0.044 | 2.43 | 0.018* |
| Dom Rank | 0.104 | 0.054 | 1.92 | 0.045* |
| Interactions | ||||
| Stable:Consort | 0.086 | 0.056 | 1.55 | 0.129 |
| Stable:Friend | 0.090 | 0.042 | 2.12 | 0.028* |
| Consort:Dom Rank | −0.258 | 0.078 | −3.29 | 0.001* |
| Friend:Dom Rank | −0.204 | 0.067 | −3.04 | 0.002* |
Legend as in Table 1
Consort status
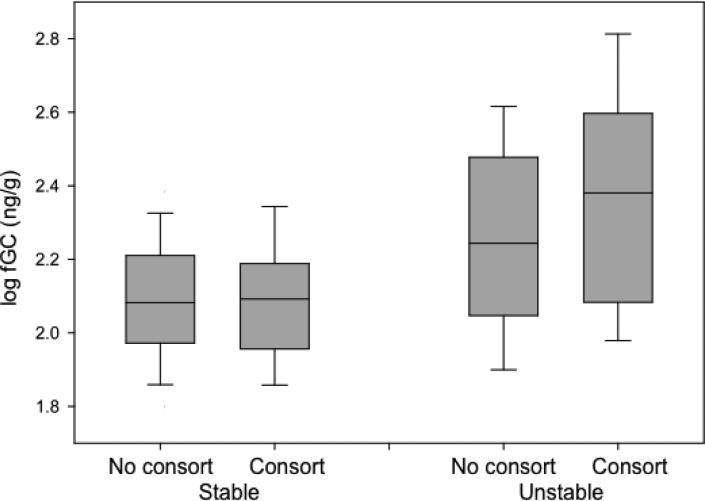
In both the full and reduced model there was an interaction between stability/instability and consort status (Tables 1 and 2), though this interaction was not significant in the reduced model. Both consorting males (N = 112, t = 2.67, P = 0.0004) and non-consorting males (N = 542, t = 5.35, P = 0.0001) experienced increases in fGC levels during unstable periods; however, consorting males experienced a greater increase than non-consorting males (13.2% versus 7.6%)(Fig. 1). There was also a significant interaction between male dominance rank and consort status (Tables 1 and 2), indicating that lower-ranking males had higher fGC levels than higher-ranking males when engaged in sexual consortships.
Fig. 1.
Fecal glucocorticoid (fGC) levels of non-consorting and consorting males during stable and unstable periods. Box plots show inter-quartile ranges and whiskers indictate 95% confidence intervals.
Friendship status
All seven males who formed one or more friendships with lactating females during this study were long-term residents who had mated with their friend during her conceptive cycle and who were often the infants’ sires (Moscovice et al. 2010; see also Palombit 2000). Most friends were relatively high-ranking, non-alpha individuals: 72% of 226 fecal samples from male friends were obtained from individuals who occupied ranks two through five. Only one alpha male was observed to form a friendship, and only after a tenure of nine months. Few friendships were formed by very low-ranking males, perhaps because they were not able to compete effectively for consortships.
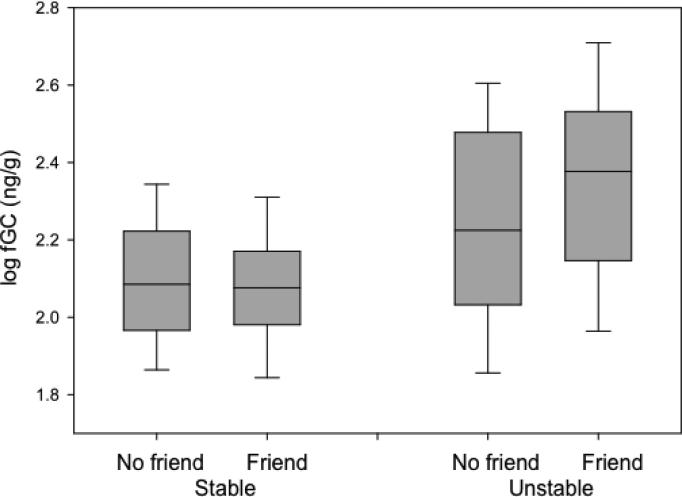
There was a highly significant interaction between stability/instability and friendship status (Tables 1 and 2)(Fig. 2). Although both males with friends (N = 227, t = 6.15, P = 0.0001) and those without friends (N = 427, t = 3.80, P = 0.0001) showed increases in fGC levels during unstable periods, males with friends showed a two-fold increase over males who did not (12.8% versus 6.2%). The significant interaction between dominance rank and friendship status (Tables 1 and 2) suggested that lower-ranking male friends tended to have higher fGC levels than higher-ranking friends.
Fig. 2.
Fecal glucocorticoid (fGC) levels of males who did or did not maintain friendships with lactating females during stable and unstable periods. Box plots show inter-quartile ranges and whiskers indicate 95% confidence intervals
We were interested in determining whether maintaining a friendship during an unstable period detracted from males’ mating opportunities. Five males were present during one or more stable and unstable periods when they both did or did not maintain friendships, allowing us to employ a within-subjects design. All five formed consortships with oestrous females while simultaneously maintaining a long-term friendship with a lactating female. To examine whether having a friendship during periods of instability might have constrained males’ ability to form consortships, we examined the proportion of days that each male was observed in a consortship while also engaged in a friendship during unstable periods as compared with stable periods. All five males spent a smaller proportion of days in consortship during unstable periods (two-tailed exact Wilcoxon signed ranks test, T = 0; P = 0.0624)(Table 3).
Table 3.
The proportion of days that males were observed in a consortship while simultaneously maintaining a friendship during stable and unstable periods
| % of days in consort while maintaining a friendship | ||
|---|---|---|
| Male | Stable periods | Unstable periods |
| BG | 100.0 | 70.8 |
| EL | 46.8 | 26.7 |
| FT | 100.0 | 62.5 |
| LO | 5.3 | 3.4 |
| NA | 60.0 | 47.4 |
Dominance rank
Although the full model did not reveal a significant interaction between stability/instability and dominance rank as predictors of fGCs (Table 1), previous studies have found that higher-ranking males experience comparatively higher fGC levels than lower-ranking males during periods of instability (Bergman et al. 2005; see also Introduction). We therefore decided to conduct a post hoc analysis of the relation between dominance rank and fGC levels during periods of stability of instability.
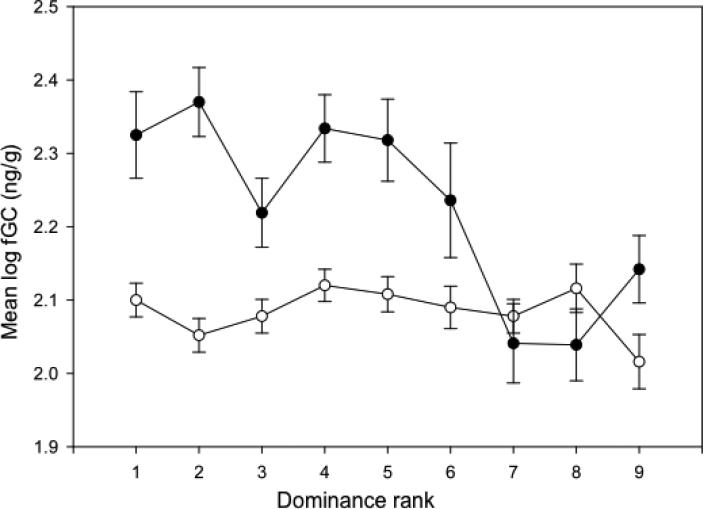
As in the previous study, there was an inverse correlation between dominance rank and fGC levels during unstable periods, with high-ranking males exhibiting higher fGC levels than low-ranking males (N = 214, t = −2.28, P = 0.025)(Fig. 3). In contrast, and again in agreement with Bergman et al. (2005), we found no relation between dominance rank and fGC levels during stable periods (N = 440, t = 0.02, P = 0.996).
Fig 3.
Mean (se) fGC levels of males of different ranks during stable (open circles) and unstable (closed circles) periods. 1= highest-ranking male
As in a previous study of yellow baboons (Gesquiere et al. 2011), the alpha male exhibited significantly higher fGC levels than the beta male, but only during stable periods (ß = −0.12074, se = 0.04401, t = −2.74, P = 0.007). During periods of instability, and in contrast to Gesquiere et al., the alpha and beta males had similarly high fGC levels (ß = 0.07125, se = 0.09460, t = 0.753, P = 0.461).
Because all high-ranking males, whether recent immigrants or not, had higher fGC levels during periods of instability, the residence status of males (recent immigrant versus longer-term resident) was not a significant predictor of fGC levels (ß = −0.071, se = 0.104, t = −0.689, P=0.535)
Discussion
Our results suggest a complex interaction between fGC concentrations and mating and parental effort in male chacma baboons. During stable periods, a male's mating success is largely determined by his ability to form consortships with oestrous females, which in turn is decided in large part by his dominance rank (Bulger 1993; Kitchen et al. 2003; Moscovice et al. 2010). Perhaps as a result, we found that lower-ranking males exhibited higher fGC levels than higher-ranking males on those occasions when they were able to obtain consortships. Instability in the top of the male dominance hierarchy, however, poses a threat to males’ mating opportunities even for high-ranking males. There was a general increase in all males’ fGC levels during unstable periods, but these increases were higher for males involved in sexual consortships.
With the exception of alpha males, whose fGC levels were higher than those of beta males, the fGC levels of high- and low-ranking males were roughly similar during periods of social stability. As in yellow baboons (Gesquiere et al. 2011), the high fGC levels demonstrated by alpha males may have reflected the stress of maintaining a rank that is subject to frequent challenges. Although Gesquiere et al. (2011) reported that the difference in fGC levels between alpha and beta males persisted even during unstable periods, this was not true in the current study. This difference may be due to fact that infanticide occurs rarely in yellow baboons (Nguyen et al. 2009), so male immigration represents little threat to current offspring.
We found an inverse correlation between male dominance rank and fGC levels during periods of instability, with high-ranking males exhibiting higher levels than lower-ranking males. This inverse correlation may have reflected the fact that high-ranking males suffered greater potential fitness costs during these periods than did low-ranking males. For immigrant males, elevated fGC levels were likely associated with the challenge of competing for and attaining alpha status. For resident males, the arrival of a dominant immigrant signaled a challenge both to their mating success (related to the potential loss of rank) and their parental success (related to the threat of infanticide). In contrast, because low-ranking males’ access to females was already restricted, upheaval in the top of the male dominance hierarchy posed little threat to their current status. These results replicate those obtained in previous studies of a number of primate species, and emphasize that threats to mating opportunities are an important source of stress for males.
Importantly, threats to males’ parental effort also had significant effects on fGC levels. During periods of instability, male friends experienced significant increases in fGC levels. The fGC levels of males with friends in these periods were roughly double those of males without friends, an increase that may reflect some of the costs that males potentially incur when they protect vulnerable infants (see also Bergman et al. 2005). Low-ranking male friends, who may be less effective at protecting infants, tended to have higher fGC levels than other males.
The increases in males’ fGC levels during periods of instability bear a striking resemblance to the increase in females’ fGC levels under the same conditions. The immigration of a potentially infanticidal male prompts significant increases in females’ fGC levels. This increase is particularly striking in lactating females, whose fitness is most at risk (Beehner et al. 2005; Engh et al. 2006; Wittig et al. 2008).
Interestingly, however, periods of instability caused the fGC levels of male and female friends to move in opposite directions. While the fGC levels of males with friends were higher than those without friends during periods of instability, the fGC levels of lactating females with friends were lower than the fGC levels of females without friends (Beehner et al. 2005; Engh et al. 2006). This difference may reflect the different costs and benefits of friendships for males and females. Although both males and females potentially derive fitness benefits through the maintenance of a friendship during periods when infants are at risk, only male friends risk serious injury when they intervene in a fight to protect an infant (see Kitchen et al. 2005).
Energy expenditure can have a strong effect on fGC levels in animals (Sapolsky 2004), and males who are challenging each other's dominance positions and access to females often engage in aggressive displays that appear to be energetically costly (Muller and Wrangham 2004). It is therefore possible that the increases in male baboons’ fGC levels observed during unstable periods were due in part to physiological stress. It is also likely, however, that these elevated fGC levels reflected the psychological stress associated with the potential loss of dominance rank and the threat of infanticide. Males’ fGC levels were generally elevated during periods of instability. Perhaps as a result, we did not detect a significant rise in males’ fGC levels in the days immediately following actual infanticide. Similarly, there was no increase in rates of male-male aggression or injury during unstable periods. Indeed, it was our impression that males often avoided aggressive encounters during periods following the immigration of a male whose fighting abilities were not yet known. A more detailed study of male aggression during a two-year period when no dominant males immigrated into the group found that, even during periods of social stability, males continued to challenge each other's rank and to fight over valuable resources (Kitchen et al. 2005). Males of all ranks compete to form and defend oestrous females (Bulger 1993; Kitchen et al. 2005). Similarly, males of all ranks form friendships with lactating females, albeit at varying frequencies, and even very low-ranking males will retaliate aggressively against high-ranking males when they attack their female friends (Palombit et al. 1997, 2000; Kitchen et al. 2005). It is also possible that mate-guarding and aggression are not as energetically costly as has sometimes been assumed. In one study of long-tailed macaques, males who engaged in mate-guarding showed no increases in levels of urinary C-peptide (a physiological indicator of energy expenditure; Deschner et al. 2008), even though they exhibited high fGC levels (Girard-Buttoz et al. 2014b).
In many socially monogamous and cooperatively breeding species, males’ mating success may be constrained by such factors as the need for bi-parental care and female-female competition. Under such conditions it may pay males to invest in current offspring, even if this comes at some expense to future mating opportunities (Maynard Smith 1977; Queller 1997; Werren et al. 1980). Our analysis suggests that this may also be true in some polygynous species. Although sample size was small, we found a tendency for males with lactating female friends to reduce their time spent in consortships during unstable periods, when the risk of infanticide was high. This reduction in time spent in consortships may have come at some cost to mating success. Thus, even in a highly polygynous mammal, males may have to balance paternal effort with mating effort. Males who invest entirely in mating effort risk losing the infants they have sired to infanticide. Males who invest in paternal care may enhance their offspring's survival, but at the cost of elevated fGC levels, the risk of injury, and the loss of mating opportunities.
Acknowledgments
We thank the Office of the President of the Republic of Botswana and the Botswana Department of Wildlife and National Parks for permission to conduct research in the Moremi Reserve. We are grateful to R. Hoffmeier, K. Seyfarth, E. Wikberg, M. Mokopi and A. Mokopi for assistance with data collection. We thank J. Beehner for comments and R. Mundry for advice on statistics. Research was supported by the National Science Foundation, the National Institutes of Health, the Leakey Foundation, the National Geographic Society and the University of Pennsylvania.
Footnotes
Ethical standards
This project complied with regulations in the Republic of Botswana (Department of Wildlife and National Parks) and in the USA (University of Pennsylvania, Animal Care and Use Committee, Protocol no.19001).
References
- Alberts SC, Buchan JC, Altmann J. Sexual selection in wild baboons: from mating opportunities to paternity success. Anim Behav. 2006;72:1177–1196. [Google Scholar]
- Altmann J. Observational study of behavior: sampling methods. Behaviour. 1974;49:229–267. doi: 10.1163/156853974x00534. [DOI] [PubMed] [Google Scholar]
- Bales KL, Kramer KM, Lewis-Reese AD, Carter CS. Effects of stress on parental care are sexually dimorphic in prairie voles. Physiol Behav. 2006;87:424–429. doi: 10.1016/j.physbeh.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Barrett GM, Shimizu K, Bardi M, Asaba S, Mori A. Endocrine correlates of rank, reproduction, and female-directed aggression in male Japanese macaques (Macaca fuscata). Horm Behav. 2002;42:85–96. doi: 10.1006/hbeh.2002.1804. [DOI] [PubMed] [Google Scholar]
- Beehner JC, Bergman TJ, Cheney DL, Seyfarth RM, Whitten PL. The effect of new alpha males on female stress in free-ranging baboons. Anim Behav. 2005;69:1211–1221. [Google Scholar]
- Beehner JC, Gesquiere L, Seyfarth RM, Cheney DL, Alberts SC, Altmann J. Testosterone related to age and life-history stages in male baboons and geladas. Horm Behav. 2009;56:472–480. doi: 10.1016/j.yhbeh.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beehner JC, Whitten PL. Modifications of a field method for faecal steroid analysis in baboons. Physiol Behav. 2004;82:269–277. doi: 10.1016/j.physbeh.2004.03.012. [DOI] [PubMed] [Google Scholar]
- Bergman TJ, Beehner JC, Cheney DL, Seyfarth RM, Whitten PL. Correlates of stress in free-ranging male chacma baboons. Anim Behav. 2005;70:703–713. [Google Scholar]
- Buchan J, Alberts SC, Silk JB, Altmann J. True paternal care in a multi-male primate society. Nature. 2003;425:179–181. doi: 10.1038/nature01866. [DOI] [PubMed] [Google Scholar]
- Bulger JB. Dominance rank and access to estrous females in male savanna baboons. Behaviour. 1993;127:67–103. [Google Scholar]
- Carlson AA, Manser MB, Young AJ, Russell AJ, Jordan NR, McNeilly AS, Clutton-Brock T. Cortisol levels are positively associated with pup-feeding rates in male meerkats. Proc R Soc Lond B. 2006;273:571–577. doi: 10.1098/rspb.2005.3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanaugh J, French JA. Post-partum variation in the expression of paternal care is unrelated to urinary steroid metabolites in marmoset fathers. Horm Behav. 2013;63:551–558. doi: 10.1016/j.yhbeh.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheney DL, Seyfarth RM, Fischer J, Beehner J Bergman T, Johnson SE, Kitchen DM, Palombit RA, Rendall D, Silk JB. Factors affecting reproduction and mortality among baboons in the Okavango Delta, Botswana. Int J Primatol. 2004;25:401–428. [Google Scholar]
- Clarke PMR, Henzi SP, Barrett L. Sexual conflict in chacma baboons (Papio hamadryas ursinus): absent males select for proactive females. Anim Behav. 2009;77:1217–1225. [Google Scholar]
- Clutton-Brock TH. The evolution of parental care. Princeton University Press; Princeton: 1991. [Google Scholar]
- Crockford C, Wittig RM, Whitten PL, Seyfarth RM, Cheney DL. Social stressors and coping mechanisms in wild female baboons (Papio hamadryas ursinus). Horm Behav. 2008;53:254–265. doi: 10.1016/j.yhbeh.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Deschner T, Kratzsch J, Hohmann G. Urinary C-peptide as a method for monitoring body mass changes in captive bonobos (Pan paniscus). Horm Behav. 2008;54:620–626. doi: 10.1016/j.yhbeh.2008.06.005. [DOI] [PubMed] [Google Scholar]
- Engh AE, Beehner JC, Bergman TJ, Whitten PL, Hoffmeier RR, Seyfarth RM, Cheney DL. Female hierarchy instability, male immigration, and infanticide increase glucocorticoid levels in female chacma baboons. Anim Behav. 2006;71:1227–1237. [Google Scholar]
- Fischer J, Kitchen DM, Seyfarth RM, Cheney DL. Baboon loud calls advertise male quality: Acoustic features and their relation to rank, age, and exhaustion. Behav Ecol Sociobiol. 2004;56:140–148. [Google Scholar]
- Gesquiere LR, Learn NH, Simao CM, Onyango PO, Alberts SC, Altmann J. Life at the top: rank and stress in wild male baboons. Science. 2011;333:357–360. doi: 10.1126/science.1207120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard-Buttoz C, Heistermann M, Rahmi E, Marzec A, Agil M, Fauzan PA, Engelhardt A. Costs of mate-guarding in wild male long-tailed macaques (Macaca fascicularis): physiological stress and aggression. Horm Behav. 2014a;66:637–648. doi: 10.1016/j.yhbeh.2014.09.003. [DOI] [PubMed] [Google Scholar]
- Girard-Buttoz C, Heistermann M, Rahmi E, Marzec A, Agil M, Fauzan PA, Engelhardt A. Mate-guarding constrains feeding activity but not energetic status of wild male long-tailed macaques (Macaca fascicularis). Behav Ecol Sociobiol. 2014b;68:583–595. doi: 10.1007/s00265-013-1673-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goymann W, Wingfield JC. Allostatic load, social status, and stress hormones - the costs of social status matter. Anim Behav. 2004;67:591–602. [Google Scholar]
- Henkel S, Heistermann M, Fischer J. Infants as costly social tools in male Barbary macaque networks. Anim Behav. 2010;79:1199–1206. [Google Scholar]
- Higham JP, Heistermann M, Maestripieri D. The endocrinology of male rhesus macaque social and reproductive status: a test of the challenge and social stress hypotheses. Behav Ecol Sociobiol. 2013;67:19–30. doi: 10.1007/s00265-012-1420-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huchard E, Alvergne A, Fejan D, Knapp LA, Cowlishaw G. More than friends? Behavioural and genetic aspects of heterosexual associations in wild chacma baboons. Behav Ecol Sociobiol. 2010;64:769–781. [Google Scholar]
- Kitchen DM, Cheney DL, Seyfarth RM. Contextual factors mediating contests between male chacma baboons in Botswana: the effect of food, friends, females. Int J Primatol. 2005;26:105–125. [Google Scholar]
- Kitchen DM, Seyfarth RM, Fischer J, Cheney DL. Loud calls as an indicator of dominance in male baboons, Papio cynocephalus ursinus. Behav Ecol Sociobiol. 2003;53:374–384. [Google Scholar]
- Maynard Smith J. Parental investment: a prospective analysis. Anim Behav. 1977;25:1–9. [Google Scholar]
- Moscovice LR, Heesen M, Di Fiore A, Seyfarth RM, Cheney DL. Paternity alone does not predict long-term investment in juveniles by male baboons. Behav Ecol Sociobiol. 2009;63:1471–1482. doi: 10.1007/s00265-009-0781-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscovice LR, DiFiore A, Crockford C, Kitchen DW, Wittig R, Seyfarth RM, Cheney DL. Hedging their bets? Male and female chacma baboons form friendships based on likelihood of paternity. Anim Behav. 2010;79:1007–1015. [Google Scholar]
- Muller MN, Wrangham RW. Dominance, cortisol and stress in wild chimpanzees (Pan troglodytes schweinfurthii). Behav Ecol Sociobiol. 2004;55:332–340. doi: 10.1007/s00265-020-02872-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen N, Van Horn RC, Alberts SC, Altmann J. “Friendships” between new mothers and adult males: adaptive benefits and determinants in wild baboons (Papio cynocephalus). Behav Ecol Sociobiol. 2009;63:1331–1344. doi: 10.1007/s00265-009-0786-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes S, Fite JE, Patera KJ, French JA. Interactions among paternal behavior, steroid hormones, and parental experience in male marmosets (Callithrix kuhlii). Horm Behav. 2001;39:70–78. doi: 10.1006/hbeh.2000.1631. [DOI] [PubMed] [Google Scholar]
- Onyango PO, Gesquiere LR, Altmann J, Alberts SC. Testosterone positively associated with both male mating effort and paternal behavior in savanna baboons (Papio cynocephalus). Horm Behav. 2013;63:430–436. doi: 10.1016/j.yhbeh.2012.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostner J, Heistermann M, Schulke O. Dominance, aggression and physiological stress in wild male Assamese macaques (Macaca assamensis). Horm Behav. 2008;54:613–619. doi: 10.1016/j.yhbeh.2008.05.020. [DOI] [PubMed] [Google Scholar]
- Palombit RA, Cheney D, Seyfarth R, Rendall D, Silk J, Johnson S, Fischer J. Male infanticide and defense of infants in chacma baboons. In: van Schaik CP, Janson CH, editors. Infanticide by males and its implications. Cambridge University Press; Cambridge: 2000. pp. 123–152. [Google Scholar]
- Palombit RA, Seyfarth RM, Cheney DL. The adaptive value of “friendships” to female baboons: experimental and observational evidence. Anim Behav. 1997;54:599–614. doi: 10.1006/anbe.1996.0457. [DOI] [PubMed] [Google Scholar]
- Queller D. Why do females care more than males? Proc R Soc Lond B. 1997;264:1555–1557. [Google Scholar]
- Sapolsky RM. Cortisol concentrations and the social significance of rank instability among wild baboons. Psychoneuroendocrinology. 1992;17:701–709. doi: 10.1016/0306-4530(92)90029-7. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Why zebras don't get ulcers. 3rd edition WH Freeman; New York: 2004. [Google Scholar]
- Setchell JM, Smite TE, Wickings J, Knapp LA. Stress, social behaviour, and secondary sexual traits in a male primate. Horm Behav. 2010;58:720–728. doi: 10.1016/j.yhbeh.2010.07.004. [DOI] [PubMed] [Google Scholar]
- Seyfarth RM. Social relationships among adult male and female baboons: II behaviour throughout the female reproductive cycle. Behaviour. 1978;64:227–247. [Google Scholar]
- Smuts BB. Sex and friendship in baboons. Aldine; New York: 1985. [Google Scholar]
- Surbeck M, Deschner T, Weltring A, Hohmann G. Social correlates of variation in urinary cortisol in wild male bonobos, (Pan paniscus). Horm Behav. 2012;62:27–35. doi: 10.1016/j.yhbeh.2012.04.013. [DOI] [PubMed] [Google Scholar]
- Trivers RL. Parental investment and sexual selection. In: Campbell B, editor. Sexual selection and the descent of man, 1871-1971. Aldine; Chicago: 1972. pp. 136–179. [Google Scholar]
- Wasser S, Hunt K, Brown J, Cooper K, Crockett C, Bechert U, Millspaugh J, Larson S, Monfort S. A generalized fecal glucocorticoid assay for use in a diverse array of nondomestic mammalian and avian species. Gen Comp Endocrinol. 2000;120:260–275. doi: 10.1006/gcen.2000.7557. [DOI] [PubMed] [Google Scholar]
- Weingrill T. Infanticide and the value of male–female relationships in mountain chacma baboons. Behaviour. 2000;137:337–359. [Google Scholar]
- Weingrill T, Lycett JE, Henzi SP. Consortship and mating success in chacma baboons (Papio cynocephalus ursinus). Ethology. 2000;106:1033–1044. [Google Scholar]
- Werren JH, Gross MR, Shine R. Paternity and the evolution of male parental care. J Theor Biol. 1980;82:619–631. doi: 10.1016/0022-5193(80)90182-4. [DOI] [PubMed] [Google Scholar]
- Wittig RM, Crockford C, Lehmann J, Whitten PL, Seyfarth RM, Cheney DL. Focused grooming networks and stress alleviation in wild female baboons. Horm Behav. 2008;54:170–177. doi: 10.1016/j.yhbeh.2008.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynne-Edwards KE, Timonin ME. Paternal care in rodents: weakening support for hormonal regulation of the transition to behavioral fatherhood in rodent animal models of biparental care. Horm Behav. 2007;52:114–121. doi: 10.1016/j.yhbeh.2007.03.018. [DOI] [PubMed] [Google Scholar]