ABSTRACT
No herpes simplex virus 2 (HSV-2) vaccine has been licensed for use in humans. HSV-2 glycoproteins B (gB) and D (gD) are targets of neutralizing antibodies and T cells, but clinical trials involving intramuscular (i.m.) injection of HSV-2 gB and gD in adjuvants have not been effective. Here we evaluated intravaginal (ivag) genetic immunization of C57BL/6 mice with a replication-defective human papillomavirus pseudovirus (HPV PsV) expressing HSV-2 gB (HPV-gB) or gD (HPV-gD) constructs to target different subcellular compartments. HPV PsV expressing a secreted ectodomain of gB (gBsec) or gD (gDsec), but not PsV expressing a cytoplasmic or membrane-bound form, induced circulating and intravaginal-tissue-resident memory CD8+ T cells that were able to secrete gamma interferon (IFN-γ) and tumor necrosis factor alpha (TNF-α) as well as moderate levels of serum HSV neutralizing antibodies. Combined immunization with HPV-gBsec and HPV-gDsec (HPV-gBsec/gDsec) vaccines conferred longer survival after vaginal challenge with HSV-2 than immunization with HPV-gBsec or HPV-gDsec alone. HPV-gBsec/gDsec ivag vaccination was associated with a reduced severity of genital lesions and lower levels of viral shedding in the genital tract after HSV-2 challenge. In contrast, intramuscular vaccination with a soluble truncated gD protein (gD2t) in alum and monophosphoryl lipid A (MPL) elicited high neutralizing antibody titers and improved survival but did not reduce genital lesions and viral shedding. Vaccination combining ivag HPV-gBsec/gDsec and i.m. gD2t-alum-MPL improved survival and reduced genital lesions and viral shedding. Finally, high levels of circulating HSV-2-specific CD8+ T cells, but not serum antibodies, correlated with reduced viral shedding. Taken together, our data underscore the potential of HPV PsV as a platform for a topical mucosal vaccine to control local manifestations of primary HSV-2 infection.
IMPORTANCE Genital herpes is a highly prevalent chronic disease caused by HSV infection. To date, there is no licensed vaccine against HSV infection. This study describes intravaginal vaccination with a nonreplicating HPV-based vector expressing HSV glycoprotein antigens. The data presented in this study underscore the potential of HPV-based vectors as a platform for the induction of genital-tissue-resident memory T cell responses and the control of local manifestations of primary HSV infection.
INTRODUCTION
Genital herpes is a common sexually transmitted disease caused by herpes simplex virus 2 (HSV-2). Worldwide, more than 500 million individuals are chronically infected by HSV-2, and the prevalence of HSV-2 infection is twice as high in women as in men (1). In the United States, the seroprevalence of HSV-2 in 14- to 49-year-olds during the 2005–2010 period was 15.7% (2). During primary infection, HSV-2 infects and replicates in epithelial cells of the genital mucosa and spreads to the regional ganglia, where it establishes a lifelong latent infection. HSV-2 can undergo reactivation and shedding from the genital mucosa, where it can cause recurrent genital lesions, which are associated with an increased risk of HIV-1 acquisition (3, 4). Shedding of HSV-2 may also be subclinical, and HSV-2 transmission can occur in the absence of lesions (5, 6). Immunosuppression is associated with an increased risk of severe disseminated disease. In addition, transmission of HSV-2 from the genital mucosae of acutely infected pregnant women to neonates can cause severe infection.
Several therapeutic and preventive interventions based on antiviral drugs, the use of condoms, abstinence, or circumcision can reduce the burden of HSV-2 infection at the individual level. However, these interventions have not controlled the HSV-2 epidemic (7). Therefore, a vaccine that could prevent primary acquisition of HSV-2 or reduce HSV-2 shedding and/or recurrent lesions in chronically infected individuals might have a substantial impact at both the individual and public health levels.
A variety of HSV-2 vaccine approaches have shown protective efficacy in animal models, including live attenuated, nonreplicating viral vector, subunit, or DNA vaccines (8–20). Recombinant soluble HSV-2 glycoprotein D (gD) combined with an aluminum salt and monophosphoryl lipid A adjuvant (alum-MPL) has been the most promising recent vaccine to undergo extensive clinical evaluation. Although it induced HSV-2 neutralizing antibodies in the sera of vaccinated subjects, this vaccine failed to confer significant protection in a phase III clinical trial (21, 22). It is therefore speculated that a successful HSV-2 vaccine should also induce a robust T cell response (23).
Infection of mice with HSV-2 has provided evidence that CD4+ or CD8+ T cells and gamma interferon (IFN-γ) can contribute to reducing the severity of primary infection, clearing virus from the nervous system, and protecting against reactivation ex vivo (24–28). More recently, it has been shown that, in contrast to circulating memory T cells, a subset of tissue-resident memory (Trm) T cells can confer immediate and enhanced protection against HSV-1 and HSV-2 infections (29–31). In humans, a subset of CD8αα T cells is induced in the genital epithelium at sites of clinical HSV-2 reactivation, and these cells persist after the lesions have healed (32, 33). The presence of these local T cells is associated with reductions in lesion severity and viral shedding (34). In mouse models, genital Trm T cells can be induced by genital immunization with live attenuated HSV-2 or by systemic immunization followed by topical application to the genital tract of immunomodulatory molecules, which can direct recently activated circulating T cells to the genital tract (29–31, 35, 36).
We previously reported an effective method for transiently transducing the cervicovaginal mucosa with a nonreplicating human papillomavirus (HPV) vector (37). Intravaginal (ivag) immunization with these HPV pseudovirus vectors (HPV PsV) expressing a model antigen induces higher numbers of antigen-specific Trm CD8+ T cells in the cervicovaginal mucosa in mice and nonhuman primates and confers greater control of local viral replication after ivag vaccinia virus challenge than intramuscular (i.m.) immunization at a remote site (38–40).
Here we generated and characterized HPV16 and HPV45 PsV expressing membrane-associated, secreted, and cytosolic forms of HSV-2 glycoprotein B (gB) and gD. We then evaluated the ivag immunogenicities of the HPV PsV constructs, including their abilities to induce cervicovaginal intraepithelial Trm CD8+ T cells and antibodies, and compared the protection conferred by this local vaccination, in a genital tract HSV-2 challenge model, with that conferred by i.m. immunization with a truncated gD protein formulated with alum and MPL.
MATERIALS AND METHODS
Design, construction, and characterization of plasmids expressing HSV-2 gB, HSV-2 gD, or reporter genes.
Plasmids encoding tomato fluorescent protein and firefly luciferase as reporter genes or as controls were generated previously and are described on the Laboratory of Cellular Oncology website (http://home.ccr.cancer.gov/Lco/plasmids.asp).
Plasmids pgB2-333 and pgD2-333 consisted of vector pcDNA3 and the gB or gD DNA fragments that were PCR amplified from HSV-2 strain 333. The entire open reading frames (ORF) were confirmed by sequencing. These HSV-2 DNA fragments were further subcloned into an HPV PsV expression plasmid. Briefly, the full-length open reading frames of gB and gD (gBfl and gDfl) were excised from the pcDNA3 constructs using restriction enzyme HindIII, the end was blunted, and the DNA was further digested using NotI. To obtain the pCI vector, pCMMf (40) was digested using XhoI, the ends were blunted, and the DNA was further digested using NotI. Purified fragments encoding gB and gD were cloned into plasmid pCI to generate plasmids pCgBfl and pCgDfl. To produce the secreted ectodomains of gB and gD (gBsec and gDsec), we generated constructs with deletions of the glycoprotein transmembrane and cytosolic domains from plasmids pCgBfl and pCgDfl by PCR using the primer pair 5′-CATACGATTCTAGAACCATGCGCGGGGGGGGCTTG-3′ and 5′-ATACTTGCGGCCGCTTAGGCGTTGGCGTCGGCGCGG-3′ for gBsec and the primer pair 5′-ACGCTATCTAGAACCATGGGGCGTTTGACCTCC-3′ and 5′-ATACTTGCGGCCGCCTAGTGGTGCGGCGCGACGTC-3′ for gDsec. To produce the cytosolic forms of gB and gD (gBcyt and gDcyt), the transmembrane and cytosolic domains were deleted from the glycoprotein constructs, and the signal peptide sequence was replaced by a Kozak sequence and an ATG codon in frame with the rest of the ORF. We generated the cytosolic forms of the glycoproteins by PCR from pCgBfl and pCgDfl constructs using primers 5′-CATACGATTCTAGAACCATGGCGGCCCCGGCGGCCC-3′ and 5′-ATACTTGCGGCCGCTTAGGCGTTGGCGTCGGCGCGG-3′ for gBcyt and primers 5′-ACGCTATCTAGAACCATGAAATACGCCTTAGCAGAC-3′ and 5′-ATACTTGCGGCCGCCTAGTGGTGCGGCGCGACGTC-3′ for gDcyt. All PCR products were cloned into a pCLucf vector backbone after digestion with restriction enzymes XbaI and NotI.
The expression of gB and gD by each plasmid was assessed by Western blotting. Briefly, 293TT cells were transfected with each pCgB and pCgD construct, or with the pCLucf plasmid as a control, using Lipofectamine 2000 (Invitrogen). Cell lysates were prepared using radioimmunoprecipitation assay (RIPA) buffer 48 h after transfection, and supernatants were obtained for the last 14 h of incubation in serum-free medium (Opti-MEM; Invitrogen) to minimize background. The expression of gB was confirmed by Western blotting under denaturing conditions with rabbit polyclonal antibody R69 (41), a gift from Gary Cohen and Roselyn Eisenberg, and gD expression was confirmed with mouse monoclonal antibody 2C10 (Abcam). The apparent molecular weights were confirmed with a molecular weight marker (MagicMark XP).
HPV vectors and HSV-2 production.
HPV vectors were produced and purified as described previously (42). Briefly, 293TT cells were cotransfected with a plasmid encoding both the L1 and L2 capsid proteins of HPV16 or HPV45 and either a plasmid encoding a reporter gene (tomato fluorescent protein or firefly luciferase) or a plasmid encoding one of the glycoprotein constructs: gBfl, gBsec, gBcyt, gDfl, gDsec, or gDcyt. After overnight incubation of cell lysates, HPV particles were purified on an OptiPrep gradient. The infectious titer of each pseudovirus preparation was determined on 293TT cells by flow cytometry and was expressed as infectious units (IU) per ml.
HSV-2 (strain R519, plaque purified from strain 333) was propagated in Vero cells as described previously (43), and the infectious titer was determined by a plaque assay on Vero cells and was expressed as PFU per ml.
Animals.
Six- to 8-week-old female C57BL/6 mice were purchased from the National Cancer Institute (NCI) and were maintained under specific-pathogen–free conditions in the animal care facilities at the NCI and the National Institute of Allergy and Infectious Diseases (NIAID). All procedures were approved by the NCI and NIAID Animal Care and Use Committees.
Immunizations and HSV-2 challenge.
For primary or single vaccination, HPV16 PsV were used; for booster vaccination, HPV45 PsV were used. HPV PsV ivag immunization was performed on anesthetized mice as described previously (40). Five days prior to immunization, C57BL/6 female mice were treated subcutaneously (s.c.) with medroxyprogesterone acetate (Depo-Provera; 3 mg; Pfizer). On the day of immunization, mice received 4% nonoxynol-9 (N9; Spectrum Chemical and Laboratory) ivag, and 5 h later, they were infected ivag with 1 × 108 or 2 × 108 IU (as indicated for each experiment) of the HPV vectors in carboxymethyl cellulose (CMC; Sigma-Aldrich).
For intranasal (i.n.) immunization, anesthetized mice received 1 μg cholera toxin (CT; List Biological Laboratories) with 20 μg of gB496–503 (NeoSystems) peptide in 10 μl phosphate-buffered saline (PBS) (5 μl per nostril).
For i.m. immunization, 3 μg of a soluble truncated recombinant gD protein (gD2t; Chiron) was adsorbed onto 50 μg Imject Alum (Thermo Scientific), mixed with 7.5 μg synthetic monophosphoryl lipid A (MPL; Invivogen), diluted in 50 μl with PBS, and injected in the quadriceps muscles of anesthetized mice.
For HSV-2 vaginal challenge, medroxyprogesterone acetate-treated mice were instilled ivag with 104 PFU of HSV-2 strain 333 in 10 μl PBS.
Immunofluorescence and confocal microscopy.
For analysis of the expression of tomato fluorescent protein in the cervicovaginal mucosa, tissues were collected 72 h after immunization and were fixed for 1 h in 4% paraformaldehyde (EMS), followed by an incubation of 24 h in 15% sucrose solution and then 24 h in 30% sucrose. Tissues were snap-frozen in tissue freezing medium (Tissue-Tek OCT; Sakura). Six-micrometer tissue sections were cut, transferred to glass slides (Superfrost Plus; VWR), and mounted with an antifade reagent (Prolong Gold with 4′,6-diamidino-2-phenylindole [DAPI]; Molecular Probes).
For analysis of CD8 and CD4 cell infiltrates, cervicovaginal tissues were collected 3 weeks after the final immunization, snap-frozen directly in Tissue-Tek compound, and processed as described previously (38). Briefly, 6-μm ethanol-fixed tissue sections were incubated with an antibody against CD16 and CD32 to block FcR (clone 24G2; BioXcell) and with normal donkey serum and were stained using Alexa Fluor 488-conjugated anti-CD8 and Alexa Fluor 594-conjugated anti-CD4 antibodies (BioLegend). Tissue sections were mounted with Prolong Gold with DAPI.
Tissue sections were analyzed at the Confocal Microscopy Core Facility, Center for Cancer Research, National Cancer Institute, NIH. Images were acquired on a Zeiss 710 confocal microscope using a 40× oil immersion objective and 364-nm, 488-nm, and 543-nm lasers. Images were analyzed using Adobe Photoshop, and color channel levels were adjusted uniformly across images.
Preparation of cell suspensions for flow cytometry analysis.
Cervicovaginal cell suspensions were obtained after mincing of the cervicovaginal tissue into small pieces using dissecting scissors. The minced tissue was incubated for 1 h at 37°C in a shaker at 250 rpm in RPMI 1640 medium with 2% fetal bovine serum (FBS; Sigma-Aldrich), 0.5 mg/ml collagenase A (Roche), and 0.1 mg/ml DNase I (Roche). Spleen cell suspensions were obtained by following the protocol described above with a 20-min collagenase A–DNase I incubation. All cell suspensions were filtered through a 70-μm cell strainer. To remove red blood cells, cell suspensions and EDTA-treated blood were incubated for 5 min at room temperature (RT) in an ammonium chloride solution, washed, and kept on ice before further analysis.
Fluorescence-activated cell sorter (FACS) analysis of CD8+ T cell responses against the HSV-2 gB immunodominant epitope gB496–503.
After FcR blocking, cell suspensions were stained for 30 min at 4°C with an allophycocyanin (APC)-conjugated H-2Kb/gB496–503 tetramer (NIH Tetramer Facility) and the following antibodies: Pacific Blue-conjugated anti-CD3 (clone 17A2), APC- and Cy7-conjugated anti-CD4 (clone RM4-5), phycoerythrin (PE)- and Cy7-conjugated anti-CD44 (clone IM7), PE-conjugated anti-CD49a (clone HMα1), PE- and Cy7-conjugated anti-CD69 (clone H1.2F3), fluorescein isothiocyanate (FITC)-conjugated anti-CD62L (clone MEL-14), peridinin chlorophyll protein (PerCP)- and Cy5.5-conjugated anti-CD103 (clone 2E7), PE-conjugated anti-CD127 (clone SB/199), PE- and Cy7-conjugated anti-CXCR3 (clone CXCR3-173) (BioLegend), FITC-conjugated anti-KLRG-1 (clone 2F1; SouthernBiotech), and Pacific Orange-conjugated anti-CD8 (clone 53.6.7; Invitrogen).
To measure intracellular cytokine content, cells were incubated for 5 h at 37°C under 5% CO2 in RPMI 1640 medium containing 10% FBS, sodium pyruvate, l-glutamine, β-mercaptoethanol, and GolgiPlug (final concentration, 1 μg/ml brefeldin A; BD Biosciences), with 1 μg/ml gB496–503 peptide. After incubation, cells were washed, FcR blocked, and stained with antibodies against CD8, CD3, and CD4 as described above; dead cells were labeled with Live/Dead yellow dye (Invitrogen); the cells were fixed and permeabilized with Cytofix/Cytoperm (BD Biosciences); and intracellular staining was performed for 30 min at 4°C with FITC-conjugated anti-IFN-γ (clone XMG1.2), PerCP- and Cy5.5-conjugated anti-interleukin 2 (anti-IL-2) (clone JES65H4), and APC-conjugated anti-tumor necrosis factor alpha (anti-TNF-α) (clone MP6-XT22) antibodies (BioLegend).
In vitro neutralization assays.
The in vitro neutralization of HPV pseudovirions has been described previously (44). Serial dilutions of serum samples from individual mice were incubated with pseudovirus expressing secreted alkaline phosphatase (SEAP) at 4°C for 1 h. The pseudovirus-serum mixtures were added to 293TT cells, and 3 days later, SEAP activity was measured in the supernatants. The 50% effective concentration (EC50) was defined using Prism software as the dilution of serum corresponding to a 50% reduction in SEAP activity.
To determine the HSV-2 neutralizing titer in serum, 2-fold serial dilutions of serum samples were incubated with HSV-2 strain 333 for 1 h at RT and were added to Vero cell monolayers for 1 h at 37°C. The virus-antibody inocula were replaced with a plaque assay overlay consisting of Vero cell growth medium containing 0.3% (vol/vol) Gamunex-C (Talecris Biotherapeutics), and cells were incubated for 48 h at 37°C. Cells were then stained with a plaque staining solution containing 10% (vol/vol) acetic acid, 60% (vol/vol) methanol, and 1% (wt/vol) crystal violet and were washed, and plaques were counted.
ELISA for measurement of gD antibodies.
Titers of IgG against HSV-2 gD in serum were determined by enzyme-linked immunosorbent assays (ELISA). Briefly, a high-binding-capacity microplate (2HB; Immulon) was coated with purified recombinant gD2t protein (50 ng/well; Chiron). Plates were blocked with 1% dry milk buffer and 0.1% FBS in PBS, and serial dilutions of serum samples were incubated in each well. Immunoglobulin binding to the plate was measured using a horseradish peroxidase (HRP)-conjugated affinity-purified goat antibody to mouse IgG (Southern Biotechnology Associates). Specific titers were defined as the reciprocals of the highest sample dilutions giving signals equal to at least 3-fold the background signal.
Titration of virus shedding.
Vaginal swabs were collected daily from day 1 to day 7 after HSV-2 genital challenge, placed in culture medium containing penicillin, streptomycin, and amphotericin B, and stored at −80°C until titration by plaque assay on Vero cells.
Statistics.
Statistical significance (P ≤ 0.05) was determined using the nonparametric Mann-Whitney U test in experiments with two independent groups. One-way analysis of variance (ANOVA) and multiple comparisons were performed for experiments with more than two groups. Spearman's coefficient (r) was calculated to determine the correlation between variables.
RESULTS
Generation and characterization of HPV PsV that target expression of HSV-2 gB and gD to different subcellular compartments.
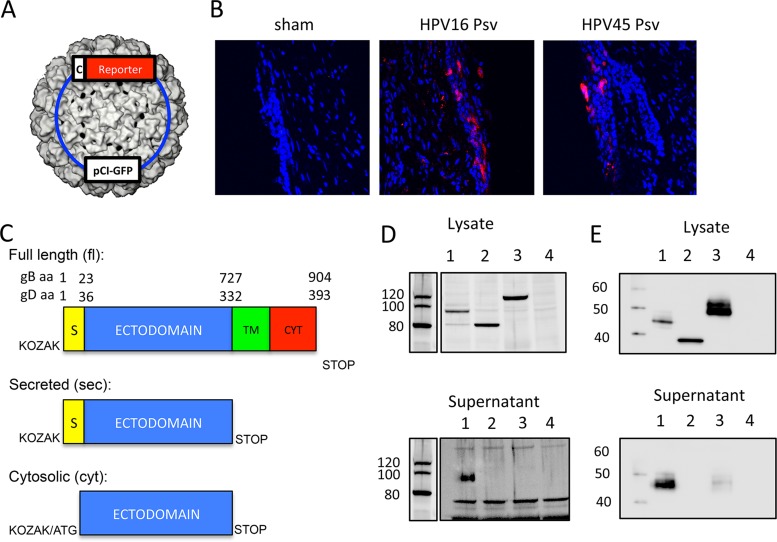
We hypothesized that targeting the accumulation of HSV-2 gB and gD to different subcellular compartments after in vivo transduction of cervicovaginal epithelial cells of mice with HPV PsV (shown in Fig. 1A as a 3-dimensional reconstruction [45]) might affect their immunogenicity. In vivo transduction of cervicovaginal epithelial cells by HPV16 and HPV45 PsV was confirmed by confocal microscopy analysis of genital tract tissue sections after intravaginal instillation of PsV expressing tomato fluorescent protein (Fig. 1B), as reported previously by Roberts et al. (37).
FIG 1.
Generation and characterization of HPV PsV expressing gB and gD constructs targeting different subcellular compartments. (A) Representation of an HPV PsV containing a typical pCI plasmid of <8 kb encoding a reporter gene expressed under the control of a cytomegalovirus promoter (c) PsV. (B) Medroxyprogesterone acetate-treated mice either were inoculated ivag with 108 IU of HPV16 or HPV45 PsV expressing tomato fluorescent protein or were sham treated with 2% carboxymethyl cellulose. (C) Representations of the constructs cloned into the pCI plasmid backbone to target the expression of HSV-2 gB and gD to different subcellular compartments. S, signal peptide; TM, transmembrane domain; CYT, cytosolic domain. (D and E) 293TT cells were transfected with pCI plasmids expressing secreted (lanes 1), cytosolic (lanes 2), and full-length (lanes 3) gB (D) or gD (E) constructs or luciferase (lanes 4), and cell lysates (50 μg protein) and serum-free supernatants (30 μl) were assayed by Western blotting using a mouse monoclonal antibody (2C10) or a rabbit polyclonal antibody (R69) to assess the presence of gB (D) or gD (E), respectively.
To convert wild-type membrane-associated gB and gD (gBfl and gDfl) into secreted (gBsec and gDsec) or cytosolic (gBcyt and gDcyt) forms, we deleted the transmembrane and cytoplasmic sequences or the transmembrane, cytoplasmic, and signal peptide sequences of gB and gD, respectively. The remaining DNA sequences were introduced into a modified expression plasmid (pCI), as depicted in Fig. 1C.
The expression of gB and gD was assessed by Western blotting of cell lysates and supernatants of 293TT cells transfected with the plasmids expressing full-length, secreted, and cytosolic forms of HSV-2 gB and gD. Full-length, secreted, and cytosolic gB and gD migrated with apparent molecular sizes of 120, 100, and 80 kDa (gB) or 50, 45, and 35 kDa (gD), respectively (Fig. 1D and E). The apparent molecular masses of the two cytosolic constructs of gB and gD in the cell lysates corresponded to their predicted molecular masses without posttranslational modification of 79 kDa and 33 kDa, respectively, strongly suggesting restricted expression of the gBcyt and gDcyt constructs in the cytosolic compartment (Fig. 1D and E). While gB and gD were detected in the supernatants of cells transfected with the secreted constructs, they were not seen in the supernatants of cells transfected with the cytosolic constructs or the full-length construct for gB (Fig. 1D and E). A faint band of the same apparent molecular mass as the gD secreted form was detected in the supernatant of cells transfected with the full-length construct (Fig. 1E), in agreement with a previous report that a fraction of full-length gD is subject to proteolytic cleavage and release of the ectodomain into the supernatant of HSV-2-infected cells (46).
CD8+ T cell responses after intravaginal immunization with HPV PsV that target the expression of HSV-2 gB to different cellular compartments.
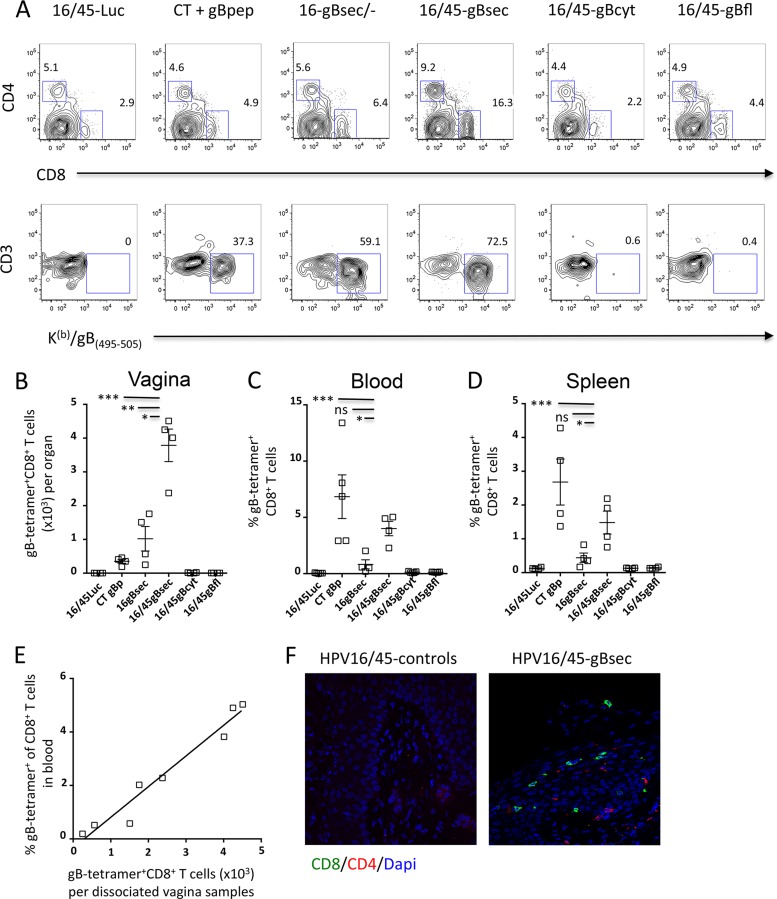
We used C57BL/6 mice for immunogenicity studies because this haplotype permits the measurement of CD8+ T cell responses against the immunodominant epitope gB496–503 restricted to H-2Kb (Kb) (47, 48). C57BL/6 mice were immunized ivag with HPV16 and HPV45 PsV (108 IU) in a prime-boost regimen to ensure that the immune response to the booster dose was not reduced by the induction of HPV16 neutralizing antibodies from the priming dose. One month after the last immunization, we used tetramer probes to detect CD8+ T cells specific for the gB496–503 epitope (referred to here as Kb/gB496 tetramer-positive CD8+ T cells) in the blood, spleen, and genital tract. As a positive control, a group of mice received 20 μg of the gB496–503 peptide with 1 μg of CT given intranasally (i.n.), which has been reported to induce strong systemic CD8+ T cell responses (49). Only ivag immunization of mice with HPV-gBsec elicited detectable Kb/gB496 tetramer-positive CD8+ T cells in cervicovaginal mucosa cell suspensions (Fig. 2A and B). While the percentage of Kb/gB496 tetramer-positive CD8+ T cells in the cervicovaginal mucosa cell suspensions was slightly higher after a booster immunization than after HPV-gBsec priming immunization alone (means, 63.6% versus 41.3%, respectively), the total number of Kb/gB496 tetramer-positive CD8+ T cells in the cervicovaginal mucosa was 4 times higher after the booster immunization. Importantly, prime-boost ivag immunization with HPV-gBsec resulted in 10 times more Kb/gB496 tetramer-positive CD8+ T cells in the cervicovaginal mucosa than prime-boost immunization with i.n. CT plus gB496–503 peptide. In contrast, prime-boost i.n. immunization with CT plus gB496–503 peptide induced higher frequencies of Kb/gB496 tetramer-positive CD8+ T cells in blood (mean, 6.8%) and spleen (mean, 2.7%) than prime-boost ivag immunization with HPV-gBsec (mean frequencies, 4.0% in blood and 1.5% in spleen) (Fig. 2C and D). As with the CD8+ T cell responses observed in the cervicovaginal mucosa, HPV-gBsec ivag prime-boost immunization induced higher frequencies of Kb/gB496 tetramer-positive CD8+ T cells in blood and in spleen than HPV-gBsec ivag priming immunization only. Interestingly, a strong correlation was observed between blood and genital CD8+ T cell responses, whether the mice received only the priming dose or the prime-boost regimen (r = 0.93; P = 0.0007) (Fig. 2E). In the HPV-gBsec group, the total number of CD8+ T cells was increased by 20-fold after ivag prime-boost immunization, whereas we observed only a 2-fold increase at most in CD4+ T cells (data not shown). In agreement with these results, immunofluorescence staining with antibodies against CD8 and CD4 confirmed the intraepithelial distribution of CD8+ cells and of some CD4+ T cells in the cervicovaginal mucosa after ivag immunization with the HPV-gBsec vector, in contrast to observations for animals treated with the HPV control vector (Fig. 2F).
FIG 2.
HPV PsV expressing secreted gB induce preferentially HSV-specific CD8+ T cells in the genital tract. Medroxyprogesterone acetate-treated mice (4 per group) were immunized ivag with 108 IU of HPV16 expressing gBsec, gBcyt, or gBfl, followed 1 month later by an ivag booster immunization with 108 IU of HPV45 expressing the same construct. As a positive control, a group of mice was immunized i.n. twice, 1 month apart, with 20 μg of the minimal immunodominant peptide gB496–503 admixed with 1 μg CT. As a negative control, a group of mice was immunized with 108 IU of HPV16 expressing luciferase, followed 1 month later by a second immunization with 108 IU of HPV45 expressing luciferase. (A) FACS analysis plots of expression of CD8 and CD4 by cervicovaginal cells and expression of CD3 and the Kb/gB496 tetramer by cervicovaginal CD8+ T cells. (B to D) Data are expressed as means ± standard errors of the means and are representative of the results of 3 independent experiments. Asterisks indicate significant differences (*, P < 0.05; **, P < 0.01; ***, P < 0.001) by one-way ANOVA and multiple comparisons. (E) Correlation between Kb/gB496 tetramer-positive CD8+ T cells in blood and Kb/gB496 tetramer-positive cervicovaginal CD8+ T cells. (F) Tissue sections of the cervicovaginal mucosae of mice immunized with a control HPV vector or with HPV-gBsec were collected 4 weeks after the booster and were stained with anti-CD8 (green) and anti-CD4 (red) antibodies and with DAPI (blue).
Together, these data indicate that the secreted ectodomain of gB expressed by cervicovaginal epithelial cells after HPV PsV immunization is much more immunogenic for T cells than the cytoplasmic or membrane-bound form of the protein and, in particular, that it can induce robust CD8+ T cell responses in the cervicovaginal epithelium. Therefore, all subsequent immunization experiments were carried out with the secreted ectodomain constructs, gBsec and gDsec.
Surface marker expression by HSV-2-specific CD8+ T cells after ivag immunization with HPV-gBsec.
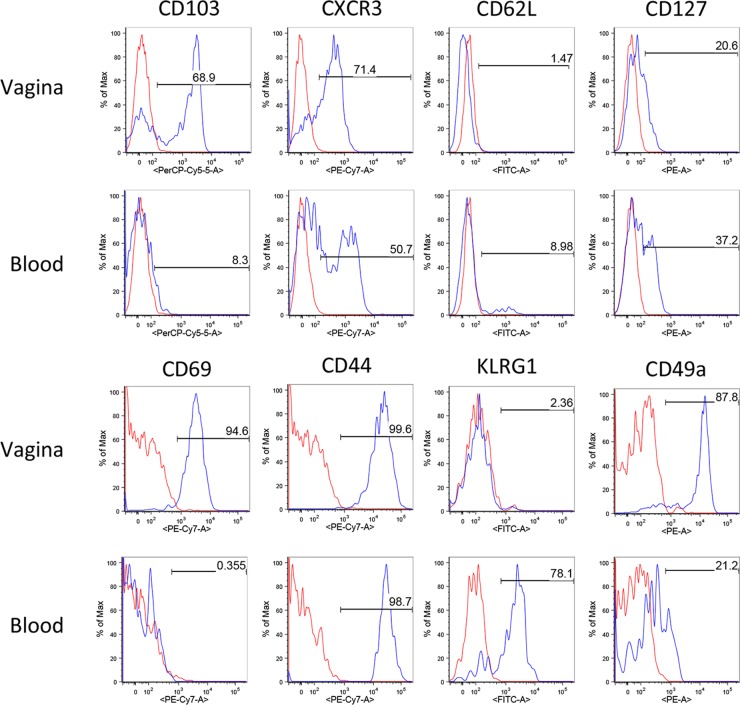
We performed phenotypic analyses by flow cytometry of HSV-2 gB-specific CD8+ T cells in order to define which type of memory cells was induced by ivag HPV-gBsec immunization. The expression of various surface molecules involved in T cell migration, activation, and function was measured on Kb/gB496 tetramer-positive CD8+ T cells in blood and cervicovaginal cell suspensions 1 month after the last immunization. In all of the tissues analyzed, HSV-specific CD8+ T cells induced by ivag HPV-gBsec immunization displayed an effector or effector/memory phenotype characterized by low expression of CD62L but heterogeneous expression of CD127 (Fig. 3). There is considerable evidence that tissue-resident memory CD8+ T cells and other intraepithelial lymphocytes selectively express the integrin CD103 and that its expression is associated with improved protection in several infectious models, including HSV-1 models (50). CD103 was selectively upregulated by HSV-specific CD8+ T cells in cervicovaginal cell suspensions but not in blood. CXCR3, which is the receptor for chemokines CXCL9 and CXCL10, is involved in the migration of memory CD8+ T cells into inflamed tissue and was recently associated with the differentiation of tissue-resident memory CD8+ T cells (51). Expression of CXCR3 was detected on the majority (70%) of cervicovaginal HSV-specific CD8+ T cells and on half of HSV-specific CD8+ T cells in blood induced by ivag HPV-gBsec vaccination. Although expression of CD69 is usually associated with recent activation of antigen-specific CD8+ T cells, all genital HSV-specific CD8+ T cells still expressed CD69 1 month after the final ivag HPV-gBsec immunization. However, CD69 was not expressed by HSV-specific CD8+ T cells in blood (Fig. 3). Interestingly, KLRG-1 was expressed by most HSV-specific CD8+ T cells in blood but was not expressed by HSV-specific cervicovaginal CD8+ T cells. As expected, CD44 was expressed by both cervicovaginal and blood HSV-specific CD8+ T cells. Finally, CD49a (α1 integrin) was expressed by HSV-specific cervicovaginal CD8+ T cells and, to a lower extent, by HSV-specific CD8+ T cells in blood (Fig. 3).
FIG 3.
Surface marker expression by Kb/gB496 tetramer-positive CD8+ T cells after prime-boost ivag immunization with HPV-gBsec. Representative plots show the expression of CD103, CXCR3, CD62L, CD127, CD69, CD44, KLRG-1, and CD49a by Kb/gB496 tetramer-positive CD8+ T cells from the cervicovaginal mucosal and blood cell suspensions obtained 4 weeks after the ivag booster immunization with HPV-gBsec. Data for antibody staining are shown in blue, and data for the unstained negative control are shown in red. Data are representative of the results of 3 independent experiments.
Intravaginal immunization with HPV-gBsec elicits the production of HSV-specific IFN-γ- and TNF-α-secreting systemic and cervicovaginal CD8+ T cells.
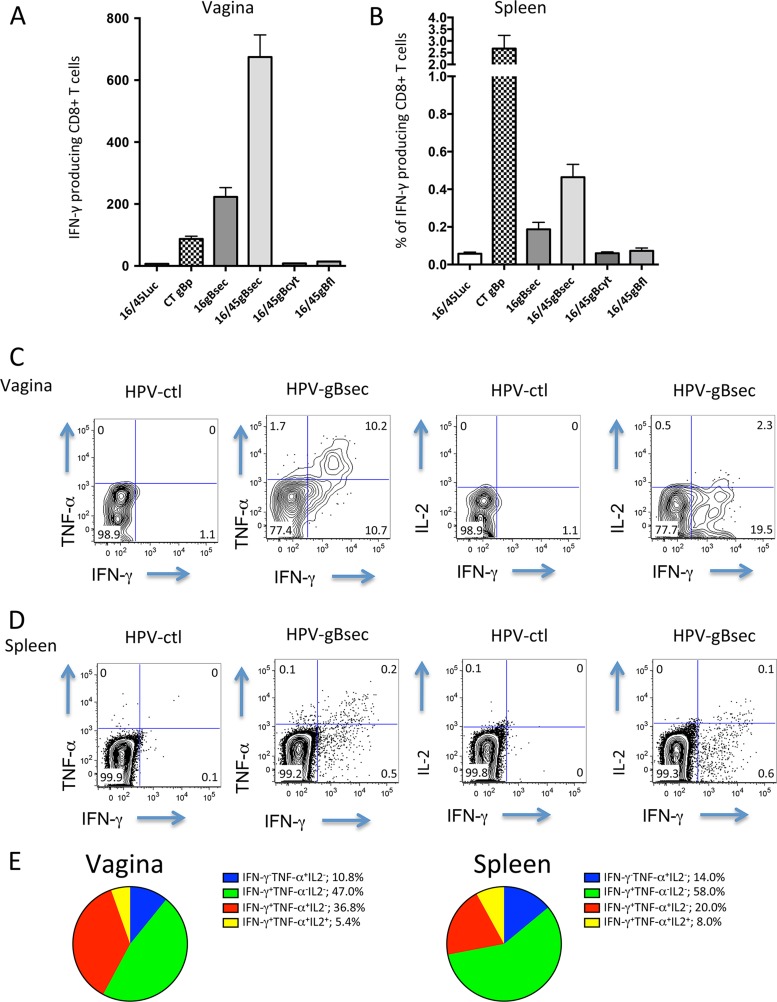
The production of multiple cytokines in memory CD8+ T cells is often associated with better function and quality of the response (52). In particular, IFN-γ and TNF-α production by local T cells has been shown to contribute to protection in the context of HSV-2 primary infection or in experimental vaccination settings (26–28). Spleen and genital tract cell suspensions were obtained 4 weeks after the last ivag immunization with HPV-gBsec, HPV-gBcyt, HPV-gBfl, or the HPV control or 4 weeks after the last i.n. immunization with CT plus gB496–503. The cell suspensions were incubated for 5 h with 1 μM gB496–503 peptide, and cytokine production in CD8+ T cells was measured by intracellular staining with antibodies against IFN-γ, TNF-α, and IL-2, followed by flow cytometry analysis. The number of IFN-γ-producing CD8+ T cells was higher in the cervicovaginal mucosa after ivag prime-boost immunization with HPV-gBsec than after i.n. immunization with CT plus the gB496–503 peptide (Fig. 4A). In contrast, the frequency of IFN-γ-producing CD8+ T cells in the spleen was higher after i.n. immunization with CT plus the gB496–503 peptide than after ivag immunization with HPV-gBsec (Fig. 4B). In agreement with the data shown in Fig. 2, neither HPV-gBcyt nor HPV-gBfl ivag vaccination induced IFN-γ-producing cells upon in vitro stimulation with the gB496–503 peptide.
FIG 4.
Cytokine production by CD8+ T cells induced after ivag immunization with HPV-gBsec, gBcyt, or gBfl. Medroxyprogesterone acetate-treated mice (4 per group) were immunized ivag with 1 × 108 IU of HPV16 expressing gBsec, gBcyt, or gBfl, followed 1 month later by an ivag booster immunization with 108 IU of HPV45 expressing the same glycoprotein. Another group of mice was immunized i.n. twice, 1 month apart, with 20 μg of the minimal immunodominant peptide gB496–503 admixed with 1 μg CT. As a control, a group of mice was immunized with 108 IU of HPV16 expressing luciferase, followed 1 month later by a second immunization with 108 IU of HPV45 expressing luciferase. (A and B) Levels of IFN-γ-producing cells in the vagina (A) and spleen (B) measured by intracellular cytokine staining after in vitro stimulation with the gB496–503 peptide 4 weeks after the final immunization. (C and D) Representative plots of IFN-γ and TNF-α production, and of IFN-γ and IL-2 production, in cells from the vagina (C) and spleen (D). (E) Pie diagrams of production of multiple cytokines by cervicovaginal and spleen CD8+ T cells in mice immunized with HPV-gBsec.
The frequency of CD8+ T cells producing at least one cytokine in response to in vitro stimulation with gB496–503 (responding CD8+ T cells) was higher in cervicovaginal suspensions (21%) than in the spleen (0.7%) (Fig. 4C and D). Cervicovaginal or spleen CD8+ T cells from HPV control-immunized mice did not produce any cytokine in response to in vitro stimulation with gB496–503 (Fig. 4C and D). In the cervicovaginal mucosae of mice immunized with HPV-gBsec, 47% of the responding CD8+ T cells produced IFN-γ only, 11% produced TNF-α only, 37% produced both IFN-γ and TNF-α, and 5% produced IFN-γ, TNF-α, and IL-2 simultaneously (Fig. 4E). Responding CD8+ T cells in the spleen produced a slightly narrower range of cytokines than those in the cervicovaginal mucosa; the majority (72%) of the responding splenic CD8+ T cells produced only one cytokine, IFN-γ or TNF-α. These data indicate that gB-specific cervicovaginal CD8+ T cells and, to a lesser extent, gB-specific CD8+ T cells in the spleen induced by ivag HPV-gBsec immunization can produce multiple cytokines for antiviral defense.
Combined ivag immunization with HPV-gBsec/gDsec vaccines confers enhanced survival after HSV-2 vaginal challenge over that obtained by immunization with HPV-gBsec or HPV-gDsec alone.
We evaluated whether ivag immunization with HPV vectors expressing gBsec or gDsec alone or combined could confer protection in the mouse HSV-2 lethal genital challenge model. Medroxyprogesterone acetate-treated mice (n, 10 per group) were immunized ivag with 108 IU of HPV16-gBsec or HPV16-gDsec alone or with a mixture of HPV16-gBsec and HPV16-gDsec (108 IU each), followed 1 month later by 108 IU of HPV45 expressing the respective antigens. As expected, none of the sham-treated (2% CMC) mice survived the genital challenge with 104 PFU HSV-2 strain 333, and sham-treated mice started to die by day 9 after HSV-2 genital challenge. Only 20% or 30% of the mice immunized ivag with HPV-gBsec or HPV-gDsec, respectively, survived the HSV-2 genital challenge (P, 0.284 and 0.048 for comparison to sham-treated mice). Interestingly, combining both HSV-2 glycoproteins in the HPV PsV conferred higher protection (50% survival) than either sham treatment (P = 0.003) or immunization with HPV-gBsec (P = 0.069) or HPV-gDsec (P = 0.093) alone (Fig. 5). These results suggest that HPV vectors can confer partial protection against HSV-2 lethal genital challenge and that delivering more than one antigen can enhance this protection.
FIG 5.
Combined immunization with HPV vectors expressing gB and gD induces enhanced protection against HSV-2 vaginal challenge. Medroxyprogesterone acetate-treated mice (n, 10/group) were immunized ivag with HPV16 expressing gBsec, gDsec, or a mixture of both (each at 108 IU), followed 1 month later by ivag booster immunization with HPV45 expressing the respective antigens at 108 IU. As a control, a group of mice was sham treated (2% CMC) twice, 1 month apart. The graph shows the survival of mice challenged ivag 1 month after the last immunization with 104 PFU of HSV-2 strain 333. Statistical significance was measured by a log rank (Mantel-Cox) test (*, P ≤ 0.05; **, P ≤ 0.01; ns, nonsignificant).
Immunogenicity of simultaneous ivag immunization with HPV-gBsec/gDsec vectors and i.m. immunization with gD2t protein in alum and MPL.
A soluble truncated gD protein (gD2t) in alum-MPL was the basis of an HSV-2 vaccine tested recently in a phase III clinical trial. This vaccine induced HSV-2 neutralizing antibodies in serum, though with low titers, and provided no protection against genital HSV-2 (21, 22). In the guinea pig HSV-2 genital model, immunization with gD2t in alum and MPL induced high titers of neutralizing antibodies in serum and conferred full survival upon challenge but did not fully control viral replication during acute infection (53). We speculated that combining a vaccine that induces high HSV-2 neutralizing antibody titers with our HPV PsV to induce high numbers of intraepithelial HSV-specific memory CD8+ T cells might confer enhanced protection against disease and improve control of local replication of the virus. Medroxyprogesterone acetate-treated mice (n, 10/group) were immunized ivag with a mixture of HPV16-gBsec and HPV16-gDsec (2 × 108 IU each) and were boosted 1 month later with 2 × 108 IU each of HPV45-gBsec and HPV45-gDsec (HPV-gBsec/gDsec). Another group of mice was immunized i.m. twice, 1 month apart, with 3 μg gD2t and 7.5 μg MPL adsorbed on 50 μg of alum (gD2t-alum-MPL). A third group of mice was prime-boost immunized ivag with HPV-gBsec/gDsec and i.m. with gD2t-alum-MPL simultaneously. For control purposes, a group of mice was prime-boost immunized ivag with HPV16 and HPV45 PsV expressing red fluorescent protein (RFP). As expected, i.m. immunization with gD2t-alum-MPL induced high HSV-2 gD-specific antibody titers and high neutralizing antibody titers, whereas ivag immunization with HPV-gBsec/gDsec induced modest levels of HSV-2 gD and HSV-2 neutralizing antibodies in the serum (Fig. 6A and B). Interestingly, the HPV-gBsec/gDsec vaccine, which delivers a small amount of HPV capsid protein (2 μg L1) to the vaginal tract in the absence of adjuvant, induced high titers of HPV type-specific neutralizing activity in the serum (Table 1).
FIG 6.
Immunogenicity of simultaneous ivag immunization with HPV-gBsec/gDsec and i.m. immunization with gD2t with alum and MPL. Medroxyprogesterone acetate-treated mice (n, 10/group) were immunized twice, 1 month apart, with 3 μg gD2t with 50 μg alum and 7.5 μg MPL (gD2t/A/MPL). A second group of mice was immunized ivag with a mixture of 2 × 108 IU of HPV16-gBsec and 2 × 108 IU of HPV16-gDsec, followed 1 month later by ivag booster immunization with HPV45 expressing the respective antigens (2 × 108 IU each) (HPV-gBs/gDs). A third group of mice was immunized simultaneously with HPV-gBsec/gDsec ivag and gD2t-alum-MPL i.m. As a control, a group of mice was prime-boost immunized ivag with HPV16 and HPV45 expressing RFP. (A) gD-specific IgG antibody titers were measured in serum samples. Each bar represents the geometric mean of the endpoint titers of serum samples (error bars, 95% confidence intervals). Symbols represent results for individual serum samples. (B) HSV-2 neutralization titers of serum samples. Data are expressed as means ± standard deviations. (C) Flow cytometry analysis of the Kb/gB496 tetramer in peripheral blood CD8+ T cells after priming and booster immunization. Each set of symbols connected by a line represents the results for an individual mouse after primary immunization (open circles) and booster immunization (open squares).
TABLE 1.
HPV16 and HPV45 neutralization activities in serum after ivag immunizationa with HPV PsV
| Immunization | Geometric mean neutralization titerb (95% CI) in serum samples |
|
|---|---|---|
| HPV16 | HPV45 | |
| HPV control | 862.6 (232–3,202) | 348 (699–221) |
| gD2t | <40 (N/A) | <40 (N/A) |
| HPV-gBsec/gDsec | 1,936 (942–3,552) | 1,087 (367–3,214) |
| HPV-gBsec/gDsec + gD2t | 1,879 (994–3,552) | 1,301 (509–3,321) |
Mice were primed and boosted 1 month apart, and serum was collected 4 weeks after each immunization.
Expressed as the 50% inhibitory concentration, determined using GraphPad Prism. 95% CI, 95% confidence interval; N/A, not applicable.
The two groups receiving HPV-gBsec/gDsec had similar frequencies of circulating HSV-specific CD8+ T cells after a single immunization (Fig. 6C). However, after the second immunization, the group receiving HPV-gBsec/gDsec ivag alone displayed a pronounced increase in circulating gB-specific CD8+ T cells that was not observed in the group receiving HPV-gBsec/gDsec ivag combined with i.m. gD2t-alum-MPL. The latter data suggest that i.m. immunization with gD2t-alum-MPL might have interfered with the boosting of gB-specific CD8+ T cells by the HPV vectors given ivag (Fig. 6C).
Intravaginal immunization with the HPV-gBsec/gDsec vaccine, but not i.m. immunization with gD2t-alum-MPL, reduces the severity of genital lesions and viral shedding after HSV-2 genital challenge.
As expected, none of the mice immunized with the control HPV vector survived the lethal HSV-2 genital challenge with 104 PFU of HSV-2 strain 333, whereas 80% of the mice immunized i.m. with gD2t-alum-MPL survived (Fig. 7A). Sixty percent of the mice immunized ivag with HPV-gBsec/gDsec survived, while 90% of mice immunized with a combination of ivag HPV-gBsec/gDsec and i.m. gD2t-alum-MPL survived. Interestingly, there was no difference in HSV-2 titers in genital swabs between HPV control-treated mice and gD2t-alum-MPL i.m.-immunized mice (Fig. 7B), despite high levels of neutralizing activity in the sera of the latter group (Fig. 6B). Mice immunized ivag with HPV-gBsec/gDsec, whether alone or combined with i.m. gD2t in alum-MPL, had lower levels of viral shedding in genital swabs than HPV control-immunized mice (Fig. 7B).
FIG 7.
Protection induced by combined immunization with HPV-gBsec/gDsec ivag and gD2t-alum-MPL i.m. Medroxyprogesterone acetate-treated mice (n, 10/group) were immunized twice, 1 month apart, with 3 μg gD2t with 50 μg alum and 7.5 μg MPL (gD2t/A/MPL). A second group of mice was primed ivag with a mixture of 108 IU of HPV16-gBsec and 108 IU of HPV16-gDsec, followed 1 month later by ivag booster immunization with HPV45 expressing the respective antigens (108 IU each). A third group of mice was primed simultaneously with a mixture of 108 IU of HPV16-gBsec and 108 IU of HPV16-gDsec given ivag and gD2t-alum-MPL given i.m., followed 1 month later by booster immunization with HPV45 expressing 108 IU of each of the respective antigens given ivag and with gD2t-alum-MPL given i.m. As a control, a fourth group of mice was prime-boost immunized ivag with HPV16 and HPV45 expressing RFP. Mice were challenged ivag 1 month after the last immunization with 104 PFU of HSV-2 strain 333. (A) Survival of mice after HSV-2 challenge. Statistical significance was measured by a log rank (Mantel-Cox) test. (B) Viral shedding measured at the indicated days in genital swabs by plaque assay titration. Means ± standard deviations are shown. Statistical analysis by two-way ANOVA (P = 0.02) was followed by multiple-comparison analysis. Symbols indicate significant differences between the HPV control and HPV-gBsec/gDsec (*, P < 0.05) or HPV-gBsec/gDsec plus gD2t-A-MPL (#, P < 0.05; ##, P < 0.01). (C) Representative pictures of local manifestations of pathology in mice infected with HSV-2. (D) Percentages of mice that did not survive (filled bars), that survived but developed genital disease (hatched bars), or that survived and had no genital disease (open bars).
The development of genital disease is a hallmark of primary human HSV-2 infection and can be characterized by redness, swelling, hair loss, and genital lesions (Fig. 7C). All mice in the groups immunized ivag with the HPV control vector or i.m. with gD2t in alum-MPL developed mild to severe genital disease (Fig. 7C). Strikingly, 40% of mice immunized ivag with HPV-gBsec/gDsec (with or without i.m. gD2t-alum-MPL) did not develop genital disease and survived (Fig. 7D).
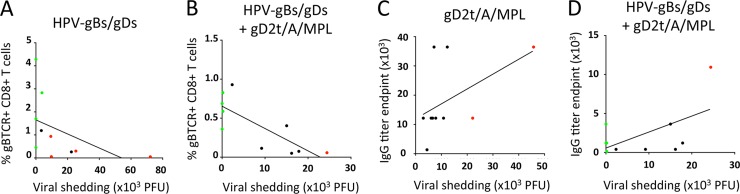
Based on these findings, we defined three subgroups of mice: those that did not survive, those that survived but developed genital disease, and those that survived and were free of genital disease. Within the group immunized i.m. with gD2t-alum-MPL, there was no difference in viral shedding over time between the subgroup of mice that did not survive and the subgroup that survived with genital disease (Fig. 8A and B). Similarly, in the groups immunized ivag with HPV-gBsec/gDsec, with or without i.m. gD2t-alum-MPL, there was no difference in viral shedding over time between the subgroup that did not survive and the subgroup that survived with genital disease. However, for the subgroups of mice that survived free of genital disease, viral titers in the genital swabs remained under 10 PFU per ml over time (Fig. 8C and D). Finally, the frequency of circulating gB-specific CD8+ T cells was inversely correlated with viral shedding in the genital swabs of mice immunized ivag with HPV-gBsec/gDsec (r = −0.78; P = 0.005) or immunized ivag with HPV-gBsec/gDsec and i.m. with gD2t-alum-MPL (r = −0.69; P = 0.014) (Fig. 9A and B), whereas serum gD-specific antibody titers did not correlate or showed a weak positive correlation with viral shedding in groups immunized i.m. with gD2t-alum-MPL only (r = 0.57; P = 0.05) or i.m. with gD2t-alum-MPL and ivag with HPV-gBsec/gDsec (r = 0.15; P = 0.34) (Fig. 9C and D). Similarly, HSV-2 neutralizing antibody titers did not correlate with lower viral shedding (data not shown).
FIG 8.
The level of HSV-2 shedding in the vagina is correlated with the severity of the genital disease. Mice were vaccinated and challenged as described in the legend to Fig. 7. (A to D) Shedding of the challenge virus was measured by taking vaginal swabs on the indicated days and titrating by plaque assays in Vero cells. Mice were divided into three subgroups according to the severity of infection: those that did not survive (red curves), those that survived but developed genital disease (black curves), and those that survived free of genital disease (green curves).
FIG 9.
The frequency of circulating gB-specific CD8+ T cells is inversely correlated with challenge virus shedding in the vaginal tract. Mice were vaccinated, challenged, and divided into three subgroups: those that did not survive (red symbols), those that survived but developed genital disease (black symbols), and those that survived free of genital disease (green symbols). (A and B) Frequencies of Kb/gB496 tetramer-positive CD8+ T cells in blood versus viral titers in genital swabs for groups immunized ivag with HPV-gBsec/gDsec with or without i.m. immunization with gD2t-alum-MPL. (C and D) Graphs show gD-specific IgG antibody titers versus viral titers in genital swabs for groups immunized i.m. with gD2t-alum-MPL with or without ivag immunization with HPV-gBsec/gDsec.
Together, these results indicate that ivag immunization with HPV-gBsec/gDsec is more effective than i.m. immunization with gD2t-alum-MPL at reducing genital disease and genital virus shedding after challenge with HSV-2.
DISCUSSION
Our study shows that transducing cervicovaginal epithelial cells in mice with HPV vectors expressing the gB and gD glycoprotein ectodomains of HSV-2 can preferentially induce HSV-specific intraepithelial-tissue-resident memory CD8+ T cells with a polyfunctional phenotype, together with modest levels of HSV-2 neutralizing antibodies. Secretion of gB was crucial to prime a CD8+ T cell response. Since gB expression in cultured cells was similar for the secreted, cytosolic, and membrane constructs, it is unlikely that a putative in vivo difference in expression levels accounted for the observed differences in immunogenicity. We have shown previously that CD4+ T cells are crucial for the priming of CD8+ T cells upon HPV vector ivag vaccination (38), so we speculate that secretion of gB and gD may facilitate the induction of CD4+ T helper cells, which, in turn, allow the priming of naïve CD8+ T cells. Our results are consistent with those of studies showing that secreted, but not intracellular, ovalbumin was able to induce CD4+ T cells upon i.n. immunization with an adenoviral vector (54) and that a secreted form of gD expressed by a DNA HSV vaccine induced a more potent IgG response than nonsecreted constructs (55).
HPV PsV have several characteristics that make them attractive vehicles for generating tissue-resident T cells after ivag delivery. First, they have a natural and highly restricted tropism for the genital epithelium, and the transduced gene(s) is only transiently expressed in the epithelium. Second, no HPV genes are expressed from the pseudogenomes, so there is no potential reduction in immunity to the target antigen because of immunodominance of an HPV viral antigen. Third, the recent cessation of an HIV trial (HVTN 502) highlighted the risk of potentiating HIV infection through the induction of HIV-susceptible CD4+ T cells (56). Because ivag genetic vaccination with HPV vectors results in highly skewed CD8+ T cell responses, it should minimize the risk of inducing HIV-susceptible target CD4+ T cells in the cervicovaginal mucosa. Occasionally, we observed a <2-fold increase in the level of CD4+ T cells in HPV vector ivag-immunized groups, which is much less pronounced than the 20-fold increase in the level of CD8+ T cells. Also, HPV vector ivag immunization did not induce expression of HIV coreceptor α4β7 by cervicovaginal CD4+ T cells (data not shown) (57, 58), in contrast to marked induction of this coreceptor after vaccination with an adenovirus 5 vector (56). Finally, there are many human and animal papillomavirus types that are distinct serotypes. Therefore, the induction of neutralizing antibodies by the priming dose of vaccine can easily be overcome by boosting with a heterologous type, as demonstrated here (38).
The mouse model of genital herpes disease has been useful for studying the roles played by innate and adaptive immunity in protection during primary infection or the mechanisms of protection conferred by vaccination; however, it has limitations (59). Vaginal HSV-2 infection in the mouse does not lead to recurrent genital lesions. Consequently, the primary endpoint of many mouse challenge studies has been protection from lethal HSV infection. Protection from death appears to be primarily antibody mediated (60, 61), in agreement with our finding that i.m. injection of HSV-2 gD in alum-MPL induced high titers of HSV-2 neutralizing antibodies and strong protection from lethal challenge, whereas ivag immunization with HPV-gDsec/gBsec induced lower neutralizing antibody titers and less protection from lethal challenge. However, death is an extremely rare consequence of human HSV-2 infection; thus, this endpoint would not be appropriate for clinical trials of HSV-2 vaccines.
Genital lesions and viral shedding are frequent and important manifestations of HSV-2 infection in humans, and therefore, these parameters, if they are predictive of what happens in people, may be more relevant endpoints for preclinical animal studies of prophylactic HSV vaccines. It is striking that i.m. gD2t in alum-MPL provided no protection against these endpoints, despite inducing high levels of serum neutralizing antibodies. If these interpretations are correct, our findings are consistent with the negative results of the recent clinical trial of an HSV-2 gD subunit vaccine, which did not prevent HSV-2 infection or disease (22). Interestingly, the HSV-2 vaccine results contrast with those observed in preclinical and clinical trials of the HPV virus-like particle (VLP) vaccine, where serum neutralizing antibodies are believed to be the principal mediator of sterilizing immunity to genital HPV infection. It is currently unclear why serum neutralizing antibodies can protect against death due to HSV infection but not against genital lesions or shedding, whereas serum antibodies can induce strong protection against genital HPV infection. Possible explanations include the facts that HSV has a large number of glycoproteins and capsid proteins relative to HPV, that HSV infects the external epithelial cells while HPV infects the cells at the basement membrane, and that HSV establishes latency and reactivates from neurons while HPV does not.
In contrast to i.m. injection of gD2t protein, ivag instillation of HPV PsV expressing secreted gB and gD induced strong protection against genital disease and virus shedding in a substantial percentage of mice. The direct correlation between the induction of gB-specific CD8+ T cell responses after genital immunization, but not systemic antibody responses after i.m. immunization, and shedding of virus from the vagina supports the hypothesis that these T cells are the primary effectors of local protection. In agreement with this conjecture, intravital microscopy studies have shown that intraepithelial CD8+ T cells translocate extensively through the lower layers of stratified squamous epithelium (as found in the mouse vaginal tract) and arrest when they contact keratinocytes that present their cognate peptide/major histocompatibility class I (MHC-I) complex (62).
Several strategies might be employed to increase the level of protection with ivag PsV vaccination. Our finding that the combination of gB and gD ectodomains conferred better protection than each antigen given separately suggests that including additional HSV antigens may improve the breadth of the immune response induced by vaccination. In addition, a recent study showed that adding the immediate early gene ICP27 to a DNA vaccine expressing gB and gD dramatically increased the efficacy of the vaccine (63, 64). Since the induction of T cells that can interrupt initial genital epithelial infection, rather than a response that is limited to neutralizing antibody induction, is the main goal of our vaccine strategy, it will be important to evaluate HSV-2 genes that are expressed very early after infection so as to favor a rapid response to virus-infected cells prior to the expression of viral immune evasion genes.
Other strategies designed to preferentially generate local T cell responses to HSV-2 have been reported. A live attenuated HSV-2 vaccine with the thymidine kinase gene deleted (HSV-TK), delivered topically, is considered among the most effective experimental HSV vaccines. This vaccine induced sterilizing immunity to genital infection that was mediated by CD4+ and CD8+ T cells producing IFN-γ (28, 36, 65). Recent studies showed that topical immunization with live attenuated HSV-1-TK induced a pool of long-lived tissue-resident memory CD8+ T cells close to the site of initial vaccination that was able to respond rapidly and to confer enhanced protection against subsequent HSV-1 skin infection (29, 51). However, this attenuated vaccine has generated potential safety concerns because it can establish latent infection in the regional ganglia, and the vaccine strain is resistant to most antivirals (66). Other studies have shown that, compared with systemic vaccination alone, the combination of systemic vaccination followed by either local inflammation or topically administered chemotactic molecules can redirect recently activated circulating HSV-2-specific CD8+ T cells to the genital tract and confer better control of viral replication in HSV-2 genital infection models (30, 31). However, although this “prime-pull” strategy can recruit T cells into the genital tract, it does not lead to their local amplification. In contrast, intravaginal boosting with HPV PsV can induce substantial local proliferation of antigen-specific tissue-resident CD8+ T cells in response to local antigen production in the transduced keratinocytes (38).
Based on the findings reported in this study, we believe that intravaginal delivery of HPV PsV expressing secreted versions of multiple HSV-2 genes is an attractive strategy to pursue in developing an HSV-2 vaccine for women. While this study has concentrated on a prophylactic vaccine, the ability to preferentially induce intraepithelial CD8+ T cells might also be useful for a therapeutic HSV-2 vaccine. This possibility could be evaluated in the guinea pig model of genital HSV-2 infection, which, unlike the mouse model, results in spontaneous recurrent vesicular genital lesions.
ACKNOWLEDGMENTS
This work was supported by the intramural research programs of the National Institute of Allergy and Infectious Diseases and the National Cancer Institute, Center for Cancer Research, NIH.
REFERENCES
- 1.Looker KJ, Garnett GP, Schmid GP. 2008. An estimate of the global prevalence and incidence of herpes simplex virus type 2 infection. Bull World Health Organ 86:805A–812A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bradley H, Markowitz LE, Gibson T, McQuillan GM. 2014. Seroprevalence of herpes simplex virus types 1 and 2—United States, 1999–2010. J Infect Dis 209:325–333. doi: 10.1093/infdis/jit458. [DOI] [PubMed] [Google Scholar]
- 3.Koelle DM, Corey L. 2008. Herpes simplex: insights on pathogenesis and possible vaccines. Annu Rev Med 59:381–395. doi: 10.1146/annurev.med.59.061606.095540. [DOI] [PubMed] [Google Scholar]
- 4.Freeman EE, Weiss HA, Glynn JR, Cross PL, Whitworth JA, Hayes RJ. 2006. Herpes simplex virus 2 infection increases HIV acquisition in men and women: systematic review and meta-analysis of longitudinal studies. AIDS 20:73–83. doi: 10.1097/01.aids.0000198081.09337.a7. [DOI] [PubMed] [Google Scholar]
- 5.Schiffer JT, Wald A, Selke S, Corey L, Magaret A. 2011. The kinetics of mucosal herpes simplex virus-2 infection in humans: evidence for rapid viral-host interactions. J Infect Dis 204:554–561. doi: 10.1093/infdis/jir314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koelle DM, Benedetti J, Langenberg A, Corey L. 1992. Asymptomatic reactivation of herpes simplex virus in women after the first episode of genital herpes. Ann Intern Med 116:433–437. doi: 10.7326/0003-4819-116-6-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnston C, Koelle DM, Wald A. 2011. HSV-2: in pursuit of a vaccine. J Clin Invest 121:4600–4609. doi: 10.1172/JCI57148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang X, Chentoufi AA, Dasgupta G, Nesburn AB, Wu M, Zhu X, Carpenter D, Wechsler SL, You S, BenMohamed L. 2009. A genital tract peptide epitope vaccine targeting TLR-2 efficiently induces local and systemic CD8+ T cells and protects against herpes simplex virus type 2 challenge. Mucosal Immunol 2:129–143. doi: 10.1038/mi.2008.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wizel B, Persson J, Thorn K, Nagy E, Harandi AM. 2012. Nasal and skin delivery of IC31-adjuvanted recombinant HSV-2 gD protein confers protection against genital herpes. Vaccine 30:4361–4368. doi: 10.1016/j.vaccine.2012.02.019. [DOI] [PubMed] [Google Scholar]
- 10.Veselenak RL, Shlapobersky M, Pyles RB, Wei Q, Sullivan SM, Bourne N. 2012. A Vaxfectin-adjuvanted HSV-2 plasmid DNA vaccine is effective for prophylactic and therapeutic use in the guinea pig model of genital herpes. Vaccine 30:7046–7051. doi: 10.1016/j.vaccine.2012.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Skoberne M, Cardin R, Lee A, Kazimirova A, Zielinski V, Garvie D, Lundberg A, Larson S, Bravo FJ, Bernstein DI, Flechtner JB, Long D. 2013. An adjuvanted herpes simplex virus 2 subunit vaccine elicits a T cell response in mice and is an effective therapeutic vaccine in guinea pigs. J Virol 87:3930–3942. doi: 10.1128/JVI.02745-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shlapobersky M, Marshak JO, Dong L, Huang ML, Wei Q, Chu A, Rolland A, Sullivan S, Koelle DM. 2012. Vaxfectin-adjuvanted plasmid DNA vaccine improves protection and immunogenicity in a murine model of genital herpes infection. J Gen Virol 93:1305–1315. doi: 10.1099/vir.0.040055-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Orr MT, Orgun NN, Wilson CB, Way SS. 2007. Recombinant Listeria monocytogenes expressing a single immune-dominant peptide confers protective immunity to herpes simplex virus-1 infection. J Immunol 178:4731–4735. doi: 10.4049/jimmunol.178.8.4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morrison LA, Da Costa XJ, Knipe DM. 1998. Influence of mucosal and parenteral immunization with a replication-defective mutant of HSV-2 on immune responses and protection from genital challenge. Virology 243:178–187. doi: 10.1006/viro.1998.9047. [DOI] [PubMed] [Google Scholar]
- 15.Meignier B, Jourdier TM, Norrild B, Pereira L, Roizman B. 1987. Immunization of experimental animals with reconstituted glycoprotein mixtures of herpes simplex virus 1 and 2: protection against challenge with virulent virus. J Infect Dis 155:921–930. doi: 10.1093/infdis/155.5.921. [DOI] [PubMed] [Google Scholar]
- 16.Manickan E, Francotte M, Kuklin N, Dewerchin M, Molitor C, Gheysen D, Slaoui M, Rouse BT. 1995. Vaccination with recombinant vaccinia viruses expressing ICP27 induces protective immunity against herpes simplex virus through CD4+ Th1+ T cells. J Virol 69:4711–4716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoshino Y, Pesnicak L, Dowdell KC, Lacayo J, Dudek T, Knipe DM, Straus SE, Cohen JI. 2008. Comparison of immunogenicity and protective efficacy of genital herpes vaccine candidates herpes simplex virus 2 dl5-29 and dl5-29-41L in mice and guinea pigs. Vaccine 26:4034–4040. doi: 10.1016/j.vaccine.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gallichan WS, Rosenthal KL. 1995. Specific secretory immune responses in the female genital tract following intranasal immunization with a recombinant adenovirus expressing glycoprotein B of herpes simplex virus. Vaccine 13:1589–1595. doi: 10.1016/0264-410X(95)00100-F. [DOI] [PubMed] [Google Scholar]
- 19.Dutton JL, Li B, Woo WP, Marshak JO, Xu Y, Huang ML, Dong L, Frazer IH, Koelle DM. 2013. A novel DNA vaccine technology conveying protection against a lethal herpes simplex viral challenge in mice. PLoS One 8:e76407. doi: 10.1371/journal.pone.0076407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Da Costa X, Kramer MF, Zhu J, Brockman MA, Knipe DM. 2000. Construction, phenotypic analysis, and immunogenicity of a UL5/UL29 double deletion mutant of herpes simplex virus 2. J Virol 74:7963–7971. doi: 10.1128/JVI.74.17.7963-7971.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Belshe RB, Leone PA, Bernstein DI, Wald A, Levin MJ, Stapleton JT, Gorfinkel I, Morrow RL, Ewell MG, Stokes-Riner A, Dubin G, Heineman TC, Schulte JM, Deal CD, Herpevac Trial for Women . 2012. Efficacy results of a trial of a herpes simplex vaccine. N Engl J Med 366:34–43. doi: 10.1056/NEJMoa1103151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Awasthi S, Belshe RB, Friedman HM. 2014. Better neutralization of herpes simplex virus type 1 (HSV-1) than HSV-2 by antibody from recipients of GlaxoSmithKline HSV-2 glycoprotein D2 subunit vaccine. J Infect Dis 210:571–575. doi: 10.1093/infdis/jiu177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ouwendijk WJ, Laing KJ, Verjans GM, Koelle DM. 2013. T-cell immunity to human alphaherpesviruses. Curr Opin Virol 3:452–460. doi: 10.1016/j.coviro.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simmons A, Tscharke DC. 1992. Anti-CD8 impairs clearance of herpes simplex virus from the nervous system: implications for the fate of virally infected neurons. J Exp Med 175:1337–1344. doi: 10.1084/jem.175.5.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu T, Khanna KM, Chen X, Fink DJ, Hendricks RL. 2000. CD8+ T cells can block herpes simplex virus type 1 (HSV-1) reactivation from latency in sensory neurons. J Exp Med 191:1459–1466. doi: 10.1084/jem.191.9.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iijima N, Linehan MM, Zamora M, Butkus D, Dunn R, Kehry MR, Laufer TM, Iwasaki A. 2008. Dendritic cells and B cells maximize mucosal Th1 memory response to herpes simplex virus. J Exp Med 205:3041–3052. doi: 10.1084/jem.20082039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Svensson A, Tunback P, Nordstrom I, Shestakov A, Padyukov L, Eriksson K. 2012. STAT4 regulates antiviral gamma interferon responses and recurrent disease during herpes simplex virus 2 infection. J Virol 86:9409–9415. doi: 10.1128/JVI.00947-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Milligan GN, Bernstein DI, Bourne N. 1998. T lymphocytes are required for protection of the vaginal mucosae and sensory ganglia of immune mice against reinfection with herpes simplex virus type 2. J Immunol 160:6093–6100. [PubMed] [Google Scholar]
- 29.Gebhardt T, Wakim LM, Eidsmo L, Reading PC, Heath WR, Carbone FR. 2009. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat Immunol 10:524–530. doi: 10.1038/ni.1718. [DOI] [PubMed] [Google Scholar]
- 30.Mackay LK, Stock AT, Ma JZ, Jones CM, Kent SJ, Mueller SN, Heath WR, Carbone FR, Gebhardt T. 2012. Long-lived epithelial immunity by tissue-resident memory T (TRM) cells in the absence of persisting local antigen presentation. Proc Natl Acad Sci U S A 109:7037–7042. doi: 10.1073/pnas.1202288109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shin H, Iwasaki A. 2012. A vaccine strategy that protects against genital herpes by establishing local memory T cells. Nature 491:463–467. doi: 10.1038/nature11522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu J, Peng T, Johnston C, Phasouk K, Kask AS, Klock A, Jin L, Diem K, Koelle DM, Wald A, Robins H, Corey L. 2013. Immune surveillance by CD8αα+ skin-resident T cells in human herpes virus infection. Nature 497:494–497. doi: 10.1038/nature12110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhu J, Koelle DM, Cao J, Vazquez J, Huang ML, Hladik F, Wald A, Corey L. 2007. Virus-specific CD8+ T cells accumulate near sensory nerve endings in genital skin during subclinical HSV-2 reactivation. J Exp Med 204:595–603. doi: 10.1084/jem.20061792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schiffer JT, Swan D, Al Sallaq R, Magaret A, Johnston C, Mark KE, Selke S, Ocbamichael N, Kuntz S, Zhu J, Robinson B, Huang ML, Jerome KR, Wald A, Corey L. 2013. Rapid localized spread and immunologic containment define herpes simplex virus-2 reactivation in the human genital tract. eLife 2:e00288. doi: 10.7554/eLife.00288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gillgrass AE, Tang VA, Towarnicki KM, Rosenthal KL, Kaushic C. 2005. Protection against genital herpes infection in mice immunized under different hormonal conditions correlates with induction of vagina-associated lymphoid tissue. J Virol 79:3117–3126. doi: 10.1128/JVI.79.5.3117-3126.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McDermott MR, Smiley JR, Leslie P, Brais J, Rudzroga HE, Bienenstock J. 1984. Immunity in the female genital tract after intravaginal vaccination of mice with an attenuated strain of herpes simplex virus type 2. J Virol 51:747–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roberts JN, Buck CB, Thompson CD, Kines R, Bernardo M, Choyke PL, Lowy DR, Schiller JT. 2007. Genital transmission of HPV in a mouse model is potentiated by nonoxynol-9 and inhibited by carrageenan. Nat Med 13:857–861. doi: 10.1038/nm1598. [DOI] [PubMed] [Google Scholar]
- 38.Çuburu N, Graham BS, Buck CB, Kines RC, Pang YY, Day PM, Lowy DR, Schiller JT. 2012. Intravaginal immunization with HPV vectors induces tissue-resident CD8+ T cell responses. J Clin Invest 122:4606–4620. doi: 10.1172/JCI63287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gordon SN, Kines RC, Kutsyna G, Ma ZM, Hryniewicz A, Roberts JN, Fenizia C, Hidajat R, Brocca-Cofano E, Çuburu N, Buck CB, Bernardo ML, Robert-Guroff M, Miller CJ, Graham BS, Lowy DR, Schiller JT, Franchini G. 2012. Targeting the vaginal mucosa with human papillomavirus pseudovirion vaccines delivering simian immunodeficiency virus DNA. J Immunol 188:714–723. doi: 10.4049/jimmunol.1101404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Graham BS, Kines RC, Corbett KS, Nicewonger J, Johnson TR, Chen M, LaVigne D, Roberts JN, Çuburu N, Schiller JT, Buck CB. 2010. Mucosal delivery of human papillomavirus pseudovirus-encapsidated plasmids improves the potency of DNA vaccination. Mucosal Immunol 3:475–486. doi: 10.1038/mi.2010.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bender FC, Whitbeck JC, Lou H, Cohen GH, Eisenberg RJ. 2005. Herpes simplex virus glycoprotein B binds to cell surfaces independently of heparan sulfate and blocks virus entry. J Virol 79:11588–11597. doi: 10.1128/JVI.79.18.11588-11597.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Buck CB, Pastrana DV, Lowy DR, Schiller JT. 2004. Efficient intracellular assembly of papillomaviral vectors. J Virol 78:751–757. doi: 10.1128/JVI.78.2.751-757.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang K, Kappel JD, Canders C, Davila WF, Sayre D, Chavez M, Pesnicak L, Cohen JI. 2012. A herpes simplex virus 2 glycoprotein D mutant generated by bacterial artificial chromosome mutagenesis is severely impaired for infecting neuronal cells and infects only Vero cells expressing exogenous HVEM. J Virol 86:12891–12902. doi: 10.1128/JVI.01055-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pastrana DV, Buck CB, Pang YY, Thompson CD, Castle PE, FitzGerald PC, Kruger Kjaer S, Lowy DR, Schiller JT. 2004. Reactivity of human sera in a sensitive, high-throughput pseudovirus-based papillomavirus neutralization assay for HPV16 and HPV18. Virology 321:205–216. doi: 10.1016/j.virol.2003.12.027. [DOI] [PubMed] [Google Scholar]
- 45.Trus BL, Roden RB, Greenstone HL, Vrhel M, Schiller JT, Booy FP. 1997. Novel structural features of bovine papillomavirus capsid revealed by a three-dimensional reconstruction to 9 Å resolution. Nat Struct Biol 4:413–420. doi: 10.1038/nsb0597-413. [DOI] [PubMed] [Google Scholar]
- 46.Murata T, Goshima F, Takakuwa H, Nishiyama Y. 2002. Excretion of herpes simplex virus type 2 glycoprotein D into the culture medium. J Gen Virol 83:2791–2795. [DOI] [PubMed] [Google Scholar]
- 47.Hanke T, Graham FL, Rosenthal KL, Johnson DC. 1991. Identification of an immunodominant cytotoxic T-lymphocyte recognition site in glycoprotein B of herpes simplex virus by using recombinant adenovirus vectors and synthetic peptides. J Virol 65:1177–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wallace ME, Keating R, Heath WR, Carbone FR. 1999. The cytotoxic T-cell response to herpes simplex virus type 1 infection of C57BL/6 mice is almost entirely directed against a single immunodominant determinant. J Virol 73:7619–7626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Porgador A, Staats HF, Faiola B, Gilboa E, Palker TJ. 1997. Intranasal immunization with CTL epitope peptides from HIV-1 or ovalbumin and the mucosal adjuvant cholera toxin induces peptide-specific CTLs and protection against tumor development in vivo. J Immunol 158:834–841. [PubMed] [Google Scholar]
- 50.Carbone FR, Mackay LK, Heath WR, Gebhardt T. 2013. Distinct resident and recirculating memory T cell subsets in non-lymphoid tissues. Curr Opin Immunol 25:329–333. doi: 10.1016/j.coi.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 51.Mackay LK, Rahimpour A, Ma JZ, Collins N, Stock AT, Hafon ML, Vega-Ramos J, Lauzurica P, Mueller SN, Stefanovic T, Tscharke DC, Heath WR, Inouye M, Carbone FR, Gebhardt T. 2013. The developmental pathway for CD103+CD8+ tissue-resident memory T cells of skin. Nat Immunol 14:1294–1301. doi: 10.1038/ni.2744. [DOI] [PubMed] [Google Scholar]
- 52.Seder RA, Darrah PA, Roederer M. 2008. T-cell quality in memory and protection: implications for vaccine design. Nat Rev Immunol 8:247–258. doi: 10.1038/nri2274. [DOI] [PubMed] [Google Scholar]
- 53.Hoshino Y, Pesnicak L, Dowdell KC, Burbelo PD, Knipe DM, Straus SE, Cohen JI. 2009. Protection from herpes simplex virus (HSV)-2 infection with replication-defective HSV-2 or glycoprotein D2 vaccines in HSV-1-seropositive and HSV-1-seronegative guinea pigs. J Infect Dis 200:1088–1095. doi: 10.1086/605645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Henning P, Gustafsson T, Flach CF, Hua YJ, Strombeck A, Holmgren J, Lindholm L, Yrlid U. 2011. The subcellular location of antigen expressed by adenoviral vectors modifies adaptive immunity but not dependency on cross-presenting dendritic cells. Eur J Immunol 41:2185–2196. doi: 10.1002/eji.201041009. [DOI] [PubMed] [Google Scholar]
- 55.Higgins TJ, Herold KM, Arnold RL, McElhiney SP, Shroff KE, Pachuk CJ. 2000. Plasmid DNA-expressed secreted and nonsecreted forms of herpes simplex virus glycoprotein D2 induce different types of immune responses. J Infect Dis 182:1311–1320. doi: 10.1086/315879. [DOI] [PubMed] [Google Scholar]
- 56.Hu H, Eller MA, Zafar S, Zhou Y, Gu M, Wei Z, Currier JR, Marovich MA, Kibuuka HN, Bailer RT, Koup RA, Robb ML, Michael NL, Kim JH, Ratto-Kim S. 2014. Preferential infection of human Ad5-specific CD4 T cells by HIV in Ad5 naturally exposed and recombinant Ad5-HIV vaccinated individuals. Proc Natl Acad Sci U S A 111:13439–13444. doi: 10.1073/pnas.1400446111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McKinnon LR, Nyanga B, Chege D, Izulla P, Kimani M, Huibner S, Gelmon L, Block KE, Cicala C, Anzala AO, Arthos J, Kimani J, Kaul R. 2011. Characterization of a human cervical CD4+ T cell subset coexpressing multiple markers of HIV susceptibility. J Immunol 187:6032–6042. doi: 10.4049/jimmunol.1101836. [DOI] [PubMed] [Google Scholar]
- 58.Cicala C, Martinelli E, McNally JP, Goode DJ, Gopaul R, Hiatt J, Jelicic K, Kottilil S, Macleod K, O'Shea A, Patel N, Van Ryk D, Wei D, Pascuccio M, Yi L, McKinnon L, Izulla P, Kimani J, Kaul R, Fauci AS, Arthos J. 2009. The integrin α4β7 forms a complex with cell-surface CD4 and defines a T-cell subset that is highly susceptible to infection by HIV-1. Proc Natl Acad Sci U S A 106:20877–20882. doi: 10.1073/pnas.0911796106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Marshak JO, Dong L, Koelle DM. 2014. The murine intravaginal HSV-2 challenge model for investigation of DNA vaccines. Methods Mol Biol 1144:305–327. doi: 10.1007/978-1-4939-0428-0_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dudley KL, Bourne N, Milligan GN. 2000. Immune protection against HSV-2 in B-cell-deficient mice. Virology 270:454–463. doi: 10.1006/viro.2000.0298. [DOI] [PubMed] [Google Scholar]
- 61.Openshaw H, Asher LV, Wohlenberg C, Sekizawa T, Notkins AL. 1979. Acute and latent infection of sensory ganglia with herpes simplex virus: immune control and virus reactivation. J Gen Virol 44:205–215. doi: 10.1099/0022-1317-44-1-205. [DOI] [PubMed] [Google Scholar]
- 62.Ariotti S, Beltman JB, Chodaczek G, Hoekstra ME, van Beek AE, Gomez-Eerland R, Ritsma L, van Rheenen J, Marée AF, Zal T, de Boer RJ, Haanen JB, Schumacher TN. 2012. Tissue-resident memory CD8+ T cells continuously patrol skin epithelia to quickly recognize local antigen. Proc Natl Acad Sci U S A 109:19739–19744. doi: 10.1073/pnas.1208927109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bright H, Perez DL, Christy C, Cockle P, Eyles JE, Hammond D, Khodai T, Lang S, West K, Loudon PT. 2012. The efficacy of HSV-2 vaccines based on gD and gB is enhanced by the addition of ICP27. Vaccine 30:7529–7535. doi: 10.1016/j.vaccine.2012.10.046. [DOI] [PubMed] [Google Scholar]
- 64.Haynes JR, Arrington J, Dong L, Braun RP, Payne LG. 2006. Potent protective cellular immune responses generated by a DNA vaccine encoding HSV-2 ICP27 and the E. coli heat labile enterotoxin. Vaccine 24:5016–5026. doi: 10.1016/j.vaccine.2006.03.046. [DOI] [PubMed] [Google Scholar]
- 65.Milligan GN, Bernstein DI. 1997. Interferon-gamma enhances resolution of herpes simplex virus type 2 infection of the murine genital tract. Virology 229:259–268. doi: 10.1006/viro.1997.8441. [DOI] [PubMed] [Google Scholar]
- 66.Coen DM, Kosz-Vnenchak M, Jacobson JG, Leib DA, Bogard CL, Schaffer PA, Tyler KL, Knipe DM. 1989. Thymidine kinase-negative herpes simplex virus mutants establish latency in mouse trigeminal ganglia but do not reactivate. Proc Natl Acad Sci U S A 86:4736–4740. doi: 10.1073/pnas.86.12.4736. [DOI] [PMC free article] [PubMed] [Google Scholar]