ABSTRACT
Natural hosts of simian immunodeficiency virus (SIV), such as African green monkeys (AGMs), do not progress to AIDS when infected with SIV. This is associated with an absence of a chronic type I interferon (IFN-I) signature. It is unclear how the IFN-I response is downmodulated in AGMs. We longitudinally assessed the capacity of AGM blood cells to produce IFN-I in response to SIV and herpes simplex virus (HSV) infection. Phenotypes and functions of plasmacytoid dendritic cells (pDCs) and other mononuclear blood cells were assessed by flow cytometry, and expression of viral sensors was measured by reverse transcription-PCR. pDCs displayed low BDCA-2, CD40, and HLA-DR expression levels during AGM acute SIV (SIVagm) infection. BDCA-2 was required for sensing of SIV, but not of HSV, by pDCs. In acute infection, AGM peripheral blood mononuclear cells (PBMCs) produced less IFN-I upon SIV stimulation. In the chronic phase, the production was normal, confirming that the lack of chronic inflammation is not due to a sensing defect of pDCs. In contrast to stimulation by SIV, more IFN-I was produced upon HSV stimulation of PBMCs isolated during acute infection, while the frequency of AGM pDCs producing IFN-I upon in vitro stimulation with HSV was diminished. Indeed, other cells started producing IFN-I. This increased viral sensing by non-pDCs was associated with an upregulation of Toll-like receptor 3 and IFN-γ-inducible protein 16 caused by IFN-I in acute SIVagm infection. Our results suggest that, as in pathogenic SIVmac infection, SIVagm infection mobilizes bone marrow precursor pDCs. Moreover, we show that SIV infection modifies the capacity of viral sensing in cells other than pDCs, which could drive IFN-I production in specific settings.
IMPORTANCE The effects of HIV/SIV infections on the capacity of plasmacytoid dendritic cells (pDCs) to produce IFN-I in vivo are still incompletely defined. As IFN-I can restrict viral replication, contribute to inflammation, and influence immune responses, alteration of this capacity could impact the viral reservoir size. We observed that even in nonpathogenic SIV infection, the frequency of pDCs capable of efficiently sensing SIV and producing IFN-I was reduced during acute infection. We discovered that, concomitantly, cells other than pDCs had increased abilities for viral sensing. Our results suggest that pDC-produced IFN-I upregulates viral sensors in bystander cells, the latter gaining the capacity to produce IFN-I. These results indicate that in certain settings, cells other than pDCs can drive IFN-I-associated inflammation in SIV infection. This has important implications for the understanding of persistent inflammation and the establishment of viral reservoirs.
INTRODUCTION
Type I interferons (IFN-I) play an important role in human immunodeficiency virus (HIV) and simian immunodeficiency virus (SIV) infections in humans and macaques (MACs). IFN-I can limit viral replication through several mechanisms, including upregulation of restriction factors (1), maturation of antigen-presenting cells (2), activation of NK cells (3), and induction of apoptosis in infected cells (4). Indeed, exogenous administration of IFN-α in chronically infected HIV patients decreases viremia, often by around 75% (4, 5). Similarly, IFN-α administration in the chronic phase of SIVagm (African green monkey) or SIVsmm (sootey mangabey monkey) infection reduced viral loads (6, 7). In one study, it suppressed the viral rebound in HIV patients upon antiretroviral treatment interruption (8). Moreover, IFN-I blockage at the time of SIVmac exposure leads to an increased viral reservoir and accelerated progression to AIDS (9). However, IFN-I production can also be detrimental: IFN-I can contribute to chronic immune activation (IA) (10), induce apoptosis of uninfected bystander cells (11), and drive cells into a short-lived effector cell differentiation program (12). Interferon-stimulated gene (ISG) expression is associated with disease progression in HIV infection (12–15). Evidence for the detrimental role of chronic IFN-I responses has come from the natural hosts of SIV, such as African green monkeys (AGMs) and sooty mangabeys (SMs) (16). These natural hosts have asymptomatic SIV infections, despite viremia levels similar to those found in HIV/SIVmac infections. Nonpathogenic SIV infection in natural hosts is associated with a lack of chronic inflammation (17, 18). This is despite a robust inflammatory response, exemplified by an increase in ISG expression and production of cytokines, such as interleukin-15 (IL-15), during acute infection (6, 19, 20). However, this inflammatory response is downmodulated by the end of the acute phase of infection. Peak IFN-I levels have been found to be lower in the plasma and lymph node cells of SIV-infected AGMs than in MACs, indicating differences in IFN-I production between the two species in vivo (20, 21). Nonetheless, exogenous injection of IFN-I at the peak of IFN-I production did not alter the ISG profile observed in AGMs (6). Moreover, AGM and SM plasmacytoid dendritic cells (pDCs) produced high IFN-I levels upon in vitro stimulation with synthetic Toll-like receptor 7 or 9 (TLR7/9) ligands, SIV, or herpes simplex virus 1 (HSV-1) (20–23).
pDCs are responsible for the majority of IFN-I production in blood upon in vitro stimulation with HIV/SIV or HSV (24–26). In chronically infected HIV patients, pDCs express increased levels of IFN-I mRNA and protein compared to healthy individuals (27, 28). pDCs sense HIV mainly through endosomal TLR7, although a role for TLR9 has not been excluded (29, 30). Blocking endosomal signaling decreased IFN-I levels and IA in treated immunological nonresponders (31). In acute SIV infections, pDCs are the main IFN-I-producing cells in lymph nodes (21, 24, 32).
HIV/SIV infections have an impact on pDC distribution, phenotype, and function. It has been shown in SIVmac and SIVagm models that activated pDCs are rapidly recruited from the circulation to lymph nodes (6, 23, 33). Simultaneously, precursor pDCs rapidly egress from the bone marrow during acute SIVmac infection (24, 33). Precursor pDCs have a diminished capacity to produce IFN-I upon viral stimulation (24, 34). In line with this, human pDCs were found to produce less IFN-I upon HSV stimulation during late acute HIV infection, i.e., Fiebig stages III and IV (35, 36). This could also be explained by pDCs becoming refractory after HIV encounter (26). However, another study using purified pDCs reported an increased capacity to produce IFN-I upon AT2-inactivated HIV stimulation during postacute HIV infection (Fiebig stages V and VI) (37). In vitro, HIV-1 is contained in an early endosomal compartment of pDCs, preventing pDC maturation in association with a continued IFN-I-producing capacity (38, 39). AGM peripheral blood mononuclear cells (PBMCs) and lymph node cells collected during acute SIV infection mounted a stronger IFN-I response upon HSV stimulation than cells from uninfected animals (23). Thus, contradictory data exist on the IFN-I-producing capacity of pDCs during acute HIV/SIV infections. The capacity of natural host pDCs to produce IFN-I in response to SIV during acute infection has not been investigated.
In this study, we investigated the effects of SIV infection on AGM pDC function to identify events that could explain the different inflammatory profiles found in asymptomatic SIVagm infection compared to pathogenic HIV/SIV infections. We assessed the IFN-I-producing capacity of pDCs collected during SIVagm infection in response to in vitro stimulation with SIV and HSV. Our data suggest that precursor pDCs, with a decreased IFN-I-producing capacity, circulate during acute SIVagm infection. We found that low BDCA-2 expression on pDCs could specifically block the sensing of SIV. Furthermore, we discovered that cells other than pDCs increased their capacity to sense virus during acute infection.
MATERIALS AND METHODS
Ethics statement.
All animals were housed in the facilities of the Commissariat à l'Energie Atomique (CEA), Fontenay-aux-Roses, France (permit number A 92-032-02) or of the Institut Pasteur, Paris, France (permit number A 78-100-3). All experimental procedures were conducted in strict accordance with the international European guidelines 2010/63/UE for protection of animals used for experimentation and other scientific purposes (French decree 2013-118). The CEA complies with Standards for Human Care and Use of Laboratory Animals of the U.S. Office for Laboratory Animal Welfare under OLAW assurance number A5826-01. All animal experimental protocols were approved by the Ethical Committee of Animal Experimentation (CETEA-DSV, IDF, France; notification numbers 10-051b and 12-006).
Animals and sample collection.
Twenty-four African green monkeys (Chlorocebus sabaeus) of Caribbean origin and 10 Chinese rhesus macaques (Macaca mulatta) were used. Blood was collected by venipuncture into tubes with sodium heparin that were then shipped to Institut Pasteur. Fifteen AGMs were infected via intravenous inoculation with 250 50% tissue culture infective doses (TCID50) of SIVagm.sab92018 and displayed high viremia levels, as described previously (6, 40). For eight AGMs, blood was drawn at days 2, 4, 7, 9, 11, 14, 25, 31, 59, 85, 122, 183, 241, and 354 postinfection (p.i.). Three more AGMs were sampled at days 7, 14, 31, and 65 p.i. Another four AGMs were sampled at days 2, 4, 7, 9, 11, 14, 28, 43, and 58 p.i.
Stimulations.
PBMCs were isolated from whole blood in sodium heparin after 1:1 dilution in phosphate-buffered saline (PBS; Life Technologies), using lymphocyte separation medium (Eurobio). Cells were spun down at 1,100 × g for 10 min in 50-ml Leucosep tubes (Greiner Bio-one), and buffy coats were collected. Residual red blood cells were lysed for 6 min at room temperature in a sterile filtered buffer consisting of 1 g/liter potassium bicarbonate, 8.3 g/liter ammonium chloride (both from Sigma), and 1 mM EDTA (pH 8; Life Technologies). Cells were cultured at 0.5 × 106 cells/well in 24-well plates (Costar) at 37°C, 5% CO2 and at 106 cells/ml in RPMI 1640 plus Glutamax medium (Life Technologies) with 10% heat-inactivated fetal calf serum (FCS; PAA or Eurobio) and penicillin-streptomycin (Life Technologies). SIVagm.sab92018 or SIVmac251 was added at a concentration of 1,500 ng/ml p27 (5 × 106 TCID50/ml for SIVagm), and HSV-1 was added at 106 TCID50/ml. Importantly, when exposing PBMCs in vitro to HSV, the majority of IFN-I comes from pDCs. For this reason, HSV has been, and still is, frequently used to measure the capacity of pDCs to produce IFN-α or IFN-I (24, 35, 36, 41, 42). A151, a gift from Olivier Schwartz (Institut Pasteur, Paris, France), and G-ODN were added to the cells simultaneously with the virus to block the TLR7 and TLR9 pathways, respectively. Sodium azide was removed from antibodies against IFN-αR2 (MMHAR-2; Pestka Biological Laboratories) and BDCA-2 (AC144; Miltenyi) by using PD Minitrap G-25 columns (GE Healthcare Life Sciences), followed by preincubation with these antibodies for 1 hour at 37°C before stimulation with virus. Previously frozen plasma in EDTA from five AGMs per time point was pooled for incubations (4 h at 37°C) with PBMCs. pDCs were depleted from PBMCs by using anti-BDCA-2 beads and magnetic stands (Miltenyi) according to the manufacturer's instructions. PBMCs were mock stimulated in medium for each of the experiments to assess spontaneous production of IFN-I.
Functional IFN-α assay.
Bioactive IFN-I was quantified as described earlier (23, 43). In short, Mardin-Darby bovine kidney (MDBK) cells were incubated with UV-inactivated supernatants for 18 h, after which the cytopathic effect of vesicular stomatic virus was determined using the CellTiter 96 AQueous nonradioactive cell proliferation assay (Promega). The 50% inhibitory concentrations (IC50s) were calculated by normalization to a standard using R, version 2.15.3, and the drc package (44).
Flow cytometry.
The following antibodies were used: CD3 (SP34-2), HLA-DR (L243), CD123 (7G3), and CD45 (D058-1283) (all from BD Biosciences); CD20 (2H7; eBioscience); BDCA-2 (AC144) and IFN-α2 (LT27:295; both from Miltenyi). FcR blocking reagent (Miltenyi) was used to block unspecific antibody binding. For intracellular staining, cells were stimulated for 2 h, after which brefeldin A was added and cells were incubated for another 4 h at 37°C. Then, cells were labeled with surface-binding antibodies for 10 min at room temperature. After fixation with 4% paraformaldehyde for 6 min at 37°C, cells were permeabilized using 10% saponin and incubated with anti-IFN-α antibody for 15 min at room temperature. Events were collected on an LSR-II flow cytometer (BD) running FACS Diva 6.0 software (BD) and analyzed with the FlowJo 9.4.10 program (TreeStar). Anti-mouse compensation beads (BD Biosciences) were used to define compensation levels.
Quantification of relative gene expression.
mRNA levels were quantified using reverse transcription-PCR as described previously (17), on a 7500 real-time PCR machine (Applied Biosystems). Briefly, mRNA was reverse transcribed with the high-capacity cDNA reverse transcription kit (Life Technologies), followed by quantitative PCR in triplicate with TaqMan gene expression assays (Life Technologies). The expression of each gene was normalized against that of 18S rRNA, and relative expression levels were calculated using the ΔΔCT method.
Statistical analyses.
Statistical inference analyses were performed using Prism 5.0 (GraphPad). For paired testing of multiple groups without missing data, a Friedman test, followed by Dunn's multiple-comparison test, was employed. In cases with missing data, the nonparametric Wilcoxon matched-pairs signed-rank test (Wilcoxon) was used to test paired observations with no multiple-testing correction. The correlations between two continuous variables were assessed with the nonparametric Spearman test.
RESULTS
SIVagm infection impacts the IFN-I-producing capacity during the acute phase.
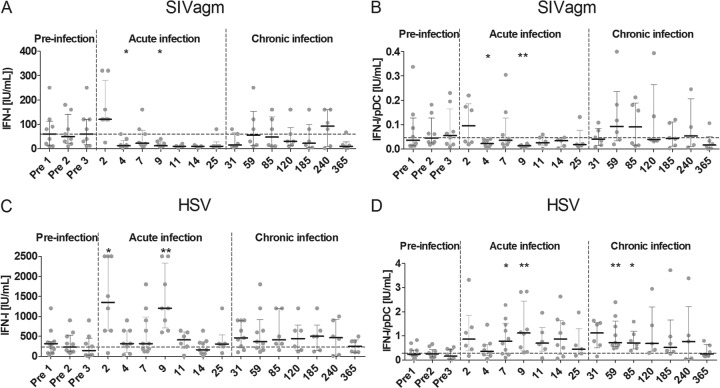
To determine the effect of SIV infection on AGM pDC function in vivo, we collected PBMCs at different stages before and after infection and stimulated them with SIVagm in vitro. We compared IFN-I production to that following stimulation with another virus, HSV. PBMCs were collected at three time points before infection to get a stable baseline. To be able to get information on the earliest and later events after infection, cells were collected and stimulated at seven time points during acute infection and seven time points during chronic infection. Spontaneous IFN-I production by mock-stimulated PBMCs was never observed, even at days 2 and 9 p.i., when high IFN-I levels were present in the plasma. The AGM pDC response to in vitro SIV stimulation diminished during acute SIV infection, starting at day 4 p.i. (Fig. 1A). This diminished response persisted throughout acute infection until day 31 p.i. The median IFN-I level upon stimulation with SIVagm before infection was 78 IU/ml, which decreased to 10 IU/ml at the nadir, on days 11, 14, and 25 p.i. Statistically significant decreases were found at days 4 and 9 p.i. (Wilcoxon, P = 0.03 and P = 0.02, respectively), compared to the preinfection values. As pDCs have been reported to be depleted from blood in acute HIV-1, SIVmac, and SIVagm infections (33, 45), we corrected for pDC counts (Fig. 1B). Correction for pDC numbers did not change the observed decreased responsiveness to SIV during acute infection. The median of 0.057 IU/ml IFN-I per pDC before infection decreased to 0.022 IU/ml at day 4 p.i. (Wilcoxon; P = 0.04) and 0.014 IU/ml at day 9 p.i. (Wilcoxon, P = 0.008). The decrease observed in the acute phase was temporary, as the median IFN-I level in the chronic phase (after day 25 p.i.) returned to close to preinfection levels, 0.044 IU/ml per pDC.
FIG 1.
Dynamics of IFN-I-producing capacity during SIVagm infection. We stimulated AGM PBMCs collected before and during the course of SIVagm infection and measured IFN-I production in the supernatant. The x axis displays the day postinfection at which cells were isolated and stimulated with SIV (A and B) or HSV (C and D). The y axis shows the total IFN-I produced after 18 h of stimulation (A and C) or the same IFN-I production corrected for the number of plasmacytoid dendritic cells among the PBMCs (B and D). The dashed horizontal line shows the median for all preinfection time point stimulations. The dashed vertical lines separate the stages of infection. Symbols represent individual animals (n = 5 to 11 for each time point). *, Wilcoxon, P < 0.05; **, Wilcoxon, P < 0.01. The P values indicate significant differences compared to the preinfection baseline.
Upon in vitro stimulation with HSV of PBMCs collected in acute SIVagm infection samples, we observed an increase in the levels of IFN-I produced compared to levels before infection (Fig. 1C). At days 2 and 9 p.i., there was a significant increase in IFN-I production, with levels of 1,350 IU/ml (Wilcoxon, P = 0.02) and 1,200 IU/ml IFN-I (Wilcoxon, P = 0.008), respectively, compared to the median preinfection level of 239 IU/ml IFN-I. When correcting for pDC numbers, we observed a more sustained increase in IFN-I production by PBMCs throughout the acute and early chronic phases of infection (Fig. 1D). So, while the IFN-I response to SIV was decreased, the responsiveness to HSV was increased during acute SIVagm infection.
An altered capacity to sense virus during acute SIVagm infection.
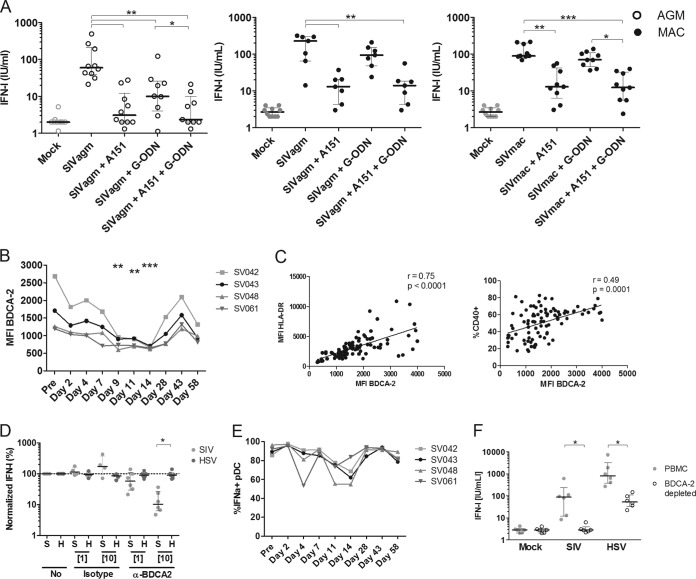
In order to understand the mechanisms underlying this differential response to SIV and HSV, we first investigated the hypothesis that the SIV-sensing machinery of AGM pDCs is specifically altered by SIV infection, leading to a decreased capacity to respond to SIV, but not to HSV. HIV and HSV are mainly sensed by two distinct receptors in human pDCs: TLR7 and TLR9, respectively (29, 30). We investigated whether the sensing of SIV by AGM pDCs also occurs through endosomal TLRs, by stimulating PBMCs of healthy AGMs with SIV in the presence of antagonists to TLR7 (A151) and/or to TLR9 (G-ODN) (Fig. 2A). Blocking TLR7 and/or TLR9 decreased the response to SIV by AGM PBMCs. IFN-I levels decreased from 60 IU/ml to 3 IU/ml and 10 IU/ml (Wilcoxon, P = 0.002 and P = 0.004) after blocking of TLR7 and TLR9, respectively. Given the stronger-than-expected inhibition upon TLR9 blockage, we repeated the experiment using MAC PBMCs, which were stimulated with either SIVagm or SIVmac. For MAC PBMCs, the response to both viruses (median of 147 IU/ml IFN-I) was lost upon blocking of TLR7 (median of 14 IU/ml IFN-I), while it was not significantly inhibited upon blocking of TLR9 (median of 89 IU/ml) (Fig. 2A). Since MAC pDC responses were not affected by TLR9 inhibition, irrespective of the SIV isolate used, it is unlikely that SIVagm is sensed more broadly than SIVmac. Altogether, AGM pDCs sense SIV through endosomal TLRs, as reported for MAC and humans.
FIG 2.
Molecules involved in SIV sensing by AGM plasmacytoid dendritic cells. (A) PBMCs from 10 noninfected AGMs (open circles) and 7 to 9 MACs (closed circles) were stimulated with mock (gray) or SIVagm or SIVmac (black). A151 (5 μg/ml) and G-ODN (0.5 μM) were added at the same time as SIV to inhibit the TLR7 and TLR9 pathway, respectively. (B) Longitudinal follow-up of MFI levels of BDCA-2 on pDCs during early SIVagm infection. BDCA-2 expression was followed for four AGMs. (C) Expression levels of CD40 and HLA-DR were measured simultaneously on AGM pDCs. pDCs were defined as CD20− HLA-DR+ CD123+ BDCA2+ cells. r = Spearman's correlation. (D) anti-BDCA-2 or irrelevant IgG1 antibodies were added to PBMCs of six uninfected AGMs for 1 h at concentrations of 1 μg/ml and 10 μg/ml. After stimulation with SIVagm (S; light gray) or HSV-1 (H; dark gray) for 18 h, we measured IFN-I levels in the supernatants. IFN-I levels were normalized to IFN-I produced after no incubation with antibodies. The dotted line represents no difference compared to stimulation without antibodies. (E) Percentage of IFN-α2+ pDCs upon in vitro HSV stimulation at different time points of SIVagm infection. PBMCs were collected before and after SIVagm infection, and pDCs were analyzed for IFN-α2 production after in vitro stimulation. pDCs were defined as CD3− HLA-DR+ CD123+ cells. (F) BDCA-2-expressing cells were depleted to remove pDCs from PBMCs (n = 6 AGMs). Then, depleted (open circles) and whole (gray, filled circles) PBMCs were stimulated with SIV or HSV, after which IFN-I was measured in the supernatant. Symbols represent individual animals. *, Wilcoxon, P < 0.05; **, Wilcoxon, P < 0.01; ***, Wilcoxon, P < 0.001.
BDCA-2 has been reported to be involved in attachment of HIV virions to the pDC surface (46). It has been shown that the ratio of precursor pDCs expressing low levels of BDCA-2 is increased during acute SIVmac infection (24, 34). If BDCA-2 were involved in the efficacy of endocytosis and sensing, lowered expression would specifically affect SIV but not HSV. We therefore evaluated the expression and function of BDCA-2 with respect to SIV and HSV sensing. BDCA-2 levels on AGM pDCs were lower during acute infection than before infection (mean fluorescent intensity [MFI] of 1,486 preinfection, to an MFI of 660 at day 14 p.i.; Wilcoxon, P < 0.01) (Fig. 2B). This lower BDCA-2 MFI in the acute phase probably reflected the circulation of precursor pDCs, expressing low BDCA-2 levels, as the BDCA-2 MFI closely correlated with the percentage of CD40+ pDCs (Spearman, r = 0.49, P = 0.0001) and HLA-DR expression on pDCs (Spearman, r = 0.75, P < 0.0001) (Fig. 2C). Indeed, these molecules are expressed at lower levels on precursor pDCs (34). To assess the role of BDCA-2 in SIV sensing by pDCs, we stimulated AGM PBMCs with SIV and HSV in the presence of BDCA-2-blocking antibodies (Fig. 2D). Blocking BDCA-2 prevented the response to SIVagm, but not to HSV, in a dose-dependent manner. An isotype control did not alter the IFN-I response to either SIV or HSV. At the end of the acute phase, BDCA-2 expression was similar to preinfection levels, concomitant with the recovery of IFN-I production upon SIV stimulation in the chronic phase of SIVagm infection. Thus, the decreased response to SIV correlated with a decrease in BDCA-2 on pDCs and an increase in the frequency of precursor pDCs in blood.
Non-pDCs produce IFN-I upon HSV stimulation due to a positive IFN-I feedback loop in vivo.
A higher ratio of precursor pDCs should impact both SIV and HSV responses. In order to further investigate the contrasting observations of an increased IFN-I response upon HSV stimulation, despite an increased circulation of precursor pDCs during acute SIVagm infection, PBMCs collected during acute SIV infection were stimulated in vitro with HSV and analyzed by intracellular flow cytometric staining for pDC-associated IFN-α production (Fig. 2E). A median of 91% IFN-α2+ pDCs upon stimulation with HSV was observed in healthy animals. During infection, the percentage of IFN-α2+ pDCs in response to HSV stimulation fell to a nadir of 65% at day 14 p.i. After the transition from the acute to the chronic phase of infection, AGM pDCs regained their capacity to produce IFN-I upon HSV stimulation. A nonparametric multiple-comparison test showed this change was significant (Friedman, P = 0.001).
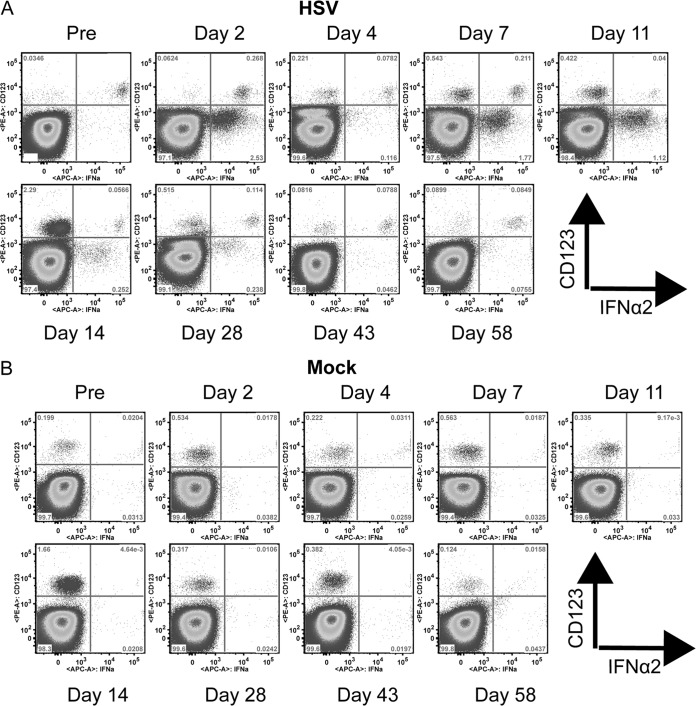
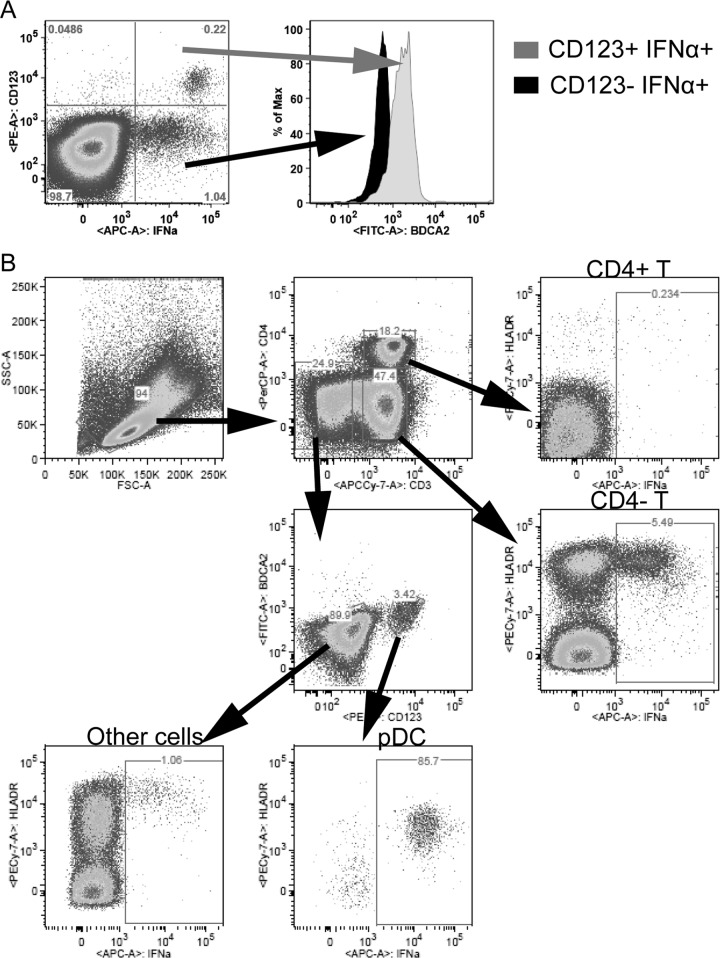
Although a decreased number of pDCs were producing IFN-I, a greater amount of IFN-I was detected in the supernatant of HSV-stimulated PBMCs collected during acute infection (Fig. 1C). To better understand this increased IFN-I production upon HSV stimulation, we searched for other cellular sources of IFN-I. After stimulation with HSV before infection, pDCs were IFN-α2 positive, while CD123− cells were not (Fig. 3A). To confirm that pDCs were the predominant producers of IFN-I upon HSV and SIV stimulation in healthy animals, we depleted BDCA-2+ cells from PBMCs to remove pDCs and stimulated them with SIV or HSV (Fig. 2F). This led to a 96.7% and 93.5% reduction in IFN-I production by pDC-depleted PBMCs upon stimulation with SIV and HSV, respectively (Wilcoxon, P = 0.031). In the absence of stimulation, no IFN-I production was observed at any time before and during infection (Fig. 3B). After infection, the percentage of IFN-α-positive pDCs decreased, while up to 3% of CD123− cells, i.e., non-pDCs, produced IFN-α2 at days 2, 7, 11, and 14 p.i. (Fig. 3A). It is possible that pDCs downregulated CD123 in culture while producing IFN-α. However, as up to 10 times more CD123− cells were producing IFN-α than there were pDCs among PBMCs, only a minor fraction of these cells could be pDCs. Furthermore, the IFN-α MFI was higher in the CD123+ cell population, supporting that the CD123− cells were not pDCs. To verify that these CD123− cells were not pDCs, we studied BDCA-2 expression on the cell populations producing IFN-α (Fig. 4A). The CD123+ cells expressed BDCA-2, unlike the CD123− cells, confirming that the CD123+ cells were pDCs. A further characterization of the PBMCs stimulated with HSV showed that the majority of the CD123− IFN-α2+ cells were CD4− T cells, but other HLA-DR+ cells also produced IFN-α2 (Fig. 4B).
FIG 3.
Non pDC-associated IFN-α production. Dot plots show CD123 and IFN-α2 expression on whole PBMCs collected throughout SIVagm infection. One representative AGM out of four analyzed is shown. (A) Cells after stimulation with HSV. (B) Mock-stimulated cells.
FIG 4.
(A) Identification of cell types producing IFN-I upon HSV stimulation. (A) BDCA-2 expression on IFN-α2+ cells. PBMCs from a representative animal stimulated with HSV at day 2 p.i. are shown. From the CD123+ IFN-α+ (gray) and the CD123− IFN-α+ (black) quadrants, the expression of BDCA-2 is shown. (B) PBMCs were gated for distinct cell populations to identify cell types that produced IFN-α2. PBMCs from one AGM at day 2 p.i. are shown. A total of 5.5% of the CD4− T cells, 85.7% of the pDCs, and 1% of non-T, non-pDCs were IFN-α+ after HSV stimulation. All IFN-α-producing cells expressed high levels of HLA-DR. The identified cells producing IFN-α in this animal and at this time point are representative of data from days 2, 9, and 11 postinfection.
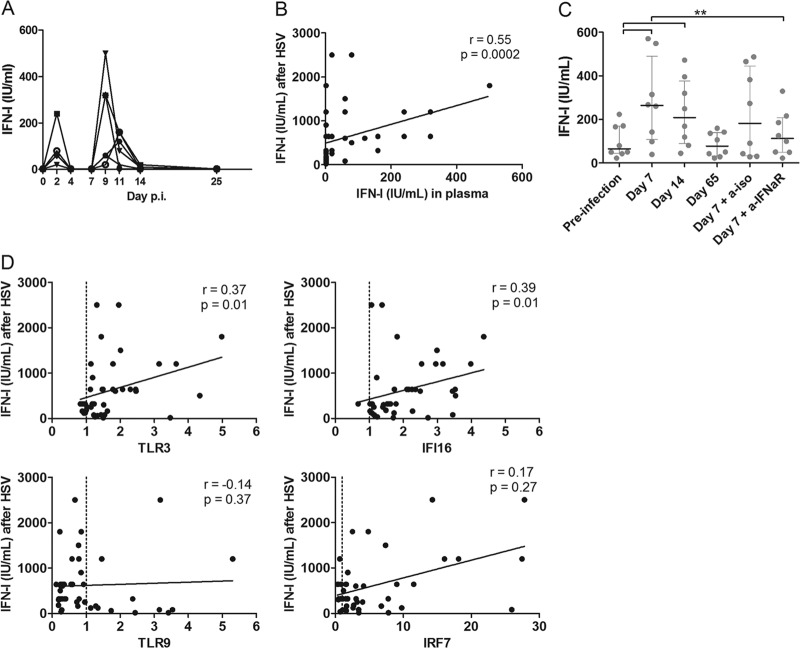
This substantial non-pDC-associated IFN-I production was surprising. A positive association between the levels of IFN-I in the plasma and the amount of IFN-I produced after stimulation with HSV-1 was observed during the acute phase (Spearman, r = 0.58, P < 0.001) (Fig. 5A and B). We therefore hypothesized that IFN-I induced a positive auto-feedback loop. To test this hypothesis, PBMCs from healthy AGMs were incubated with plasma collected at various time points of SIVagm infection and then stimulated with HSV (Fig. 5C). Preincubation with plasma collected during acute SIVagm infection increased the IFN-I response to HSV by 4-fold (Wilcoxon, P = 0.008) compared to preincubation with plasma from healthy animals. In contrast to acute-phase plasma, plasma from animals in the chronic phase of infection did not increase the IFN-I response to HSV. To confirm that IFN-I mediated this increased response to HSV, we blocked the IFN-α receptor 2 (IFN-αR2) on AGM PBMCs, incubated these cells with acute-phase plasma, and subsequently stimulated the cells with HSV. Blocking with anti-IFN-αR2, but not with an isotype antibody, prevented the increase in IFN-I production (Wilcoxon, P = 0.008). Therefore, IFN-I was required for this increased response to HSV.
FIG 5.
Auto-feedback loop of IFN-I during acute SIV infection of AGMs. (A) IFN-I levels present in the plasma of eight acutely SIV-infected AGMs. (B) Correlation between IFN-I levels in the plasma of AGMs during acute SIVagm infection in vivo and the amount of IFN-I produced upon in vitro HSV stimulation. (C) Plasma collected from healthy AGMs, acutely infected AGMs, and chronically infected AGMs was diluted to 5% in RPMI and added to PBMCs of eight healthy AGMs. After 4 h of incubation, cells were stimulated with HSV-1, and after another 18 h, supernatants were collected for IFN-I quantification. PBMCs were incubated with anti-IFN-αR2 or with an irrelevant antibody as a negative control at 10 μg/ml for 1 h to block IFN-α signaling before adding plasma. Symbols represent individual animals. (D) Correlation between mRNA levels of known HSV sensors in PBMCs of AGMs during acute SIVagm infection in vivo and the amount of IFN-I produced upon in vitro HSV stimulation of the same PBMCs. **, Wilcoxon, P < 0.01.
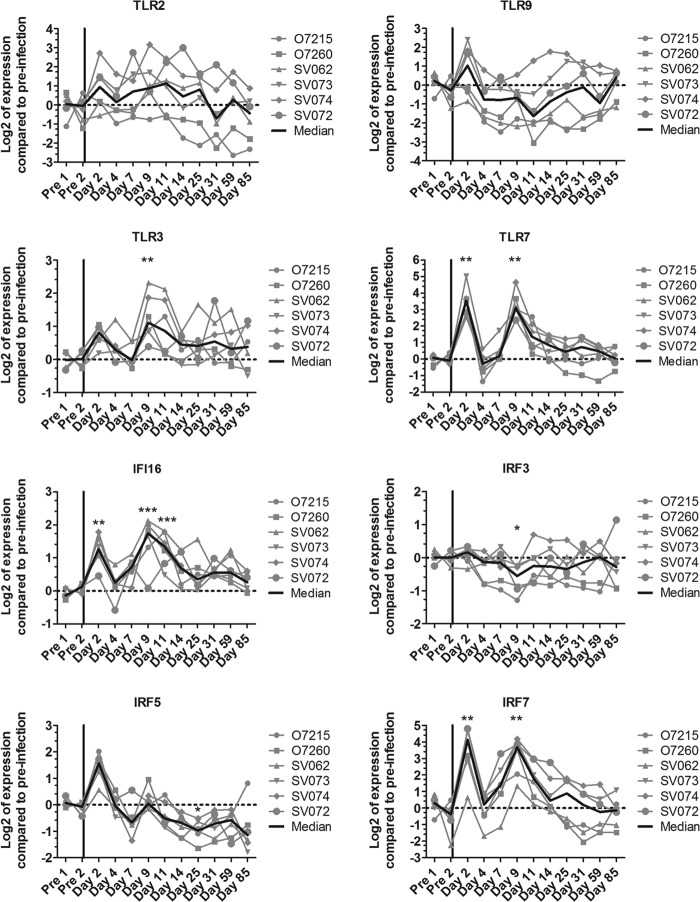
As IFN-αR signaling increased the response to HSV, we studied whether ISGs, and other molecules, involved in HSV sensing were increased during acute SIV infection. Expression levels of four known HSV sensors (TLR2, TLR3, TLR9, and IFI16) and three molecules associated with IFN-I production (IFN regulatory factor 3 [IRF3], IRF5, and IRF7) were measured in PBMCs during acute SIVagm infection (Fig. 6). Expression levels of TLR3, TLR7, IFI16, and IRF7 were increased during acute SIVagm infection (Fig. 6). In contrast, expression levels of TLR2, TLR9, IRF3, and IRF5 were not increased, or even were decreased, during acute SIVagm infection (Fig. 6). TLR3 and IFI16 mRNA levels correlated with the amount of IFN-I produced upon HSV stimulation (Fig. 5D) (Spearman, r = 0.37, P = 0.01 and r = 0.39, P = 0.01, respectively). In contrast, IRF3, IRF5, IRF7, TLR2, and TLR9 expression levels were not associated with the IFN-I response upon HSV stimulation (Fig. 5D and data not shown). These data show that SIV infection induces an upregulation of at least two sensors in PBMCs via IFN-I, which could cause cells other than pDCs to increase their sensitivity for pathogen-associated molecular patterns and to produce IFN-I upon viral encounter.
FIG 6.
Gene expression profiles of genes involved in HSV sensing and IFN-I responses. mRNA levels were measured in PBMCs from six animals (the same animals as for Fig. 5) during SIVagm infection. The gray lines indicate individual animals, and the black lines show medians for all animals. *, Wilcoxon, P < 0.05; **, Wilcoxon, P < 0.01; ***, Wilcoxon, P < 0.001. The P values indicate a significant difference compared to the preinfection baseline.
DISCUSSION
During acute SIV infection, AGM PBMCs produced less IFN-I upon in vitro stimulation with SIV but more upon stimulation with HSV, compared to preinfection levels. Blocking BDCA-2 reduced the in vitro IFN-I response to SIV, but not to HSV. During acute infection, we observed lower BDCA-2 expression on pDCs, which could have thus specifically impacted SIV sensing. It has been shown previously that BDCA-2 can bind the HIV envelope and that BDCA-2 recognition leads to antigen presentation by pDCs (46, 47). Here, we have shown that BDCA-2 is also possibly involved in the sensing of HIV/SIV by pDCs, a novel finding. An earlier report by Pritschet et al. found a crucial role for CD4, but not BDCA-2, in HIV endocytosis, although those confocal microscopy experiments were not quantitative (48). It is possible that BDCA-2, by binding HIV, increases the efficiency of CD4-dependent HIV endocytosis (49). Moreover, our data suggest an egress of precursor pDCs during acute SIVagm infection, similar to what has been reported for SIVmac infection (24, 33). As precursor pDCs are less efficient in producing IFN-I (34), this could also explain the decreased response to SIV.
While IFN-I production increased upon stimulation of PBMCs with HSV during the acute phase, the percentage of pDCs that produced IFN-I in response to HSV decreased. Surprisingly, cells other than pDCs, notably, T cells and also CD3− HLA-DR+ CD123− cells, started producing IFN-I upon in vitro stimulation with HSV during acute SIVagm infection. The increased sensing of HSV by non-pDCs was due to a positive auto-feedback loop of IFN-I, which is transiently present at high levels in the plasma during the acute phase of HIV/SIV infections and also in patients in late-stage infection. While our data do not exclude that other factors might be required in addition to IFN-I for this enhanced viral sensing, it is unlikely that SIV particles in the plasma induced the increased IFN-I response, as plasma from chronically infected animals did not increase responsiveness. The increased response to HSV was associated with increased expression levels of the viral sensors TLR3 and IFI16. These data are in line with a study that showed that TLR3-mediated sensing dominates the IFN-I response during in vivo HSV infection in mice after the initial pDC-associated IFN-I responses (50).
Since most of the cells that were producing IFN-I were CD4−, it is possible that SIV could not be sensed by these cells; this would explain, at least partially, why stimulation with SIV did not lead to increased IFN-I responses during acute infection (Fig. 1A and 3B). The IFN-I production by non-pDCs upon ex vivo stimulation with HSV is in agreement with immunohistochemistry data from the spleens of late-stage HIV-infected patients, where IFN-α colocalized only with a few pDCs and rather with other cells, including T and B lymphocytes, myeloid DCs, and macrophages (51). It is possible that in the context of high IFN-I production, as observed in late-stage HIV-infected patients with high viral loads, other cells gain this capacity. In such late-stage, immunocompromised patients, reactivated latent viruses, such as cytomegalovirus, a member of the Herpesviridae family, could trigger this non-pDC-associated IFN-α production (52). Moreover, the expanded gut virome and microbial translocation observed in SIVmac, but not SIVagm, infection could contribute to chronic IA by triggering IFN-I responses in cells normally not associated with these responses (53, 54).
Such increased sensitivity, which we observed in nonpathogenic SIVagm infection, might also occur in HIV and SIVmac infections, which may have gone unnoticed in earlier studies, as most studies using viral stimuli other than SIV did not analyze such early and numerous time points as we have included here (23, 35, 36). On the other hand, the increase of interferon-induced viral sensors could be concomitant with IFN-I-inducible restriction factors in these cells and confer a relative resistance of certain cell subpopulations to infection.
Collectively, our data show that SIVagm infection strongly impacts AGM pDC function and dynamics during acute SIV infection, similar to what happens in pathogenic HIV and SIVmac infections (24, 33). These modifications normalize, however, after acute SIVagm infection. The previously reported low level of ISG expression during the chronic phase of SIVagm infection is therefore, as suggested earlier, not a reflection of a lack of IFN-I-producing capacity by AGM pDCs (20, 22). Furthermore, these results revealed an upregulation of viral sensors in response to the existing inflammatory environment in cells that are not classically involved in IFN-I-associated inflammation. The concomitant increase of ISGs might contribute to the persistent inflammation in chronic HIV infection and/or to resistance of target cells to infection. These data suggest that these IFN-I-producing non-pDCs should be considered in future studies in exploring the establishment of viral reservoirs and immune activation.
ACKNOWLEDGMENTS
This work was supported by Agence Nationale de Recherches sur le Sida et les Hépatites Virales (M.M.T.), Fondation AREVA (M.M.T.), and Fondation Total (F.B.S.). S.J. was supported by a scholarship from the Ministère de l'Enseignement Supérieur et de la Recherche, and G.P., D.K., A.S.L., and M.P. were supported by Sidaction.
We are grateful to Christophe Joubert, Benoît Delache, Jean-Marie Héliès, Patrick Flament, and the staff of the CEA animal facilities for outstanding work in animal care. We also thank the staff of the Institut Pasteur animal facility. We appreciate the excellent technical assistance of Claire Torres, Julie Morin, Aurélien Corneau, and the TIPIV staff of the CEA. We also acknowledge the state-of-the-art National Center for Infectious Disease Models and Innovative Therapies. Rémi Cheynier kindly provided samples. Alison Isaacs read and commented on the manuscript. Olivier Schwartz provided reagents and helpful discussions. We are thankful to Bruno Vaslin and Timothée Bruel for discussions.
We declare that we do not have a commercial or other association that might pose a conflict of interest.
REFERENCES
- 1.Kirchhoff F. 2010. Immune evasion and counteraction of restriction factors by HIV-1 and other primate lentiviruses. Cell Host Microbe 8:55–67. doi: 10.1016/j.chom.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 2.Fonteneau J-F, Larsson M, Beignon A-S, McKenna K, Dasilva I, Amara A, Liu Y-J, Lifson JD, Littman DR, Bhardwaj N. 2004. Human immunodeficiency virus type 1 activates plasmacytoid dendritic cells and concomitantly induces the bystander maturation of myeloid dendritic cells. J Virol 78:5223–5232. doi: 10.1128/JVI.78.10.5223-5232.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Romagnani C, Della Chiesa M, Kohler S, Moewes B, Radbruch A, Moretta L, Moretta A, Thiel A. 2005. Activation of human NK cells by plasmacytoid dendritic cells and its modulation by CD4+ T helper cells and CD4+ CD25hi T regulatory cells. Eur J Immunol 35:2452–2458. doi: 10.1002/eji.200526069. [DOI] [PubMed] [Google Scholar]
- 4.Karpov AV. 2001. Endogenous and exogenous interferons in HIV-infection. Eur J Med Res 6:507–524. [PubMed] [Google Scholar]
- 5.Asmuth DM, Murphy RL, Rosenkranz SL, Lertora JJ, Kottilil S, Cramer Y, Chan ES, Schooley RT, Rinaldo CR, Thielman N, Li XD, Wahl SM, Shore J, Janik J, Lempicki RA, Simpson Y, Pollard RB. 2010. Safety, tolerability, and mechanisms of antiretroviral activity of pegylated interferon Alfa-2a in HIV-1-monoinfected participants: a phase II clinical trial. J Infect Dis 201:1686–1696. doi: 10.1086/652420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jacquelin B, Petitjean G, Kunkel D, Liovat AS, Jochems SP, Rogers KA, Ploquin MJ, Madec Y, Barre-Sinoussi F, Dereuddre-Bosquet N, Lebon P, Le Grand R, Villinger F, Muller-Trutwin M. 2014. Innate immune responses and rapid control of inflammation in African green monkeys treated or not with interferon-alpha during primary SIVagm infection. PLoS Pathog 10:e1004241. doi: 10.1371/journal.ppat.1004241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vanderford TH, Slichter C, Rogers KA, Lawson BO, Obaede R, Else J, Villinger F, Bosinger SE, Silvestri G. 2012. Treatment of SIV-infected sooty mangabeys with a type-I IFN agonist results in decreased virus replication without inducing hyperimmune activation. Blood 119:5750–5757. doi: 10.1182/blood-2012-02-411496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Azzoni L, Foulkes AS, Papasavvas E, Mexas AM, Lynn KM, Mounzer K, Tebas P, Jacobson JM, Frank I, Busch MP, Deeks SG, Carrington M, O'Doherty U, Kostman J, Montaner LJ. 2013. Pegylated interferon alfa-2a monotherapy results in suppression of HIV type 1 replication and decreased cell-associated HIV DNA integration. J Infect Dis 207:213–222. doi: 10.1093/infdis/jis663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sandler NG, Bosinger SE, Estes JD, Zhu RT, Tharp GK, Boritz E, Levin D, Wijeyesinghe S, Makamdop KN, Del Prete GQ, Hill BJ, Timmer JK, Reiss E, Yarden G, Darko S, Contijoch E, Todd JP, Silvestri G, Nason M, Norgren RB Jr, Keele BF, Rao S, Langer JA, Lifson JD, Schreiber G, Douek DC. 2014. Type I interferon responses in rhesus macaques prevent SIV infection and slow disease progression. Nature 511:601–605. doi: 10.1038/nature13554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hervas-Stubbs S, Perez-Gracia JL, Rouzaut A, Sanmamed MF, Le Bon A, Melero I. 2011. Direct effects of type I interferons on cells of the immune system. Clin Cancer Res 17:2619–2627. doi: 10.1158/1078-0432.CCR-10-1114. [DOI] [PubMed] [Google Scholar]
- 11.Herbeuval JP, Boasso A, Grivel JC, Hardy AW, Anderson SA, Dolan MJ, Chougnet C, Lifson JD, Shearer GM. 2005. TNF-related apoptosis-inducing ligand (TRAIL) in HIV-1-infected patients and its in vitro production by antigen-presenting cells. Blood 105:2458–2464. doi: 10.1182/blood-2004-08-3058. [DOI] [PubMed] [Google Scholar]
- 12.Sedaghat AR, German J, Teslovich TM, Cofrancesco J Jr, Jie CC, Talbot CC Jr, Siliciano RF. 2008. Chronic CD4+ T-cell activation and depletion in human immunodeficiency virus type 1 infection: type I interferon-mediated disruption of T-cell dynamics. J Virol 82:1870–1883. doi: 10.1128/JVI.02228-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liovat AS, Rey-Cuille MA, Lecuroux C, Jacquelin B, Girault I, Petitjean G, Zitoun Y, Venet A, Barre-Sinoussi F, Lebon P, Meyer L, Sinet M, Muller-Trutwin M. 2012. Acute plasma biomarkers of T cell activation set-point levels and of disease progression in HIV-1 infection. PLoS One 7:e46143. doi: 10.1371/journal.pone.0046143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meier A, Chang JJ, Chan ES, Pollard RB, Sidhu HK, Kulkarni S, Wen TF, Lindsay RJ, Orellana L, Mildvan D, Bazner S, Streeck H, Alter G, Lifson JD, Carrington M, Bosch RJ, Robbins GK, Altfeld M. 2009. Sex differences in the Toll-like receptor-mediated response of plasmacytoid dendritic cells to HIV-1. Nat Med 15:955–959. doi: 10.1038/nm.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rotger M, Dalmau J, Rauch A, McLaren P, Bosinger SE, Martinez R, Sandler NG, Roque A, Liebner J, Battegay M, Bernasconi E, Descombes P, Erkizia I, Fellay J, Hirschel B, Miro JM, Palou E, Hoffmann M, Massanella M, Blanco J, Woods M, Gunthard HF, de Bakker P, Douek DC, Silvestri G, Martinez-Picado J, Telenti A. 2011. Comparative transcriptomics of extreme phenotypes of human HIV-1 infection and SIV infection in sooty mangabey and rhesus macaque. J Clin Invest 121:2391–2400. doi: 10.1172/JCI45235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sodora DL, Allan JS, Apetrei C, Brenchley JM, Douek DC, Else JG, Estes JD, Hahn BH, Hirsch VM, Kaur A, Kirchhoff F, Muller-Trutwin M, Pandrea I, Schmitz JE, Silvestri G. 2009. Toward an AIDS vaccine: lessons from natural simian immunodeficiency virus infections of African nonhuman primate hosts. Nat Med 15:861–865. doi: 10.1038/nm.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kornfeld C, Ploquin MJ, Pandrea I, Faye A, Onanga R, Apetrei C, Poaty-Mavoungou V, Rouquet P, Estaquier J, Mortara L, Desoutter JF, Butor C, Le Grand R, Roques P, Simon F, Barre-Sinoussi F, Diop OM, Muller-Trutwin MC. 2005. Antiinflammatory profiles during primary SIV infection in African green monkeys are associated with protection against AIDS. J Clin Invest 115:1082–1091. doi: 10.1172/JCI200523006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liovat AS, Jacquelin B, Ploquin MJ, Barre-Sinoussi F, Muller-Trutwin MC. 2009. African non human primates infected by SIV: why don't they get sick? Lessons from studies on the early phase of non-pathogenic SIV infection. Curr HIV Res 7:39–50. doi: 10.2174/1570162097848546. [DOI] [PubMed] [Google Scholar]
- 19.Bosinger SE, Li Q, Gordon SN, Klatt NR, Duan L, Xu L, Francella N, Sidahmed A, Smith AJ, Cramer EM, Zeng M, Masopust D, Carlis JV, Ran L, Vanderford TH, Paiardini M, Isett RB, Baldwin DA, Else JG, Staprans SI, Silvestri G, Haase AT, Kelvin DJ. 2009. Global genomic analysis reveals rapid control of a robust innate response in SIV-infected sooty mangabeys. J Clin Invest 119:3556–3572. doi: 10.1172/JCI40115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jacquelin B, Mayau V, Targat B, Liovat AS, Kunkel D, Petitjean G, Dillies MA, Roques P, Butor C, Silvestri G, Giavedoni LD, Lebon P, Barre-Sinoussi F, Benecke A, Muller-Trutwin MC. 2009. Nonpathogenic SIV infection of African green monkeys induces a strong but rapidly controlled type I IFN response. J Clin Invest 119:3544–3555. doi: 10.1172/JCI40093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Campillo-Gimenez L, Laforge M, Fay M, Brussel A, Cumont MC, Monceaux V, Diop O, Levy Y, Hurtrel B, Zaunders J, Corbeil J, Elbim C, Estaquier J. 2010. Nonpathogenesis of simian immunodeficiency virus infection is associated with reduced inflammation and recruitment of plasmacytoid dendritic cells to lymph nodes, not to lack of an interferon type I response, during the acute phase. J Virol 84:1838–1846. doi: 10.1128/JVI.01496-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bosinger SE, Johnson ZP, Folkner KA, Patel N, Hashempour T, Jochems SP, Del Rio Estrada PM, Paiardini M, Lin R, Vanderford TH, Hiscott J, Silvestri G. 2013. Intact type I Interferon production and IRF7 function in sooty mangabeys. PLoS Pathog 9:e1003597. doi: 10.1371/journal.ppat.1003597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diop OM, Ploquin MJ, Mortara L, Faye A, Jacquelin B, Kunkel D, Lebon P, Butor C, Hosmalin A, Barre-Sinoussi F, Muller-Trutwin MC. 2008. Plasmacytoid dendritic cell dynamics and alpha interferon production during Simian immunodeficiency virus infection with a nonpathogenic outcome. J Virol 82:5145–5152. doi: 10.1128/JVI.02433-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bruel T, Dupuy S, Demoulins T, Rogez-Kreuz C, Dutrieux J, Corneau A, Cosma A, Cheynier R, Dereuddre-Bosquet N, Le Grand R, Vaslin B. 2014. Plasmacytoid dendritic cell dynamics tune interferon-alfa production in SIV-infected cynomolgus macaques. PLoS Pathog 10:e1003915. doi: 10.1371/journal.ppat.1003915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferbas JJ, Toso JF, Logar AJ, Navratil JS, Rinaldo CR Jr. 1994. CD4+ blood dendritic cells are potent producers of IFN-alpha in response to in vitro HIV-1 infection. J Immunol 152:4649–4662. [PubMed] [Google Scholar]
- 26.Tilton JC, Manion MM, Luskin MR, Johnson AJ, Patamawenu AA, Hallahan CW, Cogliano-Shutta NA, Mican JM, Davey RT Jr, Kottilil S, Lifson JD, Metcalf JA, Lempicki RA, Connors M. 2008. Human immunodeficiency virus viremia induces plasmacytoid dendritic cell activation in vivo and diminished alpha interferon production in vitro. J Virol 82:3997–4006. doi: 10.1128/JVI.01545-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lehmann C, Harper JM, Taubert D, Hartmann P, Fatkenheuer G, Jung N, van Lunzen J, Stellbrink HJ, Gallo RC, Romerio F. 2008. Increased interferon alpha expression in circulating plasmacytoid dendritic cells of HIV-1-infected patients. J Acquir Immune Defic Syndr 48:522–530. doi: 10.1097/QAI.0b013e31817f97cf. [DOI] [PubMed] [Google Scholar]
- 28.Lehmann C, Lafferty M, Garzino-Demo A, Jung N, Hartmann P, Fatkenheuer G, Wolf JS, van Lunzen J, Romerio F. 2010. Plasmacytoid dendritic cells accumulate and secrete interferon alpha in lymph nodes of HIV-1 patients. PLoS One 5:e11110. doi: 10.1371/journal.pone.0011110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beignon AS, McKenna K, Skoberne M, Manches O, DaSilva I, Kavanagh DG, Larsson M, Gorelick RJ, Lifson JD, Bhardwaj N. 2005. Endocytosis of HIV-1 activates plasmacytoid dendritic cells via Toll-like receptor-viral RNA interactions. J Clin Invest 115:3265–3275. doi: 10.1172/JCI26032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lepelley A, Louis S, Sourisseau M, Law HK, Pothlichet J, Schilte C, Chaperot L, Plumas J, Randall RE, Si-Tahar M, Mammano F, Albert ML, Schwartz O. 2011. Innate sensing of HIV-infected cells. PLoS Pathog 7:e1001284. doi: 10.1371/journal.ppat.1001284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Piconi S, Parisotto S, Rizzardini G, Passerini S, Terzi R, Argenteri B, Meraviglia P, Capetti A, Biasin M, Trabattoni D, Clerici M. 2011. Hydroxychloroquine drastically reduces immune activation in HIV-infected, antiretroviral therapy-treated immunologic nonresponders. Blood 118:3263–3272. doi: 10.1182/blood-2011-01-329060. [DOI] [PubMed] [Google Scholar]
- 32.Harris LD, Tabb B, Sodora DL, Paiardini M, Klatt NR, Douek DC, Silvestri G, Muller-Trutwin M, Vasile-Pandrea I, Apetrei C, Hirsch V, Lifson J, Brenchley JM, Estes JD. 2010. Downregulation of robust acute type I interferon responses distinguishes nonpathogenic simian immunodeficiency virus (SIV) infection of natural hosts from pathogenic SIV infection of rhesus macaques. J Virol 84:7886–7891. doi: 10.1128/JVI.02612-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brown KN, Wijewardana V, Liu X, Barratt-Boyes SM. 2009. Rapid influx and death of plasmacytoid dendritic cells in lymph nodes mediate depletion in acute simian immunodeficiency virus infection. PLoS Pathog 5:e1000413. doi: 10.1371/journal.ppat.1000413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martin-Martin L, Almeida J, Hernandez-Campo PM, Sanchez ML, Lecrevisse Q, Orfao A. 2009. Immunophenotypical, morphologic, and functional characterization of maturation-associated plasmacytoid dendritic cell subsets in normal adult human bone marrow. Transfusion 49:1692–1708. doi: 10.1111/j.1537-2995.2009.02170.x. [DOI] [PubMed] [Google Scholar]
- 35.Kamga I, Kahi S, Develioglu L, Lichtner M, Maranon C, Deveau C, Meyer L, Goujard C, Lebon P, Sinet M, Hosmalin A. 2005. Type I interferon production is profoundly and transiently impaired in primary HIV-1 infection. J Infect Dis 192:303–310. doi: 10.1086/430931. [DOI] [PubMed] [Google Scholar]
- 36.Malleret B, Maneglier B, Karlsson I, Lebon P, Nascimbeni M, Perie L, Brochard P, Delache B, Calvo J, Andrieu T, Spreux-Varoquaux O, Hosmalin A, Le Grand R, Vaslin B. 2008. Primary infection with simian immunodeficiency virus: plasmacytoid dendritic cell homing to lymph nodes, type I interferon, and immune suppression. Blood 112:4598–4608. doi: 10.1182/blood-2008-06-162651. [DOI] [PubMed] [Google Scholar]
- 37.Sabado RL, O'Brien M, Subedi A, Qin L, Hu N, Taylor E, Dibben O, Stacey A, Fellay J, Shianna KV, Siegal F, Shodell M, Shah K, Larsson M, Lifson J, Nadas A, Marmor M, Hutt R, Margolis D, Garmon D, Markowitz M, Valentine F, Borrow P, Bhardwaj N. 2010. Evidence of dysregulation of dendritic cells in primary HIV infection. Blood 116:3839–3852. doi: 10.1182/blood-2010-03-273763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lo CC, Schwartz JA, Johnson DJ, Yu M, Aidarus N, Mujib S, Benko E, Hyrcza M, Kovacs C, Ostrowski MA. 2012. HIV delays IFN-alpha production from human plasmacytoid dendritic cells and is associated with SYK phosphorylation. PLoS One 7:e37052. doi: 10.1371/journal.pone.0037052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O'Brien M, Manches O, Sabado RL, Baranda SJ, Wang Y, Marie I, Rolnitzky L, Markowitz M, Margolis DM, Levy D, Bhardwaj N. 2011. Spatiotemporal trafficking of HIV in human plasmacytoid dendritic cells defines a persistently IFN-alpha-producing and partially matured phenotype. J Clin Invest 121:1088–1101. doi: 10.1172/JCI44960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Diop OM, Gueye A, Dias-Tavares M, Kornfeld C, Faye A, Ave P, Huerre M, Corbet S, Barre-Sinoussi F, Muller-Trutwin MC. 2000. High levels of viral replication during primary simian immunodeficiency virus SIVagm infection are rapidly and strongly controlled in African green monkeys. J Virol 74:7538–7547. doi: 10.1128/JVI.74.16.7538-7547.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chung E, Amrute SB, Abel K, Gupta G, Wang Y, Miller CJ, Fitzgerald-Bocarsly P. 2005. Characterization of virus-responsive plasmacytoid dendritic cells in the rhesus macaque. Clin Diagn Lab Immunol 12:426–435. doi: 10.1128/CDLI.12.3.426-435.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ferbas J, Navratil J, Logar A, Rinaldo C. 1995. Selective decrease in human immunodeficiency virus type 1 (HIV-1)-induced alpha interferon production by peripheral blood mononuclear cells during HIV-1 infection. Clin Diagn Lab Immunol 2:138–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ankel H, Westra DF, Welling-Wester S, Lebon P. 1998. Induction of interferon-alpha by glycoprotein D of herpes simplex virus: a possible role of chemokine receptors. Virology 251:317–326. doi: 10.1006/viro.1998.9432. [DOI] [PubMed] [Google Scholar]
- 44.Ritz C, Streibig JC. 2005. Bioassay analysis using R. J Stat Softw 12:1–22. [Google Scholar]
- 45.Pacanowski J, Kahi S, Baillet M, Lebon P, Deveau C, Goujard C, Meyer L, Oksenhendler E, Sinet M, Hosmalin A. 2001. Reduced blood CD123+ (lymphoid) and CD11c+ (myeloid) dendritic cell numbers in primary HIV-1 infection. Blood 98:3016–3021. doi: 10.1182/blood.V98.10.3016. [DOI] [PubMed] [Google Scholar]
- 46.Martinelli E, Cicala C, Van Ryk D, Goode DJ, Macleod K, Arthos J, Fauci AS. 2007. HIV-1 gp120 inhibits TLR9-mediated activation and IFN-α secretion in plasmacytoid dendritic cells. Proc Natl Acad Sci U S A 104:3396–3401. doi: 10.1073/pnas.0611353104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dzionek A, Sohma Y, Nagafune J, Cella M, Colonna M, Facchetti F, Gunther G, Johnston I, Lanzavecchia A, Nagasaka T, Okada T, Vermi W, Winkels G, Yamamoto T, Zysk M, Yamaguchi Y, Schmitz J. 2001. BDCA-2, a novel plasmacytoid dendritic cell-specific type II C-type lectin, mediates antigen capture and is a potent inhibitor of interferon alpha/beta induction. J Exp Med 194:1823–1834. doi: 10.1084/jem.194.12.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pritschet K, Donhauser N, Schuster P, Ries M, Haupt S, Kittan NA, Korn K, Pohlmann S, Holland G, Bannert N, Bogner E, Schmidt B. 2012. CD4- and dynamin-dependent endocytosis of HIV-1 into plasmacytoid dendritic cells. Virology 423:152–164. doi: 10.1016/j.virol.2011.11.026. [DOI] [PubMed] [Google Scholar]
- 49.Ries M, Pritschet K, Schmidt B. 2012. Blocking type I interferon production: a new therapeutic option to reduce the HIV-1-induced immune activation. Clin Dev Immunol 2012:534929. doi: 10.1155/2012/534929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Swiecki M, Wang Y, Gilfillan S, Colonna M. 2013. Plasmacytoid dendritic cells contribute to systemic but not local antiviral responses to HSV infections. PLoS Pathog 9:e1003728. doi: 10.1371/journal.ppat.1003728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nascimbeni M, Perie L, Chorro L, Diocou S, Kreitmann L, Louis S, Garderet L, Fabiani B, Berger A, Schmitz J, Marie JP, Molina TJ, Pacanowski J, Viard JP, Oksenhendler E, Beq S, Abehsira-Amar O, Cheynier R, Hosmalin A. 2009. Plasmacytoid dendritic cells accumulate in spleens from chronically HIV-infected patients but barely participate in interferon-alpha expression. Blood 113:6112–6119. doi: 10.1182/blood-2008-07-170803. [DOI] [PubMed] [Google Scholar]
- 52.Gianella S, Strain MC, Rought SE, Vargas MV, Little SJ, Richman DD, Spina CA, Smith DM. 2012. Associations between virologic and immunologic dynamics in blood and in the male genital tract. J Virol 86:1307–1315. doi: 10.1128/JVI.06077-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Handley SA, Thackray LB, Zhao G, Presti R, Miller AD, Droit L, Abbink P, Maxfield LF, Kambal A, Duan E, Stanley K, Kramer J, Macri SC, Permar SR, Schmitz JE, Mansfield K, Brenchley JM, Veazey RS, Stappenbeck TS, Wang D, Barouch DH, Virgin HW. 2012. Pathogenic simian immunodeficiency virus infection is associated with expansion of the enteric virome. Cell 151:253–266. doi: 10.1016/j.cell.2012.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Klatt NR, Harris LD, Vinton CL, Sung H, Briant JA, Tabb B, Morcock D, McGinty JW, Lifson JD, Lafont BA, Martin MA, Levine AD, Estes JD, Brenchley JM. 2010. Compromised gastrointestinal integrity in pigtail macaques is associated with increased microbial translocation, immune activation, and IL-17 production in the absence of SIV infection. Mucosal Immunol 3:387–398. doi: 10.1038/mi.2010.14. [DOI] [PMC free article] [PubMed] [Google Scholar]