Abstract
Background: Respiratory viruses are the leading cause of respiratory tract infections among children and are responsible for causing morbidity and mortality worldwide. This study was performed to detect viruses in children with respiratory infections and describe their epidemiology and clinical characteristics.
Methods: In this descriptive cross sectional study, throat swabs and wash specimens from 202 children younger than six years of age with diagnosis of a respiratory tract infection from a total of 897 specimens were evaluated using multiplex PCR method.
Results: Respiratory viruses were detected in 92 children: respiratory synsytial virus, 16.8%; influenza virus, 5.4%; parainfluenza virus, 8.4%; adenovirus, 14.4% and human metapneumo virus 0.49% with male predominance and higher distribution in children younger than 1 year of age with preference in the cold months of year. The clinical presentations of all detected viruses were almost similar.
Conclusion: In the present study, nine different respiratory viruses were detected. RSV causes the great majority of respiratory virus infections in children. There was no significant difference in epidemiologic patterns of these viruses in comparison to other studies.
Keywords: Children, Epidemiology, Iran, Respiratory viruses
Introduction
Among children, respiratory tract infections (RTI) are a major cause of morbidity and mortality worldwide especially in developing countries (1,2). Respiratory syncytial virus (RSV), influenza virus (IFV), parainfluenza virus (PIV), adenovirus (AdV) and the recently discovered human metapneumovirus (hMPV) likely account for a majority of respiratory tract illnesses (3).The most prevalent presentations in RTI include sore throat, headache and myalgia (4). Studies assessing the relationship between clinical symptoms and respiratory infections yielded conflicting results (5). In temperate climate regions, RTI display a defined seasonality occurring mainly during the winter (2).
The aim of this study was to determine, by a sensitive method, the frequency, epidemiology and clinical characteristics of respiratory viruses among young children. This might help towards better management and treatment of infected children.
Methods
Specimen collection
This cross sectional study was performed on specimens collected between 2008 and 2009 in National Influenza Center of Iran. Throat swabs and washes were collected from 897 patients with respiratory illness and transported to the National Influenza Center, School of Public Health, Tehran University of Medical Sciences. A questionnaire was alsocompleted. Two hundred and two, out of 897, specimens belonged to children less than 6 years of age were selected.
Nucleic Acid extraction
RNA extraction and cDNA synthesis: RNA was extracted from 150 μL of samples using the Neucleospin viral RNA (Neucleospin RNA extraction kit, Macherey-Nagel) according to the manufactures instruction. cDNA synthesis was carried out in 30 μl reaction mixture containing 6 μL of 5X RT Buffer, 2.5 μL of mixed dNTPs (2.5μm each), 1 μL of RT-MULV enzyme (Fermentase), 2.5 μL Random hexamer (Fermentase), 0.5 μLRNase Inhibitor (Fermentase) and 17.5 μL RNA template, the mixture was incubated in 22° C for 10 min, 37° C for 45 min and 94° C for 5 min.
DNA extraction
DNA was extracted from 150 μL of samples using the Neucleospin viral DNA (Neucleospin DNA extraction kit, Macherey-Nagel) according to the manufactures instruction.
Primers
Primers targeted specifically the nucleocapsid gene of RSV (6), the Matrix protein gene of hMPV (7), the hemagglutinin and neuraminidase gene of PIV-1,2 (8) and PIV-3 (9) the phosphoprotein gene of PIV-4 (10) hemagglutinin(HA) gene of IFV A and B (11) and hexon gene of AdV (12).
PCRs
Three multiplex RT-PCR methods, targeting eight respiratory viruses, were developed. Multiplex PCR I for RSV and hMPV, multiplex PCR II for PIV1-4 and multiplex PCR III for Flu A and B were performed. The protocols are available upon request.
Uniplex hemi nested PCR was done for detection of adenovirus. Briefly, 5 μL of the nucleic acid extraction was added to 45 μL of reaction mixture containing 10X PCR buffer, 4μL of Mgcl2 (50mM), 2.5μL of dNTP mix (10mM), 20 pmol of each degenerate primer pair and 0.4μL of taq polymerase. Temperature and time profiles were as follows: 1 cycle at 94° C for 3 min, followed by 30 cycles of 94° for 1 min, 50° C for 1 min, 68° C for 1 min, and final incubation at 68°C for 50 min. Then nested reaction was performed with 20 pmol of the another degenerate primer pair as above.
Results
Detection of respiratory viruses
A total of 92 viruses were detected in throat swab and wash specimens from 202 children under the age of 6 with respiratory infection: RSV in 34(16.8%), AdV in 29(14.4%), PIV1-4 in 17(8.4%), IFV in 11(5.4%) and hMPV in 1(0.49%) case. Of the 34 RSV positive children, 9(4.45%) had co-infection with other viruses: 1 with hMPV, 4 with PIV1, 3 with AdV and 1 with IFV. Two of the PIV-1 positive children had a co-infection with PIV-3. No viruses could be detected in specimens from 110(54.4%) children.
Epidemiology and clinical characteristics in children with viral respiratory infections
The proportions of the patients aged <12, 12-24, 25-36, 37-48, 49-60 and 61-72 months were 16.3, 10.89, 2.97, 4.45, 3.46 and 6.93% respectively. Figure 1 shows age distribution of the children according to the different viruses. Among the patients there were 47(23.26%) males and 45(22.27%) females with a sex ratio of 1.04:1. Female sex was more frequently associated with RSV, PIV-1, IFV and hMPV in contrast AdV was detected mostly in males (Fig.2). Figure 3 and figure 4 show seasonal and monthly distribution according to the different viruses, respectively. In analyses of the clinical signs and symptoms in affected children, fever (53.6%) myalgia (49.8%) and cough (38.4%) were common clinical findings. Sore throat was observed in 25.3% of the patients. There were no headache and malaise found in these patients (Fig.5).
Fig.1 .
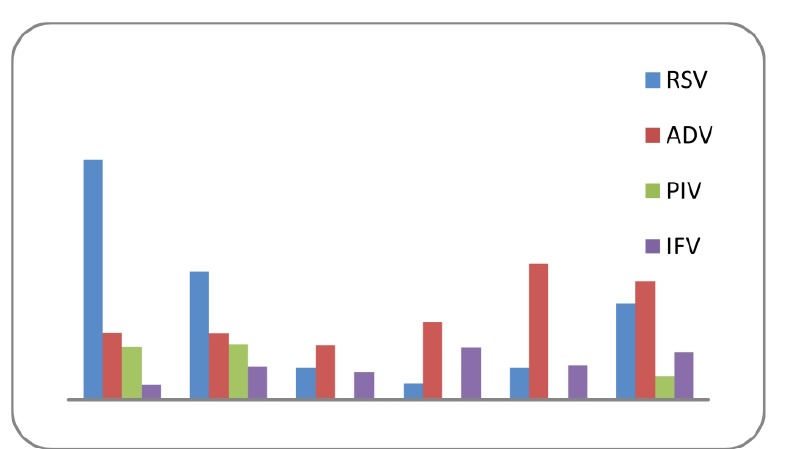
Age distribution of the children with acute viral respiratory tract infections
Fig. 2 .
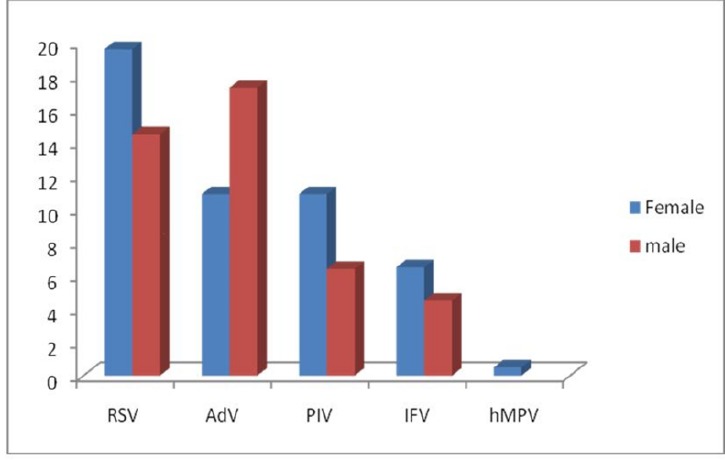
Sex distribution of the children with acute viral respiratory tract infections
Fig. 3 .
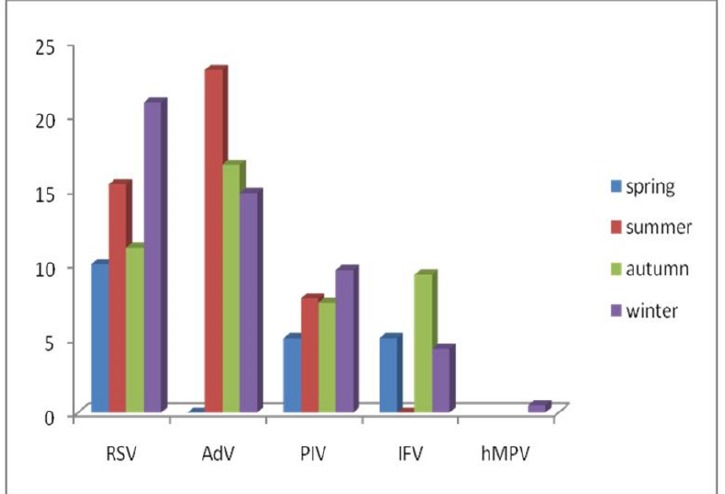
Seasonal distribution of the children with acute viral respiratory tract infections
Fig. 4 .
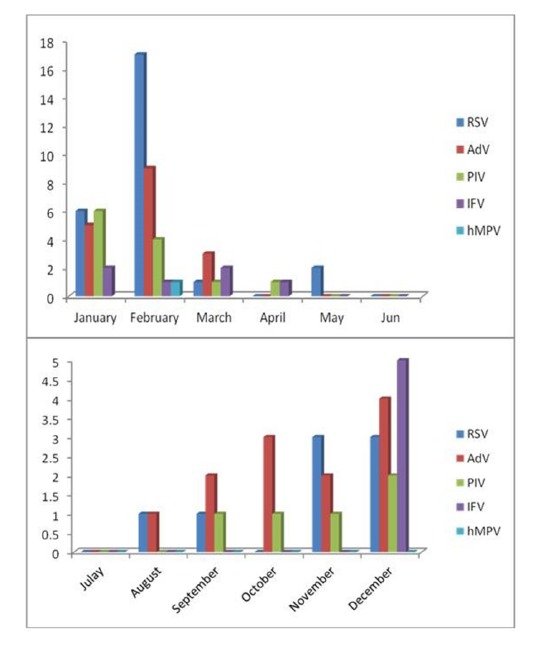
Monthly distribution of the children with acute viral respiratory tract infections
Fig. 5 .
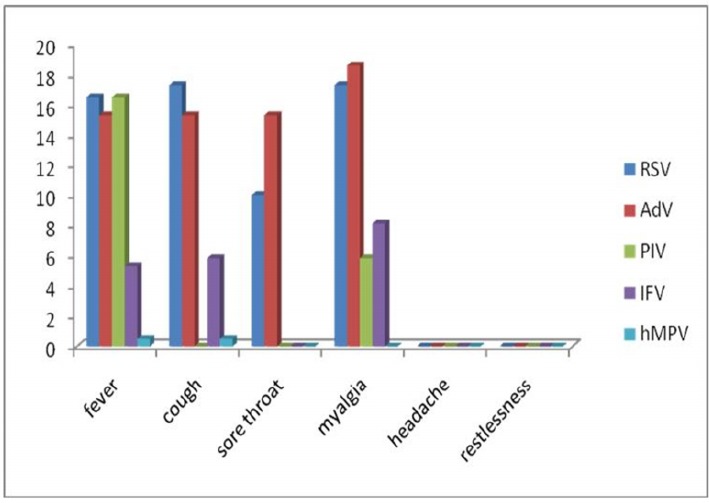
Distribution of clinical characteristics inchildren with acute viral respiratory tract infections
Discussion
The circulation of different respiratory viruses during the same period of the year makes it very difficult to detect their individual contribution to the global respiratory disease. Moreover, the pattern of the respiratory virus activity seems to change within the different age groups with different demographic characteristics (13). In each community, the epidemiology of respiratory viruses has to be determined prior to the implementation of novel and costly therapies. In the present study RSV was the most frequently demonstrated virus accounted for 16.8% of all viruses detected. Our results were similar to those from other countries (14,15) and emphasize the role of RSV as the leading cause of ARTI in infants and young children. Meanwhile RSV was mostly found in female infants less than one year of age with the typical seasonal pattern with a peak incidence during February (16). Fever, cough and myalgia were the common clinical signs and symptoms in these patients. One study in the united states showed RSV is responsible for 1% child hospitalization, 70% of which during the first year of life (17).
Adenoviruses produce lower respiratory illness in all seasons with a tendency to peak in mid-winter (18,19).In this study, AdVs followed by RSV were the most frequently detected viral agents in our patients (14.4%) which occurred mostly in the summer and winter months (August, September and February) in male children in all age groups. Fever, cough, sore throat and myalgia were the common clinical characteristics of them.
Human parainfluenza viruses are important respiratory pathogens and a major cause of croup and pneumonia in infants (20). Herein, hPIV1 and 3 were the most prevalent hPIVs similar to other studies (21) and had high peak between January and February at children younger than 2 years of age which showed preference in females. The most frequently symptoms were fever and myalgia.
Among all respiratory virus infections because of the pandemics and annual epidemics, influenza viruses are a center of concern. In the present study influenza viruses were detected in 5.4% of the children and similar to the other studies they were in all age groups during the cold months (December to March) (22). Female dominance had been seen in these children with fever, myalgia and cough.
HMPV was first identified in 2001 in children with respiratory tract disease (23). We detected 1 hMPV in 2 year old female child with RSV infection who had Kawasaki syndrome and hospitalized in the pediatric ward. Studies have reported high co-infection rates with these two viruses (24).Similar to the Catherine M. report, which showed hMPV distribution frequently in the winter months (25), we detected the only hMPV of this study on February. Fever and cough were the clinical signs and symptoms of this child. RTIs are common in young children, decrease in frequency with age, and predominate in boys (26,27). This age distribution was the same in the present study which demonstrated a higher incidence of RTI in children younger than 1 year old. There was male predominance in this study but it was not significant. The relative frequency of each viral agent and the pattern of occurrence were similar to those described in previous reports (28,29).It is difficult to formulate a single theory to explain the epidemiology of viral infections at different times of the year. The most appropriate theory may be that, by staying indoors in cold weather, families promote the spread of virus infections which rely on the droplet transmission within confined spaces (30).Susceptible children could acquire the virus from older children in whom manifestations of viral respiratory tract infections are mild (31).
Conclusion
In summary, respiratory virus infections (RVI) are a common cause of morbidity and mortality worldwide. Viral co infections and recently discovered viruses are involved in a significant percentage of acute RVI. Studies are being conducted to determine whether dual respiratory virus infections carry an increased risk for adverse outcomes or increased hospital stays for pediatric patients. Better understanding of the epidemiology of RVI may be used for timely specific antiviral therapy, prophylactic and vaccination. This study described the demographic and clinical presentations of specific RVI in 202 children under the 6 years of age in Iran. Monitoring is necessary in a broader population and for a longer period of time in order to better delineate the clinical and epidemiological behavior of respiratory viruses in children. Although routine surveillance of respiratory viruses might not seem to be cost-effective, continuous monitoring of ARTI etiology could be a useful means of planning the resources necessary for the development of new vaccines and antiviral agents.
Acknowledgments
We would like to thank the entire staff of the National Influenza centre, School of Public Health Tehran University of Medical Sciences. This study is funded by Tehran University of Medical Sciences (Number: 240.2694).
This study is funded byTehran University of Medical Sciences (Number: 240.2694).
Cite this article as: Shatizadeh S, Yavarian J, Rezaie F, Mahmoodi M, Naseri M, Mokhtari Azad T. Epidemiological and clinical evaluation of children with respiratory virus infections. Med J Islam Repub Iran 2014 (22 September). Vol. 28:102.
References
- 1.Caetano Jd Jdo R, Bordin IA, Puccini RF, Peres Cd Cde A. Factors associated to hospitalization of children under five years of age, Brazil. Rev Saude Publica. 2002;36:285–91. doi: 10.1590/s0034-89102002000300005. [DOI] [PubMed] [Google Scholar]
- 2.Shek LPC, Lee BW. Epidemiology and seasonality of respiratory tract virus infections in the tropics. Paediatr Respir Rev. 2003;4:105–11. doi: 10.1016/s1526-0542(03)00024-1. [DOI] [PubMed] [Google Scholar]
- 3.Kesebir D, Vazques M, Weibel C, Shapiro ED, Ferguson D, Landry ML. et al. Human bocavirus infection in young children in the united states: molecular epidemiological profile and clinical characteristics of a newly emerging respiratory virus. JID. 2006;194:1276–282. doi: 10.1086/508213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rani AT, Salwa B, Hussein A. Epidemiology and clinical characteristics of respiratory syncytial virus infections in Jordan. J Trop Pediatrics. 2006;52:282–87. doi: 10.1093/tropej/fml002. [DOI] [PubMed] [Google Scholar]
- 5.Fleming DM, KW Cross. Respiratory syncytial virus or influenza? Lancet. 1993;342:1507–510. doi: 10.1016/s0140-6736(05)80082-0. [DOI] [PubMed] [Google Scholar]
- 6.Zlateva KT, Lemey P, Vandamme AM, Ranst MV. Molecular Evolution and Circulation Patterns of Human Respiratory Syncytial Virus Subgroup A: Positively Selected Sites in the Attachment G Glycoprotein. J virol. 2004;78:4675–83. doi: 10.1128/JVI.78.9.4675-4683.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bellau-Pujol S, Vabret A, Legrand A, Dina J, Gouarin S, Petitjean-Lecherbonnier J. et al. Development of three multiplex RT-PCR assays for the detection of 12 respiratory RNAviruses. J Virol Methods. 2005;126:53–63. doi: 10.1016/j.jviromet.2005.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Templeton KE, Scheltinga SA, Beersma MFC, Kroes AC, Claas EC. Rapid and Sensitive Method Using Multiplex Real-Time PCR for Diagnosis of Infections by Influenza A and Influenza B Viruses, Respiratory Syncytial Virus, and Parainfluenza Viruses 1, 2, 3, and 4. J clin Microbiol. 2004;42:1564–69. doi: 10.1128/JCM.42.4.1564-1569.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Erdman DD, Weinberg GA, Edwards KM, Walker FJ, Anderson BC, Winter J. et al. GeneScan Reverse Transcription-PCR Assay for Detection of Six Common Respiratory Viruses in Young Children Hospitalized with Acute Respiratory Illness. J clin Microbiol. 2003;41:4298–4303. doi: 10.1128/JCM.41.9.4298-4303.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aguilar JC, Perez-Brena MP, Garcia ML, Cruz N, Erdman DD, Echevarría JE. Detection and Identification of Human Parainfluenza Viruses 1, 2, 3, and 4 in Clinical Samples of Pediatric Patients by Multiplex Reverse Transcription-PCR. J Clin Microbiol. 2000;38:1191–195. doi: 10.1128/jcm.38.3.1191-1195.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mokhtari-Azad T, Rezaie-Khollari F, Nadji AR, Salimi V, Noroozbabaie Z, M Naseri. et al. Comparison of Multiplex Nested RT-PCR with Virus Isolation for Detection of Influenza Viruses A and B. IJPH. 2007;36:1–7. [Google Scholar]
- 12.Casas I, Avellon A, Mosquera M, Jabado O, Echevarria JE, Campos RH. et al. Molecular Identification of Adenoviruses in Clinical Samples by Analyzing a Partial Hexon Genomic Region. J Clin Microbiol. 2005;43:6176–182. doi: 10.1128/JCM.43.12.6176-6182.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coiras MT, Aguilar JC, Garcia ML, Casas I, Perez-Brena P. Simultaneous Detection of Fourteen Respiratory Viruses in Clinical Specimens by Two Multiplex ReverseTranscription Nested-PCR Assays. J Med Virol. 2004;72:484–95. doi: 10.1002/jmv.20008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yeolekar LR, Damle RG, Kamat AN, Khude MR, Simha V. et al. Respiratory viruses in acute respiratory tract infections in Western India. Indian J Pediatr. 2008;75:341–45. doi: 10.1007/s12098-008-0035-4. [DOI] [PubMed] [Google Scholar]
- 15.Stempel H, Martin E, Kuypers J, Englund J, Zerr D. Multiple viral respiratory pathogens in children with bronchiolitis. Acta paediatr. 2008;98:123–26. doi: 10.1111/j.1651-2227.2008.01023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alonso A, Andres JM, Garmendia JR, Diez I, Gil JM, Ardura J. Bronchiolitis due to respiratory syncytial virus in hospitalized children: a study of seasonal rhythm. Acta Paediatr. 2007;96:731–35. doi: 10.1111/j.1651-2227.2007.00266.x. [DOI] [PubMed] [Google Scholar]
- 17.Calegari T, Queiroz D A.O, Yokosawa J, Silveira H L, Costa L F, Oliveira TF. et al. Clinical-epidemiological evaluation of respiratory syncytial virus infection in children attended in a public hospital in midwestern Brazil. BJID. 2005;9:156–61. doi: 10.1590/s1413-86702005000200006. [DOI] [PubMed] [Google Scholar]
- 18.Choi EW, Lee HJ, Kim SJ, Eun BW, Kim NH, Lee JA. et al. The Association of Newly Identified Respiratory Viruses with Lower Respiratory Tract Infections in Korean Children, 2000–2005. Clin Infect Dis. 2006;43:585–92. doi: 10.1086/506350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kusel MHM, de Klerk NH, Nicholas H, Patrick G H, Kebadze T, Johnston SL, Sly PD. Role of Respiratory Viruses in Acute Upper and Lower Respiratory Tract Illness in the First Year of Life: A Birth Cohort Study. Pediatr Infect Dis J. 2006;26:680–86. doi: 10.1097/01.inf.0000226912.88900.a3. [DOI] [PubMed] [Google Scholar]
- 20.Malekshahi SS, Azad TM, Yavarian J, Shahmahmoodi S, Naseri M, Rezaei F. Molecular detection of respiratory viruses in children with acute respiratory disease in Iran. PIDJ. 2010;29:931–33. doi: 10.1097/inf.0b013e3181e2062e. [DOI] [PubMed] [Google Scholar]
- 21.Fry AM, Curns AT, Harbour K, Hutwagner L, Holman RC, Anderson L. Seasonal Trends of Human Parainfluenza Viral Infections: United States, 1990–2004. Clin Infect Dis. 2006;43:1016–22. doi: 10.1086/507638. [DOI] [PubMed] [Google Scholar]
- 22.Larcher C, Jeller V, Fischer H, Huemer HP. Prevalence of respiratory viruses, including newly identified viruses, in hospitalized children in Austria . Eur J Clin Microbiol Infect Dis. 2006;25:681–86. doi: 10.1007/s10096-006-0214-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chan PKS, Tam JS, Lam CW, Chan E, Wu A, Li CK, Buckley TA. et al. Human metapneumovirus detection in patients with severe acute respiratory syndrome. Emerg Infect Dis. 2003;9:1058–63. doi: 10.3201/eid0909.030304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McNamara PS, Flanagan BF, Smyth RL, Hart CA. Impact of Human Metapneumovirus and Respiratory Syncytial Virus Co-Infection in Severe Bronchiolitis. Pediatr Pulmonol. 2007;42:740–43. doi: 10.1002/ppul.20649. [DOI] [PubMed] [Google Scholar]
- 25.Manoha C, Espinosa S, Aho SL, Huet F, Pothier P. Epidemiological and clinical features of hMPV, RSV and RVs infections in young children. J Clin Virol. 2007;38:221–26. doi: 10.1016/j.jcv.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 26.Tsai HP, Kuo PH, Liu CC, Wang JR. Respiratory viral infections among pediatric inpatients and outpatients in Taiwan from 1997 to 1999. J Clin Microbiol. 2001;39:111–18. doi: 10.1128/JCM.39.1.111-118.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Venegas AR, Bautista OP, Sansores RH. Clinical manifestations of influenza A (H1N1) Hot Topics Respir Med. 2010;13:15–21. [Google Scholar]
- 28.Tsai HN, Kuo P, Liu CC, Wang JR. Respiratory Viral Infections among Pediatric Inpatients and Outpatients in Taiwan from 1997 to 1999. J Clin Microbiol. 2001;39:111–18. doi: 10.1128/JCM.39.1.111-118.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cashat-Cruz M, Morales-Aguirre JJ, Mendoza-Azpiri M. Respiratory tract infections in children in developing countries. Semin Pediatr Infect Dis. 2005;16:84–92. doi: 10.1053/j.spid.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 30.Hall CB. Respiratory Syncytial Virus and ParainfluenzaVirus. N Engl J Med. 2001;344:1917–28. doi: 10.1056/NEJM200106213442507. [DOI] [PubMed] [Google Scholar]
- 31.McNamara PS, Smyth RL. The pathogenesis of respiratory syncytial virus disease in childhood. British Medical Bulletin. 2002;61:13–28. doi: 10.1093/bmb/61.1.13. [DOI] [PubMed] [Google Scholar]


