Abstract
The effects of aging were traditionally thought to be immutable, particularly evident in the loss of plasticity and cognitive abilities occurring in the aged central nervous system (CNS). However, it is becoming increasingly apparent that extrinsic systemic manipulations such as exercise, caloric restriction, and changing blood composition by heterochronic parabiosis or young plasma administration can partially counteract this age-related loss of plasticity in the aged brain. In this review, we discuss the process of aging and rejuvenation as systemic events. We summarize genetic studies that demonstrate a surprising level of malleability in organismal lifespan, and highlight the potential for systemic manipulations to functionally reverse the effects of aging in the CNS. Based on mounting evidence, we propose that rejuvenating effects of systemic manipulations are mediated, in part, by blood-borne ‘pro-youthful’ factors. Thus, systemic manipulations promoting a younger blood composition provide effective strategies to rejuvenate the aged brain. As a consequence, we can now consider reactivating latent plasticity dormant in the aged CNS as a means to rejuvenate regenerative, synaptic, and cognitive functions late in life, with potential implications even for extending lifespan.
Keywords: aging, cognition, heterochronic parabiosis, regeneration, rejuvenation
Until recently, the aging process – the gradual detrimental effect of time on an organism that leads to death – was considered irreversible (Lopez-Otin et al. 2013). However, research over the last 30 years has challenged this assumption, providing compelling evidence that the aging process can be affected by several factors, including the genetic composition of the organism (Kenyon et al. 1993; Kenyon 2010), as well as the experiences the organism has with its environment (Apfeld and Kenyon 1999; Alcedo and Kenyon 2004; Libert and Pletcher 2007; Jeong et al. 2012). These findings indicate that aging is not a deterministic process, but is instead plastic, potentially availing itself to manipulation by means available to the fields of biology and medicine. The malleability of the aging process raises the exciting possibility that harnessing this plasticity may provide a means to slow or even reverse the aging process itself and rejuvenate physiological systems.
Aging at its core can be thought of as a systemic event. Indeed, the effects of aging do not occur in a targeted and isolated manner, but rather functionally alter tissues throughout the body (systemic aging), albeit at different rates (Lopez-Otin et al. 2013). With this in mind, individual tissues exhibit different levels of sensitivity and resilience to aging (Rando 2006). In particular, evidence suggests that the central nervous system (CNS) is especially vulnerable to the effects of aging (Mattson and Magnus 2006), experiencing a gradual loss in the ability to physically and functionally adapt to new experiences with age (Mahncke et al. 2006). Consequently, aging in the CNS results in decreased regenerative capacity for repair (Rando 2006) and impaired maintenance of synaptic and cognitive functions (Morrison and Baxter 2012). This detrimental influence of aging on the CNS is particularly alarming when considering the role of the CNS in regulating overall homeostasis (Alcedo et al. 2013a). Functionally, the CNS not only integrates sensory information from the external environment but also responds to changes from within through communication with the systemic environment, collectively regulating important physiological processes including growth, metabolism, and reproduction (Libert and Pletcher 2007; Jeong et al. 2012; Alcedo et al. 2013a). More recently, the interactions of the CNS with the systemic environment have even been implicated in directly regulating organismal lifespan (Zhang et al. 2013). Thus, the CNS exhibits a unique duality not reflected in other tissues, serving as both a vulnerable site to the effects of aging while also acting as a potential master regulator of systemic aging itself (Fig.1).
Fig 1.
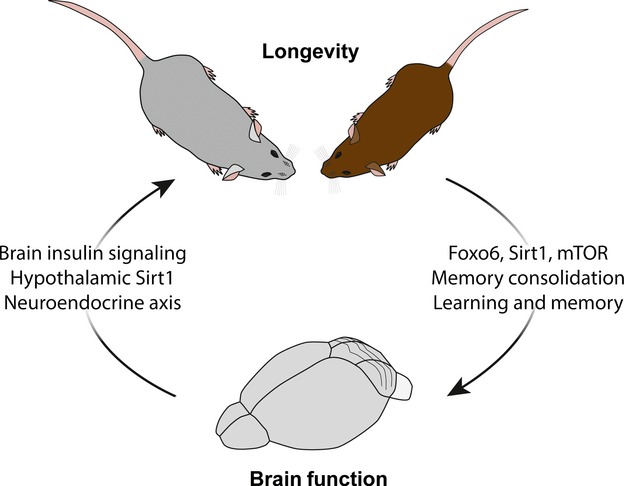
Interplay between lifespan regulation and brain function. Schematic illustration depicts the unique duality of the brain to be both responsive to systemic lifespan regulation as well as serve as a central regulator of lifespan. Genetic studies have identified the FoxO family of transcription factors, Sirtuins and mTOR signaling pathway as molecular regulators that both promote longevity and mediate critical brain functions known to undergo age-related impairments such as learning and memory. Conversely, the brain has also been demonstrated to promote longevity through neuronal regulation, particularly via the hypothalamus region.
As with many aspects of the aging process, the loss of plasticity in the aged CNS was traditionally thought to be immutable (Merzenich et al. 2014). However, it is becoming increasingly evident that changes in the aged systemic environment through systemic manipulations such as exercise, caloric restriction (CR), and heterochronic parabiosis (in which blood composition is altered by connecting circulatory systems of young and aged animals) can partially counteract the age-related loss of plasticity in the aged CNS. Correspondingly, it is feasible to consider reactivating latent plasticity dormant in the aged CNS as a means to rejuvenate regenerative, synaptic, and cognitive functions late in life, with potential implications for extending lifespan. In this review, we will discuss the process of aging and rejuvenation as systemic events, drawing attention to the potential for systemic manipulations to functionally reverse the effects of aging in the CNS. First, we will highlight genetic studies that demonstrate a surprising level of malleability in organismal lifespan, the high degree of interplay between lifespan regulation and brain function, and how the brain may, in turn, be a key regulator of lifespan (Fig.1). Second, we will review evidence for rejuvenation, focusing on several systemic manipulations – exercise, CR, parabiosis, and plasma administration (Fig.2) – and their ability to restore regenerative capacity throughout the body, as well as synaptic plasticity and cognitive function in the CNS (Table1).
Fig 2.
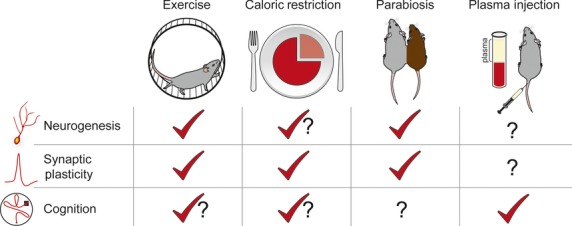
Currently known rejuvenating effects of systemic manipulations on the aged brain. Schematic illustration depicts individual systemic manipulations [exercise, caloric restriction (CR), heterochronic parabiosis, and young plasma administration] and their respective effect on each of the three main areas of rejuvenation (neurogenesis, synaptic plasticity, and cognitive function). Known enhancements are denoted by a red checkmark, contradictory reports are denoted as a red checkmark with a question mark, and unknown effects are denoted with a question mark.
Table 1.
Summary of systemic manipulations and their rejuvenating effects on the aged CNS
| Exercise | Caloric restriction | Heterochronic parabiosis | Plasma injection | |||||
|---|---|---|---|---|---|---|---|---|
| Neurogenesis & regeneration | + neurogenesis in DG |
|
|
|||||
| Synaptic plasticity |
|
|
|
+ plasticity-related genes | Villeda et al. (2014) | |||
| Cognitive function | ||||||||
| Spatial learning & memory |
|
|
+ spatial learning and memory | Villeda et al. (2014) | ||||
| Non-spatial |
|
|
+ odor discrimination | Katsimpardi et al. (2014) |
|
|||
+ increase; = no change; − decrease.
Plasticity of aging: lifespan as a genetic program
In the mid-1990s, researchers began to seriously entertain the notion that a single gene could exert significant influence over organismal lifespan (Kenyon et al. 1993; Kenyon 2011). Since then, numerous studies have substantiated the notion that individual genes can determine how long an organism lives (Kenyon 2010), and opened up the possibility that a process as complex as aging could be manipulated at the molecular level. Although many more genes have been identified, the most well-studied gene families shown to influence longevity, either through anti-aging or pro-aging effects, are the insulin receptor signaling pathway (van Heemst 2010; Fernandez and Torres-Aleman 2012), FoxO family of transcription factors (Partridge and Bruning 2008; Kenyon 2010; Webb and Brunet 2014), Sirtuins (Michan and Sinclair 2007; Baur et al. 2012), and mechanistic target of rapamycin (mTOR) (Johnson et al. 2013). A significant part of our understanding of these genes and their influence on aging come from genetic studies in model organisms such as yeast, worms, and flies, and have more recently begun to be validated in higher organisms such as mice (Lopez-Otin et al. 2013), suggesting the conservation of these pathways for aging across phylogeny. The effects of single gene manipulations on lifespan have previously been reviewed in greater detail (Michan and Sinclair 2007; Partridge and Bruning 2008; van Heemst 2010; Johnson et al. 2013); however, as several of these pathways will be mentioned throughout this review we will briefly describe them here.
Many of the genes shown to play a role in determining lifespan are involved in cellular processes that contribute to the aging of tissues, including oxidative stress resistance, glucose metabolism, and energy homeostasis, whose regulation ultimately lead to the survival or death of an organism (Lopez-Otin et al. 2013). The first molecular pathway regulating lifespan to be identified was the Insulin/IGF-1/FoxO pathway (Kenyon et al. 1993), an important signaling system that regulates energy homeostasis throughout the body (Fernandez and Torres-Aleman 2012). Whole organism mutations in a number of the insulin signaling pathway genes leading to reduced insulin signaling extend lifespan in worms, flies, and mice (Tatar et al. 2003; Kenyon 2005). In contrast, factors that act downstream of Insulin/IGF-1 signaling, such as the FoxO transcription factors, when up-regulated, act as pro-longevity signals that extend lifespan (Lin et al. 1997; Ogg et al. 1997) and also delay the onset and progression of age-related phenotypes (Partridge and Bruning 2008). Another important set of molecular mediators of longevity are the Sirtuins, a collection of histone deacetylase genes involved in oxidative metabolism and stress resistance (Michan and Sinclair 2007; Baur et al. 2012). Increased expression of several of the Sirtuins, such as Sirt1 (Satoh et al. 2013) and Sirt6 (Kanfi et al. 2012), extend lifespan in model organisms from yeast to mice (Sinclair et al. 1997; Kaeberlein et al. 1999; Tissenbaum and Guarente 2001; Michan and Sinclair 2007; Kanfi et al. 2012; Satoh et al. 2013). Lastly, another genetic pathway shown to regulate lifespan is the mTOR pathway. mTOR was originally discovered as a target of the immunosuppressant rapamycin (Heitman et al. 1991) and plays an important role in integrating multiple signals involved in cellular energy (Hay and Sonenberg 2004; Kapahi and Zid 2004). More recently, reduced activity of the mTOR pathway, often times via rapamycin administration, was also shown to promote longevity in model organisms (Vellai et al. 2003; Jia et al. 2004; Kapahi et al. 2004; Kaeberlein et al. 2005; Harrison et al. 2009). Collectively, the identification of such individual genes capable of modulating organismal lifespan first challenged long-held dogma of aging as immutable, and introduced the notion that systemic aging could indeed be itself a plastic event.
Plasticity of aging: interplay between lifespan regulation and brain function
Many of the pro-longevity signaling pathways have more recently also been shown to play important roles in higher level brain function (Hoeffer and Klann 2010; Michan et al. 2010; Garelick and Kennedy 2011; Salih et al. 2012; Herskovits and Guarente 2014), providing evidence for a connection between the plasticity of aging and the potential plasticity of the aged CNS. For example, FoxO transcription factors have been implicated in regulating CNS function. In particular, FoxO6, which is most highly expressed in the hippocampus, was recently shown to play a role in memory consolidation in mice (Salih et al. 2012). The authors found that in the absence of FoxO6, mice were unable to consolidate the memory trace in contextual fear conditioning and novel object recognition tasks (Salih et al. 2012). Similarly, Sirt1 was recently also shown to be essential for normal cognitive function and synaptic plasticity in adult mice (Michan et al. 2010). Specifically, in the absence of Sirt1, mice displayed deficits in learning and memory processes such as immediate memory, classical conditioning, and spatial learning paradigms, while over-expression of Sirt1 enhanced these behaviors (Michan et al. 2010). Furthermore, these results were validated in an independent study that suggested beneficial effects of Sirt1 on cognition were mediated through a microRNA (miR-134)-dependent mechanism (Gao et al. 2010). Lastly, multiple lines of evidence have implicated mTOR signaling as an important molecular pathway for learning and memory (Hoeffer and Klann 2010; Garelick and Kennedy 2011; Halloran et al. 2012). Most of these studies showing the importance of mTOR signaling in memory are elicited via rapamycin treatment (Hoeffer and Klann 2010; Halloran et al. 2012). However, there are also genetic studies that validate the conclusion that mTOR signaling plays a role in learning and memory, although many genetic manipulations involve upstream or downstream targets of mTOR (Antion et al. 2008; Hoeffer and Klann 2010) as global genetic deletion of mTOR is embryonic lethal (Gangloff et al. 2004). Nonetheless, rapamycin treatment prevents memory consolidation across multiple brain regions including the hippocampus, auditory cortex, gustatory cortex, amygdala, and prefrontal cortex in rodents (Garelick and Kennedy 2011). Interestingly, in the FoxO (Salih et al. 2012) and Sirtuin (Gao et al. 2010; Michan et al. 2010) studies mentioned above, the behavioral results were paralleled by changes in synaptic (long-term potentiation and long-term depression) or structural plasticity (spine density) in brain regions such as the hippocampus that are particularly vulnerable to the effects of aging. This suggests a possible parallel role for these signaling pathways in promoting longevity and potentially facilitating functional improvements in age-related cognitive dysfunction. This body of work not only demonstrated that longevity genes regulate critical CNS functions but also offered the first evidence that just as organismal lifespan is malleable so too could CNS functions prove to be amenable to rejuvenation.
Plasticity of aging: the brain as a central regulator of lifespan
The CNS is intimately associated with nearly every physiological system throughout the body (Alcedo et al. 2013a; Dietrich and Horvath 2013), placing it in a unique position to be influenced by and potentially influence systemic aging. Up to now, we have mainly considered how genetic manipulations of pro-longevity genes highlight plasticity in the aging process. However, considering aging is associated with a loss of function across multiple tissues, and the intimate association of the CNS with these tissues, we now address the mounting evidence for the CNS as a central regulator of systemic aging.
Some of the first evidence for the role of the CNS in controlling systemic aging came from cell-specific manipulations of longevity genes in model organisms such as worms, flies, and mice. For example, restoring insulin signaling in neurons, but not muscle or intestine, was sufficient to increase the lifespan of worms (Wolkow et al. 2000). In flies, manipulation of signaling molecules that impact FoxO activity improved neuronal stress response capabilities to reactive oxygen species and extended overall lifespan (Lee et al. 2009). In addition, mutations in genes that affect the ability of gustatory and olfactory sensory neurons to transmit systemic information about nutrient sources to the CNS, likely affecting metabolism, also regulated lifespan in worms and flies (Apfeld and Kenyon 1999; Alcedo and Kenyon 2004; Libert and Pletcher 2007; Libert et al. 2007; Jeong et al. 2012). There is now growing evidence that the same molecular pathways observed to regulate lifespan in the invertebrate CNS are also important regulators of aging in the mammalian CNS. In mice, reduction of brain insulin receptor substrate extended lifespan by 18% (Taguchi et al. 2007), an effect that is nearly identical to the changes in lifespan of mice that have heterozygous deletions of the insulin signaling substrate throughout the entire body (Bartke 2007; Taguchi et al. 2007). In addition, mice with homozygous deletions of the insulin signaling substrate die in utero; however, mice with homozygous deletion of this substrate specifically in the brain show a 14% increase in lifespan (Bartke 2007; Taguchi et al. 2007). These results demonstrate that, while complete loss of insulin signaling is detrimental to an organism, maintaining just one copy of this gene specifically in the CNS can significantly influence lifespan regulation promoting organismal longevity even in mammals (Bartke 2007). Together, these studies suggest that manipulation of longevity genes specifically in the CNS is sufficient to counteract the effects of aging at a systemic level.
More recently, aging research in mice has now begun to hone in on a particular region of the brain, the hypothalamus, as the potential CNS mediator of lifespan regulation (Tang and Cai 2013; Zhang et al. 2013). The hypothalamus is a region of the brain comprised of a diverse set of nuclei and is located between the thalamus and brainstem. Despite its small size, the hypothalamus receives input from nearly every tissue in the body, and by regulating endocrine signaling via hormones, plays a crucial role in many physiological processes including growth, metabolism, and reproduction to name a few (Dietrich and Horvath 2013). Thus, the hypothalamus serves as a well-established hub for communication between the CNS and the systemic environment. Indeed, it is the ability to both sense and influence tissues throughout the body that first indicated the hypothalamus as being well poised to regulate systemic aging. For example, studies have shown that brain over-expression of the pro-longevity gene Sirt1, specifically enriched in the hypothalamus, could extend lifespan in male and female mice (Satoh et al. 2013). Moreover, increased hypothalamic Sirt1 expression also improved age-related phenotypes including improvement of physical activity, oxygen consumption, sleep quality, and body temperature when compared to age-matched controls (Satoh et al. 2013). As with invertebrate studies, these data highlight the intimate interplay between pro-longevity mechanisms and CNS-mediated lifespan regulation. Independent studies have now also shown that the hypothalamus can directly regulate systemic aging itself through the immune-neuroendocrine axis (Zhang et al. 2013). Specifically, investigators showed that reducing inflammation in the hypothalamic immune cells (microglia) could change the hypothalamus response to the systemic environment extending lifespan and reducing aging-associated pathologies (Zhang et al. 2013). The authors also provide a potential mechanistic link between age-related changes in hypothalamic inflammation and systemic levels of gonadotropin-release hormone. In addition, manipulations of the immune-neuroendocrine axes also yielded significant enhancements in the CNS, including increased neurogenesis and improved cognition in aged mice (Zhang et al. 2013). While future studies remain necessary to better understand how age-related changes in the hypothalamus influence lifespan regulation, collectively these studies support the hypothesis that the CNS plays a central role in modulating systemic aging (Bartke 2007; Alcedo et al. 2013a; Gabuzda and Yankner 2013; Satoh et al. 2013; Tang and Cai 2013; Zhang et al. 2013).
Taken as a whole, studies in invertebrates and mammals highlight the malleability of the aging process and demonstrate the involvement of the CNS in regulating lifespan (Boulianne 2001; Alcedo et al. 2013a,b; Zhang et al. 2013), raising the possibility that influencing the communication between the CNS and the systemic environment could prove one effective strategy for counteracting the effects of aging (Tang and Cai 2013).
Reversal of aging: systemic manipulations as mediators of rejuvenation
Manipulating individual genes within the CNS may represent one approach with potential to counteract the effects of aging (Bowers et al. 2011). However, targeted manipulations of genes, specifically within specialized populations of neurons in regions such as the hypothalamus, prove challenging at this point (Kay 2011; Wanisch et al. 2013). While reversal of aging by the CNS appears distant, alternative strategies to rejuvenate aged tissues through a more systemic approach may prove equally effective in counteracting the aging process. In this manner, broad changes in the aged systemic environment, rather than a central regulator, may provide the means for rejuvenation. Indeed, systemic manipulations such as exercise, CR, and changes in blood composition by heterochronic parabiosis or young plasma administration (Fig.2) have already yielded much promise in their ability to combat signs of aging in both peripheral tissues and the CNS. Specifically, to date, these different systemic manipulations have been shown to ameliorate impairments in the regenerative capacity of aged tissues, as well as synaptic plasticity and even cognitive functions in the aged CNS. While exercise, CR, and changes in old blood composition have not been shown to ameliorate all of the same set of age-related impairments or to the same degree, evidence that a systemic approach to rejuvenation is plausible continues to mount. It should be noted that the field of rejuvenation research remains in its infancy and questions such how systemic manipulations can rewind the aging clock remain poorly understood.
Reversal of aging: rejuvenation of regeneration by systemic manipulations
One feature of aging that appears to be common among tissues is the loss of regenerative capacity (Conboy and Rando 2005; Rando 2006; Jones and Rando 2011). Critical for regeneration throughout life is the maintenance of endogenous tissue-specific adult stem/progenitor cells, which have the ability to self-renew and produce new cells in adult tissue (Weissman 2000; Conboy and Rando 2005; Rando 2006; Jones and Rando 2011). With age comes functional failure of adult stem/progenitor cells, serving as one potential contributor to the regenerative decline of both peripheral tissues and the CNS during aging (Conboy and Rando 2005; Jones and Rando 2011). For example, the decline in muscle regeneration is common in the elderly, and likely increases the chance of accidents and the ability to perform manual tasks (Grounds 1998; Conboy and Rando 2005). Studies suggest that this decline in skeletal muscle function and mass with age is mediated by the inability of muscle satellite cells (progenitors) to regenerate muscle fibers (Conboy and Rando 2005). Similarly, in the blood, dysfunctional regeneration because of aging of hematopoietic stem cells has been linked to inefficient immune responses, anemia, and leukemia in the elderly (Corey et al. 2007; Ergen and Goodell 2010). Interestingly, the adult CNS was traditionally thought to be devoid of significant regenerative capacity. However, we now know that specific brain regions including the subventricular zone (SVZ) lining the lateral ventricle and the subgranular zone (SGZ) of the dentate gyrus in the hippocampus harbor adult neural stem/progenitor cells capable of generating neurons (neurogenesis) (Eriksson et al. 1998; Ming and Song 2011; Lee et al. 2012). Consistent with peripheral tissues, the levels of adult neurogenesis also decline with age (Kuhn et al. 1996; Bondolfi et al. 2004; Lee et al. 2012). While adult neurogenesis has been shown to regulate cognitive functions in young adults (Dupret et al. 2007; Clelland et al. 2009; Nakashiba et al. 2012), data still remain inconclusive and predominantly correlative with regard to decreased neurogenesis and cognitive dysfunction in the elderly (Lee et al. 2012). Notwithstanding, this does not preclude the possibility that increasing adult neurogenesis in the aged brain may facilitate cognitive improvements in the elderly (Lee et al. 2012). Correspondingly, strategies that enhance regeneration in both peripheral tissues and the CNS may prove efficacious at counteracting the deleterious effects of aging on tissue function as they arise with age (Conboy and Rando 2012). Here, we will review present evidence for rejuvenation of regeneration by systemic manipulations that include exercise, CR, and heterochronic parabiosis (Table1).
Exercise
Physical exercise increases blood delivery to most tissues and leads to changes in the systemic environment (Trejo et al. 2001; Fabel et al. 2003; Cotman et al. 2007). Interestingly, numerous studies have documented rejuvenating effects of exercise on the functional and regenerative capacity of peripheral tissues and CNS in animal models (van Praag et al. 1999a,b, 2005; Shefer et al. 2010; Baker et al. 2011; Valero et al. 2012). Moreover, exercise has even been associated with a reduced incidence of developing classical age-related diseases including cardiovascular disease, type II diabetes, osteoporosis, macular degeneration, and dementia (Larson et al. 2006; Warburton et al. 2006; Mora et al. 2007; Lawson et al. 2014). Considering loss of tissue regeneration during aging is thought to contribute to decreased tissue function (Jones and Rando 2011; Conboy and Rando 2012), it is consistent with the concept of rejuvenation that the beneficial effects of exercise extend to enhancements in adult stem/progenitor cells in old age. Outside the CNS, exercise can promote hematopoiesis (regeneration of blood cells) in the aging systemic environment (Baker et al. 2011), and increase the proliferative capacity of aged skeletal muscle stem cells, including satellite (Shefer et al. 2010) and mesenchymal stem cells (Valero et al. 2012). Recent studies have shown the effects of exercise on molecular and cellular processes that play major roles in stem cell function both in in vitro and in vivo. For example, exercise induces autophagy (clearance of cellular debris) (He et al. 2012), which protects hematopoietic stem cells from metabolic stress and contributes to their lifelong maintenance (Warr et al. 2013). Exercise is also associated with increased telomere length in humans (LaRocca et al. 2010), which may aid in counteracting the effects of telomere shortening on replicative senescence (Allsopp and Weissman 2002; Flores et al. 2005). On a molecular level, exercise leads to regulation of telomere-associated genes and microRNA expression (Chilton et al. 2014), both important for stem cell self-renewal and differentiation (Lee et al. 1998; Gangaraju and Lin 2009; Jaskelioff et al. 2011).
The beneficial effects of exercise extend beyond peripheral tissues to also include the brain. In particular, increased running in aged mice has been shown to enhance neural progenitor proliferation and neurogenesis (van Praag et al. 2005; Kronenberg et al. 2006; Wu et al. 2008; Marlatt et al. 2012) to a level comparable to that observed in young animals. Because of the blood–brain barrier, it was traditionally thought that the beneficial effects of exercise on the CNS were not orchestrated through systemic changes in the periphery. However, recent studies suggest that the effects of exercise are, in part, mediated by changes in the systemic environment. Investigations looking at magnetic resonance imaging (MRI) measurements of cerebral blood volume in the hippocampus have demonstrated that exercise selectively increased the cerebral blood volume of the dentate gyrus, correlating with post-mortem increase in neurogenesis (Pereira et al. 2007). From a molecular perspective, elevated systemic levels of circulating growth factors such as vascular endothelial growth factor and insulin-like growth factor 1 (IGF-1) in blood elicited by increased exercise have been shown to mediate, in part, enhancements in neurogenesis (Trejo et al. 2001; Fabel et al. 2003). Coincidently, circulating levels of IGF-1 decrease with age and the restoration to levels resembling a younger systemic environment up-regulate neurogenesis and improve learning and memory (Lichtenwalner et al. 2001; Darnaudery et al. 2006).
Together, these studies show that a systemic manipulation such as exercise can rejuvenate adult stem cell function across tissues. In addition, it indicates that targeting the systemic environment could prove to be an effective strategy to reverse the functional impairments of aging on the aged CNS.
Caloric restriction
Another systemic manipulation shown to counteract the age-induced effects on tissue regeneration is CR, a reduction of 20–40% of caloric intake without malnutrition. The ability of CR to counteract aging was initially characterized as an extension of studies investigating the pro-longevity effects of CR on lifespan, a phenomenon conserved across phylogeny (Fontana et al. 2010; Libert and Guarente 2013). This effect likely results from increasing glucose metabolism, reducing oxidative stress and the ability of cells to counteract DNA damage, as well as influencing aspects of the aging immune and neuroendocrine system (Fusco and Pani 2013). More recently, CR has been shown to rejuvenate tissue regeneration in aged organisms (Mazzoccoli et al. 2014), similar to the effects of exercise. A number of studies have shown rejuvenating effects of CR on the decline of hematopoietic stem cell function (Chambers et al. 2007; Ertl et al. 2008; Grymula et al. 2014). Rejuvenation of regeneration was also observed in skeletal muscle, in which short-term CR in aged animals increased muscle stem cell availability and activity when compared to ad libitum fed mice (Cerletti et al. 2012). In addition, there are also beneficial effects of CR on intestinal stem cells. Specifically, a 35% increase in intestinal Olfm4-positive progenitor cells was observed in mice under CR compared to ad libitum fed controls, indicating that CR promotes intestinal stem cell self-renewal and preservation (Yilmaz et al. 2012).
The effects of both short-term and long-term CR on rejuvenation of regeneration are also found in the CNS. Early studies observed an increase in proliferating cells in the dentate gyrus after a 30% reduction of caloric intake for 3 months in rats (Lee et al. 2000) and for 2 weeks in mice (Lee et al. 2002). However, different from peripheral tissues, this effect of CR on increased cell number in the brain resulted from a reduction in cell death, rather than an increase in cell proliferation (Lee et al. 2000, 2002). In addition, only Bromodeoxyuridine (BrdU), a marker of cell proliferation, was quantified making it difficult to conclude that this increase in BrdU-positive cells translates into increased levels of neurogenesis (Lee et al. 2000, 2002). Similar effects were also observed when mice were calorie restricted over an extended duration of 3–11 months, although in this study the authors found that CR led to an increase in glial cells and not neurons (Bondolfi et al. 2004). More recently, prolonged exposure of mice to CR (40% decrease in caloric intake maintained for 10–12 months) was shown to reduce age-related decrease in neural progenitor cell divisions in the aging brain (Park et al. 2013). As a whole, because different experimental paradigms of CR were used and the level of detail with which proliferating cells were characterized varied, the effect of CR on mammalian adult neurogenesis is difficult to ascertain with clarity. Future studies are therefore needed to better understand the effect CR plays in counteracting the age-related decline in adult neurogenesis. Nevertheless, CR illustrates that an additional systemic manipulation independent of exercise can also affect regenerative capacity in the aged brain.
Heterochronic parabiosis
Lastly, rejuvenation of tissue regeneration in aging organisms has also been observed after heterochronic parabiosis. First introduced in 1864 by Dr Paul Bert, parabiosis is a surgical procedure by which two animals are physically connected. The procedure and its historical context have previously been described in greater detail (Conboy et al. 2013; Eggel and Wyss-Coray 2014). Of particular interest is the effects of the blood of young animals on the physiology and behavior of older animals. Through the use of the heterochronic parabiosis model, it has now been shown that exposure to young blood can rejuvenate the regenerative capacity of peripheral tissues and CNS in aged animals.
Several studies have shown that aged mice exposed to a young systemic environment exhibit reduced signs of biological aging in cardiovascular, skeletal, and digestive systems. In skeletal muscle, old heterochronic parabionts exposed to young blood exhibited increased muscle progenitor cell activity leading to enhanced regeneration and reduced fibrotic responses compared to old isochronic parabionts (Conboy et al. 2005; Brack et al. 2007). Liver tissue isolated from old heterochronic parabionts was also found to have more youthful levels of hepatocyte rejuvenation (Conboy et al. 2005). Furthermore, the rejuvenating effects of young blood have also been observed on both cardiovascular (Loffredo et al. 2013) and metabolic (Salpeter et al. 2013) systems in aging mice, although in these studies the effect of young blood on stem cells function was not investigated. In particular, young blood can reverse age-related cardiac hypertrophy in old heterochronic parabionts (Loffredo et al. 2013) and reverse the age-related decline in pancreatic beta cell replication thought to contribute to the development of type II diabetes (Salpeter et al. 2013). Together, these studies show that changing the composition of the aged systemic environment to a more youthful state can reverse the regenerative and functional decline in aged peripheral tissues. Indeed, these studies point to the existence of ‘pro-youthful’ factors in young blood that have begun to be identified in recent studies. For instance, restoring systemic levels of the growth differentiation factor 11 (GDF11), a member of the transforming growth factor β superfamily (McPherron et al. 2009), to a more youthful state reversed age-related skeletal muscle and cardiovascular impairments in mice (Loffredo et al. 2013; Sinha et al. 2014). The authors found that daily administration of recombinant GDF11 into aged mice increased muscle satellite cell frequency and function, as well as improved muscle function (Sinha et al. 2014). Similarly, treatment of old mice with recombinant GDF11 reversed age-related cardiac hypertrophy (Loffredo et al. 2013). Another study also found that increasing levels of oxytocin, a circulating hormone, in aged mice enhanced muscle regeneration by increasing muscle stem cell activation and proliferation (Elabd et al. 2014).
The rejuvenating effects of young blood have now also been observed in the aged CNS of old mice. In a mouse model of demyelination, exposure of old mice to a youthful systemic environment increases myelination in the spinal cord of old heterochronic parabionts by recruiting young peripheral monocytes and promoting differentiation of oligodendrocyte progenitor cells (Ruckh et al. 2012). These findings are of particular significance given that nerve demyelination occurring in the elderly functional impairs muscle strength and sensory discrimination (Verdu et al. 2000) while contributing to axonal degeneration (Edgar and Nave 2009). In the hippocampus, old heterochronic parabionts exposed to a young systemic environment exhibited increased neurogenesis in the dentate gyrus, indicated by increased BrdU-positive proliferating cells, Sox2-positive progenitors, and Doublecortin-positive newly born neurons (Villeda et al. 2011). More recently, an independent study also corroborated the rejuvenating effects of young blood on adult neurogenesis in the SVZ of old heterochronic parabionts (Katsimpardi et al. 2014). Specifically, they found that young blood increased Ki67-positive proliferating cells in the SVZ, enhanced olfactory neurogenesis, and facilitated vasculature remodeling in neurogenic regions of old mice. Interestingly, the authors found that recombinant GDF11 administration could, in part, mimic the beneficial effects of young blood on neurogenesis and vasculature remodeling (Katsimpardi et al. 2014). Consistent with other functions of the transforming growth factor-β superfamily, GDF11 appears to have pleiotropic qualities. Indeed, during development, GDF11 participates in patterning of the CNS and has been found to inhibit embryonic neurogenesis (Hastings and Gould 2003; Wu et al. 2003; Wu and Hill 2009). Surprisingly, in the context of aging it seems to promote plasticity of the CNS (Katsimpardi et al. 2014). Identification of this one factor, GDF11, is an exciting finding; however, the effects of young blood on rejuvenation of regenerative capacity are not fully recapitulated (Laviano 2014), suggesting the existence of multiple ‘pro-youthful’ factors. Given the rejuvenating effects of young blood have now been observed in neurogenic zones across multiple brain regions, this supports the possibility of global rejuvenating effects that may extend throughout the aged brain.
Reversal of aging: rejuvenation of cognitive functions by systemic manipulations
Thus far, we have focused on the ability of systemic manipulations to rejuvenate regenerative capacity, highlighting the recent body of work in the CNS focused on adult neurogenesis. However, functionally the relevance of the age-related decline in adult neurogenesis on cognitive impairments in the aged brain remains obfuscated (Lee et al. 2012). Given the hardship age-related cognitive impairments cause on the elderly, it is critical to consider the significant impact systemic manipulations could have if rejuvenating effects extend to higher order cognitive functions. Currently, there are now a number of studies demonstrating a potential role for exercise, CR, and young blood plasma administration in ameliorating age-related cognitive impairments in learning and memory. However, at present, the exact effects of exercise and CR on cognitive function remain ambiguous.
Exercise
Numerous studies have observed positive effects of exercise on cognition in both animal models and humans (Hawkins et al. 1992; Kramer et al. 1999; Radak et al. 2001; van Praag et al. 2005; van Uffelen et al. 2008a; O'Callaghan et al. 2009; Kumar et al. 2012; Marlatt et al. 2012; Snigdha et al. 2014). In aged mice, exposure to wheel running for 1 month resulted in better learning acquisition and memory retention in the Morris water maze task (van Praag et al. 2005). When aged rats exercised for 10–12 weeks, they were observed to perform better on cued and object recognition tasks when compared to non-exercise aged-matched controls (Kumar et al. 2012). Lastly, regular swimming exercise in aged rats was also associated with better performance on both short- and long-term memory measured using the passive avoidance test (Radak et al. 2001). Consistent with findings in rodents, canines exposed to short- and long-term exercise paradigms demonstrated enhanced cognitive function in discrimination and spatial learning and memory tasks, as well as object location and reversal learning (Snigdha et al. 2014). In humans, one study reported that 60- to 85-year-old individuals who underwent 10 weeks of exercise performed better in a dual-task performance task compared to the non-exercise control group (Hawkins et al. 1992). Furthermore, a second study also showed that aerobic exercise for 6 months enhanced executive function in 60- to 75-year-old adults (Kramer et al. 1999). It should be noted, however, that some contradictory studies argue against the beneficial effects of exercise, reporting that increased exercise does not modify spatial memory (Barnes et al. 1991) or ameliorate spatial memory deficits (Kumar et al. 2012) in aged rats. In older human adults with mild cognitive impairments increased exercise was also not observed to improve age-related memory and attention impairments (van Uffelen et al. 2008a,b).
Caloric restriction
Several studies in animal models have observed beneficial effects of various forms of CR on a variety of cognitive functions such as motor learning and hippocampal-dependent memory (Pitsikas and Algeri 1992; Fontan-Lozano et al. 2007; Witte et al. 2009; Dal-Pan et al. 2011; Ma et al. 2014; Talhati et al. 2014). During long-term CR applied for 30 weeks, adult mice were shown to have improved spatial learning and memory in the Morris water maze task (Ma et al. 2014). In a second study, short-term CR was also shown to enhance memory persistence in a discriminative avoidance task in young adult animals when applied before training and in aged animals when applied before testing (Talhati et al. 2014), indicating age-dependent differences in the effectiveness of CR. In primates, chronic CR also resulted in improved working memory (Dal-Pan et al. 2011). In humans, one study found that CR improved verbal memory scores by 20% in 60-year-old males and females (Witte et al. 2009). Although a majority of studies have observed beneficial effects of CR on cognitive function, a number of studies have put into question the efficacy and extent to which CR can ameliorate age-related cognitive impairments (Beatty et al. 1987; Bond et al. 1989; Yanai et al. 2004; Martin et al. 2007). For instance, long-term CR in aged rats resulted in deficits in spatial discrimination tasks compared to ad libitum age-matched control groups (Yanai et al. 2004). Similarly, short-term CR was shown to impair memory consolidation and exert no effect on memory retrieval in aged mice (Talhati et al. 2014). In humans, while CR improved verbal memory (Witte et al. 2009), no beneficial effects were detected for other types of age-related memory impairments. In addition, a randomized controlled study initiated by the National Institute of Aging found that after 6 months of CR, there were no obvious effects on verbal memory scores, visual retention, or attention when compared to control groups (Martin et al. 2007). However, the age group of participants in this study had a range of 25–45 years of age, raising the question of whether an effect would be present in older humans.
Despite the contradictory results described above, as a whole, the literature supports the notion that systemic manipulations such as exercise, and to some extent CR, elicit beneficial effects on cognitive impairments (Kramer and Erickson 2007; Hillman et al. 2008). Moreover, the disparities between studies investigating the effects of exercise and CR on ameliorating age-related cognitive impairments, to some degree, may likely reflect experimental design differences, including treatment duration, age of initiation, treatment regiment, and experimental group composition such as gender and weight (Kramer and Erickson 2007; van Uffelen et al. 2008a; Witte et al. 2009). Therefore, future studies and comparisons should strive to standardize these parameters to better understand the contribution that these systemic manipulations may have on preventing age-related cognitive impairments.
Heterochronic parabiosis and young plasma administration
Excitingly, recent research has begun to investigate the beneficial effects of changing old blood composition to a more youthful systemic environment on age-related impairments in cognition. Old mice administered young plasma over a period of less than a month demonstrated improvements in hippocampal-dependent learning and memory in water maze and contextual fear conditioning tasks compared with old mice receiving age-matched old plasma (Villeda et al. 2014). Another study found that old mice exposed to a youthful systemic environment by heterochronic parabiosis also showed enhancements in their ability to discriminate odors (Katsimpardi et al. 2014). Whether increased discriminability found in this study reflects an improvement of cognitive faculties such as learning and memory remains to be determined. Nonetheless, together, these studies show that exposure of an old mouse to a young systemic environment can reverse behavioral and cognitive impairments. These studies also demonstrate the potential for future therapeutic avenues aimed at restoring CNS functions by promoting a more youthful systemic environment. It is exciting to postulate that perhaps similar to heterochronic parabiosis, changes in the systemic environment occurring as a result of exercise or CR may actually be changing the composition of old blood back toward a more youthful state. Indeed, it would be interesting to compare the blood composition between old animals undergoing these different systemic manipulations. Altogether, however, these data all suggest that maintaining high systemic levels of ‘pro-youthful’ factors in the periphery has the capacity to counteract age-related functional impairments in the aged CNS.
At a mechanistic level, evidence now exists that beneficial effects of young blood on cognitive function may be mediated, in part, independent of stem cell rejuvenation by enhancements in alternative forms of brain plasticity. Indeed, three correlates of synaptic plasticity were increased in old heterochronic parabionts: synaptic strength, dendritic spine density, and plasticity-related gene expression (Villeda et al. 2014). Extracellular electrophysiological recordings in hippocampal slices of old heterochronic parabionts revealed enhanced long-term potentiation, a process involving long-term strengthening between neurons (Bliss and Collingridge 1993), compared to old isochronic parabionts (Villeda et al. 2014). In addition, mature hippocampal granule cell neurons also exhibited structural enhancements in dendritic spine density, a proposed neuronal locus of plasticity (Sala and Segal 2014), after exposure to a young systemic environment (Villeda et al. 2014). Consistent with these results, microarray analysis of hippocampal tissue from old heterochronic parabionts identified expression profiles indicative of enhanced synaptic plasticity. In particular, activation of the cyclic AMP response element binding protein (Creb) signaling pathway was observed to mediate in part the rejuvenating effects of young blood on mature neurons in the hippocampus of heterochronic parabionts (Villeda et al. 2014). Moreover, Creb activation specifically in mature hippocampal neurons was also demonstrated to mediate in part cognitive rejuvenation after young plasma administration (Villeda et al. 2014). Notably, findings observed with heterochronic parabiosis and young plasma administration are consistent with previous reports in which synaptic plasticity and expression of plasticity-related molecules are also enhanced in the aged brain by other systemic manipulations including exercise and CR (Eckles-Smith et al. 2000; Fontan-Lozano et al. 2007; O'Callaghan et al. 2009; Marlatt et al. 2012). Interestingly, the structural and molecular rejuvenating effects of young blood by heterochronic parabiosis and young plasma administration were primarily observed in the dentate gyrus and not CA1 region of the hippocampus, suggesting that ‘pro-youthful’ factors may elicit molecular changes selectively within the CNS regions susceptible to the effects of aging (Villeda et al. 2014).
Taken together, these studies are the first to highlight the rejuvenating effect a young systemic environment exerts on higher level cognitive function in the old brain (Katsimpardi et al. 2014; Villeda et al. 2014). Excitingly, observing rejuvenation of plasticity at a molecular, structural, and functional level now opens the possibility that global age-related impairments in plasticity can be restored throughout the aging brain. However, whether enhancements of young blood extend beyond neurogenic regions such as the hippocampus to other areas of the brain lacking regenerative capacity such as the neocortex remains unknown. Thus, future studies are now needed to determine the extent to which age-related functional impairments throughout the CNS can be reversed.
Conclusion
In this review, we have discussed how a growing body of work focusing on genetic and even CNS regulation of lifespan has demonstrated the plasticity of aging and its amenability to modulation (Fig.1). This work has raised the exciting possibility that we can tap into this latent plasticity as a means to counteract or even reverse signs of systemic aging. As our understanding of this plasticity grows, the more we can expect it to guide the development of strategies aimed at reversing age-related impairments. One such strategy that has emerged from our understanding of the plasticity of aging is the possibility for rejuvenation through external manipulations that alter the systemic environment (Fig.2). Here, we have highlighted the effects of exercise, CR, heterochronic parabiosis, and young plasma administration in rejuvenating multiple forms of plasticity throughout the aging body, with particular emphasis on the CNS (Table1).
To date, the rejuvenating effects of exercise and heterochronic parabiosis have been attributed, at least in part, to changes in the composition of the systemic environment, suggesting the presence of ‘pro-youthful’ factors. Indeed, elevated systemic levels of vascular endothelial growth factor and IGF-1 during exercise mediate enhancements in neurogenesis (Trejo et al. 2001; Fabel et al. 2003). Moreover, restoring systemic levels of IGF-1 to more youthful levels in aged animals also enhances neurogenesis and improves learning and memory (Lichtenwalner et al. 2001; Darnaudery et al. 2006). Similarly, heterochronic parabiosis studies have demonstrated that restoring systemic levels of GDF11 to resemble a more youthful systemic environment also elicits enhancements in neurogenesis and neurovasculature remodeling in aged mice (Katsimpardi et al. 2014). It is remarkable that ‘pro-youthful’ factors, such as GDF11, are being identified (Loffredo et al. 2013; Katsimpardi et al. 2014; Sinha et al. 2014); however, it should be noted that at present the effects of young blood have not been fully recapitulated, pointing to the existence of additional ‘pro-youthful’ factors (Laviano 2014). While it is critical that future studies continue to explore the rejuvenating effects of ‘pro-youthful’ factors, alternative strategies for rejuvenation should also be considered. Indeed, heterochronic parabiosis and old plasma administration studies have pointed to the existence of ‘pro-aging’ factors as negative regulators of regenerative capacity in peripheral tissues (Conboy et al. 2005; Brack et al. 2007) and the CNS (Villeda et al. 2011; Katsimpardi et al. 2014), as well as cognitive function (Villeda et al. 2011). Correspondingly, it has been proposed that mitigating the systemic levels of ‘pro-aging’ factors in old blood may also provide an effective approach to rejuvenate aging phenotypes (Villeda and Wyss-Coray 2013; Laviano 2014; Villeda et al. 2014). Together, these findings position systemic manipulations altering either ‘pro-youthful’ or ‘pro-aging’ factors as exciting new approaches for accessing the plasticity of aging required for tissue and cognitive rejuvenation. Cumulatively, this body of work also provides support for future investigations to test whether the beneficial effects of systemic manipulations extend beyond normal aging to reverse cellular and cognitive decline in those suffering from age-related degenerative disorders such as Alzheimer's disease.
Acknowledgments and conflict of interest disclosure
We would like to thank Gregor Bieri for designing and generating schematic images. In addition, we thank Dr Kris Bouchard for his insightful comments and review of the manuscript, as well as the entire Villeda lab for critically reading the manuscript. This work was supported by grants from UCSF PBBR, Sandler Foundation (S.A.V.), UCSF-CTSI (UL1-TR000004, S.A.V.), NIH Director's Independence Award (DP5-OD12178, S.A.V.).
All experiments were conducted in compliance with the ARRIVE guidelines. The authors have no conflict of interest to declare.
References
- Alcedo J, Kenyon C. Regulation of C. elegans longevity by specific gustatory and olfactory neurons. Neuron. 2004;41:45–55. doi: 10.1016/s0896-6273(03)00816-x. [DOI] [PubMed] [Google Scholar]
- Alcedo J, Flatt T, Pasyukova EG. Neuronal inputs and outputs of aging and longevity. Front. Genet. 2013a;4:71. doi: 10.3389/fgene.2013.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcedo J, Flatt T, Pasyukova EG. The role of the nervous system in aging and longevity. Front. Genet. 2013b;4:124. doi: 10.3389/fgene.2013.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allsopp RC, Weissman IL. Replicative senescence of hematopoietic stem cells during serial transplantation: does telomere shortening play a role? Oncogene. 2002;21:3270–3273. doi: 10.1038/sj.onc.1205314. [DOI] [PubMed] [Google Scholar]
- Antion MD, Merhav M, Hoeffer CA, Reis G, Kozma SC, Thomas G, Schuman EM, Rosenblum K, Klann E. Removal of S6K1 and S6K2 leads to divergent alterations in learning, memory, and synaptic plasticity. Learn. Mem. 2008;15:29–38. doi: 10.1101/lm.661908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apfeld J, Kenyon C. Regulation of lifespan by sensory perception in Caenorhabditis elegans. Nature. 1999;402:804–809. doi: 10.1038/45544. [DOI] [PubMed] [Google Scholar]
- Baker JM, De Lisio M, Parise G. Endurance exercise training promotes medullary hematopoiesis. FASEB J. 2011;25:4348–4357. doi: 10.1096/fj.11-189043. [DOI] [PubMed] [Google Scholar]
- Barnes CA, Forster MJ, Fleshner M, Ahanotu EN, Laudenslager ML, Mazzeo RS, Maier SF, Lal H. Exercise does not modify spatial memory, brain autoimmunity, or antibody response in aged F-344 rats. Neurobiol. Aging. 1991;12:47–53. doi: 10.1016/0197-4580(91)90038-l. [DOI] [PubMed] [Google Scholar]
- Bartke A. Aging: all in the head? Cell Metab. 2007;6:153–154. doi: 10.1016/j.cmet.2007.08.007. [DOI] [PubMed] [Google Scholar]
- Baur JA, Ungvari Z, Minor RK, Le Couteur DG, de Cabo R. Are sirtuins viable targets for improving healthspan and lifespan? Nat. Rev. Drug Discov. 2012;11:443–461. doi: 10.1038/nrd3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty WW, Clouse BA, Bierley RA. Effects of long-term restricted feeding on radial maze performance by aged rats. Neurobiol. Aging. 1987;8:325–327. doi: 10.1016/0197-4580(87)90071-6. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Bond NW, Everitt AV, Walton J. Effects of dietary restriction on radial-arm maze performance and flavor memory in aged rats. Neurobiol. Aging. 1989;10:27–30. doi: 10.1016/s0197-4580(89)80007-7. [DOI] [PubMed] [Google Scholar]
- Bondolfi L, Ermini F, Long JM, Ingram DK, Jucker M. Impact of age and caloric restriction on neurogenesis in the dentate gyrus of C57BL/6 mice. Neurobiol. Aging. 2004;25:333–340. doi: 10.1016/S0197-4580(03)00083-6. [DOI] [PubMed] [Google Scholar]
- Boulianne GL. Neuronal regulation of lifespan: clues from flies and worms. Mech. Ageing Dev. 2001;122:883–894. doi: 10.1016/s0047-6374(01)00245-7. [DOI] [PubMed] [Google Scholar]
- Bowers WJ, Breakefield XO, Sena-Esteves M. Genetic therapy for the nervous system. Hum. Mol. Genet. 2011;20:R28–R41. doi: 10.1093/hmg/ddr110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brack AS, Conboy MJ, Roy S, Lee M, Kuo CJ, Keller C, Rando TA. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science. 2007;317:807–810. doi: 10.1126/science.1144090. [DOI] [PubMed] [Google Scholar]
- Cerletti M, Jang YC, Finley LW, Haigis MC, Wagers AJ. Short-term calorie restriction enhances skeletal muscle stem cell function. Cell Stem Cell. 2012;10:515–519. doi: 10.1016/j.stem.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers SM, Shaw CA, Gatza C, Fisk CJ, Donehower LA, Goodell MA. Aging hematopoietic stem cells decline in function and exhibit epigenetic dysregulation. PLoS Biol. 2007;5:e201. doi: 10.1371/journal.pbio.0050201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chilton WL, Marques FZ, West J, Kannourakis G, Berzins SP, O'Brien BJ, Charchar FJ. Acute Exercise leads to regulation of telomere-associated genes and microRNA expression in immune cells. PLoS ONE. 2014;9:e92088. doi: 10.1371/journal.pone.0092088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clelland CD, Choi M, Romberg C, et al. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science. 2009;325:210–213. doi: 10.1126/science.1173215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conboy IM, Rando TA. Aging, stem cells and tissue regeneration: lessons from muscle. Cell Cycle. 2005;4:407–410. doi: 10.4161/cc.4.3.1518. [DOI] [PubMed] [Google Scholar]
- Conboy IM, Rando TA. Heterochronic parabiosis for the study of the effects of aging on stem cells and their niches. Cell Cycle. 2012;11:2260–2267. doi: 10.4161/cc.20437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005;433:760–764. doi: 10.1038/nature03260. [DOI] [PubMed] [Google Scholar]
- Conboy MJ, Conboy IM, Rando TA. Heterochronic parabiosis: historical perspective and methodological considerations for studies of aging and longevity. Aging Cell. 2013;12:525–530. doi: 10.1111/acel.12065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corey SJ, Minden MD, Barber DL, Kantarjian H, Wang JC, Schimmer AD. Myelodysplastic syndromes: the complexity of stem-cell diseases. Nat. Rev. Cancer. 2007;7:118–129. doi: 10.1038/nrc2047. [DOI] [PubMed] [Google Scholar]
- Cotman CW, Berchtold NC, Christie LA. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci. 2007;30:464–472. doi: 10.1016/j.tins.2007.06.011. [DOI] [PubMed] [Google Scholar]
- Dal-Pan A, Pifferi F, Marchal J, Picq JL, Aujard F, Consortium R. Cognitive performances are selectively enhanced during chronic caloric restriction or resveratrol supplementation in a primate. PLoS ONE. 2011;6:e16581. doi: 10.1371/journal.pone.0016581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnaudery M, Perez-Martin M, Belizaire G, Maccari S, Garcia-Segura LM. Insulin-like growth factor 1 reduces age-related disorders induced by prenatal stress in female rats. Neurobiol. Aging. 2006;27:119–127. doi: 10.1016/j.neurobiolaging.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Dietrich MO, Horvath TL. Hypothalamic control of energy balance: insights into the role of synaptic plasticity. Trends Neurosci. 2013;36:65–73. doi: 10.1016/j.tins.2012.12.005. [DOI] [PubMed] [Google Scholar]
- Dupret D, Fabre A, Dobrossy MD, et al. Spatial learning depends on both the addition and removal of new hippocampal neurons. PLoS Biol. 2007;5:e214. doi: 10.1371/journal.pbio.0050214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckles-Smith K, Clayton D, Bickford P, Browning MD. Caloric restriction prevents age-related deficits in LTP and in NMDA receptor expression. Brain Res. Mol. Brain Res. 2000;78:154–162. doi: 10.1016/s0169-328x(00)00088-7. [DOI] [PubMed] [Google Scholar]
- Edgar JM, Nave KA. The role of CNS glia in preserving axon function. Curr. Opin. Neurobiol. 2009;19:498–504. doi: 10.1016/j.conb.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Eggel A, Wyss-Coray T. A revival of parabiosis in biomedical research. Swiss Med. Wkly. 2014;144:w13914. doi: 10.4414/smw.2014.13914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elabd C, Cousin W, Upadhyayula P, Chen RY, Chooljian MS, Li J, Kung S, Jiang KP, Conboy IM. Oxytocin is an age-specific circulating hormone that is necessary for muscle maintenance and regeneration. Nat. Commun. 2014;5:4082. doi: 10.1038/ncomms5082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ergen AV, Goodell MA. Mechanisms of hematopoietic stem cell aging. Exp. Gerontol. 2010;45:286–290. doi: 10.1016/j.exger.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nat. Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- Ertl RP, Chen J, Astle CM, Duffy TM, Harrison DE. Effects of dietary restriction on hematopoietic stem-cell aging are genetically regulated. Blood. 2008;111:1709–1716. doi: 10.1182/blood-2007-01-069807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabel K, Fabel K, Tam B, Kaufer D, Baiker A, Simmons N, Kuo CJ, Palmer TD. VEGF is necessary for exercise-induced adult hippocampal neurogenesis. Eur. J. Neuorsci. 2003;18:2803–2812. doi: 10.1111/j.1460-9568.2003.03041.x. [DOI] [PubMed] [Google Scholar]
- Fernandez AM, Torres-Aleman I. The many faces of insulin-like peptide signalling in the brain. Nat. Rev. Neurosci. 2012;13:225–239. doi: 10.1038/nrn3209. [DOI] [PubMed] [Google Scholar]
- Flores I, Cayuela ML, Blasco MA. Effects of telomerase and telomere length on epidermal stem cell behavior. Science. 2005;309:1253–1256. doi: 10.1126/science.1115025. [DOI] [PubMed] [Google Scholar]
- Fontana L, Partridge L, Longo VD. Extending healthy life span–from yeast to humans. Science. 2010;328:321–326. doi: 10.1126/science.1172539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontan-Lozano A, Saez-Cassanelli JL, Inda MC, De los Santos-Arteaga M, Sierra-Dominguez SA, Lopez-Lluch G, Delgado-Garcia JM, Carrion AM. Caloric restriction increases learning consolidation and facilitates synaptic plasticity through mechanisms dependent on NR2B subunits of the NMDA receptor. J. Neurosci. 2007;27:10185–10195. doi: 10.1523/JNEUROSCI.2757-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusco S, Pani G. Brain response to calorie restriction. Cell. Mol. Life Sci. 2013;70:3157–3170. doi: 10.1007/s00018-012-1223-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabuzda D, Yankner BA. Physiology: inflammation links ageing to the brain. Nature. 2013;497:197–198. doi: 10.1038/nature12100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangaraju VK, Lin H. MicroRNAs: key regulators of stem cells. Nat. Rev. Mol. Cell Biol. 2009;10:116–125. doi: 10.1038/nrm2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangloff YG, Mueller M, Dann SG, et al. Disruption of the mouse mTOR gene leads to early postimplantation lethality and prohibits embryonic stem cell development. Mol. Cell. Biol. 2004;24:9508–9516. doi: 10.1128/MCB.24.21.9508-9516.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J, Wang WY, Mao YW, et al. A novel pathway regulates memory and plasticity via SIRT1 and miR-134. Nature. 2010;466:1105–1109. doi: 10.1038/nature09271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garelick MG, Kennedy BK. TOR on the brain. Exp. Gerontol. 2011;46:155–163. doi: 10.1016/j.exger.2010.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grounds MD. Age-associated changes in the response of skeletal muscle cells to exercise and regeneration. Ann. N. Y. Acad. Sci. 1998;854:78–91. doi: 10.1111/j.1749-6632.1998.tb09894.x. [DOI] [PubMed] [Google Scholar]
- Grymula K, Piotrowska K, Sluczanowska-Glabowska S, et al. Positive effects of prolonged caloric restriction on the population of very small embryonic-like stem cells: hematopoietic and ovarian implications. J. Ovarian Res. 2014;7:68. doi: 10.1186/1757-2215-7-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halloran J, Hussong SA, Burbank R, et al. Chronic inhibition of mammalian target of rapamycin by rapamycin modulates cognitive and non-cognitive components of behavior throughout lifespan in mice. Neuroscience. 2012;223:102–113. doi: 10.1016/j.neuroscience.2012.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison DE, Strong R, Sharp ZD, et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–395. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings NB, Gould E. Neurons inhibit neurogenesis. Nat. Med. 2003;9:264–266. doi: 10.1038/nm0303-264. [DOI] [PubMed] [Google Scholar]
- Hawkins HL, Kramer AF, Capaldi D. Aging, exercise, and attention. Psychol. Aging. 1992;7:643–653. doi: 10.1037//0882-7974.7.4.643. [DOI] [PubMed] [Google Scholar]
- Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev. 2004;18:1926–1945. doi: 10.1101/gad.1212704. [DOI] [PubMed] [Google Scholar]
- He C, Bassik MC, Moresi V, et al. Exercise-induced BCL2-regulated autophagy is required for muscle glucose homeostasis. Nature. 2012;481:511–515. doi: 10.1038/nature10758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Heemst D. Insulin, IGF-1 and longevity. Aging Dis. 2010;1:147–157. [PMC free article] [PubMed] [Google Scholar]
- Heitman J, Movva NR, Hall MN. Targets for cell cycle arrest by the immunosuppressant rapamycin in yeast. Science. 1991;253:905–909. doi: 10.1126/science.1715094. [DOI] [PubMed] [Google Scholar]
- Herskovits AZ, Guarente L. SIRT1 in neurodevelopment and brain senescence. Neuron. 2014;81:471–483. doi: 10.1016/j.neuron.2014.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillman CH, Erickson KI, Kramer AF. Be smart, exercise your heart: exercise effects on brain and cognition. Nat. Rev. Neurosci. 2008;9:58–65. doi: 10.1038/nrn2298. [DOI] [PubMed] [Google Scholar]
- Hoeffer CA, Klann E. mTOR signaling: at the crossroads of plasticity, memory and disease. Trends Neurosci. 2010;33:67–75. doi: 10.1016/j.tins.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaskelioff M, Muller FL, Paik JH, et al. Telomerase reactivation reverses tissue degeneration in aged telomerase-deficient mice. Nature. 2011;469:102–106. doi: 10.1038/nature09603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong DE, Artan M, Seo K, Lee SJ. Regulation of lifespan by chemosensory and thermosensory systems: findings in invertebrates and their implications in mammalian aging. Front. Genet. 2012;3:218. doi: 10.3389/fgene.2012.00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia K, Chen D, Riddle DL. The TOR pathway interacts with the insulin signaling pathway to regulate C. elegans larval development, metabolism and life span. Development. 2004;131:3897–3906. doi: 10.1242/dev.01255. [DOI] [PubMed] [Google Scholar]
- Johnson SC, Rabinovitch PS, Kaeberlein M. mTOR is a key modulator of ageing and age-related disease. Nature. 2013;493:338–345. doi: 10.1038/nature11861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DL, Rando TA. Emerging models and paradigms for stem cell ageing. Nat. Cell Biol. 2011;13:506–512. doi: 10.1038/ncb0511-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaeberlein M, McVey M, Guarente L. The SIR2/3/4 complex and SIR2 alone promote longevity in Saccharomyces cerevisiae by two different mechanisms. Genes Dev. 1999;13:2570–2580. doi: 10.1101/gad.13.19.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaeberlein M, Powers RW, 3rd, Steffen KK, et al. Regulation of yeast replicative life span by TOR and Sch9 in response to nutrients. Science. 2005;310:1193–1196. doi: 10.1126/science.1115535. [DOI] [PubMed] [Google Scholar]
- Kanfi Y, Naiman S, Amir G, Peshti V, Zinman G, Nahum L, Bar-Joseph Z, Cohen HY. The sirtuin SIRT6 regulates lifespan in male mice. Nature. 2012;483:218–221. doi: 10.1038/nature10815. [DOI] [PubMed] [Google Scholar]
- Kapahi P, Zid B. TOR pathway: linking nutrient sensing to life span. Sci. Aging Knowledge Environ. 2004;2004:PE34. doi: 10.1126/sageke.2004.36.pe34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapahi P, Zid BM, Harper T, Koslover D, Sapin V, Benzer S. Regulation of lifespan in Drosophila by modulation of genes in the TOR signaling pathway. Curr. Biol. 2004;14:885–890. doi: 10.1016/j.cub.2004.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsimpardi L, Litterman NK, Schein PA, et al. Vascular and neurogenic rejuvenation of the aging mouse brain by young systemic factors. Science. 2014;344:630–634. doi: 10.1126/science.1251141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay MA. State-of-the-art gene-based therapies: the road ahead. Nat. Rev. Genet. 2011;12:316–328. doi: 10.1038/nrg2971. [DOI] [PubMed] [Google Scholar]
- Kenyon C. The plasticity of aging: insights from long-lived mutants. Cell. 2005;120:449–460. doi: 10.1016/j.cell.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Kenyon CJ. The genetics of ageing. Nature. 2010;464:504–512. doi: 10.1038/nature08980. [DOI] [PubMed] [Google Scholar]
- Kenyon C. The first long-lived mutants: discovery of the insulin/IGF-1 pathway for ageing. Philos. Trans. R. Soc. Lond., B, Biol. Sci. 2011;366:9–16. doi: 10.1098/rstb.2010.0276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon C, Chang J, Gensch E, Rudner A, Tabtiang R. A C. elegans mutant that lives twice as long as wild type. Nature. 1993;366:461–464. doi: 10.1038/366461a0. [DOI] [PubMed] [Google Scholar]
- Kramer AF, Erickson KI. Capitalizing on cortical plasticity: influence of physical activity on cognition and brain function. Trends Cogn. Sci. 2007;11:342–348. doi: 10.1016/j.tics.2007.06.009. [DOI] [PubMed] [Google Scholar]
- Kramer AF, Hahn S, Cohen NJ, et al. Ageing, fitness and neurocognitive function. Nature. 1999;400:418–419. doi: 10.1038/22682. [DOI] [PubMed] [Google Scholar]
- Kronenberg G, Bick-Sander A, Bunk E, Wolf C, Ehninger D, Kempermann G. Physical exercise prevents age-related decline in precursor cell activity in the mouse dentate gyrus. Neurobiol. Aging. 2006;27:1505–1513. doi: 10.1016/j.neurobiolaging.2005.09.016. [DOI] [PubMed] [Google Scholar]
- Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J. Neurosci. 1996;16:2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Rani A, Tchigranova O, Lee WH, Foster TC. Influence of late-life exposure to environmental enrichment or exercise on hippocampal function and CA1 senescent physiology. Neurobiol. Aging. 2012;33:828. doi: 10.1016/j.neurobiolaging.2011.06.023. . e821–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaRocca TJ, Seals DR, Pierce GL. Leukocyte telomere length is preserved with aging in endurance exercise-trained adults and related to maximal aerobic capacity. Mech. Ageing Dev. 2010;131:165–167. doi: 10.1016/j.mad.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson EB, Wang L, Bowen JD, McCormick WC, Teri L, Crane P, Kukull W. Exercise is associated with reduced risk for incident dementia among persons 65 years of age and older. Ann. Intern. Med. 2006;144:73–81. doi: 10.7326/0003-4819-144-2-200601170-00004. [DOI] [PubMed] [Google Scholar]
- Laviano A. Young blood. N. Engl. J. Med. 2014;371:573–575. doi: 10.1056/NEJMcibr1407158. [DOI] [PubMed] [Google Scholar]
- Lawson EC, Han MK, Sellers JT, Chrenek MA, Hanif A, Gogniat MA, Boatright JH, Pardue MT. Aerobic exercise protects retinal function and structure from light-induced retinal degeneration. J. Neurosci. 2014;34:2406–2412. doi: 10.1523/JNEUROSCI.2062-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HW, Blasco MA, Gottlieb GJ, Horner JW, 2nd, Greider CW, DePinho RA. Essential role of mouse telomerase in highly proliferative organs. Nature. 1998;392:569–574. doi: 10.1038/33345. [DOI] [PubMed] [Google Scholar]
- Lee J, Duan W, Long JM, Ingram DK, Mattson MP. Dietary restriction increases the number of newly generated neural cells, and induces BDNF expression, in the dentate gyrus of rats. J. Mol. Neurosci. 2000;15:99–108. doi: 10.1385/JMN:15:2:99. [DOI] [PubMed] [Google Scholar]
- Lee J, Seroogy KB, Mattson MP. Dietary restriction enhances neurotrophin expression and neurogenesis in the hippocampus of adult mice. J. Neurochem. 2002;80:539–547. doi: 10.1046/j.0022-3042.2001.00747.x. [DOI] [PubMed] [Google Scholar]
- Lee KS, Iijima-Ando K, Iijima K, Lee WJ, Lee JH, Yu K, Lee DS. JNK/FOXO-mediated neuronal expression of fly homologue of peroxiredoxin II reduces oxidative stress and extends life span. J. Biol. Chem. 2009;284:29454–29461. doi: 10.1074/jbc.M109.028027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SW, Clemenson GD, Gage FH. New neurons in an aged brain. Behav. Brain Res. 2012;227:497–507. doi: 10.1016/j.bbr.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libert S, Guarente L. Metabolic and neuropsychiatric effects of calorie restriction and sirtuins. Annu. Rev. Physiol. 2013;75:669–684. doi: 10.1146/annurev-physiol-030212-183800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libert S, Pletcher SD. Modulation of longevity by environmental sensing. Cell. 2007;131:1231–1234. doi: 10.1016/j.cell.2007.12.002. [DOI] [PubMed] [Google Scholar]
- Libert S, Zwiener J, Chu X, Vanvoorhies W, Roman G, Pletcher SD. Regulation of Drosophila life span by olfaction and food-derived odors. Science. 2007;315:1133–1137. doi: 10.1126/science.1136610. [DOI] [PubMed] [Google Scholar]
- Lichtenwalner RJ, Forbes ME, Bennett SA, Lynch CD, Sonntag WE, Riddle DR. Intracerebroventricular infusion of insulin-like growth factor-I ameliorates the age-related decline in hippocampal neurogenesis. Neuroscience. 2001;107:603–613. doi: 10.1016/s0306-4522(01)00378-5. [DOI] [PubMed] [Google Scholar]
- Lin K, Dorman JB, Rodan A, Kenyon C. daf-16: an HNF-3/forkhead family member that can function to double the life-span of Caenorhabditis elegans. Science. 1997;278:1319–1322. doi: 10.1126/science.278.5341.1319. [DOI] [PubMed] [Google Scholar]
- Loffredo FS, Steinhauser ML, Jay SM, et al. Growth differentiation factor 11 is a circulating factor that reverses age-related cardiac hypertrophy. Cell. 2013;153:828–839. doi: 10.1016/j.cell.2013.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Zhao Z, Wang R, Zhang X, Zhang J, Dong W, Xu B, Zhang J. Caloric restriction can improve learning ability in C57/BL mice via regulation of the insulin-PI3K/Akt signaling pathway. Neurol. Sci. 2014;35:1381–1386. doi: 10.1007/s10072-014-1717-5. [DOI] [PubMed] [Google Scholar]
- Mahncke HW, Bronstone A, Merzenich MM. Brain plasticity and functional losses in the aged: scientific bases for a novel intervention. Prog. Brain Res. 2006;157:81–109. doi: 10.1016/S0079-6123(06)57006-2. [DOI] [PubMed] [Google Scholar]
- Marlatt MW, Potter MC, Lucassen PJ, van Praag H. Running throughout middle-age improves memory function, hippocampal neurogenesis, and BDNF levels in female C57BL/6J mice. Dev. Neurobiol. 2012;72:943–952. doi: 10.1002/dneu.22009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin CK, Anton SD, Han H, York-Crowe E, Redman LM, Ravussin E, Williamson DA. Examination of cognitive function during six months of calorie restriction: results of a randomized controlled trial. Rejuvenation Res. 2007;10:179–190. doi: 10.1089/rej.2006.0502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Magnus T. Ageing and neuronal vulnerability. Nat. Rev. Neurosci. 2006;7:278–294. doi: 10.1038/nrn1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzoccoli G, Tevy MF, Borghesan M, Delle Vergini MR, Vinciguerra M. Caloric restriction and aging stem cells: the stick and the carrot? Exp. Gerontol. 2014;50:137–148. doi: 10.1016/j.exger.2013.10.014. [DOI] [PubMed] [Google Scholar]
- McPherron AC, Huynh TV, Lee SJ. Redundancy of myostatin and growth/differentiation factor 11 function. BMC Dev. Biol. 2009;9:24. doi: 10.1186/1471-213X-9-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merzenich MM, Van Vleet TM, Nahum M. Brain plasticity-based therapeutics. Front. Hum. Neurosci. 2014;8:385. doi: 10.3389/fnhum.2014.00385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michan S, Sinclair D. Sirtuins in mammals: insights into their biological function. Biochem. J. 2007;404:1–13. doi: 10.1042/BJ20070140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michan S, Li Y, Chou MM, et al. SIRT1 is essential for normal cognitive function and synaptic plasticity. J. Neurosci. 2010;30:9695–9707. doi: 10.1523/JNEUROSCI.0027-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming GL, Song H. Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron. 2011;70:687–702. doi: 10.1016/j.neuron.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora S, Cook N, Buring JE, Ridker PM, Lee IM. Physical activity and reduced risk of cardiovascular events: potential mediating mechanisms. Circulation. 2007;116:2110–2118. doi: 10.1161/CIRCULATIONAHA.107.729939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison JH, Baxter MG. The ageing cortical synapse: hallmarks and implications for cognitive decline. Nat. Rev. Neurosci. 2012;13:240–250. doi: 10.1038/nrn3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashiba T, Cushman JD, Pelkey KA, et al. Young dentate granule cells mediate pattern separation, whereas old granule cells facilitate pattern completion. Cell. 2012;149:188–201. doi: 10.1016/j.cell.2012.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Callaghan RM, Griffin EW, Kelly AM. Long-term treadmill exposure protects against age-related neurodegenerative change in the rat hippocampus. Hippocampus. 2009;19:1019–1029. doi: 10.1002/hipo.20591. [DOI] [PubMed] [Google Scholar]
- Ogg S, Paradis S, Gottlieb S, Patterson GI, Lee L, Tissenbaum HA, Ruvkun G. The Fork head transcription factor DAF-16 transduces insulin-like metabolic and longevity signals in C. elegans. Nature. 1997;389:994–999. doi: 10.1038/40194. [DOI] [PubMed] [Google Scholar]
- Park JH, Glass Z, Sayed K, et al. Calorie restriction alleviates the age-related decrease in neural progenitor cell division in the aging brain. Eur. J. Neuorsci. 2013;37:1987–1993. doi: 10.1111/ejn.12249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge L, Bruning JC. Forkhead transcription factors and ageing. Oncogene. 2008;27:2351–2363. doi: 10.1038/onc.2008.28. [DOI] [PubMed] [Google Scholar]
- Pereira AC, Huddleston DE, Brickman AM, et al. An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proc. Natl Acad. Sci. USA. 2007;104:5638–5643. doi: 10.1073/pnas.0611721104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitsikas N, Algeri S. Deterioration of spatial and nonspatial reference and working memory in aged rats: protective effect of life-long calorie restriction. Neurobiol. Aging. 1992;13:369–373. doi: 10.1016/0197-4580(92)90110-j. [DOI] [PubMed] [Google Scholar]
- van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc. Natl Acad. Sci. USA. 1999a;96:13427–13431. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat. Neurosci. 1999b;2:266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- van Praag H, Shubert T, Zhao C, Gage FH. Exercise enhances learning and hippocampal neurogenesis in aged mice. J. Neurosci. 2005;25:8680–8685. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radak Z, Kaneko T, Tahara S, Nakamoto H, Pucsok J, Sasvari M, Nyakas C, Goto S. Regular exercise improves cognitive function and decreases oxidative damage in rat brain. Neurochem. Int. 2001;38:17–23. doi: 10.1016/s0197-0186(00)00063-2. [DOI] [PubMed] [Google Scholar]
- Rando TA. Stem cells, ageing and the quest for immortality. Nature. 2006;441:1080–1086. doi: 10.1038/nature04958. [DOI] [PubMed] [Google Scholar]
- Ruckh JM, Zhao JW, Shadrach JL, van Wijngaarden P, Rao TN, Wagers AJ, Franklin RJ. Rejuvenation of regeneration in the aging central nervous system. Cell Stem Cell. 2012;10:96–103. doi: 10.1016/j.stem.2011.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sala C, Segal M. Dendritic spines: the locus of structural and functional plasticity. Physiol. Rev. 2014;94:141–188. doi: 10.1152/physrev.00012.2013. [DOI] [PubMed] [Google Scholar]
- Salih DA, Rashid AJ, Colas D, et al. FoxO6 regulates memory consolidation and synaptic function. Genes Dev. 2012;26:2780–2801. doi: 10.1101/gad.208926.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salpeter SJ, Khalaileh A, Weinberg-Corem N, Ziv O, Glaser B, Dor Y. Systemic regulation of the age-related decline of pancreatic beta-cell replication. Diabetes. 2013;62:2843–2848. doi: 10.2337/db13-0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh A, Brace CS, Rensing N, Cliften P, Wozniak DF, Herzog ED, Yamada KA, Imai S. Sirt1 extends life span and delays aging in mice through the regulation of Nk2 homeobox 1 in the DMH and LH. Cell Metab. 2013;18:416–430. doi: 10.1016/j.cmet.2013.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shefer G, Rauner G, Yablonka-Reuveni Z, Benayahu D. Reduced satellite cell numbers and myogenic capacity in aging can be alleviated by endurance exercise. PLoS ONE. 2010;5:e13307. doi: 10.1371/journal.pone.0013307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair DA, Mills K, Guarente L. Accelerated aging and nucleolar fragmentation in yeast sgs1 mutants. Science. 1997;277:1313–1316. doi: 10.1126/science.277.5330.1313. [DOI] [PubMed] [Google Scholar]
- Sinha M, Jang YC, Oh J, et al. Restoring systemic GDF11 levels reverses age-related dysfunction in mouse skeletal muscle. Science. 2014;344:649–652. doi: 10.1126/science.1251152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snigdha S, de Rivera C, Milgram NW, Cotman CW. Exercise enhances memory consolidation in the aging brain. Front. Aging Neurosci. 2014;6:3. doi: 10.3389/fnagi.2014.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi A, Wartschow LM, White MF. Brain IRS2 signaling coordinates life span and nutrient homeostasis. Science. 2007;317:369–372. doi: 10.1126/science.1142179. [DOI] [PubMed] [Google Scholar]
- Talhati F, Patti CL, Zanin KA, et al. Food restriction increases long-term memory persistence in adult or aged mice. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2014;50:125–136. doi: 10.1016/j.pnpbp.2013.12.007. [DOI] [PubMed] [Google Scholar]
- Tang Y, Cai D. Hypothalamic inflammation and GnRH in aging development. Cell Cycle. 2013;12:2711–2712. doi: 10.4161/cc.26054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatar M, Bartke A, Antebi A. The endocrine regulation of aging by insulin-like signals. Science. 2003;299:1346–1351. doi: 10.1126/science.1081447. [DOI] [PubMed] [Google Scholar]
- Tissenbaum HA, Guarente L. Increased dosage of a sir-2 gene extends lifespan in Caenorhabditis elegans. Nature. 2001;410:227–230. doi: 10.1038/35065638. [DOI] [PubMed] [Google Scholar]
- Trejo JL, Carro E, Torres-Aleman I. Circulating insulin-like growth factor I mediates exercise-induced increases in the number of new neurons in the adult hippocampus. J. Neurosci. 2001;21:1628–1634. doi: 10.1523/JNEUROSCI.21-05-01628.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Uffelen JG, Chin APMJ, Hopman-Rock M, van Mechelen W. The effects of exercise on cognition in older adults with and without cognitive decline: a systematic review. Clin. J. Sport Med. 2008a;18:486–500. doi: 10.1097/JSM.0b013e3181845f0b. [DOI] [PubMed] [Google Scholar]
- van Uffelen JG, Chinapaw MJ, van Mechelen W, Hopman-Rock M. Walking or vitamin B for cognition in older adults with mild cognitive impairment? A randomised controlled trial. Br. J. Sports Med. 2008b;42:344–351. doi: 10.1136/bjsm.2007.044735. [DOI] [PubMed] [Google Scholar]
- Valero MC, Huntsman HD, Liu J, Zou K, Boppart MD. Eccentric exercise facilitates mesenchymal stem cell appearance in skeletal muscle. PLoS ONE. 2012;7:e29760. doi: 10.1371/journal.pone.0029760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vellai T, Takacs-Vellai K, Zhang Y, Kovacs AL, Orosz L, Muller F. Genetics: influence of TOR kinase on lifespan in C. elegans. Nature. 2003;426:620. doi: 10.1038/426620a. [DOI] [PubMed] [Google Scholar]
- Verdu E, Ceballos D, Vilches JJ, Navarro X. Influence of aging on peripheral nerve function and regeneration. J. Peripher. Nerv. Syst. 2000;5:191–208. doi: 10.1046/j.1529-8027.2000.00026.x. [DOI] [PubMed] [Google Scholar]
- Villeda SA, Wyss-Coray T. The circulatory systemic environment as a modulator of neurogenesis and brain aging. Autoimmun. Rev. 2013;12:674–677. doi: 10.1016/j.autrev.2012.10.014. [DOI] [PubMed] [Google Scholar]
- Villeda SA, Luo J, Mosher KI, et al. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature. 2011;477:90–94. doi: 10.1038/nature10357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villeda SA, Plambeck KE, Middeldorp J, et al. Young blood reverses age-related impairments in cognitive function and synaptic plasticity in mice. Nat. Med. 2014;20:659–663. doi: 10.1038/nm.3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanisch K, Kovac S, Schorge S. Tackling obstacles for gene therapy targeting neurons: disrupting perineural nets with hyaluronidase improves transduction. PLoS ONE. 2013;8:e53269. doi: 10.1371/journal.pone.0053269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warburton DE, Nicol CW, Bredin SS. Health benefits of physical activity: the evidence. CMAJ. 2006;174:801–809. doi: 10.1503/cmaj.051351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warr MR, Binnewies M, Flach J, Reynaud D, Garg T, Malhotra R, Debnath J, Passegue E. FOXO3A directs a protective autophagy program in haematopoietic stem cells. Nature. 2013;494:323–327. doi: 10.1038/nature11895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb AE, Brunet A. FOXO transcription factors: key regulators of cellular quality control. Trends Biochem. Sci. 2014;39:159–169. doi: 10.1016/j.tibs.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman IL. Translating stem and progenitor cell biology to the clinic: barriers and opportunities. Science. 2000;287:1442–1446. doi: 10.1126/science.287.5457.1442. [DOI] [PubMed] [Google Scholar]
- Witte AV, Fobker M, Gellner R, Knecht S, Floel A. Caloric restriction improves memory in elderly humans. Proc. Natl Acad. Sci. USA. 2009;106:1255–1260. doi: 10.1073/pnas.0808587106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolkow CA, Kimura KD, Lee MS, Ruvkun G. Regulation of C. elegans life-span by insulinlike signaling in the nervous system. Science. 2000;290:147–150. doi: 10.1126/science.290.5489.147. [DOI] [PubMed] [Google Scholar]
- Wu MY, Hill CS. Tgf-beta superfamily signaling in embryonic development and homeostasis. Dev. Cell. 2009;16:329–343. doi: 10.1016/j.devcel.2009.02.012. [DOI] [PubMed] [Google Scholar]
- Wu HH, Ivkovic S, Murray RC, Jaramillo S, Lyons KM, Johnson JE, Calof AL. Autoregulation of neurogenesis by GDF11. Neuron. 2003;37:197–207. doi: 10.1016/s0896-6273(02)01172-8. [DOI] [PubMed] [Google Scholar]
- Wu CW, Chang YT, Yu L, Chen HI, Jen CJ, Wu SY, Lo CP, Kuo YM. Exercise enhances the proliferation of neural stem cells and neurite growth and survival of neuronal progenitor cells in dentate gyrus of middle-aged mice. J. Appl. Physiol. 2008;105:1585–1594. doi: 10.1152/japplphysiol.90775.2008. [DOI] [PubMed] [Google Scholar]
- Yanai S, Okaichi Y, Okaichi H. Long-term dietary restriction causes negative effects on cognitive functions in rats. Neurobiol. Aging. 2004;25:325–332. doi: 10.1016/S0197-4580(03)00115-5. [DOI] [PubMed] [Google Scholar]
- Yilmaz OH, Katajisto P, Lamming DW, et al. mTORC1 in the Paneth cell niche couples intestinal stem-cell function to calorie intake. Nature. 2012;486:490–495. doi: 10.1038/nature11163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Li J, Purkayastha S, Tang Y, Zhang H, Yin Y, Li B, Liu G, Cai D. Hypothalamic programming of systemic ageing involving IKK-beta, NF-kappaB and GnRH. Nature. 2013;497:211–216. doi: 10.1038/nature12143. [DOI] [PMC free article] [PubMed] [Google Scholar]


