ABSTRACT
Human respiratory syncytial virus (RSV) lower respiratory tract infection can result in inflammation and mucus plugging of airways. RSV strain A2-line19F induces relatively high viral load and mucus in mice. The line 19 fusion (F) protein harbors five unique residues compared to the non-mucus-inducing strains A2 and Long, at positions 79, 191, 357, 371, and 557. We hypothesized that differential fusion activity is a determinant of pathogenesis. In a cell-cell fusion assay, line 19 F was more fusogenic than Long F. We changed the residues unique to line 19 F to the corresponding residues in Long F and identified residues 79 and 191 together as responsible for high fusion activity. Surprisingly, mutation of residues 357 or 357 with 371 resulted in gain of fusion activity. Thus, we generated RSV F mutants with a range of defined fusion activity and engineered these into recombinant viruses. We found a clear, positive correlation between fusion activity and early viral load in mice; however, we did not detect a correlation between viral loads and levels of airway mucin expression. The F mutant with the highest fusion activity, A2-line19F-K357T/Y371N, induced high viral loads, severe lung histopathology, and weight loss but did not induce high levels of airway mucin expression. We defined residues 79/191 as critical for line 19 F fusion activity and 357/371 as playing a role in A2-line19F mucus induction. Defining the molecular basis of the role of RSV F in pathogenesis may aid vaccine and therapeutic strategies aimed at this protein.
IMPORTANCE Human respiratory syncytial virus (RSV) is the most important lower respiratory tract pathogen of infants for which there is no vaccine. Elucidating mechanisms of RSV pathogenesis is important for rational vaccine and drug design. We defined specific amino acids in the fusion (F) protein of RSV strain line 19 critical for fusion activity and elucidated a correlation between fusion activity and viral load in mice. Further, we identified two distinct amino acids in F as contributing to the mucogenic phenotype of the A2-line19F virus. Taken together, these results illustrate a role for RSV F in virulence.
INTRODUCTION
Human respiratory syncytial virus (hRSV according to the International Committee on Taxonomy of Viruses; often abbreviated RSV) is a negative-sense, nonsegmented, and single-stranded RNA virus in the Paramyxoviridae family. RSV is the most important viral lower respiratory tract pathogen for infants, causing 16 times more hospitalizations than influenza virus in children under 1 year old (1). There is currently no vaccine licensed to prevent RSV infections; there is also a need for additional therapies, and further knowledge of RSV pathogenesis will enhance efforts toward these goals.
Strains of RSV exhibit differential pathogenesis in the BALB/c mouse model of RSV disease (2–4). A strain called line 19 was originally isolated in 1967 and then passaged in primary chick kidney cells, as well as primary chick lung cells, prior to 72 passages in human MRC-5 fibroblast cells (5). Line 19 exhibits enhanced pathogenesis in mice compared to that in commonly studied laboratory strains A2 and Long (2, 3). This virus strain was sequenced and found to encode 10 amino acids differing from RSV strain Long, six of which are located in the fusion (F) protein (3). Of those six amino acids, five remained unique to line 19 F when the sequence was additionally compared to the A2 F protein (3). Furthermore, these five point mutations are exclusive to line 19 F compared to virtually all other complete RSV F protein sequences in GenBank, suggesting that line 19 is derived from a unique passage history (see Fig. S1 in the supplemental material). None of the residues unique to line 19 F are located in the currently described RSV F antigenic sites (6). Based on the overall striking degree of identity of strains line 19 and Long (16 nucleotide and 10 amino acid differences), it is likely that line 19 is a laboratory passage variant strain derived from the Long strain.
Chimeric strain A2-line19F, composed of the F protein of strain line 19 in the genetic background of strain A2, displays disease symptoms in BALB/c mice that more closely mimic human disease than those of other RSV strains (3). Features of A2-line19F pathogenesis in BALB/c mice include high levels of airway goblet cell hyperplasia, a relatively high peak viral load, airway hyperreactivity, and increased breathing effort compared to that caused by the A2 strain and a chimeric virus harboring the F protein of the Long strain in an A2 strain genetic background (3, 7). Airway mucin induction leading to airway plugging is considered an important sequelae of RSV disease in infants (8, 9). The F mutations unique to line 19 are located at F positions 79 in the F2 subunit, 191 in heptad repeat A, 357 and 371 in the F1 subunit, and 557 in the cytoplasmic tail. We hypothesize that these residues singly or in combination contribute to the differential pathogenesis of chimeric RSV strains A2-line19F and A2-LongF by a fusion activity-based mechanism.
The RSV F protein, like other paramyxovirus F proteins, is responsible for virus-cell fusion as well as for fusion of infected cells with neighboring cells (10). A generally accepted model for paramyxovirus fusion involves insertion of a hydrophobic fusion peptide into the opposing cell membrane in conjunction with major conformational changes, which lead to refolding into a postfusion conformation and membrane merger (6, 11–14). Studies have defined domains and specific residues important for fusion activity of RSV F (15–20); however, the impact of fusion activity on paramyxovirus pathogenesis is not well understood. A pair of reports on Sendai virus demonstrated residues in heptad repeat A of F that enhance or dampen fusion activity and modulate pathogenesis (21, 22). A hyperfusogenic mutation in Sendai virus F resulted in greater pathogenesis and mortality than the wild-type virus in the DBA/2 mouse model used, while a hypofusogenic mutation resulted in lessened mortality and pathogenesis compared to those of the wild-type virus (21).
The A2-line19F BALB/c mouse model of RSV pathogenesis recapitulates airway mucin induction seen in human disease. Using this model and reverse genetics, we changed, singly or in combinations, the unique F residues in line 19 F to the corresponding residues in the Long strain F, a laboratory strain that is nonmucogenic. We identified residues in F that modulate fusion activity. Higher fusion activity in vitro correlated with greater lung viral loads in mice. We also identified F residues that contribute to airway mucin expression and found that mucin induction did not correlate with fusion activity or viral load, suggesting an alternative mechanism for this aspect of pathogenesis. Defining RSV F residues that affect fusion and pathogenesis may guide targeted therapies and rational vaccine designs.
MATERIALS AND METHODS
Cells and mice.
HEp-2 and 293T cells were obtained from ATCC and cultured in minimal essential medium (MEM) containing 10% fetal bovine serum (FBS) and 1 μg/ml penicillin, streptomycin, and amphotericin B (PSA). BEAS-2B bronchial epithelial cells were cultured in RPMI containing 10% FBS and 1 μg/ml PSA, as described previously (4). BSR-T7/5 cells were a gift from Ursula Buchholz (National Institutes of Health, Bethesda, MD) and Karl-Klaus Conzelmann (Ludwig-Maximilians University Munich, Munich, Germany) and were cultured in Glasgow's minimal essential medium (GMEM) containing 10% FBS and 1 μg/ml PSA and supplemented with 1 mg/ml Geneticin every other passage, as described previously (23).
Seven-week-old female BALB/c mice were obtained from the Jackson Laboratory (Bar Harbor, ME). Mice were housed in specific-pathogen-free facilities, and all animal procedures were conducted according to the guidelines of the Emory University Institutional Animal Care and Use Committee.
Plasmids.
Human codon bias-optimized line 19 F and Long F cDNAs were obtained from GeneArt (Life Technologies, Grand Island, NY) and cloned into pcDNA3.1+ expression vector (Life Technologies). Sequences were based on GenBank nucleotide accession numbers FJ614813 (line 19) and FJ614815 (Long) (3). Site-directed mutagenesis was used to mutate residues 79, 191, 357, 371, and 557 in the pcDNA-line19F plasmid to the corresponding residues in the Long F sequence. Plasmids used to express codon-optimized RSV N, P, M2-1, and L were described (24). DSP1-7 and DSP8-11 plasmids were a gift from Naoyuki Kondo (25).
A bacterial artificial chromosome (BAC), which harbors the A2-line19F antigenomic cDNA, was previously described (24). We generated mutants of the A2-line19F virus strain at positions F79, F191, F357, F371, F557, F79/191, and F357/371 by using the recombination-mediated mutagenesis method we described previously (24). Sequences of primers used to amplify galK with RSV homology arms and oligonucleotides homologous to RSV antigenome flanking nucleotides undergoing mutagenesis are available upon request. M79I/R191K and K357T/Y371N double mutants were made by sequential mutagenesis of the BAC at each residue. The RSV F sequence of each BAC was confirmed to contain only the desired mutation prior to proceeding with virus rescue.
Rescue of recombinant viruses.
Recombinant RSV rescue using the BAC constructs and codon-optimized helper plasmids has been described (24). Briefly, each BAC was cotransfected into BSR-T7/5 cells with helper plasmids encoding codon-optimized RSV N, P, M2-1, and L using Lipofectamine 2000 (Life Technologies). Transfected cells were passaged until visible cytopathic effect affected a majority of the cells. The transfected cells were then scraped in their medium and frozen. The harvested material was passaged onto HEp-2 cells at 37°C to generate master and working stocks, as described elsewhere (4). Viral RNA was extracted from working stock samples using the QIAamp viral RNA minikit (Qiagen, Valencia, CA), and F-specific RNA was reverse transcribed using primer F-r (3). F cDNA was amplified using primers F-f and F-r, followed by sequencing to confirm that only the desired mutations were present (3). All viruses rescued in BSR-T7/5 cells, but after 4 failed attempts to grow at 37°C, A2-line19F-K357T/Y371N master and working stocks were grown at 32°C to generate virus titers high enough for experimentation. High-titer (1 × 108 PFU/ml) working stocks of A2-line19F-K357T/Y371N were generated similarly to virus stocks grown at 32°C, except, at the time of harvest, medium was removed until only 5 ml of growth medium remained in the T-175 flask. Cells were scraped in that 5 ml and then sonicated, centrifuged, and aliquoted as previously described (4).
Generation of F protein models.
F structure models are based on the recently published pre- or postfusion F coordinates in the Protein Data Bank (PDB; accession numbers 4JHW and 3RRR) (6, 11). Mac PyMol and UCSF Chimera programs were used for modeling and presentation (26).
Multistep virus growth curves.
Subconfluent BEAS-2B cells in 6-well plates were infected at a multiplicity of infection (MOI) of 0.01 with each virus at room temperature with rocking for 1 h. After the hour of incubation, the inoculum was removed and cells were washed with phosphate-buffered saline (PBS) before 2 ml RPMI supplemented with 10% FBS and 1 μg/ml PSA was added to each well. At 12, 24, 48, 72, 96, or 120 h postinfection, one well of cells infected with each virus was scraped in the medium, aliquoted, and frozen at −80°C. After all samples were collected, viral titers were determined by immunodetection plaque assay, as previously described (4).
Dual-split protein fusion assay.
The dual-split protein (DSP) cell-cell fusion assay was previously described (15, 23). Nearly confluent 293T cells in 6-well plates were transfected using Lipofectamine 2000 with 2 μg each of either DSP1-7 and F expression construct or with DSP8-11 in the presence of the fusion inhibitor BMS-433771 (provided by Jin Hong, Alios Biopharma, San Francisco, CA) (27). Twenty-four hours posttransfection, DSP1-7/F-containing cells were washed in PBS and harvested by pipetting in medium containing EnduRen live-cell substrate (Promega, Madison, WI). DSP8-11-transfected cells were similarly harvested. Equal volumes of each set of cells were mixed and aliquoted into four wells each of an opaque 96-well plate or clear 96-well plate. The plates were incubated at 37°C, and luciferase activity in the opaque plate was recorded on a TopCount Luminescence counter (PerkinElmer, Waltham, MA) at 4, 6, and 8 h post-cell plating. In addition, images of green fluorescent protein (GFP)-expressing syncytia were captured at 8 h post-cell plating in the clear plate.
F protein total and surface expression determination.
Nearly confluent 293T cells in 6-well plates were transfected using Lipofectamine 2000 with 2 μg of each F construct in the presence of BMS-433771 RSV F inhibitor. Twenty-four hours posttransfection, cells were washed in PBS and harvested for either Western blotting or flow cytometry. For Western blots, cells were lysed in 200 μl radioimmunoprecipitation assay buffer (RIPA; Sigma-Aldrich, St. Louis, MO) buffer containing Halt protease inhibitor cocktail (Thermo Scientific, Waltham, MA). Lysates were cleared by centrifugation at 12,000 × g for 5 min and frozen until use. Lysates were loaded onto 10% SDS-PAGE gels and separated via gel electrophoresis. Proteins were transferred to polyvinylidene fluoride (PVDF; Bio-Rad, Hercules, CA) membranes and detected using an RSV F-specific monoclonal antibody, motavizumab (generously provided by Nancy Ulbrandt, MedImmune), and a horseradish peroxidase (HRP)-conjugated anti-human secondary antibody (Jackson ImmunoResearch, West Grove, PA) (28). SuperSignal West Femto chemiluminescent substrate (Thermo Scientific) was used for signal detection. Antibodies were stripped and membranes reprobed with anti-GAPDH clone 6C5 (Life Technologies) and HRP-conjugated anti-mouse secondary antibody (Jackson ImmunoResearch). For flow cytometry, the transfected 293T cells were harvested in PBS by pipetting from the well and transferred to fluorescence-activated cell sorting (FACS) tubes. Cells were washed in PBS containing 2% FBS and 0.1% sodium azide and stained using the RSV F-specific monoclonal antibody palivizumab and a phycoerythrin (PE)-conjugated anti-human secondary antibody or human IgG1, K isotype control (both from Southern Biotech, Birmingham, AL) (29). Flow cytometric analysis was performed using a Becton Dickinson LSRII flow cytometer, and data were analyzed using FlowJo software (Tree Star Inc., Ashland, OR).
In vivo viral load determination, pathology, and mucus induction.
For viral load determination, histopathology, and mucus induction, 7-week-old BALB/c mice were infected with 105 PFU (hypofusogenic viruses) or 106 PFU (hyperfusogenic viruses) of virus. On day 1, 2, 4, 6, or 8 postinfection, mice were euthanized and the left lung was harvested for viral load. Lungs were homogenized using a mini-beadbeater, and virus was titrated by immunodetection plaque assay as previously described (4). For quantification of airway mucin and histopathology analysis, 7-week-old female BALB/c mice were infected with 106 PFU of virus or mock infected. On day 2, 4, or 8 postinfection, both lungs were harvested and fixed in 10% formalin overnight. Following embedding in paraffin blocks, 5-μm-thick sections of each pair of lungs were mounted onto slides and stained with periodic acid-Schiff (PAS) stain or hematoxylin and eosin (H&E). A pathologist blinded to the experimental groups scored the H&E-stained slides for histopathological changes. Scoring for eosinophil recruitment (numbers surrounding arterioles) was as follows: 1 = 1 to 10, 2 = 11 to 30, 3 = 21 to 30, 4 = 31 to 40. Perivascular cuffing was scored as follows: 1 = 1 cell layer, 2 = 2 to 4 cell layers, 3 = 5 to 9 cell layers, 4 = 10+ cell layers. Interstitial pneumonia was scored as based on how thick the alveolar septa appeared, with each score representing the number of leukocyte thickness. Slides were scanned using a Mirax-Midi microscope (Zeiss, Thornwood, NY), as described previously (4). PAS staining was quantified by annotation using HistoQuant software (3D Histotech, Budapest, Hungary), as previously described (4).
For weight monitoring, groups of 10 8-week-old BALB/c mice were infected with 5 × 106 (data not shown) or 1 × 106 PFU A2-line19F or various doses of A2-line19F-K357T/Y371N (5 × 105, 1 × 106, 5 × 106, or 1 × 107 PFU) or mock infected. Weights were measured daily, and body weight was calculated as a percentage of day 0 preinfection weight. On day 8 postinfection, mice were euthanized and left lungs were harvested for homogenization by a mini-beadbeater. Right lungs were sectioned and PAS stained to determine mucin induction. Homogenates from the groups of 5 × 106 PFU were used in a mouse 20-plex Luminex assay (Invitrogen). Assays were performed as directed by the manufacturer, and detection was performed on a Luminex 200 analyzer. Day 1 postinfection lung homogenates from the hyperfusogenic virus viral load time course (see above) were pooled and used in an enzyme-linked immunosorbent assay (ELISA) to determine interferon alpha (IFN-α) levels. PBL Assay Science (Piscataway, NJ) mouse interferon alpha ELISA was performed as directed by the manufacturer's instructions.
RESULTS
In vitro fusion activity.
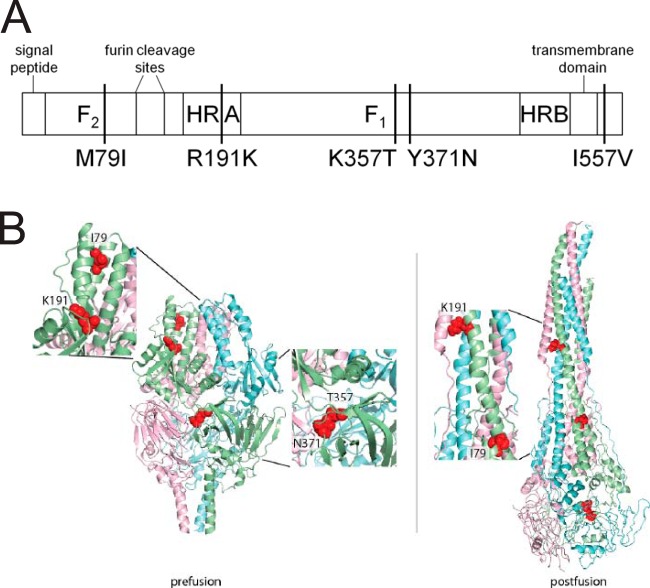
Previous work showed that RSV expressing the F protein of strain line 19 exhibits greater viral load and airway mucin induction in BALB/c mice than RSV expressing the F protein of strain Long and identified five amino acids unique to the line 19 F protein compared to the F proteins of RSV strains A2 and Long (Fig. 1A) (3). We sought to understand how the line 19 F protein was responsible for the differences in pathogenesis between strains A2-line19F and A2-LongF. Modeling the unique line 19 F residues on the recently crystallized structures of pre- or postfusion RSV F posits residues 79 and 191 at a lateral face of the prefusion F head, which undergoes major conformational changes during the fusion process (Fig. 1B) (6, 11). In contrast, residues 357 and 371 are predicted to reside in close proximity in the base of the F head that remains conformationally intact during F refolding. Accordingly, the F rearrangements do not reposition these residues relative to each other in the structural models. Based on these predictions, we also chose to study 79/191 and 357/371 double mutants due to the location of these residues in the pre- and postfusion structures of F (Fig. 1B).
FIG 1.
Amino acid residues unique to line 19 F. (A) Schematic of RSV F, with the major domains and line 19 unique residues indicated. Residues are identified by the amino acid in line 19 F, the position in F, and the amino acid in A2 and Long F (e.g., M79I is methionine in line 19 F and isoleucine in A2 and Long F at position 79). (B) Pre- and postfusion structures of RSV strain A2 F, with residues unique to line 19 F indicated.
In order to test our hypothesis that the F proteins from line 19 and Long strains have different fusion activities, we employed a dual-reporter cell-to-cell fusion assay (15, 23). We first determined if the line 19 and Long F proteins exhibit any differences in total or surface F expression. We measured total F expression by Western blotting (Fig. 2A) and surface F expression by flow cytometry (Fig. 2B). While total and surface expression of Long F was slightly greater than line 19 F, these differences were not statistically significant (Fig. 2A and B). We quantified the cell-to-cell fusion activity of these two proteins using a dual-split protein (DSP) fusion assay. The DSP assay was initially described for use in HIV research (30) but has been used for determining RSV fusion activity (15, 23). In brief, one DSP plasmid expresses the amino terminal portion of Renilla luciferase as well as a portion of GFP, while the other DSP plasmid expresses the remaining portions of both proteins. Major benefits to this system are that the F proteins themselves are not modified by attachment of reporter constructs and that fusion is monitored both visually and quantitatively. When cell content mixing occurs via F-mediated fusion, the two DSPs associate and both GFP expression and luciferase activity are measurable. Using this assay, we found that line 19 F had higher fusion activity than Long F (Fig. 2C and D), consistent with our primary hypothesis that fusion activity of line 19 F is a determinant of pathogenesis.
FIG 2.
F expression and cell-cell fusion activity. (A) 293T cells were transfected with F mutant plasmids in the presence of the fusion inhibitor BMS-433771, and lysates were harvested 24 h posttransfection for F-specific Western blotting. A representative F blot is shown with a GAPDH loading control. The bar graph shows expression as a percentage of line 19 F for three experiments combined. (B) Surface expression of the F mutants determined by flow cytometry of 293T cells harvested 24 h posttransfection with F mutant plasmids in the presence of BMS-433771. The histogram displays results from a single experiment, and the bar graph shows expression as a percentage of line 19 F from three experiments combined. (C and D) F expression plasmids were transfected into 293T cells in the presence of fusion inhibitor, and cell-cell fusion activity was quantified by luciferase activity or monitored by GFP expression. Representative results are shown for luciferase activity (C) and GFP expression (D). Luciferase activity was measured at 4, 6, and 8 h post-cell mixing, indicated from left to right by the three bars for each F construct. Cross bars in panel C indicate groups significantly different compared to line 19 F. *, P < 0.05 by one-way analysis of variance (ANOVA) and Tukey multiple-comparison test. (D) Images of GFP-expressing cells were taken 8 h post-cell mixing.
We next determined which residue differences between line 19 F and Long F are critical for the differential fusion activity observed by changing the residues in the line 19 F protein to the corresponding residues in Long F. We found no significant differences from line 19 F in total or surface expression of the F mutants (Fig. 2A and B). Western blots also indicated no deficiencies in cleavage of the F mutants (data not shown). Quantification of the cell-cell fusion activity of these mutants revealed that the M79I, R191K, Y371N, and I557V mutations had no effect on the cell-to-cell fusion activity of line 19 F (Fig. 2C). The M79I/R191K double mutant displayed fusion activity lower than that of line 19 F, similar to that of Long F, indicating that these residues in combination are important for the fusion activity of line 19 F (Fig. 2C and D). The K357T mutation increased the fusion activity of line 19 F (Fig. 2C and D). This finding was an unexpected gain of function. Additionally, the K357T/Y371N double mutant exhibited even greater fusion activity than the K357T mutant. Taken together, we determined that residues M79 and R191 in combination contribute to the high fusion activity of line 19 F compared to Long F. We also identified residues K357 and K357/Y371 as dampening line 19 F fusion activity relative to that of residues T357 and T357/N371 in F.
Viral replication in vitro and in BALB/c mice.
We focused our further studies on those mutants that altered line 19 F fusion activity by generating recombinant viruses in the A2-line19F backbone with the K357T, M79I/R191K, or K357T/Y371N mutations. We tested viral growth at a low MOI in a human bronchial epithelial cell line, BEAS-2B. A2-LongF exhibited an initial delay in replication but reached peak viral titers similar to A2-line19F (Fig. 3). A2-line19F-K357T and A2-line19F-M79I/R191K replicated similarly to A2-line19F (Fig. 3). We did not test the A2-line19F-K357T/Y371N double mutant for growth in BEAS-2B at 37°C, because growth at 32°C was required to generate the stocks for this virus (see Materials and Methods). The A2-line19F-K357T/Y371N mutant generated large syncytia in vitro, and we used a lower growth temperature in order to limit fusion and increase yield.
FIG 3.
Virus replication in BEAS-2B cells. BEAS-2B cells were infected at an MOI of 0.01 with each virus, and cells were harvested at the designated time points for viral titer quantification. Input virus titer is represented as the zero time point. A composite of three experiments is shown. There were no significant differences between viruses at each time point by one-way ANOVA.
We investigated how modulation of line19 F fusion activity affected lung viral load in BALB/c mice. We infected mice with either the highly fusogenic viruses, A2-line19F-K357T and A2-line19F-K357T/Y371N, or the minimally fusogenic viruses, A2-line19F-M79I/R191K and A2-LongF, and quantified viral load relative to A2-line19F. Based on peak virus stock titers obtainable in cell culture, we used a lower dose (1 × 105 PFU/mouse) of the minimally fusogenic viruses than of the highly fusogenic viruses (1 × 106 PFU/mouse). The A2-line19F-K357T/Y371N mutant, which displayed the highest F protein fusion activity, exhibited lung viral loads greater than A2-line19F and A2-line19F-K357T on days 1 and 2 postinfection, while both highly fusogenic mutants (K357T and K357T/Y371N mutants) had lung viral loads greater than that of A2-line19F on day 4 postinfection (Fig. 4A). Conversely, the viruses with the F proteins exhibiting the lowest fusion activities, A2-line19F-M79I/R191K and A2-LongF, had lower lung viral loads than A2-line19F on days 1 and 2 postinfection (Fig. 4B). Interestingly, the A2-line19F-M79I/R191K mutant did not have a reduced viral load relative to that of A2-line19F on day 4 postinfection, indicating that fusion activity does not correlate with peak viral loads (Fig. 4B). Sequence analysis of A2-line19F-M79I/R191K isolated from these lungs at day 4 revealed that the F gene did not revert to parental line 19. There was no difference in viral clearance of any of the strains, which were cleared (as measured by plaque assay) by day 8 postinfection (data not shown). Taken together, these RSV fusion mutant viruses show that in vitro fusion activity correlates with early, but not peak, lung viral loads in BALB/c mice.
FIG 4.
Lung viral load in BALB/c mice. (A and B) BALB/c mice were inoculated with 106 (A) or 105 (B) PFU of the indicated viruses, and left lungs were harvested on day 1, 2, or 4, postinfection. Viral titers were determined by immunodetection plaque assay. The dotted lines represent the limit of detection. Groups significantly different compared to A2-line19F are indicated by cross bars. Data are combined from three independent experiments, each with 4 or 5 mice per group. (A) *, P < 0.05; **, P < 0.001, by one-way ANOVA with Tukey multiple-comparison test; (B) *, P < 0.01; **, P < 0.001, by one-way ANOVA with Tukey multiple-comparison test.
Airway mucin expression in BALB/c mice.
We investigated the ability of these viruses to induce airway mucin expression by infecting BALB/c mice and performing periodic acid-Schiff (PAS) stains on lung sections. PAS stains mucin and mucin-producing cells, and PAS positivity per airway can be quantified as a measure of mucus induction, as previously published (4, 23). As previously reported, A2-line19F induced greater airway mucus than A2-Long F (Fig. 5A and C) (3). Both viruses A2-line19F-K357T and A2-line19F-M79I/R191K induced greater airway mucus than A2-line19F (Fig. 5A and C). Thus, fusion activity and viral load did not correlate with levels of airway mucin expression. The virus displaying the greatest fusion activity and viral load (A2-line19F-K357T/Y371N) induced levels of mucus lower than that of A2-line19F and similar to that in mock-infected mice (Fig. 5B and D), indicating that residues 357 and 371 act in concert to promote airway mucin expression in this model, though the mechanism is currently unknown. Taken together, these data suggest that neither early viral load nor peak viral load are critical determinants of airway mucin induction in the RSV BALB/c model.
FIG 5.
Airway mucus induction in BALB/c mice. BALB/c mice were inoculated with 105 (A and C) or 106 (B and D) PFU of the indicated viruses, and whole lungs were harvested at day 8 postinfection. Lungs were fixed, sectioned, and stained with periodic acid-Schiff (PAS). (C and D) Magenta coloring indicates PAS-positive airway cells. (A and B) Mucus induction was quantified using 3D Histech software as the percentage of airway positive for PAS staining. (A) Cross bars indicate groups with percentages significantly lower than those of A2-line19F, and asterisks indicate groups with percentages significantly greater than those of A2-line19F. *, P < 0.05; **, P < 0.001. (B) Cross bars indicate groups significantly with percentages lower than those of A2-line19F. Data are a combination of 3 (A) or 2 (B) experiments, and images in panels C and D are representative of experiments in panels A and B, respectively. All data were normalized using an arcsine transformation and analyzed using a one-way ANOVA with Bonferroni multiple-comparison test.
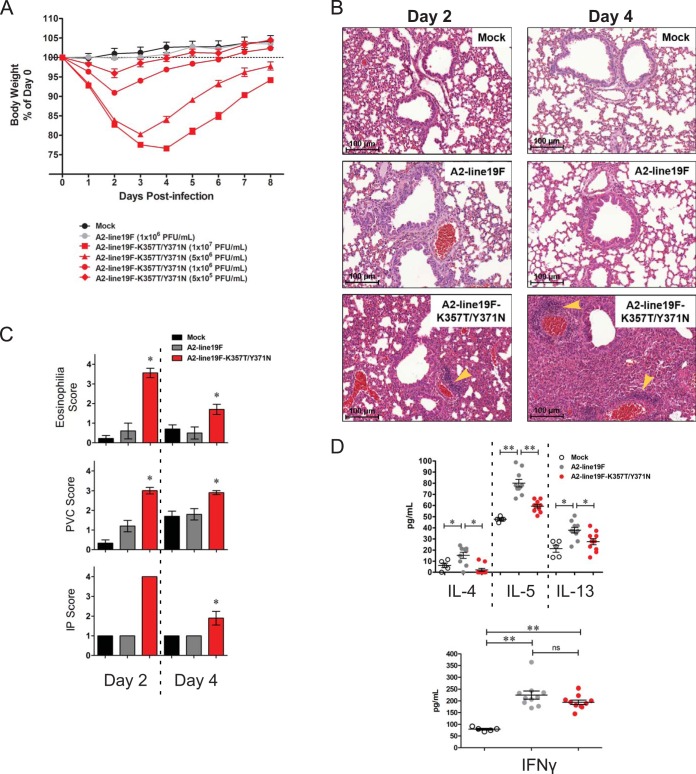
As we observed a marked increase in early viral load in A2-line19F-K357T/Y371N-infected mice compared to A2-line19F-infected mice, we analyzed other parameters of pathogenesis in mice infected with this hyperfusogenic mutant. We infected BALB/c mice with various doses of A2-line19F-K357T/Y371N, a single dose of A2-line19F, or a mock inoculum. By day 2 postinfection, all A2-line19F-K357T/Y371N-infected mice lost significantly more weight than mock-infected or A2-line19F-infected mice (Fig. 6A). This weight loss and subsequent weight recovery in A2-line19F-K357T/Y371N-infected mice was dose dependent (Fig. 6A). On day 8 postinfection, we harvested the lungs of the mice infected with multiple doses of A2-line19F-K357T/Y371N and performed PAS staining and found no differences in mucin induction between the groups (data not shown), suggesting that the initial viral inoculum is not directly responsible for the induction of mucin.
FIG 6.
Pathogenesis of A2-line19F-K357T/Y371N. (A) Groups of 10 BALB/c mice were infected with the indicated doses of A2-line19F or A2-line19F-K357T/Y371N or mock infected, and their weights were monitored daily. (B) Hematoxylin and eosin (H&E)-stained lung sections from BALB/c mice infected with 106 PFU of A2-line19F or A2-line19F-K357T/Y371N or mock infected. Lungs were harvested on day 2 or 4 postinfection. Yellow arrowheads indicate areas of perivascular cuffing (PVC). (C) H&E-stained lung tissues from the infection in panel B were scored by a pathologist blinded to the groups. Scoring for eosinophilia indicates numbers around arterioles. PVC, as indicated in panel B, describes thickness of the leukocyte layer surrounding the vasculature. Interstitial pneumonia (IP) score reflects thickened alveolar septa. See Materials and Methods for a description of the individual scoring guidelines. *, P < 0.05 by one-way ANOVA and Tukey multiple-comparison test. (D) Luminex assay performed on lung homogenates harvested day 8 postinfection from mice infected with 5 × 105 PFU A2-line19F or A2-line19F-K357T/Y371N or mock infected. *, P < 0.05; **, P < 0.01, by one-way ANOVA and Tukey multiple-comparison test.
Although A2-line19F-K357T/Y371N displayed reduced airway mucin induction compared to that of A2-line19F on day 8 postinfection, mice infected with A2-line19F-K357T/Y371N exhibited enhanced lung lesions on days 2 and 4 postinfection (Fig. 6B and C). A2-line19F-K357T/Y371N-infected mice had higher levels of eosinophils, perivascular cuffing (PVC), and interstitial pneumonia (IP) than A2-line19F- or mock-infected mice on days 2 and 4 postinfection (Fig. 6C). The enhanced scores reflect greater numbers of eosinophils surrounding arterioles and thickened leukocyte layers surrounding the vasculature and expanding the alveolar septa. The IP and PVC were characterized by infiltration of neutrophils, monocytes, and lymphocytes, with eosinophils also present in the PVC.
A2-line19F was previously shown to induce the Th2 cytokine interleukin-13 (IL-13), which mediates RSV-induced airway mucus expression (2, 3). Using a multiplex Luminex assay, we measured the levels of the Th2 cytokines IL-4, IL-5, and IL-13 in lung homogenates from mock-, A2-line19F-, or A2-line19F-K357T/Y371N-infected mice on day 8 postinfection. Levels of these Th2 cytokines were lower in A2-line19F-K357T/Y371N lung homogenates than in A2-line19F homogenates, while there was no difference between the groups in levels of the Th1 cytokine interferon gamma (Fig. 6D). Although IL-10 was previously shown to be important in regulating a myriad of cytokines during RSV infection (reviewed in reference 31), we found no differences in IL-10 or IL-12p70 levels induced in A2-line19F- and A2-line19F-K357T/Y371N-infected mice (data not shown). Lower IL-13 levels correlate with the lower levels of airway mucus induced by the A2-line19F-K357T/Y371N virus at this time point (Fig. 5B and D). Although A2-line19F-K357T/Y371N induced eosinophils early after infection, the eosinophil numbers abated by day 8 (data not shown), correlating with the lack of IL-5 at this time point. These data show that infection of mice with the hyperfusogenic A2-line19F-K357T/Y371N results in high viral load and pathogenicity but an altered host response compared to that of A2-line19F.
Because the differences in A2-line19F/A2-line19F-K357T and A2-line19F-K357T/Y371N were established by day 2 postinfection (Fig. 6), we hypothesized that differences in innate immune responses could be responsible for the altered pathogenesis. To this end, we measured interferon alpha levels by ELISA in lung homogenates from day 1 postinfection but found no significant differences between the A2-line19F-K357T- and A2-line19F-K357T/Y371N-infected groups (data not shown). These results do not rule out differential innate immune recognition as the cause of altered pathogenesis between the strains but suggest that the differences are not mediated by type I interferon.
DISCUSSION
We utilized RSV strain A2-line19F to identify amino acids critical to the fusion activity of the RSV F protein and showed that modulating fusion activity can result in changing the dynamics of viral load in BALB/c mice. We used an in vitro cell-to-cell fusion assay to identify residues 79/191 in line 19 F as directly contributing to its fusion activity. We hypothesize that the location of these residues in the F structure impacts their influence on fusion activity. Both residues are located in regions that undergo conformational changes during the prefusion to postfusion transition (Fig. 1B). A recent report highlighted F residue 66, which lies in the same loop as residue 79, as contributing to the fusion activity of RSV strain A2 F (17). In that study, pathogenesis was not tested, but fusogenicity of A2 F could be increased or decreased depending on the residue at position 66 (17). Based on this recent work along with our results and reports on other paramyxovirus proteins, the loop region of F2 containing residue 79 appears to be involved in the mechanism of fusion (17, 32, 33). An individual residue in F2 was implicated in the differential fusogenicity of wild-type measles virus compared to the Edmonston strain of measles virus (33). In 2007, Gardner and Dutch demonstrated that in non-Pneumovirus paramyxoviruses, a conserved region in F2 encompassing residue 79 drastically affected fusion activity in vitro (32). In addition, for human parainfluenza virus 5, they postulated a potential interaction of this region with heptad repeat A (32). Our data are consistent with these findings. A single mutation of RSV line 19 F residue 79 in F2 and single mutation of residue 191 in heptad repeat A had no effect on fusion, but mutating both residues resulted in a decrease in fusion activity of line 19 F. In our study, decreasing the fusion activity of A2-line19F by M79I and R191K mutations affected the day 1 and 2 viral loads in mice, suggesting that a certain threshold of fusion activity is important for early pulmonary infection.
We found that residues F357 or F357 together with F371 impacted RSV fusion activity. Mutating F357 from the lysine (K) in line 19 to the threonine (T) in Long unexpectedly resulted in a gain in fusion activity. Based on the core position of this residue in the F structure (Fig. 1B), our current working hypothesis is that this residue functions as an important determinant for overall conformational stability of the F protein. In our study, we mutated this residue from a basic amino acid, lysine, to uncharged, polar threonine. We speculate that the K-to-T mutation destabilizes the prefusion conformation of RSV F, resulting in a more readily triggered (hyperfusogenic) F protein. The possibility of residue 357 interacting with other internal F residues is implied by our in vitro cell-to-cell fusion assay results with a K357T/Y371N mutant F, which exhibited an even greater hyperfusogenic phenotype. In contrast to the low fusion activity of A2-line19F-M79I/R191K, the highly fusogenic A2-line19-K357T/Y371N exhibited greater viral load than A2-line19F on days 1 and 2 postinfection, leading us to the conclusion that RSV in vitro fusion activity is a determinant of early viral load in mice. The higher viral load, more severe lung lesions, and weight loss caused by A2-line19F-K357T/Y371N make it potentially useful as an RSV challenge strain. Viral load, lung lesions, and weight loss peaked concomitantly at day 4 postinfection with this mutant, whereas RSV viral load typically peaks before disease manifestations in mice infected with other RSV strains. Thus, A2-line19F-K357T/Y371N may be practical for efficacy studies.
Based on several reports implicating viral load as a predictor of disease severity in infants (34–37), we hypothesized that in mice, viruses exhibiting high fusion would also exhibit high viral load and be those that induced severe disease. Using mucus expression alone as a marker of pathogenesis, we found this not to be the case, but when we investigated pathogenesis parameters in addition to mucus, the hypothesis was supported. Neither early nor peak viral load correlated with mucus induction. The A2-line19F-K357T/Y371N mutant virus, with the highest viral load at all time points tested, induced mucus levels similar to those in mock-infected mice (Fig. 4A and 5B). While these results do not support viral load predicting mucus induction in BALB/c mice, the data do implicate residues K357 and Y371 together as critical for mucus induction by A2-line19F. It is possible that a threshold of viral load is necessary to induce airway mucin expression but that the high viral loads of A2-line19F-K357T/Y371N downregulate a pathway leading to airway mucin expression that lower viral loads fail to downregulate.
In contrast to early lung viral load, the mucus response to RSV did not correlate with F protein activity and likely involves orchestrated innate and adaptive immune responses. RSV strain line 19-induced airway mucin expression in mice has been shown to be modulated by immune regulators, such as CXCR2 (38), IL-12 (39), Toll-like receptor 3 (TLR3) (40), CCR1 (41), MyD88 (42), IL-25 (43), and Beclin-1 (44). In our study, compared to A2-line19F, the hypofusogenic A2-line19F-M79I/R191K had lower early viral loads, recovered to a peak viral load equivalent to that of A2-line19F, and induced slightly but significantly more airway mucin expression than A2-line19F. Our working hypothesis is that early innate immune responses in part dictate the later IL-13-dependent mucus response. We speculate that lower antiviral innate responses to A2-line19F-M79I/R191K pave the way for a somewhat exacerbated mucus response.
In the case of hyperfusogenic A2-line19F-K357T/Y371N, which establishes high early viral loads and lung lesions, our working hypothesis is that robust innate immune responses prime for lower Th2 cytokines we observed on day 8 postinfection. We initially suspected that the Th1 cytokine IFN-γ may be elevated in A2-line19F-K357T/Y371N-infected mice, but this was not the case. We are currently investigating what type of innate immune regulators might be differentially activated by A2-line19F-K357T/Y371N compared to A2-line19F. Initial studies measuring early type I interferon levels point away from a type I interferon-mediated difference. Additional studies are needed to characterize the host response to this pathogenic RSV strain. In the case of A2-line19F-K357T, a mutant with higher fusion activity than that of A2-line19F but not as high as that of the 357/371 mutant, we observed higher peak viral loads and higher mucin expression levels than those of A2-line19F. As the early (day 1 and day 2) viral loads of the K357T mutant did not differ significantly from those of A2-line19F, we speculate that the innate response to this mutant is not qualitatively altered and that the exacerbated mucus response may be due to elevated peak antigen load and a quantitative Th2 response. Thus, our model posits that a substantial peak viral load is required for airway mucin expression, consistent with A2-LongF being nonmucogenic, but that early host responses to a more virulent strain (A2-line19F-K357T/Y371N) can set the stage for lower Th2 cytokine and mucin expression.
Single amino acid mutations in F resulting in modulated fusion activity and alterations in pathogenesis are not unprecedented for paramyxoviruses. A study published in 2010 showed that mutation of a single amino acid in Sendai virus F, which created a hyperfusogenic mutant, was also linked to enhanced pulmonary inflammation in vivo (21). A more recent report on parainfluenza virus 5 described a mechanism by which a single amino acid mutation in F causing greater fusion activity also resulted in differential recognition of the virus by the complement pathway (45). We showed that two amino acid changes in F dramatically altered the host response to RSV. Furthermore, we identified regions of F (residues 79/191 and 357/371) outside the 5 described antigenic sites (6) that modulate F function and RSV pathogenesis. This work highlights the need for additional studies on the role of F in RSV pathogenesis, specifically how small sequence changes can cause large alterations in pathogenesis.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by NIH grants 1R01AI087798 (to M.L.M.) and 1U19AI095227 (to R.S.P.), the Children's Center for Immunology and Vaccines, and public health service grant AI083402 from the NIH/NIAID (to R.K.P.).
We thank Carla Shoffeitt for histology technical assistance. We thank Nancy Ulbrandt (MedImmune LLC) for the motavizumab monoclonal antibody. We thank Ursula Buchholz and Karl-Klaus Conzelmann for the BSR-T7/5 cells. We thank Naoyuki Kondo and Zene Matsuda for the DSP1-7/8-11 plasmids. We thank Jin Hong at Alios Biopharma for the BMS-433771 fusion inhibitor. We also thank the Emory Children's Pediatric Research Center flow cytometry core and immunology core, which are supported by Children's Healthcare of Atlanta.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JVI.02472-14.
REFERENCES
- 1.Zhou H, Thompson WW, Viboud CG, Ringholz CM, Cheng PY, Steiner C, Abedi GR, Anderson LJ, Brammer L, Shay DK. 2012. Hospitalizations associated with influenza and respiratory syncytial virus in the United States, 1993-2008. Clin Infect Dis 54:1427–1436. doi: 10.1093/cid/cis211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lukacs NW, Moore ML, Rudd BD, Berlin AA, Collins RD, Olson SJ, Ho SB, Peebles RS Jr. 2006. Differential immune responses and pulmonary pathophysiology are induced by two different strains of respiratory syncytial virus. Am J Pathol 169:977–986. doi: 10.2353/ajpath.2006.051055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moore ML, Chi MH, Luongo C, Lukacs NW, Polosukhin VV, Huckabee MM, Newcomb DC, Buchholz UJ, Crowe JE Jr, Goleniewska K, Williams JV, Collins PL, Peebles RS Jr. 2009. A chimeric A2 strain of respiratory syncytial virus (RSV) with the fusion protein of RSV strain line 19 exhibits enhanced viral load, mucus, and airway dysfunction. J Virol 83:4185–4194. doi: 10.1128/JVI.01853-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stokes KL, Chi MH, Sakamoto K, Newcomb DC, Currier MG, Huckabee MM, Lee S, Goleniewska K, Pretto C, Williams JV, Hotard A, Sherrill TP, Peebles RS Jr, Moore ML. 2011. Differential pathogenesis of respiratory syncytial virus (RSV) clinical isolates in BALB/c mice. J Virol 85:5782–5793. doi: 10.1128/JVI.01693-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Herlocher ML, Ewasyshyn M, Sambhara S, Gharaee-Kermani M, Cho D, Lai J, Klein M, Maassab HF. 1999. Immunological properties of plaque purified strains of live attenuated respiratory syncytial virus (RSV) for human vaccine. Vaccine 17:172–181. doi: 10.1016/S0264-410X(98)00155-8. [DOI] [PubMed] [Google Scholar]
- 6.McLellan JS, Chen M, Leung S, Graepel KW, Du X, Yang Y, Zhou T, Baxa U, Yasuda E, Beaumont T, Kumar A, Modjarrad K, Zheng Z, Zhao M, Xia N, Kwong PD, Graham BS. 2013. Structure of RSV fusion glycoprotein trimer bound to a prefusion-specific neutralizing antibody. Science 340:1113–1117. doi: 10.1126/science.1234914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boyoglu-Barnum S, Gaston KA, Todd SO, Boyoglu C, Chirkova T, Barnum TR, Jorquera P, Haynes LM, Tripp RA, Moore ML, Anderson LJ. 2013. A respiratory syncytial virus (RSV) anti-G protein F(ab′)2 monoclonal antibody suppresses mucous production and breathing effort in RSV rA2-line19F-infected BALB/c mice. J Virol 87:10955–10967. doi: 10.1128/JVI.01164-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aherne W, Bird T, Court SD, Gardner PS, McQuillin J. 1970. Pathological changes in virus infections of the lower respiratory tract in children. J Clin Pathol 23:7–18. doi: 10.1136/jcp.23.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson JE, Gonzales RA, Olson SJ, Wright PF, Graham BS. 2007. The histopathology of fatal untreated human respiratory syncytial virus infection. Mod Pathol 20:108–119. doi: 10.1038/modpathol.3800725. [DOI] [PubMed] [Google Scholar]
- 10.Collins PL, Crowe JE Jr. 2007. Respiratory syncytial virus and metapneumovirus, p 1601-1646. In Knipe DM, Howley PM (ed), Fields virology, vol 2 Lippincott, Williams, and Wilkins, Philadelphia, PA. [Google Scholar]
- 11.McLellan JS, Yang Y, Graham BS, Kwong PD. 2011. Structure of respiratory syncytial virus fusion glycoprotein in the postfusion conformation reveals preservation of neutralizing epitopes. J Virol 85:7788–7796. doi: 10.1128/JVI.00555-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yin HS, Wen X, Paterson RG, Lamb RA, Jardetzky TS. 2006. Structure of the parainfluenza virus 5 F protein in its metastable, prefusion conformation. Nature 439:38–44. doi: 10.1038/nature04322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Swanson KA, Settembre EC, Shaw CA, Dey AK, Rappuoli R, Mandl CW, Dormitzer PR, Carfi A. 2011. Structural basis for immunization with postfusion respiratory syncytial virus fusion F glycoprotein (RSV F) to elicit high neutralizing antibody titers. Proc Natl Acad Sci U S A 108:9619–9624. doi: 10.1073/pnas.1106536108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steinhauer DA, Plemper RK. 2012. Structure of the primed paramyxovirus fusion protein. Proc Natl Acad Sci U S A 109:16404–16405. doi: 10.1073/pnas.1214903109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baviskar PS, Hotard AL, Moore ML, Oomens AG. 2013. The respiratory syncytial virus fusion protein targets to the perimeter of inclusion bodies and facilitates filament formation by a cytoplasmic tail-dependent mechanism. J Virol 87:10730–10741. doi: 10.1128/JVI.03086-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gonzalez-Reyes L, Ruiz-Arguello MB, Garcia-Barreno B, Calder L, Lopez JA, Albar JP, Skehel JJ, Wiley DC, Melero JA. 2001. Cleavage of the human respiratory syncytial virus fusion protein at two distinct sites is required for activation of membrane fusion. Proc Natl Acad Sci U S A 98:9859–9864. doi: 10.1073/pnas.151098198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lawlor HA, Schickli JH, Tang RS. 2013. A single amino acid in the F2 subunit of respiratory syncytial virus fusion protein alters growth and fusogenicity. J Gen Virol 94(Part 12):2627–2635. doi: 10.1099/vir.0.055368-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li P, Mc LRHW, Brown G, Sugrue RJ. 2007. Functional analysis of the N-linked glycans within the fusion protein of respiratory syncytial virus. Methods Mol Biol 379:69–83. doi: 10.1007/978-1-59745-393-6_5. [DOI] [PubMed] [Google Scholar]
- 19.Martin D, Calder LJ, Garcia-Barreno B, Skehel JJ, Melero JA. 2006. Sequence elements of the fusion peptide of human respiratory syncytial virus fusion protein required for activity. J Gen Virol 87:1649–1658. doi: 10.1099/vir.0.81715-0. [DOI] [PubMed] [Google Scholar]
- 20.Zimmer G, Trotz I, Herrler G. 2001. N-glycans of F protein differentially affect fusion activity of human respiratory syncytial virus. J Virol 75:4744–4751. doi: 10.1128/JVI.75.10.4744-4751.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luque LE, Bridges OA, Mason J N, Boyd KL, Portner A, Russell CJ. 2010. Residues in the heptad repeat a region of the fusion protein modulate the virulence of Sendai virus in mice. J Virol 84:810–821. doi: 10.1128/JVI.01990-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luque LE, Russell CJ. 2007. Spring-loaded heptad repeat residues regulate the expression and activation of paramyxovirus fusion protein. J Virol 81:3130–3141. doi: 10.1128/JVI.02464-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stokes KL, Currier MG, Sakamoto K, Lee S, Collins PL, Plemper RK, Moore ML. 2013. The respiratory syncytial virus fusion protein and neutrophils mediate the airway mucin response to pathogenic respiratory syncytial virus infection. J Virol 87:10070–10082. doi: 10.1128/JVI.01347-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hotard AL, Shaikh FY, Lee S, Yan D, Teng MN, Plemper RK, Crowe JE Jr, Moore ML. 2012. A stabilized respiratory syncytial virus reverse genetics system amenable to recombination-mediated mutagenesis. Virology 434:129–136. doi: 10.1016/j.virol.2012.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ishikawa H, Meng F, Kondo N, Iwamoto A, Matsuda Z. 2012. Generation of a dual-functional split-reporter protein for monitoring membrane fusion using self-associating split GFP. Protein Eng Des Sel 25:813–820. doi: 10.1093/protein/gzs051. [DOI] [PubMed] [Google Scholar]
- 26.Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE. 2004. UCSF Chimera—a visualization system for exploratory research and analysis. J Comput Chem 25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 27.Cianci C, Langley DR, Dischino DD, Sun Y, Yu KL, Stanley A, Roach J, Li Z, Dalterio R, Colonno R, Meanwell NA, Krystal M. 2004. Targeting a binding pocket within the trimer-of-hairpins: small-molecule inhibition of viral fusion. Proc Natl Acad Sci U S A 101:15046–15051. doi: 10.1073/pnas.0406696101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abarca K, Jung E, Fernandez P, Zhao L, Harris B, Connor EM, Losonsky GA. 2009. Safety, tolerability, pharmacokinetics, and immunogenicity of motavizumab, a humanized, enhanced-potency monoclonal antibody for the prevention of respiratory syncytial virus infection in at-risk children. Pediatr Infect Dis J 28:267–272. doi: 10.1097/INF.0b013e31818ffd03. [DOI] [PubMed] [Google Scholar]
- 29.Johnson S, Oliver C, Prince GA, Hemming VG, Pfarr DS, Wang SC, Dormitzer M, O'Grady J, Koenig S, Tamura JK, Woods R, Bansal G, Couchenour D, Tsao E, Hall WC, Young JF. 1997. Development of a humanized monoclonal antibody (MEDI-493) with potent in vitro and in vivo activity against respiratory syncytial virus. J Infect Dis 176:1215–1224. doi: 10.1086/514115. [DOI] [PubMed] [Google Scholar]
- 30.Kondo N, Miyauchi K, Meng F, Iwamoto A, Matsuda Z. 2010. Conformational changes of the HIV-1 envelope protein during membrane fusion are inhibited by the replacement of its membrane-spanning domain. J Biol Chem 285:14681–14688. doi: 10.1074/jbc.M109.067090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lotz MT, Peebles RS Jr. 2012. Mechanisms of respiratory syncytial virus modulation of airway immune responses. Curr Allergy Asthma Rep 12:380–387. doi: 10.1007/s11882-012-0278-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gardner AE, Dutch RE. 2007. A conserved region in the F(2) subunit of paramyxovirus fusion proteins is involved in fusion regulation. J Virol 81:8303–8314. doi: 10.1128/JVI.00366-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Plemper RK, Compans RW. 2003. Mutations in the putative HR-C region of the measles virus F2 glycoprotein modulate syncytium formation. J Virol 77:4181–4190. doi: 10.1128/JVI.77.7.4181-4190.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Devincenzo JP. 2004. Natural infection of infants with respiratory syncytial virus subgroups A and B: a study of frequency, disease severity, and viral load. Pediatr Res 56:914–917. doi: 10.1203/01.PDR.0000145255.86117.6A. [DOI] [PubMed] [Google Scholar]
- 35.DeVincenzo J P, El Saleeby CM, Bush AJ. 2005. Respiratory syncytial virus load predicts disease severity in previously healthy infants. J Infect Dis 191:1861–1868. doi: 10.1086/430008. [DOI] [PubMed] [Google Scholar]
- 36.DeVincenzo JP, Wilkinson T, Vaishnaw A, Cehelsky J, Meyers R, Nochur S, Harrison L, Meeking P, Mann A, Moane E, Oxford J, Pareek R, Moore R, Walsh E, Studholme R, Dorsett P, Alvarez R, Lambkin-Williams R. 2010. Viral load drives disease in humans experimentally infected with respiratory syncytial virus. Am J Respir Crit Care Med 182:1305–1314. doi: 10.1164/rccm.201002-0221OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.El Saleeby CM, Bush AJ, Harrison LM, Aitken J A, Devincenzo JP. 2011. Respiratory syncytial virus load, viral dynamics, and disease severity in previously healthy naturally infected children. J Infect Dis 204:996–1002. doi: 10.1093/infdis/jir494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miller AL, Strieter RM, Gruber AD, Ho SB, Lukacs NW. 2003. CXCR2 regulates respiratory syncytial virus-induced airway hyperreactivity and mucus overproduction. J Immunol 170:3348–3356. doi: 10.4049/jimmunol.170.6.3348. [DOI] [PubMed] [Google Scholar]
- 39.Tekkanat KK, Maassab H, Berlin AA, Lincoln PM, Evanoff HL, Kaplan MH, Lukacs NW. 2001. Role of interleukin-12 and stat-4 in the regulation of airway inflammation and hyperreactivity in respiratory syncytial virus infection. Am J Pathol 159:631–638. doi: 10.1016/S0002-9440(10)61734-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rudd BD, Smit JJ, Flavell RA, Alexopoulou L, Schaller MA, Gruber A, Berlin AA, Lukacs NW. 2006. Deletion of TLR3 alters the pulmonary immune environment and mucus production during respiratory syncytial virus infection. J Immunol 176:1937–1942. doi: 10.4049/jimmunol.176.3.1937. [DOI] [PubMed] [Google Scholar]
- 41.Miller AL, Gerard C, Schaller M, Gruber AD, Humbles AA, Lukacs NW. 2006. Deletion of CCR1 attenuates pathophysiologic responses during respiratory syncytial virus infection. J Immunol 176:2562–2567. doi: 10.4049/jimmunol.176.4.2562. [DOI] [PubMed] [Google Scholar]
- 42.Rudd BD, Schaller MA, Smit JJ, Kunkel SL, Neupane R, Kelley L, Berlin AA, Lukacs NW. 2007. MyD88-mediated instructive signals in dendritic cells regulate pulmonary immune responses during respiratory virus infection. J Immunol 178:5820–5827. doi: 10.4049/jimmunol.178.9.5820. [DOI] [PubMed] [Google Scholar]
- 43.Petersen BC, Dolgachev V, Rasky A, Lukacs NW. 2014. IL-17E (IL-25) and IL-17RB promote respiratory syncytial virus-induced pulmonary disease. J Leukoc Biol. 95:809–815. doi: 10.1189/jlb.0913482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reed M, Morris SH, Jang S, Mukherjee S, Yue Z, Lukacs NW. 2013. Autophagy-inducing protein beclin-1 in dendritic cells regulates CD4 T cell responses and disease severity during respiratory syncytial virus infection. J Immunol 191:2526–2537. doi: 10.4049/jimmunol.1300477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Johnson JB, Schmitt AP, Parks GD. 2013. Point mutations in the paramyxovirus F protein that enhance fusion activity shift the mechanism of complement-mediated virus neutralization. J Virol 87:9250–9259. doi: 10.1128/JVI.01111-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.