ABSTRACT
Skin keratinocytes represent a primary entry site for herpes simplex virus 1 (HSV-1) in vivo. The cellular proteins nectin-1 and herpesvirus entry mediator (HVEM) act as efficient receptors for both serotypes of HSV and are sufficient for disease development mediated by HSV-2 in mice. How HSV-1 enters skin and whether both nectin-1 and HVEM are involved are not known. We addressed the impact of nectin-1 during entry of HSV-1 into murine epidermis and investigated the putative contribution of HVEM. Using ex vivo infection of murine epidermis, we showed that HSV-1 entered the basal keratinocytes of the epidermis very efficiently. In nectin-1-deficient epidermis, entry was strongly reduced. Almost no entry was observed, however, in nectin-1-deficient keratinocytes grown in culture. This observation correlated with the presence of HVEM on the keratinocyte surface in epidermis and with the lack of HVEM expression in nectin-1-deficient primary keratinocytes. Our results suggest that nectin-1 is the primary receptor in epidermis, while HVEM has a more limited role. For primary murine keratinocytes, on which nectin-1 acts as a single receptor, electron microscopy suggested that HSV-1 can enter both by direct fusion with the plasma membrane and via endocytic vesicles. Thus, we concluded that nectin-1 directs internalization into keratinocytes via alternative pathways. In summary, HSV-1 entry into epidermis was shown to strongly depend on the presence of nectin-1, but the restricted presence of HVEM can potentially replace nectin-1 as a receptor, illustrating the flexibility employed by HSV-1 to efficiently invade tissue in vivo.
IMPORTANCE Herpes simplex virus (HSV) can cause a range of diseases in humans, from uncomplicated mucocutaneous lesions to life-threatening infections. The skin is one target tissue of HSV, and the question of how the virus overcomes the protective skin barrier and penetrates into the tissue to reach its receptors is still open. Previous studies analyzing entry into cells grown in vitro revealed nectin-1 and HVEM as HSV receptors. To explore the contributions of nectin-1 and HVEM to entry into a natural target tissue, we established an ex vivo infection model. Using nectin-1- or HVEM-deficient mice, we demonstrated the distinct involvement of nectin-1 and HVEM for HSV-1 entry into epidermis and characterized the internalization pathways. Such advances in understanding the involvement of receptors in tissue are essential preconditions for unraveling HSV invasion of skin, which in turn will allow the development of antiviral reagents.
INTRODUCTION
Herpes simplex viruses (HSV) are ubiquitous human pathogens which can cause a range of diseases, from mild, uncomplicated mucocutaneous lesions to life-threatening infections. HSV-1 is dominantly associated with orofacial infections and encephalitis, whereas HSV-2 more likely causes genital infections. To enter its human host, HSV must come into contact with mucosal surfaces, skin, or the cornea. During initial exposure on mucosa or skin, HSV targets epidermal keratinocytes and establishes a primary infection in the epithelium. Cellular entry of HSV relies on the interaction of several viral glycoproteins with various cell surface receptors (1, 2). The envelope glycoprotein D (gD) is essential for the entry process, and only after gD binding to a receptor is fusion with a cellular membrane induced (3). The major gD receptors mediating entry into mouse and human cells are herpesvirus entry mediator (HVEM) and nectin-1 (4–6). HVEM is a member of the tumor necrosis factor receptor superfamily which can activate either proinflammatory or inhibitory signaling pathways (7), while nectin-1 is an immunoglobulin-like cell adhesion molecule (8). A further gD receptor is 3-O-sulfated heparan sulfate, which may also play a role in HSV-1 entry into various cell types (9, 10).
Although gD interactions with either HVEM or nectin-1 have been studied extensively in different cell lines, little is known about the receptors that mediate uptake of HSV-1 in vivo at the natural sites of virus infection. Based on expression patterns, HVEM is thought to act as the principal receptor for HSV on lymphoid cells, with nectin-1 playing the same role on epithelial and neuronal cells (4). Mice are widely used as an animal model for studies of HSV skin, mucosal, and corneal infections, and the murine homologs of HVEM and nectin-1 support HSV entry (11). One exploratory focus was on infection by HSV-2 via the intravaginal route in various mouse models (12). When nectin-1/HVEM double-knockout (double-KO) mice were infected intravaginally with HSV-2, nearly no signs of viral lesions were detected, demonstrating that disease development requires expression of either nectin-1 or HVEM (13). Infection studies with a gD mutant unable to bind HVEM suggest that gD-HVEM interactions suppress innate defenses during murine intravaginal infection with HSV-2, which may highlight an additional function of HVEM (14). Another exploratory focus of HSV infection was on an ocular model where infection followed scarification of the murine cornea. This method is clearly distinct from the natural entry route of HSV, but these studies helped us to understand pathogenesis. Expression of nectin-1 and HVEM was observed in the epithelium of the murine cornea (15, 16), and infection studies with HSV-1 in KO mice indicated attenuated disease in the absence of either HVEM or nectin-1 (17). In contrast, HSV-2 does not require HVEM to cause disease in corneal infections, suggesting that the HVEM requirement depends on the serotype (18). In all these mouse models, the impacts of the HSV receptors nectin-1 and HVEM were correlated with disease development. The contributions of individual receptors to the initiation of infection in various tissues, however, are less clear.
We established an ex vivo infection model of murine epidermal sheets to explore the invasion route of HSV-1 into epidermal tissue at the cellular level. Our focus is on the identification of the cells and molecular determinants that mediate initial entry into tissue. Here we determined the epidermis-specific roles of both nectin-1 and HVEM as receptors for HSV-1 infection and investigated the internalization pathway. The outermost layer of skin is the epidermis, which contains appendages such as hair follicles and sebaceous glands. The interfollicular epidermis consists of a multilayered epithelium, including a basal layer, a granular layer, and an outer, cornified layer of dead, keratinized cells. Keratinocytes are the major constituent of the epidermis, and its stratified structure is the result of epidermal differentiation that allows keratinocytes of the basal layer to migrate into the upper layers (19). Nectin-1, -2, -3, and -4 are Ca2+-independent cell-cell adhesion molecules involved in the formation of adherens junctions in keratinocytes. In particular, they play important roles in cell-cell junctions that are dynamic and require remodeling (8). Nectin-1 is the most abundant, being expressed throughout the murine epidermis, from the basal layer to the cornified layer. Nectin-2 and -4 are expressed in the lower levels, while nectin-3 is not expressed in murine epidermis (20). In contrast, the expression pattern of HVEM in the epidermis is less well characterized.
Here we investigated whether nectin-1 plays an exclusive role as a receptor during HSV-1 entry into murine epidermis. Our results indicate that the initial HSV-1 entry into tissue strongly depends on the presence of nectin-1 in murine epidermis. HVEM can also serve as a receptor in epidermis, although less efficiently than nectin-1, based on the small number of HVEM-expressing cells.
MATERIALS AND METHODS
Mice, preparation of murine skin, and isolation of murine keratinocytes.
Breeding was performed with Pvrl−/− and Pvrl+/− mice (21), and genotypes of homozygous animals were confirmed by a duplex PCR assay with primer pairs for nectin-1 (forward primer, 5′-AGCTGTTTCCGTCTGGTGTC-3′; wild-type [wt] reverse primer, 5′-AGGGGTGGAGTTAAGATGAGG-3′; and neomycin resistance cassette reverse primer, 5′-CTTGGCTGGACGTAAACTCC-3′) as described previously (21).
Murine epidermal sheets were taken from back skin of wt Pvrl+/+ (C57BL/6), Pvrl+/−, or Pvrl−/− newborn mice, named wt, heterozygous, or nectin-1-deficient mice, respectively, throughout the text. One to 3 days after birth, mice were decapitated, and skin pieces of about 15 mm in diameter were taken to prepare epidermal sections or whole mounts as described previously (22). Epidermal sheets were taken from tail skin from adult mice (1 to 3 months), since the long embedded hair follicles on the back impeded separation of intact epidermis. After incubation for 30 min at 37°C with 5 mg/ml dispase II (Roche) in phosphate-buffered saline (PBS), the epidermis was washed three times in PBS, gently removed from the underlying dermis as an intact sheet by use of forceps, and used immediately for infection studies. Epidermal sheets were also prepared from newborn or adult tail skin of HVEM KO mice (23). For gentle cell dissociation, the epidermal sheets were incubated with the basal side on TrypLE Select cell dissociation enzyme (Life Technologies) for 30 min at room temperature. These cell suspensions were used to isolate keratinocytes or extract RNA.
Murine primary keratinocytes isolated from newborn epidermis were cultured on collagen-coated dishes (collagen G; Merck Millipore) in the presence of 3T3 fibroblasts as feeder cells at 32°C. In brief, primary keratinocytes were maintained in keratinocyte culture medium (Dulbecco's modified Eagle's medium [DMEM]/Ham's F-12 [3.5:1.1]; Merck Millipore) containing 50 μM calcium ions, 10% fetal calf serum (FCS; calcium-free), penicillin (400 IU/ml), streptomycin (50 μg/ml), adenine (1.8 × 10−4 M), glutamine (300 μg/ml), hydrocortisone (0.5 μg/ml), epidermal growth factor (EGF) (10 ng/ml), cholera enterotoxin (10−10 M), insulin (5 μg/ml), and ascorbic acid (0.05 mg/ml) in the presence of mitomycin C-treated 3T3 fibroblasts (strain J2).
Cells, viruses, and plasmids.
HaCaT (24) and hTERT RPE-1 (ATCC) cells were maintained in DMEM/high glucose/GlutaMAX (Life Technologies) and DMEM/F-12/GlutaMAX (Life Technologies), respectively, containing 10% FCS, penicillin (100 IU/ml), and streptomycin (100 μg/ml). Infection studies were performed with purified preparations of HSV-1 wt strain 17 as described previously (25). In brief, virus inoculum was added to the cells at 37°C, defining time point 0. For electron microscopy (EM) studies, primary keratinocytes were incubated with HSV-1 at 800 PFU/cell for 1 h at 4°C, followed by incubation at 37°C for 10 or 30 min to allow uptake. EM studies of epidermal sheets were performed at approximately 1,500 PFU/cell, and preadsorption was allowed for 2 h at 4°C.
Prior to infection, murine primary keratinocytes were seeded without 3T3 fibroblasts and cultivated overnight on collagen-coated dishes (collagen G; Merck Millipore) at 32°C.
Virus titers were determined by plaque assays on Vero-B4 cells. Epidermal sheets were washed at 1 h postinfection (p.i.), and the titer of cell-released virus (CRV) was analyzed as indicated.
The expression vector pSC386, encoding human HVEM (26), was used for transient overexpression. Plasmid EGFP-N1 (Clontech) was used as a control.
Ethics statement.
The preparation of epidermal sheets and cells from sacrificed animals was carried out in strict accordance with the recommendations of the Guide of Landesamt für Natur, Umwelt and Verbraucherschutz, Northrhine-Westphalia (Germany). The study was approved by LANUV NRW (approval number 8.84-02.05.20.13.018).
Transient expression.
Murine primary keratinocytes (∼3 × 105) were transfected with 1 μg of plasmid pSC386 or EGFP-N1 by use of X-tremeGENE HP transfection reagent (Roche). At 21 h posttransfection, cells were infected with HSV-1 at 20 PFU/cell.
RNA preparation and reverse transcription-PCR (RT-PCR).
TRIzol reagent (Life Technologies) was used to extract RNA from keratinocytes directly after dissociation from murine epidermis by TrypLE Select (Life Technologies) treatment or from primary murine keratinocytes that were cultivated for 1 day in the absence of 3T3 fibroblasts. cDNAs were synthesized from total RNA by using SuperScript II reverse transcriptase (Life Technologies), and PCR was performed with native Taq DNA polymerase (Life Technologies) and the following primer pairs: (i) nectin-1 primers (forward, 5′-ACTGGTTTCTGGAGCGCGAGG-3′; and reverse, 5′-CTCGTAGGGAGGCAGCACGGA-3′), (ii) nectin-2 primers (forward, 5′-AGCTGGGCCGAACGAACTGATC-3′; and reverse, 5′-AGCCACAACTGTGCCATCCAGG-3′), (iii) nectin-3 primers (forward, 5′-GAGGCGGCAAAGCACAACTT-3′; and reverse, 5′-GGATGATGAACTGCAACAGTCTGTG-3′), (iv) nectin-4 primers (forward, 5′-GGCAGCTTTCAGGCACGGAT-3′; and reverse, 5′-GGCACCAGATGGAACTCTGAAG-3′), (v) HVEM primers (forward, 5′-TGAAGCAGGTCTGCAGTGAG-3′; and reverse, 5′-GCTGTTGGTCCCACGTCTTA-3′), and (vi) glyceraldehyde-3-phosphate dehydrogenase (GAPDH) primers (forward, 5′-TGATGACATCAAGAAGGTGGTGAAG-3′; and reverse, 5′-TCCTTGGAGGCCATGTGGGCCAT-3′).
Immunocytochemistry and antibodies.
Murine epidermal whole mounts from wt or nectin-1-deficient mice were fixed with 3.4% formaldehyde as described previously (27) and then stained overnight with mouse anti-ICP0 (monoclonal antibody [MAb] 11060) (28) diluted 1:60, followed by washing with PBS-0.2% Tween 20 for 4 h, overnight incubation with anti-mouse immunoglobulin G (AF488) (Life Technologies) and DAPI (4′,6-diamidino-2-phenylindole), and washing with PBS-0.2% Tween 20 for 4 h, all at room temperature. Murine epidermal cryosections from wt or nectin-1-deficient mice were embedded in Tissue Tek (Sakura) and frozen in liquid nitrogen; 10-μm frozen cross sections were then cut using a CM3050 (Leica) cryomicrotome. The tissue sections were fixed with 0.5% formaldehyde for 10 min at room temperature, blocked with 5% normal goat serum in PBS for 30 min at room temperature, and then stained for 60 min with mouse anti-ICP0 (11060) (28) diluted 1:60, followed by incubation with AF488-conjugated anti-mouse IgG (Life Technologies) and DAPI for 45 min at room temperature.
Murine primary keratinocytes grown on collagen G (Merck Millipore)-coated coverslips were fixed with 2% formaldehyde for 10 min at room temperature, permeabilized with 0.5% NP-40 for 10 min at room temperature, and then costained for 60 min with mouse anti-ICP0 (11060) (28) diluted 1:60 and/or rabbit polyclonal anti-mouse keratin 14 (AF64; Covance) diluted 1:10,000 (room temperature), followed by incubation with secondary antibodies and DAPI for 45 min at room temperature. Staining of F-actin was performed with tetramethyl rhodamine isothiocyanate (TRITC)-conjugated phalloidin (Sigma) for 15 min at room temperature. Phagocytosis assays were performed with sulfate-modified polystyrene, fluorescent orange latex beads (2 μm) (L1153; Sigma). Murine primary keratinocytes, HaCaT cells, and RPE-1 cells were incubated with latex beads (4 × 106 per well in a 24-well plate) for 24 h. Cells were fixed and stained with TRITC-conjugated phalloidin and DAPI.
Microscopy was performed using a Leica DM IRB/E microscope linked to a Leica TCS-SP/5 confocal unit and a Zeiss Axiophot microscope. Images were assembled using Photoshop (version CS2; Adobe) and Corel Draw (version 9.0; Corel Corporation).
Transmission electron microscopy.
Infected cells and epidermal sheets were prepared for electron microscopy as described previously (29, 30). Ultrathin sections of keratinocytes or semithin sections of epidermal sheets were cut, stained with uranyl acetate and lead citrate, and analyzed in a JEOL 1200 EX II or Zeiss 902A electron microscope, respectively.
Fluorescence-activated cell sorter (FACS) analysis.
Murine epidermal sheets from wt or nectin-1-deficient mice were infected for 3 h, followed by incubation on TrypLE Select (Life Technologies) for 15 min at room temperature and 15 min at 37°C or on enzyme-free cell dissociation solution (CDS) (Sigma) for 15 min at room temperature, 15 min at 37°C, and 15 min at room temperature to gently dissociate keratinocytes. The dissociated keratinocytes were dispersed by gentle pipetting and filtered one time (TrypLE Select) or two times (CDS) through a 40-μm cell strainer (BD) to remove cell clumps and debris. To stain surface receptors, cells were kept in PBS-5% FCS and incubated on ice for 45 min with an Armenian hamster MAb against murine HVEM (clone HMHV-1B18; diluted 1:200) (BioLegend), a mouse anti-nectin-1 (CK41) antibody (diluted 1:100) (31), or the following isotype controls: for HVEM, Armenian hamster IgG (eBioscience); and for nectin-1, mouse IgG1 (Life Technologies). This was followed by incubation with the secondary antibody, i.e., anti-Armenian hamster IgG–phycoerythrin (PE) (eBioscience) diluted 1:50 or anti-mouse IgG–Cy5 (Jackson ImmunoResearch Laboratories Inc.) diluted 1:100, for 30 min on ice. To visualize ICP0, cells were fixed with 3.7% formaldehyde for 10 min at room temperature, permeabilized with 0.2% saponin for 15 min on ice, and then incubated on ice for 35 min with mouse anti-ICP0 (11060) (28) diluted 1:30 in 0.2% saponin or with isotype control mouse IgG2b (Life Technologies), followed by incubation on ice for 30 min with anti-mouse IgG–Cy5 (Jackson ImmunoResearch Laboratories Inc.) diluted 1:20 in 0.2% saponin. Samples were analyzed by using a FACSCanto II flow cytometer (BD) and FACSDiva (version 6.1.3; BD) and FlowJo (version 7.6.3; Tree Star) software.
RESULTS
Entry of HSV-1 into nectin-1-deficient epidermis.
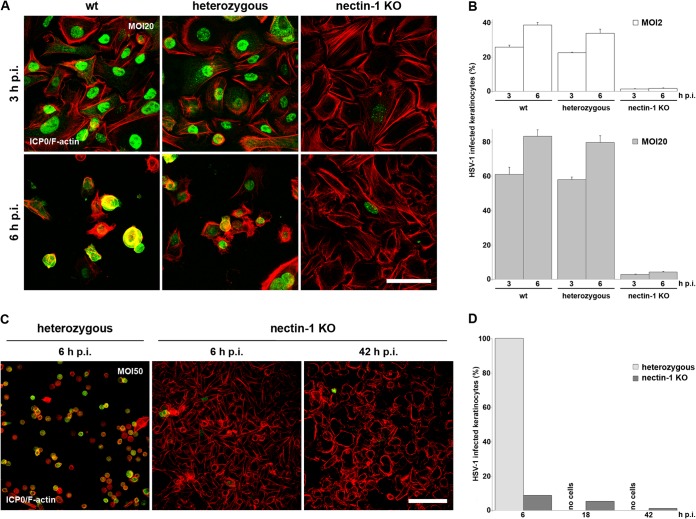
To address the role of nectin-1 as a receptor for HSV-1 in the epidermis, we investigated the skin of mice lacking nectin-1. Nectin-1-deficient mice have no obvious skin phenotype, which may be explained by the expression of multiple nectins, such as nectin-2 and -4, leading to functional redundancy (20). We used mice which carry a null mutation in the nectin-1-encoding gene, Pvrl1 (Pvrl1−/−) (21). These are viable and fertile but manifest microphthalmia and defective amelogenesis of the incisor teeth (21). We infected murine epidermal sheets ex vivo to compare the efficiencies of HSV-1 entry in the absence and presence of nectin-1. Skin was prepared from backs of newborn mice or from the tails of adult mice, and the epidermis was separated from the dermis by dispase treatment. The epidermal sheets were submerged in virus-containing medium, and infection was visualized at various times p.i. by staining infected cells with an antibody directed against the HSV-1 immediate early protein ICP0. The visualization of ICP0 expression was used as a marker for successful HSV-1 entry. The cellular localization of ICP0 passes through distinct phases during early infection; ICP0 in nuclear foci indicates an early stage of viral gene expression which is followed by relocalization of ICP0 to the cytoplasm (32). Previously, we demonstrated that infection of epidermal sheets is initially restricted to the basal keratinocyte layer (32). When we infected epidermal whole mounts from wt mice with 50 to 100 PFU/cell, ICP0 was visible in the cytoplasm of all cells of the basal keratinocyte layer by 3 h p.i., indicating very efficient entry (Fig. 1B and C). In contrast, a greatly reduced number of infected cells was detectable in the basal layer of nectin-1-deficient epidermis (Fig. 1B and C). The efficiency of entry varied across the interfollicular epidermis, with ∼10% infection in some areas and up to ∼30% in others (Fig. 1B and C). Furthermore, ICP0 was only in the nucleus in most infected cells at 3 h p.i., suggesting that entry was slower in this case than in wt epidermis (Fig. 1B and C). In addition to the interfollicular epidermis, different regions of the hair follicles were infected. In adult (1-month-old) wt mice, hair germs and parts of the outer root sheath of hair follicles were strongly infected, while in nectin-1-deficient mice, individual infected cells were found in the hair germs and rarely in the outer root sheaths (Fig. 1A and C). Staining of the sebaceous glands (SG) underneath the hair shaft was shown to be unspecific (data not shown).
FIG 1.
HSV-1 enters murine wt or nectin-1-deficient epidermis. (A) Scheme illustrating epidermal whole mounts, showing the visible surface of the basal keratinocyte layer with hair follicles. The epidermis was prepared either from newborn back skin or from adult tail skin (1-month-old mice). Abbreviations: DHF, developing hair follicle; HF, hair follicle; IFE, interfollicular epidermis; ORS, outer root sheath; SG, sebaceous gland. (B and C) Epidermal sheets of wt or nectin-1-deficient mice from newborn skin or from adult tail skin were infected with HSV-1 at 100 PFU/cell. Epidermal whole mounts showing the basal keratinocyte layer and developing hair follicles (B) or hair follicles from adult epidermis (C) were stained with anti-ICP0 (green) at 3 h p.i. to visualize infected cells. Counterstaining of the nuclei with DAPI (blue) demonstrates that all cells in wt epidermis were infected, while the number of infected cells in nectin-1-deficient epidermis was reduced. In nectin-1-deficient epidermis, areas with high and low densities of infected cells are shown. (D) Newborn epidermis from wt mice was infected at 0.1, 1, 10, or 100 PFU/cell and stained with anti-ICP0 (green), which demonstrates the preferential infection of developing hair follicles. (E) Three, 9, 12, or 18 h after infection at 100 PFU/cell, sections of wt and nectin-1 KO epidermises were stained with anti-ICP0 (green) and with DAPI (blue) as a nuclear counterstain, illustrating the spread of infection in suprabasal layers. Confocal projections and merged images are shown. Bars, 25 μm.
To compare the efficiencies of entry into epidermis for adult and newborn mice, we also infected epidermal whole mounts of nectin-1-deficient newborn mice. These mice showed an even greater reduction in the number of infected cells in the interfollicular epidermis compared to nectin-1-deficient adult epidermis, with most infected cells present in the developing hair follicles (DHF) (Fig. 1B). To determine the reason for the higher infection rate in the densely packed developing hair follicles, we infected the epidermises of newborn wt mice at different virus doses. At 0.1 PFU/cell, ICP0-expressing cells were found almost exclusively at the developing hair follicles, while cells in the interfollicular epidermis expressed ICP0 only at a higher multiplicity of infection (MOI) (Fig. 1D). These results demonstrate that keratinocytes in the developing hair follicles are more accessible to HSV-1 infection than those in the interfollicular epidermis in both wt and nectin-1-deficient newborn mice. We concluded that the receptor(s) is expressed in both the interfollicular epidermis and the hair follicles of newborn and adult mice. Thus, the reduced number of infected cells in newborn nectin-1-deficient epidermis mirrors that seen in adult epidermis.
When wt epidermis was infected for longer than 3 h (9, 12, and 18 h), we observed increasing numbers of ICP0-expressing cells in the suprabasal layers, indicating that entry was not restricted to the basal keratinocyte layer but that the virus was capable of spreading to other cells in the tissue (Fig. 1E). Virus production was confirmed by determining the virus titer at 48 h p.i. (Fig. 2). To analyze whether nectin-1 deficiency interferes with the spread of infection to suprabasal layers, we visualized ICP0 at various times p.i. As expected, at 3 h p.i., only a few infected cells were detected in the basal layer. There was a slight increase in the number of infected basal keratinocytes at 9 h p.i., but infection of the suprabasal layer was not observed until 12 and 18 h p.i. and involved relatively few cells, suggesting that viral spread was limited in the absence of nectin-1 (Fig. 1E). This is in line with the reduced virus production in nectin-1-deficient compared to wt epidermis (Fig. 2).
FIG 2.
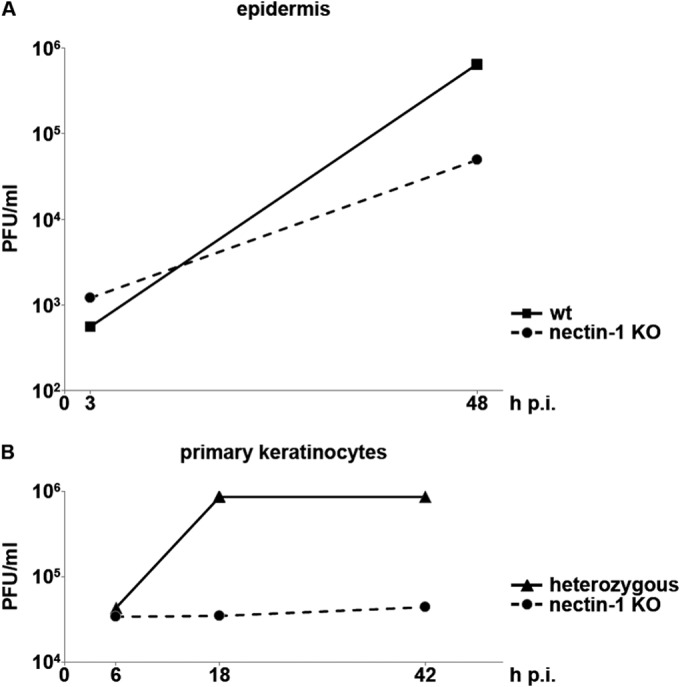
HSV-1 production in murine epidermal sheets and primary keratinocytes. (A) Epidermal sheets from wt or nectin-1-deficient adult tail skin were infected with HSV-1 at 1 PFU/cell. The virus titer was determined at 3 h and 48 h p.i. in at least 2 independent experiments and demonstrated the reduced virus production in nectin-1-deficient epidermis. The titer at 3 h p.i. represents the input virus. (B) Heterozygous or nectin-1-deficient primary keratinocytes were infected at 50 PFU/cell. At 6, 18, and 42 h p.i., the virus titer was determined in at least 2 independent experiments and demonstrated the loss of virus production in nectin-1-deficient primary keratinocytes.
In summary, the efficiency of infection was strongly reduced in the absence of nectin-1, indicating that nectin-1 serves as the major receptor in the basal keratinocyte layer of the epidermis. Nectin-1 also facilitated virus spread to suprabasal layers of the epidermis. However, a limited number of cells allowed HSV-1 entry and viral spread even in the absence of nectin-1, suggesting the presence of an alternative receptor(s) in a subpopulation of basal keratinocytes in the epidermis.
Entry of HSV-1 into nectin-1-deficient keratinocytes.
To further characterize the role of nectin-1 and possible alternative receptors in the basal layer of the epidermis, we isolated cells from epidermal sheets of newborn mice to take primary keratinocytes in culture. After 2 or 3 passages in culture, primary keratinocytes of wt, heterozygous (Pvrl1+/−), and nectin-1-deficient (Pvrl1−/−) mice were infected with HSV-1 at 20 PFU/cell. At 3 h p.i., ICP0 was present in approximately 60% of the cells from wt and heterozygous mice but only approximately 3% of the nectin-1-deficient keratinocytes (Fig. 3A and B). Even at 6 h p.i., the number of infected nectin-1-deficient cells showed only a slight increase, to 5% of cells, while ∼80% of wt keratinocytes expressed ICP0 at that time point (Fig. 3A and B). Increasing the multiplicity of infection to 50 PFU/cell did not increase the numbers of ICP0-expressing cells in the absence of nectin-1 at 6 h p.i., and this number even declined by 42 h p.i. (Fig. 3C and D), suggesting that the few infected cells died and detached without infecting neighboring cells. This suggestion is supported by the observation that keratinocytes isolated from heterozygous mice showed a notable cytopathic effect (CPE) at 6 h p.i. (Fig. 3C) and were completely detached by 18 h p.i. (Fig. 3D). Furthermore, analysis of virus growth showed an increase in titer in primary keratinocytes from heterozygous mice but no virus production in nectin-1-deficient keratinocytes (Fig. 2). The observation that efficiencies of infection were nearly identical in primary keratinocytes from wt and heterozygous mice was confirmed by infection studies at a low MOI (Fig. 3B).
FIG 3.
HSV-1 enters murine wt and heterozygous primary keratinocytes but not nectin-1-deficient cells. (A) wt, heterozygous, and nectin-1-deficient primary keratinocytes infected with HSV-1 at 20 PFU/cell were stained with anti-ICP0 (green) at 3 or 6 h p.i. F-actin was visualized with TRITC-phalloidin (red). While all wt and heterozygous cells expressed ICP0 at 3 h p.i., ICP0 was rarely found in nectin-1-deficient cells, even at 6 h p.i. (B) wt, heterozygous, and nectin-1-deficient primary keratinocytes were infected at 2 or 20 PFU/cell. The percentages of infected wt, heterozygous, and nectin-1-deficient primary keratinocytes from at least three independent experiments demonstrate that only very few primary keratinocytes show ICP0 expression in the absence of nectin-1. Results are means and standard deviations. (C) Heterozygous and nectin-1-deficient primary keratinocytes infected at 50 PFU/cell were stained with anti-ICP0 (green) and phalloidin (F-actin; red) at 6 or 42 h p.i. The high MOI resulted in a CPE in heterozygous cells at 6 h p.i., while ICP0 expression was rarely visible in nectin-1-deficient cells at 6 and 42 h p.i. Confocal projections and merged images are shown. Bars, 50 μm (A) or 100 μm (C). (D) At 6, 18, and 42 h p.i., the number of infected cells was determined in at least 2 independent experiments. No heterozygous cells were present on the dishes at 18 h and 42 h p.i., because of the strong CPE.
Taken together, the very small number of ICP0-expressing cells and the failure of the virus to propagate indicate an almost complete block of entry in the absence of nectin-1. These results support nectin-1 as the sole receptor in wt primary keratinocytes and suggest the lack of an alternative receptor.
Nectin-1 and HVEM expression in epidermis and primary keratinocytes.
We next investigated the presence of nectin family members in nectin-1-deficient mice by RT-PCR. We confirmed the absence of nectin-1 mRNAs in the epidermis (Fig. 4A). However, a weak signal was visible for nectin-1-deficient primary keratinocyte cultures (Fig. 4A and B). Control experiments confirmed that this signal was due to the 3T3 fibroblasts which were cocultured as feeder layers prior to the infection experiments (Fig. 4C). Nectin-2, -3, and -4 were expressed both in epidermis and in primary keratinocytes, and this expression was independent of the presence of nectin-1 (Fig. 4A). Nectin-2 serves as a receptor for various mammalian alphaherpesviruses, but the specificity of murine nectin-2 is restricted to pseudorabies virus (PRV) (9, 33). Nectin-3 and -4 exhibit no known receptor activity for alphaherpesviruses. Thus, it is unlikely that other nectins can serve as alternative receptors for nectin-1.
FIG 4.
Nectins and HVEM are expressed in epidermal sheets, but only nectins are expressed in primary keratinocytes. (A and B) RNAs were isolated from wt or nectin-1-deficient newborn epidermis or from wt or nectin-1-deficient primary keratinocytes. (A) Results of RT-PCR demonstrate expression of nectin-1, -2, -3, and -4 in epidermis and primary keratinocytes. (B) In contrast, HVEM expression was detected only in epidermis. As a control, GAPDH transcripts are shown. The water controls (H2O) contained no cDNA. (C) RNAs were isolated from 3T3 fibroblasts, primary keratinocytes cultured with 3T3 fibroblasts, and primary keratinocytes from which 3T3 fibroblasts were washed out. The results of RT-PCR demonstrate HVEM expression in fibroblasts and cocultured keratinocytes, but only minor amounts were detected when fibroblasts were washed out, indicating that keratinocytes do not express HVEM. Nectin-1 expression is shown as a control. (D) Nectin-1-deficient primary keratinocytes transfected with a plasmid expressing HVEM (pSC386) prior to infection with HSV-1 at 20 PFU/cell showed an increased number of infected cells compared to that for untransfected cells. The number of infected cells was determined at 3 h p.i. in 3 independent experiments. (E) Nectin-1-deficient primary keratinocytes were transfected with plasmids expressing HVEM (pSC386) or GFP (EGFP-N1). After infection at 20 PFU/cell, cells were stained with anti-ICP0 (red) and anti-keratin 14 (green), which demonstrated that ICP0 was expressed only in wt untransfected and HVEM-transfected nectin-1-deficient keratinocytes. In contrast, untransfected and GFP-transfected cells were not infected in the absence of nectin-1. Confocal projections are shown. Bars, 100 μm.
The best candidate as an alternative receptor for HSV-1 is HVEM. As a member of the tumor necrosis factor receptor family, HVEM is expressed mainly on lymphoid cells but is also found on fibroblasts and endothelial and epithelial cells (6, 34, 35). To determine whether the presence of HVEM correlated with the susceptibility of keratinocytes to entry, we investigated HVEM expression by RT-PCR. After dissociation of epidermal sheets from wt or nectin-1-deficient mice, HVEM was detected in epidermis both in the presence and in the absence of nectin-1 (Fig. 4B). In contrast, no HVEM was detected in primary keratinocytes (Fig. 4B). These results suggest the downregulation of HVEM in keratinocytes when they were taken into culture.
In summary, HVEM was present in murine epidermis, where a subpopulation of cells was infected in the absence of nectin-1, while in nectin-1-deficient primary keratinocytes, where HSV-1 ICP0 expression was nearly absent, HVEM was not detected.
Since the protein half-life of HVEM is not known, we cannot directly correlate the downregulation of HVEM transcription with the loss of HVEM protein in primary keratinocytes of low passage number. Maybe the small numbers of ICP0-expressing cells resulted from HVEM protein that was still present on some cells. We therefore performed infection studies in nectin-1-deficient keratinocytes that were kept in culture for at least 15 days. Irrespective of the time in culture, a small number of ICP0-expressing cells was still detected (data not shown), suggesting that HVEM was not responsible for HSV-1 entry into these nectin-1-deficient primary keratinocytes.
As a control, we confirmed that HVEM can facilitate entry by transfection of an HVEM-expressing plasmid into nectin-1-deficient primary keratinocytes. While untransfected cells or cells transfected with enhanced green fluorescent protein (EGFP)-expressing plasmids showed little or no ICP0 expression following exposure to HSV-1, transient overexpression of HVEM resulted in a significant number of ICP0-expressing cells (Fig. 4D and E). These results suggest that overexpressed HVEM can rescue HSV-1 entry into primary nectin-1-deficient keratinocytes.
Surface expression of HVEM and nectin in epidermis.
If HVEM serves as a receptor in the epidermis, it should be expressed on the surfaces of keratinocytes in the epidermis. To investigate surface expression of HVEM in comparison to nectin-1, we performed FACS analysis. After dissociation of epidermal sheets from newborn wt mice, the HVEM-expressing population represented approximately 17% of the analyzed cells (Fig. 5A). Comparable percentages were found on cells from epidermis of adult tail skin (data not shown). Analysis of nectin-1-deficient newborn epidermis revealed a population of approximately 10% HVEM-expressing cells (Fig. 5A). Staining was performed with a monoclonal antibody against murine HVEM (clone HMHV-1B18); the specificity of this antibody was shown by analysis of HVEM-deficient epidermis, in which the antibody detected no HVEM-expressing cells (Fig. 5B). HVEM expression on cells from wt or nectin-1-deficient epidermis was detected only when cells were dissociated from epidermis by use of an enzyme-free cell dissociation solution (CDS). This procedure is considerably less efficient than dissociation with enzyme-based reagents, such as TrypLE Select, which caused some variations in the number of HVEM-expressing cells detected. In summary, HVEM was present on the surface for a subpopulation of keratinocytes in the epidermises of wt and nectin-1-deficient mice.
FIG 5.
HVEM is expressed on the surface of wt or nectin-1-deficient epidermis. (A and B) Cells were dissociated from newborn wt or nectin-1- or HVEM-deficient epidermis by CDS treatment and stained with hamster anti-HVEM or an isotype control antibody. FACS analyses showed that 17% and 10% of the gated cells expressed HVEM (blue lines) on the surfaces of cells in wt and nectin-1 KO epidermises, respectively. (C) To analyze nectin-1 surface expression, cells were dissociated from newborn wt or nectin-1-deficient epidermis by TrypLE Select treatment and then stained with anti-nectin-1. FACS analyses demonstrated nectin-1 (blue lines) surface expression on 70% of the gated cells in wt epidermis. (D) Epidermal sheets infected with HSV-1 at 100 PFU/cell, dissociated with TrypLE Select at 3 h p.i., and stained with anti-nectin-1 show that nectin-1 disappears after infection. (E) As a control, ICP0 expression was compared in mock-infected wt epidermis (black line) and wt epidermis infected for 3 h (red line), demonstrating that ICP0 was present in 50% of the cells. Representative profiles from at least 2 (B), 3 (D), or 5 (A, C, and E) independent experiments are shown.
As a control, surface expression of nectin-1 was investigated on cells dissociated from wt epidermis by use of TrypLE Select. Staining with the anti-nectin-1 monoclonal antibody CK41 revealed that nectin-1 was detected on approximately 70% of the analyzed cells, indicating that it was present on most keratinocytes in wt epidermis (Fig. 5C). CK41 antibody did not recognize any epitopes in nectin-1-deficient epidermis, thereby confirming the specificity of the antibody (Fig. 5C). Infection of wt epidermis resulted in a loss of detectable surface nectin-1 (Fig. 5D), while ICP0 expression was detected in approximately 50% of the analyzed cells (Fig. 5E). Downregulation of cell surface nectin-1, mediated by binding of gD during HSV-1 entry, has been reported (36, 37). Thus, we suggest that nectin-1, which is present on most keratinocytes in wt epidermis, is very efficiently internalized upon HSV-1 entry.
Uptake of HSV-1 into primary keratinocytes and epidermis.
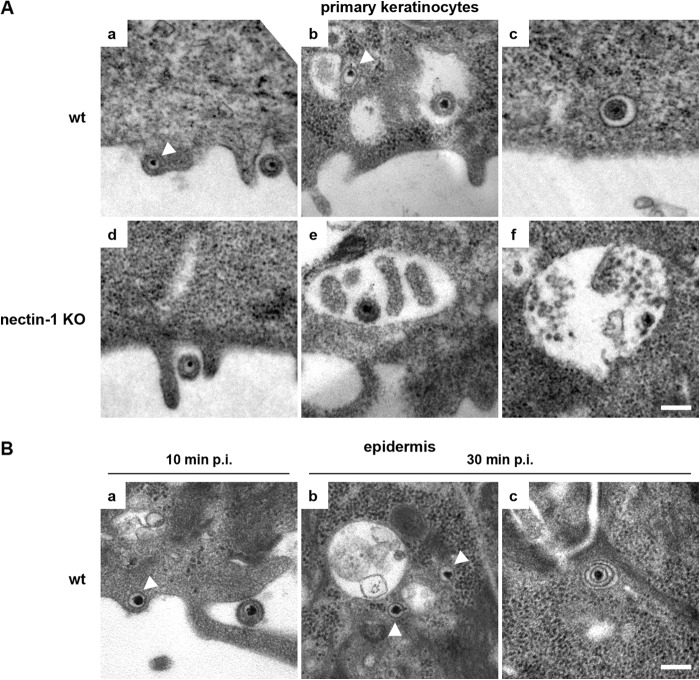
Based on the observations that HVEM was absent in cultured keratinocytes and that nectin-1 deficiency led to an almost complete block of infection, we assume that nectin-1 serves as the sole receptor in primary murine keratinocytes. To address HSV-1 internalization under these circumstances, we analyzed virus uptake by using electron microscopy. Ten minutes after infection of wt primary keratinocytes, we observed particles attached to the cell surface, frequently at cytoplasmic extensions of the plasma membrane. Free capsids were seen in the cytoplasm, often just underneath the plasma membrane, which suggests direct fusion of the viral envelope (Fig. 6A, panel a). One notable feature was the presence of large vesicles, some containing enveloped virus particles. Interestingly, free capsids were sometimes visible in close proximity to such vesicles (Fig. 6A, panel b). In addition, virus particles were occasionally seen in vesicles with a membrane closely surrounding the particle (Fig. 6A, panel c). In nectin-1-deficient primary keratinocytes, attached virus particles were present at the cell surface, as expected (Fig. 6A, panel d). As seen for wt cells, we observed large vesicles containing virus particles (Fig. 6A, panel e), some of which might have been damaged (Fig. 6A, panel f). In contrast to wt primary keratinocytes, we never observed any free capsids in the cytoplasm of nectin-1-deficient cells. These results suggest that the loss of infection in the absence of nectin-1 correlates with the absence of free capsids in the cytoplasm. Virus uptake into vesicles appears to be nectin-1 independent, but release of the capsids from vesicles by fusion with the vesicle membrane and direct fusion of the viral envelope with the plasma membrane may be blocked in the absence of nectin-1.
FIG 6.
Characterization of HSV-1 uptake into murine primary keratinocytes or epidermal sheets. (A) EM analyses of wt or nectin-1-deficient primary keratinocytes infected for 10 min show particles on the cell surface (a and d), free cytoplasmic capsids (indicated by arrowheads) (a and b), and enveloped particles in vesicles (b, c, e, and f). No cytoplasmic capsids are visible in nectin-1-deficient cells, but only particles in vesicles. (B) EM analyses of wt epidermal sheets infected for 10 or 30 min show a particle on the surface (a), free cytoplasmic capsids (indicated by arrowheads) (a and b), and an enveloped particle in a vesicle (c), supporting the presence of uptake mechanisms comparable to those in primary keratinocytes. Bars, 0.2 μm.
To further address the formation of large vesicles, we investigated the efficiency of potential phagocytic uptake in primary murine keratinocytes. Phagocytosis is defined as an uptake mechanism for particles of >0.5 μm (38). To investigate the efficiency of phagocytic uptake in primary keratinocytes, we examined the uptake of fluorescently labeled latex beads (2 μm). Beads were internalized by all primary murine keratinocytes and accumulated at perinuclear regions (Fig. 7). As a positive control, we analyzed RPE cells, which are known for extremely efficient phagocytic uptake, and we observed perinuclear accumulation of the beads in all cells (Fig. 7). As an additional control, we analyzed HaCaT cells and detected efficient uptake in only a limited number of cells (Fig. 7). Our results demonstrate that phagocytic uptake takes place in murine primary keratinocytes with a high efficiency, which presumably explains the prominence of large virus-containing vesicles in both wt and nectin-1-deficient keratinocytes.
FIG 7.
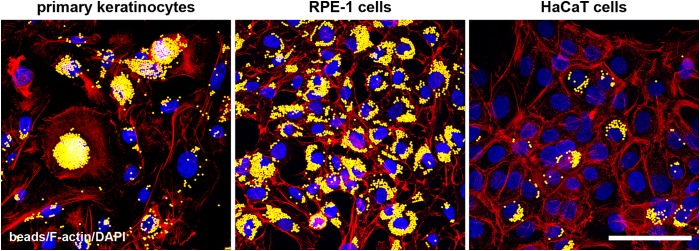
Uptake of latex beads into epithelial cells. Murine primary keratinocytes, RPE-1 cells, and HaCaT cells were incubated with latex beads (yellow). Visualization of F-actin (red) and nuclei (blue) demonstrates the internalization of the beads and their accumulation in primary keratinocytes. The very efficient uptake visible in RPE cells is shown as a control and is compared to the less efficient uptake in HaCaT cells. Confocal projections and merged images are shown. Bar, 50 μm.
We also analyzed virus uptake into epidermal sheets from wt mice, in which nectin-1 and potentially HVEM act as receptors for HSV-1. At 10 min p.i., we observed virions at the cell surface of keratinocytes, representing the basal layer in the epidermal sheets. In addition, free capsids were detected just underneath the plasma membrane, suggesting direct fusion of the viral envelope with the plasma membrane (Fig. 6B, panel a). By 30 min p.i., the majority of virus particles in the cytoplasm were free capsids, which were sometimes found in the vicinity of vesicles (Fig. 6B, panel b). Particles in vesicles were rarely detected, although some intact virions in small vesicles were seen (Fig. 6B, panel c), and large vesicles themselves were less prominent than in cultured keratinocytes.
In summary, these observations suggest that HSV-1 uses alternative pathways to enter cultured murine keratinocytes, where nectin-1 acts as the sole receptor. In epidermal sheets with nectin-1 serving as the major receptor, similar observations were made. Thus, we assume that nectin-1 promotes both direct fusion with the plasma membrane and fusion after uptake via vesicles into murine keratinocytes.
DISCUSSION
The cellular entry of HSV-1 is well studied in cultured cell lines, but the molecular mechanisms on which entry into epidermis depends are still unknown. Here we identified the receptor(s) and characterized the uptake mechanisms contributing to the entry of HSV-1 into murine epidermis.
Ex vivo infection of murine epidermal sheets revealed nectin-1 as a major but not exclusive receptor for entry of HSV-1 into keratinocytes. Since HSV-1 does not enter a host via the cornified layer of skin, we prepared epidermal sheets by removing the dermal layer. This allowed the virus to access the basal layer of keratinocytes and to spread to suprabasal layers. While HSV-1 was able to enter all the basal keratinocytes of wt epidermis, infection of nectin-1-deficient epidermis was much more limited. The few infected cells were detected mainly in groups, suggesting that only some areas of the interfollicular epidermis allowed entry. In addition, the germs of wt hair follicles were infected very efficiently, while nectin-1-deficient hair germs showed only sporadically infected cells. Thus, we assume that the localization of infected cells in nectin-1-deficient epidermis reflects sporadic expression of an alternative receptor.
Since nectin-1/HVEM double-KO mice show almost no signs of disease development after HSV-2 infection (13, 39), we decided to investigate HVEM as a potential alternative receptor in the epidermis. HVEM transcripts were present in the keratinocytes of newborn and adult epidermis in both the presence and absence of nectin-1. Interestingly, no HVEM RNA was found in primary keratinocytes that were kept in culture for at least 5 days, suggesting that HVEM expression became downregulated after isolation from newborn epidermis. The lack of HVEM transcripts correlates with our observation that HSV-1 entry into primary keratinocytes was almost completely prevented but could be restored by transfecting them with an HVEM-expressing plasmid. The situation in cultured keratinocytes is clearly distinct from that in epidermis, where a reduced number of cells supported infection and HVEM transcripts were readily detected. Interestingly, although nectin-1 was detected on the surfaces of nearly all keratinocytes from dissociated epidermal sheets, HVEM was present on only a subpopulation of them, which presumably explains, at least partially, its minor role in infection. The widespread occurrence of nectin-1 is in line with previous results demonstrating that it is present at cell-cell contacts in the epidermal layers and at the tips of developing hair follicles in mouse fetal epidermis (40). In contrast, the expression of HVEM on skin keratinocytes has not been described so far. Immunofluorescence studies would be helpful to further characterize and compare the HVEM expression pattern with that of nectin-1 in the various epidermal layers; however, our tested antibodies against HVEM or nectin-1 gave no reliable staining pattern in the epidermis. From our results, we conclude that HVEM can serve as an alternative receptor in the epidermis and that, in the absence of nectin-1, HVEM leads to a rather limited and slower infection. The high efficiency of nectin-1 as a receptor for HSV-1 was confirmed by infection studies with HVEM-deficient epidermis, which demonstrated numbers of infected cells comparable to those in wt epidermis (Fig. 8). The observation that nectin-1 serves as the major receptor for HSV-1 in epidermis, with HVEM acting in a minor, possibly supplementary role, is in line with recent studies demonstrating that a deficiency of nectin-1 decreased the occurrence of viral lesions in murine vaginas after HSV-2 inoculation, while HVEM deficiency had no effect (13).
FIG 8.

Efficiency of HSV-1 infection in epidermal sheets in the presence and absence of HVEM. Epidermal sheets were prepared from adult wt and HVEM-deficient tail skin and infected with HSV-1 at 50 to 100 PFU/cell. At 3 h p.i., epidermal whole mounts stained with anti-ICP0 (green) and DAPI (DNA; blue) demonstrated comparable efficiencies of infection in the presence and absence of HVEM. Abbreviations: HF, hair follicle; IFE, interfollicular epidermis; SG, sebaceous gland. Bar, 50 μm.
The identification of the receptors involved in HSV-1 entry into the epidermis is an important precondition to better understand the uptake mechanism(s) into keratinocytes. Since nectin-1 acts as the major receptor for HSV-1 in epidermis, we expected that the observed nectin-1 downregulation from the cell surface would correlate with the mode of HSV-1 uptake. Recent studies led to the conclusion that HSV-1 uses a nectin-1 internalization pathway for endocytic uptake (37). However, our EM studies of epidermal sheets revealed capsids just underneath the plasma membrane in addition to enveloped virus particles in vesicles, suggesting that HSV-1 can enter both by direct fusion with the plasma membrane and by endocytosis. During uptake into primary murine keratinocytes, we again observed cytoplasmic capsids underneath the plasma membrane and particles in vesicles, although vesicle formation appeared more prominent than the case in epidermal sheets. Surprisingly, large vesicles containing virus particles were also present in nectin-1-deficient keratinocytes, where a block of infection was observed. Since no capsids were observed in the cytoplasm, we assume that internalization via such vesicles leads to lysosomal degradation. One possibility is that in the absence of nectin-1, virus particles get trapped in phagocytic vesicles (41), which appeared to be prominent in primary keratinocytes. Non-receptor-mediated uptake of HSV-1 is in line with previous studies demonstrating that CHO cells, which lack any known gD receptor, can internalize HSV-1 (42). In contrast, in the receptor-negative murine melanoma cell line B78, virions were found only on the cell surface (43).
We concluded that when nectin-1 is present, HSV-1 most likely enters via fusion with the plasma membrane, but probably also by endocytic uptake and membrane fusion, which results in rapid release of capsids into the cytoplasm. How these alternative pathways correlate with nectin-1 internalization has still to be shown. The dual internalization mode in murine keratinocytes is in line with our results for human keratinocytes, where HSV-1 uptake occurs by direct fusion with the plasma membrane and by endocytic vesicles, in a process which requires dynamin and is dependent on cholesterol (27).
In summary, we characterized HSV-1 entry into murine epidermis, where nectin-1 acts as the major receptor, with HVEM playing a minor role both in initial uptake of free virus and in viral spread. We assume that only a limited number of keratinocytes in the epidermal layers express HVEM on the surface, while nectin-1 is present on most cells. Maybe HVEM plays a role not only as a receptor but also in modulating the host immune response by interfering with natural ligands (44). In our working model, ex vivo infection allowed the virus to access the epidermal layers from the basal side and to reach nectin-1 as the major receptor. Determining how HSV-1 gains access to the cell-cell adhesion molecule nectin-1 in intact epidermal layers will require further experimentation.
ACKNOWLEDGMENTS
We thank Sonja Kropp for assistance with breeding of the HVEM KO mice, Beatrix Martiny for technical assistance with the preparations of EM samples, Jennifer Kaltenberg, Julia Etich, and Bent Brachvogel for help with FACS analysis and for providing antibodies, Claudia Uthoff-Hachenberg for help with antibody preparations, and Roselyn Eisenberg and Gary Cohen for providing the plasmid pSC386. We are grateful to Roger Everett for the antibodies against ICP0. Additional thanks go to Markus Plomann and Mats Paulsson for discussions and advice.
This research was supported by the German Research Foundation (grant KN536/16-2) and the Köln Fortune Program/Faculty of Medicine, University of Cologne. F.J.R. is funded by the UK Medical Research Council.
REFERENCES
- 1.Heldwein EE, Krummenacher C. 2008. Entry of herpesviruses into mammalian cells. Cell Mol Life Sci 65:1653–1668. doi: 10.1007/s00018-008-7570-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Connolly SA, Jackson JO, Jardetzky TS, Longnecker R. 2011. Fusing structure and function: a structural view of the herpesvirus entry machinery. Nat Rev Microbiol 9:369–381. doi: 10.1038/nrmicro2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eisenberg RJ, Atanasiu D, Cairns TM, Gallagher JR, Krummenacher C, Cohen GH. 2012. Herpes virus fusion and entry: a story with many characters. Viruses 4:800–832. doi: 10.3390/v4050800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Montgomery RI, Warner MS, Lum BJ, Spear PG. 1996. Herpes simplex virus-1 entry into cells mediated by a novel member of the TNF/NGF receptor family. Cell 87:427–436. doi: 10.1016/S0092-8674(00)81363-X. [DOI] [PubMed] [Google Scholar]
- 5.Geraghty RJ, Krummenacher C, Cohen GH, Eisenberg RJ, Spear PG. 1998. Entry of alphaherpesviruses mediated by poliovirus receptor-related protein 1 and poliovirus receptor. Science 280:1618–1620. doi: 10.1126/science.280.5369.1618. [DOI] [PubMed] [Google Scholar]
- 6.Krummenacher C, Baribaud F, Ponce de Leon M, Baribaud I, Whitbeck JC, Xu R, Cohen GH, Eisenberg RJ. 2004. Comparative usage of herpesvirus entry mediator A and nectin-1 by laboratory strains and clinical isolates of herpes simplex virus. Virology 322:286–299. doi: 10.1016/j.virol.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 7.Steinberg MW, Cheung TC, Ware CF. 2011. The signaling networks of the herpesvirus entry mediator (TNFRSF14) in immune regulation. Immunol Rev 244:169–187. doi: 10.1111/j.1600-065X.2011.01064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takai Y, Ikeda W, Ogita H, Rikitake Y. 2008. The immunoglobulin-like cell adhesion molecule nectin and its associated protein afadin. Annu Rev Cell Dev Biol 24:309–342. doi: 10.1146/annurev.cellbio.24.110707.175339. [DOI] [PubMed] [Google Scholar]
- 9.Shukla D, Rowe CL, Dong Y, Racaniello VR, Spear PG. 1999. The murine homolog (Mph) of human herpesvirus entry protein B (HveB) mediates entry of pseudorabies virus but not herpes simplex virus types 1 and 2. J Virol 73:4493–4497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O'Donnell CD, Kovacs M, Akhtar J, Valyi-Nagy T, Shukla D. 2010. Expanding the role of 3-O sulfated heparan sulfate in herpes simplex virus type-1 entry. Virology 397:389–398. doi: 10.1016/j.virol.2009.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yoon M, Zago A, Shukla D, Spear PG. 2003. Mutations in the N termini of herpes simplex virus type 1 and 2 gDs alter functional interactions with the entry/fusion receptors HVEM, nectin-2, and 3-O-sulfated heparan sulfate but not with nectin-1. J Virol 77:9221–9231. doi: 10.1128/JVI.77.17.9221-9231.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parr MB, Kepple L, McDermott MR, Drew MD, Bozzola JJ, Parr EL. 1994. A mouse model for studies of mucosal immunity to vaginal infection by herpes simplex virus type 2. Lab Invest 70:369–380. [PubMed] [Google Scholar]
- 13.Taylor JM, Lin E, Susmarski N, Yoon M, Zago A, Ware CF, Pfeffer K, Miyoshi J, Takai Y, Spear PG. 2007. Alternative entry receptors for herpes simplex virus and their roles in disease. Cell Host Microbe 2:19–28. doi: 10.1016/j.chom.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoon M, Kopp SJ, Taylor JM, Storti CS, Spear PG, Muller WJ. 2011. Functional interaction between herpes simplex virus type 2 gD and HVEM transiently dampens local chemokine production after murine mucosal infection. PLoS One 6:e16122. doi: 10.1371/journal.pone.0016122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Valyi-Nagy T, Sheth V, Clement C, Tiwari V, Scanlan P, Kavouras JH, Leach L, Guzman-Hartman G, Dermody TS, Shukla D. 2004. Herpes simplex virus entry receptor nectin-1 is widely expressed in the murine eye. Curr Eye Res 29:303–309. doi: 10.1080/02713680490516756. [DOI] [PubMed] [Google Scholar]
- 16.Kovacs SK, Tiwari V, Prandovszky E, Dosa S, Bacsa S, Valyi-Nagy K, Shukla D, Valyi-Nagy T. 2009. Expression of herpes virus entry mediator (HVEM) in the cornea and trigeminal ganglia of normal and HSV-1 infected mice. Curr Eye Res 34:896–904. doi: 10.3109/02713680903184250. [DOI] [PubMed] [Google Scholar]
- 17.Karaba AH, Kopp SJ, Longnecker R. 2011. Herpesvirus entry mediator and nectin-1 mediate herpes simplex virus 1 infection of the murine cornea. J Virol 85:10041–10047. doi: 10.1128/JVI.05445-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karaba AH, Kopp SJ, Longnecker R. 2012. Herpesvirus entry mediator is a serotype specific determinant of pathogenesis in ocular herpes. Proc Natl Acad Sci U S A 109:20649–20654. doi: 10.1073/pnas.1216967109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Watt FM. 2001. Stem cell fate and patterning in mammalian epidermis. Curr Opin Genet Dev 11:410–417. doi: 10.1016/S0959-437X(00)00211-2. [DOI] [PubMed] [Google Scholar]
- 20.Wakamatsu K, Ogita H, Okabe N, Irie K, Tanaka-Okamoto M, Ishizaki H, Ishida-Yamamoto A, Iizuka H, Miyoshi J, Takai Y. 2007. Up-regulation of loricrin expression by cell adhesion molecule nectin-1 through Rap1-ERK signaling in keratinocytes. J Biol Chem 282:18173–18181. doi: 10.1074/jbc.M611159200. [DOI] [PubMed] [Google Scholar]
- 21.Barron MJ, Brookes SJ, Draper CE, Garrod D, Kirkham J, Shore RC, Dixon MJ. 2008. The cell adhesion molecule nectin-1 is critical for normal enamel formation in mice. Hum Mol Genet 17:3509–3520. doi: 10.1093/hmg/ddn243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Braun KM, Niemann C, Jensen UB, Sundberg JP, Silva-Vargas V, Watt FM. 2003. Manipulation of stem cell proliferation and lineage commitment: visualisation of label-retaining cells in wholemounts of mouse epidermis. Development 130:5241–5255. doi: 10.1242/dev.00703. [DOI] [PubMed] [Google Scholar]
- 23.Wang Y, Subudhi SK, Anders RA, Lo J, Sun Y, Blink S, Wang Y, Liu X, Mink K, Degrandi D, Pfeffer K, Fu YX. 2005. The role of herpesvirus entry mediator as a negative regulator of T cell-mediated responses. J Clin Invest 115:711–717. doi: 10.1172/JCI22982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boukamp P, Petrussevska RT, Breitkreutz D, Hornung J, Markham A, Fusenig NE. 1988. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J Cell Biol 106:761–771. doi: 10.1083/jcb.106.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schelhaas M, Jansen M, Haase I, Knebel-Mörsdorf D. 2003. Herpes simplex virus type 1 exhibits a tropism for basal entry in polarized epithelial cells. J Gen Virol 84:2473–2484. doi: 10.1099/vir.0.19226-0. [DOI] [PubMed] [Google Scholar]
- 26.Connolly SA, Landsburg DJ, Carfi A, Wiley DC, Eisenberg RJ, Cohen GH. 2002. Structure-based analysis of the herpes simplex virus glycoprotein D binding site present on herpesvirus entry mediator HveA (HVEM). J Virol 76:10894–10904. doi: 10.1128/JVI.76.21.10894-10904.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rahn E, Petermann P, Hsu MJ, Rixon FJ, Knebel-Mörsdorf D. 2011. Entry pathways of herpes simplex virus type 1 into human keratinocytes are dynamin- and cholesterol-dependent. PLoS One 6:e25464. doi: 10.1371/journal.pone.0025464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Everett RD, Cross A, Orr A. 1993. A truncated form of herpes simplex virus type 1 immediate-early protein Vmw110 is expressed in a cell type dependent manner. Virology 197:751–756. doi: 10.1006/viro.1993.1651. [DOI] [PubMed] [Google Scholar]
- 29.Roberts AP, Abaitua F, O'Hare P, McNab D, Rixon FJ, Pasdeloup D. 2009. Differing roles of inner tegument proteins pUL36 and pUL37 during entry of herpes simplex virus type 1. J Virol 83:105–116. doi: 10.1128/JVI.01032-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bechtel M, Keller MV, Bloch W, Sasaki T, Boukamp P, Zaucke F, Paulsson M, Nischt R. 2012. Different domains in nidogen-1 and nidogen-2 drive basement membrane formation in skin organotypic cocultures. FASEB J 26:3637–3648. doi: 10.1096/fj.11-194597. [DOI] [PubMed] [Google Scholar]
- 31.Krummenacher C, Baribaud I, Ponce de Leon M, Whitbeck JC, Lou H, Cohen GH, Eisenberg RJ. 2000. Localization of a binding site for herpes simplex virus glycoprotein D on herpesvirus entry mediator C by using antireceptor monoclonal antibodies. J Virol 74:10863–10872. doi: 10.1128/JVI.74.23.10863-10872.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Petermann P, Haase I, Knebel-Mörsdorf D. 2009. Impact of Rac1 and Cdc42 signaling during early herpes simplex virus type 1 infection of keratinocytes. J Virol 83:9759–9772. doi: 10.1128/JVI.00835-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Warner MS, Geraghty RJ, Martinez WM, Montgomery RI, Whitbeck JC, Xu R, Eisenberg RJ, Cohen GH, Spear PG. 1998. A cell surface protein with herpesvirus entry activity (HveB) confers susceptibility to infection by mutants of herpes simplex virus type 1, herpes simplex virus type 2, and pseudorabies virus. Virology 246:179–189. doi: 10.1006/viro.1998.9218. [DOI] [PubMed] [Google Scholar]
- 34.Kwon BS, Tan KB, Ni J, Oh KO, Lee ZH, Kim KK, Kim YJ, Wang S, Gentz R, Yu GL, Harrop J, Lyn SD, Silverman C, Porter TG, Truneh A, Young PR. 1997. A newly identified member of the tumor necrosis factor receptor superfamily with a wide tissue distribution and involvement in lymphocyte activation. J Biol Chem 272:14272–14276. doi: 10.1074/jbc.272.22.14272. [DOI] [PubMed] [Google Scholar]
- 35.Hung SL, Cheng YY, Wang YH, Chang KW, Chen YT. 2002. Expression and roles of herpesvirus entry mediators A and C in cells of oral origin. Oral Microbiol Immunol 17:215–223. doi: 10.1034/j.1399-302X.2002.170403.x. [DOI] [PubMed] [Google Scholar]
- 36.Stiles KM, Milne RS, Cohen GH, Eisenberg RJ, Krummenacher C. 2008. The herpes simplex virus receptor nectin-1 is down-regulated after trans-interaction with glycoprotein D. Virology 373:98–111. doi: 10.1016/j.virol.2007.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stiles KM, Krummenacher C. 2010. Glycoprotein D actively induces rapid internalization of two nectin-1 isoforms during herpes simplex virus entry. Virology 399:109–119. doi: 10.1016/j.virol.2009.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Groves E, Dart AE, Covarelli V, Caron E. 2008. Molecular mechanisms of phagocytic uptake in mammalian cells. Cell Mol Life Sci 65:1957–1976. doi: 10.1007/s00018-008-7578-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kopp SJ, Karaba AH, Cohen LK, Banisadr G, Miller RJ, Muller WJ. 2013. Pathogenesis of neonatal herpes simplex 2 disease in a mouse model is dependent on entry receptor expression and route of inoculation. J Virol 87:474–481. doi: 10.1128/JVI.01849-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Okabe N, Ozaki-Kuroda K, Nakanishi H, Shimizu K, Takai Y. 2004. Expression patterns of nectins and afadin during epithelial remodeling in the mouse embryo. Dev Dyn 230:174–186. doi: 10.1002/dvdy.20033. [DOI] [PubMed] [Google Scholar]
- 41.Kinchen JM, Ravichandran KS. 2008. Phagosome maturation: going through the acid test. Nat Rev Mol Cell Biol 9:781–795. doi: 10.1038/nrm2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nicola AV, Straus SE. 2004. Cellular and viral requirements for rapid endocytic entry of herpes simplex virus. J Virol 78:7508–7517. doi: 10.1128/JVI.78.14.7508-7517.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Milne RSB, Nicola AV, Whitbeck JC, Eisenberg RJ, Cohen GH. 2005. Glycoprotein D receptor-dependent, low-pH-independent endocytic entry of herpes simplex virus type 1. J Virol 79:6655–6663. doi: 10.1128/JVI.79.11.6655-6663.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stiles KM, Whitbeck JC, Lou H, Cohen GH, Eisenberg RJ, Krummenacher C. 2010. Herpes simplex virus glycoprotein D interferes with binding of herpesvirus entry mediator to its ligands through downregulation and direct competition. J Virol 84:11646–11660. doi: 10.1128/JVI.01550-10. [DOI] [PMC free article] [PubMed] [Google Scholar]