Abstract
Purpose
To evaluate transient, large visual acuity (VA) decreases, termed sporadic vision loss, during anti-vascular endothelial growth factor treatment for neovascular age-related macular degeneration (AMD).
Design
Cohort within a randomized clinical trial.
Methods
Setting
Comparison of AMD Treatments Trials (CATT).
Study Population
1185 CATT patients.
Main Outcome Measures
incidence of sporadic vision loss and odds ratio (OR) for association with patient and ocular factors. Sporadic vision loss was a decline of ≥ 15 letters from the previous visit, followed by a return at the next visit to no more than 5 letters worse than the visit before the VA loss.
Results
There were 143 sporadic vision loss events in 122/1185 (10.3%) patients. Mean VA at two years for those with and without sporadic vision loss was 58.5 (~20/63) and 68.4 (~20/40) letters, respectively (P < 0.001). Among patients treated pro re nata, no injection was given for 27.6% (27/98) of sporadic vision loss events. Multivariate analysis demonstrated that baseline predictors for sporadic vision loss included worse baseline VA (OR 2.92, 95%CI:1.65–5.17 for ≤ 20/200 compared with ≥ 20/40), scar (OR 2.21, 95%CI:1.22–4.01), intraretinal foveal fluid on optical coherence tomography (OR 1.80, 95%CI:1.11–2.91), and medical history of anxiety (OR 1.90, 95%CI:1.12–3.24) and syncope (OR 2.75, 95%CI:1.45–5.22). Refraction decreased the likelihood of sporadic vision loss (OR 0.62, 95%CI:0.42–0.91).
Conclusions
Approximately 10% of CATT patients had sporadic vision loss. Baseline predictors included AMD-related factors and factors independent of AMD. These data are relevant for clinicians in practice and those involved in clinical trials.
Introduction
Visual acuity (VA) has been the primary outcome measure for every major clinical trial for neovascular age-related macular degeneration (AMD).1–7 Previous studies have established that VA measurement administered under a standard protocol that includes refraction provides a reliable outcome measure.8,9 Still, VA scores can be affected by multiple factors, some of which have little to do with the condition of the eye. Health issues that are not primarily ocular, such as depression and neurological disease, can impact VA measurement or visual function.10–17 In addition, clinicians occasionally see patients in follow-up who have a worse VA measurement without any change on clinical exam.
As part of their analysis of vision loss during the Minimally Classic/Occult Trial of the Anti-VEGF Antibody Ranibizumab in the Treatment of Neovascular AMD (MARINA) and Anti-VEGF Antibody for the Treatment of Predominantly Classic Choroidal Neovascularization in AMD (ANCHOR) trials, Wolf et al. identified patients that had acute loss of ≥ 15 letters within any one month period.18 106 (13.9%) of 758 ranibizumab treated patients experienced an acute loss of vision during the first year, and several had more than one episode of acute vision loss. While they concluded that continued treatment was beneficial, there was no clear relationship between patient characteristics and acute vision loss, including an analysis of study eye adverse events (AEs) or serious adverse events (SAEs). In addition to progressive AMD disease, it is possible that other factors were involved in some of these acute vision loss events.
Given that significant resources are devoted to studying a treatment’s effects on VA in AMD patients, we have sought further understanding of factors that influence this outcome measurement. The Comparison of Age-Related Macular Degeneration Treatments Trials (CATT) was a 2 year study that evaluated the efficacy of ranibizumab compared with bevacizumab, as well as monthly compared with as needed treatments.6,19 The CATT database provides an unprecedented opportunity to investigate AMD patients as it expands on MARINA and ANCHOR data, providing treatment regimen, drug, and optical coherence tomography (OCT) correlations. We previously reported the frequency of sustained VA loss and its associated factors within CATT.20 Here, we report similarly for sporadic VA loss within CATT. Rather than studying patients with only an acute loss of ≥ 15 letters, we were interested in patients who had a decline of ≥ 15 letters from the previous visit, followed by a return of vision at the next visit. While changes of 5 and occasionally 10 letters are within test-retest variability,9 little is known about the causes of transient VA losses of ≥15 letters for AMD patients.
Methods
This study was a secondary analysis of a cohort within a randomized clinical trial (CATT). Previous CATT reports provide a detailed summary of the CATT study design.6,19 CATT is registered with http://www.clinicaltrials.gov (NCT00593450). Design features relevant to this report are described here.
Study Patients
Study patients provided written informed consent to participate in CATT. The Institutional Review Board of each study site prospectively approved the CATT study protocol, and the study is in accordance with the Health Insurance Portability and Accountability Act regulations. The inclusion criteria were age ≥ 50 years, untreated choroidal neovascularization (CNV) from AMD in the study eye, VA of 20/25 to 20/320, and neovascularization or its sequelae at the foveal center. Baseline medical history was obtained from all patients.
Patients were randomized at study entry to one of four treatment arms: ranibizumab monthly, bevacizumab monthly, ranibizumab pro re nata (PRN), and bevacizumab PRN. At 1 year, study patients in the monthly groups were randomized again 1:1 to continued monthly treatment or PRN treatment. PRN treatment was given when there were signs of active neovascularization, defined as fluid on OCT, hemorrhage, decreased VA compared with the prior visit, or leakage or increased lesion size on fluorescein angiography.
All patients had monthly VA measurements using an electronic VA testing system by certified VA examiners who were masked to the patients’ treatment assignment.9 Protocol refraction before measurement of VA was required at baseline and weeks 4, 12, 24, 36, 52, 64, 76, 88, and 104. For efficiency, refractions were not routinely performed at every visit
Imaging Procedures
Stereoscopic, color fundus photography and fluorescein angiography were performed by certified photographers at baseline, 52 weeks, and 104 weeks. Stratus (version 4.0 or higher) time domain OCT systems (Carl Zeiss Meditec, Dublin, California) were used for first year visits and most second year visits. Spectral domain OCT images were obtained for 23% of second year visits. OCT images were obtained monthly in the PRN arms. Certified technicians masked to the patients’ treatment assignment followed standardized procedures and performed OCT imaging with macular thickness maps and fast macular thickness maps. OCT scans were independently analyzed by two certified OCT readers at the CATT OCT Reading Center, and photographs were analyzed by two certified readers at the CATT Photography Reading Center. Details about image acquisition and analysis by the reading centers are previously described.21–23
Data Analysis
Sporadic vision loss required VA data from three consecutive visits (i.e., VA1, VA2, and VA3). Sporadic vision loss was defined as a decline of ≥ 15 letters from the previous visit (i.e., VA1 – VA2 ≥ 15 letters), followed by a return at the next visit to no more than 5 letters worse than the visit before the VA loss (i.e., VA3 - VA1 ≥ -5 letters). 5 letters was chosen for the latter part of this definition since 89% of test-retest electronic VA measurements reportedly are within 5 letters.9 Sporadic vision loss of 30 letters was defined as a decline of ≥ 30 letters from the previous visit, followed by a return at the next visit to no more than 5 letters worse than the visit before the VA loss.
The incidence of sporadic vision loss loss was calculated as the proportion of eyes with sporadic vision loss within 2 years among all CATT patients. Mean VA during the study was compared between eyes with sporadic vision loss and all other study eyes. VA, fundus photograph features, and OCT features were compared at 2 years between eyes with and without sporadic vision loss. As noted, the two group t-test or the paired t-test was used for comparison of means. Fisher’s exact test or McNemar’s test was used for comparison of proportions.
For the 27 events of sporadic vision loss that did not coincide with an injection, investigators for these events were queried about the possible cause of vision loss, whether new hemorrhage at the macula was present, and why no injection was given.
For the evaluation of baseline medical history associations with sporadic vision loss, we focused on neurological and psychological histories because of their potential effects on visual function measurements.10–17 Additionally, a Functional Comorbidity Index was used to determine if patients with more comorbidities in their baseline history had an increased risk for sporadic vision loss. The Functional Comorbidity Index is an established measure of comorbid disease that correlates with physical function as the outcome of interest.24 This index contains 18 items such as visual impairment, congestive heart failure, arthritis, asthma, depression, anxiety, and neurological disease. The Functional Comorbidity Index is scored by summing the number of specific comorbidities in a patient’s medical history. A score of 0 indicates no relevant comorbidities, while a score of 18 indicates the highest number of comorbid illnesses.
The association of sporadic vision loss and non-ocular SAEs was investigated using non-ocular SAEs reported within 30 days (before or after) of the time of sporadic vision loss events. These timeframes were chosen as we were interested in knowing if sporadic vision loss has an association with a patient that is still recovering from a recent systemic SAE or that is becoming systemically ill and about to have an SAE. To investigate these potential associations, we matched sporadic vision loss patients (cases) with patients without sporadic vision loss (controls). The matching criteria were: drug, regimen, age (±3 years), Functional Comorbidity Index score (±2 points), and the number of visits with measured VA. To maximize the use of the controls, we allowed one case to have more than one control if available (i.e., 1:n matching).
The evaluation of factors associated with sporadic vision loss was first performed by univariate analysis using repeated measures logistic regression models to accommodate patients with more than one event of sporadic vision loss. Multivariate analyses started with the factors with a P < 0.20 in univariate analysis, and the final multivariate analysis model was developed using a backward selection procedure by keeping only predictors with a P < 0.05, with the exception of the drug and regimen groups. Adjusted odds ratios (OR) of sporadic vision loss and the 95% confidence intervals (95% CI) were calculated from the final multivariate logistic regression model for repeated measures. All data analyses were performed using SAS v9.2 (SAS Inc., Cary, NC). Two-sided P < 0.05 was considered statistically significant.
Results
Incidence and Visual Acuity
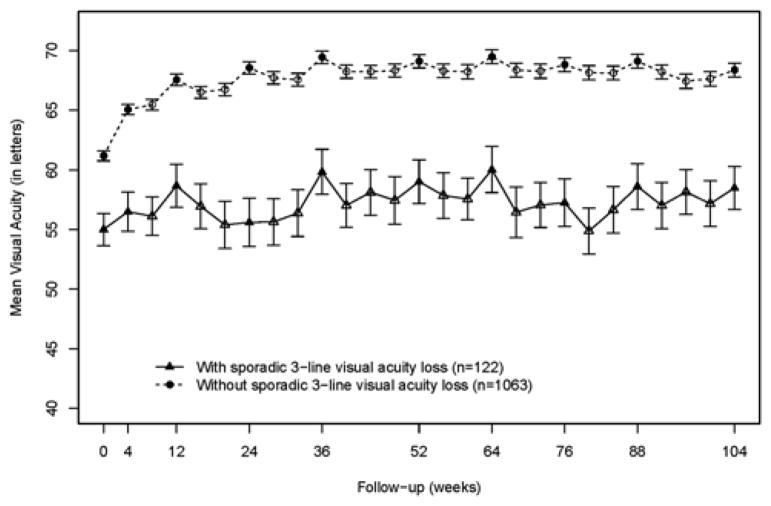
Over 2 years, 122 (10.3%) of the 1185 patients had at least one event of sporadic vision loss. There were 143 sporadic vision loss events. 102 (83.6%) of 122 patients had only one sporadic vision loss event. 19 (15.6%) had 2 events, and 1 patient (0.82%) had 4 events. There were 10 patients (0.8%) of the 1185 patients who developed sporadic vision loss of 30 letters, including one patient who had two events of this. The time to first sporadic vision loss event was evenly distributed across the entire duration of the study. For 59 (41.3%) of 143 sporadic vision loss events, the patient had a VA of 20/40 or better at the study visit preceding the sporadic vision loss. At all time points throughout the study, the patients with sporadic vision loss had a worse mean VA than patients without sporadic vision loss (Figure).
Figure.
Mean (+/− standard error) visual acuity over 2 years among patients with and without sporadic vision loss in the Comparison of Age-Related Macular Degeneration Treatments Trials. Study visits with refraction are represented by solid symbols.
Optical Coherence Tomography Features and Injections Around the Time of Sporadic Vision Loss Among Eyes Treated Pro Re Nata
Sporadic vision loss events occurred 98 times in 83 eyes treated according to the PRN dosing regimen. OCT analysis of the 98 events showed that the mean retinal thickness was 169 μm at the visit before the sporadic vision loss, 183 μm at the time of sporadic vision loss, and 151 μm at the visit afterwards (Table 1). Among all CATT patients, a change in OCT retinal thickness had a weak correlation with a change in VA (data not shown), and only 13 (13.3%) of these 98 sporadic vision loss events among eyes treated PRN coincided with an increase of retinal thickness of 50 μm or more. Foveal fluid was seen in 25 (25.5%) events before the sporadic vision loss, 37 (37.8%) at the time of sporadic vision loss, and 17 (17.4%) afterwards. Subretinal fluid at the fovea was seen in 12 (12.2%) events before the sporadic vision loss, 14 (14.3%) at the time of sporadic vision loss, and 5 (5.1%) afterwards.
Table 1.
Comparison of Treatment Status and Optical Coherence Tomography Features Before, At, and After Sporadic Vision Loss Among Eyes Treated Pro Re Nata in the Comparison of Age-Related Macular Degeneration Treatments Trials (83 eyes, 98 events)a- patients in pro re nata arm for 2 years or switchers in the 2nd year
| 4 Weeks Before Sporadic Vision Loss | At Sporadic Vision Loss | 4 Weeks After Sporadic Vision Loss | P Valuea (At vs Before Sporadic Vision Loss) | P Valuea (At vs After Sporadic Vision Loss) | P Valuea (Before vs After Sporadic Vision Loss) | |
|---|---|---|---|---|---|---|
| Events with injections in pro re nata groups, n(%) | 43 (43.9%) | 71 (72.4%) | 38 (38.8%) | <0.001 | <0.001 | 0.45 |
| Retinal thickness at foveal center (microns) | ||||||
| <120 | 24 (24.5%) | 21 (21.4%) | 28 (28.6%) | 0.28 | 0.006 | 0.43 |
| 120 to 212 | 55 (56.1%) | 50 (51.0%) | 58 (59.2%) | |||
| >212 | 17 (17.4%) | 25 (25.5%) | 12 (12.2%) | |||
| Mean (SE) | 169 (8) | 183 (10) | 151 (6) | 0.15 | <0.001 | 0.007 |
| Retinal fluid at foveal center | ||||||
| No | 69 (70.4%) | 59 (60.2%) | 79 (80.6%) | 0.08 | <0.001 | 0.07 |
| Yes | 25 (25.5%) | 37 (37.8%) | 17 (17.4%) | |||
| Sub-retinal fluid at foveal center | ||||||
| No | 82 (83.7%) | 80 (81.6%) | 90 (91.8%) | 0.78 | 0.007 | 0.01 |
| Yes | 12 (12.2%) | 14 (14.3%) | 5 (5.1%) |
SE = standard error
The totals may not add to 98 because of missing values in less than 5%.
McNemar test for comparing proportions, Paired t-test for comparing means.
Of the 98 events among eyes treated PRN, 43 (43.9%) had a study injection at the prior visit, and 71 (72.4%) had an injection at the visit when sporadic vision loss was noted. Among the 27 patients that were not treated at the time of sporadic vision loss, 6 (22.2%) had intraretinal or subretinal fluid at the fovea. Investigators were queried about these 27 events, and responses for 21 of these events were received. No identifiable cause for vision loss was found for 11 of these 21 events. For the remaining events, the cause of sporadic vision loss was thought to be related to a change in systemic health (3 of 21), progression of non-neovascular AMD (3/21), dry eyes (2/21), cataract (1/21), and increased subretinal fluid from neovascular AMD (1/21). The one patient that that had increased subretinal fluid refused treatment on that day, and the other patients were not treated because the investigator did not think there were signs of neovascular AMD activity. None of the responding investigators indicated that there was new hemorrhage at the macula.
Two Year Visual Acuity and Morphologic Features Associated with Sporadic Vision Loss
113 patients that had sporadic vision loss were available for data analysis of two year VA and morphology. At two years, the mean VA of sporadic vision loss patients was 58.5 letters (~20/63) as compared with 68.4 letters (~20/40) for those patients without sporadic vision loss (P < 0.001) (Table 2). The mean VA change from baseline was 3.1 letters for patients with sporadic vision loss compared with 6.7 letters for patients without sporadic vision loss (P = 0.03). 44 (38.9%) of 113 patients with sporadic vision loss were 20/40 or better as compared with 610 (66.2%) of 921 patients without sporadic vision loss (P < 0.001). 56 (49.6%) of 113 sporadic vision loss patients had a scar as compared with 371 (40.3%) of 921 patients without sporadic vision loss (P = 0.04). 16 (14.2%) of 113 sporadic vision loss patients had no pathology at the foveal center compared with 188 (20.4%) of 921 patients without sporadic vision loss (P = 0.02). Also, patients with sporadic vision loss had a larger total area of CNV lesion (9.97 mm2 vs. 8.04 mm2, P = 0.02). The presence of geographic atrophy was not significantly associated with sporadic vision loss (P = 0.27). OCT analysis showed that the percent with fluid and the mean retinal thickness were not associated with sporadic vision loss (P > 0.05).
Table 2.
Comparison of Visual Acuity and Morphology Features at Year 2 Between Eyes With and Without Sporadic Visual Acuity Loss in the Comparison of Age-Related Macular Degeneration Treatments Trials (N=1034)
| Visual Acuity and Morphology features at Year 2 | With Sporadic Vision Loss (N=113) | Without Sporadic Vision Loss (N=921) | P Valuea |
|---|---|---|---|
| Visual Acuity at Year 2 | |||
| 20/40 or better | 44 (38.9%) | 610 (66.2%) | <0.001 |
| Worse than 20/40 | 69 (61.1%) | 311 (33.8%) | |
| Mean (SE) | 58.5 (1.8) | 68.4 (0.6) | <0.001 |
| Mean (SE) Change from Baseline | 3.05 (1.88) | 6.74 (0.53) | 0.03 |
| Features of fundus photographs/Fluorescein angiogram | n (%) | n (%) | |
| Scar anywhere | 56 (49.6%) | 371 (40.3%) | 0.04 |
| Geographic atrophy anywhere | 28 (24.8%) | 188 (20.4%) | 0.27 |
| Pathology in foveal center | |||
| No pathology | 16 (14.2%) | 188 (20.4%) | 0.02 |
| Fluid | 3 (2.6%) | 30 (3.3%) | |
| CNV/SPED | 15 (13.3%) | 166 (18.0%) | |
| Scar | 38 (33.6%) | 191 (20.7%) | |
| Geographic atrophy | 11 (9.7%) | 52 (5.7%) | |
| RPE tear | 1 (0.9%) | 8 (0.9%) | |
| Other | 29 (25.7%) | 286 (31.1%) | |
| Total area of CNV lesion (mm2): | |||
| Mean (SE) | 9.97 (0.85) | 8.04 (0.27) | 0.02 |
| Mean (SE) Change from Baseline | 2.46 (0.84) | 1.86 (0.22) | 0.39 |
| OCT Features | |||
| Intra-retinal fluid: Yes (%) | 66 (60.6%) | 460 (51.8%) | 0.10 |
| Sub-retinal fluid: Yes (%) | 29 (27.9%) | 325 (36.9%) | 0.08 |
| Sub-RPE fluid: Yes (%) | 32 (32.3%) | 332 (38.2%) | 0.27 |
| Retinal thickness at foveal center (microns) | <0.001 | ||
| <120 | 43 (38.7%) | 203 (22.4%) | |
| 120–212 | 50 (45.1%) | 593 (65.5%) | |
| > 212 | 18 (16.2%) | 110 (12.1%) | |
| Mean (SE) | 147 (8) | 162 (3) | 0.06 |
| Mean (SE) Change from baseline | −92 (13) | −53 (4) | 0.004 |
| Sub-retinal tissue complex thickness at foveal | |||
| center (microns): | |||
| Mean (SE) | 124 (9) | 128 (4) | 0.77 |
| Mean (SE) Change from Baseline | −100 (15) | −80 (5) | 0.21 |
SE= standard error, CNV=choroidal neovascularization, SPED=serous retinal pigment epithelial detachment; RPE=retinal pigment epithelium
The totals may not add to 113 or 921 due to missing values in less than 5%.
Fisher’s Exact test for comparing proportions, and two groups t-test for comparing means.
Association of Serious Adverse Events with Sporadic Vision Loss
There were 11 events (10 patients) of sporadic vision loss of 30 letters, which met criteria for an ocular SAE. The causes reported by the investigator were: related to AMD (5/12), central retinal vein occlusion (1/12), and possibly related to systemic health condition (3/12). There was no clear cause stated for the vision loss in 2/12 of these patients. Furthermore, an evaluation of ocular and systemic AEs did not show any significant associations (data not shown).
Using a matched case control approach, we also evaluated whether a non-ocular SAE within 30 days (before or after) was associated with sporadic vision loss. Among 94 matched case-control pairs that met criteria for analysis, 6 (6.4%) of 94 patients with sporadic vision loss had a non-ocular SAE within 30 days compared with 9 (4.5%) of 199 matched controls without sporadic vision loss (P = 0.48). Similarly, 47 (38.5%) of 122 patients with sporadic vision loss had a non-ocular SAE during the 2 years of the trial compared with 377 (35.5%) of 1063 patients without sporadic vision loss (P = 0.55).
Baseline Medical History and Ocular Predictors of Sporadic Vision Loss
The univariate analysis (Supplemental Table 1, Supplemental Material at AJO.com) showed that a baseline neurological history and a baseline psychological history were risk factors for sporadic vision loss. 65 (11.9%) of 546 patients with a neurological history had sporadic vision loss compared with 57 (8.9%) of 639 patients without a neurological history (P = 0.04). 32 (13.8%) of 232 patients with a psychological history had sporadic vision loss compared with 90 (9.4%) of 953 patients without a psychological history (P = 0.02). Within the broad category of psychological disorders, subcategory analysis showed an “anxiety” history for 12 (9.8%) of 122 sporadic vision loss patients and only 43 (4.1%) of 1063 patients without sporadic vision loss (P = 0.01) (Supplemental Table 2, Supplemental Material at AJO.com). Additionally, the neurological history subcategory of “syncope” was present for 10 (8.2%) of 122 patients with sporadic vision loss compared with 28 (2.6%) of 1063 patients without sporadic vision loss (P = 0.004). Of note, the one patient that had 2 events of sporadic vision loss of 30 letters had a baseline history including early Alzheimer’s disease and anxiety with hallucinations; for both of these events, the investigator did not find an ocular cause and thought that the medical history played a role. To further analyze whether patients with more comorbidities in their medical history had an increased risk for sporadic vision loss events, we applied a Functional Comorbidity Index to the data.24 However, there was no significant association between the Functional Comorbidity Index values and sporadic vision loss.
Univariate analysis of baseline ocular and OCT features are provided in the online supplement (Supplemental Tables 3–4, Supplemental Material at AJO.com).
In multivariate analysis, history of a psychological disorder (OR 1.52, 95% CI: 1.03–2.25) was an independent predictor (Table 3). Further analysis of psychological subcategories demonstrated that an anxiety history was the driving force for this association (OR 1.90, 95% CI: 1.12–3.24). While a neurological history was not a significant independent predictor, further subcategory analysis showed that a syncope history was an independent predictor (OR 2.75, 95% CI: 1.45–5.22). Other independent baseline predictors for sporadic vision loss included: worse baseline VA (OR 2.92, 95% CI: 1.65–5.17 for baseline VA of 20/200–20/320 compared with 20/25–20/40), baseline scar (OR 2.21; 95% CI: 1.22–4.01), and OCT presence of foveal intra-retinal fluid (OR 1.80; 95% CI: 1.11–2.91) (Table 3). Drug or treatment regimen was not associated with sporadic vision loss (P > 0.10).
Table 3.
Multivariate Analysis For Baseline Predictors of Sporadic Vision Loss in the Comparison of Age-Related Macular Degeneration Treatments Trials (N=1152)
| Baseline Characteristics | Patients at Baseline, N | Sporadic Vision Loss in 2 Years, n (%) | Odds Ratio (95% CI) | P Valuea |
|---|---|---|---|---|
| Baseline visual acuity in study eye | ||||
| 20/25–40 | 410 | 29 (7.1%) | 1.00 | 0.002 |
| 20/50–80 | 431 | 41 (9.5%) | 1.43 (0.89, 2.27) | |
| 20/100–160 | 233 | 32 (13.7%) | 1.88 (1.15, 3.08) | |
| 20/200–320 | 78 | 18 (23.1%) | 2.92 (1.65, 5.17) | |
| Refraction when visual acuity measured (by visit) | ||||
| No | 17877 | 107 (0.6%) | 1.00 | 0.01 |
| Yes | 9153 | 34 (0.4%) | 0.62 (0.42, 0.91) | |
| Psychological disorder | ||||
| No | 922 | 88 (9.5%) | 1.00 | 0.03 |
| Yes | 230 | 32 (13.9%) | 1.52 (1.03, 2.25) | |
| Baseline fibrotic or atrophic scar | ||||
| No | 1108 | 109 (9.8%) | 1.00 | 0.009 |
| Yes | 44 | 11 (25.0%) | 2.21 (1.22, 4.01) | |
| Intra-retinal fluid | ||||
| No Fluid | 275 | 20 (7.3%) | 1.00 | 0.04 |
| Fluid not in fovea center | 315 | 28 (8.9%) | 1.33 (0.76, 2.34) | |
| Fluid in fovea center | 562 | 72 (12.8%) | 1.80 (1.11, 2.91) | |
| Drug | ||||
| Ranibizumab | 584 | 64 (11.0%) | 1.00 | 0.41 |
| Bevacizumab | 568 | 56 (9.9%) | 0.86 (0.61, 1.23) | |
| Regimen | ||||
| Monthly | 303 | 32 (10.6%) | 1.00 | 0.14 |
| Switched | 266 | 22 (8.3%) | -- | |
| Pro re nata | 583 | 66 (11.3%) | 1.32 (0.91, 1.92) | |
Patients (n=33) with missing data in any of variables in the final multivariate model were excluded from analysis.
CI = confidence interval.
From generalized linear model using generalized estimating equation to account for correlation from multiple events of sporadic visual acuity loss in some eyes.
Initial model includes: baseline VA of study eye, lesion type, fibrotic or atrophic scar, retinal fluid, psychological disorder, neurological disorder, refraction status, drug and regimen.
Association of Refraction Status with Sporadic Vision Loss
Refractions were performed approximately every 3 months, and multivariate analysis showed that refraction decreased the likelihood of sporadic vision loss (OR 0.62; 95% CI: 0.42–0.91) (Table 3). As seen in the Figure, refractions generally gave a small but consistent VA boost for all patients. After anti-VEGF therapy stabilized the vision (after 12 weeks), refractions were associated with a mean VA score 1.21 letters (95% CI: 1.00–1.42) better than visits without refraction.
Discussion
Over 2 years of monthly visits, 122 (10.3%) of 1185 CATT patients had sporadic vision loss and these patients had less VA gains at two years compared with patients without sporadic vision loss. There were significant associations of sporadic vision loss with worse baseline vision, and this is consistent with the increased variability of vision measurements with lower acuities.9 Furthermore, there were some AMD-related associations such as the presence of a scar or other pathology at the fovea. These findings can explain the lower mean VA gains of this subgroup. Still, for those patients who did have OCTs around the time of sporadic vision loss, the average increase in mean retinal thickness was only 14 microns more compared with the visit prior to the sporadic vision loss event. While some patients may have had acute worsening of the disease, many others did not, and this caused the average change in mean retinal thickness to be modest. We attempted to further correlate sporadic vision loss events with changes in OCT morphology. However, only 13 of 98 sporadic vision loss events among eyes treated PRN correlated with a ≥ 50 μm change in retina thickness. Thus, it should be emphasized that there were many cases in which the cause of sporadic vision loss was not clearly linked to AMD.
Data from the eyes treated PRN further support the conclusion that many cases of sporadic vision loss were not directly linked to worsening of AMD. Of particular interest is the finding that investigators did not give an injection for 27 (27.6%) of 98 sporadic vision loss events in eyes treated PRN, even though vision loss was an indication for PRN treatment. 6 (22.2%) of these 27 patients had fluid at the fovea based on OCT reading center evaluation, and this also was a treatment indication. It was previously reported that approximately 30% of patients in PRN groups did not receive an injection even though the reading center found fluid on the OCT.6 For these untreated sporadic vision loss cases with fluid, the investigator may not have noticed a small amount of fluid or, less likely, thought that the fluid was not significant enough to warrant treatment. When investigators for these 27 events were queried, 11 of the 21 responses indicated that there was no identifiable cause and 3 indicated that it may be related to a change in systemic health. Only 1 of 21 responses indicated that there was a worsening of neovascular AMD. Our data suggests that there were other causes for sporadic vision loss including a low baseline VA, syncope history, anxiety history, or absence of refraction.
Previous reports have highlighted the role that depression plays on visual function in AMD patients.10–13 While we did not find that a baseline history of depression specifically is associated with sporadic vision loss, our multivariate analysis showed that a psychiatric history generally increases the odds of sporadic vision loss. Further analysis showed that a history of anxiety, rather than depression, was the driving force behind the significance of a psychiatric history. Additionally, the neurological subcategory of syncope was a significant predictor of sporadic vision loss. Among the elderly population, the most common causes of syncope are orthostatic hypotension, volume depletion, cardiovascular events, vasovagal reflex, and idiopathic.25,26 These data suggest that acute changes in mental health as well as those factors that lead to syncope may lead to sporadic vision loss. One may wonder if patients who are “sicker” overall at baseline are more likely to have sporadic vision loss, but we could not find a clear association of this through our use of a Functional Comorbidity Index. We also could not find any associations between SAEs or AEs with sporadic vision loss. This is consistent with Wolf’s analysis of acute vision loss in the MARINA and ANCHOR studies,18 although they looked at ocular adverse events and did not specifically focus on transient vision loss. Given the paramount importance of vision measurements, it may be worthwhile for investigators to consider these findings when enrolling patients for clinical trials. This is emphasized by a study patient with a history of anxiety and hallucinations who had two events of sporadic vision loss of 30 letters.
In an effort to increase efficiency, some clinical trials do not perform refracted VA at every study visit. This data from CATT showed that refraction slightly boosted the mean VA measurements, and absence of refraction was associated with sporadic vision loss. Although the vision difference on average was small, the data demonstrate the important role of study visit refractions. Some studies have defined visits with refractions and a protocol stipulation for the visits without routine refractions. If the VA has changed by ten or more letters since the last visit, then a refraction should be performed.27
There are several limitations of this secondary analysis. In the CATT, OCT was not required at every visit for the monthly treatment patients. Thus, we had OCT data from the time of all sporadic vision loss events for PRN treated patients but not for monthly treated patients. Fundus photos were performed only at the baseline, 1 year, and 2 years visits. Although we recognize that an image characteristic at the last study visit may not have been present at the time of the sporadic vision loss event, we did investigate the differences to understand why sporadic vision loss patients had a lower mean VA at two years. It should be noted that we cannot exclude the possibility of hemorrhage at the macula at the time of sporadic vision loss in some patients since photos were not available at every visit. However, investigators for the 27 events in PRN eyes that were not treated were queried about the sporadic vision loss and the decision not to treat. None of the responses indicated that there was new hemorrhage at the macula. While hemorrhage at the macula could explain some of the 143 events of sporadic vision loss, the data suggests that there were several other factors involved in sporadic vision loss as well.
In summary, approximately 10% of CATT patients had a sporadic vision loss event during the trial, and 27.6% of sporadic vision loss events in PRN groups did not coincide with an injection. While there is some expected relationship between acute worsening of AMD and sporadic vision loss, there certainly were other associations with these aberrant VA measurements, including worse baseline vision, psychiatric history, syncope history, and lack of refraction. We believe that these data are valuable for clinicians, those planning clinical trials, and trial investigators.
Supplementary Material
Acknowledgments
Supported by cooperative agreements U10 EY017823, U10 EY017825, U10 EY017826, and U10 EY017828 from the National Eye Institute, National Institutes of Health, Department of Health and Human Services.
Biography

Dr. Benjamin Kim is an Assistant Professor of Ophthalmology at the Scheie Eye Institute of the University of Pennsylvania. Dr. Kim completed his residency training at the Massachusetts Eye and Ear Infirmary and a fellowship in medical and surgical retina at the Wilmer Eye Institute. Dr. Kim’s interests are in clinical trials, age-related macular degeneration, and diabetes.
Credit Roster for the Comparison of AMD Treatments Trials Clinical Centers (Ordered by Number of Patients Enrolled)
Certified Roles at Clinical Centers
Clinic Coordinator (CC), Data Entry Staff (DE), Participating Ophthalmologist (O), Ophthalmic Photographer (OP); Optical Coherent Tomography Technician (OCT), Principal Investigator (PI), Refractionist (R), Visual Acuity Examiner (VA)
VitreoRetinal Surgery, PA (Edina, MN)
David F. Williams, MD (PI); Sara Beardsley, COA (VA/R); Steven Bennett, MD (O); Herbert Cantrill, MD (O); Carmen Chan-Tram, COA (VA/R); Holly Cheshier, CRA, COT, OCTC (OP); Kathyrn Damato, COT (VA); John Davies, MD (O); Sundeep Dev, MD (O); Julianne Enloe, CCRP, COA (CC); Gennaro Follano (OP/OCT); Peggy Gilbert, COA (VA/R); Jill Johnson, MD (O); Tori Jones, COA (OCT); Lisa Mayleben, COMT (CC/VA/R/OCT); Robert Mittra, MD (O); Martha Moos, COMT, OSA (VA/R); Ryan Neist, COMT (VA/R); Neal Oestreich, COT (CC); Polly Quiram, MD (O); Robert Ramsay, MD (O); Edwin Ryan, MD (O); Stephanie Schindeldecker, OA (VA/R); John Snater, COA (VA); Trenise Steele, COA (VA); Dwight Selders, COA (VA/R); Jessica Tonsfeldt, AO (OP/OCT); Shelly Valardi, COT (VA/R).
Texas Retina Associates (Dallas, TX)
Gary Edd Fish, MD (PI); Hank A. Aguado, CRA (OP/OCT); Sally Arceneaux (CC/VA/R); Jean Arnwine (CC); Kim Bell, COA (VA/R); Tina Bell (CC/OCT); Bob Boleman (OP); Patricia Bradley, COT (CC); David Callanan, MD (O); Lori Coors, MD (O); Jodi Creighton, COA (VA/R); Timothy Crew, COA (OCT); Kimberly Cummings (OP/OCT); Christopher Dock (OCT); Karen Duignan, COT (VA/R); Dwain Fuller, MD (O); Keith Gray (OP/OCT); Betsy Hendrix, COT, ROUB (OCT); Nicholas Hesse (OCT); Diana Jaramillo, COA (OCT); Bradley Jost, MD (O); Sandy Lash (VA/R); Laura Lonsdale, CCRP (DE); Michael Mackens (OP/OCT); Karin Mutz, COA (CC); Michael Potts (VA/R); Brenda Sanchez (VA/R); William Snyder, MD (O); Wayne Solley, MD (O); Carrie Tarter (VA/R); Robert Wang, MD (O); Patrick Williams, MD (O).
Southeastern Retina Associates (Knoxville, TN)
Stephen L. Perkins, MD (PI); Nicholas Anderson, MD (O); Ann Arnold, COT (VA/R); Paul Blais (OP/OCT); Joseph Googe, MD (O); Tina T. Higdon, (CC); Cecile Hunt (VA/R); Mary Johnson, COA (VA/R); James Miller, MD (O); Misty Moore (VA/R); Charity K. Morris, RN (CC); Christopher Morris (OP/OCT); Sarah Oelrich, COT (OP/OCT); Kristina Oliver, COA (VA/R); Vicky Seitz, COT (VA/R); Jerry Whetstone (OP/OCT).
Retina Vitreous Consultants (Pittsburgh, PA)
Bernard H. Doft (PI); Jay Bedel, RN, (CC); Robert Bergren, MD (O); Ann Borthwick (VA/R); Paul Conrad, MD, PHD (O); Amanda Fec (OCT); Christina Fulwylie (VA/R); Willia Ingram (DE); Shawnique Latham (VA/R); Gina Lester (VA/R); Judy Liu, MD (O); Louis Lobes, MD (O); Nicole M. Lucko, (CC); Holly Mechling (CC); Lori Merlotti, MS, CCRC (CC); Keith McBroom (OCT); Karl Olsen, MD (O); Danielle Puskas, COA (VA/R); Pamela Rath, MD (O); Maria Schmucker (CC); Lynn Schueckler (OCT); Christina Schultz (CC/VA/R); Heather Shultz (OP/OCT); David Steinberg, CRA (OP/OCT); Avni Vyas, MD (O); Kim Whale (VA/R); Kimberly Yeckel, COA, COT (VA/R).
Ingalls Memorial Hospital/Illinois Retina Associates (Harvey, IL)
David H. Orth, MD (PI); Linda S. Arredondo, RN (CC/VA); Susan Brown (VA/R); Barbara J. Ciscato (CC/VA); Joseph M. Civantos, MD (O); Celeste Figliulo (VA/R); Sohail Hasan, MD (O); Belinda Kosinski, COA (VA/R); Dan Muir (OP/OCT); Kiersten Nelson (OP/OCT); Kirk Packo, MD (O); John S. Pollack, MD (O); Kourous Rezaei, MD (O); Gina Shelton (VA); Shannya Townsend-Patrick (OP/OCT); Marian Walsh, CRA (OP/OCT).
West Coast Retina Medical Group, Inc. (San Francisco, CA)
H. Richard McDonald, MD (PI); Nina Ansari (VA/R/OCT); Amanda Bye, (OP/OCT); Arthur D. Fu, MD (O); Sean Grout (OP/OCT); Chad Indermill (OCT); Robert N. Johnson, MD (O); J. Michael Jumper, MD (O); Silvia Linares (VA/R); Brandon J. Lujan, MD (O); Ames Munden (OP/OCT); Meredith Persons (CC); Rosa Rodriguez (CC); Jennifer M. Rose (CC); Brandi Teske, COA (VA/R); Yesmin Urias (OCT); Stephen Young (OP/OCT).
Retina Northwest, P.C. (Portland, OR)
Richard F. Dreyer, MD (PI); Howard Daniel (OP/OCT); Michele Connaughton, CRA (OP/OCT); Irvin Handelman, MD (O); Stephen Hobbs (VA/R/OCT); Christine Hoerner (OP/OCT); Dawn Hudson (VA/R/OCT); Marcia Kopfer, COT (CC/VA/R/OCT); Michael Lee, MD (O); Craig Lemley, MD (O); Joe Logan, COA (OP/OCT); Colin Ma, MD (O); Christophe Mallet (VA/R); Amanda Milliron (VA/R); Mark Peters, MD (O); Harry Wohlsein, COA (OP).
Retinal Consultants Medical Group, Inc. (Sacramento, CA)
Joel A. Pearlman, MD, PHD (PI); Margo Andrews (OP/OCT); Melissa Bartlett (OCT); Nanette Carlson (CC/OCT); Emily Cox (VA/R); Robert Equi, MD (O); Marta Gonzalez (VA/R/OCT); Sophia Griffin (OP/OCT); Fran Hogue (VA/R); Lance Kennedy (OP/OCT); Lana Kryuchkov (OCT); Carmen Lopez (VA/R); Danny Lopez (OP/OCT); Bertha Luevano (VA/R); Erin McKenna, (CC); Arun Patel, MD (O); Brian Reed, MD (O); Nyla Secor (CC/OCT); Iris R. Sison (CC); Tony Tsai, MD (O); Nina Varghis, (CC); Brooke Waller (OCT); Robert Wendel, MD (O); Reina Yebra (OCT).
Retina Vitreous Center, PA (New Brunswick, NJ)
Daniel B. Roth, MD (PI); Jane Deinzer, RN (CC/VA/R); Howard Fine, MD MHSC (O); Flory Green (VA/R); Stuart Green, MD (O); Bruce Keyser, MD (O); Steven Leff, MD (O); Amy Leviton (VA/R); Amy Martir (OCT); Kristin Mosenthine (VA/R/OCT); Starr Muscle, RN (CC); Linda Okoren (VA/R); Sandy Parker (VA/R); Jonathan Prenner, MD (O); Nancy Price (CC); Deana Rogers (OP/OCT); Linda Rosas (OP/OCT); Alex Schlosser (OP/OCT); Loretta Studenko (DE); Thea Tantum (CC); Harold Wheatley, MD (O).
Vision Research Foundation/Associated Retinal Consultants, P.C. (Royal Oak, MI)
Michael T. Trese, MD (PI); Thomas Aaberg, MD (O); Tina Bell (VA/R/OP/OCT); Denis Bezaire, CRA (OP/OCT); Craig Bridges, CRA (OP/OCT); Doug Bryant, CRA (OP/OCT); Antonio Capone, MD (O); Michelle Coleman, RN (CC); Christina Consolo, CRA, COT (OP/OCT); Cindy Cook, RN (CC); Candice DuLong (VA/R); Bruce Garretson, MD (O); Tracy Grooten (VA/R); Julie Hammersley, RN (CC); Tarek Hassan, MD (O); Heather Jessick (OP/OCT); Nanette Jones (VA/R/OP/OCT); Crystal Kinsman (VA/R); Jennifer Krumlauf (VA/R); Sandy Lewis, COT (VA/R/OP/OCT); Heather Locke (VA/R); Alan Margherio, MD (O); Debra Markus, COT (CC/VA/R/OP/OCT); Tanya Marsh, COA (OP/OCT); Serena Neal (CC); Amy Noffke, MD (O); Kean Oh, MD (O); Clarence Pence (OP/OCT); Lisa Preston (VA/R); Paul Raphaelian, MD (O); Virginia R. Regan, RN, CCRP (VA/R); Peter Roberts (OP/OCT); Alan Ruby, MD (O); Ramin Sarrafizadeh, MD, PHD (O); Marissa Scherf (OP/OCT); Sarita Scott (VA/R); Scott Sneed, MD (O); Lisa Staples (CC); Brad Terry (VA/R/OP/OCT); Matthew T. Trese (OCT); Joan Videtich, RN (VA/R); George Williams, MD (O); Mary Zajechowski, COT, CCRC (CC/VA/R).
Barnes Retina Institute (St. Louis, MO)
Daniel P. Joseph, MD (PI); Kevin Blinder, MD (O); Lynda Boyd, COT (VA/R); Sarah Buckley (OP/OCT); Meaghan Crow (VA/R); Amanda Dinatale, (OCT); Nicholas Engelbrecht, MD (O); Bridget Forke (OP/OCT); Dana Gabel (OP/OCT); Gilbert Grand, MD (O); Jennifer Grillion-Cerone (VA/R); Nancy Holekamp, MD (O); Charlotte Kelly, COA (VA/R); Ginny Nobel, COT (CC); Kelly Pepple (VA/R); Matt Raeber, (OP/OCT); P. Kumar Rao, MD (O); Tammy Ressel, COT (VA/R); Steven Schremp (OCT); Merrilee Sgorlon (VA/R); Shantia Shears, MA (CC); Matthew Thomas, MD (O); Cathy Timma (VA/R); Annette Vaughn,(OP/OCT); Carolyn Walters, COT (CC/VA/R); Rhonda Weeks, CRC (CC/VA/R); Jarrod Wehmeier (OP/OCT); Tim Wright (OCT).
The Retina Group of Washington (Chevy Chase, MD)
Daniel M. Berinstein, MD (PI); Aida Ayyad (VA/R); Mohammed K. Barazi, MD (O); Erica Bickhart (CC/VA/R); Tracey Brady (OCT); Lisa Byank, MA (CC); Alysia Cronise, COA (VA/R); Vanessa Denny (VA/R); Courtney Dunn (VA/R); Michael Flory (OP/OCT); Robert Frantz (OP/OCT); Richard A. Garfinkel, MD (O); William Gilbert, MD (O); Michael M. Lai, MD, PHD (O); Alexander Melamud, MD (O); Janine Newgen (VA/R); Shamekia Newton (CC); Debbie Oliver (CC); Michael Osman, MD (O); Reginald Sanders, MD (O); Manfred von Fricken, MD (O).
Retinal Consultants of Arizona (Phoenix, AZ)
Pravin Dugel, MD (PI); Sandra Arenas (CC); Gabe Balea (OCT); Dayna Bartoli (OP/OCT); John Bucci (OP/OCT); Jennifer A. Cornelius (CC); Scheleen Dickens, (CC); Don Doherty (OP/OCT); Heather Dunlap, COA (VA/R); David Goldenberg, MD (O); Karim Jamal, MD (O); Norma Jimenez (OP/OCT); Nicole Kavanagh (VA/R); Derek Kunimoto, MD (O); John Martin (OP/OCT); Jessica Miner, RN (VA/R); Sarah Mobley, CCRC (CC/VA/R); Donald Park, MD (O); Edward Quinlan, MD (O); Jack Sipperley, MD (O); Carol Slagle (R); Danielle Smith (OP/OCT); Miguelina Yafchak (OCT); Rohana Yager, COA (OP/OCT).
Casey Eye Institute (Portland, OR)
Christina J. Flaxel, MD (PI); Steven Bailey, MD (O); Peter Francis, MD, PHD (O); Chris Howell, (OCT); Thomas Hwang, MD (O); Shirley Ira, COT (VA/R); Michael Klein, MD (O); Andreas Lauer, MD (O); Teresa Liesegang, COT (CC/VA/R); Ann Lundquist, (CC/VA/R); Sarah Nolte (DE); Susan K. Nolte (VA/R); Scott Pickell (OP/OCT); Susan Pope, COT (VA/R); Joseph Rossi (OP/OCT); Mitchell Schain (VA/R); Peter Steinkamp, MS (OP/OCT); Maureen D. Toomey (CC/VA/R); Debora Vahrenwald, COT (VA/R); Kelly West (OP/OCT).
Emory Eye Center (Atlanta, GA)
Baker Hubbard, MD (PI); Stacey Andelman, MMSC, COMT (CC/VA/R); Chris Bergstrom, MD (O); Judy Brower, COMT (CC/VA/R); Blaine Cribbs, MD (O); Linda Curtis (VA/R); Jannah Dobbs (OP/OCT); Lindreth DuBois, MED, MMSC, CO, COMT (CC/VA/R); Jessica Gaultney (OCT); Deborah Gibbs, COMT, CCRC (VA/R); Debora Jordan, CRA (OP/OCT); Donna Leef, MMSC, COMT (VA/R); Daniel F. Martin, MD (O); Robert Myles, CRA (OP); Timothy Olsen, MD (O); Bryan Schwent, MD (O); Sunil Srivastava, MD (O); Rhonda Waldron, MMSC, COMT, CRA, RDMS (OCT).
Charlotte Eye, Ear, Nose & Throat Associates/Southeast Clinical Research (Charlotte, NC)
Andrew N. Antoszyk, MD (PI); Uma Balasubramaniam, COA (OCT); Danielle Brooks, CCRP (VA/R); Justin Brown, MD (O); David Browning, MD, PHD (O); Loraine Clark, COA (OP/OCT); Sarah Ennis, CCRC (VA/R); Susannah Held (OCT); Jennifer V. Helms, CCRC,(CC); Jenna Herby, CCRC (CC); Angie Karow, CCRP (VA/R); Pearl Leotaud, CRA (OP/OCT); Caterina Massimino (OCT); Donna McClain, COA (OP/OCT); Michael McOwen, CRA (OP/OCT); Jennifer Mindel, CRA, COA (OP/OCT); Candace Pereira, CRC (CC); Rachel Pierce, COA (VA/R); Michele Powers (OP/OCT); Angela Price, MPH, CCRC (CC); Jason Rohrer (CC); Jason Sanders, MD (O).
California Retina Consultants (Santa Barbara, CA)
Robert L. Avery, MD (PI); Kelly Avery (VA/R); Jessica Basefsky (CC/OCT); Liz Beckner (OP); Alessandro Castellarin, MD (O); Stephen Couvillion, MD (O); Jack Giust (CC/OCT); Matthew Giust (OP); Maan Nasir, MD (O); Dante Pieramici, MD (O); Melvin Rabena (VA/R); Sarah Risard (VA/R/OCT/DE); Robert See, MD (O); Jerry Smith (VA/R); Lisha Wan (VA/R).
Mayo Clinic (Rochester, MN)
Sophie J. Bakri, MD (PI); Nakhleh Abu-Yaghi, MD (O); Andrew Barkmeier, MD (O); Karin Berg, COA (VA/R); Jean Burrington, COA (VA/R); Albert Edwards, MD (O); Shannon Goddard, COA (OP/OCT); Shannon Howard (VA/R); Raymond Iezzi, MD (O); Denise Lewison, COA (OP/OCT); Thomas Link, CRA (OP/OCT); Colin A. McCannel, MD (O); Joan Overend (VA/R); John Pach, MD (O); Margaret Ruszczyk, CCRP (CC); Ryan Shultz, MD (O); Cindy Stephan, COT (VA/R); Diane Vogen (CC).
Dean A. McGee Eye Institute (Oklahoma City, OK)
Reagan H. Bradford Jr, MD (PI); Vanessa Bergman, COA, CCRC (CC); Russ Burris (OP/OCT); Amanda Butt, CRA (OP/OCT); Beth Daniels, COA (CC); Connie Dwiggins, CCRC (CC); Stephen Fransen, MD (O); Tiffany Guerrero (CC/DE); Darin Haivala, MD (O); Amy Harris (CC); Sonny Icks (CC/DE); Ronald Kingsley, MD (O); Lena Redden (VA/R); Rob Richmond (OP/OCT); Brittany Ross (VA/R); Kammerin White, CCRC (VA/R); Misty Youngberg, COA, CCRC (VA/R).
Ophthalmic Consultants of Boston (Boston, MA)
Trexler M. Topping, MD (PI); Steve Bennett (OCT); Sandy Chong (VA/R); Mary Ciotti, COA (CC); Tina Cleary, MD (O); Emily Corey (VA/R); Dennis Donovan (OP/OCT); Albert Frederick, MD (O); Lesley Freese (CC/VA/R); Margaret Graham (OP/OCT); Natalya Gud, COA (VA/R); Taneika Howard (VA/R); Mike Jones (OP/OCT); Michael Morley, MD (O); Katie Moses (VA/R); Jen Stone (VA/R); Robin Ty, COA (VA/R); Torsten Wiegand, PHD, MD (O); Lindsey Williams (CC); Beth Winder (CC).
Tennessee Retina, P.C. (Nashville, TN)
Carl C. Awh, MD (PI); Michelle Amonette (OCT); Everton Arrindell, MD (O); Dena Beck (OCT); Brandon Busbee, MD (O); Amy Dilback (OP/OCT); Sara Downs (VA/R); Allison Guidry, COA (VA/R); Gary Gutow, MD (O); Jackey Hardin (VA/R); Sarah Hines, COA (CC); Emily Hutchins (VA/R); Kim LaCivita, MA (OP/OCT); Ashley Lester (OP/OCT); Larry Malott (OP/OCT); MaryAnn McCain, RN, CNOR (CC); Jayme Miracle (VA/R); Kenneth Moffat, MD (O); Lacy Palazzotta (VA/R); Kelly Robinson, COA (VA/R); Peter Sonkin, MD (O); Alecia Travis (OP/OCT); Roy Trent Wallace, MD (O); Kelly J. Winters, COA (CC); Julia Wray (OP/OCT).
Retina Associates Southwest, P.C. (Tucson, AZ)
April E. Harris, MD (PI); Mari Bunnell (OCT); Katrina Crooks (VA/R); Rebecca Fitzgerald, CCRC (CC/OCT); Cameron Javid, MD (O); Corin Kew (VA/R); Erica Kill, VAE (VA/R); Patricia Kline (VA/R); Janet Kreienkamp (VA/R); Maricruz Martinez (CC/OCT); Roy Ann Moore, OMA (CC/OCT); Egbert Saavedra, MD (O); LuAnne Taylor, CSC (CC/OCT); Mark Walsh, MD (O); Larry Wilson (OP).
Midwest Eye Institute (Indianapolis, IN)
Thomas A. Ciulla, MD (PI); Ellen Coyle, COMT (VA/R); Tonya Harrington, COA (VA/R); Charlotte Harris, COA (VA/OCT); Cindi Hood (OCT); Ingrid Kerr, COA (VA/R); Raj Maturi, MD (O); Dawn Moore (OCT); Stephanie Morrow, COA (OP); Jennifer Savage, COA (VA); Bethany Sink, COA (CC/VA/R); Tom Steele, CRA (OP); Neelam Thukral, CCRC (CC/OCT); Janet Wilburn, COA (CC).
National Ophthalmic Research Institute (Fort Myers, FL)
Joseph P. Walker, MD (PI); Jennifer Banks (VA/R); Debbie Ciampaglia (OP/OCT); Danielle Dyshanowitz (VA/R); Jennifer Frederick, CRC (CC); A. Tom Ghuman, MD (O); Richard Grodin, MD (O); Cheryl Kiesel, CCRC (CC); Eileen Knips, RN, CCRC, CRA (OP/OCT); Jonathan McCue (VA/R); Maria Ortiz (VA/R); Crystal Peters, CCRC (CC); Paul Raskauskas, MD (O); Etienne Schoeman (OP/OCT); Ashish Sharma, MD (O); Glenn Wing, MD (O), Rebecca Youngblood (CC).
University of Wisconsin Madison (Madison, WI)
Suresh R. Chandra, MD (PI); Michael Altaweel, MD (O); Barbara Blodi, MD (O); Kathryn Burke, BA (VA/R); Kristine A. Dietzman, (CC); Justin Gottlieb, MD (O); Gene Knutson (OP/OCT); Denise Krolnik (OP/OCT); T. Michael Nork, MD (O); Shelly Olson (VA/R); John Peterson, CRA (OP/OCT); Sandra Reed (OP/OCT); Barbara Soderling (VA/R); Guy Somers (VA/R); Thomas Stevens, MD (O); Angela Wealti, (CC).
Duke University Eye Center (Durham, NC)
Srilaxmi Bearelly, MD (PI); Brenda Branchaud (VA/R); Joyce W. Bryant, COT, CPT (CC/VA/R); Sara Crowell (CC/VA); Sharon Fekrat, MD (O); Merritt Gammage (OP/OCT); Cheala Harrison, COA (VA/R); Sarah Jones (VA); Noreen McClain, COT, CPT, CCRC (VA/R); Brooks McCuen, MD (O); Prithvi Mruthyunjaya, MD (O); Jeanne Queen, CPT (OP/OCT); Neeru Sarin, MBBS (VA/R); Cindy Skalak, RN, COT (VA/R); Marriner Skelly, CRA (OP/OCT); Ivan Suner, MD (O); Ronnie Tomany (OP/OCT); Lauren Welch (OP/OCT).
University of California-Davis Medical Center (Sacramento, CA)
Susanna S. Park, MD, PHD (PI); Allison Cassidy (VA/R); Karishma Chandra (OP/OCT); Idalew Good (VA/R); Katrina Imson (CC); Sashi Kaur (OP/OCT); Helen Metzler, COA, CCRP (CC/VA/R); Lawrence Morse, MD, PHD (O); Ellen Redenbo, ROUB (OP/OCT); Marisa Salvador (VA/R); David Telander, MD (O); Mark Thomas, CRA (OCT); Cindy Wallace, COA (CC).
University of Louisville School of Medicine, KY (Louisville, KY)
Charles C. Barr, MD (PI); Amanda Battcher (VA/R); Michelle Bottorff, COA (CC/OCT); Mary Chasteen (VA/R); Kelly Clark (VA/R); Diane Denning, COT (OCT); Debra Schoen (OP); Amy Schultz (OP); Evie Tempel, CRA, COA (OP); Lisa Wheeler, COT (VA/R); Greg K. Whittington, MPS, PSY (CC).
Retina Associates of Kentucky (Lexington, KY)
Thomas W. Stone, MD (PI); Todd Blevins (OP/OCT); Michelle Buck, COT, (VA/R/OCT); Lynn Cruz, COT (CC); Wanda Heath (VA/R); Diana Holcomb (VA/R); Rick Isernhagen, MD (O); Terri Kidd, COA (OCT); John Kitchens, MD (O); Cathy Sears, CST, COA (VA/R); Ed Slade, CRA, COA (OP/OCT); Jeanne Van Arsdall, COA (VA/R); Brenda VanHoose, COA (VA/R); Jenny Wolfe, RN (CC); William Wood, MD (O).
Colorado Retina Associates (Denver, CO)
John Zilis, MD (PI); Carol Crooks, COA (VA/R); Larry Disney (VA/R); Mimi Liu, MD (O); Stephen Petty, MD (O); Sandra Sall, ROUB, COA (CC/VA/R/OP/OCT).
University of Iowa Hospitals & Clinics (Iowa City, IA)
James C. Folk, MD (PI); Tracy Aly, CRA (OP/OCT); Abby Brotherton (VA); Douglas Critser, CRA (OP/OCT); Connie J. Hinz, COT, CCRC (CC/VA/R); Stefani Karakas, CRA (OP/OCT); Valerie Kirschner (VA); Cheyanne Lester (VA/R); Cindy Montague, CRA (OP/OCT); Stephen Russell, MD (O); Heather Stockman (VA/R); Barbara Taylor, CCRC (VA/R); Randy Verdick, FOPS (OP/OCT), Jean Walshire (CC).
Retina Specialists (Towson, MD)
John T. Thompson, MD (PI) ; Barbara Connell (VA/R); Maryanth Constantine (CC); John L. Davis Jr (VA/R); Gwen Holsapple (VA/R); Lisa Hunter (OP/OCT); C. Nicki Lenane (CC/VA/R/OP/OCT); Robin Mitchell (CC); Leslie Russel, CRA (OP/OCT); Raymond Sjaarda, MD (O).
Retina Consultants of Houston (Houston, TX)
David M. Brown, MD (PI); Matthew Benz, MD (O); Llewellyn Burns (OCT); JoLene G. Carranza, COA, CCRC (CC); Richard Fish, MD (O); Debra Goates (VA/R); Shayla Hay (VA/R); Theresa Jeffers, COT (VA/R); Eric Kegley, CRA, COA (OP/OCT); Dallas Kubecka (VA/R); Stacy McGilvra (VA/R); Beau Richter (OCT); Veronica Sneed, COA (VA/R); Cary Stoever (OCT); Isabell Tellez (VA/R); Tien Wong, MD (O).
Massachusetts Eye and Ear Infirmary/Harvard Vanguard Medical Associates (Boston, MA)
Ivana Kim, MD (PI); Christopher Andreoli, MD (O); Leslie Barresi, CRA, COA, OCT-C (VA/OP/OCT); Sarah Brett (OP); Charlene Callahan (OP); Karen Capaccioli (OCT); William Carli, COA (VA/R/OCT); Matthew Coppola, COA (VA); Nicholas Emmanuel (CC); Claudia Evans, OD (VA/R); Anna Fagan, COA (VA/R); Marcia Grillo (OCT); John Head, CRA, OCT-C (OP/OCT); Troy Kieser, COA, OCT-C (CC/VA/R); Elaine Lee, COA (VA); Ursula Lord, OD (VA/R); Edward Miretsky (CC); Kate Palitsch (OP/OCT); Todd Petrin, RN (OCT); Liz Reader (CC); Svetlana Reznichenko, COA (VA); Mary Robertson, COA (VA); Justin Smith, OD (VA/R); Demetrios Vavvas, MD, PHD (O).
Palmetto Retina Center (West Columbia, SC)
John Wells, MD (PI); Cassie Cahill (VA/R); W. Lloyd Clark, MD (O); Kayla Henry (VA/R); David Johnson, MD (O); Peggy Miller (CC/VA/R); LaDetrick Oliver, COT (OP/OCT); Robbin Spivey (OP/OCT); Tiffany Swinford (VA/R); Mallie Taylor (CC).
Retina and Vitreous of Texas (Houston, TX)
Michael Lambert, MD (PI); Kris Chase (OP/OCT); Debbie Fredrickson, COA (VA/R); Joseph Khawly, MD, FACS (O); Valerie Lazarte (VA/R); Donald Lowd (OP/OCT); Pam Miller (CC); Arthur Willis, MD (O).
Long Island Vitreoretinal Consultants (Great Neck, NY)
Philip J. Ferrone, MD (PI); Miguel Almonte (OCT); Rachel Arnott, (CC); Ingrid Aviles (VA/R/OCT); Sheri Carbon (VA/R); Michael Chitjian (OP/OCT); Kristen DAmore (CC); Christin Elliott (VA/R); David Fastenberg, MD (O); Barry Golub, MD (O); Kenneth Graham, MD (O); AnnMarie Lavorna (CC); Laura Murphy (VA/R); Amanda Palomo (VA/R); Christina Puglisi (VA/R); David Rhee, MD (O); Juan Romero, MD (O); Brett Rosenblatt, MD (O); Glenda Salcedo (OP/OCT); Marianne Schlameuss, RN (CC); Eric Shakin, MD (O); Vasanti Sookhai (VA/R).
Wills Eye Institute (Philadelphia, PA)
Richard Kaiser, MD (PI); Elizabeth Affel, MS, OCT-C (OCT); Gary Brown, MD (O); Christina Centinaro (CC); Deborah Fine, COA (OCT); Mitchell Fineman, MD (O); Michele Formoso (CC); Sunir Garg, MD (O); Lisa Grande (VA/R); Carolyn Herbert (VA/R); Allen Ho, MD (O); Jason Hsu, MD (O); Maryann Jay (OCT); Lisa Lavetsky (OCT); Elaine Liebenbaum (OP); Joseph Maguire, MD (O); Julia Monsonego (OP/OCT); Lucia O’Connor (OCT); Lisa Pierce (CC); Carl Regillo, MD (O); Maria Rosario (DE); Marc Spirn, MD (O); James Vander, MD (O); Jennifer Walsh (VA/R).
Ohio State University Eye Physicians & Surgeons-Retina Division (Dublin, OH)
Frederick H. Davidorf, MD (PI); Amanda Barnett (OP/OCT); Susie Chang, MD (O); John Christoforidis, MD (O); Joy Elliott (CC); Heather Justice (VA/R); Alan Letson, MD (O); Kathryne McKinney, COMT (CC); Jeri Perry, COT (VA/R); Jill A. Salerno, COA (CC); Scott Savage (OP); Stephen Shelley (OCT).
Retina Associates of Cleveland (Beachwood, OH)
Lawrence J. Singerman, MD (PI);Joseph Coney, MD (O); John DuBois (OP/OCT); Kimberly DuBois, LPN, CCRP, COA (VA/R); Gregg Greanoff, CRA (OP/OCT); Dianne Himmelman, RN, CCRC (CC); Mary Ilc, COT (VA/R); Elizabeth Mcnamara (VA/R/OP); Michael Novak, MD (O); Scott Pendergast, MD (O); Susan Rath, PA-C (CC); Sheila Smith-Brewer, CRA (OP/OCT); Vivian Tanner, COT, CCRP (VA/R); Diane E. Weiss, RN, (CC); Hernando Zegarra, MD (O).
Retina Group of Florida (Fort Lauderdale, FL)
Lawrence Halperin, MD (PI); Patricia Aramayo (OCT); Mandeep Dhalla, MD (O); Brian Fernandez, MD (OP/OCT); Cindy Fernandez, MD (CC); Jaclyn Lopez (CC); Monica Lopez (OCT); Jamie Mariano, COA (VA/R); Kellie Murphy, COA (OCT); Clifford Sherley, COA (VA/R); Rita Veksler, COA (OP/OCT).
Retina-Vitreous Associates Medical Group (Beverly Hills, CA)
Firas Rahhal, MD (PI); Razmig Babikian (DE); David Boyer, MD (O); Sepideh Hami (DE); Jeff Kessinger (OP/OCT); Janet Kurokouchi (CC); Saba Mukarram (VA/R); Sarah Pachman (VA/R); Eric Protacio (OCT); Julio Sierra (VA/R); Homayoun Tabandeh, MD, MS, FRCP (O); Adam Zamboni (VA/R).
Elman Retina Group, P.A. (Baltimore, MD)
Michael Elman, MD (PI); Jennifer Belz (CC); Tammy Butcher (CC); Theresa Cain (OP/OCT); Teresa Coffey, COA (VA/R); Dena Firestone (VA/R); Nancy Gore (VA/R); Pamela Singletary (VA/R); Peter Sotirakos (OP/OCT); JoAnn Starr (CC).
University of North Carolina at Chapel Hill (Chapel Hill, NC)
Travis A. Meredith, MD (PI); Cassandra J. Barnhart, MPH (CC/VA/R); Debra Cantrell, COA (VA/R/OP/OCT); RonaLyn Esquejo-Leon (OP/OCT); Odette Houghton, MD (O); Harpreet Kaur (VA/R); Fatoumatta NDure, COA (CC).
Ophthalmologists Enrolling Patients but No Longer Affiliated with a CATT Center
Ronald Glatzer, MD (O); Leonard Joffe, MD (O); Reid Schindler, MD (O).
Resource Centers
Chairman’s Office (Cleveland Clinic, Cleveland, OH)
Daniel F. Martin, MD (Chair); Stuart L. Fine, MD (Vice-Chair; University of Colorado, Denver, CO); Marilyn Katz (Executive Assistant).
Coordinating Center (University of Pennsylvania, Philadelphia, PA)
Maureen G. Maguire, PhD (PI); Mary Brightwell-Arnold, SCP (Systems Analyst); Ruchira Glaser, MD (Medical Monitor); Judith Hall (Protocol Monitor); Sandra Harkins (Staff Assistant); Jiayan Huang, MS (Biostatistician); Alexander Khvatov, MS (Systems Analyst); Kathy McWilliams, CCRP (Protocol Monitor); Susan K. Nolte (Protocol Monitor); Ellen Peskin, MA, CCRP (Project Director); Maxwell Pistilli, MS, Med (Biostatistician); Susan Ryan (Financial Administrator); Allison Schnader (Administrative Coordinator); Gui-Shuang Ying, PhD (Senior Biostatistician).
OCT Reading Center (Duke University, Durham, NC)
Glenn Jaffe, MD (PI); Jennifer Afrani-Sakyi (CATT PowerPoint Presentations); Brannon Balsley (OCT Technician Certifications); Linda S. Bennett (Project Manager); Adam Brooks (Reader/SD-Reader); Adrienne Brower-Lingsch (Reader); Lori Bruce (Data Verification); Russell Burns (Senior Technical Analyst/Senior Reader/SD Reader/OCT Technician Certifications); Dee Busian (Reader); John Choong (Reader); Lindsey Cloaninger (Reader Reliability Studies/ Document Creation/CATT PPT Files); Francis Char DeCroos (Research Associate); Emily DuBois (Data Entry); Mays El-Dairi (Reader/SD-Reader); Sarah Gach (Reader); Katelyn Hall (Project Manager/Reader Reliability Studies/ Data Verification/Document Creation); Terry Hawks (Reader); ChengChenh Huang (Reader); Cindy Heydary (Senior Reader/Quality Assurance Coordinator/SD Reader/Data Verification); Alexander Ho (Reader, Transcription); Shashi Kini (Data Entry/Transcription); Michelle McCall (Data Verification); Daaimah Muhammad (Reader Feedback); Jayne Nicholson (Data Verification); Jeanne Queen (Reader/SD-Reader); Pamela Rieves (Transcription); Kelly Shields (Senior Reader); Cindy Skalak (Reader); Adam Specker (Reader); Sandra Stinnett (Biostatistician); Sujatha Subramaniam (Reader); Patrick Tenbrink (Reader); Cynthia Toth, MD (Director of Grading); Aaron Towe (Reader); Kimberly Welch (Data Verification); Natasha Williams (Data Verification); Katrina Winter (Senior Reader); Ellen Young (Senior Project Manager).
Fundus Photograph Reading Center (University of Pennsylvania, Philadelphia, PA)
Juan E. Grunwald, MD (PI); Judith Alexander (Director); Ebenezer Daniel, MBBS, MS, MPH, PhD (Director); Elisabeth Flannagan (Administrative Coordinator); E. Revell Martin (Reader); Candace Parker (Reader); Krista Sepielli (Reader); Tom Shannon (Systems Analyst); Claressa Whearry (Data Coordinator).
National Eye Institute, National Institutes of Health
Maryann Redford, DDS, MPH (Program Officer).
Committees
Executive Committee
Daniel F. Martin, MD (chair); Robert L. Avery, MD; Sophie J. Bakri, MD; Ebenezer Daniel, MBBS, MS, MPH; Stuart L. Fine, MD; Juan E. Grunwald, MD; Glenn Jaffe, MD, Marcia R. Kopfer, BS, COT; Maureen G. Maguire, PhD; Travis A. Meredith, MD; Ellen Peskin, MA, CCRP; Maryann Redford, DDS, MPH; David F. Williams, MD.
Operations Committee
Daniel F. Martin, MD (chair); Linda S. Bennett; Ebenezer Daniel, MBBS, MS, MPH; Frederick L. Ferris III, MD; Stuart L. Fine, MD; Juan E. Grunwald, MD; Glenn Jaffe, MD; Maureen G. Maguire, PhD; Ellen Peskin, MA, CCRP; Maryann Redford, DDS, MPH; Cynthia Toth, MD.
Clinic Monitoring Committee
Ellen Peskin, MA, CCRP (chair); Mary Brightwell-Arnold, SCP; Joan DuPont; Maureen G. Maguire, PhD; Kathy McWilliams, CCRP; Susan K. Nolte.
Data and Safety Monitoring Committee
Lawrence M. Friedman, MD (chair); Susan B. Bressler, MD; David L. DeMets, PhD; Martin Friedlander, MD, PhD; Mark W. Johnson, MD; Anne Lindblad, PhD; Douglas W. Losordo, MD, FACC; Franklin G. Miller, PhD.
Footnotes
ALL AUTHORS HAVE COMPLETED AND SUBMITTED THE ICMJE FORM FOR DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST. The authors indicate the following financial disclosures: BJK has been on advisory boards for Thrombogenics, Eyetech, and Allergan. The other authors have no financial disclosures.
Design and conduct of the study (BJK, GSY, MGM); collection and management of data (GSY, JH, NEL); analysis, and interpretation of data (BJK, GSY, JH, NEL, MGM); preparation, review, approval of manuscript (BJK, GSY, JH, MGM).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Argon laser photocoagulation for senile macular degeneration. Results of a randomized clinical trial. Arch Ophthalmol. 1982;100(6):912–918. doi: 10.1001/archopht.1982.01030030920003. [DOI] [PubMed] [Google Scholar]
- 2.Bressler NM Treatment of Age-Related Macular Degeneration with Photodynamic Therapy Study Group. Photodynamic therapy of subfoveal choroidal neovascularization in age-related macular degeneration with verteporfin: two-year results of 2 randomized clinical trials-tap report 2. Arch Ophthalmol. 2001;119(2):198–207. [PubMed] [Google Scholar]
- 3.Gragoudas ES, Adamis AP, Cunningham ET, Jr, Feinsod M, Guyer DR VISiON Clinical Trial Group. Pegaptanib for neovascular age-related macular degeneration. N Engl J Med. 2004;351(27):2805–2816. doi: 10.1056/NEJMoa042760. [DOI] [PubMed] [Google Scholar]
- 4.Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1419–1431. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 5.Brown DM, Kaiser PK, Michels M, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1432–1444. doi: 10.1056/NEJMoa062655. [DOI] [PubMed] [Google Scholar]
- 6.Comparison of Age-related Macular Degeneration Treatments Trials Research Group. Martin DF, Maguire MG, et al. Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results. Ophthalmology. 2012;119(7):1388–1398. doi: 10.1016/j.ophtha.2012.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heier JS, Brown DM, Chong V, et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012;119(12):2537–2548. doi: 10.1016/j.ophtha.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 8.Blackhurst DW, Maguire MG. Reproducibility of refraction and visual acuity measurement under a standard protocol. The Macular Photocoagulation Study Group. Retina. 1989;9(3):163–169. [PubMed] [Google Scholar]
- 9.Beck RW, Moke PS, Turpin AH, et al. A computerized method of visual acuity testing: adaptation of the early treatment of diabetic retinopathy study testing protocol. Am J Ophthalmol. 2003;135(2):194–205. doi: 10.1016/s0002-9394(02)01825-1. [DOI] [PubMed] [Google Scholar]
- 10.Rovner BW, Casten RJ, Massof RW, Leiby BE, Tasman WS Wills Eye AMD Study. Psychological and cognitive determinants of vision function in age-related macular degeneration. Arch Ophthalmol. 2011;129(7):885–890. doi: 10.1001/archophthalmol.2011.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rovner BW, Casten RJ, Tasman WS. Effect of depression on vision function in age-related macular degeneration. Arch Ophthalmol. 2002;120(8):1041–1044. doi: 10.1001/archopht.120.8.1041. [DOI] [PubMed] [Google Scholar]
- 12.Zhang X, Bullard KM, Cotch MF, et al. Association between depression and functional vision loss in persons 20 years of age or older in the United States, NHANES 2005–2008. JAMA Ophthalmol. 2013;131(5):573–581. doi: 10.1001/jamaophthalmol.2013.2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carriere I, Delcourt C, Daien V, et al. A prospective study of the bi-directional association between vision loss and depression in the elderly. J Affect Disord. 2013;151(1):164–170. doi: 10.1016/j.jad.2013.05.071. [DOI] [PubMed] [Google Scholar]
- 14.Armstrong RA. Visual symptoms in Parkinson's disease. Parkinsons Dis. 2011;2011:908306. doi: 10.4061/2011/908306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kirby E, Bandelow S, Hogervorst E. Visual impairment in Alzheimer's disease: a critical review. J Alzheimers Dis. 2010;21(1):15–34. doi: 10.3233/JAD-2010-080785. [DOI] [PubMed] [Google Scholar]
- 16.Jackson GR, Owsley C. Visual dysfunction, neurodegenerative diseases, and aging. Neurol Clin. 2003;21(3):709–728. doi: 10.1016/s0733-8619(02)00107-x. [DOI] [PubMed] [Google Scholar]
- 17.Moschos MM, Markopoulos I, Chatziralli I, et al. Structural and functional impairment of the retina and optic nerve in Alzheimer's disease. Curr Alzheimer Res. 2012;9(7):782–788. doi: 10.2174/156720512802455340. [DOI] [PubMed] [Google Scholar]
- 18.Wolf S, Holz FG, Korobelnik JF, et al. Outcomes following three-line vision loss during treatment of neovascular age-related macular degeneration: subgroup analyses from MARINA and ANCHOR. Br J Ophthalmol. 2011;95(12):1713–1718. doi: 10.1136/bjophthalmol-2011-300471. [DOI] [PubMed] [Google Scholar]
- 19.Group CR, Martin DF, Maguire MG, et al. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med. 2011;364(20):1897–1908. doi: 10.1056/NEJMoa1102673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ying GS, Kim BJ, Maguire MG, et al. Sustained visual acuity loss in the Comparison of AMD Treatments Trials (CATT) JAMA Ophthalmol. doi: 10.1001/jamaophthalmol.2014.1019. (forthcoming) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ying GS, Huang J, Maguire MG, et al. Baseline predictors for one-year visual outcomes with ranibizumab or bevacizumab for neovascular age-related macular degeneration. Ophthalmology. 2013;120(1):122–129. doi: 10.1016/j.ophtha.2012.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grunwald JE, Daniel E, Ying GS, et al. Photographic assessment of baseline fundus morphologic features in the Comparison of Age-Related Macular Degeneration Treatments Trials. Ophthalmology. 2012;119(8):1634–1641. doi: 10.1016/j.ophtha.2012.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DeCroos FC, Toth CA, Stinnett SS, et al. Optical coherence tomography grading reproducibility during the Comparison of Age-related Macular Degeneration Treatments Trials. Ophthalmology. 2012;119(12):2549–2557. doi: 10.1016/j.ophtha.2012.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Groll DL, To T, Bombardier C, Wright JG. The development of a comorbidity index with physical function as the outcome. J Clin Epidemiol. 2005;58(6):595–602. doi: 10.1016/j.jclinepi.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 25.Kenny RA, Bhangu J, King-Kallimanis BL. Epidemiology of syncope/collapse in younger and older Western patient populations. Prog Cardiovasc Dis. 2013;55(4):357–363. doi: 10.1016/j.pcad.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 26.Khera S, Palaniswamy C, Aronow WS, et al. Predictors of mortality, rehospitalization for syncope, and cardiac syncope in 352 consecutive elderly patients with syncope. J Am Med Dir Assoc. 2013;14(5):326–330. doi: 10.1016/j.jamda.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 27.Age-Related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol. 2001;119(10):1417–1436. doi: 10.1001/archopht.119.10.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.