Abstract
Rats with chronic inhibition of GABA synthesis by infusion of l-allyglycine, a glutamic acid decarboxylase inhibitor, into their dorsomedial/perifornical hypothalamus are anxious and exhibit panic-like cardio-respiratory responses to treatment with intravenous (i.v.) sodium lactate (NaLac) infusions, in a manner similar to what occurs in patients with panic disorder. We previously showed that either NMDA receptor antagonists or metabotropic glutamate receptor type 2/3 receptor agonists can block such a NaLac response, suggesting that a glutamate mechanism is contributing to this panic-like state. Using this animal model of panic, we tested the efficacy of CBiPES and THIIC, which are selective group II metabotropic glutamate type 2 receptor allosteric potentiators (at 10–30mg/kg i.p.), in preventing NaLac-induced panic-like behavioral and cardiovascular responses. The positive control was alprazolam (3mg/kg i.p.), a clinically effective anti-panic benzodiazepine. As predicted, panic-prone rats given a NaLac challenge displayed NaLac-induced panic-like cardiovascular (i.e. tachycardia and hypertensive) responses and “anxiety” (i.e. decreased social interaction time) and “flight” (i.e. increased locomotion) -associated behaviors; however, systemic injection of the panic-prone rats with CBiPES, THIIC or alprazolam prior to the NaLac dose blocked all NaLac-induced panic-like behaviors and cardiovascular responses. These data suggested that in a rat animal model, selective group II metabotropic glutamate type 2 receptor allosteric potentiators show an anti-panic efficacy similar to alprazolam.
Keywords: CBiPES, THIIC, panic, anxiety, alprazolam, glutamate receptor, GABA, hypothalamus, social interaction, stress, pre-clinical study, sodium lactate, tachycardia, hypertension, anti-panic drugs
Introduction
Panic disorder is a severe anxiety disorder characterized by recurring “spontaneous” panic attacks (Weissman and Merikangas, 1986; Kessler et al., 1994; Hirschfeld, 1996; Katon et al., 2002) that, although considered spontaneous, can be consistently provoked in the majority of panic disorder patients by using mild interoceptive stimuli such as intravenous (i.v.) infusions of 0.5M sodium lactate (NaLac) (Liebowitz et al., 1984) or inhalation of 7% CO2 (Gorman et al., 1994) as challenges. This suggests that the threshold for some interoceptive stimuli to be able to induce a panic response is reduced in patients with panic disorder, which has led to the hypothesis that the initial pathology in these patients appears to be an alteration somewhere in the central neural pathways that are critical sites for responding to disruptive interoceptive stimuli and regulating normal panic responses.
Although a number of limbic and autonomic regulatory centers have been implicated in the pathophysiology of panic disorder (Gorman et al., 2000; Shekhar et al., 2003), the dorsomedial hypothalamus (DMH) is one such panic-related site, which not only coordinates autonomic and behavioral responses for a variety of homeostatic mechanisms (Bernardis and Bellinger, 1998; DiMicco et al., 2002; Chou et al., 2003), but is also an important efferent target of circumventricular organs (CVOs) that are critical for sensing changes in interoceptive-associated plasma parameters such as NaLac (Shekhar and Keim, 1997; Molosh et al., 2010). In light of this, Shekhar and colleagues developed an animal model of panic disorder, where chronically disrupting the inhibitory GABAergic activity (where GABA stands for gamma-aminobutyric acid) by infusion of the GABA synthesis inhibitor l-allylglycine (l–AG) into the dorsomedial/perifornical hypothalamus (DMH/PeF) produces rats that have heightened anxiety and display panic-like responses following exposure to i.v. infusions of 0.5 M NaLac (Shekhar et al., 1996; Shekhar and Keim, 1997; Johnson and Shekhar, 2006; Johnson et al., 2008; Johnson et al., 2010). Consistent with this, panic disorder patients do demonstrate deficits in central GABA concentrations (Goddard et al., 2001) and a hyperactive hypothalamus during anticipatory anxiety (Boshuisen et al., 2002). Additionally, benzodiazepine agonists (which enhance GABA activity) attenuate NaLac-induced panic attacks in panic patients (Pohl et al., 1994), as well as NaLac-induced panic-like responses in panic-prone rats (Shekhar and Keim, 2000; Johnson et al., 2010). Because they are rapid and effective, benzodiazepines have been the most widely used class of drugs for the treatment of acute anxiety since the 1960s, yet there are significant and common side effects, such as sedation, memory impairment and dependence (Nutt et al., 2002; Baldwin et al., 2005; Bandelow et al., 2008; Cloos and Ferreira, 2009); therefore, there is a great need for newer, rapidly effective anxiolytic agents without the typical benzodiazepine side effects.
Recent evidence suggests that there is a balance between tonic GABAergic inhibition and the glutamate-mediated excitation within the DMH/PeF that makes rats vulnerable to NaLac-induced panic responses (Shekhar and Keim, 2000; Johnson and Shekhar, 2006). Under basal conditions, the activity of DMH neurons is regulated by a tonic GABAergic inhibition of the excitatory glutamatergic drive (Soltis and DiMicco, 1991). However, when the GABAergic inhibitory tone is reduced with l-AG infusions, the glutamate-mediated excitation appears to become prominent, leading to chronic activation and a panic-prone state (Johnson and Shekhar, 2006). In light of this, there is increasing recognition that glutamatergic mechanisms may offer a potential avenue for developing new treatment for anxiety problems such as panic disorder (Gorman, 2003).
In one review, the two Group II metabotropic glutamate receptors (mGluR2/3) are shown to be activated by excess synaptic glutamate (Nicoletti et al., 2010), while conversely, they negatively modulate presynaptic glutamate neurotransmission (Pin and Duvoisin, 1995). This suggests that mGluR2/3 potentiators could possibly reduce excessive synaptic glutamate release in panic-prone rats, to reduce panic vulnerability without altering basal glutamate release (Johnson et al., 2005). Consistent with this hypothesis, mGluR2/3 agonists do reduce anxiety-associated behaviors in rodents (Monn et al., 1997; Helton et al., 1998; Shekhar and Keim, 2000; Walker and Davis, 2002) and also have anxiolytic properties in humans (Grillon, 2003; Schoepp et al., 2003) without any of the benzodiazepine-associated side effects, such as sedation (Schoepp et al., 2003). Anxiolytic-like properties have also been observed for several mGluR2 potentiators, including CBiPES (N-(4′-cyano-biphenyl-3-yl)-N-(3-pyridinylmethyl)-(ethanesulfonamide hydrochloride)), which readily reverses stress-induced changes in body temperature (Johnson et al., 2005). Recently, we found evidence of the antidepressant and anxiolytic-like effects of the mGluR2 potentiator designated as THIIC (having the formula N-(4-{[3-hydroxy-4-(2-methylpropanoyl)-2-(trifluoromethyl)phenoxy]methyl}benzyl)-1-methyl-1H-imidazole-4-carboxamide), including the reversal of stress-induced hyperthermia and in decreased marble-burying behavior and efficacy, in the mouse forced swim test and DRL–72 test in the rat, respectively (Fell et al., 2011). Data generated with mGluR2 knockout mice clearly suggest that these effects are mediated via the mGluR2 receptor. Also, in vitro data indicate that mGlu2R2 potentiators suppress glutamate release under conditions of enhanced, but not normal, glutamate release (Johnson et al., 2005) indicating that this kind of therapy could be an advantageous approach to stabilizing glutamatergic tone with reduced side effect liability. To further test our excessive glutamate-based hypothesis, in this study panic-prone rats were pretreated with either vehicle, the two mGlu2 potentiators (CBiPES and THIIC), or the benzodiazepine alprazolam prior to exerting a panic provocation with NaLac.
Methods and materials
Animals and housing conditions
All experiments were conducted on adult male Sprague-Dawley rats (300–350 g), which were purchased from Harlan Laboratories and were housed individually, in plastic cages under standard environmental conditions (22 °C; 12/12 light/dark cycle; lights on at 7:00 am) for 7–10 days prior to the surgical manipulations. Food and water were provided ad libitum. Animal care procedures were conducted in accordance with the NIH Guidelines for the Care and Use of Laboratory Animals (US National Institute of Health Publication #80–23), revised in 1996, and the guidelines of the IUPUI Institutional Animal Care and Use Committee.
Surgical procedures, cardiovascular and locomotor activity assessment, and osmotic minipump infusions
Prior to and during surgery, rats were anesthetized with a nose cone connected to an isoflurane system (MGX Research Machine; Vetamic, Rossville, IN). Rats were surgically fitted with femoral arterial catheters for measurement of mean arterial blood pressure (MAP) and heart rate (HR), and with femoral venous catheters for i.v. infusions, as previously described (Shekhar et al., 1996).
Cardiovascular responses (i.e. MAP and HR) were measured by a femoral arterial line connected to a telemetric probe, which contained a pressure transducer (Cat. no. C50–PXT, Data Science International (DSI), St. Paul, MN). The C50–PXT probes also assess general motor activity. DSI Dataquest software was used to monitor and record MAP, HR and general motor activity, which was recorded continuously in the freely-moving conscious rats and were expressed as a 20-min time course. Data reported for each rat are: changes in MAP, HR and motor activity from the average of the baseline (5 min prior to the NaLac infusion, from t = −5 min to t = −1 min).
After 3 days of recovery, the rats were tested for baseline cardiovascular responses to lactate (see below). Following baseline testing, rats were anesthetized as stated previously and 26 gauge T-shaped cannulae (Cat. no. 3260PG, Plastics One Inc., Roanoake, VA) were directed at the cardio-excitatory regions of the DMH/PeF (Samuels et al., 2004), based on coordinates (from bregma: 1.2 mm posterior, +2.1 mm lateral, +9.1 mm ventral; adjusted for approaching at a 10 degree angle toward the midline, with the stereotaxic incisor bar elevated 5 mm above the interaural line) and cemented into place, as described previously (Shekhar et al., 1996). The 22-gauge side arm was then attached, using PE–60 tubing, to an osmotic minipump (prefilled with l–AG solution) and sutured into place subcutaneously, at the nape of the neck (DURECT Corporation, Model no. 2002). The concentration of the solutions was such that 3.5 nmol/0.5µl per hour of l–AG was continuously infused into the DMH/PeF region, for the remainder of the given experiment.
Previous studies determined that the dose of l–AG that we utilized here reduces local GABA concentrations by approximately 60%, following unilateral infusions (Abshire et al., 1988; Shekhar et al., 1996; Shekhar and Keim, 1997; Shekhar et al., 2006), and was supported by immunohistochemistry (Johnson and Shekhar, 2006) and increased anxiety-like behavior (i.e. as measured by the social interaction (SI) test and elevated plus-maze (EPM)), without increasing the cardiorespiratory responses (Shekhar et al., 2006; Johnson et al., 2008).
Social interaction test to measure anxiety responses
The social interaction (SI) test is a fully validated test of experimental anxiety-like behavior in rats (File, 1980) and the procedure, as used in our laboratory, has been described previously (Sanders and Shekhar, 1995; Shekhar and Katner, 1995). The apparatus itself consists of a solid wooden box with an open roof approximately 0.9 m long × 0.9 m wide, with walls 0.3 m high. All behavioral tests are videotaped by a camera located above the box. The “experimental” rat and an unfamiliar “partner” rat are both allowed to individually habituate to the box for a 5-min period, 24 h prior to each SI test. During the SI test, the two rats are placed together in the center of the box, so that the total duration (in seconds) of non-aggressive physical contact (grooming, sniffing, crawling over and under, etc.) initiated by the “experimental” rat is quantified over a 5-min period. A baseline SI test was performed 72+ h after i.v. catheterization, but prior to osmotic minipump implantation. Another SI test was performed 5 days following minipump infusions, and also immediately following either saline control or sodium lactate infusions. Videotaped sessions were scored at a later time (by SDF), whom was blind to any drug treatment.
Experiment 1: Effects on 1–AG infusion into the DMH/PeF on the local tissue concentrations of GABA and glutamate
Adult male rats were made panic-prone by the 5-day unilateral infusions of l–AG into the DMH/PeF, as described above. On day 5, rats were anaesthetized with isoflurane and then decapitated; their brains were removed and frozen in isopentane that was pre-cooled (−50°C) on dry ice; then sectioned coronally at 300 µm thickness on a cryostat, placed on glass slides that were pre-cooled on dry ice prior to the micropunching of tissue from the hypothalamus. Next, the DMH tissue was dissected with a Microtome tissue micropunch (inside diameter = 0.51mm) from the ipsilateral and contralateral DMH, for use in high performance liquid chromotography (HPLC) analysis of the GABA concentrations in order to determine the effectiveness of the l–AG pumps, and of glutamate to determine if this 1–AG pre-treatment led to an increase in glutamate concentrations. Once micropunched, the remaining tissue samples were frozen at −70°C.
GABA analysis
During extraction, 500 µl of 0.5M perchloric acid was added to each sample and the samples were left to stand at 4°C for at least 24 h. Samples were then sonicated on ice for 15 sec and centrifuged at 14,000 × g for 10 min using a microfuge. The supernatant was decanted and alkalized with 36 µl of 10 M NaOH, briefly vortexed, and stored at 4°C until assayed for GABA content. GABA content in the samples was measured by HPLC with electrochemical detection following o-phthalaldehyde/sulfite derivatization (Bourdelais and Kalivas, 1991). Briefly, samples were loaded into an autosampler and 20 µl of sample was mixed with 20 µl of derivatizing reagent. After a period for a 5 min reaction, samples were automatically injected onto a C–18 column (cat. no. HR–80, ESA) with a mobile phase of 100 mM monosodium ortho-phosphate and 25% methanol, at a pH of 4.6 and at 40°C. GABA was detected at E2 set at 50 nA using a Coulochem II coulometric detector with potential settings of: guard cell +700mv, E1 +400mV, and E2 +650mV. Peak areas were calculated and values determined by comparison with a standard curve.
Glutamate analysis
Glutamate levels in tissue extracts were measured by HPLC separation and electrochemical detection of the o-phthaldialdehyde (OPA)-mercaptoethanol derivative, using a modification of the method used by Donzanti and Yamamoto (1988). Briefly, 10 µl of supernatant from the tissue extract was transferred into assay tubes and placed in an autosampler tray at 4°C. Ten 10 µl of 10 µM homoserine standard was added to each sample, then the sample was derivitized by the addition and mixing of 20 µl of an OPA/β-mercaptoethanol reagent. At 90 seconds after the addition of the reagent, 10 µl of the sample-reagent mixture was injected onto an HPLC column (3µm, C–18 column, 150 × 2mm). Separations were carried out isocratically, with a mobile phase containing 0.1 M sodium phosphate dibasic (pH 6.75) and 25% methanol, at a flow rate of 410 µl/min and a column temperature of 40°C. Electrochemical detection was performed by an ESA Coulochem II system with a dual electrode, set at 300 mV on channel 1, to preoxidize undesirable analytes and 600 mV on channel 2 for oxidization of the glutamate derivative. Peak areas were calculated based on standard curves and adjusted for the value of the internal standard.
Experiment 2: Systemic injections of CBiPES or alprazolam
5 days following l–AG infusion onset, in a counterbalanced design, sets of six rats each received an intraperitoneal (i.p.) injection of either CBiPES, 30mg/kg in 0.2ml/100g volume of 10% DMSO in ddH2O; THIIC, 10 or 30 mg/kg; alprazolam 3 mg/kg; Tocris, in 0.2ml/100g of 10% DMSO in ddH2O; or control vehicle (0.2ml/100g volume of 10% DMSO in ddH2O) 30 min prior to a NaLac challenge.
Experiment 3: Systemic injections of THIIC
Five days following onset of l–AG infusion, in a counterbalanced design, sets of six rats each received an i.p. injection of either vehicle control (0.2ml/100g volume DMSO), 10mg/kg THIIC, or 30mg/kg THIIC in 0.2ml/100g volume DMSO, exactly 30 min prior to the NaLac challenge.
Histology
Following experiments, all rats were anesthetized and decapitated; their brains were removed and frozen, then sectioned (30µm) and placed on slides; and these were stained with Neutral Red dye for determination of injection cannulae placements.
Statistical analyses
Each dependent variable for in vivo analyses (i.e. SI duration, activity, HR and MAP) was analyzed using a one-way ANOVA with repeated measures with drug treatment as the main factor and time in repeated measures, as well. In the presence of significant main effects, between subject post-hoc tests were conducted using a parametric Tukey’s test, and the within-subjects time effects were assessed using a Dunnett’s one-way analysis, with the time just prior to the i.v. infusion used as the control. Each dependent variable for ex-vivo analyses (i.e. GABA and glutamate concentrations) was analyzed using a one-tailed paired t-test. Statistical significance was accepted with p < 0.05. All statistical analyses were carried out using SPSS 13.0 (SPSS Inc., IL, USA) and Systat 5.02 for Windows (SYSTAT Inc., Evanston, IL, USA), plus all graphs were generated using SigmaPlot 2001 for Windows (SPSS Inc., IL, USA), while the figure-plate illustrations were done using CorelDraw version 12 for Windows.
Results
Experiment 1: Effects of 1–AG in the DMH/PeF on local tissue concentrations of GABA and glutamate
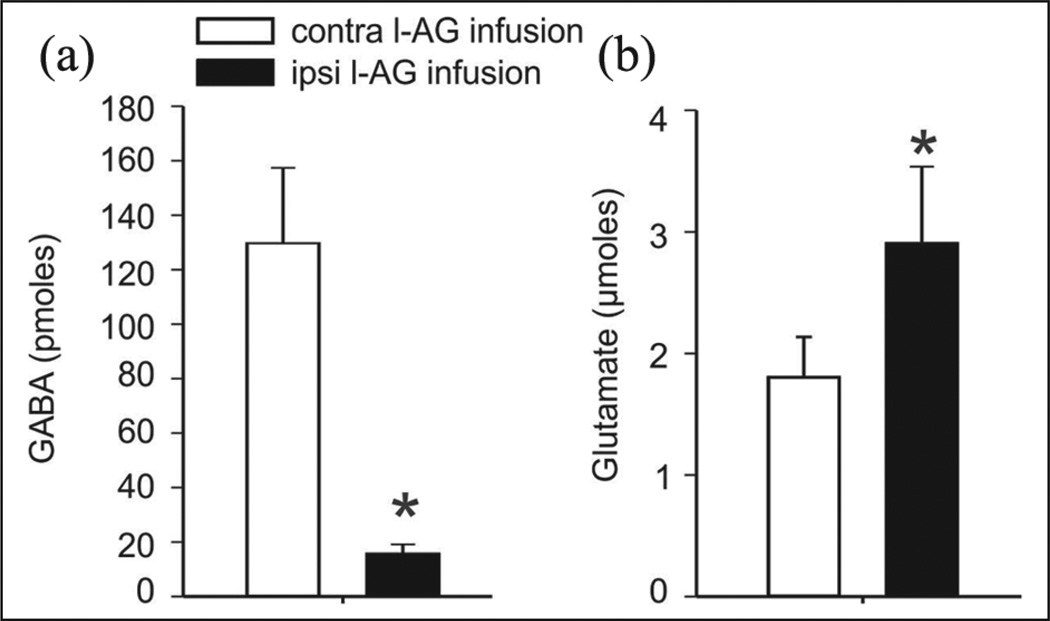
Unilateral infusions of a GABA synthesis inhibitor (l–AG) into the DMH/PeF, over 5 days, reduces GABA (t(6) = 5.6, p <0.001) as seen in Figure 1(a) and increases glutamate (t(6) = 2.0, p <0.05), as seen in Figure 1(b), as per analysis of 300uM micro-punches obtained from frozen coronal sections of the DMH/PeF ipsilateral to the infusion site, as compared to the contralateral control site, which is indicated by the * symbol (n = 7/group).
Figure 1.
Unilateral infusions of a GABA synthesis inhibitor, l-allylglycine (l–AG), into the DMH/PeF over 5 days: (a) reduces GABA (t(6)= 5.6, p < 0.001, left graph) and increases (b) glutamate (t(6)= 2.0, p < 0.05, right graph), as assessed in 300uM micro-punches from frozen rat coronal sections of the DMH/PeF ipsilateral to the infusion site, as compared to the contralateral control site, and as indicated by the symbol * (n = 7/group; p < 0.05, in a one-tailed paired t-test).
GABA: gamma-aminobutyric acid; DMH/PeF: dorsomedial/perifornical hypothalamus.
Experiment 2: Effects of CBiPES or alprazolam on NaLac-induced anxiety-like social behavior and panic-associated cardio-excitatory responses in panic-prone rats
Anxiety-associated behavior
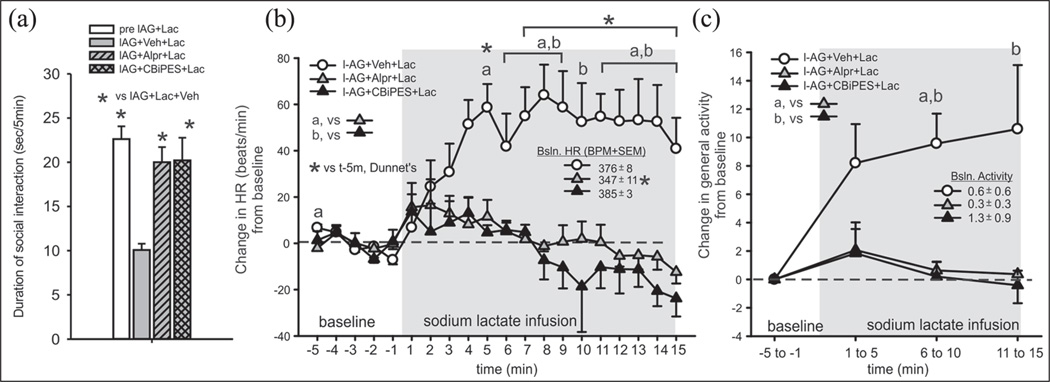
An ANOVA with a Tukey’s posthoc test revealed significant differences between the groups (treatment effect F(3,23)= 11.8, p <0.001, n = 6/group), as seen in Figure 2(a), where the alprazolam or the CBiPES drug blocked NaLac + l–AG-induced anxiety behavior (i.e. reductions in social interaction times).
Figure 2.
Systemic injections of the benzodiazepine Alprazolam or CBiPES attenuates lactate-induced changes (from 5 min baseline) in (a) anxiety-associated behavior (reduced SI), (b) tachycardia, and (c) general locomotor activity in rats made panic-prone with chronic reductions of GABA synthesis in the DMH/PeF (i.e. by local l-AG infusion). There was no significant baseline HR or general locomotor activity between groups (see legends on figures with baseline HR +/− SEM).
Gray shaded area represents the duration of intravenous (i.v.) lactate infusions. The a, b and c symbols in Figures (b) and (c) and the * symbol in Figure 2(b) indicate between-subject differences using a Tukey’s HSD posthoc test that was protected with an ANOVA. * indicates within-subject time points that are significantly different than at the −5 min pre-lactate infusion time point, using a one-way Dunnett’s posthoc test (n= 6,6,6 and 5 for each respective group).
GABA: gamma-aminobutyric acid; DMH/PeF: dorsomedial/perifornical hypothalamus; I-AG: l-allylglycine; Veh: vehicle control; Lac: lactate; Alpr: Alprazolam; CBiPES: (N-(4′-cyano-biphenyl-3-yl)-N-(3-pyridinylmethyl)-(ethanesulfonamide hydrochloride)); HR: heart rate; HSD: honestly significant difference; t: time; Bsln: baseline; BPM: beats per minute; SEM: Standard error of the mean; SI: social interaction.
Cardiovascular activity responses
An overall ANOVA with time as a repeated measure detected a treatment by time interaction for the change in HR (F(38,266) = 4.8, p <0.001), as seen in Figure 2(b), and MAP (F(38,266) = 2.9, p <0.001, data not shown) when comparing the l–AG + NaLac + vehicle group to the l–AG + NaLac + CBiPES or 20mg groups, when looking at all time-points over the 20 min period. A within-subjects analysis of HR and MAP changes revealed that lactate increased HR (F(19,100) = 4.6, p < 0.002) and MAP (F(19,100) = 2.4, p < 0.005) from the baseline, over time, in only the l–AG +NaLac + vehicle-treated rats (see the asterisk on graphs). A radio-telemetry probe battery malfunctioned (evidenced by HR, and MAP data that was outside the physiological range) prior to the CBiPES injection; therefore, there was n = 6 for the vehicle group, n = 6 for the alprazolam group and n = 5 for the CBiPES group. There was no significant difference in baseline (5 min prior to intravenous infusion) MAP (F(3,22)= 0.6, p = 0.594) between groups (see legends on figures with baseline data +/− SEM); however, an ANOVA with a subsequent Tukey's posthoc test did reveal that prior to NaLac infusion the baseline HR was significantly lower in the alprazolam-treated rats, as compared to all other groups (F(3,19) = 5.2, p = 0.009).
General motor activity responses
Although a within-group, over time ANOVA did not detect an increase in motor activity from baseline following the lactate challenge (F(3,20) = 2.9, p = 0.061), between-group analyses detected that the l–AG + NaLac + vehicle group did have higher locomotor activity post NaLac exposure, than the other groups did at the 5–10 min (F(2,14) = 13.6, p < 0.001) and 10–15 min (F(2,14) = 4.7, p < 0.05) interval post NaLac challenge (see Figure 2(c) for details from subsequent Tukey’s posthoc tests). There were also no significant differences in the baseline (5 min prior to intravenous infusion) activity (F(3,22)= 0.4, p = 0.721) between groups (see the legends on figures, with baseline data +/− SEM).
Experiment 3: Effects of THIIC on NaLac-induced anxiety-like social behavior and panic-associated cardio-excitatory responses in panic-prone rats
Anxiety-associated behavior
An ANOVA with a Tukey’s post-hoc test revealed that there were significant differences between groups of six individuals, with a treatment effect (F(2,15)= 6.2, p = 0.004) seen between Figure 1(a) and Figure 3(a), where the THIIC drug blocked the NaLac + l–AG -induced anxiety behavior (i.e. there were reductions in social interaction times observed).
Figure 3.
(a) Systemic injections of the highest dose of THIIC attenuated NaLac-induced anxiety behavior (as measured by reduced social interaction times) in rats made panic-prone with chronic reductions of GABA synthesis in the DMH/PeF (i.e. local l-AG infusions). The * symbol in Figure 3 (a) indicates between-subject differences using a Tukey’s posthoc test protected with an ANOVA. (b) Systemic injections of both doses of the THIIC attenuated NaLac-induced tachycardia in rats made panic-prone with chronic reductions of GABA synthesis in the DMH/PeF (i.e. l-AG infusions). (c) NaLac did not elicit an increase in general motor activity. There was no significant baseline HR or general locomotor activity between groups (see Figure legends for baseline HR and activity +/− SEM).
Symbols a and b in Figure 3(b) indicate the between-subject differences, using a Tukey’s posthoc test protected with an ANOVA. The * symbol in Figure 3(b) indicates within-subject time points that are significantly different that the 1 min pre-NaLac infusion time point, using a one-way Dunnett’s posthoc test.
THIIC: N-(4-{[3-hydroxy-4-(2-methylpropanoyl)-2-(trifluoromethyl)phenoxy]methyl}benzyl)-1-methyl-1H-imidazole-4-carboxamide); NaLac: sodium lactate; GABA: gamma-aminobutyric acid; DMH/PeF: dorsomedial/perifornical hypothalamus; I-AG: l-allyglycine; HR: heart rate; Lac: lactate; Veh: control vehicle; BsIn: baseline; BPM: beats per minute.
Cardiovascular activity responses
An overall ANOVA with time as a repeated measure did not detect a treatment by time interaction (F(38,266)= 1.4, p = 0.058) or a time effect (F(19,266)= 1.1, p = 0.356) for the change in HR, when comparing the l–AG + NaLac + vehicle group to the l–AG + NaLac + either 10 or 30mg/kg THIIC groups, if looking at all the time points over the 20-min period. Nor was there an overall within-subject treatment effect seen (F(2,14)= 2.7, p = 0.100); however, if the analysis for HR is restricted to looking only at the 5-min baseline and post-NaLac time points of 6–15min, then there was a significant treatment × time interaction (F(28,196)= 1.9, p = 0.006), as seen in Figure 3(b). A within-subject analysis of HR changes revealed that NaLac increased HR from baseline over time in only l–AG + NaLac + vehicle -treated rats (F(19,95)= 3.7, p < 0.001), as is also seen in Figure 3(b). No significant change in MAP (F(19,380)= 0.6, p = 0.975) was noted (data not shown). A radio-telemetry probe battery malfunctioned (evidenced by HR data that was outside the physiological range) prior to the 10mg/kg THIIC injection; therefore, n = 6 rats were in the vehicle group, only n = 5 in the 10mg/kg group and n = 6 for the 30mg/kg group, for HR. Also, no significant differences in baseline (5 min prior to intravenous infusion) HR (F(2,17)= 0.2, p = 0.795) nor MAP (F(2,17)= 0.3, p = 0.773) between groups (see legends on figures, with baseline data +/− SEM).
General motor activity responses
No significant change in locomotor activity (F(9,60) = 1.4, p = 0.223) was noted, as is shown in Figure 3(c). No significant differences in baseline (5 min prior to intravenous infusion) activity (F(2,17)= 0.04, p = 0.964) between groups occurred, either (see Figure legends with baseline data +/− SEM).
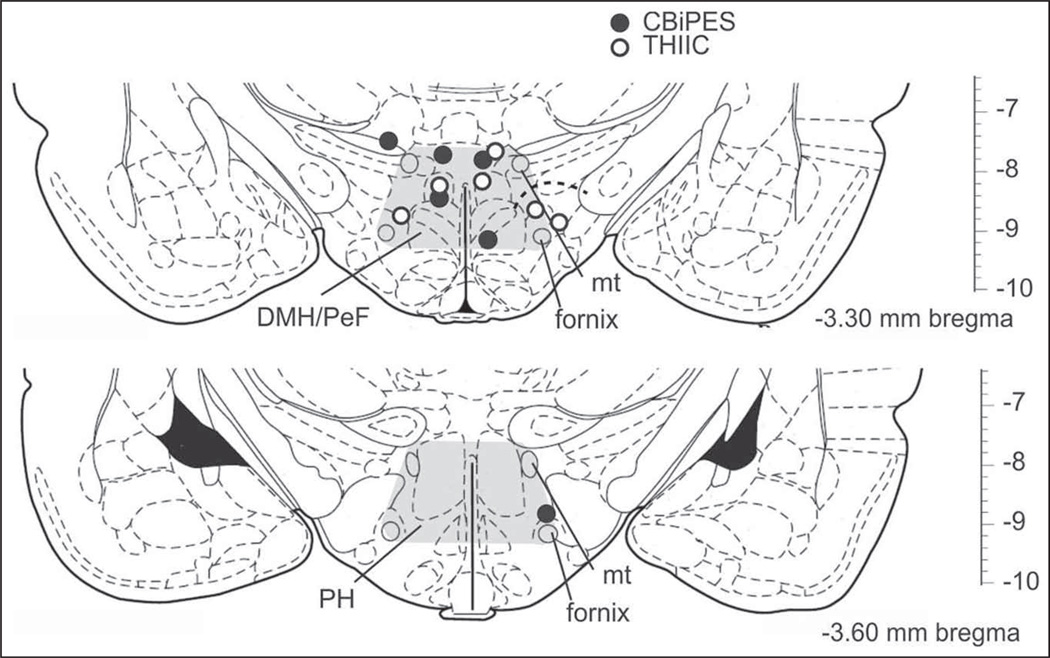
Osmotic minipump probe placement
All minipump cannulae placements resided in regions of the DMH/PeF and posterior hypothalamus (PH), known to be cardioexcitatory, as is seen in Figure 4 (Samuels et al., 2004; Shekhar and Keim, 1997). Although there was some tissue damage at the site of implantation, this damage was minimal and it was observed equally in the control and experimental groups, with the majority of cells in the DMH/PeF/PH still remaining intact, surrounding the infusion site and histologically staining with methyl green (data not shown).
Figure 4.
Coronal brain sections of the rat that illustrate the rostrocaudal distribution of unilateral placement of a minipump filled with the glutamic amino acid decarboxylase inhibitor l-allylglycine (l-AG, as represented by circles) from experiments testing the drugs CBiPES and alprazolam, denoted by black circles, and THIIC, denoted by open white circles. Numbers at bottom right of illustrations represent the distance from bregma.
PH: posterior hypothalamus; DMH: dorsomedial hypothalamus; PeF: perifornical hypothalamus; mt: mammillothalamic tract.
Discussion
Extensive previous studies demonstrate that chronic inhibition of GABA synthesis in the DMH/PeF leads to a panic-prone state in rats. We previously demonstrated that subsequent increases in local glutamatergic activity are in part responsible for this state (Johnson and Shekhar, 2006). In this study, we obtained more direct confirmation that blocking of GABA synthesis in the DMH/PeF not only decreased local GABA concentrations, but this decrease in GABA was accompanied by an increase in local glutamate concentrations. This was not surprising, since antagonizing GABA synthesis in the DMH/PeF would decrease the conversion of glutamate to GABA locally, so it is a likely explanation for the increased glutamate tone. We also previously showed that pre-injecting NMDA receptor antagonists into the DMH/PeF (to inhibit the postsynaptic effects of glutamate) does block NaLac-induced panic responses (Johnson and Shekhar, 2006). Thus, the increase in local glutamatergic tone within the DMH/PeF, as well as other postsynaptic sites, supports the use of a mGluR 2 potentiator such as CBiPES or THIIC in subsequent experiments, to see whether they could reduce the consequences of the excess synaptic glutamate, thus reduce vulnerability to feeling panic. In the present study, we found that the mGluR2 potentiators CBiPES or THIIC, when given as a pre-treatment, were each as effective a pre-treatment as the anti-panic drug benzodiazepine alprazolam in blocking NaLac-induced anxiety-like behavior, as well as panic-like increases in cardioexcitation, suggesting that the mGluR 2 potentiators could possibly become clinically effective antipanic agents in humans. The effective doses used in the present study correlated with potency in other animal models for treatment of anxiety/depression in both the rat and mouse (Johnson et al., 2005; Fell et al., 2011). Rats that were pre-treated with alprazolam had significantly lower baseline HRs prior to NaLac challenge than those rats given either the control vehicle, CBiPES or THIIC, which suggested that mGluR 2 potentiators may have fewer of the kind of side effects associated with benzodiazepines. These data are consistent with a previous study where pre-treating panic-prone rats with LY354740, an mGluR2/3 agonist, attenuated NaLac-induced panic responses (Shekhar and Keim, 2000); however, in addition to limiting the release of glutamate, a review mentions that LY354740 is also known to alter postsynaptic excitability through actions at the group II mGluR3 (Swanson et al., 2005). Our present findings suggested that blocking the release of glutamate by a presynaptic mechanism through treatment with mGluR2 potentiators may be therapeutic in treating panic disorder. This novel approach would not only be a significant improvement over therapy with benzodiazepines such as alprazolam, but this treatment may potentially have even fewer side-effects than the less selective mGlu2/3 agonists.
The present report adds to a wide variety of preclinical and clinical studies which suggest that panic vulnerability could partially be explained by glutamate-mediated hyperexcitation in brain regions such as the DMH/PeF, or other panic-generating sites such as the amygdala (Sajdyk and Shekhar, 2000; Shekhar et al., 2003) or the dorsal periaqueductal gray (DPAG), which is proposed to be a critical locus for the “suffocation alarm system” in response to NaLac or hypoxia (Schimitel et al., 2012) and, if hyperactive, could contribute to what Klein terms “false suffocation alarm,” to trigger panic attacks (Klein, 1993; Schenberg et al., 2001). In light of this, there is increasing recognition that glutamatergic mechanisms may be a potential avenue to develop for new treatments for anxiety problems such as panic disorder (Gorman, 2003). For instance, the noncompetitive NMDA antagonist MK–801 (Xie and Commissaris, 1992) and the competitive NMDA antagonist AP–7 (Plaznik et al., 1994) are both shown to reduce anxiety-like and panic-associated behavior in rats. Still, although these previously reviewed preclinical trials with NMDA receptor antagonists appear to reduce anxiety, human clinical trials with NMDA receptor antagonists (e.g. Dizolcilpine (MK-801), Cerestat (CNS-1102), Selfotel (CGS–19755) and D-CPPene) demonstrated they are not a realistic therapy, in light of their numerous undesired side-effects (Parsons et al., 1999). The adverse side effects that are most commonly reported are the ability of certain NMDA receptor antagonists to induce psychotomimetic effects (e.g. phencyclidine) and having sedative effects at high doses (e.g. ketamine). These adverse effects are unfortunate, because NMDA antagonists have shown promising therapeutic applications for acute (e.g. stroke and trauma) and chronic (e.g. Alzheimer’s and Parkinson’s disease) neurodegeneration disorders, anxiety, depression and chronic pain (Parsons et al., 1999).
According to a review by Swanson and Schoepp (2002), more recent preclinical research had greater success, by targeting mGluRs to treat anxiety with fewer side effects. The mGluR family of glutamate G-protein-coupled receptors are classified into three major groups, based on molecular structure, function and pharmacology: group I (mGluR 1 and 5), group II (mGluR 2 and 3) and group III (mGluR 4,6–8) receptors (Nicoletti et al., 2010). The mGluR 2/3 potentiators such as LY354740 or LY379268 have shown anxiolytic action in several anxiety models, such as: fear induced potentiated startle (Monn et al., 1997; Helton et al., 1998; Walker and Davis, 2002), elevated plus maze (Monn et al., 1997; Helton et al., 1998), and in addition, LY354740 reduces NaLac-induced panic responses in panic-prone rats (Shekhar and Keim, 2000). Furthermore, in humans the mGlu2/3 potentiatiors have anxiolytic properties in fear-potentiated startle (Grillon, 2003) and CO2-induced panic attacks (Schoepp et al., 2003). However, because the mGlu 3 receptors are located postsynaptically and also on glia, it suggests that targeting of mGlu 2 receptors, which are activated by an excess synaptic glutamate (Nicoletti et al., 2010) to negatively modulate presynaptic glutamate neurotransmission (Pin and Duvoisin, 1995) would potentially be more selective in reducing an excessive synaptic glutamate release in the DMH/PeF of panic-prone rats, to reduce panic vulnerability without altering the basal glutamate release (Johnson et al., 2005). Our current study data clearly show that the mGluR2 potentiators used were as effective as a benzodiazepine in blocking the full spectrum of NaLac-induced panic responses in panic-prone rats. Clinical studies will be needed to determine whether mGluR2 potentiators such as CBiPES or THIIC can effectively treat severe human anxiety disorders, such as panic disorder, with fewer side effects than those associated with other clinically-approved anxiolytics.
The distribution of mGlu 2 receptors may give insight into where CBiPES may exert its panicolytic effects without the adverse side-effects that are associated with NMDA receptor antagonists. The mGluR2 mRNA (Ohishi et al., 1993a, 1993b) and protein (Neki et al., 1996; Petralia et al., 1996) are present in the hypothalamus and are particularly dense in limbic (e.g. basolateral amygdala) and cortical areas in rats, as well as in the brainstem structures through which NaLac may drive the autonomic (e.g. raphe, reticular nuclei) and respiratory (e.g. lateral parabrachial nucleus) responses in panic-prone rats (Johnson et al., 2008). Within the hypothalamus, the mGluR2 mRNA (Gu et al., 2008) and mGluR2/3 protein (Petralia et al., 1996) is dense in the zona incerta, which is found dorsal to the DMH/PeF region. Therefore, some of the panicolytic properties of the mGluR 2 potentiators used here could derive from reducing excess glutamate in the DMH/PeF region of panic-prone rats, as well as efferent targets of the DMH/PeF (see the hypothetical illustration in Figure 5). Consistent with this hypothesis, we previously showed that local injections of NMDA glutamate receptor antagonists into the DMH/PeF of panic-prone rats prior to a NaLac challenge blocks the NaLac-induced panic responses (Johnson and Shekhar, 2006). The anxiolytic effects of NMDA receptor antagonists in the DMH/PeF may be state dependent, because under normal conditions, when the tonic GABAergic inhibition is intact in the DMH/PeF, reducing glutamate excitation with direct injections of glutamate receptor antagonists into the DMH/PeF will increase exploratory behavior, but have no effect on anxiety-related behavior, as is measured by an elevated plus-maze test or Vogel conflict test (Jardim and Guimaraes, 2004).
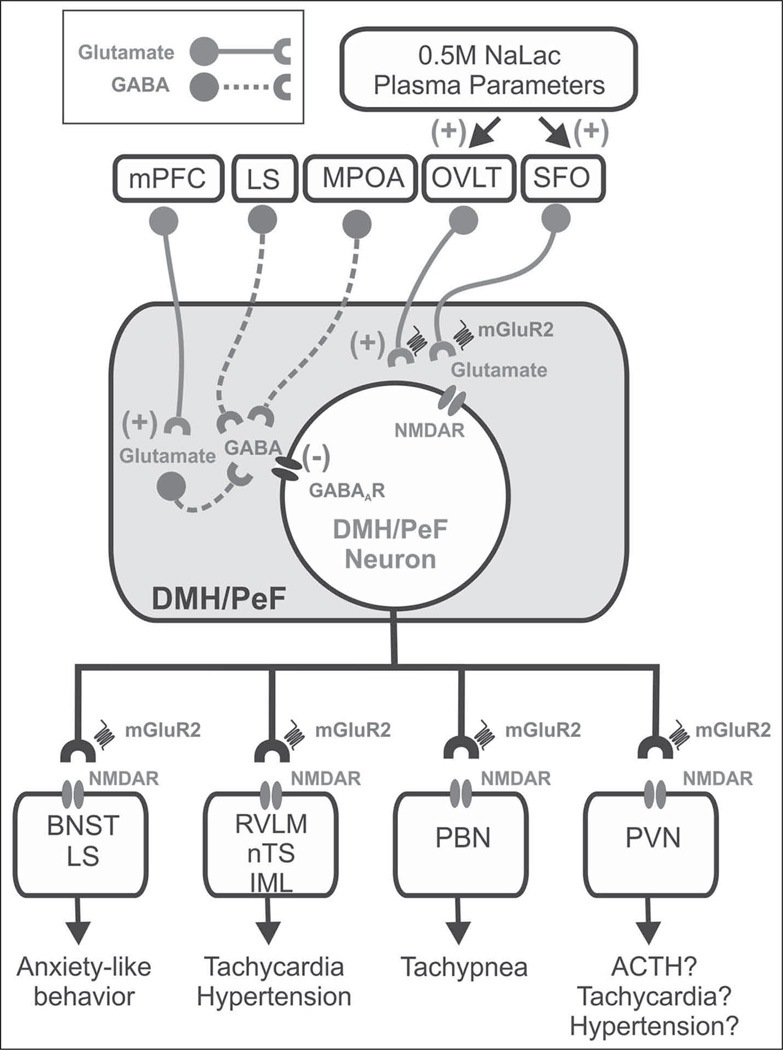
Figure 5.
Summary schema of glutamatergic and GABAergic input into the DMH/PeF and important efferent brain regions implicated in the regulation of panic-like responses. GABAergic and glutamatergic neurons are represented by circles attached to dashed or solid lines, respectively. Sources of GABA include local interneurons, which are tonically driven by glutamatergic input from mPFC (Bard and Mountcastle, 1948) as well as efferent brain regions such as the LS and mPOA (Feldblum et al., 1993; Risold and Swanson, 1997; Thompson and Swanson, 1998; Grob et al., 2003; Molosh et al., 2010). Glutamatergic input which excites the DMH/PeF region appears to arise from afferent sites such as CVOs (e.g. MnPO/OVLT and SFO (Richard and Bourque, 1992; Grob et al., 2003; Molosh et al., 2010)). Afferent targets of the DMH/PeF that are implicated in the regulation of panic-like responses are also listed (Johnson et al., 2008; Chamberlin and Saper, 1994; Thompson et al., 1996; Fontes et al., 2001; Chen et al., 2004). The Group II mGluRs 2 and 3 are predominantly expressed presynpatically (Shigemoto et al., 1997) and they negatively modulate glutamate neurotransmission (Pin and Duvoisin, 1995), acting on postsynaptic NMDAR (Johnson and Shekhar, 2006).
ACTH: adrenocorticotropic hormone; BNST: bed nucleus of the stria terminalis; CVO: circumventricular organ; DMH/PeF: dorsomedial/perifornical hypothalamus; GABA: gamma-aminobutyric acid; Glu: glutamatergic; GluR2: glutamate receptor 2; IML: intermediolateral cell column of spinal cord; LC: locus coeruleus; LS: lateral septum; mPFC: medial prefrontal cortex; mGluR: metabotropic glutamate receptor; MnPO: median preoptic nucleus; mPOA: medial preoptic area; NaLac: sodium lactate; NMDAR: N-methyl-D-aspartic acid receptor; nTS: nucleus of solitary tract; OVLT: organum vasculosum lamina terminalis; PBN: parabrachial nucleus; PVN: paraventricular hypothalamic nucleus; RVLM: rostroventrolateral medulla; SFO: subfornical organ.
Alternatively, the panicolytic effects of the CBiPES or THIIC compounds may be due to blocking of the NaLac-induced cardioexcitatory activity, by inhibiting peripheral cardiovascular activity. An argument against this is that beta-blockers, which reduce NaLac-induced cardioexcitatory (HR and MAP) responses in patients with panic disorder (Price et al., 1995) or after the DMH/PeF activation model of panic (Shekhar and Katner, 1995), do not block self-reported panic responses in humans (Price et al., 1995) nor anxiety-associated behavior in rats (Shekhar and Katner, 1995).
In conclusion, our present studies confirmed that disrupting GABA synthesis in a panic-generating hypothalamic site (i.e. DMH/PeF) produced panic-prone rats, which also coincided with an increase in local glutamate activity. Furthermore, pre-treating these panic-prone rats with one of the selective group II mGlu receptor agonists like CBiPES, THIIC, or the currently-used benzodiazepine alprazolam did block NaLac-induced panic-like responses, as was indicated by the increased social anxiety, increased locomotor activity and cardio-excitation. These animal study data suggest that mGluR2 potentiators could become a novel anti-panic drug that is as effective as alprazolam, but acts without some of the adverse effects typical of benzodiazepines.
Acknowledgments
Funding
This work was supported by Eli Lilly & Co. and the NIH (R01 grant number MH52619), awarded to AS; as well as by Anxiety Disorders Association of America (Junior Faculty Research Award) awarded to PLJ, the NIH (Student LRP) awarded to PLJ and the National Alliance for Schizophrenia and Depression (Young Investigators Award) awarded to PLJ.
Footnotes
Conflict of interest
None declared.
References
- Abshire VM, Hankins KD, Roehr KE, et al. Injection of L-allylglycine into the posterior hypothalamus in rats causes decreases in local GABA which correlate with increases in heart rate. Neuropharmacol. 1988;27:1171–1177. doi: 10.1016/0028-3908(88)90013-5. [DOI] [PubMed] [Google Scholar]
- Baldwin DS, Anderson IM, Nutt DJ, et al. Evidence-based guidelines for the pharmacological treatment of anxiety disorders: Recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2005;19:567–596. doi: 10.1177/0269881105059253. [DOI] [PubMed] [Google Scholar]
- Bandelow B, Zohar J, Hollander E, et al. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for the pharmacological treatment of anxiety, obsessive-compulsive and post-traumatic stress disorders - first revision. World J Biol Psychiatry. 2008;9:248–312. doi: 10.1080/15622970802465807. [DOI] [PubMed] [Google Scholar]
- Bard P, Mountcastle VB. Some forebrain mechanisms involved in the expression of rage with special reference to suppression of angry behavior. Res Publ Assoc Res Nerv Ment Dis. 1948;27:362–404. [PubMed] [Google Scholar]
- Bernardis LL, Bellinger LL. The dorsomedial hypothalamic nucleus revisited: 1998 Update. Proc Soc Exp Biol Med. 1998;218:284–306. doi: 10.3181/00379727-218-44296. [DOI] [PubMed] [Google Scholar]
- Boshuisen ML, Ter Horst GJ, Paans AM, et al. rCBF differences between panic disorder patients and control subjects during anticipatory anxiety and rest. Biol Psy. 2002;52:126–135. doi: 10.1016/s0006-3223(02)01355-0. [DOI] [PubMed] [Google Scholar]
- Bourdelais A, Kalivas PW. High sensitivity HPLC assay for GABA in brain dialysis studies. J Neurosci Meth. 1991;39:115–121. doi: 10.1016/0165-0270(91)90077-d. [DOI] [PubMed] [Google Scholar]
- Chamberlin NL, Saper CB. Topographic organization of respiratory responses to glutamate microstimulation of the parabrachial nucleus in the rat. J Neurosci. 1994;14:6500–6510. doi: 10.1523/JNEUROSCI.14-11-06500.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T, Hui R, Dong YX, et al. Endomorphin 1- and endomorphin 2-like immunoreactive neurons in the hypothalamus send axons to the parabrachial nucleus in the rat. Neurosci Lett. 2004;357:139–142. doi: 10.1016/j.neulet.2003.12.079. [DOI] [PubMed] [Google Scholar]
- Chou TC, Scammell TE, Gooley JJ, et al. Critical role of dorsomedial hypothalamic nucleus in a wide range of behavioral circadian rhythms. J Neurosci. 2003;23:10691–10702. doi: 10.1523/JNEUROSCI.23-33-10691.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloos JM, Ferreira V. Current use of benzodiazepines in anxiety disorders. Curr Opin Psych. 2009;22:90–95. doi: 10.1097/YCO.0b013e32831a473d. [DOI] [PubMed] [Google Scholar]
- DiMicco JA, Samuels BC, Zaretskaia MV, et al. The dorsomedial hypothalamus and the response to stress: Part renaissance, part revolution. Pharmacol Biochem Behav. 2002;71:469–480. doi: 10.1016/s0091-3057(01)00689-x. [DOI] [PubMed] [Google Scholar]
- Donzanti BA, Yamamoto BK. An improved and rapid HPLC-EC method for the isocratic separation of amino acid neurotransmitters from brain tissue and microdialysis perfusates. Life Sci. 1988;43:913–922. doi: 10.1016/0024-3205(88)90267-6. [DOI] [PubMed] [Google Scholar]
- Feldblum S, Erlander MG, Tobin AJ. Different distributions of GAD65 and GAD67 mRNAs suggest that the two glutamate decarboxylases play distinctive functional roles. J Neurosci Res. 1993;34:689–706. doi: 10.1002/jnr.490340612. [DOI] [PubMed] [Google Scholar]
- File SE. The use of social interaction as a method for detecting anxiolytic activity of chlordiazepoxide-like drugs. J Neurosci Meth. 1980;2:219–238. doi: 10.1016/0165-0270(80)90012-6. [DOI] [PubMed] [Google Scholar]
- Fontes MA, Tagawa T, Polson JW, et al. Descending pathways mediating cardiovascular response from dorsomedial hypothalamic nucleus. Am J Physiol Heart Circ Physiol. 2001;280:H2891–H2901. doi: 10.1152/ajpheart.2001.280.6.H2891. [DOI] [PubMed] [Google Scholar]
- Goddard AW, Mason GF, Almai A, et al. Reductions in occipital cortex GABA levels in panic disorder detected with 1h-magnetic resonance spectroscopy. Arch Gen Psych. 2001;58:556–561. doi: 10.1001/archpsyc.58.6.556. [DOI] [PubMed] [Google Scholar]
- Gorman JM. New molecular targets for antianxiety interventions. J Clin Psych. 2003;64:28–35. [PubMed] [Google Scholar]
- Gorman JM, Kent JM, Sullivan GM, et al. Neuroanatomical hypothesis of panic disorder, revised. Am J Psych. 2000;157:493–505. doi: 10.1176/appi.ajp.157.4.493. [DOI] [PubMed] [Google Scholar]
- Gorman JM, Papp LA, Coplan JD, et al. Anxiogenic effects of CO2 and hyperventilation in patients with panic disorder. Am J Psych. 1994;151:547–553. doi: 10.1176/ajp.151.4.547. [DOI] [PubMed] [Google Scholar]
- Grillon C, Cordova J, Levine LR, et al. Anxiolytic effects of a novel group II metabotropic glutamate receptor agonis (LY354740) in the fear-potentiated startle paradigm in humans. Psychopharmacology (Berl) 2003;168:446–454. doi: 10.1007/s00213-003-1444-8. [DOI] [PubMed] [Google Scholar]
- Grob M, Trottier JF, Drolet G, et al. Characterization of the neurochemical content of neuronal populations of the lamina terminalis activated by acute hydromineral challenge. Neurosci. 2003;122:247–257. doi: 10.1016/j.neuroscience.2003.07.005. [DOI] [PubMed] [Google Scholar]
- Gu G, Lorrain DS, Wei H, et al. Distribution of metabotropic glutamate 2 and 3 receptors in the rat forebrain: Implication in emotional responses and central disinhibition. Brain Res. 2008;1197:47–62. doi: 10.1016/j.brainres.2007.12.057. [DOI] [PubMed] [Google Scholar]
- Helton DR, Tizzano JP, Monn JA, et al. Anxiolytic and side-effect profile of LY354740: A potent, highly selective, orally active agonist for group II metabotropic glutamate receptors. J Pharmacol Exp Ther. 1998;284:651–660. [PubMed] [Google Scholar]
- Hirschfeld RMA. Panic disorder: Diagnosis, epidemiology, and clinical course. J Clin Psych. 1996;57:3–8. [PubMed] [Google Scholar]
- Jardim MC, Guimaraes FS. Role of glutamate ionotropic receptors in the dorsomedial hypothalamic nucleus on anxiety and locomotor behavior. Pharmacol Biochem Behav. 2004;79:541–546. doi: 10.1016/j.pbb.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Johnson MP, Barda D, Britton TC, et al. Metabotropic glutamate 2 receptor potentiators: Receptor modulation, frequency-dependent synaptic activity, and efficacy in preclinical anxiety and psychosis model(s) Psychopharmacol. 2005;179:271–283. doi: 10.1007/s00213-004-2099-9. [DOI] [PubMed] [Google Scholar]
- Johnson PL, Shekhar A. Panic-prone state induced in rats with GABA dysfunction in the dorsomedial hypothalamus is mediated by NMDA receptors. J Neurosci. 2006;26:7093–7104. doi: 10.1523/JNEUROSCI.0408-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PL, Truitt WA, Fitz SD, et al. Neural pathways underlying lactate-induced panic. Neuropsychopharmacol. 2008;33:2093–2107. doi: 10.1038/sj.npp.1301621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PL, Truitt W, Fitz SD, et al. A key role for orexin in panic anxiety. Nat Med. 2010;16:111–115. doi: 10.1038/nm.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katon WJ, Roy-Byrne P, Russo J, et al. Cost-effectiveness and cost offset of a collaborative care intervention for primary care patients with panic disorder. Arch Gen Psych. 2002;59:1098–1104. doi: 10.1001/archpsyc.59.12.1098. [DOI] [PubMed] [Google Scholar]
- Kessler RC, McGonagle KA, Zhao S, et al. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Arch Gen Psych. 1994;51:8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- Klein DF. False suffocation alarms, spontaneous panics, and related conditions. Arch Gen Psych. 1993;50:306–317. doi: 10.1001/archpsyc.1993.01820160076009. [DOI] [PubMed] [Google Scholar]
- Liebowitz MR, Gorman J, Fyer A, et al. Biological accompaniments of lactate-induced panic. Psychopharmacol Bull. 1984;20:43–44. [PubMed] [Google Scholar]
- Molosh AI, Johnson PL, Fitz SD, et al. Changes in central sodium and not osmolarity or lactate induce panic-like responses in a model of panic disorder. Neuropsychopharmacol. 2010;35:1333–1347. doi: 10.1038/npp.2010.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monn JA, Valli MJ, Massey SM, et al. Design, synthesis, and pharmacological characterization of (+)-2-aminobicyclo[3.1.0] hexane-2,6-dicarboxylic acid (LY354740): A potent, selective, and orally active group 2 metabotropic glutamate receptor agonist possessing anticonvulsant and anxiolytic properties. J Med Chem. 1997;40:528–537. doi: 10.1021/jm9606756. [DOI] [PubMed] [Google Scholar]
- Neki A, Ohishi H, Kaneko T, et al. Pre- and postsynaptic localization of a metabotropic glutamate receptor, mGluR2, in the rat brain: An immunohistochemical study with a monoclonal antibody. Neurosci Lett. 1996;202:197–200. doi: 10.1016/0304-3940(95)12248-6. [DOI] [PubMed] [Google Scholar]
- Nicoletti F, Bockaert J, Collingridge GL, et al. Metabotropic glutamate receptors: From the workbench to the bedside. Neuropharmacology. 2010;60:1017–1041. doi: 10.1016/j.neuropharm.2010.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutt DJ, Ballenger JC, Sheehan D, et al. Generalized anxiety disorder: Comorbidity, comparative biology and treatment. Int J Neuropsychopharmacol. 2002;5:315–325. doi: 10.1017/S1461145702003048. [DOI] [PubMed] [Google Scholar]
- Ohishi H, Shigemoto R, Nakanishi S, et al. Distribution of the messenger RNA for a metabotropic glutamate receptor, mGluR2, in the central nervous system of the rat. Neurosci. 1993a;53:1009–1018. doi: 10.1016/0306-4522(93)90485-x. [DOI] [PubMed] [Google Scholar]
- Ohishi H, Shigemoto R, Nakanishi S, et al. Distribution of the mRNA for a metabotropic glutamate receptor (mGluR3) in the rat brain: An in situ hybridization study. J Comp Neurol. 1993b;335:252–266. doi: 10.1002/cne.903350209. [DOI] [PubMed] [Google Scholar]
- Parsons CG, Danysz W, Quack G. Memantine is a clinically well tolerated N-methyl-D-aspartate (NMDA) receptor antagonist: A review of preclinical data. Neuropharmacol. 1999;38:735–767. doi: 10.1016/s0028-3908(99)00019-2. [DOI] [PubMed] [Google Scholar]
- Petralia RS, Wang YX, Niedzielski AS, et al. The metabotropic glutamate receptors, mGluR2 and mGluR3, show unique postsynaptic, presynaptic and glial localizations. Neurosci. 1996;71:949–976. doi: 10.1016/0306-4522(95)00533-1. [DOI] [PubMed] [Google Scholar]
- Pin JP, Duvoisin R. The metabotropic glutamate receptors: Structure and functions. Neuropharmacol. 1995;34:1–26. doi: 10.1016/0028-3908(94)00129-g. [DOI] [PubMed] [Google Scholar]
- Plaznik A, Palejko W, Nazar M, et al. Effects of antagonists at the NMDA receptor complex in two models of anxiety. Eur Neuropsychopharmacol. 1994;4:503–512. doi: 10.1016/0924-977x(94)90299-2. [DOI] [PubMed] [Google Scholar]
- Pohl R, Balon R, Bechou R, et al. Lactate-induced anxiety after imapramine and diazepam treatment. Anxiety. 1994;1:54–63. doi: 10.1002/anxi.3070010204. [DOI] [PubMed] [Google Scholar]
- Price LH, Goddard AW, Barr LC, et al. Pharmacological challenges in anxiety disorders. In: Bloom FE, Kupfer DJ, editors. Psychopharmacology: The fourth generation of progression. New York: Raven; 1995. pp. 1311–1323. [Google Scholar]
- Richard D, Bourque CW. Synaptic activation of rat supraoptic neurons by osmotic stimulation of the organum vasculosum lamina terminalis. Neuroendocrinol. 1992;55:609–611. doi: 10.1159/000126174. [DOI] [PubMed] [Google Scholar]
- Risold PY, Swanson LW. Connections of the rat lateral septal complex. Brain Res Rev. 1997;24:115–195. doi: 10.1016/s0165-0173(97)00009-x. [DOI] [PubMed] [Google Scholar]
- Sajdyk TJ, Shekhar A. Sodium lactate elicits anxiety in rats after repeated GABA receptor blockade in the basolateral amygdala. Eur J Pharmacol. 2000;394:265–273. doi: 10.1016/s0014-2999(00)00128-x. [DOI] [PubMed] [Google Scholar]
- Samuels BC, Zaretsky DV, DiMicco JA. Dorsomedial hypothalamic sites where disinhibition evokes tachycardia correlate with location of raphe-projecting neurons. Am J Physiol Regul Integr Comp Physiol. 2004;287:R472–R478. doi: 10.1152/ajpregu.00667.2003. [DOI] [PubMed] [Google Scholar]
- Sanders SK, Shekhar A. Regulation of anxiety by GABAA receptors in the rat amygdala. Pharmacol Biochem Behav. 1995;52:701–706. doi: 10.1016/0091-3057(95)00153-n. [DOI] [PubMed] [Google Scholar]
- Schenberg LC, Bittencourt AS, Sudre EC, et al. Modeling panic attacks. Neurosci Biobehav Rev. 2001;25:647–659. doi: 10.1016/s0149-7634(01)00060-4. [DOI] [PubMed] [Google Scholar]
- Schimitel FG, De Almeida GM, Pitol DN, et al. Evidence of a suffocation alarm system within the periaqueductal gray matter of the rat. Neurosci. 2012;200:59–73. doi: 10.1016/j.neuroscience.2011.10.032. [DOI] [PubMed] [Google Scholar]
- Schoepp DD, Wright RA, Levine LR, et al. LY354740, an mGlu2/3 receptor agonist as a novel approach to treat anxiety/stress. Stress. 2003;6:189–197. doi: 10.1080/1025389031000146773. [DOI] [PubMed] [Google Scholar]
- Shekhar A, Johnson PL, Sajdyk TJ, et al. Angiotensin-II is a putative neurotransmitter in lactate-induced panic-like responses in rats with disruption of GABAergic inhibition in the dorsomedial hypothalamus. J Neurosci. 2006;26:9205–9215. doi: 10.1523/JNEUROSCI.2491-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shekhar A, Katner JS. Dorsomedial hypothalamic GABA regulates anxiety in the social interaction test. Pharmacol Biochem Behav. 1995;50:253–258. doi: 10.1016/0091-3057(94)00307-5. [DOI] [PubMed] [Google Scholar]
- Shekhar A, Keim SR. The circumventricular organs form a potential neural pathway for lactate sensitivity: Implications for panic disorder. J Neurosci. 1997;17:9726–9735. doi: 10.1523/JNEUROSCI.17-24-09726.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shekhar A, Keim SR. LY354740, a potent group II metabotropic glutamate receptor agonist prevents lactate-induced panic-like response in panic-prone rats. Neuropharmacol. 2000;39:1139–1146. doi: 10.1016/s0028-3908(99)00215-4. [DOI] [PubMed] [Google Scholar]
- Shekhar A, Keim SR, Simon JR, et al. Dorsomedial hypothalamic GABA dysfunction produces physiological arousal following sodium lactate infusions. Pharmacol Biochem Behav. 1996;55:249–256. doi: 10.1016/s0091-3057(96)00077-9. [DOI] [PubMed] [Google Scholar]
- Shekhar A, Sajdyk TJ, Gehlert DR, et al. The amygdala, panic disorder, and cardiovascular responses. Ann NY Acad Sci. 2003;985:308–325. doi: 10.1111/j.1749-6632.2003.tb07090.x. [DOI] [PubMed] [Google Scholar]
- Shigemoto R, Kinoshita A, Wada E, et al. Differential presynaptic localization of metabotropic glutamate receptor subtypes in the rat hippocampus. J Neurosci. 1997;17:7503–7522. doi: 10.1523/JNEUROSCI.17-19-07503.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson CJ, Bures M, Johnson MP, et al. Metabotropic glutamate receptors as novel targets for anxiety and stress disorders. Nature Rev. 2005;4:131–144. doi: 10.1038/nrd1630. [DOI] [PubMed] [Google Scholar]
- Swanson CJ, Schoepp DD. The group II metabotropic glutamate receptor agonist (−)-2-oxa-4-aminobicyclo[3.1.0.]hexane-4,6-dicarboxylate (LY379268) and clozapine reverse phencyclidine-induced behaviors in monoamine-depleted rats. J Pharmacol Exp Ther. 2002;303:919–927. doi: 10.1124/jpet.102.038422. [DOI] [PubMed] [Google Scholar]
- Thompson RH, Canteras NS, Swanson LW. Organization of projections from the dorsomedial nucleus of the hypothalamus: A PHA-L study in the rat. J Comp Neurol. 1996;376:143–173. doi: 10.1002/(SICI)1096-9861(19961202)376:1<143::AID-CNE9>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Thompson RH, Swanson LW. Organization of inputs to the dorsomedial nucleus of the hypothalamus: A reexamination with Fluorogold and PHAL in the rat. Brain Res Rev. 1998;27:89–118. doi: 10.1016/s0165-0173(98)00010-1. [DOI] [PubMed] [Google Scholar]
- Walker DL, Davis M. The role of amygdala glutamate receptors in fear learning, fear-potentiated startle, and extinction. Pharmacol Biochem Behav. 2002;71:379–392. doi: 10.1016/s0091-3057(01)00698-0. [DOI] [PubMed] [Google Scholar]
- Weissman MM, Merikangas KR. The epidemiology of anxiety and panic disorders: An update. J Clin Psych. 1986;47:11–17. [PubMed] [Google Scholar]
- Xie Z, Commissaris RL. Anxiolytic-like effects of the noncompetitive NMDA antagonist MK 801. Pharmacol Biochem Behav. 1992;43:471–477. doi: 10.1016/0091-3057(92)90178-i. [DOI] [PubMed] [Google Scholar]