ABSTRACT
Previous animal model experiments have shown a correlation between interferon gamma (IFN-γ) expression and both survival from infection with attenuated rabies virus (RABV) and reduction of neurological sequelae. Therefore, we hypothesized that rapid production of murine IFN-γ by the rabies virus itself would induce a more robust antiviral response than would occur naturally in mice. To test this hypothesis, we used reverse engineering to clone the mouse IFN-γ gene into a pathogenic rabies virus backbone, SPBN, to produce the recombinant rabies virus designated SPBNγ. Morbidity and mortality were monitored in mice infected intranasally with SPBNγ or SPBN(−) control virus to determine the degree of attenuation caused by the expression of IFN-γ. Incorporation of IFN-γ into the rabies virus genome highly attenuated the virus. SPBNγ has a 50% lethal dose (LD50) more than 100-fold greater than SPBN(−). In vitro and in vivo mouse experiments show that SPBNγ infection enhances the production of type I interferons. Furthermore, knockout mice lacking the ability to signal through the type I interferon receptor (IFNAR−/−) cannot control the SPBNγ infection and rapidly die. These data suggest that IFN-γ production has antiviral effects in rabies, largely due to the induction of type I interferons.
IMPORTANCE Survival from rabies is dependent upon the early control of virus replication and spread. Once the virus reaches the central nervous system (CNS), this becomes highly problematic. Studies of CNS immunity to RABV have shown that control of replication begins at the onset of T cell entry and IFN-γ production in the CNS prior to the appearance of virus-neutralizing antibodies. Moreover, antibody-deficient mice are able to control but not clear attenuated RABV from the CNS. We find here that IFN-γ triggers the early production of type I interferons with the expected antiviral effects. We also show that engineering a lethal rabies virus to express IFN-γ directly in the infected tissue reduces rabies virus replication and spread, limiting its pathogenicity in normal and immunocompromised mice. Therefore, vector delivery of IFN-γ to the brain may have the potential to treat individuals who would otherwise succumb to infection with rabies virus.
INTRODUCTION
Rabies virus (RABV) is the type species of the Lyssavirus genus in the Rhabdoviridae family. Its small, negative-stranded RNA genome contains only five true genes (1, 2). Although relatively simple, this zoonotic virus has a devastating impact worldwide. The majority of human rabies deaths occur in children in the developing world, and it is estimated that at least 55,000 humans die of rabies each year in Africa and Asia alone (3).
Although RABV infection historically has been viewed as a death sentence once the virus reaches the brain, there is a small but growing number of humans who have survived rabies even though the virus entered the brain (4, 5). Due to such cases and to research using animal models of RABV infection (6–8), many believe that the immune system may be capable of clearing RABV from the brain without causing irreparable immunopathology. It is clear, however, that therapeutic intervention will be necessary in the vast majority of cases. Some theoretical or experimental rabies treatments involve slowing virus replication and/or spread by induction of hypothermia (9), the highly controversial use of therapeutic coma (5), enhancing immune cell entry into the central nervous system (CNS) (10), superinfection with an attenuated RABV (11, 12), and systemic or intrathecal administration of antiviral drugs and interferons (IFN) (13). For decades while many researchers have been studying the effects of type I IFN (predominantly IFN-α and -β) during RABV infection, our work has focused primarily on the role(s) of IFN-γ during RABV clearance from the CNS.
IFN-γ is a pleiotropic cytokine and is the only known form of type II interferon. Although originally discovered by its ability to “interfere” with virus infection (14), its immunomodulatory functions were quickly recognized, and it is now widely accepted that IFN-γ has many important functions in both innate and adaptive immunity. Some of these include upregulation of adhesion molecules, activation of macrophages and NK cells, T cell activation and differentiation, upregulation of major histocompatibility complex (MHC) molecules, and antibody isotype switching, as well as induction of reactive oxygen species and reactive nitrogen intermediates (15). These are merely a sample of what IFN-γ induces as it is known to affect the expression of hundreds of genes (16). Additionally, cross talk between type I and type II IFN has been shown (17), suggesting IFN-γ can amplify its antiviral effects via the induction of type I IFN. Furthermore, it is known that IFN-γ can potentiate the action of type I IFN (18) and act synergistically with them (19). Since its discovery, researchers and clinicians have been trying to harness IFN-γ for its potential therapeutic effects. It has been tested experimentally as an adjuvant in vaccines (20, 21) and as a treatment in animal models of disease, such as tuberculosis (22), each with various degrees of success. Currently the only FDA-approved uses for IFN-γ in humans are treatments for chronic granulomatous disease (23) and osteopetrosis (24), but IFN-γ is also prescribed by veterinarians to treat canine atopic dermatitis (25). These clinical uses of IFN-γ highlight its pleiotropic nature as the mechanisms of action in these diseases are through enhanced innate immunity, activation of osteoclasts, and suppression of the adaptive immune response, respectively.
Our previous work has shown that IFN-γ mRNA expression in the brains of mice infected with attenuated RABV strongly correlates with blood-brain barrier (BBB) permeability changes and clearance of RABV from the CNS (6, 26). Recent experiments confirm the importance of IFN-γ during RABV clearance from the CNS, supporting the concept that IFN-γ is at the center of the signaling pathway that alters tight-junction protein expression and leads to increased BBB permeability and survival of RABV infection (27). These rabies-specific data, as well as the more commonly known roles of IFN-γ in the innate and adaptive arms of the immune response, suggest that therapeutic enhancement of IFN-γ expression in the CNS during a rabies infection may prevent virus spread and aid the clearance of RABV from CNS tissues through a number of mechanisms. Therefore, to more precisely determine the effects of IFN-γ expression in the CNS on pathogenic RABV infection, we engineered a pathogenic RABV to express the murine IFN-γ gene. We report that RABV-expressed IFN-γ highly attenuates a pathogenic RABV in mice and that this attenuation is due, at least in part, to upregulation of type I IFN.
MATERIALS AND METHODS
Mice.
Female Swiss Webster mice were purchased from Taconic Farms (Germantown, NY). Female C57BL/6J mice and IFN-γ−/− mice on a C57BL/6 background were purchased from Jackson Laboratories (Bar Harbor, ME). WT129 and IFNAR−/− mice were from investigator breeding colonies maintained at Thomas Jefferson University and were originally provided by Michel Aguet (28). All mouse experiments were approved by the Thomas Jefferson University Institutional Animal Care and Use Committee.
Cell lines.
BSR cells (a BHK-21 clone) (29) grown in Dulbecco's modified Eagle's medium (DMEM) (Mediatech, Manassas, VA) supplemented with 10% fetal bovine serum (FBS) (Atlanta Biologicals, Flowery Ranch, GA) were used to grow virus stocks. Mouse neuroblastoma (NA) cells were grown in RPMI medium 1640 (Mediatech, Manassas, VA) and used for growth curves, virus titers, and immunofluorescence. Mouse astrocytoma (AS) cells and monocyte lineage (MC) cells were grown in DMEM supplemented with 10% FBS and were used for growth curves.
Viruses.
SPBN, the prototype recombinant RABV, was derived from the SADB19 strain (30, 31). Although SPBN is often referred to as a vaccine vector, when administered to mice intranasally (i.n.), it is highly pathogenic (32).
Construction of recombinant viruses.
The recombinant RABVs SPBN(−) and SPBNγ were engineered as described previously (33). For SPBNγ, murine IFN-γ DNA was PCR amplified from mRNA extracted from RABV-infected mouse brain tissue using the custom primers (IDT, Coralville, IA) described below and DeepVent polymerase (NEB, Ipswich, MA). The forward primer, 5′ATAGAATTCCGTACGAAGATGAACGCTACACACTGCATCTTGGCT3′, contains a BsiWI restriction site (boldface), the start codon (underlined), and the gene-specific sequence (italic). The reverse primer, 5′ATTCTCTAGATAGCTAGCTCAGCAGCGACTCCTTTTCCGCTTCCT3′, contains an NheI restriction site (boldface), a stop codon (underlined), and the gene-specific sequence (italic). The resultant IFN-γ DNA was cloned into the pSPBN plasmid, creating the pSPBNγ plasmid. Standard transformation and transfection methods were used to complete the virus rescue of SPBNγ as outlined above.
Growth curves.
NA, AS, or MC cells were infected at a multiplicity of infection (MOI) of 0.01 for multistep and 1 for single-step growth curves. One hundred-microliter supernatant aliquots were taken at 0, 24, 48, and 72 h postinfection (hpi). Virus titers from the supernatants were calculated as described below.
Virus titers.
To determine virus titers, NA cells were seeded into 96-well plates, grown for 2 days, and then infected with virus in serial 10-fold dilutions. Two days postinfection (dpi), the cells were fixed with 80% acetone and stained with fluorescein isothiocyanate (FITC)-conjugated anti-RABV RNP antibody (Fujirebio Diagnostics, Malvern, PA). Virus titers of triplicate samples were determined using a fluorescence microscope.
Immunofluorescent staining.
NA cells were grown in culture well slides for 24 h and then infected with RABV at an MOI of 0.1. Twenty-four hours later, cells were stained for the presence of RABV and mouse IFN-γ using FITC-conjugated anti-RABV RNP antibody and phycoerythrin (PE)-conjugated anti-mouse IFN-γ antibody (Sigma, St. Louis, MO), respectively. Slides were mounted using ProLong Gold with 4′,6-diamidino-2-phenylindole (DAPI) (Life Technologies, Eugene, OR). Photos were taken using a DM6000B fluorescence microscope (Leica, Buffalo Grove, IL).
Infection of mice.
Groups of mice were infected under isoflurane (Vedco, St. Joseph, MO) anesthesia: intracranially (i.c.) with 103 focus-forming units (FFU) in 10 μl phosphate-buffered saline (PBS) or intranasally (i.n.) with 103 to 106 FFU in 20 μl PBS. Mice were observed for at least 30 days for appearance of clinical signs of rabies, such as limb paralysis, tremors, and weight loss. Mice were humanely euthanized when moribund or after losing greater than 30% of starting body weight.
IFN-γ treatment.
Cells were treated with 10, 100, or 1,000 U of recombinant mouse IFN-γ (BD Biosciences, San Jose, CA) at 24 and 48 h postinfection by addition to the culture medium.
ELISA.
Fifty-microliter aliquots of virus-infected supernatants were assayed for IFN-γ using an OptEIA mouse IFN-γ kit II (BD Biosciences, San Jose, CA) or for IFN-α using a mouse IFN-alpha Platinum ELISA kit (eBioscience, Vienna, Austria) per the manufacturers' instructions. The plates were then scanned in a Synergy H1 plate reader (BioTek, Winooski, VT), and absorbance at 450 nm was recorded.
qPCR.
Total RNA (RNAtot) was extracted from brain samples using a Qiagen RNeasy kit (Qiagen, Valencia, CA) and modified Qiagen protocol previously described (6). Briefly, tissues were homogenized by aspiration through a sterile 20-gauge needle approximately 15 times in TriReagent (MRC, Cincinnati, OH). Homogenized samples were spun in a Heraeus Biofuge Pico benchtop centrifuge (Thermo Scientific, Langenselbold, Germany) at 12,000 rpm for 15 min at room temperature. The aqueous layer was removed and mixed with an equal portion of 70% ethanol and then added to the Qiagen spin column. All subsequent steps are outlined in the Qiagen manual, including the optional on-column DNase I digestion, which was used. After RNAtot extraction, cDNA was prepared using oligo(dT15) primers, deoxynucleoside triphosphate (dNTP), and Moloney murine leukemia virus reverse transcriptase (Promega, Madison, WI) as described previously (6). Finally, each sample was analyzed for the presence of specific mRNAs by quantitative PCR (qPCR) using a Bio-Rad iCycler, iQ Supermix, or iQ Sybr (Bio-Rad, Hercules, CA) and primer and probe sets (IDT, Coralville, IA) designed as follows: (i) CD4, F (forward), AGGTCTCGCTTCAGTTTGCT, R (reverse), AGCCACTTTCATCACCACCA, and Pr (probe), TGGCAACCTGACTCTGACTCTGGACA; (ii) CD8, F, CATCCTGCTTCTGCTGGCATT, R, TGGGCGCTGATCATTTGTGAAA, and Pr, TGTGTGCGGAGGAGAGCCCGAATTCA; (iii) CD19, F, GAGCTCAGAGCCATGAAACA, R, CAAGGTTGGAGTCGTTCTCA, and Pr, CCAGACAGCGAGGAGGGCTCTGAAT; (iv) IFN-γ, F, AGCAACAACATAAGCGTCATT, R, CCTCAAAACTTGGCAATACTCA, and Pr, ACCTTCTTCAGCAACAGCAAGGGC; (v) SPBN-N, F, AGAAGGGAATTGGGCTCTG, R, TGTTTTGCCCGGATATTTTG, and Pr, CGTCCTTAGTCGGTCTTCTCT; (vi) L13, F CTACAGTGAGATACCACACCAAG, R, TGGACTTGTTTCGCCTCCTG, and Pr, ATCCACAAGAAAGTGGCTCGCACCAT; (vii) IFN-α, F, ATTTTGGATTCCCCTTGGAG, and R, TGATGGAGGTCATTGCAGAA; and (viii) IFN-β, F, CACAGCCCTCTCCATCAACT, and R, GCAACCACCACTCATTCTGA.
Statistical analysis.
All calculations were performed using GraphPad Prism 5.01 (GraphPad Software, San Diego, CA), with the exception of the Habel test for potency (34).
RESULTS
Recombinant rabies virus expresses murine IFN-γ.
Recombinant RABVs were constructed using the pathogenic SPBN backbone vector. Figure 1A shows a wild-type RABV genome, while panel C depicts the IFN-γ-expressing SPBN, termed SPBNγ. To control for any changes in replication efficiency that might occur due to increased size of the RABV genome, we used the previously constructed SPBN(−) (Fig. 1B). SPBN(−) has an inactivated tumor necrosis factor alpha (TNF-α) gene in lieu of murine IFN-γ.
FIG 1.

RABV constructs. (A) Negative-stranded RNA genome of the wild-type (WT) parental vector, SADB19, which was used as the backbone to construct two RABV strains used for all subsequent experiments. The control virus, SPBN(−), which contains a cytokine gene (red cross-hatching), inactivated by the substitution of STOP codons for the initial seven START codons, is shown in panel B. Panel C represents the interferon gamma-expressing RABV, SPBNγ. N, nucleoprotein; P, phosophoprotein; M, matrix protein; G, glycoprotein; L, large catalytic subunit of the viral polymerase; TGA, STOP codon; γ, murine IFN-γ gene.
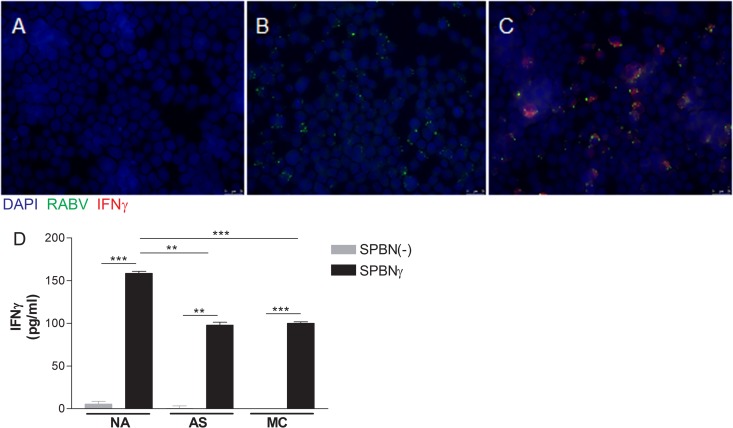
To ensure that SPBNγ can produce murine IFN-γ, we infected NA cells grown on culture well slides with SPBNγ or SPBN(−) at an MOI of 0.1. Twenty-four hours postinfection (hpi), the cells were fixed, permeabilized, and stained for evidence of RABV infection and murine IFN-γ production. Figure 2 shows the results of immunostaining for RABV and IFN-γ of uninfected (Fig. 2A), SPBN(−)-infected (Fig. 2B), and SPBNγ-infected (Fig. 2C) NA cells. While cells infected with SPBN(−) are positive only for RABV, the SPBNγ-infected cells stained positive for both RABV and IFN-γ, demonstrating that our recombinant SPBNγ produces IFN-γ. We next used ELISA to analyze the supernatants of NA, AS, and MC cells infected with SPBNγ or SPBN(−) for the presence of IFN-γ. Figure 2D shows that by 12 hpi, significant amounts of IFN-γ are produced and secreted into the supernatants of all cell types infected with SPBNγ but not SPBN(−).
FIG 2.
IFN-γ production. NA cells were infected at an MOI of 0.1 and stained 24 h later for the presence of RABV and IFN-γ (A to C). NA, AS, and MC cells were infected at an MOI of 1, and the supernatants were analyzed by IFN-γ-specific ELISA 12 hpi. (A) Uninfected control NA cells; (B) SPBN(−)-infected cells; (C) SPBNγ-infected cells; (D) IFN-γ concentration in supernatants. All photos are at a magnification of ×280. Significance was determined by Student's t test. **, P ≤ 0.01; ***, P ≤ 0.001.
Virus-encoded IFN-γ has differential effects on RABV replication in vitro.
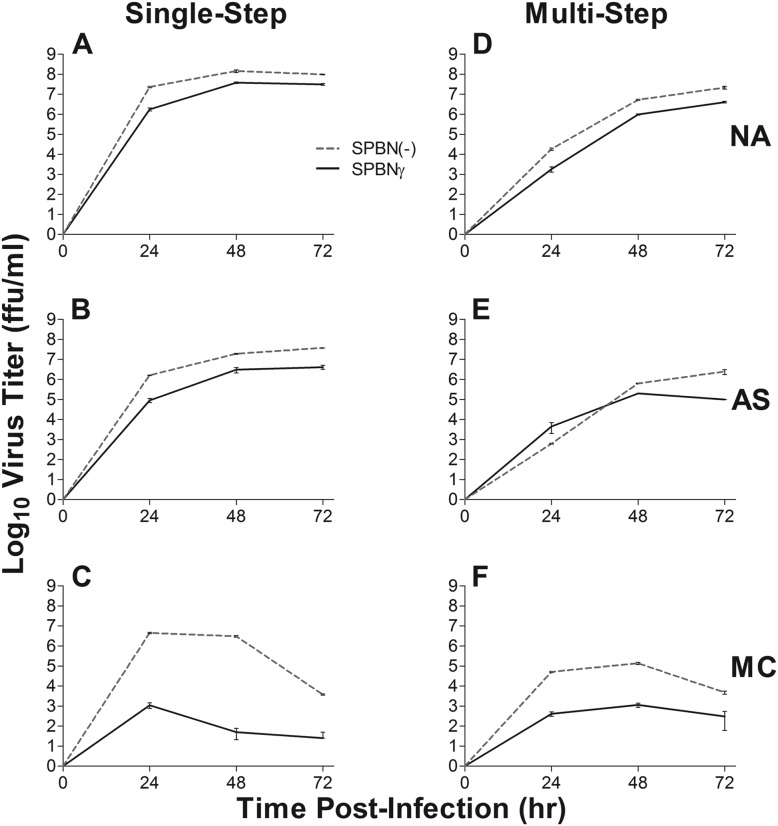
To determine the effects of IFN-γ expression on RABV replication in relevant cell types, we infected NA, AS, and MC cells with SPBNγ or SPBN(−) (Fig. 3). As shown in Fig. 3A and D, there is less than a 1-log10 difference in virus replication and spread between SPBNγ and SPBN(−) in NA cells by 48 hpi. Both viruses grow to relatively high titers (>107 FFU/ml). In AS cells, there is a similar result with less than a 1-log10 difference in growth by 48 hpi in the single-step growth curve (Fig. 3B). IFN-γ production may slow the spread of SPBNγ in AS cells, however, as there is a greater than 1-log10 difference between the virus titers at 72 hpi (Fig. 3E). In contrast to the minor differences seen in NA and AS cell lines, the replication rate and spread of SPBNγ in MC cells are several log10 values lower than those of SPBN(−) (Fig. 3C and F). The antiviral mechanisms in the MC cells appear to be very rapidly activated, as this multilog difference in replication is evident as early as 24 hpi. This significant decrease in SPBNγ replication compared to SPBN(−) indicates that the IFN-γ produced by SPBNγ and measured by ELISA (Fig. 2D) is indeed functional. Although SPBNγ replication is hampered in MC cells, substantial numbers of progeny virions (∼103 FFU/ml) were still produced, indicating that even in an immune cell line highly responsive to IFN-γ, SPBNγ is able replicate and spread.
FIG 3.
Virus growth curves. NA, AS, and MC cell lines were infected with SPBN(−) or SPBNγ RABV at an MOI of 1 for single-step growth curves (A to C) and an MOI of 0.01 for multistep growth curves (D to F). Supernatant virus titers were measured at the indicated times postinfection.
IFN-γ attenuates pathogenic RABV.
After demonstrating the ability of SPBNγ to replicate well in permissive cells and produce IFN-γ in vitro, we examined the pathogenicity of SPBN(−) and SPBNγ in vivo. Groups of 10 Swiss Webster mice were infected i.n. with 103 to 106 FFU of SPBNγ or SPBN(−). Mice were monitored for mortality and morbidity for 30 days. The resultant survivorships (Fig. 4A and C) clearly demonstrate the attenuation of the pathogenic SPBN vector by the addition of IFN-γ to its genome. The most striking difference in pathogenicity occurs at 105 FFU, where 90% of SPBNγ mice survive infection, while 100% of mice infected with SPBN(−) succumb within 15 days, but SPBNγ is significantly attenuated at all doses compared to SPBN(−). To quantify the attenuation of pathogenicity due to IFN-γ, we calculated the 50% lethal doses (LD50) of SPBNγ and SPBN(−) and found greater than a 2-log10 difference between the viruses (Fig. 4B). It should be noted that the exact difference could not be calculated because infection with all doses of SPBN(−) killed more than 50% of the mice. Thus, the expression of IFN-γ from the RABV genome decreased the pathogenicity of SPBN by more than 100-fold when administered i.n.
FIG 4.
In vivo mouse infection. Groups of 10 Swiss Webster mice were infected i.n. with SPBNγ or SPBN(−) RABV and monitored for mortality. Panel A shows absolute survival proportions for each dose of virus used to calculate the LD50 in panel B. Panel C is the Kaplan-Meier plot indicating both death and time to death. The LD50 was calculated by the Habel test for potency. Significant difference between survival curves was determined by the Mantel-Cox test. **, P ≤ 0.01; ***, P ≤ 0.001; #, LD50 for SPBN(−) could not be accurately calculated because all SPBN(−) doses killed more than 50% of mice.
SPBNγ replication is diminished while IFN-γ expression is elevated in the CNS of infected mice.
Having demonstrated that IFN-γ expression by RABV has differential effects on virus replication in various cell types in vitro, we next infected groups of C57BL/6 mice with SPBNγ or control SPBN(−) to quantify virus replication and IFN-γ gene expression in the CNS. At 10 dpi, viral nucleoprotein (N) RNA expression in both the cerebellum (CB) and cortex (CX) is approximately 10-fold lower in mice infected with SPBNγ than in those infected with SPBN(−) (Fig. 5A). Despite the reduced replication, the cerebellum and cortex of the SPBNγ-infected mice showed approximately 10-fold greater IFN-γ mRNA levels than in SPBN(−)-infected mice (Fig. 5B). Taken together, these data show that at 10 dpi, SPBNγ induces greater than 100-fold more IFN-γ expression per viral N message in the CNS of mice than SPBN(−) (Fig. 5C).
FIG 5.
IFN-γ expression and RABV replication in the brain. Groups of five C57BL/6 mice were infected i.n. with SPBNγ or SPBN(−) RABV. Ten days postinfection, brains were analyzed for the presence of specific mRNA. Panel A shows copies of viral nucleoprotein (N) mRNA, while panel B shows copies of IFN-γ mRNA. Panel C shows IFN-γ/N expression illustrated as the fold change in the brains of SPBNγ-infected compared to control SPBN(−)-infected mice. All copy numbers have been normalized to the L13 housekeeping gene. Significance was determined by the Mann-Whitney test. **, P ≤ 0.01.
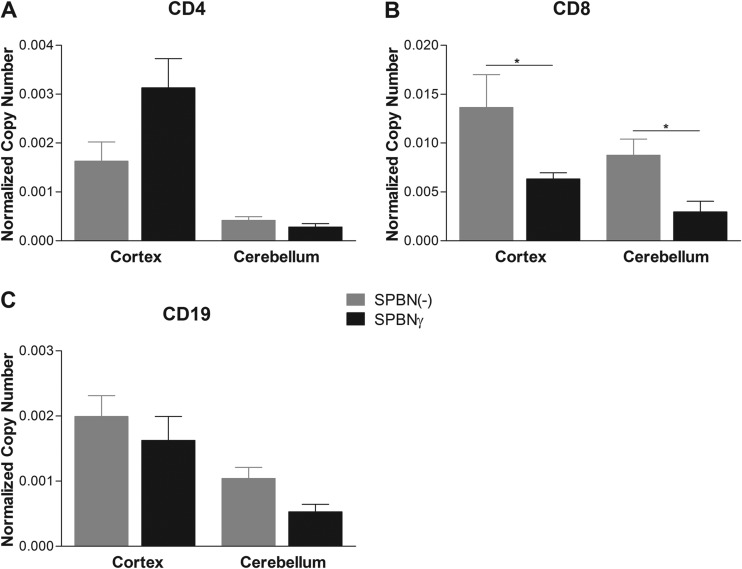
SPBNγ infection does not increase immune cell infiltration into the CNS.
In other animal models, immune cell infiltration into the CNS has been shown to be essential for RABV clearance (e.g., see references 8 and 35). To determine whether enhanced immune cell infiltration could play a role in the attenuation of SPBNγ, we measured the expression of mRNA specific for CD4+, CD8+, and CD19+ cells as an estimation of T and B cell influx into the CNS of mice infected with SPBNγ or SPBN(−) (Fig. 6). At 10 dpi, we found no significant increase in immune cell markers in the brains of SPBNγ-infected compared to SPBN(−)-infected mice. In fact, the SPBN(−)-infected mice showed increased expression of T cell marker CD8 in both the cerebellum (CB) and cortex (CX).
FIG 6.
Adaptive immune cell markers in the brain. Groups of five C57BL/6 mice were infected i.n. with SPBNγ or SPBN(−) RABV. Ten days postinfection, brains were analyzed for the presence of specific mRNA. Normalized mRNA copy numbers of CD4, CD8, and CD19 cells in the brains of RABV-infected mice are depicted in panels A, B, and C, respectively. All copy numbers are normalized to the L13 housekeeping gene. Significance was determined by the Mann-Whitney test. *, P ≤ 0.05.
SPBNγ attenuation occurs in the absence of endogenous IFN-γ or antibodies.
Previously it has been suggested that IFN-γ-producing T cells as well as virus-neutralizing antibodies (VNA) are important for the clearance of RABV from the brain (36, 37). To determine if endogenously produced IFN-γ or VNA is essential for SPBNγ attenuation, we infected groups of IFN-γ−/− mice, having no endogenous IFN-γ, and groups of JHD−/− mice, lacking functional B cells and antibodies, with SPBNγ or SPBN(−). At 16 dpi, 100% of SPBNγ-infected IFN-γ−/− mice were alive compared to only 11% of those infected with SPBN(−) (Fig. 7A). We continued to monitor the mice until 35 dpi, at which time 75% of IFN-γ−/− mice infected with SPBNγ remained alive, while only 11% of SPBN(−)-infected mice survived. As seen in Fig. 7B, SPBN(−)-infected IFN-γ−/− mice also begin to lose weight earlier than their SPBNγ-infected counterparts.
FIG 7.
Infection of IFN-γ−/− and JHD−/− mice. Groups of 8 to 9 IFN-γ−/− mice (A and B) and groups of 13 to 14 JHD−/− mice (C and D) were infected i.n. with 105 FFU of SPBN(−) or SPBNγ and monitored for mortality and morbidity for 35 and 32 days, respectively. The survival curves are shown in panels A and C. Weight loss as an indicator of infection is shown in panels B and D. Significant differences between survival curves were determined by the Mantel-Cox test. Significant differences in weight loss were determined by Student's t test. **, P ≤ 0.01; ***, P ≤ 0.001.
The importance of antibody to RABV clearance from the brain is well established. Therefore, we infected JHD−/− mice to determine if IFN-γ overexpression could prevent or delay death in the absence of antibody. Infection of JHD−/− mice yields a similar outcome to that seen with IFN-γ−/− mice. By 17 dpi, all SPBN(−)-infected JHD−/− mice succumbed to rabies virus infection, while 100% of those infected with SPBNγ remained alive (Fig. 7C). By the end of the experiment, at 32 dpi, 92% of JHD−/− mice infected with SPBNγ survived, despite having no functional, circulating antibodies. As can be expected, the mice infected with SPBN(−) lost weight faster than those infected with SPBNγ (Fig. 7D). Although most of the mice were sacrificed at day 32, we continued to monitor five SPBNγ-infected JHD−/− mice. Surprisingly, 5 of 5 of these mice survived for 75 days, 4 of 5 survived 87 days, and 3 of 5 remained alive for 88 days, at which time this experiment was concluded (data not shown). Thus, even this severe immune deficit does not prevent long-term control of SPBNγ infection by the host.
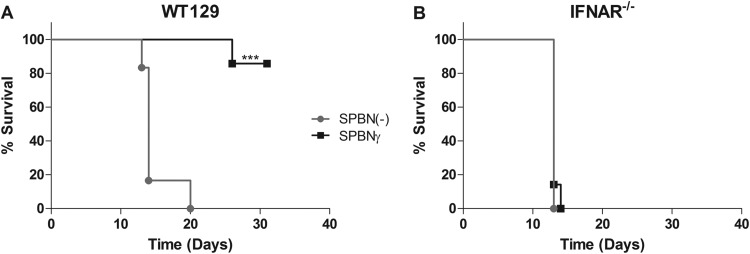
IFNAR−/− mice do not survive SPBNγ infection.
IFNAR−/− mice are unresponsive to type I IFN because the IFNAR1 chain of the heterodimeric receptor is knocked out (28). Based on knowledge that IFN-γ can potentiate type I IFN effects as well as act synergistically with type I IFN, we infected groups of IFNAR−/− mice, as well as their background control WT129 mice, with SPBNγ or SPBN(−) to determine the role of type I IFN in attenuation of SPBNγ (Fig. 8). One hundred percent of IFNAR−/− mice died from infection with SPBNγ by 14 dpi, and this was not significantly different from the control SPBN(−)-infected IFNAR−/− mice, which all died by 13 dpi (Fig. 8B). The WT129 background does not significantly alter the strong attenuation of SPBNγ seen in Swiss Webster mice, as 86% of SPBNγ-infected WT129 mice survived for 31 days, while 100% of these animals infected with SPBN(−) died (Fig. 8A).
FIG 8.
Infection of IFNAR−/− mice. Groups of 6 to 7 WT129 (A) or IFNAR−/− (B) mice were infected i.n. with 105 FFU of SPBNγ or SPBN(−) and monitored for mortality and morbidity. Significance was determined by the Mantel-Cox test. ***, P ≤ 0.001.
SPBNγ induces early type I IFN expression in vitro and in vivo.
There is substantial overlap in the regulation of type I and type II IFN (38). The early or increased expression of IFN-γ by SPBNγ may increase the expression of type I IFN, thereby enhancing the innate immune response to RABV and protecting mice from death during SPBNγ infection. To test this theory, we infected flasks of MC cells with SPBNγ or SPBN(−) and measured virus- and IFN-specific mRNA levels in these cells at various time points postinfection. Infection of MC cells with SPBNγ led to an early and sustained overexpression of IFN-γ (Fig. 9A) and induced a significant increase in IFN-α expression compared to SPBN(−) infection from 6 to 48 hpi (Fig. 9B). This effect was also seen when measuring IFN-β induction (Fig. 9C); however, IFN-β expression continued to rise in the SPBN(−)-infected MC cells, becoming significantly higher in these cultures by 24 hpi. The increase in type I IFN expression in SPBN(−)-infected MC cells during the later time points is most likely the result of more rapid virus replication, as evidenced by the viral N message levels (Fig. 9A to C). SPBNγ replication remained very low throughout the experiment but induced relatively high type I and type II IFN expression. The addition of IFN-γ to cultures of SPBN(−)-infected MC cells reduced viral replication (Fig. 9D) in concert with increasing IFN-α levels (Fig. 9E) at early time points. Elevated IFN-α levels, which were only seen in the presence of both IFN-γ and virus infection, were particularly evident at 24 h of culture (Fig. 9F).
FIG 9.
In vitro induction of type I interferon. MC cells were infected with SPBNγ or SPBN(−) at an MOI of 10. In panels A, B, and C, cultures were analyzed at the indicated time points for levels of mRNA specific for RABV N versus IFN-γ, IFN-α, and IFN-β. Bars represent normalized IFN mRNA levels plotted on the left y axis, and lines represent normalized viral N mRNA expression plotted on the right y axis. IFN-γ was added to the cell cultures in panels D, E, and F, and virus titers (D) as well as IFN-α levels (E and F) were assessed. Panel F shows IFN-α levels in the cultures at 24 hpi. Significant differences in cytokine levels between SPBN(−)- and SPBNγ-infected cultures (A to C) and control versus treated cultures (F) were determined by Student's t test.
To determine if SPBNγ also induces higher levels of type I IFN in the brain than SPBN(−), we infected groups of C57BL/6 mice with either virus. We sacrificed the mice at early time points during infection and analyzed the samples by qPCR for the presence of viral RNA as well as type I and type II IFN mRNA. It is widely held that IFN expression levels increase as virus replication increases (39). In fact, mice dying from RABV and other related viruses often have highest levels of circulating IFN just before death (40). Therefore, we compared IFN expression levels to the amount of virus replication in the olfactory bulb (OB) (Fig. 10A, D, and G), cerebellum (CB) (Fig. 10B, E, and H), and cerebral cortex (CX) (Fig. 10C, F, and I) of infected mice. After normalization of the IFN message levels to RABV N mRNA, it is clear that SPBNγ induces significantly higher levels of IFN-γ (Fig. 10A to C) and type I IFN (Fig. 10D to I) per viral message than does SPBN(−). Relative levels of IFN-γ, IFN-α, and IFN-β expression were significantly increased in SPBNγ-infected OB at 4, 6, and 8 dpi compared to SPBN(−)-infected OB (Fig. 10A, D, and G). In the CB and CX, expression of type I IFN was significantly increased at 6 and 8 dpi (Fig. 10E, F, H, and I). No SPBNγ virus was detected in these samples at day 4 postinfection, likely due to the time required for the spread of SPBNγ to those brain regions from the OB. The relative level of IFN-γ was significantly increased in SPBNγ-infected CB at 8 dpi (Fig. 10B) and both 6 and 8 dpi in the CX (Fig. 10C) compared to that in SPBN(−)-infected tissues.
FIG 10.
In vivo induction of type I interferon. Groups of five C57BL/6 mice were infected i.n. with 105 FFU of SPBNγ or SPBN(−), and their brain sections were analyzed for the gene products denoted above. Expression levels are normalized to the L13 housekeeping gene. Significant differences were determined by the Mann-Whitney test. *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001.
DISCUSSION
IFN-γ is a pleiotropic cytokine capable of modulating both the innate and adaptive immune responses and, as hypothesized, the addition of murine IFN-γ to the genome of SPBN highly attenuated the pathogenicity of this virus in mice. To determine which IFN-γ-inducible antiviral mechanism(s) led to the strong attenuation of the RABV, we infected cell lines relevant to RABV infection as well as wild-type mice and knockout mice with specific immune deficits. Together the data support the concept that IFN-γ production by SPBNγ attenuates the virus by enhancing the production of type I IFNs early in the infection. Thus, IFN-γ, an important product of adaptive immunity, inhibits RABV replication by activating well-established innate antiviral mechanisms.
We previously showed a significant correlation exists among IFN-γ expression, BBB permeability, and RABV clearance from the CNS (6, 26). The changes in BBB permeability are necessary to allow immune effectors to enter the CNS and eliminate the RABV infection. Various studies suggest that the mechanism involves IFN-γ-mediated induction of radicals (26, 41), which culminates in the alteration of tight-junction proteins (27). In the present study, however, we did not observe an increase in BBB permeability due to IFN-γ overexpression. While pathogenic when administered intranasally, the control SPBN(−) virus, unlike wild-type RABV (42), induces BBB permeability and allows immune cells to infiltrate the brain. In fact, we showed that SPBN(−) infection resulted in a significant increase in immune cell markers in some brain regions compared to SPBNγ infection, despite having much lower levels of IFN-γ expression. This seemingly paradoxical result is most likely the result of decreased SPBNγ replication necessitating the influx of fewer immune cells to clear the infection, compared to the SPBN(−) infection. Since SPBN(−) is pathogenic, despite triggering functional changes in the BBB and immune cell invasion into the infected CNS, we can conclude that viral IFN-γ expression has additional therapeutic effects on RABV infection.
Since it is known that production of VNA is important for protection against RABV infection and IFN-γ is capable of affecting the humoral adaptive response against viruses, we studied SPBNγ infection in JHD−/− mice, which have no functional B cells and produce no antibodies. Interestingly, the substantial attenuation of SPBNγ is not the result of earlier or greater production of antibodies, as evidenced by the survival of 92% of SPBNγ-infected B-cell-deficient mice 32 dpi. In actuality, addition of IFN-γ to a vaccine RABV strain could even suppress VNA induction due to lower viral replication. Our data support the concept that circulating antibody may not be the best indicator of vaccine efficacy as IFN-γ could be affecting memory in T cells or other cell types that are not evident when judging vaccine efficacy only by the induction of serum VNA (43). It should be noted that antibody is not required for long-term survival from RABV in the context of IFN-γ overexpression in our experiments. Nevertheless, these data do not refute the numerous reports that VNA are essential for the clearance of RABV in the absence of such therapeutic intervention.
Previous findings suggest that IFN-γ-producing T cells play an important role in the clearance of RABV (6, 26, 36, 44), as well as other neurotropic viruses (45, 46), from the CNS of mice. Additionally, little is known about the role of NK cell-derived IFN-γ during RABV infection. To determine if the cellular source of IFN-γ is essential for a therapeutic effect, we infected IFN-γ−/− mice with SPBN(−) or SPBNγ. We found that the majority of SPBNγ-infected mice survived, while SPBN(−)-infected IFN-γ−/− mice did not. Since we used i.n. infection and RABVs primarily infect neurons, it is not likely that infected T or NK cells were a major source of RABV-encoded IFN-γ in the CNS. Therefore, during SPBNγ infection of mice, the production of IFN-γ by classical immune cells does not appear to be essential for survival. Furthermore, the survival of SPBNγ-infected IFN-γ−/− mice demonstrates that only virus-encoded IFN-γ is necessary for protection, and endogenous IFN-γ production by NK or T cells is not required. Together, these IFN-γ−/− and JHD−/− data show that attenuation of RABV by IFN-γ is profound in adult mice and is not dependent upon the early production of VNA or an immune source of IFN-γ.
Analysis of our in vitro monocyte infection experiments leads us to consider that the stimulation of type I IFN expression by IFN-γ contributes to survival of SPBNγ infection. Experiments performed over the last several decades have questioned the importance of type I IFN during RABV infection. Although some early experiments found type I IFN expression to be inconsequential to the fate of the RABV-infected animal (39) or equivocal (47), other reports suggest an important role for type I IFN (7, 48). Measuring IFN levels late in the course of infection as well as focusing on survival as an estimation of IFN efficacy may have contributed to the confusion as to the importance of type I IFN. One of the early experiments showed that there are two waves of type I IFN expression during wild-type RABV infection (40). Very early expression of type I IFN is important for survival, while the second wave of expression is not therapeutic and is likely the result of uncontrolled RABV replication (40). More recent work has demonstrated that type I IFN can impede replication and spread of a highly neuroinvasive RABV both in vitro and in vivo and can delay or reduce mortality but cannot prevent death altogether (48). Furthermore, an engineered RABV-based HIV vaccine vector expressing IFN-β is attenuated by its IFN-β production (49). Still other experiments have shown that attenuated RABV induces high levels of type I IFN, while wild-type RABV do not (7). Since type I IFN signaling is important for protection against infection, it is not surprising that the RABV itself dedicates substantial resources to blocking type I IFN signaling. Both the N and P genes of RABV express products that interfere with type I IFN signaling (50, 51), and mutation of P can attenuate the virus (52). The blockade of type I IFN by RABV is a “leaky” process, however, and the host response is able to impede replication and spread to some degree despite this immunoevasion strategy (48).
The attenuation of SPBNγ is profound in a number of wild-type mouse strains as well as those with severe immune deficits. When we infected IFNAR−/− mice with SPBNγ, however, the overexpression of IFN-γ could not compensate for this immune deficiency, and the mice quickly died. This result again underscores the importance of type I IFN and suggests the mechanism by which SPBNγ is attenuated. Together, our in vitro and in vivo data showing highly increased type I IFN expression from cell lines and brain tissue infected with SPBNγ over SPBN(−), as well as the lack of attenuation of SPBNγ in IFNAR−/− mice, suggest that expression of IFN-γ induces the expression of type I IFN and that enhancement of type I IFN signaling plays a vital role in the attenuation of SPBNγ.
Although IFN-γ induces hundreds of genes involved in other antiviral mechanisms, we have shown that a major mechanism of SPBNγ attenuation is through the induction of type I IFN. This rapid type I IFN expression only occurs when an IFN-γ-responsive cell is exposed to the virus. This mechanism may circumvent the IFN evasion tactics of the RABV, leading to control of virus replication and spread and, ultimately, virus clearance.
Altogether, our results indicate that the expression of IFN-γ by RABV renders it attenuated even in mice with severe immune deficits. The fact that the replication and spread of RABV in the CNS are controlled by immune mechanisms long before neutralizing antibody is produced has long been enigmatic in rabies, and understanding the process may hold the key to therapy. Our work shows that IFN-γ produced early in the immune response to RABV likely uses innate mechanisms to control the infection, such that the later appearance of antibody can clear the infection.
ACKNOWLEDGMENTS
We thank Bernhard Dietzschold for critical review of the manuscript.
This work was supported by National Institutes of Health grants R01AI093369 and U01AI083045 to D.C.H. and R01AI093666 to M.F.
REFERENCES
- 1.Holloway BP, Obijeski JF. 1980. Rabies virus-induced RNA synthesis in BHK21 cells. J Gen Virol 49:181–195. doi: 10.1099/0022-1317-49-1-181. [DOI] [PubMed] [Google Scholar]
- 2.Tordo N, Poch O, Ermine A, Keith G, Rougeon F. 1988. Completion of the rabies virus genome sequence determination: highly conserved domains among the L (polymerase) proteins of unsegmented negative-strand RNA viruses. Virology 165:565–576. doi: 10.1016/0042-6822(88)90600-9. [DOI] [PubMed] [Google Scholar]
- 3.Knobel DL, Cleaveland S, Coleman PG, Fèvre EM, Meltzer MI, Miranda MEG, Shaw A, Zinsstag J, Meslin F-X. 2005. Re-evaluating the burden of rabies in Africa and Asia. Bull World Health Organ 83:360–368. [PMC free article] [PubMed] [Google Scholar]
- 4.Hattwick MAW. 1972. Recovery from rabies: a case report. Ann Intern Med 76:931. doi: 10.7326/0003-4819-76-6-931. [DOI] [PubMed] [Google Scholar]
- 5.Willoughby RE Jr, Tieves KS, Hoffman GM, Ghanayem NS, Amlie-Lefond CM, Schwabe MJ, Chusid MJ, Rupprecht CE. 2005. Survival after treatment of rabies with induction of coma. N Engl J Med 352:2508–2514. doi: 10.1056/NEJMoa050382. [DOI] [PubMed] [Google Scholar]
- 6.Phares TW, Kean RB, Mikheeva T, Hooper DC. 2006. Regional differences in blood-brain barrier permeability changes and inflammation in the apathogenic clearance of virus from the central nervous system. J Immunol 176:7666–7675. doi: 10.4049/jimmunol.176.12.7666. [DOI] [PubMed] [Google Scholar]
- 7.Wang ZW, Sarmento L, Wang Y, Li X-Q, Dhingra V, Tseggai T, Jiang B, Fu ZF. 2005. Attenuated rabies virus activates, while pathogenic rabies virus evades, the host innate immune responses in the central nervous system. J Virol 79:12554–12565. doi: 10.1128/JVI.79.19.12554-12565.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roy A, Hooper DC. 2007. Lethal silver-haired bat rabies virus infection can be prevented by opening the blood-brain barrier. J Virol 81:7993–7998. doi: 10.1128/JVI.00710-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jackson AC. 2011. Therapy of human rabies. Adv Virus Res 79:365–375. doi: 10.1016/B978-0-12-387040-7.00017-2. [DOI] [PubMed] [Google Scholar]
- 10.Roy A, Phares TW, Koprowski H, Hooper DC. 2007. Failure to open the blood-brain barrier and deliver immune effectors to central nervous system tissues leads to the lethal outcome of silver-haired bat rabies virus infection. J Virol 81:1110–1118. doi: 10.1128/JVI.01964-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baer GM, Shaddock JH, Williams LW. 1975. Prolonging morbidity in rabid dogs by intrathecal injection of attenuated rabies vaccine. Infect Immun 12:98–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Faber M, Li J, Kean RB, Hooper DC, Alugupalli KR, Dietzschold B. 2009. Effective preexposure and postexposure prophylaxis of rabies with a highly attenuated recombinant rabies virus. Proc Natl Acad Sci U S A 106:11300–11305. doi: 10.1073/pnas.0905640106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Warrell MJ, White NJ, Looareesuwan S, Phillips RE, Suntharasamai P, Chanthavanich P, Riganti M, Fisher-Hoch SP, Nicholson KG, Manatsathit S. 1989. Failure of interferon alfa and tribavirin in rabies encephalitis. BMJ 299:830–833. doi: 10.1136/bmj.299.6703.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wheelock EF. 1965. Interferon-like virus-inhibitor induced in human leukocytes by phytohemagglutinin. Science 149:310–311. doi: 10.1126/science.149.3681.310. [DOI] [PubMed] [Google Scholar]
- 15.Schroder K, Hertzog PJ, Ravasi T, Hume DA. 2004. Interferon-gamma: an overview of signals, mechanisms and functions. J Leukoc Biol 75:163–189. doi: 10.1189/jlb.0603252. [DOI] [PubMed] [Google Scholar]
- 16.Der SD, Zhou A, Williams BR, Silverman RH. 1998. Identification of genes differentially regulated by interferon alpha, beta, or gamma using oligonucleotide arrays. Proc Natl Acad Sci U S A 95:15623–15628. doi: 10.1073/pnas.95.26.15623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsumoto M, Tanaka N, Harada H, Kimura T, Yokochi T, Kitagawa M, Schindler C, Taniguchi T. 1999. Activation of the transcription factor ISGF3 by interferon-gamma. Biol Chem 380:699–703. [DOI] [PubMed] [Google Scholar]
- 18.Improta T, Pine R, Pfeffer LM. 1992. Interferon-gamma potentiates the antiviral activity and the expression of interferon-stimulated genes induced by interferon-alpha in U937 cells. J Interferon Res 12:87–94. doi: 10.1089/jir.1992.12.87. [DOI] [PubMed] [Google Scholar]
- 19.Levy DE, Lew DJ, Decker T, Kessler DS, Darnell JE Jr. 1990. Synergistic interaction between interferon-alpha and interferon-gamma through induced synthesis of one subunit of the transcription factor ISGF3. EMBO J 9:1105–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Giavedoni L, Ahmad S, Jones L, Yilma T. 1997. Expression of gamma interferon by simian immunodeficiency virus increases attenuation and reduces postchallenge virus load in vaccinated rhesus macaques. J Virol 71:866–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haq K, Elawadli I, Parvizi P, Mallick AI, Behboudi S, Sharif S. 2011. Interferon-γ influences immunity elicited by vaccines against very virulent Marek's disease virus. Antiviral Res 90:218–226. doi: 10.1016/j.antiviral.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 22.Mata-Espinosa DA, Mendoza-Rodríguez V, Aguilar-León D, Rosales R, López-Casillas F, Hernández-Pando R. 2008. Therapeutic effect of recombinant adenovirus encoding interferon-gamma in a murine model of progressive pulmonary tuberculosis. Mol Ther 16:1065–1072. doi: 10.1038/mt.2008.69. [DOI] [PubMed] [Google Scholar]
- 23.Marciano BE, Wesley R, De Carlo ES, Anderson VL, Barnhart LA, Darnell D, Malech HL, Gallin JI, Holland SM. 2004. Long-term interferon-gamma therapy for patients with chronic granulomatous disease. Clin Infect Dis 39:692–699. doi: 10.1086/422993. [DOI] [PubMed] [Google Scholar]
- 24.Prescrire International. 2006. Interferon gamma-1b: new indication. Severe malignant osteopetrosis: too many unknowns. Prescrire Int 15:179–180. [PubMed] [Google Scholar]
- 25.Yasukawa K, Saito S, Kubo T, Shibasaki Y, Yamaoka K, Hachimura H, Kuyama T, Amimoto A, Kumata T, Kitahara Y, Takenaka M, Matsumura H, Uno T, Uchino T, Takehara K, Nishida K, Kadoya M, Sato M, Kato K, Matsumoto K, Saito S, Shimoda T. 2010. Low-dose recombinant canine interferon-γ for treatment of canine atopic dermatitis: an open randomized comparative trial of two doses. Vet Dermatol 21:42–49. doi: 10.1111/j.1365-3164.2009.00764.x. [DOI] [PubMed] [Google Scholar]
- 26.Phares TW, Fabis MJ, Brimer CM, Kean RB, Hooper DC. 2007. A peroxynitrite-dependent pathway is responsible for blood-brain barrier permeability changes during a central nervous system inflammatory response: TNF-alpha is neither necessary nor sufficient. J Immunol 178:7334–7343. doi: 10.4049/jimmunol.178.11.7334. [DOI] [PubMed] [Google Scholar]
- 27.Chai Q, He WQ, Zhou M, Lu H, Fu ZF. 2014. Enhancement of blood-brain barrier permeability and reduction of tight junction protein expression are modulated by chemokines/cytokines induced by rabies virus infection. J Virol 88:4698–4710. doi: 10.1128/JVI.03149-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Müller U, Steinhoff U, Reis LF, Hemmi S, Pavlovic J, Zinkernagel RM, Aguet M. 1994. Functional role of type I and type II interferons in antiviral defense. Science 264:1918–1921. [DOI] [PubMed] [Google Scholar]
- 29.Macpherson I, Stoker M. 1962. Polyoma transformation of hamster cell clones—an investigation of genetic factors affecting cell competence. Virology 16:147–151. [DOI] [PubMed] [Google Scholar]
- 30.Conzelmann KK, Cox JH, Schneider LG, Thiel HJ. 1990. Molecular cloning and complete nucleotide sequence of the attenuated rabies virus SAD B19. Virology 175:485–499. [DOI] [PubMed] [Google Scholar]
- 31.Schnell MJ, Mebatsion T, Conzelmann KK. 1994. Infectious rabies viruses from cloned cDNA. EMBO J 13:4195–4203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pulmanausahakul R, Faber M, Morimoto K, Spitsin S, Weihe E, Hooper DC, Schnell MJ, Dietzschold B. 2001. Overexpression of cytochrome c by a recombinant rabies virus attenuates pathogenicity and enhances antiviral immunity. J Virol 75:10800–10807. doi: 10.1128/JVI.75.22.10800-10807.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Faber M, Bette M, Preuss MAR, Pulmanausahakul R, Rehnelt J, Schnell MJ, Dietzschold B, Weihe E. 2005. Overexpression of tumor necrosis factor alpha by a recombinant rabies virus attenuates replication in neurons and prevents lethal infection in mice. J Virol 79:15405–15416. doi: 10.1128/JVI.79.24.15405-15416.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.World Health Organization. 1996. Laboratory techniques in rabies, 4th ed. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 35.Kuang Y, Lackay SN, Zhao L, Fu ZF. 2009. Role of chemokines in the enhancement of BBB permeability and inflammatory infiltration after rabies virus infection. Virus Res 144:18–26. doi: 10.1016/j.virusres.2009.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hooper DC, Morimoto K, Bette M, Weihe E, Koprowski H, Dietzschold B. 1998. Collaboration of antibody and inflammation in clearance of rabies virus from the central nervous system. J Virol 72:3711–3719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dietzschold B, Kao M, Zheng YM, Chen ZY, Maul G, Fu ZF, Rupprecht CE, Koprowski H. 1992. Delineation of putative mechanisms involved in antibody-mediated clearance of rabies virus from the central nervous system. Proc Natl Acad Sci U S A 89:7252–7256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Platanias LC. 2005. Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat Rev Immunol 5:375–386. doi: 10.1038/nri1604. [DOI] [PubMed] [Google Scholar]
- 39.Lodmell DL, Wiedbrauk DL, Ewalt LC. 1989. Interferon induced within the central nervous system during infection is inconsequential as a mechanism responsible for murine resistance to street rabies virus. J Gen Virol 70:473–478. [DOI] [PubMed] [Google Scholar]
- 40.Marcovistz R, Galabru J, Tsiang H, Hovanessian AG. 1986. Neutralization of interferon produced early during rabies virus infection in mice. J Gen Virol 67:387–390. [DOI] [PubMed] [Google Scholar]
- 41.Fabis MJ, Phares TW, Kean RB, Koprowski H, Hooper DC. 2008. Blood-brain barrier changes and cell invasion differ between therapeutic immune clearance of neurotrophic virus and CNS autoimmunity. Proc Natl Acad Sci U S A 105:15511–15516. doi: 10.1073/pnas.0807656105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roy A, Hooper DC. 2008. Immune evasion by rabies viruses through the maintenance of blood-brain barrier integrity. J Neurovirol 14:401–411. doi: 10.1080/13550280802235924. [DOI] [PubMed] [Google Scholar]
- 43.Talaat KR, Luke CJ, Khurana S, Manischewitz J, King LR, McMahon BA, Karron RA, Lewis KDC, Qin J, Follmann DA, Golding H, Neuzil KM, Subbarao K. 2014. A live attenuated influenza A(H5N1) vaccine induces long-term immunity in the absence of a primary antibody response. J Infect Dis 209:1860–1869. doi: 10.1093/infdis/jiu123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Irwin DJ, Wunner WH, Ertl HC, Jackson AC. 1999. Basis of rabies virus neurovirulence in mice: expression of major histocompatibility complex class I and class II mRNAs. J Neurovirol 5:485–494. [DOI] [PubMed] [Google Scholar]
- 45.Griffin DE. 2010. Recovery from viral encephalomyelitis: immune-mediated noncytolytic virus clearance from neurons. Immunol Res 47:123–133. doi: 10.1007/s12026-009-8143-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Patterson CE, Lawrence DMP, Echols LA, Rall GF. 2002. Immune-mediated protection from measles virus-induced central nervous system disease is noncytolytic and gamma interferon dependent. J Virol 76:4497–4506. doi: 10.1128/JVI.76.9.4497-4506.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chopy D, Pothlichet J, Lafage M, Megret F, Fiette L, Si-Tahar M, Lafon M. 2011. Ambivalent role of the innate immune response in rabies virus pathogenesis. J Virol 85:6657–6668. doi: 10.1128/JVI.00302-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chopy D, Detje CN, Lafage M, Kalinke U, Lafon M. 2011. The type I interferon response bridles rabies virus infection and reduces pathogenicity. J Neurovirol 17:353–367. doi: 10.1007/s13365-011-0041-6. [DOI] [PubMed] [Google Scholar]
- 49.Faul EJ, Wanjalla CN, McGettigan JP, Schnell MJ. 2008. Interferon-β expressed by a rabies virus-based HIV-1 vaccine vector serves as a molecular adjuvant and decreases pathogenicity. Virology 382:226–238. doi: 10.1016/j.virol.2008.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brzózka K, Finke S, Conzelmann K-K. 2005. Identification of the rabies virus alpha/beta interferon antagonist: phosphoprotein P interferes with phosphorylation of interferon regulatory factor 3. J Virol 79:7673–7681. doi: 10.1128/JVI.79.12.7673-7681.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Masatani T, Ito N, Ito Y, Nakagawa K, Abe M, Yamaoka S, Okadera K, Sugiyama M. 2013. Importance of rabies virus nucleoprotein in viral evasion of interferon response in the brain. Microbiol Immunol 57:511–517. doi: 10.1111/1348-0421.12058. [DOI] [PubMed] [Google Scholar]
- 52.Rieder M, Brzozka K, Pfaller CK, Cox JH, Stitz L, Conzelmann K-K. 2011. Genetic dissection of interferon-antagonistic functions of rabies virus phosphoprotein: inhibition of interferon regulatory factor 3 activation is important for pathogenicity. J Virol 85:842–852. doi: 10.1128/JVI.01427-10. [DOI] [PMC free article] [PubMed] [Google Scholar]