ABSTRACT
Replication-competent adenoviral (RC-Ad) vectors generate exceptionally strong gene-based vaccine responses by amplifying the antigen transgenes they carry. While they are potent, they also risk causing adenovirus infections. More common replication-defective Ad (RD-Ad) vectors with deletions of E1 avoid this risk but do not replicate their transgene and generate markedly weaker vaccine responses. To amplify vaccine transgenes while avoiding production of infectious progeny viruses, we engineered “single-cycle” adenovirus (SC-Ad) vectors by deleting the gene for IIIa capsid cement protein of lower-seroprevalence adenovirus serotype 6. In mouse, human, hamster, and macaque cells, SC-Ad6 still replicated its genome but prevented genome packaging and virion maturation. When used for mucosal intranasal immunization of Syrian hamsters, both SC-Ad and RC-Ad expressed transgenes at levels hundreds of times higher than that of RD-Ad. Surprisingly, SC-Ad, but not RC-Ad, generated higher levels of transgene-specific antibody than RD-Ad, which notably climbed in serum and vaginal wash samples over 12 weeks after single mucosal immunization. When RD-Ad and SC-Ad were tested by single sublingual immunization in rhesus macaques, SC-Ad generated higher gamma interferon (IFN-γ) responses and higher transgene-specific serum antibody levels. These data suggest that SC-Ad vectors may have utility as mucosal vaccines.
IMPORTANCE This work illustrates the utility of our recently developed single-cycle adenovirus (SC-Ad6) vector as a new vaccine platform. Replication-defective (RD-Ad6) vectors produce low levels of transgene protein, which leads to minimal antibody responses in vivo. This study shows that replicating SC-Ad6 produces higher levels of luciferase and induces higher levels of green fluorescent protein (GFP)-specific antibodies than RD in a permissive Syrian hamster model. Surprisingly, although a replication-competent (RC-Ad6) vector produces more luciferase than SC-Ad6, it does not elicit comparable levels of anti-GFP antibodies in permissive hamsters. When tested in the larger rhesus macaque model, SC-Ad6 induces higher transgene-specific antibody and T cell responses. Together, these data suggest that SC-Ad6 could be a more effective platform for developing vaccines against more relevant antigens. This could be especially beneficial for developing vaccines for pathogens for which traditional replication-defective adenovirus vectors have not been effective.
INTRODUCTION
Adenoviruses (Ads) are robust vectors for gene-based vaccination (1–13; for reviews, see references 14 and 15). Current adenovirus vectors fall broadly into two categories: replication defective and replication competent. Replication-competent Ad (RC-Ad) vectors can undergo a normal viral life cycle, including genome replication and progeny virion production. Because they replicate not only their genome but also any transgene they carry, they can mediate robust transgene expression and vaccine responses. While this provides potency, it also potentially exposes health care workers and vaccine recipients to adenovirus infection and pathogenesis.
Replication-defective Ads (RD-Ads) were engineered by deleting pivotal early E1A and E1B genes in the viruses (14, 15). This disables early viral gene transcription, genome replication, and the production of infectious progeny viruses. While these vectors are safer, they unfortunately produce significantly less transgene protein and lower-level immune responses because transgenes are not amplified by genome replication.
To amplify transgenes but avoid infectious progeny virus production, we recently developed “single-cycle” Ad (SC-Ad) vectors (16). In these vectors, a pivotal viral gene is deleted that ablates functional virion production but maintains the ability of the virus to replicate its DNA. In the first application, we deleted the minor IIIa capsid protein in the context of low-seroprevalence adenovirus serotype 6 viruses that still carry E1 genes (SC-Ad6). We demonstrated that SC-Ad6 replicates its genome and transgenes as well as RC-Ad and that both expressed their transgene proteins at 100 times the level of RD-Ad in human cells in vitro (16). While SC-Ad was as potent as RC-Ad in vitro, when they were compared in vivo in mice, the improvement over RD-Ad was found to be attenuated relative to the amplification observed in human cells.
Mice are not a permissive host for adenovirus infection (17). Given this, in this study, we tested the transduction and vaccine potential of SC-Ad6 in more permissive Syrian hamsters and rhesus macaques. We compared each of the Ad6 vectors for the ability to replicate their genomes and amplify transgene expression in vitro and in vivo. We then tested their ability to drive gene-based immune responses in vivo after single mucosal vaccination in permissive Syrian hamsters and rhesus macaques.
MATERIALS AND METHODS
Cell culture.
Syrian hamster kidney (HaK) cells were purchased from the American Type Culture Collection (ATCC, Manassas, VA). Rhesus FRhK4 cells were generously provided by Yasuhiro Ikeda, Mayo Clinic. 293-IIIa cells expressing the Ad6 IIIa protein were generated as described in reference 16. All cells were maintained in Dulbecco's modified Eagle medium supplemented with 10% fetal bovine serum (FBS; HyClone, Rockford, IL) and penicillin-streptomycin at 100 U/ml (Invitrogen).
Adenoviruses.
RD-Ad6-GL, SC-Ad6-GL, and RC-Ad6-GL virus plasmids were generated as described in reference 16 and shown in Fig. 1. Each virus has its E3 gene cassette deleted and a cytomegalovirus (CMV) green fluorescent protein-luciferase (GFP-Luc) expression cassette inserted in between fiber and E4 (Fig. 1). Viruses were rescued in 293 cells or Ad6 IIIa-expressing 293-IIIa cells and were purified by double CsCl banding. Virus was desalted in 10% sucrose/phosphate-buffered saline (PBS). Virus particle (VP) concentration was determined by optical density at 260 nm (OD260). The VP/infectious unit (IU) ratio was determined by 50% tissue culture infective dose (TCID50). Ratios were as follows: RD-Ad6-GL, 28 VPs/IU; SC-Ad6-GL, 64 VPs/IU; and RC-Ad6-GL, 22 VPs/IU.
FIG 1.
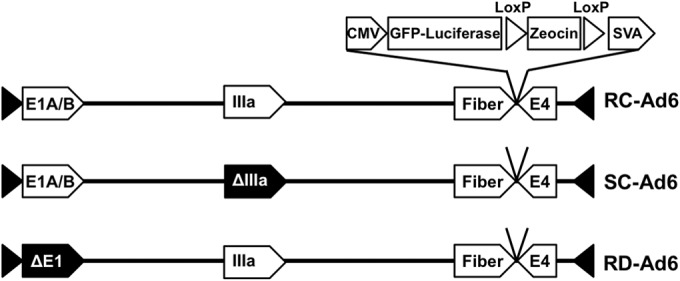
Schematic of Ad genomes expressing GFP-luciferase fusion protein. CMV, cytomegalovirus; SVA, simian virus 40 polyadenylation sequence.
In vitro vector genome quantification.
A total of 3 × 105 cells were plated in 6-well plates and infected at 100 VPs/cell. Total DNA was isolated at 2, 24, 48, and 72 h after infection using the DNeasy blood and tissue kit according to the manufacturer's protocol (Qiagen) with an RNase A digestion. Vector genomes were quantified using quantitative real-time PCR (qPCR) with primers against adenovirus hexon.
qPCR.
Concentrations of DNA samples were determined by OD260 and diluted to 20 ng/μl. Real-time PCR was performed using the Applied Biosystems Prism 7900HT sequence detection system with SDS 2.3 software. Each well contained 10 μl of Sybr green (Applied Biosystems, Warrington, United Kingdom), 3.8 μl of H2O, 0.6 μl of 10 μM F primer, 0.6 μl of 10 μM R primer, and 5 μl of sample (i.e., 20 ng of DNA/well).
In vitro luciferase assay.
To quantify luciferase expression, 1 × 103 cells were plated in black-walled 96-well plates and infected at 100 VPs/cell. At various time points, Bright-Glo luciferase reagent (Promega, Madison, WI) was added at a 1:1 ratio and luciferase activity was measured using the Beckman Coulter DTX 880 multimode detector system.
Syrian hamsters.
Female Syrian hamsters were purchased from Harlan Sprague-Dawley (Indianapolis, IN). They were housed in the Mayo Clinic Animal Facility under Association for Assessment and Accreditation of Laboratory Animal Care (AALAC) guidelines with animal use protocols approved by the Mayo Clinic Animal Use and Care Committee. All animal experiments were carried out according to the provisions of the Animal Welfare Act, PHS Animal Welfare Policy, the principles of the National Research Council's Guide for the Care and Use of Laboratory Animals (18), and the policies and procedures of the Mayo Clinic. Four groups of 5 hamsters were immunized intranasally with phosphate-buffered saline (PBS) or 1 × 1011 RD-Ad6, SC-Ad6, or RC-Ad6 VPs in 100 μl of PBS.
In vivo bioluminescence imaging.
At various time points, hamsters were anesthetized with 150 mg/kg of ketamine and 10 mg/kg of xylazine and injected intranasally with 100 μl of d-luciferin (20 mg/ml; Molecular Imaging Products, Bend, OR). Animals were imaged on the Lumazone imaging system (Photometrics, Roper Scientific, Tucson, AZ) for 10 min with 1 × 1 binning using no filters or photomultiplication. Lumazone imaging software was used to determine total light intensity per hamster (photons/second) for data analysis.
Collection of samples.
At specific time points, hamsters were anesthetized with isoflurane. Blood was collected in BD Microtainer tubes with serum separators (Becton Dickinson and Company) from their submandibular veins using 25-gauge syringes. Samples were incubated for 1 h at room temperature and centrifuged for 2 min at 13,000 × g, and serum was transferred to microcentrifuge tubes. Vaginal wash samples were obtained by applying 100 μl of sterile PBS into the vagina and aspirating the released fluid, repeating once. Samples were stored at −20°C until assayed.
Enzyme-linked immunosorbent assay (ELISA).
Immulon 4 HBX plates (Thermo, Milford, MA) were coated with 100 ng/well of green fluorescent protein (GFP) in 1× PBS (Abcam, Cambridge, MA) at room temperature or with UV-inactivated Ad6 in 1× PBS at 4°C overnight. Wells were blocked with 200 μl of blocking buffer (0.25% bovine serum albumin [BSA], 0.05% Tween 20, 1× PBS) at room temperature for 1 h. Wells were washed once with sterile water, and hamster serum (1:200), macaque plasma (1:300), or hamster vaginal wash (1:5) samples were diluted in blocking buffer in quadruplicate. Samples were added to plates and incubated at room temperature for 3 h. Wells were washed 4 times with sterile water. Rabbit anti-hamster IgG, IgM, and IgA-horseradish peroxidase (HRP; Brookwood Biomedical, Birmingham, AL) was diluted 1:10,000, or anti-macaque IgG-HRP (Non-Human Primate Reagent Resource) was diluted 1:5,000. One hundred microliters was added to each well and incubated at room temperature for 2 h. Wells were washed 4 times with sterile water. One hundred microliters of 1-Step Ultra TMB-ELISA (Thermo Fisher Scientific Inc., Rockford, IL) was added to each well. When color developed, 50 μl of 2 N H2SO4 was added to each well, and A450 was measured using the Beckman Coulter DTX 880 multimode detector system.
Rhesus macaques.
All animal experiments were approved by the Institutional Animal Care and Use Committee at The University of Texas M. D. Anderson Cancer Center and were carried out according to the provisions of the Animal Welfare Act, PHS Animal Welfare Policy, the principles of the National Research Council's Guide for the Care and Use of Laboratory Animals (18), and the policies and procedures of The University of Texas M. D. Anderson Cancer Center. Nine adult female rhesus macaques (Macaca mulatta) of Indian origin were maintained in the specific-pathogen-free breeding colony at the Michael Keeling Center for Comparative Medicine and Research of The University of Texas M. D. Anderson Cancer Center, Bastrop, TX. The chamber size for the animals was 44 (width) by 88 (height) by 160 (depth) ft. All monkeys were given water ad libitum and were fed a commercial monkey diet (Harlan). Additional enrichment was provided in the form of manipulanda, visual stimulation or auditory stimulation and combinations thereof. Animals were monitored daily, including weekends and holidays. Anesthetics and analgesics were used to minimize any discomfort, distress, pain, and injury the animal might have experienced. As an endpoint, animals were euthanized with ketamine (11 mg/kg) followed by Beuthanasia (1 ml/10 lb). The animals were anesthetized during procedures to minimize discomfort.
Sublingual rhesus macaque immunization.
Rhesus macaques were anesthetized by intravenous (i.v.) injection of ketamine (5 to 10 mg/kg) and immunized by the sublingual route. Sublingual inoculum is achieved with the animal in dorsal recumbency and the head down on back. The inoculum is slowly delivered under the tongue using an appropriate-size slip tip syringe. Visualization is achieved using a mouth gag and light source. Following inoculation, animals were observed twice a day. Groups of 3 female macaques were immunized sublingually with 1011 VPs of negative control AAV-gp130, RD-Ad6-GL, or SC-Ad6-GL at week 0.
Collection of rhesus macaque samples.
Samples were collected at each time point before any immunization or procedure. Peripheral venous blood samples were collected in EDTA. Before the separation of peripheral blood mononuclear cells (PBMCs) from the blood samples, plasma was separated and stored immediately at −80°C. PBMCs were prepared from the blood on Ficoll-Hypaque density gradients.
ELISPOT assay for detecting antigen-specific IFN-γ-producing cells.
Freshly prepared PBMCs were used for gamma interferon (IFN-γ) enzyme-linked immunosorbent spot (ELISPOT) assay as described in reference 19. PBMCs were stimulated with either GFP or luciferase proteins or with concanavalin A (ConA; 5 μg/ml) as a positive control. A total of 1 × 105 PBMCs were seeded in duplicate in 96-well polyvinylidene difluoride-backed plates (MAIP S 45; Millipore, Bedford, MA) that had previously been coated with anti-IFN-γ. The cells were incubated with antigens for 36 h at 37°C and were then removed, and the wells were washed prior to incubation with 100 μl of alkaline-phosphatase-conjugated anti-IFN-γ for 2 h at room temperature. Spots representing individual cells secreting IFN-γ were detected using 5-bromo-4-chloro-3-indolylphosphate (BCIP) and nitroblue tetrazolium (NBT). The plates were washed and the spots were counted by Zellnet Consulting (NJ). Responses in terms of IFN-γ spot-forming cells (SFCs) represented for 105 total input PBMCs were determined for individual macaques after subtraction of the background. Ten spots represent the background of the assay, as it is twice the number observed in cells cultured in the medium.
Data analysis.
Graphs were generated and statistical analyses were performed using Prism Graphical software.
RESULTS
Adenovirus 6 (Ad6) vectors with E1 intact replicate their genomes in Syrian hamster cells.
Syrian hamsters have previously been established as a model for investigating the life cycle of species C adenovirus serotype 5 (17). To confirm that species C adenovirus serotype 6 had a similar capability to undergo genome replication in this model, Syrian hamster kidney (HaK) cells were infected at 100 virus particles (VPs) per cell with Ad6 vectors that express a green fluorescent protein-luciferase (GFP-Luc) fusion protein (Fig. 1). At various time points, total DNA was isolated from the cells, followed by quantitative real-time PCR against the hexon gene (Fig. 2A). At 2 h, there were comparable numbers of genome copies of RD-, SC-, and RC-Ad6, indicating comparable infectivities. However, over 3 days the copy number of RD-Ad6 remained the same, while the genome copy numbers of SC- and RC-Ad6 continued to increase over time, peaking, respectively, 550 and 640 times higher than those of RD-Ad (P < 0.0001). Notably, SC-Ad6 copies remained constant from 2 to 3 days, while RC-Ad6 copies continued to increase to a significantly higher level than those of SC-Ad6 (P < 0.05). When transgene expression was measured by luciferase activity, it was found that the SC- and RC-Ad6 vectors expressed the transgene 38 and 73 times higher than RD-Ad6 at peak day 3 (P < 0.001 and P < 0.0001) (Fig. 2B). While SC- and RC-Ad6 produced comparable levels of luciferase at day 1, RC-Ad6 produced significantly more luciferase than SC-Ad6 at days 2 to 4, perhaps due to the additional cycles of infection (P < 0.0001, P < 0.001, and P < 0.05) (Fig. 2B).
FIG 2.
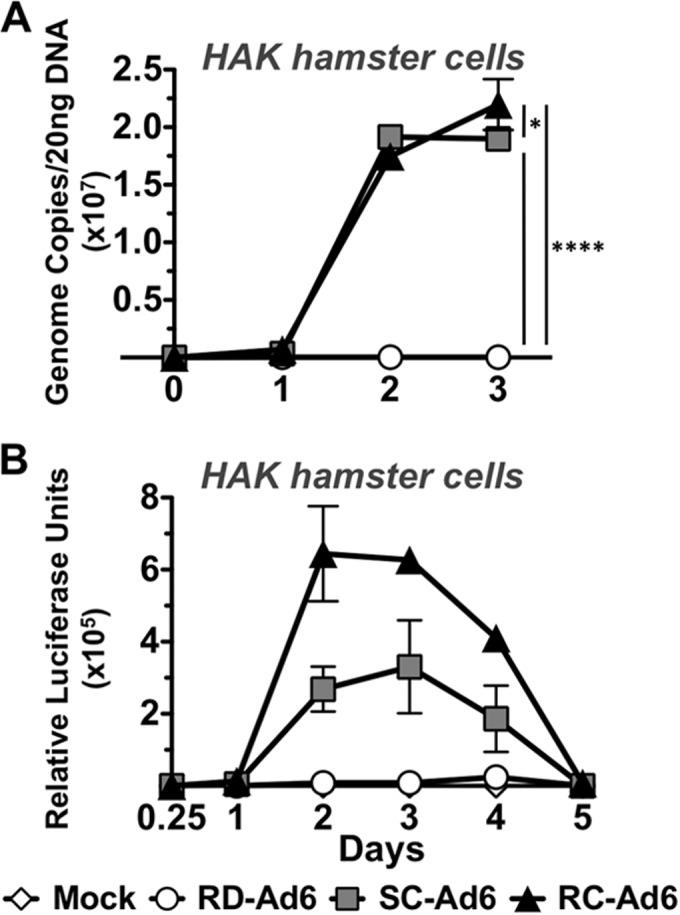
In vitro characterization of Ad vectors in Syrian hamster kidney (HaK) cells. HaK cells were infected with RD-, SC-, or RC-Ad6 at 100 VPs/cell. (A) Total DNA was collected from cell lysates at the indicated time points. Viral DNA was quantified by qPCR with primers amplifying the Ad6 hexon. (B) Luciferase expression was measured using Bright-Glo luciferase reagent at the indicated time points. Luminescence was measured using a Beckman Coulter plate reader. Data were analyzed by two-way analysis of variance (ANOVA) with Bonferroni multiple-comparison posttest. *, P < 0.05; ****, P < 0.0001.
RC- and SC-Ad6 express higher levels of luciferase in Syrian hamsters in vivo than RD-Ad6.
Female Syrian hamsters were vaccinated intranasally with 1 × 1011 VPs of RD-, SC-, or RC-Ad6, and luciferase expression was imaged over time (Fig. 3). Luciferase peaked at 3 days and declined to baseline by day 7, with SC- and RC-Ad6 expression peaking 7 and 12 times higher than RD-Ad6 (Fig. 3B). Representative images are shown for each group at day 3 (Fig. 3A).
FIG 3.
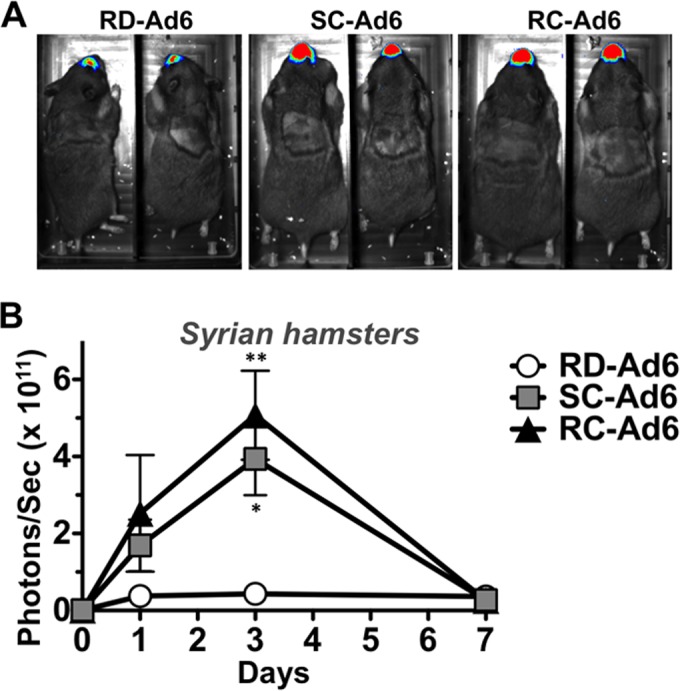
In vivo bioluminescence in Syrian hamsters. Syrian hamsters were immunized once intranasally with PBS or 1 × 1011 VPs of RD-, SC-, or RC-Ad6. At days 1, 3, and 7, luminescence was measured using a Lumazone imaging system. (A) Representative images at day 3 postvaccination. (B) Quantification of luciferase expression. Data were analyzed by two-way ANOVA with Bonferroni multiple-comparison posttest. *, P < 0.05; **, P < 0.01.
SC-Ad6 induces persistently higher levels of anti-GFP antibodies in serum and vaginal washes from Syrian hamsters.
Sera from the hamsters were collected at various times and anti-GFP antibodies were measured by ELISA (Fig. 4A). SC-Ad6 induced significantly higher antibody responses than both RD- and RC-Ad6 at 3, 6, 12, and 24 weeks postvaccination. Interestingly, antibody levels increased from 6 to 12 weeks and remained heightened at 24 weeks after this single mucosal immunization.
FIG 4.
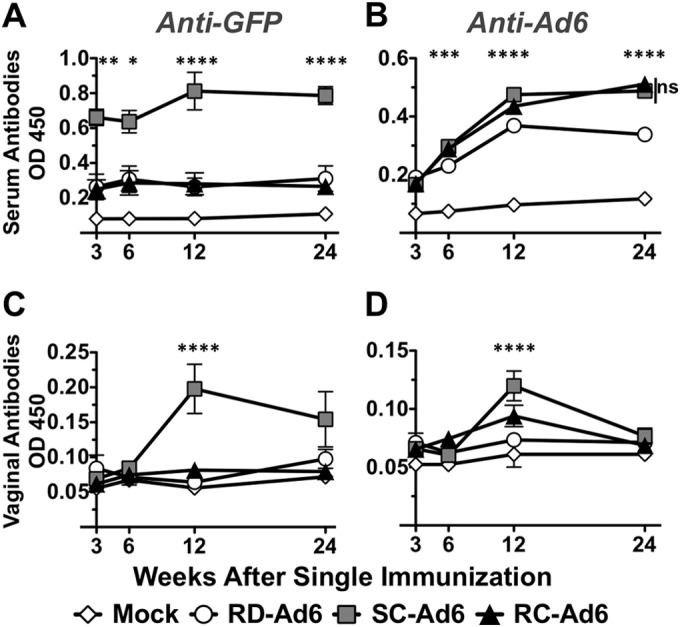
Serum and vaginal wash antibodies in Syrian hamsters following single intranasal immunization. Serum and vaginal wash samples were collected at 3, 6, 12, and 24 weeks post-single immunization. Serum anti-GFP (A) and anti-Ad6 (B) and vaginal wash anti-GFP (C) and anti-Ad6 (D) antibodies were measured by ELISA. Data were analyzed by two-way ANOVA with Bonferroni multiple-comparison posttest. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001.
Anti-GFP ELISAs were also performed on vaginal wash samples from each of the hamsters (Fig. 4C). At 3 and 6 weeks postvaccination, there were no significant levels of anti-GFP antibody in the vaginal washes for any of the vectors. However, at 12 weeks after the single immunization, there were significantly higher levels of antibody in the SC-Ad6-vaccinated animals than in the other groups (P < 0.0001). These antibodies remained elevated for 24 weeks, remaining higher than the levels generated by RD- or RC-Ad (P = 0.056).
Ad6 replicates its genome in rhesus macaque cells.
Rhesus macaques are an important model for vaccine testing for a variety of pathogens. To explore if Ad6 vectors can replicate in macaques, rhesus macaque FRhK4 kidney cells were infected with 100 VPs/cell of each vector and genome copies were measured over 72 h (Fig. 5A). Similar to the case with HaK cells, there were equivalent genome copy numbers of all three vectors at 2 h. Over 3 days, viral genome copy numbers increased to 1,543 and 1,746 times higher for SC-Ad and RC-Ad, respectively, than for RD-Ad6 (P < 0.0001). When transgene expression was measured, luciferase levels peaked at 48 or 72 h, with SC- and RC-Ad6 generating 78 and 179 times higher expression than RD-Ad6 at peak expression (Fig. 5B).
FIG 5.
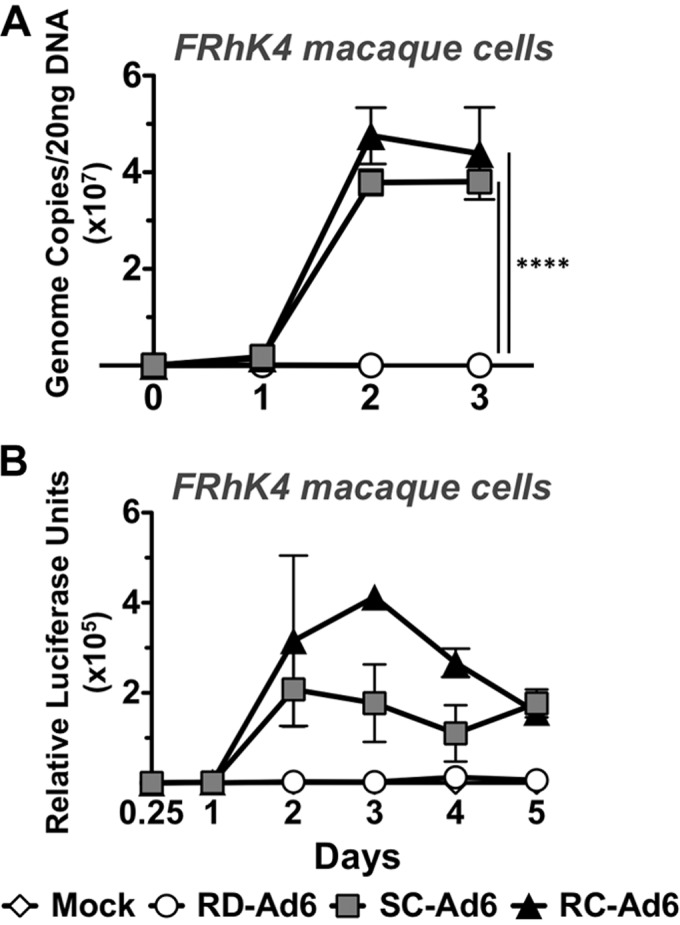
In vitro characterization of Ad vectors in rhesus macaque FRhK4 cells. FRhK4 cells were infected with RD-, SC-, or RC-Ad6 at 100 VPs/cell. (A) Total DNA was collected from cell lysates at indicated time points. Viral DNA was quantified by qPCR with primers amplifying the Ad6 hexon. (B) Luciferase expression was measured using Bright-Glo luciferase reagent at the indicated time points. Luminescence was measured using a Beckman Coulter plate reader. Data were analyzed by two-way ANOVA with Bonferroni multiple-comparison posttest. ****, P < 0.0001.
SC-Ad6 induces higher levels of anti-GFP antibodies than RD-Ad6 in rhesus macaques.
It is relatively difficult to generate systemic immune responses by the sublingual route in nonhuman primates. To compare SC-Ad to traditional RD-Ad, rhesus macaques were immunized a single time by the sublingual mucosal route with 1 × 1011 VPs of each vector or a control. Plasma samples were collected before and after the immunization, anti-GFP and anti-Ad6 antibodies were measured by ELISA, and preimmune antibody levels were subtracted for each animal (Fig. 6A and B). After only one sublingual administration, SC-Ad6 induced higher levels of anti-GFP antibody than both the control and RD-Ad6 at 9 weeks (Fig. 6A). In contrast, there was no significant difference in anti-Ad6 antibody levels between the three groups (Fig. 6B).
FIG 6.
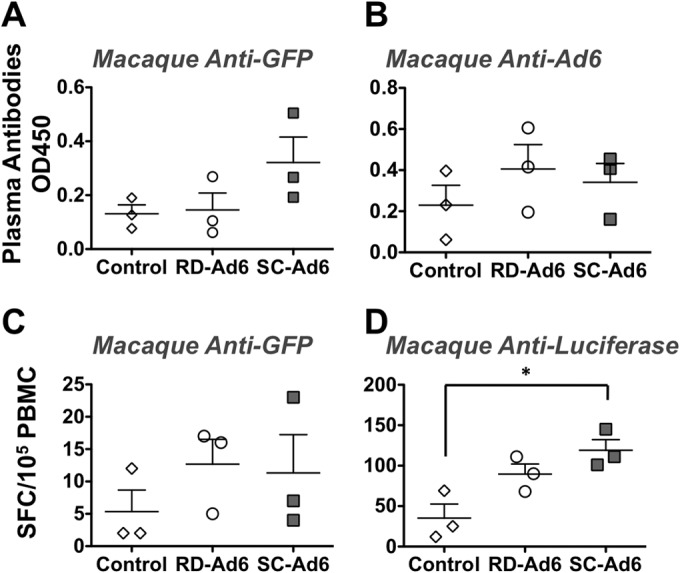
Immune responses in rhesus macaques following a single sublingual immunization. Plasma samples (9 weeks) and PBMCs (7 weeks) were collected postimmunization. Anti-GFP (A) and anti-Ad6 (B) antibodies were quantified by ELISA. Anti-GFP (C) and antiluciferase (D) CD8+ T cells were quantified by ELISPOT assay. Data were analyzed by one-way ANOVA with Bonferroni multiple-comparison posttest. *, P < 0.05.
SC-Ad6 induces higher anti-luciferase T cell responses than RD-Ad6 in rhesus macaques.
PBMCs were collected from the immunized macaques 6 weeks after immunization, and anti-luciferase and anti-GFP T cell responses were measured by ELISPOT assay (Fig. 6C and D). While there were no significant differences in anti-GFP T cell responses between the three groups (Fig. 6C), only SC-Ad6, induced significantly higher anti-luciferase T cell responses than controls (P < 0.05) (Fig. 6D).
DISCUSSION
Human adenoviruses have been potent as gene-based vaccine vectors in mouse trials but have been less effective when scaled up to larger animals and humans (1–13). Notably, most of these studies have used replication-defective Ad5 vectors, where E1 deletion renders the vector unable to amplify the one antigen gene that is delivered per viral genome to the cell. When RD-Ads and RC-Ads have been compared in nonhuman primates, the replicating vectors have generally been found to be more potent at eliciting immune responses (20).
While RC-Ads are more potent, they also put the vaccinated person and the health care worker at a finite risk of frank adenovirus infection. Given this, we engineered single-cycle SC-Ad vectors to allow genome and transgene replication but disabled their ability to produce infectious virions to avoid adenovirus infections (16).
We previously showed that in human cells, SC-Ad6 replicates its genome and transgene but produces empty, immature virus particles (16). This replication of the viral genome concomitantly replicated the transgene that the SC-Ad virus carried. This resulted in increased protein production compared to that of RD-Ad6 in human immortalized A549 and primary human airway epithelial cells, as well as in vivo in mouse livers. In primary human lung cells, the ability of SC-Ad to replicate the transgene translated into the ability to use 33-fold less vector than RD-Ad to achieve the same level of gene expression (16).
While SC-Ad amplified its genome and transgene like RC-Ad, it notably did not generate infectious progeny, highlighting its potential for greater safety relative to fully replication-competent adenovirus vaccines. In particular, an SC-Ad vaccine should not cause overt adenovirus infections in patients or health care workers. Also, this disabled platform should avoid shedding of active infectious vaccine, as has been observed with RC-Ad vaccines (21).
While mouse experiments indicated that SC-Ad was more robust than RD-Ad, mice cannot support a full adenovirus life cycle. Given this fact, in this study, we investigated the vaccine potential of SC-Ad6 in more permissive Syrian hamsters and rhesus macaques. In vitro, in Syrian hamster kidney (HaK) cells and rhesus macaque FRhK4 cells, SC-Ad6 replicated its genome equally to RC-Ad6 and produced significantly more luciferase protein than RD-Ad6. However, under conditions in which not all cells were observably infected in the first round, RC-Ad produced significantly more luciferase than SC-Ad6. This could be attributed to both a slightly higher particle/IU ratio of RC-Ad6 and the fact that RC-Ad6 may be able to undergo limited additional rounds of infection in vitro.
This trend was mirrored in vivo in Syrian hamsters after a single-dose intranasal vector delivery. SC-Ad6 produced significantly more luciferase than RD-Ad6 but less than RC-Ad6. While hamsters are thought to be relatively permissive for adenovirus, we did not observe an obvious “second cycle” of luciferase amplification by progeny virions in the RC-Ad group. This could be due to limitations in detection or due to rapid downregulation of the virus by the innate immune system. The increased transgene protein production by SC-Ad6 compared to that by RD-Ad6 correlated with increased GFP-specific antibodies in both the serum and vaginal washes for the two vectors. In serum, antibodies were observed as early as 3 weeks after vaccination with SC-Ad6 but not with the other vectors. These antibodies peaked at 12 weeks and remained elevated up to 6 months after only a single intranasal immunization.
In vaginal wash samples, GFP antibodies were not present at early time points but climbed in the SC-Ad group by 12 weeks after vaccination, and they remained elevated 6 months after vaccination. This was not observed in the RD- or RC-Ad6 group. Surprisingly, although RC-Ad6 generated significantly more luciferase than SC-Ad6, it was less effective at generating GFP antibodies than RD-Ad6 in both the serum and vaginal washes. However, comparable levels of antibodies were generated against Ad6 itself by RC-Ad6 and SC-Ad6. This indicates that similar amounts of the vectors were indeed delivered to the hamster immune system and perhaps that the RC-Ad6 vector is not spread well after the first cycle of infection. This is consistent with the absence of observed spread of luciferase after 3 days. If so, the hamster model may underestimate the efficacy of RC-Ad6 vectors.
Sublingual mucosal immunization in nonhuman primates does not generally produce strong systemic immune responses. However, SC-Ad6 generated systemic immune responses following a single sublingual vaccination. By this route, SC-Ad6 induced higher levels of serum anti-GFP antibody and anti-luciferase T cells than RD-Ad6. These data demonstrate that SC-Ad6 can drive more potent immune responses than RD-Ad6 in this nonhuman primate vaccine model by this challenging mucosal immunization route.
One question remains as to why SC-Ad6 was unexpectedly better than RC-Ad6 in hamsters. This could be an artifact related to hamsters, the specifics of these viruses and their ability to drive innate immune responses, or their GFP-Luc transgene. However, it is interesting that the immune responses against GFP-Luc were higher for SC-Ad6 in the hamsters than those generated by RC-Ad6, despite the fact that RC-Ad6 expressed more luciferase, as determined by imaging. In contrast, these two vectors generated similar amounts of anti-Ad6 antibodies. These data suggest that the better activity of SC-Ad6 may be related to how the two vectors display or express the transgene or how the immune system reacts to the infection. RC-Ad6 and SC-Ad6 mediate virtually identical expression levels in vitro in primary human airway cells (16), suggesting that the vaccine effect involves participation of the in vivo immune system. Unlike RC-Ad6, SC-Ad6 does not package its genome or the transgene it carries into viral particles (16). These unpackaged genomes may remain available in the nucleus for expression or immune stimulation or may be released as an adjuvant. Alternately, RC-Ad6 may simply produce a more inflammatory milieu during its attempt at second cycle spread, and the immune response to this may blunt vaccine potential. Regardless, SC-Ad6 appears to be robust at generating immune responses without generating potentially problematic infectious progeny viruses.
It should be emphasized that in all of these experiments, we delivered a single vaccine dose by an intranasal or sublingual mucosal route. No booster immunizations were performed. We show that a single immunization using SC-Ad6 amplified the antigen transgene to a level capable of inducing significant antibody titers and that these can persist for up to 6 months after vaccination. These data suggest that single-cycle adenovirus vaccines may have utility as mucosal and systemic vaccines.
ACKNOWLEDGMENTS
We thank Mary Barry for excellent technical assistance. We also thank Elizabeth R. Magden, the veterinarian supplying assistance with macaque handling and supplying the samples for immunoassays; Bharti Nehete and Guojun Yang for assistance with immunoassay protocols and reagents; and Shailbala Singh for technical support in immunoassays.
This work was supported by NIH/NIAID grants R01 AI096967 and R01 CA136945 and the Division of Infectious Diseases, Department of Medicine at Mayo Clinic.
REFERENCES
- 1.Lubeck MD, Davis AR, Chengalvala M, Natuk RJ, Morin J E, Molnar-Kimber K, Mason BB, Bhat BM, Mizutani S, Hung PP. 1989. Immunogenicity and efficacy testing in chimpanzees of an oral hepatitis B vaccine based on live recombinant adenovirus. Proc Natl Acad Sci U S A 86:6763–6767. doi: 10.1073/pnas.86.17.6763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheng SM, Lee SG, Ronchetti-Blume M, Virk KP, Mizutani S, Eichberg JW, Davis A, Hung PP, Hirsch VM, Chanock RM. 1992. Coexpression of the simian immunodeficiency virus Env and Rev proteins by a recombinant human adenovirus host range mutant. J Virol 66:6721–6727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Natuk RJ, Chanda PK, Lubeck MD, Davis AR, Wilhelm J, Hjorth R, Wade MS, Bhat BM, Mizutani S, Lee S. 1992. Adenovirus-human immunodeficiency virus (HIV) envelope recombinant vaccines elicit high-titered HIV-neutralizing antibodies in the dog model. Proc Natl Acad Sci U S A 89:7777–7781. doi: 10.1073/pnas.89.16.7777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buge SL, Richardson E, Alipanah S, Markham P, Cheng S, Kalyan N, Miller CJ, Lubeck M, Udem S, Eldridge J, Robert-Guroff M. 1997. An adenovirus-simian immunodeficiency virus env vaccine elicits humoral, cellular, and mucosal immune responses in rhesus macaques and decreases viral burden following vaginal challenge. J Virol 71:8531–8541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lubeck MD, Natuk R, Myagkikh M, Kalyan N, Aldrich K, Sinangil F, Alipanah S, Murthy SC, Chanda PK, Nigida SM Jr, Markham PD, Zolla-Pazner S, Steimer K, Wade M, Reitz MS Jr, Arthur LO, Mizutani S, Davis A, Hung PP, Gallo RC, Eichberg J, Robert-Guroff M. 1997. Long-term protection of chimpanzees against high-dose HIV-1 challenge induced by immunization. Nat Med; 3:651–658. doi: 10.1038/nm0697-651. [DOI] [PubMed] [Google Scholar]
- 6.Pinto AR, Fitzgerald JC, Giles-Davis W, Gao GP, Wilson J M, Ertl HC. 2003. Induction of CD8(+) T cells to an HIV-1 antigen through a prime boost regimen with heterologous E1-deleted adenoviral vaccine carriers. J Immunol 171:6774–6779. doi: 10.4049/jimmunol.171.12.6774. [DOI] [PubMed] [Google Scholar]
- 7.Vinner L, Wee EG, Patel S, Corbet S, Gao GP, Nielsen C, Wilson JM, Ertl HC, Hanke T, Fomsgaard A. 2003. Immunogenicity in Mamu-A*01 rhesus macaques of a CCR5-tropic human immunodeficiency virus type 1 envelope from the primary isolate (Bx08) after synthetic DNA prime and recombinant adenovirus 5 boost. J Gen Virol 84:203–213. doi: 10.1099/vir.0.18589-0. [DOI] [PubMed] [Google Scholar]
- 8.McCoy K, Tatsis N, Korioth-Schmitz B, Lasaro MO, Hensley SE, Lin SW, Li Y, Giles-Davis W, Cun A, Zhou D, Xiang Z, Letvin NL, Ertl HC. 2007. Effect of preexisting immunity to adenovirus human serotype 5 antigens on the immune responses of nonhuman primates to vaccine regimens based on human- or chimpanzee-derived adenovirus vectors. J Virol 81:6594–6604. doi: 10.1128/JVI.02497-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barouch DH, Pau MG, Custers JH, Koudstaal W, Kostense S, Havenga MJ, Truitt DM, Sumida SM, Kishko MG, Arthur JC, Korioth-Schmitz B, Newberg MH, Gorgone DA, Lifton MA, Panicali DL, Nabel GJ, Letvin NL, Goudsmit J. 2004. Immunogenicity of recombinant adenovirus serotype 35 vaccine in the presence of pre-existing anti-Ad5 immunity. J Immunol 172:6290–6297. doi: 10.4049/jimmunol.172.10.6290. [DOI] [PubMed] [Google Scholar]
- 10.Lemckert AA, Sumida SM, Holterman L, Vogels R, Truitt DM, Lynch DM, Nanda A, Ewald BA, Gorgone DA, Lifton MA, Goudsmit J, Havenga MJ, Barouch DH. 2005. Immunogenicity of heterologous prime-boost regimens involving recombinant adenovirus serotype 11 (Ad11) and Ad35 vaccine vectors in the presence of anti-Ad5 immunity. J Virol 79:9694–9701. doi: 10.1128/JVI.79.15.9694-9701.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nanda A, Lynch DM, Goudsmit J, Lemckert AA, Ewald BA, Sumida SM, Truitt DM, Abbink P, Kishko MG, Gorgone DA, Lifton MA, Shen L, Carville A, Mansfield KG, Havenga MJ, Barouch DH. 2005. Immunogenicity of recombinant fiber-chimeric adenovirus serotype 35 vector-based vaccines in mice and rhesus monkeys. J Virol 79:14161–14168. doi: 10.1128/JVI.79.22.14161-14168.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sumida SM, Truitt DM, Lemckert AA, Vogels R, Custers JH, Addo MM, Lockman S, Peter T, Peyerl FW, Kishko MG, Jackson SS, Gorgone DA, Lifton MA, Essex M, Walker BD, Goudsmit J, Havenga MJ, Barouch DH. 2005. Neutralizing antibodies to adenovirus serotype 5 vaccine vectors are directed primarily against the adenovirus hexon protein. J Immunol 174:7179–7185. doi: 10.4049/jimmunol.174.11.7179. [DOI] [PubMed] [Google Scholar]
- 13.Havenga M, Vogels R, Zuijdgeest D, Radosevic K, Mueller S, Sieuwerts M, Weichold F, Damen I, Kaspers J, Lemckert A, van Meerendonk M, van der Vlugt R, Holterman L, Hone D, Skeiky Y, Mintardjo R, Gillissen G, Barouch D, Sadoff J, Goudsmit J. 2006. Novel replication-incompetent adenoviral B-group vectors: high vector stability and yield in PER.C6 cells. J Gen Virol 87:2135–2143. doi: 10.1099/vir.0.81956-0. [DOI] [PubMed] [Google Scholar]
- 14.Campos SK, Barry MA. 2007. Current advances and future challenges in adenoviral vector biology and targeting. Curr Gene Ther 7:189–204. doi: 10.2174/156652307780859062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khare R, Chen CY, Weaver EA, Barry MA. 2011. Advances and future challenges in adenoviral vector pharmacology and targeting. Curr Gene Ther 11:241–258. doi: 10.2174/156652311796150363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crosby CM, Barry MA. 2014. IIIa deleted adenovirus as a single-cycle genome replicating vector. Virology 462-463:158–165. doi: 10.1016/j.virol.2014.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thomas MA, Spencer JF, Wold WS. 2007. Use of the Syrian hamster as an animal model for oncolytic adenovirus vectors. Methods Mol Med 130:169–183. doi: 10.1385/1-59745-166-5:169. [DOI] [PubMed] [Google Scholar]
- 18.National Research Council. 2011. Guide for the care and use of laboratory animals, 8th ed. National Academies Press, Washington, DC. [Google Scholar]
- 19.Nehete PN, Gambhira R, Nehete BP, Sastry KJ. 2003. Dendritic cells enhance detection of antigen-specific cellular immune responses by lymphocytes from rhesus macaques immunized with an HIV envelope peptide cocktail vaccine. J Med Primatol 32:67–73. doi: 10.1034/j.1600-0684.2003.00011.x. [DOI] [PubMed] [Google Scholar]
- 20.Peng B, Wang LR, Gomez-Roman VR, Davis-Warren A, Montefiori DC, Kalyanaraman VS, Venzon D, Zhao J, Kan E, Rowell TJ, Murthy KK, Srivastava I, Barnett SW, Robert-Guroff M. 2005. Replicating rather than nonreplicating adenovirus-human immunodeficiency virus recombinant vaccines are better at eliciting potent cellular immunity and priming high-titer antibodies. J Virol 79:10200–10209. doi: 10.1128/JVI.79.16.10200-10209.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gurwith M, Lock M, Taylor EM, Ishioka G, Alexander J, Mayall T, Ervin JE, Greenberg RN, Strout C, Treanor JJ, Webby R, Wright PF. 2013. Safety and immunogenicity of an oral, replicating adenovirus serotype 4 vector vaccine for H5N1 influenza: a randomised, double-blind, placebo-controlled, phase 1 study. Lancet Infect Dis 13:238–250. doi: 10.1016/S1473-3099(12)70345-6. [DOI] [PMC free article] [PubMed] [Google Scholar]


