Abstract
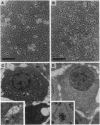
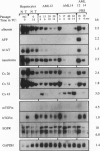
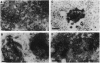
Hepatocytes are extensively used in studies of gene regulation but cannot be maintained in long-term culture as replicating, differentiated cells while remaining nontumorigenic. We have derived two hepatocyte lines from livers of transgenic mice overexpressing transforming growth factor alpha, a potent hepatocyte mitogen, which overcome these limitations. The transgenic hepatocytes were maintained for > or = 2 months in serum-supplemented primary culture and gave rise to cell lines, of which two (AML12 and AML14) have been cultured for > 1.5 years (> 80 passages). Both lines have typical hepatocyte features such as peroxisomes and bile canalicular-like structures, do not grow in soft agar, and are nontumorigenic in nude mice. Like normal hepatocytes, AML cells express high levels of mRNA for serum (albumin, alpha 1-antitrypsin, and transferrin) and gap junction (connexins 26 and 32) proteins, secrete albumin, and contain solely isozyme 5 of lactate dehydrogenase. After extensive passaging, AML12 cells continue to strongly coexpress hepatocyte connexin mRNAs but do not display nonparenchymal cell markers. Although mRNA levels for some serum proteins progressively fall, high expression in late AML12 cultures may be regained by passage in serum-free medium. The AML14 line loses expression of both differentiated markers and transgene mRNA with extended passaging, and hepatocytic traits are only partially restored by passage in serum-free medium. These differentiated, nontumorigenic cell lines should serve as models in which to study hepatocyte growth and differentiation.
Full text
PDF
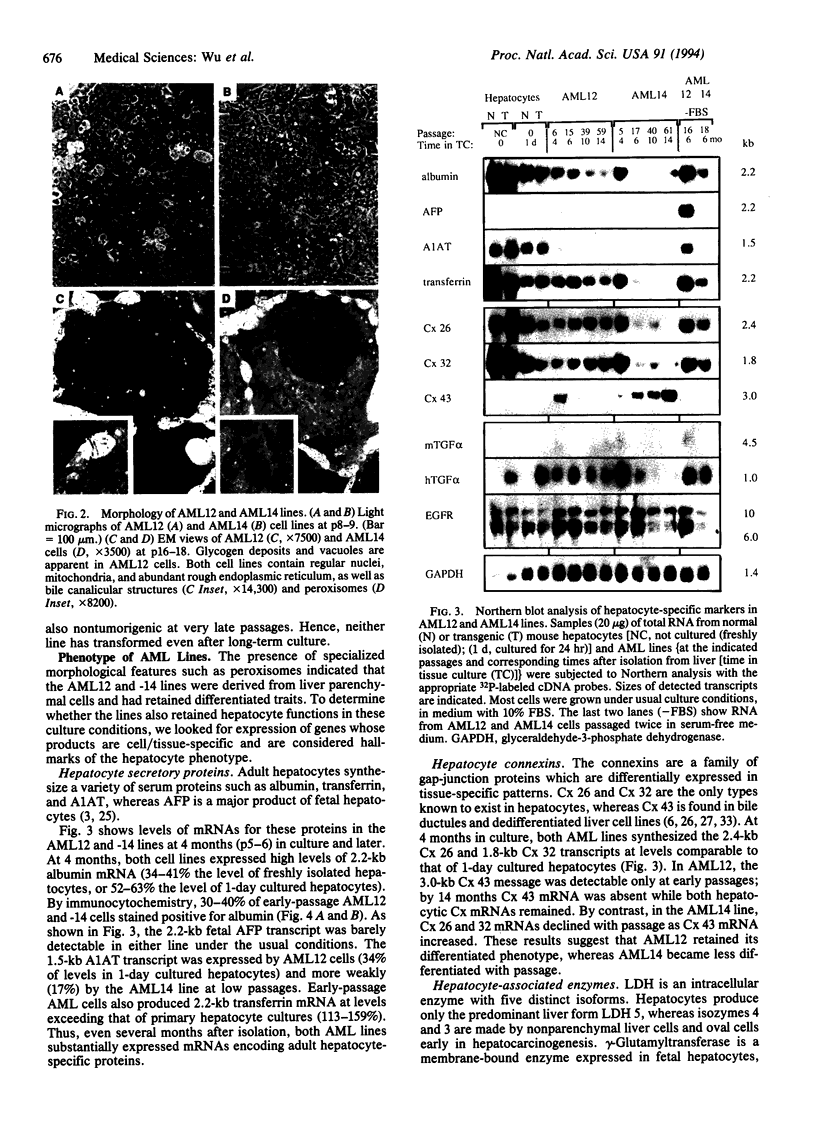


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beyer E. C., Paul D. L., Goodenough D. A. Connexin43: a protein from rat heart homologous to a gap junction protein from liver. J Cell Biol. 1987 Dec;105(6 Pt 1):2621–2629. doi: 10.1083/jcb.105.6.2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun L., Mikumo R., Fausto N. Production of hepatocellular carcinoma by oval cells: cell cycle expression of c-myc and p53 at different stages of oval cell transformation. Cancer Res. 1989 Mar 15;49(6):1554–1561. [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- DeCaprio J. A., Ludlow J. W., Figge J., Shew J. Y., Huang C. M., Lee W. H., Marsilio E., Paucha E., Livingston D. M. SV40 large tumor antigen forms a specific complex with the product of the retinoblastoma susceptibility gene. Cell. 1988 Jul 15;54(2):275–283. doi: 10.1016/0092-8674(88)90559-4. [DOI] [PubMed] [Google Scholar]
- Derman E., Krauter K., Walling L., Weinberger C., Ray M., Darnell J. E., Jr Transcriptional control in the production of liver-specific mRNAs. Cell. 1981 Mar;23(3):731–739. doi: 10.1016/0092-8674(81)90436-0. [DOI] [PubMed] [Google Scholar]
- Derynck R. Transforming growth factor alpha. Cell. 1988 Aug 26;54(5):593–595. doi: 10.1016/s0092-8674(88)80001-1. [DOI] [PubMed] [Google Scholar]
- Fausto N. Growth factors in liver development, regeneration and carcinogenesis. Prog Growth Factor Res. 1991;3(3):219–234. doi: 10.1016/0955-2235(91)90008-r. [DOI] [PubMed] [Google Scholar]
- Grisham J. W. Cell types in long-term propagable cultures of rat liver. Ann N Y Acad Sci. 1980;349:128–137. doi: 10.1111/j.1749-6632.1980.tb29521.x. [DOI] [PubMed] [Google Scholar]
- Hayner N. T., Braun L., Yaswen P., Brooks M., Fausto N. Isozyme profiles of oval cells, parenchymal cells, and biliary cells isolated by centrifugal elutriation from normal and preneoplastic livers. Cancer Res. 1984 Jan;44(1):332–338. [PubMed] [Google Scholar]
- Höhne M., Schaefer S., Seifer M., Feitelson M. A., Paul D., Gerlich W. H. Malignant transformation of immortalized transgenic hepatocytes after transfection with hepatitis B virus DNA. EMBO J. 1990 Apr;9(4):1137–1145. doi: 10.1002/j.1460-2075.1990.tb08220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhappan C., Stahle C., Harkins R. N., Fausto N., Smith G. H., Merlino G. T. TGF alpha overexpression in transgenic mice induces liver neoplasia and abnormal development of the mammary gland and pancreas. Cell. 1990 Jun 15;61(6):1137–1146. doi: 10.1016/0092-8674(90)90076-q. [DOI] [PubMed] [Google Scholar]
- Kaufmann W. K., Zhang Y., Kaufman D. G. Association between expression of transforming growth factor-alpha and progression of hepatocellular foci to neoplasms. Carcinogenesis. 1992 Aug;13(8):1481–1483. doi: 10.1093/carcin/13.8.1481. [DOI] [PubMed] [Google Scholar]
- Kioussis D., Eiferman F., van de Rijn P., Gorin M. B., Ingram R. S., Tilghman S. M. The evolution of alpha-fetoprotein and albumin. II. The structures of the alpha-fetoprotein and albumin genes in the mouse. J Biol Chem. 1981 Feb 25;256(4):1960–1967. [PubMed] [Google Scholar]
- Lee G. H., Merlino G., Fausto N. Development of liver tumors in transforming growth factor alpha transgenic mice. Cancer Res. 1992 Oct 1;52(19):5162–5170. [PubMed] [Google Scholar]
- Lee L. W., Raymond V. W., Tsao M. S., Lee D. C., Earp H. S., Grisham J. W. Clonal cosegregation of tumorigenicity with overexpression of c-myc and transforming growth factor alpha genes in chemically transformed rat liver epithelial cells. Cancer Res. 1991 Oct 1;51(19):5238–5244. [PubMed] [Google Scholar]
- Lemire J. M., Fausto N. Multiple alpha-fetoprotein RNAs in adult rat liver: cell type-specific expression and differential regulation. Cancer Res. 1991 Sep 1;51(17):4656–4664. [PubMed] [Google Scholar]
- Mead J. E., Fausto N. Transforming growth factor alpha may be a physiological regulator of liver regeneration by means of an autocrine mechanism. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1558–1562. doi: 10.1073/pnas.86.5.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlino G. T., Ishii S., Whang-Peng J., Knutsen T., Xu Y. H., Clark A. J., Stratton R. H., Wilson R. K., Ma D. P., Roe B. A. Structure and localization of genes encoding aberrant and normal epidermal growth factor receptor RNAs from A431 human carcinoma cells. Mol Cell Biol. 1985 Jul;5(7):1722–1734. doi: 10.1128/MCB.5.7.1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami H., Sanderson N. D., Nagy P., Marino P. A., Merlino G., Thorgeirsson S. S. Transgenic mouse model for synergistic effects of nuclear oncogenes and growth factors in tumorigenesis: interaction of c-myc and transforming growth factor alpha in hepatic oncogenesis. Cancer Res. 1993 Apr 15;53(8):1719–1723. [PubMed] [Google Scholar]
- Paul D., Höhne M., Pinkert C., Piasecki A., Ummelmann E., Brinster R. L. Immortalized differentiated hepatocyte lines derived from transgenic mice harboring SV40 T-antigen genes. Exp Cell Res. 1988 Apr;175(2):354–362. doi: 10.1016/0014-4827(88)90199-1. [DOI] [PubMed] [Google Scholar]
- Pfeifer A. M., Cole K. E., Smoot D. T., Weston A., Groopman J. D., Shields P. G., Vignaud J. M., Juillerat M., Lipsky M. M., Trump B. F. Simian virus 40 large tumor antigen-immortalized normal human liver epithelial cells express hepatocyte characteristics and metabolize chemical carcinogens. Proc Natl Acad Sci U S A. 1993 Jun 1;90(11):5123–5127. doi: 10.1073/pnas.90.11.5123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plenat F., Braun L., Fausto N. Demonstration of glucose-6-phosphatase and peroxisomal catalase activity by ultrastructural cytochemistry in oval cells from livers of carcinogen-treated rats. Am J Pathol. 1988 Jan;130(1):91–102. [PMC free article] [PubMed] [Google Scholar]
- Rutenburg A. M., Kim H., Fischbein J. W., Hanker J. S., Wasserkrug H. L., Seligman A. M. Histochemical and ultrastructural demonstration of gamma-glutamyl transpeptidase activity. J Histochem Cytochem. 1969 Aug;17(8):517–526. doi: 10.1177/17.8.517. [DOI] [PubMed] [Google Scholar]
- Salomon D. S., Kim N., Saeki T., Ciardiello F. Transforming growth factor-alpha: an oncodevelopmental growth factor. Cancer Cells. 1990 Dec;2(12):389–397. [PubMed] [Google Scholar]
- Sammons D. W., Sanchez E., Darlington G. J. Immuno-overlay: a method for identification of hepatoma cell colonies that secrete albumin. In Vitro. 1980 Nov;16(11):918–924. doi: 10.1007/BF02619329. [DOI] [PubMed] [Google Scholar]
- Sandgren E. P., Luetteke N. C., Qiu T. H., Palmiter R. D., Brinster R. L., Lee D. C. Transforming growth factor alpha dramatically enhances oncogene-induced carcinogenesis in transgenic mouse pancreas and liver. Mol Cell Biol. 1993 Jan;13(1):320–330. doi: 10.1128/mcb.13.1.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada N., Lee G. H., Mochizuki Y., Ishikawa T. Active proliferation of mouse hepatocytes in primary culture under defined conditions as compared to rat hepatocytes. Jpn J Cancer Res. 1988 Sep;79(9):983–988. doi: 10.1111/j.1349-7006.1988.tb00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada N., Tomomura A., Sattler C. A., Sattler G. L., Kleinman H. K., Pitot H. C. Effects of extracellular matrix components on the growth and differentiation of cultured rat hepatocytes. In Vitro Cell Dev Biol. 1987 Apr;23(4):267–273. doi: 10.1007/BF02623709. [DOI] [PubMed] [Google Scholar]
- Sirica A. E., Mathis G. A., Sano N., Elmore L. W. Isolation, culture, and transplantation of intrahepatic biliary epithelial cells and oval cells. Pathobiology. 1990;58(1):44–64. doi: 10.1159/000163564. [DOI] [PubMed] [Google Scholar]
- Stutenkemper R., Geisse S., Schwarz H. J., Look J., Traub O., Nicholson B. J., Willecke K. The hepatocyte-specific phenotype of murine liver cells correlates with high expression of connexin32 and connexin26 but very low expression of connexin43. Exp Cell Res. 1992 Jul;201(1):43–54. doi: 10.1016/0014-4827(92)90346-a. [DOI] [PubMed] [Google Scholar]
- Takagi H., Sharp R., Hammermeister C., Goodrow T., Bradley M. O., Fausto N., Merlino G. Molecular and genetic analysis of liver oncogenesis in transforming growth factor alpha transgenic mice. Cancer Res. 1992 Oct 1;52(19):5171–5177. [PubMed] [Google Scholar]
- Woodworth C., Secott T., Isom H. C. Transformation of rat hepatocytes by transfection with simian virus 40 DNA to yield proliferating differentiated cells. Cancer Res. 1986 Aug;46(8):4018–4026. [PubMed] [Google Scholar]
- Zhang J. T., Nicholson B. J. Sequence and tissue distribution of a second protein of hepatic gap junctions, Cx26, as deduced from its cDNA. J Cell Biol. 1989 Dec;109(6 Pt 2):3391–3401. doi: 10.1083/jcb.109.6.3391. [DOI] [PMC free article] [PubMed] [Google Scholar]