ABSTRACT
Human metapneumovirus (hMPV) is a respiratory paramyxovirus that is distributed worldwide and induces significant airway morbidity. Despite the relevance of hMPV as a pathogen, many aspects of the immune response to this virus are still largely unknown. In this report, we focus on the antiviral immune response, which is critical for viral clearance and disease resolution. Using in vitro and in vivo systems, we show that hMPV is able to induce expression of lambda interferon 1 (IFN-λ1), IFN-λ2, IFN-λ3, and IFN-λ4. The induction of IFN-λ expression by hMPV was dependent on interferon regulatory factor 7 (IRF-7) expression but not on IRF-3 expression. Treatment of hMPV-infected mice with IFN-λ reduced the disease severity, lung viral titer, and inflammatory response in the lung. Moreover, the IFN-λ response induced by the virus was regulated by the expression of the hMPV G protein. These results show that type III interferons (IFN-λs) play a critical protective role in hMPV infection.
IMPORTANCE Human metapneumovirus (hMPV) is a pathogen of worldwide importance. Despite the relevance of hMPV as a pathogen, critical aspects of the immune response induced by this virus remain unidentified. Interferons (IFNs), including IFN-λ, the newest addition to the interferon family, constitute an indispensable part of the innate immune response. Here, we demonstrated that IFN-λ exhibited a protective role in hMPV infection in vitro and in an experimental mouse model of infection.
INTRODUCTION
Human metapneumovirus (hMPV) was first identified in 2001 after its isolation from infants and children with lower respiratory tract infections (LTRI) of unknown etiology (1). hMPV is a negative-sense, nonsegmented RNA virus that belongs to the Paramyxoviridae family (2, 3). The clinical manifestations of hMPV infection in young children are indistinguishable from those of human respiratory syncytial virus (hRSV) infection. LRTI associated with hMPV in infants and young children is a frequent cause of hospitalization. Several studies indicate that hMPV likely accounts for 5% to 15% of LRTI hospitalizations in infants and young children and is second only to hRSV as a cause of bronchiolitis in early childhood (4–8). By the age of 5 years, most children have been infected by hMPV, and by the age of 25, virtually all adults have already been exposed to the virus (8, 9). Currently, there is no vaccine available or any other specific treatment of hMPV infection. Therefore, knowledge of the aspects of the regulation of the antiviral immune response to this virus is critical for the understanding hMPV-induced disease.
The interferon (IFN) response plays a pivotal role in shaping antiviral immune responses in the respiratory tract. The IFN system constitutes the first line of defense against viruses in mammals, and IFNs are categorized into three groups: type I (alpha/beta IFN [IFN-α/β]), type II (IFN-γ), and type III (IFN-λ). Among them, IFN-λ (lambda IFN) is the most recently described group of small helical cytokines capable of inducing an antiviral state in responsive cells (10). Therefore, their importance to specific viral infections is less known. The human genome contains at least three subtypes of IFN-λ: IFN-λ1 (interleukin-29 [IL-29]), IFN-λ2 (IL-28A), and IFN-λ3 (IL-28B) (11). More recently, an additional human isoform (IFN-λ4) which is moderately similar to IFN-λ3 has been described (12, 13). IFN-λ4 was initially described as a pseudogene, but it has been recently reported to antagonize the activity of IFN-α in response to hepatitis C virus (HCV) and is apparently expressed in only a fraction of the human population due to a widespread genetic polymorphism introducing a 5′ proximal frameshift (13). IFN-λ signals through a heterodimeric class II cytokine receptor composed of the IFN-λR1 chain, which is specific to IFN-λ, and the IL-10R2 chain, which is shared by other IL-10-related cytokines (10, 14). The activation of the IFN-λ receptor induces the transcription of many of the same genes as does the activation of IFN-α/β receptor (15). However, in contrast to the ubiquitous expression of IFN-α/β receptor complex, expression of IFN-λR1 is characteristic of epithelial cells, keratinocytes, differentiated dendritic cells (16–19), and human hepatocytes (20), limiting its activity primarily to these types of cells. We have previously reported that hMPV induces the production of IFN-λ in vitro (in dendritic cells) and in vivo (in infected mice) and that its production is dependent on the presence of MDA5 (21). There has also been an indication that IFN-λ may play a role in hMPV infection as reported in a study using mice lacking of the receptor IL-28Rα (18). Nonetheless, the role of IFN-λ in hMPV infection is still largely unknown.
Here, we assessed the role of IFN-λ in the antiviral and inflammatory response of hMPV infection using in vitro and in vivo experimental models. Our results indicate that hMPV is susceptible to the effect of recombinant IFN-λ and that its production is strongly regulated by the expression of the hMPV G protein and the transcription factor interferon regulatory factor 7 (IRF-7). Moreover, IFN-λ contributes to the control of hMPV-induced disease by influencing body weight loss, pulmonary inflammation, and lung viral titer.
MATERIALS AND METHODS
Virus stocks.
hMPV (strain CAN97-83) stock was provided by the Respiratory Virus Section, Centers for Disease Control (CDC), Atlanta, GA, with permission from Guy Boivin at the Research Center in Infectious Diseases, Regional Virology Laboratory, Laval University, Quebec City, Canada. Virus was propagated and titrated in LLC-MK2 cells (ATCC CCL7) in the presence of trypsin (Worthington, Lakewood, NJ), as described elsewhere (22, 23). The recombinant full-length hMPV (rhMPV) and the G gene deletion mutant virus (ΔG) were generated from a cDNA clone encoding the complete 13,335-nucleotide (nt) anti-genomic RNA of hMPV, as previously reported (22, 24). Briefly, the complete hMPV antigenome was cloned into pBlueScript II SK(+) vector (Agilent Technologies), constructed by sequential ligation of three cDNA fragments, as described by Biacchesi et al. (24). The original pBlueScript II SK(+) vector was digested with the BssHII restriction enzyme to remove all restriction sites located between base 619 and base 792. This vector region was replaced by a polylinker containing the following DNA sequences: BssHII-AatII-NheI-Acc65I-PmeI-NotI-T7 terminator-BssHII (GCGCGC-GACGTC-GCTAGC-GGTACC-GTTTAAAC-GCGGCCGCCTAGCATAACCCCTTGGGGCCTCTAAACGGGTC-TTGAGGGGTTTTTTG-GCGCGC). Fragment I of the cloned hMPV genome contained an AatII restriction site designed for virus cDNA assembly purposes followed by the T7 RNA polymerase promoter (T7p) and three additional, noncoding G residues before the beginning of the virus leader sequence to improve transcriptional efficiency. This DNA segment was followed by 3,038 bases of the virus sequence, including a NheI restriction site artificially created within the putative M-F intergenic region. Fragment II, containing a 3,921-bp sequence, was inserted between the NheI and the naturally occurring Acc65I restriction site. Fragment III was first assembled as part of the pcDNA 3.1 TOPO intermediate vector (Life Technologies), using an Acc65I-DraII cDNA fragment (positions 6959 to 9932) and a DraII-NotI cDNA fragment (from position 9932 up to the virus 3′ end [position 13335] followed by the hepatitis delta virus ribozyme). A ribozyme sequence was added as part of the primer sequence used for reverse transcription-PCR (RT-PCR) amplifications during fragment III assembly. The final cDNA construct was verified by sequencing. Fragment II was used as the template to generate a DNA fragment without an open reading frame (ORF) encoding G protein, using a PCR-based overlap extension technique. This modified fragment, fragment II, was subsequently used to assemble the hMPV ΔG construct. hMPV cDNA was used as the template to clone the structural genes (N, P, L, and M2-1) in the pMT-1 vector (25) (generously provided by Bernard Moss, National Institutes of Health) necessary for virus recovery, as described by Biacchesi et al. (22). Virus recovery was performed using BSR T7/5 cells (26), as described elsewhere (22, 27). Viruses were purified by the use of a 60% sucrose cushion and were not used beyond passage 5. Respiratory syncytial virus (RSV) A2 was grown in HEp-2 cells (ATCC CCL 23) and purified by polyethylene glycol precipitation, followed by centrifugation on 35% to 65% discontinuous sucrose gradients as previously described (28). The virus titers, for both recombinant viruses, were determined by a methylcellulose plaque assay, as previously reported (29, 30).
Infection of epithelial cells in vitro.
A549 cells (ATCC CCL-185C) were cultivated in F12K medium enriched with 10% fetal bovine serum (FBS) and with 1% penicillin-streptomycin. Cells were infected with hMPV or RSV at different multiplicities of infection (MOI). At the indicated time points, cells or cell supernatants were collected for subsequent analysis. In a separate set of experiments, A549 cells were pretreated with 10 to 50 ng/ml of recombinant human (rh) IFN-λ1 (rhIFN-λ1) or IFN-λ2 (both from Peprotech) or IFN-λ3 (R&D Systems) 24 h prior to infection, followed by removal of the medium. Virus was adsorbed for 2 h before replenishment with new medium containing the corresponding IFN-λ was performed.
Mice and infection protocol.
Animal care and use were conducted in accordance with the National Institutes of Health and Louisiana State University institutional guidelines. The BALB/c and C57BL/6J mice used in this work were purchased from Harlan Laboratories. IRF-3−/− and IRF-7−/− mice were generated by Tadatsugo Taniguchi (University of Tokyo, Tokyo, Japan) (31). All mice were kept under the care of the Division of Laboratory Animal Medicine Facility, Louisiana State University, Baton Rouge, LA, as previously reported (21, 30). After being subjected to light anesthesia, 8-to-10-week-old mice were infected intranasally (i.n.) with 50 μl of hMPV or RSV diluted in phosphate-buffered saline (PBS; final administered dose, 1 × 107 PFU). As a mock treatment, mice were inoculated with an equivalent volume of PBS (here referred as “mock”). In a separate set of experiments, mice were treated via intranasal inoculation with 1, 3, or 5 μg of recombinant murine (rm) IFN-λ2 (rmIFN-λ2; Peprotech) or 3 μg of rmIFN-λ3 (PBL Assay Science) at 24 h prior hMPV infection.
BAL fluid, histology, and lung tissue collection.
Mice were sacrificed by an intraperitoneal injection of ketamine and xylazine and exsanguinated via the femoral vessels as previously reported (30). To collect a bronchoalveolar lavage (BAL) fluid sample, the lungs were flushed twice with ice-cold sterile PBS (1 ml) as previously described (23, 30). Cell-free supernatants were stored at −70°C until analysis. For histological analysis, lungs were perfused and fixed in 10% buffered formalin and embedded in paraffin. Multiple 4-μm-thick sections were stained with hematoxylin & eosin (H&E) to assess lung inflammation. A blind analysis and scoring for cellular infiltration in the peribronchial and perivascular spaces were performed by a board-certified pathologist as previously described (30, 32). Briefly, inflammatory infiltrates were scored by enumerating the layers of inflammatory cells surrounding the vessels and bronchioles. Finding zero to three layers of inflammatory cells was considered normal. Finding moderate to abundant infiltrate (more than three layers of inflammatory cells surrounding 50% or more of the circumference of the vessel or bronchioles) was considered abnormal. The number of abnormal perivascular and peribronchial spaces divided by the total perivascular and peribronchial spaces was the percentage reported as the pathology score. For gene expression experiments, lung tissue was collected, snap-frozen in liquid nitrogen, and stored at −70°C until analysis.
Measurement of cytokines and IFNs.
Production of IFN-β and IFN-λ2/3 (IL-28A/IL-28B) in BAL fluid samples was determined by an enzyme-linked immunosorbent assay (ELISA), according to the manufacturer's instructions (PBL Assay Science). Levels of cytokines and chemokines in BAL fluid were determined with a Milliplex MAP 32-mouse cytokine detection system (Millipore), following the manufacturer's protocol. The range of sensitivity of the assay is 3.2 to 10,000 pg/ml.
Clinical illness score.
To evaluate the severity of illness in mice, the animals were scored daily using a standardized 1-to-5 grading system, as previously described (23, 33). In addition, daily determination of body weight was performed in order to monitor the progression of disease over the experimental period.
Lung virus titer.
Lungs were removed from infected animals at day 4 after hMPV infection. Tissue samples were homogenized in 1 ml of Dulbecco's modified Eagle's medium and centrifuged twice at 10,000 × g for 1 min at 4°C. Serial 2-fold dilutions of the supernatant were performed to determine the viral titer by plaque assay on LLC-MK2 cells under a methylcellulose overlay. Plaques were visualized after 6 days by horseradish peroxidase (HRP) staining, as previously described (22, 23).
Real-time qRT-PCR.
RNA from cells or lung tissue was extracted using an RNeasy Plus kit (Qiagen). Determination of the expression of the genes by qRT-PCR was performed by using predesigned TaqMan assays, as previously described (21). All primers and probes were obtained from Integrated DNA Technologies. Quantitative RT-PCRs (qRT-PCRs) were run on a 7900HT fast real-time PCR system following the manufacturer's suggested cycling parameters (Applied Biosystems). The comparative cycle threshold (ΔΔCT) method was used to quantitate the expression of target genes, and results were normalized to the endogenous reference (GAPDH [glyceraldehyde-3-phosphate dehydrogenase]) expression levels of transcripts from uninfected, control cells.
Statistical analyses.
Statistical analyses were performed with the InStat 3 biostatistics package (GraphPad) using one-way analysis of variance (ANOVA) to ascertain differences between groups. Results are expressed as means ± standard errors of the means.
RESULTS
Differential induction of lambda interferon expression by hMPV in vitro.
We first analyzed the expression of lambda interferon in response to hMPV infection in epithelial cells and compared it to that seen in response to RSV infection. A549 cells were infected for 24 h with either hMPV or RSV at different MOIs (0.1, 0.5, 1.0, and 3.0), and the expression of IFN-λ1, IFN-λ2/3, and IFN-λ4 was determined by qRT-PCR, using predesigned primers. The expression of IFN-β was also assessed as a comparison with type I IFN induction by hMPV. hMPV induced the expression of all four interferons assessed in the infected cells (Fig. 1A). Their induction was dose dependent, as the increasing interferon expression was directly proportional to the amount of virus added to the cells. Interestingly, hMPV infection was a stronger inducer of the interferon responses than RSV infection. Based on those results, an MOI of 3.0 was used to assess the expression of the interferon response at different time points (12, 24, 48, and 72 h). As shown in Fig. 1B, the induction of IFN-λ1, IFN-λ2/3, IFN-λ4, and IFN-β by both viruses was time dependent, as they induced marginal expression of interferon as early as 12 h, with a maximum expression level observed between 48 to 72 h after infection, except in the case of IFN-λ4 expression, which peaked at 24 h after hMPV infection. However, as previously observed (Fig. 1A), hMPV induced a stronger interferon response than RSV. Particularly in the case of IFN-λ4, hMPV (MOI of 3.0) induced a peak of an ∼4.0 ± 1.7 × 104-fold increase versus the 45 ± 25.3-fold increase observed with RSV (Fig. 1A).We also observed that production of lambda interferon was dependent on viral replication, as inactivation of the virus by UV light failed to induce its expression. Finally, in order to determine if the differences between the two viruses in the levels of IFN gene expression were due to different rates of viral replication, we analyzed RSV or hMPV N gene expression by qRT-PCR. Our data showed similar levels of viral gene expression generated in the epithelial cells infected by RSV and hMPV (Fig. 1B).
FIG 1.
IFN-λ is differentially expressed in epithelial cells in hMPV and RSV infection. The expression of IFN-β, IFN-λ1, -λ2, -λ3, and -λ4 was determined in RSV- or hMPV-infected A549 cells by qRT-PCR. (A) Cells were infected with RSV or hMPV at MOIs of 0.1, 0.5, 1, and 3. IFN expression was assessed after 24 h of infection. Data shown are representative of the results of two experiments run in duplicate. (B) Cells were infected with virus at an MOI of 3. IFN-λ and hMPV or RSV N gene expression was determined at 12, 24, 48, and 72 h after infection. Viruses were also inactivated by UV light, as previously described (30). Data shown are representative of the results of 3 independent experiments run in duplicate. The bar graphs represent means ± standard errors of the means. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
hMPV is susceptible to lambda interferons in vitro.
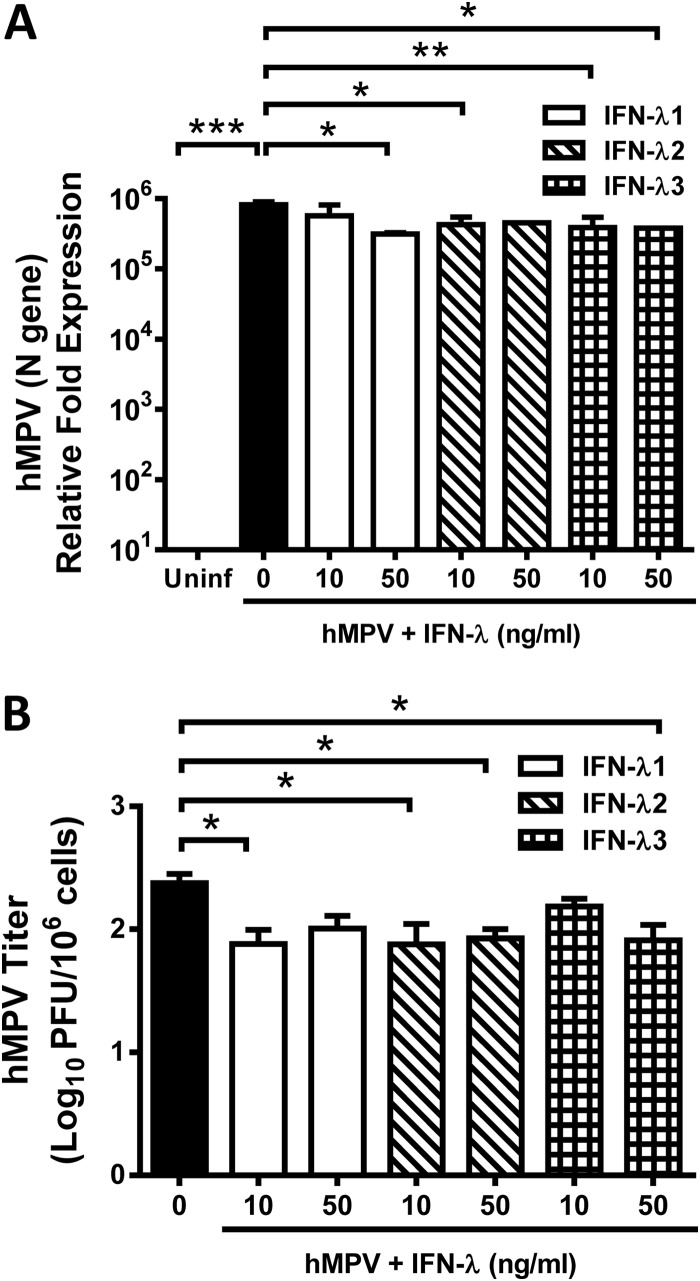
In order to investigate whether hMPV was susceptible to the reported antiviral activity of lambda interferon (14, 18, 34–36), we infected the epithelial cells with hMPV in the presence of lambda interferons. A549 cells were treated with 10 to 50 ng/ml of human recombinant IFN-λ1, IFN-λ2, or IFN-λ3 for 24 h prior to infection with hMPV, at an MOI of 3.0, and incubated for an additional 24 h. Expression of viral antigen (hMPV N gene) was determined by qRT-PCR, and the viral titer was determined by a methylcellulose plaque assay. As shown in Fig. 2A, a significant reduction of N gene expression was observed in those cells treated with lambda interferons compared to that seen with hMPV-infected, nontreated cells. Treatment of infected cells with 50 ng/ml IFN-λ1 resulted in a >60% decrease in viral antigen expression, while treatment with IFN-λ2 or IFN-λ3 decreased viral antigen expression by ∼50% with both concentrations (10 or 50 ng/ml) of interferon used. That effect was further confirmed with the determination of the release of infectious virus in the same set of samples, where we observed an ∼0.5 log10 reduction of hMPV titer when cells were infected in the presence of IFN-λ1, IFN-λ2, and IFN-λ3 (Fig. 2B).
FIG 2.
hMPV is sensitive to the effect of IFN-λ. A549 cells were treated with 10 or 50 ng/ml of human recombinant IFN-λ1, -λ2, or -λ3 for 24 h, followed by hMPV infection (MOI of 3) and replenishment of the corresponding IFN-λ for additional 24 h. (A) Expression of the hMPV N gene was determined by qRT-PCR. Uninf, uninfected. (B) Released virus titers were determined on LLC-MK2 cell monolayers by methylcellulose plaque assay. The bar graph represents PFU means ± standard errors of the means per million cells. Data shown in the graphs are the means of the results of 3 independent experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Differential induction of lambda interferon by hMPV infection and RSV infection in vivo.
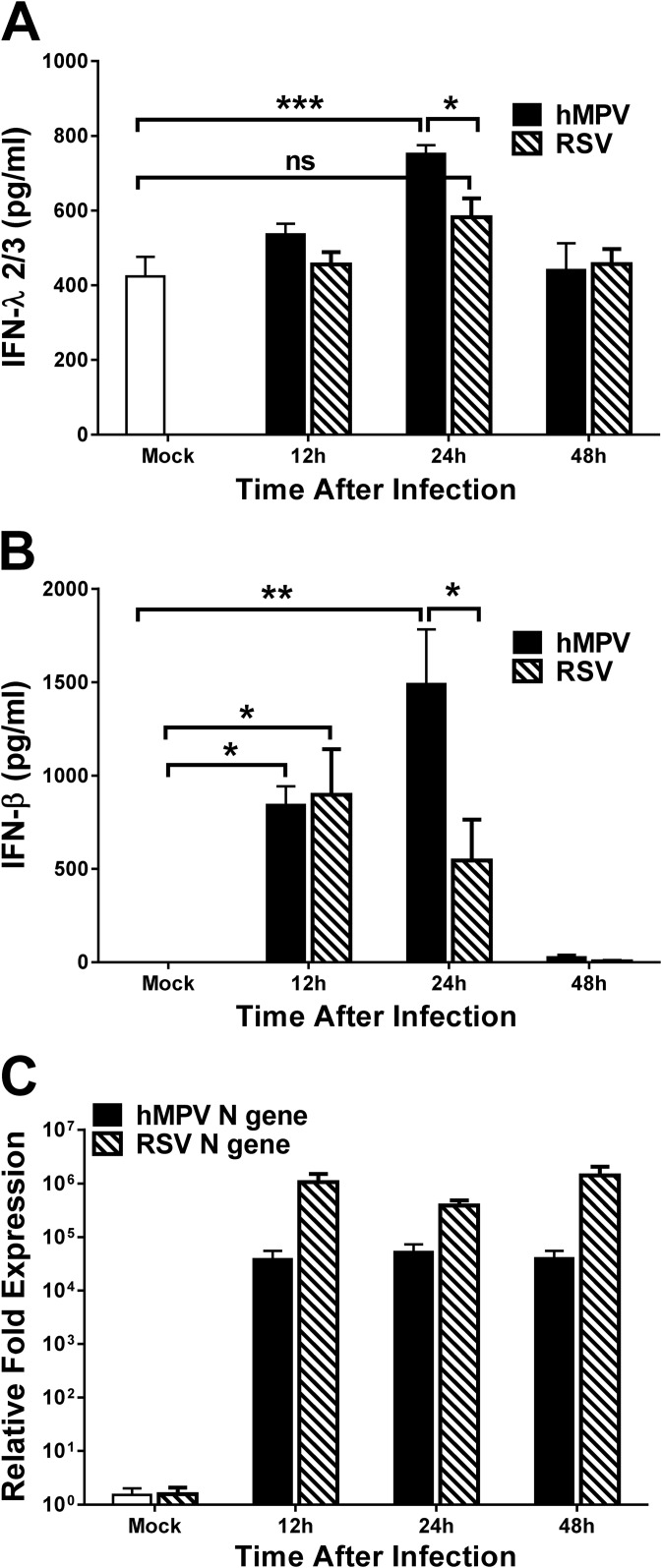
We next analyzed the lambda interferon response in infected mice. BALB/c mice were infected with hMPV, and production of lambda and beta interferon was assessed by ELISA in BAL fluid samples at 12, 24, and 48 h after infection. A separate group of mice was infected with RSV in order to compare the lambda interferon responses to these two close-related human paramyxoviruses. As shown in Fig. 3A, the production of IFN-λ2/3 in response to hMPV infection was induced by the infection as early as 12 h (535.8 ± 29 pg/ml), with a significant peak at 24 h (711.1 ± 32.4 pg/ml) and, eventually, a decrease to levels similar to those seen with mock-infected mice (440.3 ± 72 pg/ml) by 48 h after inoculation. On the other hand, production of lambda interferon was not significantly induced by RSV at any of the time points tested (Fig. 3A). Regarding the IFN-β response, our results show that at 12 h after inoculation, similar significant amounts of IFN-β were induced by the two viruses (842.2 pg/ml for hMPV and 897.6 pg/ml for RSV). However, at 24 h after hMPV infection, a significant peak of 1,129.8 ± 319 pg/ml was observed, while the production of IFN-β induced by RSV started to decrease (544.5 ± 219.8). By 48 h after inoculation, we observed a decrease in the production of IFN-β induced by both viruses (Fig. 3B). Also, we observed a high baseline level (423 ± 52 pg/ml) of IFN-λ2/3 in mock-infected mice, while IFN-β expression was almost absent (0.02 ± 0.02 pg/ml). Furthermore, the induction of IFN-β by hMPV infection was ∼4-fold higher than the induction of IFN-λ2/3 (subtracting the baseline amount of IFN-λ seen with mock-infected mice). In order to investigate whether the observed differences between hMPV and RSV in IFN production were due to differences in the viral infectivity, we analyzed N gene expression by qRT-PCR for each virus in lung samples from the same mice whose results are shown in Fig. 3A and B. Data shown in Fig. 3C indicate that RSV N gene expression was higher than that seen with hMPV-infected mice. Overall, these data indicate that hMPV induced larger amounts of IFN-λ2/3 and IFN-β than RSV in an experimental mouse model of infection.
FIG 3.
hMPV infection induces IFN-λ in vivo. (A and B) BALB/c mice were infected i.n. with 1 ×107 PFU of hMPV or RSV or were mock infected (Mock). Mice were sacrificed at the indicated time after infection, and BAL fluid and lung samples were collected. Production of IFN-λ2/3 (A) or IFN-β (B) in BAL fluid was determined by ELISA. (C) RSV or hMPV N gene expression was quantitated in lung tissue by qRT-PCR. Data are representative of the results of two independent experiments (n = 6 mice/group). The bar graphs represent means ± standard errors of the means. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ns, not significant.
Induction of lambda interferon by hMPV infection is dependent on IRF-7 expression.
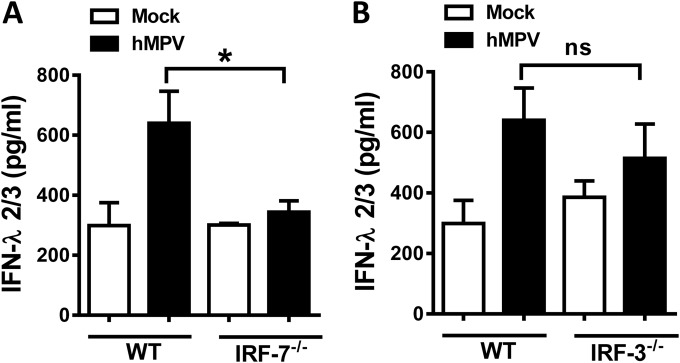
We previously reported that IFN-α/β production resulting from hMPV infection in vivo was dependent on the transcription factor IRF-7 (21). In order to define the transcriptional regulation of lambda interferon induction in hMPV infection, mice deficient in IRF-7 (IRF-7−/−) or IRF-3 (IRF-3−/−) or C57BL/6 wild-type (wt) mice were infected with hMPV for 24 h, as described in Materials and Methods. Concentrations of IFN-λ2/3 in BAL fluid samples were determined by ELISA. We observed that, in the absence of IRF-7, the production of IFN-λ2/3 was significantly reduced compared to the levels observed in the mock-infected mice. However, no significant reduction was observed when IRF-3−/− mice were infected (Fig. 4), indicating that IRF-7, but not IRF-3, regulates the lambda IFN response during hMPV infection.
FIG 4.
IFN-λ2/3 production is dependent on IRF-7 expression. IRF-7−/− (A) or IRF-3−/− (B) mice were infected i.n. with 1 × 107 PFU of hMPV or RSV or were mock infected. C57BL/6 mice were used as controls (WT). After 24 h, mice were sacrificed and BAL fluid samples were collected. Production of IFN-λ2/3 was determined by ELISA. Data are representative of the results of 3 independent experiments (n = 6 to 7 mice/group). Bar graphs represent means ± standard errors of the means. *, P < 0.05; ns, not significant.
hMPV is susceptible to treatment with lambda IFN in vivo.
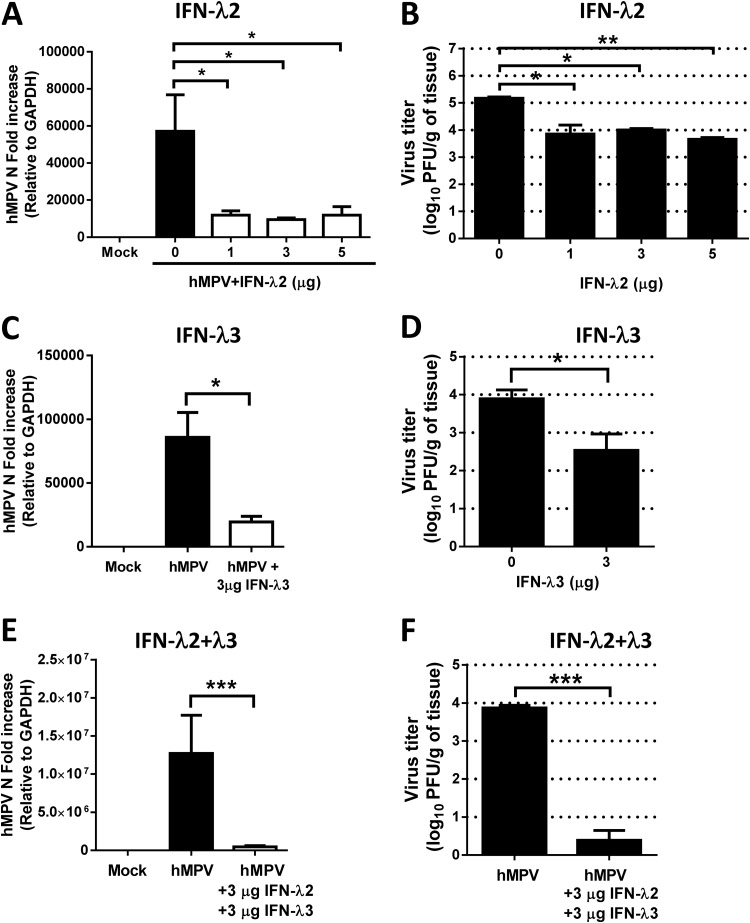
To determine the effect of lambda interferon on hMPV in vivo, we determined the viral antigen expression and lung viral titers in infected mice treated with rmIFN-λ2 or rmIFN-λ3. IFN-λ1 was not included in the in vivo analysis since IFN-λ1 is a pseudogene in mice. BALB/c mice were treated i.n. with increasing concentrations (1, 3, or 5 μg) of rmIFN-λ2 24 h prior to infection with hMPV. Lung tissue was collected at day 4 after infection, and expression of the hMPV N gene was determined. The assessment was performed using primers and a probe designed to amplify the hMPV N gene transcript. Analysis of the N gene revealed a significant (80%) decrease in hMPV N gene expression at each treatment concentration of IFN-λ2 (Fig. 5A). The antiviral effect of IFN-λ2 in hMPV infection was confirmed by lung viral titration using a methylcellulose plaque assay. As shown in Fig. 5B, treatment of mice with 1, 3, or 5 μg of IFN-λ2 significantly reduced the virus titer by more than 1 log10. A similar effect was observed when mice were treated with 3 μg of IFN-λ3 (Fig. 5C and D), indicating that hMPV is susceptible to both IFN-λ2 and IFN-λ3. Furthermore, in order to determine whether treatment with a combination of the two IFN-λ isoforms would have an additive effect, mice were treated with 3 μg of IFN-λ2 and 3 μg of IFN-λ3 prior to hMPV infection (Fig. 5E and F). Our data show that when animals were treated with IFN-λ2 at a concentration of 3 μg/mouse, the lung viral titer was decreased 1.2 log10 (Fig. 5B). A similar effect was observed when mice were treated with same amount of IFN-λ3; the titer was decreased 1.4 log10 (Fig. 5D). However, when animals were treated with a combination of the two IFN-λ isoforms, the lung viral titer was decreased 3.5 log10 (Fig. 5F) compared with the untreated hMPV-infected animals, indicating that the combination of the two IFNs (IFN-λ2 plus IFN-λ3) has a greater effect on hMPV replication than treatment with either IFN-λ separately. These data also indicate that saturation of the receptor for IFN-λ had not occurred at a concentration of 3 μg/mouse. Overall, these results confirm the in vitro findings observed in A549 epithelial cells (Fig. 2) and reveal an important role for lambda IFN in the antiviral response to hMPV infection in vivo by contributing to the viral clearance in hMPV infection.
FIG 5.
IFN-λ2 and IFN-λ3 treatment decreases hMPV lung viral titer in infected mice. BALB/c mice were treated i.n. with the indicated concentrations of murine recombinant IFN-λ2 (A and B) or IFN-λ3 (C and D) or with a combination of IFN-λ2 plus IFN-λ3 (E and F) for 24 h prior infection with 1 × 107 PFU of hMPV. Lung tissue was collected at day 4 after infection. (A, C, and E) Expression of the hMPV N gene was determined by qRT-PCR. (B, D, and F) Infectious viral particles were titrated on LLC-MK2 cell monolayers by a methylcellulose plaque assay. Data are representative of at least two experiments with similar results (n = 3 to 6 mice/group). The bar graphs represent means ± standard errors of the means. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Treatment of lambda interferon ameliorates disease severity and inflammatory response in hMPV-infected mice.
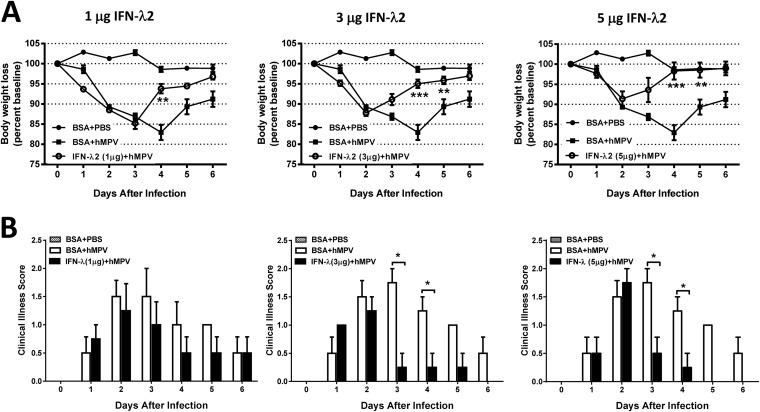
In order to assess the physiological relevance of lambda interferon in the regulation of disease severity and inflammation after treatment with IFN-λ, body weight loss, clinical illness score, pathology score, and cytokine release levels were determined. BALB/c mice were treated with increasing concentrations of rmIFN-λ2 24 h prior to i.n. infection with hMPV, as described above. Mice were monitored daily for disease symptoms and for changes in body weight. Samples for determination of cytokine levels and lung inflammation were collected at day 7 after infection. Treatment of mice with IFN-λ2 significantly reduced body weight loss; mice treated with 1 μg of IFN-λ2 recovered faster (beginning on day 4 after infection) than untreated mice, while mice treated with 3 and 5 μg of IFN-λ2 lost less weight and recovered significantly faster (beginning at day 3 and completely by day 4). In fact, those mice treated with 5 μg of IFN-λ2 fully recovered their baseline weight by day 4 after infection (Fig. 6A). A similar positive effect of IFN-λ2 was observed when the clinical illness score was determined (Fig. 6B). Overall, the beneficial effect of the treatment correlated with the dose increase of IFN-λ2.
FIG 6.
IFN-λ2 reduces disease severity in hMPV-infected mice. BALB/c mice were treated i.n. with 1, 3, or 5 μg of rmIFN-λ2 for 24 h and infected with 1 × 107 PFU of hMPV for 6 days. Mice were monitored daily for body weight loss (A) and illness score (B). Data are representative of two experiments with similar results (n = 4 mice/group). The bar graphs represent means ± standard errors of the means. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
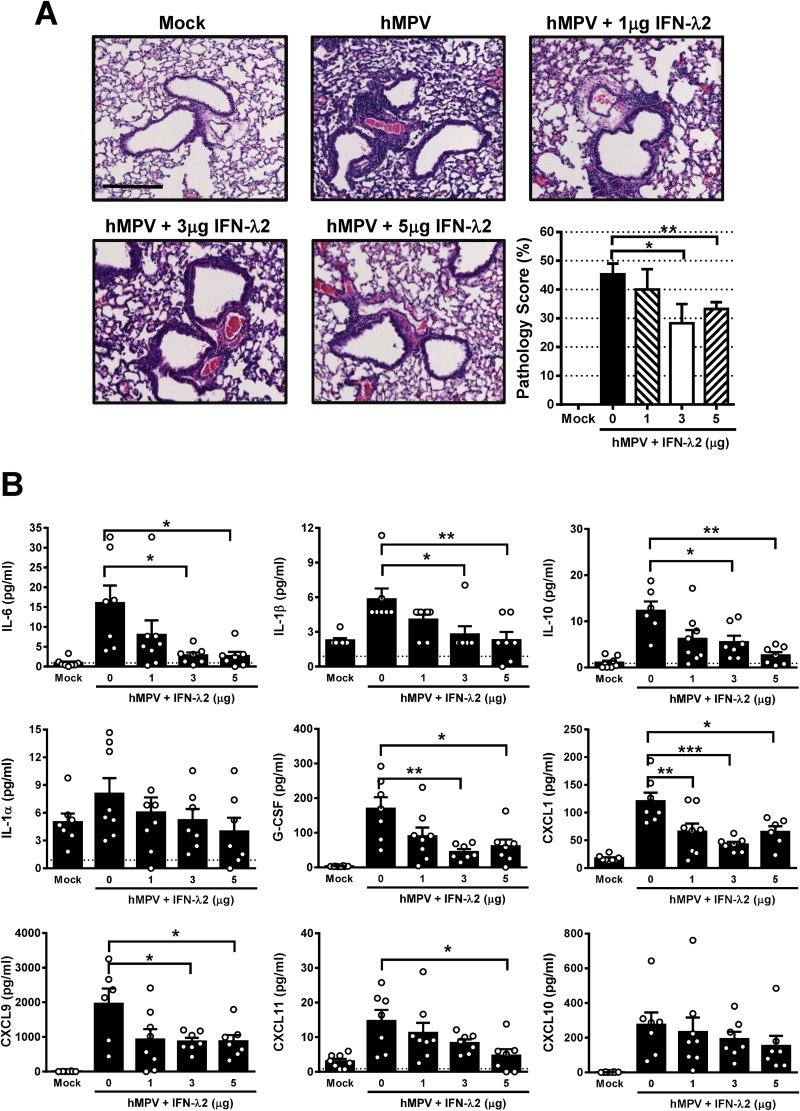
To evaluate the histological changes in the lungs of mice treated with IFN-λ2 after hMPV infection, H&E-stained lung sections, obtained at day 7 after infection (Fig. 7A), were analyzed. We observed that there was minimal cellular peribronchial and perivascular infiltration in mock-infected mice. However, in hMPV-infected, nontreated mice, significant pathology was observed, indicated by an increased cellular infiltration in the alveolar, perivascular, and peribronchoalveolar spaces. Compared to the response seen in tissue sections from mice treated with 3 and 5 μg of IFN-λ2 and infected with hMPV, that response was significantly ameliorated, as observed in the quantification of the pulmonary inflammation, indicated as the percentage of the pathology score (Fig. 7A). To define the role of IFN-λ2 in the control of the hMPV-induced cytokine response, the levels of cytokines and chemokines were assessed in BAL fluid samples from each group of mice after 7 days of infection and assessed for the levels of cytokines by the use of a multiple-cytokine-detection system. Data shown in Fig. 7B indicate that treatment of hMPV-infected mice with IFN-λ2 exhibited a significantly reduced release of proinflammatory cytokines IL-6 and IL-1β. Levels of IL-10, G-CSF, CXCL1 (KC, IL-8 homologue), CXCL9 (MIG), and CCL11 (eotaxin) were also significantly decreased by the treatment of infected mice with IFN-λ2. No significant changes in the production of IL-1α and CXCL10 (gamma interferon-induced protein 10 [IP-10]) were observed (Fig. 7B). Overall, these data indicate that IFN-λ2 treatment controls the disease severity and the inflammatory response in hMPV-infected mice.
FIG 7.
IFN-λ modulates inflammatory response in the lung after hMPV infection. BALB/c mice were treated i.n. with rmIFN-λ2 for 24 h prior to infection with 1 × 107 PFU of hMPV. At the 7-day time point, lung tissue was collected, fixed for slide preparation, and stained with H&E. (A) Representative stained lung tissue sections are presented. Scale bar, 200 μm. The graph shows pathology scores of prepared slides. (B) Cytokine and chemokine profile determined by multiplex analysis in BAL fluid samples from same mice. Data are representative of two independent experiments with similar results (n = 4 to 8 mice/group). The bar graphs represent means ± standard errors of the means. *, P < 0.05; **, P < 0.01; ***, P < 0.001. G-CSF, granulocyte colony-stimulating factor.
Induction of lambda interferon in vitro and in vivo is regulated by hMPV G protein expression.
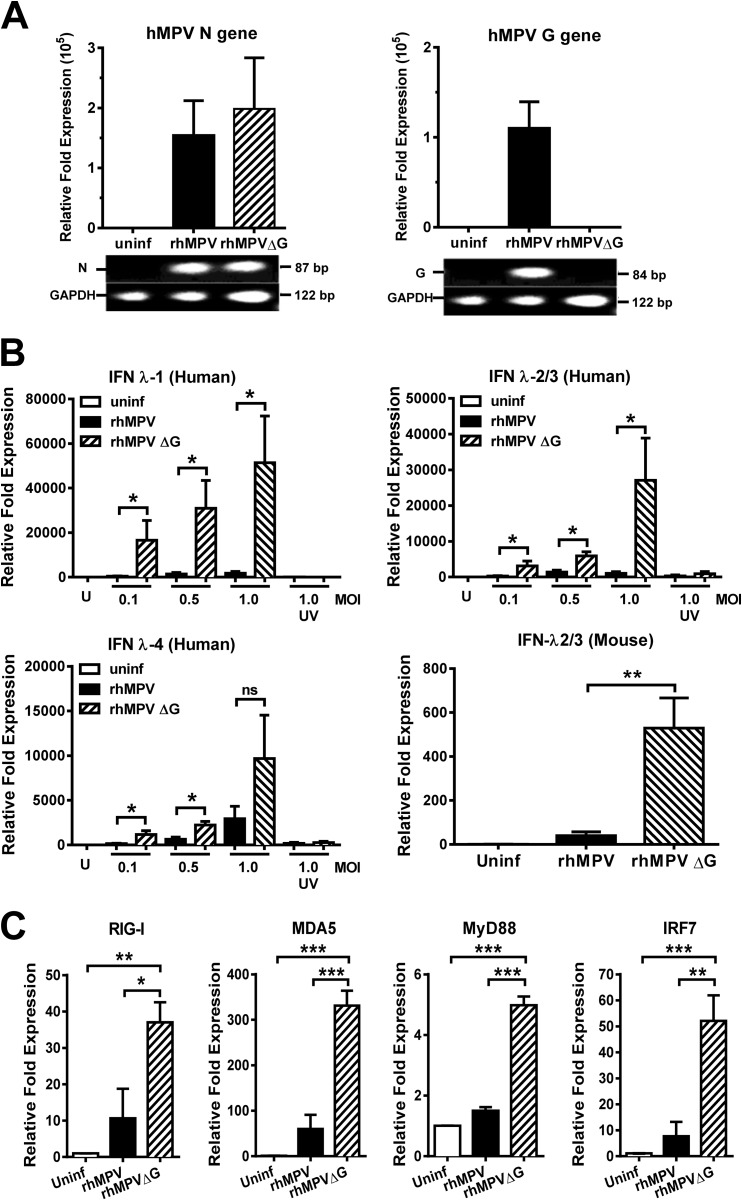
As previous studies indicated, recombinant hMPV, lacking the expression of the G protein, is immunogenic and protective against hMPV challenge (24). Moreover, G protein exerts an inhibitory effect on the IFN-α/β response (27). To define whether the G protein plays a role in the regulation of lambda IFN production by hMPV, we generated full-length recombinant hMPV (rhMPV) and a mutant hMPV lacking G protein (rhMPVΔG), as described in Materials and Methods. We first confirmed the lack of G gene expression in rhMPVΔG. A549 cells were infected with the recombinant viruses for 24 h, and expression of hMPV G and N genes was assessed by qRT-PCR. As shown in Fig. 8A, the G gene is absent in rhMPVΔG but is present in rhMPV, confirming the lack of the G gene in rhMPVΔG, as expected. On the other hand, N gene is expressed equally by the two recombinant viruses. Next, the effect of G protein on the expression of lambda interferon was assessed in vitro and in vivo. For in vitro experiments, A549 cells were infected with rhMPV or rhMPVΔG at different MOIs (0.1, 0.5, and 1.0) for 24 h, and expression of lambda interferon genes was determined by qRT-PCR. Data shown in Fig. 8B demonstrate that G protein inhibited the expression of IFN-λ1, IFN-λ2/3, and IFN-λ4, as the expression of lambda interferons was significantly increased in those cells infected with rhMPVΔG compared with the cells infected with rhMPV. Furthermore, we assessed the effect of hMPV G protein on the signaling pathway of IFN-λ in hMPV infection. We have previously observed that production of IFN-λ by hMPV is dependent on the presence of MDA5 (21) and IRF-7 (Fig. 4). Therefore, expression of several molecules of the IFN-λ signaling pathway was determined. A549 cells were infected with rhMPV or rhMPVΔG, and expression of MDA5, RIG-I, MyD88, and IRF-7 was quantified by qRT-PCR. Our data show that the presence of hMPV G protein significantly inhibited the expression of RIG-I (26.4-fold decrease), MDA5 (271.2-fold decrease), the adaptor molecule MyD88 (3.5-fold decrease), and the transcription factor IRF-7 (44.4-fold decrease), indicating that G protein targets the IFN-λ activation pathways (Fig. 8C). To assess this inhibitory effect in an in vivo system, IFN-λ2/3 expression was determined in BALB/c mice 24 h after viral infection. Our data indicate that the expression of IFN-λ2/3 in the lungs of rhMPVΔG-infected mice was 13-fold higher than the expression from those infected with the rhMPV (Fig. 8B). Together, these data indicate that hMPV G protein modulates the lambda interferon response in vitro and in vivo.
FIG 8.
hMPV G protein inhibits the expression of IFN-λ in vitro and in vivo. A549 cells were infected with full-length recombinant hMPV (rhMPV) and the recombinant mutant hMPV lacking G protein (rhMPVΔG) at different MOIs for 24 h. (A) The virus genotype was confirmed by analyzing the expression of hMPV N and G genes by qRT-PCR. (B) The expression of IFN-λ1, IFN-λ2/3, and IFN-λ4 was also determined by qRT-PCR. Data are representative of the results of three independent experiments. The effect of rhMPVΔG on the expression of IFN-λ2/3 in vivo was also determined by infecting BALB/c mice with 105 PFU of rhMPV or rhMPV ΔG. Lung tissue was collected after 24 h and analyzed for the expression of IFN-λ2/3 by qRT-PCR. Data are representative of two independent experiments with similar results (n = 4 to 6 mice/group). (C) RIG-I, MDA5, MyD88, and IRF-7 expression was determined in rhMPV- and rhMPVΔG-infected cells by qRT-PCR. Data are representative of the results of 4 independent experiments. The bar graphs represent means ± standard errors of the means. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
DISCUSSION
hMPV infection is known to induce and to be susceptible to IFN-α/β responses in vitro and in vivo, as we have previously reported (21, 23, 37–39). The newest members of the IFN family, IFN-λ1, IFN-λ2, and IFN-λ3 (also referred as IL-29, IL-28A, and IL28B, respectively), were discovered in 2003 and have been reported to be antiviral cytokines producing an effect similar to that of IFN-α/β (10). However, to date, the activity and regulation of IFN-λ in hMPV infection have not been fully elucidated. The antiviral state induced by IFN-λ appears to be specifically expressed by epithelial cells and, as a consequence, to induce protection mostly at epithelial surfaces (16, 18). In contrast to the ubiquitously expressed IFN-α/β receptor, the IFN-λ receptor is expressed mostly on epithelial cells and is composed of the unique α chain (encoded by the IFNR1 gene) and the IL-10 receptor β chain (encoded by the IL-10RB gene). In mammals, IFN-λ receptor chains are differentially induced. IL-28Rα is mostly expressed in epithelial cells (16), while IL-10Rβ is expressed in epithelial cells as well as in a wide array of other cell types (40). Therefore, IFN-λ in the lung appears to protect primarily the epithelium during viral infection, with fewer side effects than type I IFN (41) due to its more restricted distribution.
In this work, we observed that human epithelial cells (A549 cells) were able to express all four isoforms of IFN-λ, including IFN-λ4, after hMPV infection. Of note, IFN-λ2 and IFN-λ3 were the most prevalently induced IFN-λ isoforms after hMPV infection. hMPV induced stronger expression of IFN-λ2/3, IFN-λ4, and IFN-β than RSV in a dose- or time-dependent manner. Similar data were obtained using primary human bronchial/epithelial (NHBE) cells (data not shown). These data are in line with our previous observation that hMPV infection is a stronger inducer of the overall IFN-α/β response than RSV infection in vitro and in vivo (23, 37, 38). Interestingly, we observed that IFN-λ4 production was almost absent in RSV-infected cells but was highly induced by hMPV infection, while a time-dependent pattern of expression different from those seen with IFN-λ1, -λ2, and -λ3 was found. Although what the function of IFN-λ4 in hMPV-induced disease may be has not yet been defined, it has been recently reported that its presence correlated with a poor response to IFN-α treatment for chronic hepatitis C virus (HCV) infection, suggesting an antagonistic activity (13). Therefore, future studies to define the specific role of IFN-λ4 in hMPV infection are warranted.
Variable susceptibility of virus to IFN-λ isoforms in vitro has been observed for other viral infections, such as hepatitis C virus (HCV) infection (15, 42). In this study, we observed that hMPV is susceptible in vitro to the three available human isoforms of IFN-λ. However, treatment of infected cells with IFN-λ2 or IFN-λ3 at a concentration of 10 ng/ml showed a higher protective effect than IFN-λ1 treatment. At a concentration of 50 ng/ml, all three IFN-λ isoforms had a significant effect on hMPV N gene expression and release of infectious viral particles. In line with these data, an antiviral effect has been observed when human macrophages have been treated with similar concentrations of rhIFN-λ1 or IFN-λ2 (10 to 1,000 ng/ml) or rhIFN-λ3 (12.5 to 100 ng/ml) and infected with human immunodeficiency virus type 1 (HIV-1) (43, 44).
In order to investigate the role of IFN-λ in hMPV infection in vivo, we first determined the level of induction of IFN-λ2/3 in hMPV-infected mice and further compared it with that seen with mice infected with RSV (the most closely related human paramyxovirus). Mice have orthologous genes for human IFN-λ2 and IFN-λ3. However, in mice, the IFN-λ1 gene is a pseudogene (45) and expression of IFN-λ4 has not been reported. Therefore, in the in vivo experiments, only the expression of IFN-λ2/3 was assessed. The in vivo data are in line with our results determined in vitro in that hMPV induced higher expression of IFN-λ than RSV in epithelial cells, although the difference between the viruses in the levels of production of IFN-λ2/3 in vivo was rather modest. Moreover, the release of IFN-λ2/3 upon hMPV or RSV infection in mice was less than that of IFN-β. Whether the contribution of other cells in the lung could account for the production of the IFN-λ responses after RSV or hMPV infection in the respiratory tract in vivo is unclear. Although we have previously reported that hMPV is able to induce IFN-λ production in dendritic cells also (21), future studies are needed to define the main cell producing IFN-λ in vivo after hMPV and RSV infection.
Expression of IFN responses is known to be regulated by the IRF-3 and IRF-7 transcription factors. The regulation of IFN-α/β gene expression is relatively well characterized (31, 46). However, the regulation of IFN-λ expression is less known. In this work, we found that IFN-λ2/3 expression, induced by hMPV, was regulated by IRF-7 expression. On the other hand, we observed that the expression of IRF-3 did not contribute to the induction of IFN-λ2/3 production in the infected mice. These data are in line with those reported with Sendai virus (47) and influenza virus (48) infections, where IRF-7 regulated the expression of IFN-λ2/3. In fact, it has been reported that both IRF-3 and IRF-7 may regulate the induction of IFN-λ1, while IFN-λ2/3 is mostly regulated by the expression of IRF-7 (47).
When mice were treated with rmIFN-λ2 or rmIFN-λ3, we observed a significant reduction in lung virus titers. It therefore appears that IFN-λ2 and IFN-λ3 have similar levels of antiviral activity in the hMPV-infected mice. However, the combination of the two isoforms (3 μg IFN-λ2 plus 3 μg IFN-λ3) had a more potent antiviral effect on hMPV replication than treatment with 5 μg of IFN-λ2 alone. The use of rmIFN-λ2 under comparable conditions in other viral infections has had similar antiviral effects. Treatment of mice infected with rotavirus with 1 μg of IFN-λ2 mediated protection in the infected animals (36). Mice infected with herpes simplex virus 2 presented a reduced hepatic viral titer and decreased virus replication and disease progression in vaginal mucosa when treated with 5 or 10 μg of IFN-λ (35). Similarly, IFN-λ2 and IFN-λ3 protected against intranasal challenge with influenza A virus infection (49). On the other hand, transgenic hepatitis B virus (HBV) mice treated with 10 μg and 100 μg of mrIFN-λ showed rather modest inhibition of HBV replication (50). However, the antiviral effect of IFN-λ seems to be selective, as IFN-λ did not have any effect on the in vivo replication of other RNA viruses, such as encephalomyocarditis virus (EMCV) or lymphocytic choriomeningitis virus (LCMV) (35).
In this work, we demonstrated that IFN-λ2 has a positive effect on hMPV-induced disease, since treated mice exhibited reduced disease severity compared to untreated mice. A combination of body weight loss and illness score was used as a parameter to monitor disease severity. The body weight loss was significantly reduced in IFN-λ-treated mice at the recovery phase of the disease. Also, the symptoms of illness were less pronounced, indicating that IFN-λ has a beneficial effect in hMPV-induced disease. These results are consistent with those reported with other respiratory viruses, including influenza A virus, RSV, and MPV, using a mouse model lacking both IFN-λ and IFN-α receptor chains (IFNAR10/0 IL28Rα0/0) (18), where the lack of IFN-λ receptor contributed to higher body weight loss and higher hMPV gene expression (N gene) than were seen with IFNAR10/0 alone, suggesting that IFN-λ contributed to resistance to hMPV infection (18). This is similar to what has been reported with influenza A virus (49) and coronavirus infection (51) using IL28Rα0/0 and IFNAR10/0 mice.
IFN-λ treatment also alleviated the inflammatory response induced by hMPV infection. The inflammation response represents a critical host response to control viral infections in the lung. In this study, we showed that treatment of hMPV-infected mice with IFN-λ significantly reduced pulmonary inflammation, which correlates with the observed reduction of the production of inflammatory cytokines in BAL fluid samples. Production of several inflammatory mediators has been reported in infants and mice infected with hMPV (38, 52). We found that the cytokine response to hMPV infection at day 7 was altered in IFN-λ-treated mice. In the presence of IFN-λ, hMPV induced lower levels of IL-6, IL-1β, IL-10, G-CSF, CXCL1, CXCL9, and CXCL11, which correlates with the reduced inflammation in the airways of IFN-λ-treated mice. We have previously reported that, similarly to the amelioration of lung inflammation by IFN-λ treatment, treatment of mice with IFN-α also has an anti-inflammatory effect on hMPV-induced disease (23).
Regulation of the IFN response by human paramyxoviruses has been reported to be attributable to viral proteins. One of the two major proteins expressed in hMPV's envelope is the glycoprotein (G protein). The G protein is responsible for the attachment to the cell receptor. However, it is not essential for viral replication, and it has been observed that the mutant virus lacking G protein (rhMPVΔG) is able to replicate and has a protective effect in challenged animals such as hamsters (24) and nonhuman primates (53). Thus, the rhMPVΔG virus represents a potential vaccine candidate. Here, we demonstrated that a lack of G protein from hMPV results in increased expression of IFN-λ, both in vitro and in vivo. The mechanism(s) of regulation of the IFN-λ response by hMPV G protein appears to include the MDA5 pathway, as MDA5 was highly induced by hMPV infection but significantly overexpressed in rhMPVΔG-infected cells. In agreement with these data, we have previously reported that the production of IFN-λ is dependent on MDA5 expression in human dendritic cells and in infected mice (21). However, we do not rule out the possibility of a role of a Toll-like receptor (TLR) pathway in the production of IFN-λ in response to hMPV infection since the expression of MyD88 was also inhibited by the expression of the hMPV G protein. In line with our data, viral proteins from other paramyxoviruses are also known to inhibit the expression of IFN-λ. RSV nonstructural proteins (NS1 and NS2) inhibit the expression of IFN-λ1 and IFN-λ2/3 in monocyte-derived macrophages (54), and the NS2 protein of pneumonia virus (PVM) alters the expression of IFN-λ in infected mice (55). Overall, production of IFN-λ in hMPV infection appears to be regulated by expression of the attachment G protein by inhibiting at least the MDA5 pathway.
In conclusion, we present clear evidence that IFN-λ plays a key role in the control of hMPV-induced disease and antiviral and inflammatory responses and that its expression is regulated by the transcription factor IRF-7 and hMPV G protein. Thus, while many questions regarding IFN-λ remain to be answered, our data highlight the relevance of this recently discovered IFN family in protecting the respiratory tract from hMPV infection.
ACKNOWLEDGMENTS
This research work was supported by grants from the National Institute of Allergy and Infectious Diseases (R03AI081171), the National Center for Research Resources (P20RR020159-09), and the National Institute of General Medical Sciences (P20GM103458-09) of the National Institutes of Health and by a Flight Attendant Medical Research Institute YCSA grant to A.G.-P.
REFERENCES
- 1.van den Hoogen BG, de Jong JC, Groen J, Kuiken T, de Groot R, Fouchier RA, Osterhaus AD. 2001. A newly discovered human pneumovirus isolated from young children with respiratory tract disease. Nat Med 7:719–724. doi: 10.1038/89098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deffrasnes C, Hamelin ME, Boivin G. 2007. Human metapneumovirus. Semin Respir Crit Care Med 28:213–221. doi: 10.1055/s-2007-976493. [DOI] [PubMed] [Google Scholar]
- 3.Hermos CR, Vargas SO, McAdam AJ. 2010. Human metapneumovirus. Clin Lab Med 30:131–148. doi: 10.1016/j.cll.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boivin G, De Serres G, Cote S, Gilca R, Abed Y, Rochette L, Bergeron MG, Dery P. 2003. Human metapneumovirus infections in hospitalized children. Emerg Infect Dis 9:634–640. doi: 10.3201/eid0906.030017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mullins JA, Erdman DD, Weinberg GA, Edwards K, Hall CB, Walker FJ, Iwane M, Anderson LJ. 2004. Human metapneumovirus infection among children hospitalized with acute respiratory illness. Emerg Infect Dis 10:700–705. doi: 10.3201/eid1004.030555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van den Hoogen BG, van Doornum GJ, Fockens JC, Cornelissen JJ, Beyer WE, de Groot R, Osterhaus AD, Fouchier RA. 2003. Prevalence and clinical symptoms of human metapneumovirus infection in hospitalized patients. J Infect Dis 188:1571–1577. doi: 10.1086/379200. [DOI] [PubMed] [Google Scholar]
- 7.Williams JV, Harris PA, Tollefson SJ, Halburnt-Rush LL, Pingsterhaus JM, Edwards KM, Wright PF, Crowe JE Jr. 2004. Human metapneumovirus and lower respiratory tract disease in otherwise healthy infants and children. N Engl J Med 350:443–450. doi: 10.1056/NEJMoa025472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kahn JS. 2006. Epidemiology of human metapneumovirus. Clin Microbiol Rev 19:546–557. doi: 10.1128/CMR.00014-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leung J, Esper F, Weibel C, Kahn JS. 2005. Seroepidemiology of human metapneumovirus (hMPV) on the basis of a novel enzyme-linked immunosorbent assay utilizing hMPV fusion protein expressed in recombinant vesicular stomatitis virus. J Clin Microbiol 43:1213–1219. doi: 10.1128/JCM.43.3.1213-1219.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sheppard P, Kindsvogel W, Xu W, Henderson K, Schlutsmeyer S, Whitmore TE, Kuestner R, Garrigues U, Birks C, Roraback J, Ostrander C, Dong D, Shin J, Presnell S, Fox B, Haldeman B, Cooper E, Taft D, Gilbert T, Grant FJ, Tackett M, Krivan W, McKnight G, Clegg C, Foster D, Klucher KM. 2003. IL-28, IL-29 and their class II cytokine receptor IL-28R. Nat Immunol 4:63–68. doi: 10.1038/ni873. [DOI] [PubMed] [Google Scholar]
- 11.Donnelly RP, Kotenko SV. 2010. Interferon-lambda: a new addition to an old family. J Interferon Cytokine Res 30:555–564. doi: 10.1089/jir.2010.0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hamming OJ, Terczynska-Dyla E, Vieyres G, Dijkman R, Jorgensen SE, Akhtar H, Siupka P, Pietschmann T, Thiel V, Hartmann R. 2013. Interferon lambda 4 signals via the IFNlambda receptor to regulate antiviral activity against HCV and coronaviruses. EMBO J 32:3055–3065. doi: 10.1038/emboj.2013.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prokunina-Olsson L, Muchmore B, Tang W, Pfeiffer RM, Park H, Dickensheets H, Hergott D, Porter-Gill P, Mumy A, Kohaar I, Chen S, Brand N, Tarway M, Liu L, Sheikh F, Astemborski J, Bonkovsky HL, Edlin BR, Howell CD, Morgan TR, Thomas DL, Rehermann B, Donnelly RP, O'Brien TR. 2013. A variant upstream of IFNL3 (IL28B) creating a new interferon gene IFNL4 is associated with impaired clearance of hepatitis C virus. Nat Genet 45:164–171. doi: 10.1038/ng.2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kotenko SV, Gallagher G, Baurin VV, Lewis-Antes A, Shen M, Shah NK, Langer J A, Sheikh F, Dickensheets H, Donnelly RP. 2003. IFN-lambdas mediate antiviral protection through a distinct class II cytokine receptor complex. Nat Immunol 4:69–77. doi: 10.1038/ni875. [DOI] [PubMed] [Google Scholar]
- 15.Meager A, Visvalingam K, Dilger P, Bryan D, Wadhwa M. 2005. Biological activity of interleukins-28 and -29: comparison with type I interferons. Cytokine 31:109–118. doi: 10.1016/j.cyto.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 16.Sommereyns C, Paul S, Staeheli P, Michiels T. 2008. IFN-lambda (IFN-lambda) is expressed in a tissue-dependent fashion and primarily acts on epithelial cells in vivo. PLoS Pathog 4:e1000017. doi: 10.1371/journal.ppat.1000017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yin Z, Dai J, Deng J, Sheikh F, Natalia M, Shih T, Lewis-Antes A, Amrute SB, Garrigues U, Doyle S, Donnelly RP, Kotenko SV, Fitzgerald-Bocarsly P. 2012. Type III IFNs are produced by and stimulate human plasmacytoid dendritic cells. J Immunol 189:2735–2745. doi: 10.4049/jimmunol.1102038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mordstein M, Neugebauer E, Ditt V, Jessen B, Rieger T, Falcone V, Sorgeloos F, Ehl S, Mayer D, Kochs G, Schwemmle M, Gunther S, Drosten C, Michiels T, Staeheli P. 2010. Lambda interferon renders epithelial cells of the respiratory and gastrointestinal tracts resistant to viral infections. J Virol 84:5670–5677. doi: 10.1128/JVI.00272-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mennechet FJ, Uze G. 2006. Interferon-lambda-treated dendritic cells specifically induce proliferation of FOXP3-expressing suppressor T cells. Blood 107:4417–4423. doi: 10.1182/blood-2005-10-4129. [DOI] [PubMed] [Google Scholar]
- 20.Hermant P, Demarez C, Mahlakoiv T, Staeheli P, Meuleman P, Michiels T. 2014. Human but not mouse hepatocytes respond to interferon-lambda in vivo. PLoS One 9:e87906. doi: 10.1371/journal.pone.0087906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baños-Lara Mdel R, Ghosh A, Guerrero-Plata A. 2013. Critical role of MDA5 in the interferon response induced by human metapneumovirus infection in dendritic cells and in vivo. J Virol 87:1242–1251. doi: 10.1128/JVI.01213-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Biacchesi S, Skiadopoulos MH, Tran KC, Murphy BR, Collins PL, Buchholz UJ. 2004. Recovery of human metapneumovirus from cDNA: optimization of growth in vitro and expression of additional genes. Virology 321:247–259. doi: 10.1016/j.virol.2003.12.020. [DOI] [PubMed] [Google Scholar]
- 23.Guerrero-Plata A, Baron S, Poast J S, Adegboyega PA, Casola A, Garofalo RP. 2005. Activity and regulation of alpha interferon in respiratory syncytial virus and human metapneumovirus experimental infections. J Virol 79:10190–10199. doi: 10.1128/JVI.79.16.10190-10199.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Biacchesi S, Skiadopoulos MH, Yang L, Lamirande EW, Tran KC, Murphy BR, Collins PL, Buchholz UJ. 2004. Recombinant human metapneumovirus lacking the small hydrophobic SH and/or attachment G glycoprotein: deletion of G yields a promising vaccine candidate. J Virol 78:12877–12887. doi: 10.1128/JVI.78.23.12877-12887.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moss B, Elroy-Stein O, Mizukami T, Alexander WA, Fuerst TR. 1990. Product review. New mammalian expression vectors. Nature 348:91–92. [DOI] [PubMed] [Google Scholar]
- 26.Buchholz UJ, Finke S, Conzelmann KK. 1999. Generation of bovine respiratory syncytial virus (BRSV) from cDNA: BRSV NS2 is not essential for virus replication in tissue culture, and the human RSV leader region acts as a functional BRSV genome promoter. J Virol 73:251–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bao X, Liu T, Shan Y, Li K, Garofalo RP, Casola A. 2008. Human metapneumovirus glycoprotein G inhibits innate immune responses. PLoS Pathog 4:e1000077. doi: 10.1371/journal.ppat.1000077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ueba O. 1978. Respiratory syncytial virus. I. Concentration and purification of the infectious virus. Acta Med Okayama 32:265–272. [PubMed] [Google Scholar]
- 29.Kisch AL, Johnson KM. 1963. A plaque assay for respiratory syncytial virus. Proc Soc Exp Biol Med 112:583. doi: 10.3181/00379727-112-28111. [DOI] [PubMed] [Google Scholar]
- 30.Chakraborty K, Zhou Z, Wakamatsu N, Guerrero-Plata A. 2012. Interleukin-12p40 modulates human metapneumovirus-induced pulmonary disease in an acute mouse model of infection. PLoS One 7:e37173. doi: 10.1371/journal.pone.0037173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sato M, Suemori H, Hata N, Asagiri M, Ogasawara K, Nakao K, Nakaya T, Katsuki M, Noguchi S, Tanaka N, Taniguchi T. 2000. Distinct and essential roles of transcription factors IRF-3 and IRF-7 in response to viruses for IFN-alpha/beta gene induction. Immunity 13:539–548. doi: 10.1016/S1074-7613(00)00053-4. [DOI] [PubMed] [Google Scholar]
- 32.Stack AM, Malley R, Saladino RA, Montana JB, MacDonald KL, Molrine DC. 2000. Primary respiratory syncytial virus infection: pathology, immune response, and evaluation of vaccine challenge strains in a new mouse model. Vaccine 18:1412–1418. doi: 10.1016/S0264-410X(99)00399-0. [DOI] [PubMed] [Google Scholar]
- 33.Graham BS, Bunton LA, Wright PF, Karzon DT. 1991. Role of T lymphocyte subsets in the pathogenesis of primary infection and rechallenge with respiratory syncytial virus in mice. J Clin Invest 88:1026–1033. doi: 10.1172/JCI115362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ank N, Iversen MB, Bartholdy C, Staeheli P, Hartmann R, Jensen UB, Dagnaes-Hansen F, Thomsen AR, Chen Z, Haugen H, Klucher K, Paludan SR. 2008. An important role for type III interferon (IFN-lambda/IL-28) in TLR-induced antiviral activity. J Immunol 180:2474–2485. doi: 10.4049/jimmunol.180.4.2474. [DOI] [PubMed] [Google Scholar]
- 35.Ank N, West H, Bartholdy C, Eriksson K, Thomsen AR, Paludan SR. 2006. Lambda interferon (IFN-lambda), a type III IFN, is induced by viruses and IFNs and displays potent antiviral activity against select virus infections in vivo. J Virol 80:4501–4509. doi: 10.1128/JVI.80.9.4501-4509.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pott J, Mahlakoiv T, Mordstein M, Duerr CU, Michiels T, Stockinger S, Staeheli P, Hornef MW. 2011. IFN-lambda determines the intestinal epithelial antiviral host defense. Proc Natl Acad Sci U S A 108:7944–7949. doi: 10.1073/pnas.1100552108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guerrero-Plata A, Casola A, Suarez G, Yu X, Spetch L, Peeples ME, Garofalo RP. 2006. Differential response of dendritic cells to human metapneumovirus and respiratory syncytial virus. Am J Respir Cell Mol Biol 34:320–329. doi: 10.1165/rcmb.2005-0287OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guerrero-Plata A, Casola A, Garofalo RP. 2005. Human metapneumovirus induces a profile of lung cytokines distinct from that of respiratory syncytial virus. J Virol 79:14992–14997. doi: 10.1128/JVI.79.23.14992-14997.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bao X, Liu T, Spetch L, Kolli D, Garofalo RP, Casola A. 2007. Airway epithelial cell response to human metapneumovirus infection. Virology 368:91–101. doi: 10.1016/j.virol.2007.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fiorentino DF, Zlotnik A, Vieira P, Mosmann TR, Howard M, Moore KW, O'Garra A. 1991. IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th1 cells. J Immunol 146:3444–3451. [PubMed] [Google Scholar]
- 41.Miller DM, Klucher KM, Freeman J A, Hausman DF, Fontana D, Williams DE. 2009. Interferon lambda as a potential new therapeutic for hepatitis C. Ann N Y Acad Sci 1182:80–87. doi: 10.1111/j.1749-6632.2009.05241.x. [DOI] [PubMed] [Google Scholar]
- 42.Friborg J, Levine S, Chen C, Sheaffer AK, Chaniewski S, Voss S, Lemm JA, McPhee F. 2013. Combinations of lambda interferon with direct-acting antiviral agents are highly efficient in suppressing hepatitis C virus replication. Antimicrob Agents Chemother 57:1312–1322. doi: 10.1128/AAC.02239-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hou W, Wang X, Ye L, Zhou L, Yang ZQ, Riedel E, Ho WZ. 2009. Lambda interferon inhibits human immunodeficiency virus type 1 infection of macrophages. J Virol 83:3834–3842. doi: 10.1128/JVI.01773-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu MQ, Zhou DJ, Wang X, Zhou W, Ye L, Li J L, Wang YZ, Ho WZ. 2012. IFN-lambda3 inhibits HIV infection of macrophages through the JAK-STAT pathway. PLoS One 7:e35902. doi: 10.1371/journal.pone.0035902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bartlett NW, Buttigieg K, Kotenko SV, Smith GL. 2005. Murine interferon lambdas (type III interferons) exhibit potent antiviral activity in vivo in a poxvirus infection model. J Gen Virol 86:1589–1596. doi: 10.1099/vir.0.80904-0. [DOI] [PubMed] [Google Scholar]
- 46.Honda K, Yanai H, Negishi H, Asagiri M, Sato M, Mizutani T, Shimada N, Ohba Y, Takaoka A, Yoshida N, Taniguchi T. 2005. IRF-7 is the master regulator of type-I interferon-dependent immune responses. Nature 434:772–777. doi: 10.1038/nature03464. [DOI] [PubMed] [Google Scholar]
- 47.Osterlund PI, Pietila TE, Veckman V, Kotenko SV, Julkunen I. 2007. IFN regulatory factor family members differentially regulate the expression of type III IFN (IFN-lambda) genes. J Immunol 179:3434–3442. doi: 10.4049/jimmunol.179.6.3434. [DOI] [PubMed] [Google Scholar]
- 48.Crotta S, Davidson S, Mahlakoiv T, Desmet CJ, Buckwalter MR, Albert ML, Staeheli P, Wack A. 2013. Type I and type III interferons drive redundant amplification loops to induce a transcriptional signature in influenza-infected airway epithelia. PLoS Pathog 9:e1003773. doi: 10.1371/journal.ppat.1003773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mordstein M, Kochs G, Dumoutier L, Renauld JC, Paludan SR, Klucher K, Staeheli P. 2008. Interferon-lambda contributes to innate immunity of mice against influenza A virus but not against hepatotropic viruses. PLoS Pathog 4:e1000151. doi: 10.1371/journal.ppat.1000151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pagliaccetti NE, Chu EN, Bolen CR, Kleinstein SH, Robek MD. 2010. Lambda and alpha interferons inhibit hepatitis B virus replication through a common molecular mechanism but with different in vivo activities. Virology 401:197–206. doi: 10.1016/j.virol.2010.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mahlakõiv T, Ritz D, Mordstein M, DeDiego ML, Enjuanes L, Müller MA, Drosten C, Staeheli P. 2012. Combined action of type I and type III interferon restricts initial replication of severe acute respiratory syndrome coronavirus in the lung but fails to inhibit systemic virus spread. J Gen Virol 93:2601–2605. doi: 10.1099/vir.0.046284-0. [DOI] [PubMed] [Google Scholar]
- 52.Laham FR, Israele V, Casellas JM, Garcia AM, Lac Prugent CM, Hoffman SJ, Hauer D, Thumar B, Name MI, Pascual A, Taratutto N, Ishida MT, Balduzzi M, Maccarone M, Jackli S, Passarino R, Gaivironsky RA, Karron RA, Polack NR, Polack FP. 2004. Differential production of inflammatory cytokines in primary infection with human metapneumovirus and with other common respiratory viruses of infancy. J Infect Dis 189:2047–2056. doi: 10.1086/383350. [DOI] [PubMed] [Google Scholar]
- 53.Biacchesi S, Pham QN, Skiadopoulos MH, Murphy BR, Collins PL, Buchholz UJ. 2005. Infection of nonhuman primates with recombinant human metapneumovirus lacking the SH, G, or M2-2 protein categorizes each as a nonessential accessory protein and identifies vaccine candidates. J Virol 79:12608–12613. doi: 10.1128/JVI.79.19.12608-12613.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Spann KM, Tran KC, Chi B, Rabin RL, Collins PL. 2004. Suppression of the induction of alpha, beta, and lambda interferons by the NS1 and NS2 proteins of human respiratory syncytial virus in human epithelial cells and macrophages [corrected]. J Virol 78:4363–4369. doi: 10.1128/JVI.78.8.4363-4369.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Heinze B, Frey S, Mordstein M, Schmitt-Graff A, Ehl S, Buchholz UJ, Collins PL, Staeheli P, Krempl CD. 2011. Both nonstructural proteins NS1 and NS2 of pneumonia virus of mice are inhibitors of the interferon type I and type III responses in vivo. J Virol 85:4071–4084. doi: 10.1128/JVI.01365-10. [DOI] [PMC free article] [PubMed] [Google Scholar]